Abstract
The cell wall proteins of fungi are modified by N- and O-linked mannosylation and phosphomannosylation, resulting in changes to the physical and immunological properties of the cell. Glycosylation of cell wall proteins involves the activities of families of endoplasmic reticulum and Golgi-located glycosyl transferases whose activities are difficult to infer through bioinformatics. The Candida albicans MNT1/KRE2 mannosyl transferase family is represented by five members. We showed previously that Mnt1 and Mnt2 are involved in O-linked mannosylation and are required for virulence. Here, the role of C. albicans MNT3, MNT4, and MNT5 was determined by generating single and multiple MnTΔ null mutants and by functional complementation experiments in Saccharomyces cerevisiae. CaMnt3, CaMnt4, and CaMnt5 did not participate in O-linked mannosylation, but CaMnt3 and CaMnt5 had redundant activities in phosphomannosylation and were responsible for attachment of approximately half of the phosphomannan attached to N-linked mannans. CaMnt4 and CaMnt5 participated in N-mannan branching. Deletion of CaMNT3, CaMNT4, and CaMNT5 affected the growth rate and virulence of C. albicans, affected the recognition of the yeast by human monocytes and cytokine stimulation, and led to increased cell wall chitin content and exposure of β-glucan at the cell wall surface. Therefore, the MNT1/KRE2 gene family participates in three types of protein mannosylation in C. albicans, and these modifications play vital roles in fungal cell wall structure and cell surface recognition by the innate immune system.
Keywords: Cell Wall, Cell-Cell Interaction, Cytokine, Fungi, Glycoprotein Biosynthesis, Glycosylation, Innate Immunity, Pattern Recognition Receptor, Candida, Mannan
Introduction
The human pathogen Candida albicans is the most frequent cause of systemic candidosis, which is a common, life-threatening infection in immunocompromised patients (1). The C. albicans cell wall is a robust yet dynamic structure that protects the cell from changes in the extracellular environment. It is the immediate contact point with host cells and contains antigenic determinants, glycoproteins involved in the adhesion to host tissues, and most of the pathogen-associated molecular patterns that are recognized by host immune system (2). The wall is organized in an inner skeletal layer comprising chitin, β1,3- and β1,6-glucans, and an outer layer that is dominated by highly glycosylated proteins (3). These proteins are post-translationally modified with N- and/or O-linked mannans, both of which can be further elaborated with oligomannosides that are attached via phosphodiester linkages (phosphomannans). Mannans have important roles in cell wall integrity, adhesion to host cells and tissues, virulence, and the establishment of a response by immune cells (2, 4–10). The O- and N-linked mannans, along with β-glucans, represent the main C. albicans pathogen-associated molecular patterns recognized by the innate immune system (2, 11–13).
Mannan biosynthesis has been carefully characterized in Saccharomyces cerevisiae, and the main features of the pathways involved in the construction of these oligosaccharides are conserved in C. albicans. However, N- and O-linked mannans of C. albicans and S. cerevisiae have different characteristics that are relevant to immune recognition. For example, the N-linked mannan outer chain from C. albicans is usually of higher molecular weight, has a lower α1,3-mannose content, and contains β1,2-mannose residues in both the acid-labile (phosphomannan) and acid-stable fractions (14–16). Additionally, C. albicans O-linked mannans do not have α1,3-mannose caps and are homopolymers of five or more α1,2-mannose residues (8). The mannosyltransferases involved in glycosylation are usually encoded by gene families, and these have demonstrated a wide range of expansion and contraction in copy number between species (17). Because C. albicans is a significant human pathogen, the study of glycosylation pathways in this organism is important in understanding the biological properties conferred by different glycan moieties in relation to cell wall function, virulence, and immune sensing. Moreover, many fungal Golgi-located mannosyltransferases are not present in mammalian cells; hence, the study of these proteins might identify new antifungal drug targets and novel routes to immunotherapy.
The S. cerevisiae KRE2/MNT1 gene family is composed of nine members that encode Golgi Mn2+-dependent mannosyltransferases. With exception of Ktr6, they all catalyze the transfer of α-mannose residues from a GDP-Man donor to a mannoside acceptor (18). It has been demonstrated that Kre2/Mnt1, Ktr1, and Ktr3 are α1,2-mannosyltransferases involved in the addition of the second and third mannose residues to the O-linked mannan and in the proper elaboration of the N-linked mannan outer chain (19), whereas YUR1 and KTR2 encode mannosyltransferases only involved in N-linked mannosylation (20). In terms of enzyme activity, Ktr6/Mnn6 is the most diverse member of this family, because it is the main enzyme involved in the phosphomannosylation of both O- and N-liked mannans (21, 22). It is predicted that the rest of the family members Ktr4, Ktr5, and Ktr7 are mannosyltransferases, but no firm role has been assigned for these enzymes in protein glycosylation.
The homologous KRE2/MNT1 gene family in C. albicans has five members (23), of which only Mnt1 and Mnt2 have been characterized. They are α1,2-mannosyltransferases that have redundant activities in the addition of the second and third α1,2-mannose units to O-linked mannans (8, 23, 24). Phenotypic characterization of a mnt1-mnt2Δ double null mutant highlighted the importance of the correct O-linked mannan structure in cell wall assembly, adhesion to human buccal epithelial cells, and virulence in a mouse model (8). To establish the role of C. albicans MNT3, MNT4, and MNT5 in the glycobiology of this fungus, we characterized single and multiple mntΔ null mutants and performed functional complementation analyses of these genes in glycosylation mutants of S. cerevisiae. Our results indicate that none of these transferases are involved in the biosynthesis of O-linked mannan, but CaMnt4 and CaMnt5 have redundant functions in the elaboration of the N-mannan outer chain, and CaMNT3 and CaMNT5 encode enzymes with redundant phosphomannosyltransferase activity. Combinations of deletions of MNT3, MNT4, and MNT5 genes affected cell wall composition, cytokine stimulation from peripheral blood human monocytes (PBMCs),5 and virulence in a murine model of systemic candidosis. Therefore, all members of the C. albicans KRE2/MNT1 are important for interaction with the host, and this family encodes enzymes that participate in O-linked, N-linked, and phosphomannosylation.
EXPERIMENTAL PROCEDURES
Strains and Culture Conditions
All of the strains used in this work are listed in supplemental Table 1S. The strains were grown at 30 °C in YPD medium (1% (w/v) yeast extract, 2% (w/v) mycological peptone, 2% (w/v) glucose) or in SC medium (0.67% (w/v) yeast nitrogen base with ammonium sulfate without amino acids, 2% (w/v) glucose, 0.077% (w/v) complete supplement mixture minus uracil) supplemented with 50 μg/ml uridine as required. S. cerevisiae cells were grown in SC medium or in SC + GAL medium (0.67% (w/v) yeast nitrogen base with ammonium sulfate without amino acids, 0.077% (w/v) complete supplement mixture minus uracil) with added 2% (w/v) galactose and 3% (w/v) raffinose. Filamentation was induced growing the cells either at 37 °C in YPD medium with 20% (v/v) fetal calf serum or at 30 °C on solid Spider medium (25). For β-N-acetylhexosaminidase (HexNAcase) induction and metabolic labeling of glycans, the cells were grown at 30 °C in SC+GlcNAc medium (0.67% (w/v) yeast nitrogen base with ammonium sulfate without amino acids, 0.077% (w/v) complete supplement mixture minus uracil, 25 mm GlcNAc) and YP medium (1% (w/v) yeast extract, 2% (w/v) bacto peptone), respectively. The cells used for the cytokine stimulation were grown at 30 °C in Sabouraud broth (1% (w/v) mycological peptone, 4% (w/v) glucose), whereas for virulence assays NGY medium (0.1% (w/v) neopeptone, 0.4% (w/v) glucose, 0.1% (w/v) yeast extract) was used.
