Abstract
The accessory protein Vpx is encoded by lentiviruses of the human immunodeficiency virus type 2 (HIV-2) and the simian immunodeficiency SIVsm/SIVmac lineage. It is packaged into virions and is indispensable in early steps of monocyte infection. HIV-1, which does not encode Vpx, is not able to infect human monocytes, but Vpx enables infection with HIV-1. The underlying mechanism is not completely understood. In this work, we focus on Vpx-mediated intracellular postentry events as counteraction of host cell proteins. We found that Vpx binds to apolipoprotein B mRNA-editing catalytic polypeptide 3 family member A (APOBEC3A; A3A), a member of the family of cytidine deaminases, present in monocytes. This interaction led to a reduction of the steady-state protein level of A3A. A single-point mutation in Vpx (H82A) abrogated binding to A3A and single-round infection of monocytes by HIV-1. Taken together, our data indicate that lentiviral Vpx counteracts A3A in human monocytes.
Keywords: Lentivirus, Protein Degradation, Viral Protein, Virus Entry, Virus, Host-Pathogen Interaction, Protein Interaction
Introduction
Primate lentiviruses of the human immunodeficiency virus type 2 (HIV-2)5 and the simian immunodeficiency (SIVsm/SIVmac) lineage uniquely encode for the viral protein Vpx. This 13-kDa accessory protein is packaged into budding virions by interaction with the p6 domain of Gag (1) and might have a role in early infection steps in target cells. Vpx has been shown to be crucial for infection of human monocytes and monocyte-derived cells as such as macrophages and dendritic cells in vitro, but is dispensable for infection of cell lines and primary lymphocytes (2–5). In vivo, infection of monkeys by a mutant SIV lacking the vpx gene is characterized by a loss of pathogenicity (6). Predelivery of Vpx protein by virus-like particles (VLPs) to monocytes can promote replication of HIV-1, a virus that does not encode for Vpx (7). These findings indicate a myeloid-specific function of Vpx during lentivirus infection.
When transiently expressed in cell lines, the Vpx protein localizes preferentially to the nucleus (8–10). It was proposed that this property is required for trafficking of the viral preintegration complex to the nucleus of nondividing cells (11). Recently, several groups described that Vpx associates with a CUL4A·DDB1·DCAF ubiquitin ligase complex, similar to the related viral protein Vpr (4, 12, 13). Abrogation of the Vpx-DDB1 interaction or knockdown of DCAF impaired the replication of HIV-2 and SIV in macrophages (4, 12). Some observations indicate that Vpx-deficient viruses are targeted by an unknown restriction factor in early postentry steps of the replication cycle (4, 12, 13).
Postentry restrictions are best investigated for HIV-1 as, for instance, the host protein TRIM5α blocks a step at viral reverse transcription and/or uncoating in simian cells (for review, see Ref. 14). Besides TRIM5α, low molecular mass complexes of apolipoprotein B mRNA-editing catalytic polypeptide 3 family member G (APOBEC3G, A3G) was described to constitute a potent restriction against HIV-1 in peripheral resting T lymphocytes and monocytes (15), whereas other groups demonstrated contradictory results (16, 17). A study by Peng et al. (18) suggested APOBEC3A (A3A) to be involved in restriction of HIV-1 in monocytes. In the same study, the decrease of A3A expression levels during the differentiation of monocytes to macrophages has been associated with an increased susceptibility to HIV-1. Based on these findings, we hypothesize that Vpx acts antagonistically to A3A in monocytes. In this work, we demonstrate an interaction of A3A with the viral protein Vpx. In addition, we found that Vpx, but not a binding-deficient Vpx-mutant, enhances protein degradation of A3A.
EXPERIMENTAL PROCEDURES
Plasmids
Codon-optimized non-, HA-, and FLAG-tagged SIV Vpx of the isolate SIVsmm PBj1.9 (19) were cloned into pcDNA3.1. The H82A mutant was generated using the Site-directed Mutagenesis kit (Stratagene). The HA-tagged A3A construct was a gift of Bryan Cullen (20). For bacterial expression of GST fusion protein, non-codon-optimized Vpx was cloned in-frame with GST into pGEX-2T (GE Healthcare).
Cell Culture and Monocyte Isolation
293T and HeLa cells were grown in Dulbecco's modified Eagle's medium, and U937 cells were grown in RPMI 1640 medium, both containing 1 mm l-glutamine and 10% fetal calf serum. Primary human monocytes from at least five healthy donors were isolated with the Monocyte Isolation Kit II (Miltenyi) and cultured as described previously (21).