Construction of Null Mutants and Control Strains
Single null mutants were generated by the “Ura-blaster” protocol (26), whereas the “mini-Ura-blaster” method was used for the construction of multiple null mutants (27), as outlined in detail in the supplemental text. To construct reintegrant control strains, the open reading frame plus ∼980 bp of its promoter sequence and ∼650 bp of its terminator sequence was amplified and cloned into the NotI site of CIp10 as detailed in the supplemental text.
Complementation of S. cerevisiae ktr6Δ Null Mutant
The open reading frame of each member of the MNT1 gene family spanning from the first ATG codon to just before the stop codon was amplified by PCR and cloned into the pYES2.1/V5-His-TOPO® vector (Invitrogen). The constructions generated were used to transform S. cerevisiae XW44, a ktr6Δ null mutant (21). As a control, the cells were also transformed with the empty vector. C. albicans MNT gene expression was under the control of the GAL1 promoter.
Determination of Phosphomannosylation and N-Glycosylation Status
Phosphomannan content was assessed by cell affinity to Alcian Blue (AB) dye as previously described (9, 10), whereas HexNAcase electrophoretic mobility shift assays on native PAGE zymograms (9) were used to determine N-glycosylation status.
Analysis of O-Linked Mannans by Thin Layer Chromatography
Metabolic labeling of mannans, nonreductive β-elimination, and thin layer chromatography were performed as outlined in the supplemental text.
Analysis of the Cell Wall Carbohydrate Content
Cell wall β-glucan, mannan, and chitin content was determined by hydrolysis of the polymers and quantification of glucose, mannose, and glucosamine, respectively. Yeast cell walls were prepared and hydrolyzed in 2 m trifluoroacetic acid as described (10). The acid-hydrolysates were analyzed by high performance anion exchange chromatography with pulsed amperometric detection in a carbohydrate analyzer from Dionex (Surrey, UK) as described previously (28).
Calcofluor White Staining and Fluorescence Microscopy
Yeast cells growing exponentially in YPD medium were washed in phosphate-buffered saline, incubated with 25 μg/ml Calcofluor White for 30 min at room temperature, washed again in phosphate-buffered saline, and examined by phase differential interference contrast and fluorescence microscopy with a Zeiss Axioplan 2 microscope. The images were taken using the Openlab system (Openlab version 4.04; Improvision, Coventry, UK) and a Hamamatsu C4742-95 digital camera (Hamamatsu Photonics, Hamamatsu, Japan). Calcofluor White fluorescence was quantified as described (29).
Cytokine Stimulation in Human Monocytes
Isolation of PBMCs and cytokine stimulation assays were performed as outlined in detail in the supplemental text. The cytokines were quantified by enzyme-linked immunosorbent assay using commercial kits from R & D Systems (Abingdon, UK).
C. albicans cells were inactivated by incubation for 1 h at 56 °C. The blocking assays were conducted incubating PBMCs with 10 μg/ml glucan phosphate (glucan-P) for 60 min at 37 °C, before stimulation with C. albicans cells. The assays were conducted by duplicate with a total of six healthy donors. The Mann-Whitney U test was used to analyze the data, with a significance level of p < 0.05. The data are given as the means ± S.D.
Virulence Assays
The tests were conducted with six female, immunocompetent BALB/c mice/group (Harlan Sera-Lab Ltd, Loughborough, UK) as described (10).
RESULTS
Analysis and Disruption of CaMNT3, CaMNT4, and CaMNT5
The 1137-bp CaMNT3 open reading frame (GenBankTM/EBI accession Y13642), the 1443-bp CaMNT4 open reading frame (GenBankTM accession AJ277170), and the CaMNT5 1440-bp open reading frame (GenBankTM/EBI accession XM_707827) are predicted to be the orthologs of ScKTR1, ScKTR4, and ScKTR2, respectively. CaMnt3 and CaMnt4 have characteristic type II membrane protein domain structures with single transmembrane regions of 22 and 21 amino acids at their N termini, respectively, preceded by short cytosolic tails. CaMnt5 is predicted to be a multipass membrane protein, with an N-terminal tail of 29 amino acids into the lumen of the Golgi complex, a 21-amino acid transmembrane domain, and a cytoplasmic loop of 19 residues that is followed by a second transmembrane domain of 21 amino acids. CaMnt3 and CaMnt5 include the YNLCHFWSNFEI sequence found in most of the products encoded by the yeast KRE2/MNT1 family members, and CaMnt1, which has been proposed to contain the active site nucleophile (18). Like ScKtr4, CaMnt4 has a modified signature sequence (366YSTCHFWSNFEI377). They also contain the Glu, Asp, and His residues required for the nucleophilic reaction and Mn2+ binding capacity (24).
The CaMNT3, CaMNT4, and CaMNT5 genes were initially disrupted in strain CAI4 by sequential gene replacement using the Ura-blaster protocol (26). Functional redundancy was expected because of the significant degree of homology between these genes, hence double and triple null mutants were also constructed using the mini-Ura-blaster strategy (27). The triple Camnt3-Camnt4-Camnt5Δ null mutant was used as background in which a quintuple Camnt1-Camnt2-Camnt3-Camnt4-Camnt5Δ null mutant was generated by then disrupting CaMNT1-CaMNT2 genes in a further step. To avoid problems associated with ectopic expression of URA3, such as growth delay and virulence defects (30), the resulting null mutants were all transformed with plasmid CIp10 (31), reintroducing URA3 at the neutral RSP1 locus. Reintegrant control strains were generated by transformation of the null strains with CaMNT3, CaMNT4, or CaMNT5, again integrated at the RPS1 locus and under the control of their own promoters. In many of the phenotypes examined (see below) reintegration of CaMNT5 was best able to complement alterations observed in double and triple null mutant strains. For all of the experiments, strain CAI4 transformed with CIp10 was used as wild type control. The strains generated and used in this work are listed in supplemental Table 1S.
Multiple mnt Mutants Display Growth and Morphogenesis Defects
The Camnt3Δ, Camnt4Δ, and Camnt5Δ single null mutants and the Camnt3-Camnt4Δ, and Camnt4-Camnt5Δ double null strains had duplication times similar to wild type cells (1.42 ± 0.1 h) in YPD medium at 30 °C. The Camnt3-Camnt5Δ double null mutant displayed a slight defect in the doubling time (1.71 ± 0.3 h), which was restored upon complementation with either CaMNT3 or CaMNT5 (duplication times were 1.53 ± 0.2 and 1.55 ± 0.1 h, respectively). The Camnt3-Camnt4-Camnt5Δ triple null mutant had an increased duplication time of 2.34 ± 0.2 h. Reintegration of CaMNT3 or CaMNT4 partially restored the duplication time (1.84 ± 0.2 and 2.0 ± 0.1 h, respectively), whereas complementation with CaMNT5 restored it to wild type levels (1.45 ± 0.3 h). The growth defect of the Camnt1-Camnt2-Camnt3-Camnt4-Camnt5Δ quintuple null mutant was similar to that showed by the triple null mutant, with a duplication time of 2.28 ± 0.3 h. Only the triple and quintuple null mutants displayed defects in cellular morphologies, forming small aggregates, and some yeast cells were swollen. Complementation of the triple null strain with either CaMNT3 or CaMNT4 partially attenuated the clumpy phenotype, whereas cells complemented with CaMNT5 had normal cell morphology. The colony morphology of all the null mutants analyzed was similar to that displayed by wild type cells. The single and double null mutants had normal filamentation in 20% (v/v) serum and on solid Spider medium, whereas the Camnt3-Camnt4-Camnt5Δ triple null mutant had normal hypha formation in serum but delayed filamentation on solid Spider medium. In all cases the reintegrant strains showed similar phenotypes to the wild type controls. The phenotypes observed in multiple mnt, but not single, mnt mutants therefore suggest some redundancy of Mnt function.
CaMnt3 and CaMnt5 Are Involved in the Phosphomannosylation of C. albicans
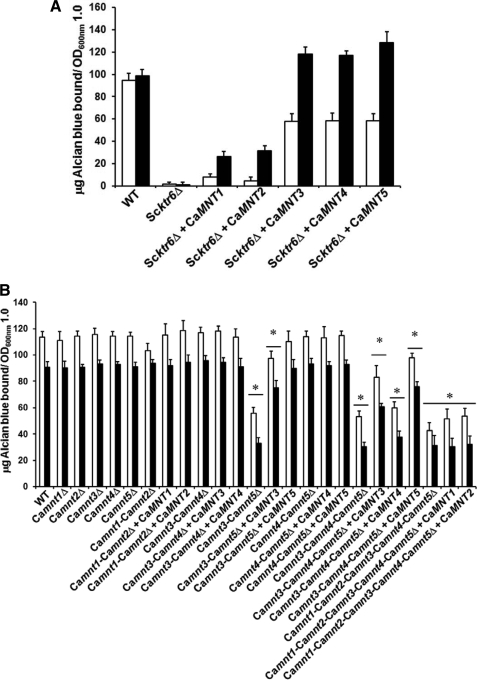
The five members of the C. albicans MNT1 gene family have over 55% similarity to ScKTR6, the S. cerevisiae mannosylphosphate transferase involved in the addition of phosphomannan to the N- and O-linked mannan (21, 22). Therefore, we tested whether members of the MNT1 gene family were involved in phosphomannan elaboration in C. albicans by functional complementation of a S. cerevisiae ktr6Δ null mutant. In these experiments the CaMNT genes were placed under the control of the galactose-inducible GAL1 promoter. Transformants were assessed for their ability to bind the cationic AB that binds to the phosphate group of phosphomannan. Complementation of Scktr6Δ with CaMNT1 and CaMNT2 only partially restored AB binding, whereas CaMNT3, CaMNT4, and CaMNT5 fully complemented the ktr6Δ null mutant (Fig. 1A). Cells growing in noninductive conditions also bound more AB than the null mutant cells, and reverse transcription-PCR assays confirmed that some transcription of CaMNT genes did occur in cells growing in presence of glucose, a negative regulator of the GAL1 promoter.
FIGURE 1.
Members of C. albicansMNT1 participate in phosphomannosylation. A, C. albicans MNT1 family members restore the ability of S. cerevisiae ktr6Δ null mutant to bind AB. S. cerevisiae BY4741 (wild type, WT), NGS1 (Scktr6Δ), NGS2 (Scktr6Δ + CaMNT1), NGS3 (Scktr6Δ + CaMNT2), NGS4, (Scktr6Δ + CaMNT3), NGS5 (Scktr6Δ + CaMNT4), and NGS6 (Scktr6Δ + CaMNT5) were grown in either SC medium (open bars) or SC medium added with 2% galactose and 3% raffinose (closed bars), and the ability of the cells to bind the AB was determined. The results obtained with all of the mutant strains are significantly different of those generated with the wild type strain (p < 0.01). B, C. albicans null mutants lacking of both CaMNT3 and CaMNT5 have a reduced ability to bind AB. C. albicans NGY152 (wild type), NGY158 (Camnt1Δ), NGY145 (Camnt2Δ), NGY146 (Camnt3Δ), NGY313 (Camnt4Δ), NGY147 (Camnt5Δ), NGY337 (Camnt1-Camnt2Δ), NGY335 (Camnt1-Camnt2Δ + CaMNT1), NGY336 (Camnt1-Camnt2Δ + CaMNT2), NGY509 (Camnt3-Camnt4Δ), NGY510 (Camnt3-Camnt4Δ + CaMNT3), NGY511 (Camnt3-Camnt4Δ + CaMNT4), NGY1227 (Camnt3-Camnt5Δ), NGY1228 (Camnt3-Camnt5Δ + CaMNT3), NGY1229 (Camnt3-Camnt5Δ + CaMNT5), NGY516 (Camnt4-Camnt5Δ), NGY517 (Camnt4-Camnt5Δ + CaMNT4), NGY518 (Camnt4-Camnt5Δ + CaMNT5), NGY527 (Camnt3-Camnt4-Camnt5Δ), NGY528 (Camnt3-Camnt4-Camnt5Δ + CaMNT3), NGY529 (Camnt3-Camnt4-Camnt5Δ + CaMNT4), NGY530 (Camnt3-Camnt4-Camnt5Δ + CaMNT5) NGY535 (Camnt1-Camnt2-Camnt3-Camnt4-Camnt5Δ), NGY536 (Camnt1-Camnt2-Camnt3-Camnt4-Camnt5Δ + CaMNT1), and NGY537 (Camnt1-Camnt2-Camnt3-Camnt4-Camnt5Δ + CaMNT2) were grown in YPD medium, and the ability to bind AB was measured before (open bars) or after β-elimination of O-linked mannans with 0.1 m NaOH (closed bars). The data represent the means ± S.D. of three independent assays performed in triplicate. *, p < 0.05.