Viral Particle Production, Monocyte Single-round Infection, and Fluorescence-activated Cell Sorter Analysis
293T cells were co-transfected with the SIV PBj1.9-derived packaging construct PBj-psi10, pMD.G coding for vesicular stomatitis virus G and the appropriate Vpx construct for generation of VLPs or with the HIV-1-EGFP-encoding plasmid pHR-CMV-EGFP, the HIV-1 packaging construct pCMVΔR8.9 and pMD.G for generation of HIV-1 particles as described before (5). Particle purification and titration of HIV-1 were described earlier (5, 21). The amount of VLPs was determined with the Lenti RT Activity kit (Cavidi) and normalized in comparison with PBj-derived vectors of known infectivity. The amount of VLPs/cells was given as m.o.i. equivalents. For single-round infection, monocytes were exposed to HIV-1-EGFP for 4 h and subjected to flow cytometry analysis 5 days after transduction. For analysis of virus replication, monocytes were infected in triplicate on day 1 after isolation with 0.5 m.o.i. of SIVsmm PBj X2 or wt strain and cultured up to day 15. Virus in the supernatant was quantified with the Lenti RT Activity kit.
Transfection and Co-immunoprecipitation Experiments
293T cells were transiently transfected with the respective plasmids using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. For transfection of HeLa FuGENE 6 (Roche Applied Science) and for transfection of U937 cells DMRIE-C (Invitrogen) were used in accordance with the manufacturers' instructions. For co-immunoprecipitation, transfected cells were lysed, sonicated, and incubated either with anti-HA beads (Roche Applied Science) or anti-FLAG beads (Sigma). After a 1-h incubation at 4 °C, the beads were washed, and associated proteins were subjected to SDS-PAGE.
GST Pulldown Assay
GST- or GST-Vpx-encoding constructs were transformed in the Escherichia coli strain BL21 DE3, and GST proteins were purified. For GST pulldown, 10 μg of recombinant protein was incubated with 20 μl of glutathione-Sepharose 4B beads (GE Healthcare) and 600 μg of 293T cell lysate for 1 h at 4 °C. The beads were washed, and isolated proteins were subjected to SDS-PAGE.
Immunoblot and Antibodies
Cells were lysed in radioimmune precipitation assay buffer and sonicated. When using cycloheximide, cells were incubated with 100 μg/ml cycloheximide (Calbiochem) as indicated before lysis. Nuclear extracts were prepared as described previously (22). Protein extracts were separated via SDS-PAGE and transferred to a nitrocellulose membrane (GE Healthcare). For detection, anti-HA (Roche Applied Science), anti-Vpx (23), anti-p27 (24), anti-laminin A (BioLegend), anti-APOBEC3A (Abgent), anti-β-actin, anti-tubulin, and anti-FLAG (all Cell Signaling Technology) were used as primary antibodies. Secondary horseradish peroxidase-conjugated anti-mouse and anti-rabbit antibodies were obtained from GE Healthcare.
Cell Staining and Microscopy
HeLa cells were seeded on a LabTek chambered coverglass (Nunc) and transfected with the indicated expression plasmids using FuGENE 6 (Roche Applied Science). After 24 h, cells were fixed, permeabilized, and blocked as described previously (25). Cells were incubated with anti-HA (Roche Applied Science) and anti-FLAG (Cell Signaling Technology) primary antibodies and stained with secondary Alexa Fluor 594 anti-rabbit, Alexa Fluor 488 anti-mouse antibodies (both from Invitrogen) and DAPI (Chemicon). Images were acquired with a ZEISS LSM Meta confocal microscope.
RESULTS
Vpx-dependent Infection of Monocytes Is Accompanied by Reduction of A3A Protein Levels
Previously, it has been shown that the Vpx-deficient SIVsmm PBj1.9 X2 but not wt failed to replicate in macaque macrophages, whereas both were able to replicate in human peripheral blood mononuclear cells and cell lines (3, 26). Before testing both viruses on monocytes, we confirmed similar replication on C8166 lymphocytes (supplemental Fig. 1).