Single mnt null mutants and the Camnt3-Camnt4Δ and Camnt4-Camnt5Δ double null mutants were not affected in AB binding; however, the Camnt3-Camnt5Δ double null mutant and the triple mutant lacking CaMNT3, CaMNT4, and CaMNT5 had a 50% reduction in AB binding (Fig. 1B). The Camnt3-Camnt5Δ double null mutant was partially and fully complemented by reintegration of CaMNT3 and CaMNT5, respectively, and CaMNT3 and CaMNT5, but not CaMNT4, partially restored AB binding in the Camnt3-Camnt4-Camnt5Δ triple null mutant (Fig. 1B). The quintuple Camnt1-Camnt2-Camnt3-Camnt4-Camnt5Δ null mutant displayed a slight reduction in the ability to bind AB, compared with the triple Camnt3-Camnt4-Camnt5Δ null mutant, and reintegration of CaMNT1 and CaMNT2 genes restored AB binding to that of the triple null mutant (Fig. 1B). When the AB binding assay was conducted using β-eliminated cells to remove O-linked glycans, most of the strains bound less AB (∼22 μg less AB/A600 nm bound; Fig. 1B), indicating the presence of a minor phosphomannan fraction attached to O-linked mannan. Binding of AB in mutants already depleted in O-mannan such as the Camnt1-Camnt2Δ and the quintuple null mutant was much less affected by β-elimination (Fig. 1B). Therefore, CaMNT3 and CaMNT5 are involved in phosphomannosylation of C. albicans N-linked mannan but not of O-mannan.
CaMnt4 and CaMnt5 Participate in N-Linked Glycosylation Pathway
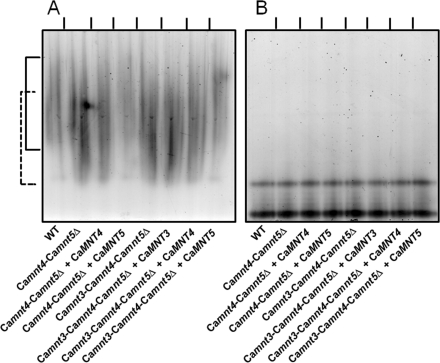
The effect of disruption of CaMNT3, CaMNT4, and CaMNT5 on the N-linked mannan structure was determined using electrophoretic mobility shift assays of secreted HexNAcase as a molecular marker (9, 10). The HexNAcase from single null mutants and Camnt3-Camnt4Δ and Camnt3-Camnt5Δ null mutants had an electrophoretic mobility similar to that in wild type cells (supplemental Fig. S1). The double Camnt4-Camnt5Δ and triple Camnt3-Camnt4-Camnt5Δ null mutants generated protein with increased electrophoretic mobility (Fig. 2A). CaMNT5 was the only gene able to partially restore the phenotype of both the double Camnt4-Camnt5Δ and the triple Camnt3-Camnt4-Camnt5Δ null mutant (Fig. 2A). Deglycosylated HexNAcase from all of the strains showed the same electrophoretic mobility (Fig. 2B). These results suggest that CaMNT4 and CaMNT5 are required for the N-linked mannosylation, and the role of CaMnt5 is epistatic over CaMnt4.
FIGURE 2.
Electrophoretic mobility shift assays of HexNAcase. The HexNAcase activity was induced as described under “Experimental Procedures,” and protein samples before (A) or after (B) deglycosylation with endoglycosidase H were separated by nondenaturing electrophoresis, and enzyme activity was revealed with naphthyl-GlcNAc and Fast Blue. The strains used were C. albicans NGY152 (wild type, WT), NGY516 (Camnt4-Camnt5Δ), NGY517 (Camnt4-Camnt5Δ + CaMNT4), NGY518 (Camnt4-Camnt5Δ + CaMNT5), NGY527 (Camnt3-Camnt4-Camnt5Δ), NGY528 (Camnt3-Camnt4-Camnt5Δ + CaMNT3), NGY529 (Camnt3-Camnt4-Camnt5Δ + CaMNT4), and NGY530 (Camnt3-Camnt4-Camnt5Δ + CaMNT5). The continuous and dashed lines indicate the electrophoretic mobility of normal and underglycosylated HexNAcase, respectively.
O-Linked mannans were labeled with d-[2-3H]mannose, β-eliminated and separated by thin layer chromatography. No significant differences were found in the size of the Ο-linked mannans from the single, double, and triple null mutants, which, as described previously (8) contained up to five mannose residues (supplemental Fig. 2S). Therefore, CaMNT3, CaMNT4, and CaMNT5 are not involved in the C. albicans O-linked mannosylation but contribute to N-linked mannosylation and phosphomannosylation.
Modifications in the Cell Wall Composition
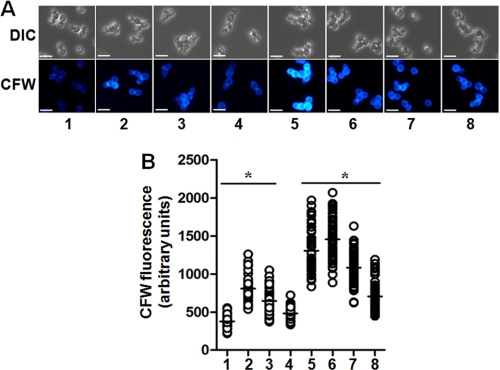
To assess the effect of the disruption of CaMNT3, CaMNT4, and/or CaMNT5 on gross cell wall composition, the content of glucose, mannose, and N-acetylglucosamine was analyzed by high performance anion exchange chromatography. The three single null mutants and the Camnt3-Camnt4Δ double null mutant had a normal cell wall carbohydrate content, but the Camnt3-Camnt5Δ double null mutant had an increased chitin content. Once again only reintegration of CaMNT5 fully complemented this phenotype (Table 1). The cell wall of Camnt4-Camnt5Δ double null mutant had reduced mannan content but increased glucan content. Complementation with CaMNT4 partially restored the phenotype, whereas integration of CaMNT5 again fully complemented the double null mutant (Table 1). The Camnt3-Camnt4-Camnt5Δ triple null mutant displayed a combined phenotype of these two double null mutants, with increased cell wall glucan and chitin and less mannan. Reintegration of CaMNT3 did not affect the phenotype of the triple null mutant, but complementation with CaMNT4 and CaMNT5 partially restored the carbohydrate content to wild type levels (Table 1). The increase in the chitin content at the cell wall of Camnt3-Camnt5Δ and Camnt3-Camnt4-Camnt5Δ null mutants was confirmed by their increased ability to bind Calcofluor White (Fig. 3).
TABLE 1.
Cell wall composition of C. albicans yeast cells lacking MNT3, MNT4, or MNT5 and reintegrant strains
The values are measured in μg/mg of cell wall dry weight ± S.D. (n = 4).
| Strain | Glucose | Mannose | Glucosamine |
|---|---|---|---|
| Wild type | 554.7 ± 28.7 | 281.0 ± 29.0 | 14.3 ± 3.8 |
| Camnt3Δ | 570.3 ± 22.4 | 277.4 ± 16.9 | 12.4 ± 7.2 |
| Camnt4Δ | 557.6 ± 19.5 | 261.7 ± 14.7 | 10.7 ± 5.8 |
| Camnt5Δ | 555.2 ± 16.8 | 266.4 ± 20.3 | 8.4 ± 3.8 |
| Camnt3-Camnt4Δ | 549.2 ± 9.1 | 259.3 ± 13.0 | 11.4 ± 8.2 |
| Camnt3-Camnt5Δ | 559.2 ± 13.4 | 248.6 ± 32.0 | 52.2 ± 22.9a |
| Camnt3-Camnt5Δ + CaMNT3 | 550.2 ± 10.2 | 256.5 ± 11.1 | 38.3 ± 8.1a |
| Camnt3-Camnt5Δ + CaMNT5 | 560.2 ± 26.9 | 260.4 ± 27.5 | 16.4 ± 2.1 |
| Camnt4-Camnt5Δ | 639.4 ± 20.2a | 185.2 ± 20.0a | 34.4 ± 19.3 |
| Camnt4-Camnt5Δ + CaMNT4 | 625.4 ± 52.3 | 174.5 ± 40.1a | 40.1 ± 23.3 |
| Camnt4-Camnt5Δ + CaMNT5 | 568.8 ± 18.5 | 252.4 ± 16.7 | 17.8 ± 1.7 |
| Camnt3-Camnt4-Camnt5Δ | 640.8 ± 16.4a | 159.7 ± 10.8a | 62.4 ± 8.7a |
| Camnt3-Camnt4-Camnt5Δ + CaMNT3 | 654.5 ± 34.2a | 166.3 ± 29.8a | 59.3 ± 8.0a |
| Camnt3-Camnt4-Camnt5Δ + CaMNT4 | 611.1 ± 40.5 | 186.8 ± 40.5a | 52.1 ± 13.5a |
| Camnt3-Camnt4-Camnt5Δ + CaMNT5 | 590.5 ± 56.5 | 211.6 ± 58.7 | 41.9 ± 3.5a |
a The p value was <0.05.
FIGURE 3.
Calcofluor White binding assays. In A, C. albicans cells were stained with Calcofluor White (CFW) and examined under differential interference contrast (DIC) or fluorescence microscopy. Scale bar, 10 μm. In B, the fluorescence associated with cells was quantified using the Openlab system. The data represent the means of three independent assays performed by duplicate, analyzing 50 cells/strain/experiment. *, p < 0.01. The strains were: column 1, NGY152 (wild type, WT); column 2, NGY1227 (Camnt3-Camnt5Δ); column 3, NGY1228 (Camnt3-Camnt5Δ + CaMNT3); column 4, NGY1229 (Camnt3-Camnt5Δ + CaMNT5); column 5, NGY527 (Camnt3-Camnt4-Camnt5Δ); column 6, NGY528 (Camnt3-Camnt4-Camnt5Δ + CaMNT3); column 7, NGY529 (Camnt3-Camnt4-Camnt5Δ + CaMNT4); and column 8, NGY530 (Camnt3-Camnt4-Camnt5Δ + CaMNT5).
Corresponding to these cell wall changes, plate assays of Camnt3-Camnt5Δ, Camnt4-Camnt5Δ, and Camnt3-Camnt4-Camnt5Δ null mutants showed that these strains had increased susceptibility to Calcofluor White, SDS, and tunicamycin, which compromise the integrity of the cell wall. In the double null mutants, reintegration of CaMNT5 again fully complemented the strains, whereas CaMNT3 and CaMNT4 only partially complemented the mutant phenotype. The triple null mutant was partially complemented with all of these MNT genes. There were no significant differences in the sensitivity of any of the mutants to Congo Red, hygromycin B, caffeine, NaCl, or KCl. These results indicate that CaMnt3, CaMnt4, and CaMnt5 activity are required for normal robustness of the cell wall and that some but not all Mnt mutations result in the activation of the cell wall salvage pathway that leads to increased chitin synthesis.
Combined Disruption of CaMNT3, CaMNT4, and CaMNT5 Affects the C. albicans-Host Interaction
Because mutants with increased duplication times or with defects in N- or O-mannosylation are known to be attenuated in virulence for mice (7–10, 32–36), we only tested the consequence of the Camnt3-Camnt5Δ double null mutant for virulence. This double null mutant was highly attenuated in virulence in a mouse model of systemic candidosis, with a mean mouse survival time of 21 days, compared with 10 days for the wild type strain. Control strains harboring single reintegrations of either CaMNT3 or CaMNT5 were not sufficient to complement the virulence defect, indicating that full virulence requires both Mnt3 and Mnt5.
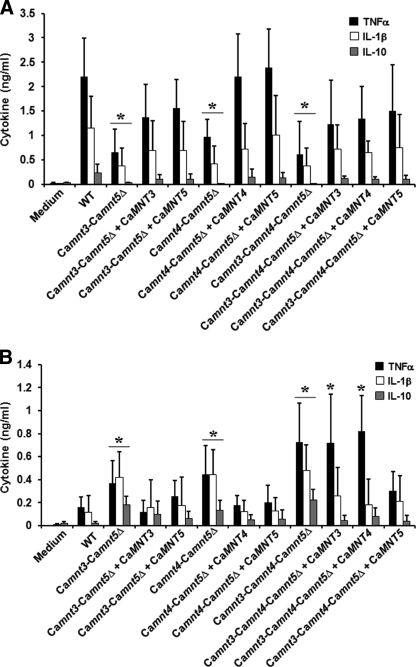
Next, we tested the ability of the null mutants to induce cytokine production by human PBMCs. We used both live and heat-killed C. albicans cells to assess dectin-1-dependent and -independent induction of cytokines (13). Single null mutants and the Camnt3-Camnt4Δ double null mutant were equally as potent in inducing cytokine production as the wild type C. albicans cells. Heat-killed Camnt3-Camnt5Δ, Camnt4-Camnt5Δ, and Camnt3-Camnt4-Camnt5Δ null mutants induced significantly less tumor necrosis factor α and interleukin 1β, 6, and 10 production (Fig. 4A and supplemental Fig. 3S). Normal cytokine production was restored in the respective reintegrant controls.
FIGURE 4.
Cytokine stimulation by C. albicansMNT1 null mutants. PBMCs were coincubated with either heat-killed (A) or live (B) yeast cells in a ratio monocyte: yeast 5: 1 for 24 h, and the cytokine concentrations were determined. The strains were: NGY152 (wild type, WT), NGY1227 (Camnt3-Camnt5Δ), NGY1228 (Camnt3-Camnt5Δ + CaMNT3), NGY1229 (Camnt3-Camnt5Δ + CaMNT5), NGY516 (Camnt4-Camnt5Δ), NGY517 (Camnt4-Camnt5Δ + CaMNT4), NGY518 (Camnt4-Camnt5Δ + CaMNT5), NGY527 (Camnt3-Camnt4-Camnt5Δ), NGY528 (Camnt3-Camnt4-Camnt5Δ + CaMNT3), NGY529 (Camnt3-Camnt4-Camnt5Δ + CaMNT4), and NGY530 (Camnt3-Camnt4-Camnt5Δ + CaMNT5). The data represent the means ± S.D. *, p < 0.05.
In contrast, live yeast cells of Camnt3-Camnt5Δ, Camnt4-Camnt5Δ, and Camnt3-Camnt4-Camnt5Δ null mutants induced significantly more cytokines than wild type cells (Fig. 4B and supplemental Fig. 3S). Reintegrant controls for the double null mutants stimulated lower cytokine levels, similar to those observed with wild type yeast cells, whereas CaMNT5 was again the only MNT gene able to complement the triple null mutant by restoring the cytokine stimulation to wild type levels (Fig. 4B and supplemental Fig. 3S).