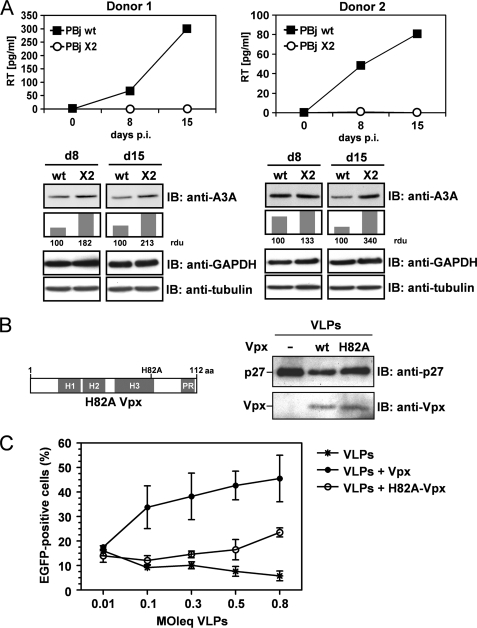
To investigate whether Vpx may be antagonistic to A3A, we performed infection experiments with SIV PBj1.9 wt and the Vpx-deficient mutant PBj1.9 X2 in human monocytes. We found replication of PBj1.9 but not of PBj1.9 X2 (Fig. 1A), and at days 8 and 15 after infection we observed reduced A3A protein levels in monocytes infected with PBj wt compared with Vpx-deficient PBj X2 (Fig. 1A).
FIGURE 1.
Vpx-dependent infection of human monocytes correlates with reduced A3A expression levels. A, human monocytes of two donors were infected with 0.5 m.o.i. of PBj1.9 wt or PBj1.9 X2. At days 8 and 15 after infection, reverse transcriptase (RT) concentration in the supernatant was determined. Subsequently, the monocytes were lysed and immunoblotted (IB) with anti-A3A and anti-tubulin antibodies. A3A protein band density was normalized to tubulin protein band density. A3A relative density units (rdu) of wt infected cells were set to 100, showing the relative increase in A3A protein band density in X2-infected cells. B: left, domain structure of Vpx and location of the H82A mutation. H indicates an α-helix, and PR indicates a proline-rich region. Right, 293T cells were transfected with the packaging construct PBj-psi10 and plasmids encoding for vesicular stomatitis virus G and Vpx or H82A-Vpx. Three days after transfection, VLPs were analyzed via immunoblotting with anti-Vpx and anti-p27 antibody. C, 1 day after isolation, primary human monocytes were incubated with different m.o.i. equivalents (MOIeq) of VLPs containing wt Vpx, H82A-Vpx, or empty VLPs. Monocytes were transduced with a m.o.i. = 8 HIV-1 vector particles harboring EGFP as reporter transgene. Monocytes were analyzed via fluorescence-activated cell sorting 5 days later.
Interestingly, viruses harboring a histidine point mutation in the Vpx gene within a nonclassical nuclear localization signal fail to replicate in macrophages, although packaging of the mutant Vpx protein into the virus particle has been shown (8). This histidine at position 82 is evolutionary highly conserved among described Vpx variants, and for further analysis we introduced a H82A-Vpx mutant (Fig. 1B, left). To analyze the postentry biological activity of Vpx and the H82A-Vpx mutant, we monitored single-round infection of monocytes by HIV-1 with increasing amounts of Vpx. Western blot analysis of Vpx-containing VLPs revealed comparable packaging of Vpx and H82A-Vpx proteins (Fig. 1B, right). Preincubation with VLPs containing Vpx efficiently enhanced monocyte single-round infection up to 45% in a dose-dependent manner (Fig. 1C). In contrast, H82A-Vpx was strongly impaired in this capability. At low m.o.i. equivalents, the infection rate of HIV-1 was comparable with those using empty VLPs for preincubation. We observed a dose response in the presence of H82A-Vpx, but the infection rate was only marginally increased at the highest m.o.i. equivalent (Fig. 1C). Performing full HIV replication experiments instead of single-round infection, we obtained similar results, as Vpx-VLPs allowed HIV-1 replication in monocytes (supplemental Fig. 2). Interestingly, we found H82A Vpx dysfunction to be donor-dependent during multiple rounds of infection (supplemental Fig. 2).