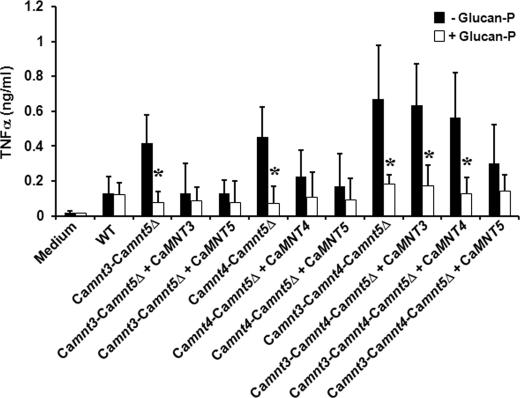
To determine whether exposure of β-glucan at the cell surface accounted for the increased cytokine stimulation by live mnt null mutants, we preincubated human PBMCs with glucan-P, a dectin-1-blocking compound (Fig. 5). Blocking of PBMCs with glucan-P, then exposing these to live yeast cells of Camnt3-Camnt5Δ, Camnt4-Camnt5Δ, and Camnt3-Camnt4-Camnt5Δ, resulted in very low tumor necrosis factor α production, similar to that stimulated by wild type cells. Therefore, cell wall reorganization in these mutants led to exposure of β-glucan at the cell wall surface, thereby increasing immune recognition via dectin-1.
FIGURE 5.
Cytokine stimulation upon blocking of dectin-1 with glucan-P. The strains and conditions are as in Fig. 4, but PBMCs were incubated with 10 μg/ml glucan-P for 60 min at 37 °C prior to stimulation with C. albicans cells. The data represent the means ± S.D. *, p < 0.05. WT, wild type.
To determine whether the different levels of cell wall phosphomannan, and therefore of acid-labile β1,2-mannose, may contribute to the differential cytokine stimulation by live and heat-killed cells, we compared cytokine stimulation with strains deficient in cell wall β1,2-mannose. A C. albicans mnn4Δ mutant, which lacks phosphomannan and β1,2-mannose units in the acid-labile N-mannan fraction (37), and a C. albicans serotype B strain, which lacks β1,2-mannose residues in the acid-stable N-mannan fraction (14), along with C. albicans mnn4Δ serotype B, which lacks all β-1,2-mannose and phosphomannan (38), induced cytokine production in amounts similar to that in wild type control cells (supplemental Fig. 4S). This indicates that phosphomannan and β1,2-mannose do not have a major role in immune recognition of C. albicans yeast cells by human PBMCs.
DISCUSSION
In this study, we describe the role of CaMNT3, CaMNT4, and CaMNT5 in cell wall glycosylation of C. albicans and the importance of the encoded mannosyltransferases in the composition and organization of the cell wall and of interactions of the fungus with the host innate immune system. Because of the significant homology of the CaMNT3–5 genes to ScKTR6, we assessed the possible role of the MNT1 gene family members in phosphomannan elaboration. Expression of CaMNT3, CaMNT4, or CaMNT5 in S. cerevisiae ktr6Δ null mutant fully restored the cell ability to bind AB, whereas CaMNT1 and CaMNT2 only partially restored phosphomannan synthesis. Phenotypic characterization of the C. albicans null mutants indicated that only CaMNT3 and CaMNT5 participate in the elaboration of phosphomannan in vivo. It has been suggested that Ktr6 is the main phosphomannosyltransferase in S. cerevisiae but that this may not be the only protein involved in elaborating the phosphomannan (39, 40). Therefore, it is possible that overexpression of mannosyltransferases such CaMnt1, CaMnt2, or CaMnt4 may affect the mannan structure by altering the normal glycan structure so it becomes a substrate for phosphomannosyltransferases within the cells. The mobility of HexNAcase was unaltered in the Camnt3-Camnt5Δ double null mutant. We infer from this that N-mannan in this mutant was of a similar molecular mass but deficient in AB binding. This reinforced the suggestion that these transferases are normally involved in phosphomannosylation rather than N-linked mannan elaboration per se. Furthermore, because the Camnt3Δ and Camnt5Δ single null mutants were not affected in AB binding, CaMnt3 and CaMnt5 are likely to have partially redundant functions in phosphomannosylation.
We found evidence for defects in N-linked mannosylation in all of the mutants that harbored disruptions in both Camnt4Δ and Camnt5Δ. This was evident in the underglycosylation of HexNAcase, which is exclusively N-linked mannosylated and has been previously utilized as a molecular marker of N-linked mannosylation defects (9, 10). Again, single null mutants did not show such alterations, indicating that CaMnt4 and CaMnt5 may have redundant functions in N-linked mannan synthesis. The electrophoretic mobility of the HexNAcase obtained from the Camnt4-Camnt5Δ double null mutant was increased, indicating altered N-mannan, but this strain was not defective in cell wall phosphomannan. However, the N-mannosylation defect was not as severe as in other glycosylation mutants where the elongation of the N-linked α1,6-mannan backbone is blocked (7, 9, 10). Hence, it is possible that the N-linked mannan in this double mutant strain had defects in either the number or length of outer chain branches in N-mannan. Because phosphomannan is normally attached to the second or third α1,2-mannose residue of side branches from the α1,6-mannan backbone (21), it seems more likely that the defect in this mutant lies in the length of the N-mannan side chains that are formed.
Only the triple Camnt3-Camnt4-Camnt5Δ null mutant had a clumpy phenotype, indicating that the lack of proper phosphomannosylation and N-linked mannosylation may affect hydrophobicity and/or cell wall composition, resulting in flocculation. Cell aggregates have also been observed upon disruption of other glycosylation genes (7–10, 41, 42).
Previous studies have established a relationship between normal glycosylation and normal cell wall structure, adherence and virulence (7–10, 23, 32–35, 37, 42–44). The Camnt3-Camnt4-Camnt5Δ null mutant had significantly altered cell wall composition and increased sensitivity to various cell wall perturbing agents. The cell wall composition profile of the Camnt4-Camnt5Δ mutant is not unusual for glycosylation mutants with strong defects in mannosylation, where the lack of mannan is compensated by increased synthesis of chitin and β-glucan resulting from the activation of the cell wall integrity pathway (7, 9, 10, 45, 46). However, the Camnt3-Camnt5Δ double null mutant did not have an altered cell wall mannan or β-glucan content but only increased chitin. This suggests that mild glycosylation defects may be overcome by salvage mechanisms that differ from those activated in mutants with severe glycosylation defects. The altered virulence phenotype of Camnt3-Camnt5Δ double null mutant was similar to some other glycosylation mutants (9, 10). It is unlikely that this phenotype is solely attributable to the partial loss of phosphomannan, because C. albicans mnn4Δ null mutants lacking all phosphomannan are not attenuated in virulence (37). We demonstrated that these cells have a weakened cell wall, and this may directly affect the cell fitness and therefore the ability to colonize and invade host tissues. This is supported by the slight increase in the duplication times of this mutant.
Recognition of the cell wall of C. albicans by the host innate immune system is an essential part of the establishment of an effective immune response against this pathogen. This interaction is governed by the pattern recognition receptors on the immune cell surface, which recognize pathogen-associated molecular patterns and trigger the production of pro- and anti-inflammatory cytokines (2). It has been demonstrated that C. albicans glycosylation mutants induce low cytokine production by human PBMCs (10, 12). Here, a significant reduction was recorded in the cytokine production by PBMCs challenged with heat-killed yeast cells of the Camnt3-Camnt5Δ, Camnt4-Camnt5Δ, and Camnt3-Camnt4-Camnt5Δ null mutants. This underlines the importance of mannosylation for the recognition of C. albicans by these immune cells. The Camnt3-Camnt5Δ double null mutant had half the phosphomannan content, and the increased cell wall chitin and heat-killed cells of this strain induced a significantly reduced cytokine response. Evidence presented here and elsewhere (12) indicates that it is unlikely that the phosphomannan changes alone can account for this observation. It is possible therefore that the increased chitin content of the walls of these mutants may block the proper recognition of C. albicans by human PBMCs or that other changes in the cell wall mannan account for the lack of cytokine stimulation in this strain. Additionally, the cell wall compensatory mechanisms are likely to include changes in the cell wall protein content, which might contribute to the poor recognition of these mutants by human PBMCs.
As reported previously, heat-killed cells have β-glucan exposed at the cell surface, and this polymer is then more accessible for recognition through dectin-1 (13). Thus heat-killed cells often induce more cytokine production than live cells (13). Interestingly, in contrast to the lower cytokine production induced by heat-killed strains, live Camnt3-Camnt5Δ, Camnt4-Camnt5Δ, and Camnt3-Camnt4-Camnt5Δ null mutants induced more cytokine production than wild type cells. This apparent discrepancy can be explained by the differential exposure of different pathogen-associated molecular patterns by heat-killed and live C. albicans. β-Glucans are exposed in both wild type and mutant heat-killed strains of C. albicans, and as a consequence, heat-killed wild type strains induce cytokines through the synergistic effects of mannans and β-glucans. This synergism may be partially lost in the null mutant strains because of the modified mannan structures, and thus heat-killed null mutant strains induce lower cytokine release from PBMCs. A different situation is found for live C albicans. Wild type live yeast cells expose almost only mannans to immune cells, and little synergism between mannans and β-glucans occurs. In comparison, live glycosylation mutant strains are likely to have both β-glucans and mannans exposed at the surface, and the partial synergism that results would be sufficient to induce a higher cytokine production compared with wild type C. albicans strains. These responses were not phosphomannan- or β1,2-mannose-dependent, an observation that is supported by previous observations demonstrating that phosphomannan-deficient C. albicans mnn4Δ cells induce normal cytokine responses in human PBMCs and dendritic cells (12, 16). The increased cytokine response induced by live null mutant cells was shown to be dectin-1-dependent, suggesting that the cell wall of these null mutants was reorganized, and β-glucan was likely to be more exposed at the cell surface.
In conclusion, MNT3 and MNT5 have redundant roles in phosphomannosylation, and MNT4 and MNT5 participate in N-mannan assembly. Despite their sequence homology to the Mnt1 and Mnt2 α-1,2 mannosyl transferases that are involved in O-linked mannan synthesis, Mnt3, Mnt4, and Mnt5 apparently do not contribute to O-linked glycosylation. The MNT1 family of glycosyl transferases as a whole is therefore involved in three types of mannosylation at the cell surface of C. albicans: O-linked, N-linked, and phosphodiester-linked. We also demonstrate that defects in specific mannosylation pathways induce different alterations in the composition, architecture, and integrity of the cell wall and result in the attenuation of virulence driven in part by alterations to the normal pattern of recognition by cells of the innate immune system.
Supplementary Material
Acknowledgments
We thank Rhian Whitton and Lynne Thomson for construction of strains.
This work was supported by Wellcome Trust Programme Grant 080088 (to N. A. R. G., A. J. P. B., and F. C. O.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental text, Table 1S, and Figs. 1S–4S.
- PBMCs
- peripheral blood human monocytes
- HexNAcase
- β-N-acetylhexosaminidase
- AB
- Alcian Blue
- glucan-P
- glucan phosphate.
REFERENCES
- 1.Pappas P. G., Rex J. H., Lee J., Hamill R. J., Larsen R. A., Powderly W., Kauffman C. A., Hyslop N., Mangino J. E., Chapman S., Horowitz H. W., Edwards J. E., Dismukes W. E. (2003) Clin. Infect. Dis. 37, 634–643 [DOI] [PubMed] [Google Scholar]
- 2.Netea M. G., Brown G. D., Kullberg B. J., Gow N. A. (2008) Nat. Rev. Microbiol. 6, 67–78 [DOI] [PubMed] [Google Scholar]
- 3.Klis F. M., de Groot P., Hellingwerf K. (2001) Med. Mycol. 39, (Suppl. 1) 1–8 [PubMed] [Google Scholar]
- 4.Calderone R. A. (1993) Trends Microbiol. 1, 55–58 [DOI] [PubMed] [Google Scholar]
- 5.Wang Y., Li S. P., Moser S. A., Bost K. L., Domer J. E. (1998) Infect. Immun. 66, 1384–1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sundstrom P. (2002) Cell Microbiol. 4, 461–469 [DOI] [PubMed] [Google Scholar]
- 7.Bates S., MacCallum D. M., Bertram G., Munro C. A., Hughes H. B., Buurman E. T., Brown A. J., Odds F. C., Gow N. A. (2005) J. Biol. Chem. 280, 23408–23415 [DOI] [PubMed] [Google Scholar]
- 8.Munro C. A., Bates S., Buurman E. T., Hughes H. B., Maccallum D. M., Bertram G., Atrih A., Ferguson M. A., Bain J. M., Brand A., Hamilton S., Westwater C., Thomson L. M., Brown A. J., Odds F. C., Gow N. A. (2005) J. Biol. Chem. 280, 1051–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bates S., Hughes H. B., Munro C. A., Thomas W. P., MacCallum D. M., Bertram G., Atrih A., Ferguson M. A., Brown A. J., Odds F. C., Gow N. A. (2006) J. Biol. Chem. 281, 90–98 [DOI] [PubMed] [Google Scholar]
- 10.Mora-Montes H. M., Bates S., Netea M. G., Díaz-Jiménez D. F., López-Romero E., Zinker S., Ponce-Noyola P., Kullberg B. J., Brown A. J., Odds F. C., Flores-Carreón A., Gow N. A. (2007) Eukaryot. Cell 6, 2184–2193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown G. D., Gordon S. (2001) Nature 413, 36–37 [DOI] [PubMed] [Google Scholar]
- 12.Netea M. G., Gow N. A., Munro C. A., Bates S., Collins C., Ferwerda G., Hobson R. P., Bertram G., Hughes H. B., Jansen T., Jacobs L., Buurman E. T., Gijzen K., Williams D. L., Torensma R., McKinnon A., MacCallum D. M., Odds F. C., Van der Meer J. W., Brown A. J., Kullberg B. J. (2006) J. Clin. Invest. 116, 1642–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gow N. A., Netea M. G., Munro C. A., Ferwerda G., Bates S., Mora-Montes H. M., Walker L., Jansen T., Jacobs L., Tsoni V., Brown G. D., Odds F. C., Van der Meer J. W., Brown A. J., Kullberg B. J. (2007) J. Infect. Dis. 196, 1565–1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shibata N., Arai M., Haga E., Kikuchi T., Najima M., Satoh T., Kobayashi H., Suzuki S. (1992) Infect. Immun. 60, 4100–4110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trinel P. A., Lepage G., Jouault T., Strecker G., Poulain D. (1997) FEBS Lett. 416, 203–206 [DOI] [PubMed] [Google Scholar]
- 16.Cambi A., Netea M. G., Mora-Montes H. M., Gow N. A., Hato S. V., Lowman D. W., Kullberg B. J., Torensma R., Williams D. L., Figdor C. G. (2008) J. Biol. Chem. 283, 20590–20599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Butler G., Rasmussen M. D., Lin M. F., Santos M. A., Sakthikumar S., Munro C. A., Rheinbay E., Grabherr M., Forche A., Reedy J. L., Agrafioti I., Arnaud M. B., Bates S., Brown A. J., Brunke S., Costanzo M. C., Fitzpatrick D. A., de Groot P. W., Harris D., Hoyer L. L., Hube B., Klis F. M., Kodira C., Lennard N., Logue M. E., Martin R., Neiman A. M., Nikolaou E., Quail M. A., Quinn J., Santos M. C., Schmitzberger F. F., Sherlock G., Shah P., Silverstein K. A., Skrzypek M. S., Soll D., Staggs R., Stansfield I., Stumpf M. P., Sudbery P. E., Srikantha T., Zeng Q., Berman J., Berriman M., Heitman J., Gow N. A., Lorenz M. C., Birren B. W., Kellis M., Cuomo C. A. (2009) Nature 459, 657–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lussier M., Sdicu A. M., Bussey H. (1999) Biochim. Biophys. Acta 1426, 323–334 [DOI] [PubMed] [Google Scholar]
- 19.Lussier M., Sdicu A. M., Bussereau F., Jacquet M., Bussey H. (1997) J. Biol. Chem. 272, 15527–15531 [DOI] [PubMed] [Google Scholar]
- 20.Lussier M., Sdicu A. M., Camirand A., Bussey H. (1996) J. Biol. Chem. 271, 11001–11008 [DOI] [PubMed] [Google Scholar]
- 21.Wang X. H., Nakayama K., Shimma Y., Tanaka A., Jigami Y. (1997) J. Biol. Chem. 272, 18117–18124 [DOI] [PubMed] [Google Scholar]
- 22.Nakayama K., Feng Y., Tanaka A., Jigami Y. (1998) Biochim. Biophys. Acta 1425, 255–262 [DOI] [PubMed] [Google Scholar]
- 23.Buurman E. T., Westwater C., Hube B., Brown A. J., Odds F. C., Gow N. A. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 7670–7675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomson L. M., Bates S., Yamazaki S., Arisawa M., Aoki Y., Gow N. A. (2000) J. Biol. Chem. 275, 18933–18938 [DOI] [PubMed] [Google Scholar]
- 25.Liu H., Köhler J., Fink G. R. (1994) Science 266, 1723–1726 [DOI] [PubMed] [Google Scholar]
- 26.Fonzi W. A., Irwin M. Y. (1993) Genetics 134, 717–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson R. B., Davis D., Enloe B. M., Mitchell A. P. (2000) Yeast 16, 65–70 [DOI] [PubMed] [Google Scholar]
- 28.Plaine A., Walker L., Da Costa G., Mora-Montes H. M., McKinnon A., Gow N. A., Gaillardin C., Munro C. A., Richard M. L. (2008) Fungal Genet. Biol. 45, 1404–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walker L. A., Munro C. A., de Bruijn I., Lenardon M. D., McKinnon A., Gow N. A. (2008) PLoS Pathog. 4, e1000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brand A., MacCallum D. M., Brown A. J., Gow N. A., Odds F. C. (2004) Eukaryot. Cell 3, 900–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murad A. M., Lee P. R., Broadbent I. D., Barelle C. J., Brown A. J. (2000) Yeast 16, 325–327 [DOI] [PubMed] [Google Scholar]
- 32.Timpel C., Strahl-Bolsinger S., Ziegelbauer K., Ernst J. F. (1998) J. Biol. Chem. 273, 20837–20846 [DOI] [PubMed] [Google Scholar]
- 33.Timpel C., Zink S., Strahl-Bolsinger S., Schröppel K., Ernst J. (2000) J. Bacteriol. 182, 3063–3071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prill S. K., Klinkert B., Timpel C., Gale C. A., Schröppel K., Ernst J. F. (2005) Mol. Microbiol. 55, 546–560 [DOI] [PubMed] [Google Scholar]
- 35.Bai C., Xu X. L., Chan F. Y., Lee R. T., Wang Y. (2006) Eukaryot. Cell 5, 238–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rieg G., Fu Y., Ibrahim A. S., Zhou X., Filler S. G., Edwards J. E., Jr. (1999) Infect. Immun. 67, 3193–3198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hobson R. P., Munro C. A., Bates S., MacCallum D. M., Cutler J. E., Heinsbroek S. E., Brown G. D., Odds F. C., Gow N. A. (2004) J. Biol. Chem. 279, 39628–39635 [DOI] [PubMed] [Google Scholar]
- 38.Singleton D. R., Masuoka J., Hazen K. C. (2005) Eukaryot. Cell 4, 639–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Odani T., Shimma Y., Tanaka A., Jigami Y. (1996) Glycobiology 6, 805–810 [DOI] [PubMed] [Google Scholar]
- 40.Jigami Y., Odani T. (1999) Biochim. Biophys. Acta 1426, 335–345 [DOI] [PubMed] [Google Scholar]
- 41.Southard S. B., Specht C. A., Mishra C., Chen-Weiner J., Robbins P. W. (1999) J. Bacteriol. 181, 7439–7448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nishikawa A., Poster J. B., Jigami Y., Dean N. (2002) J. Bacteriol. 184, 29–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Warit S., Zhang N., Short A., Walmsley R. M., Oliver S. G., Stateva L. I. (2000) Mol. Microbiol. 36, 1156–1166 [DOI] [PubMed] [Google Scholar]
- 44.Rouabhia M., Schaller M., Corbucci C., Vecchiarelli A., Prill S. K., Giasson L., Ernst J. F. (2005) Infect. Immun. 73, 4571–4580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Nobel H., Ruiz C., Martin H., Morris W., Brul S., Molina M., Klis F. M. (2000) Microbiology 146, 2121–2132 [DOI] [PubMed] [Google Scholar]
- 46.de Nobel H., van Den Ende H., Klis F. M. (2000) Trends Microbiol. 8, 344–345 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.