A3A Interacts with SIVsm Vpx
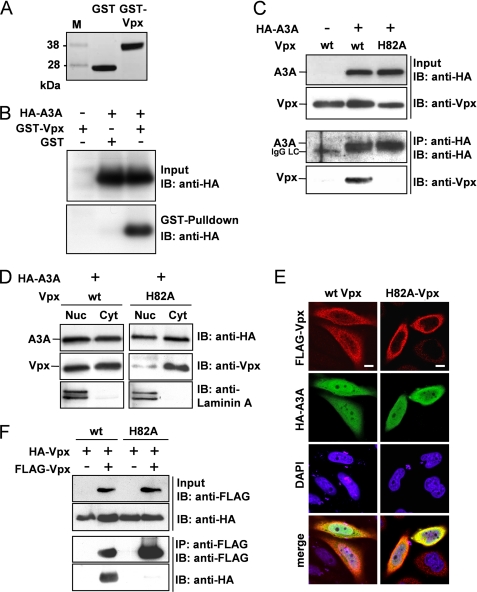
Based on findings that depletion of A3A might enhance the permissiveness of monocytes to HIV-1 (18), we investigated whether Vpx may antagonize A3A. To elucidate whether A3A and Vpx interact, a recombinant GST-Vpx fusion protein was generated (Fig. 2A). For GST pulldown experiments, GST-Vpx or GST was incubated with cell lysates of 293T cells, transiently expressing HA-tagged A3A. A3A was only pulled down by the GST-Vpx fusion protein, but not in the presence of GST, indicating an interaction of Vpx and A3A (Fig. 2B). This interaction was further supported using other HA-tagged APOBEC3 proteins (A3C and A3G) that did not interact with GST-Vpx (supplemental Fig. 3). This finding was confirmed by co-immunoprecipitation in eukaryotic cell lysates using A3A and Vpx or the mutant H82A-Vpx. Only in the presence of A3A did we detect co-precipitation of Vpx, thus supporting that Vpx interacts with A3A (Fig. 2C). Although Vpx and H82A-Vpx were expressed at comparable levels, the mutant H82A was not precipitated by A3A, indicating an abrogated interaction (Fig. 2C).
FIGURE 2.
Vpx interacts with A3A, and both proteins co-localize to the same cellular compartment. A, 10 μg of GST or GST-Vpx was incubated with glutathione-coupled Sepharose beads, and after GST pulldown recombinant proteins were separated with SDS-PAGE and stained with Coomassie Blue. B, 10 μg of GST or GST-Vpx was incubated with lysates of 293T cells transiently expressing HA-A3A and glutathione-coupled Sepharose beads. After pulldown, associated proteins were analyzed by immunoblotting (IB) using an anti-HA antibody. C, 293T cells were transiently transfected with HA-A3A and Vpx or H82A-Vpx. After HA-directed immunoprecipitation, proteins were detected with anti-Vpx and anti-HA antibodies in immunoblot analysis. IgG LC indicates the light chain of the IP antibody. D, HeLa cells were transiently transfected with HA-A3A and Vpx or H82A-Vpx. Cell lysates were separated in nuclear (Nuc) and cytoplasmic (Cyt) fractions, and equal protein amounts of the respective fractions were subjected to immunoblot analysis using anti-HA and anti-Vpx antibodies. Anti-laminin A was used as control for a nucleus-specific protein. E, HeLa cells were transfected with HA-A3A and FLAG-Vpx or FLAG-H82A-Vpx. Cellular localization was determined via confocal microscopy using anti-HA and anti-FLAG primary and fluorescent secondary antibodies. Nuclei were stained with DAPI. White bar, 10 μm. F, 293T cells were transfected with HA- and FLAG-tagged Vpx or H82A-Vpx, and FLAG-directed immunoprecipitation was performed. Cell lysates and precipitated proteins were identified with anti-HA and anti-FLAG antibodies in immunoblot analysis.
To support these findings further, we investigated a putative co-localization of A3A and Vpx proteins in HeLa cells by analyzing their subcellular distribution. HeLa cells were co-transfected with A3A and wt Vpx or H82A-Vpx expression plasmids. Subsequently, nuclei of the transfected HeLa cells were separated from the cytoplasm, and equal protein amounts of the respective fractions were analyzed by immunoblot analysis. Laminin A was used as nuclear marker protein. Similar to wt Vpx, A3A was detected in the cytoplasm as well as in the nuclear fraction, whereas H82A-Vpx was observed predominantly in the cytoplasm (Fig. 2D). To verify these data, we performed laser-scanning microscopy and observed a heterogeneous distribution of A3A and Vpx either localizing to the nucleus, to the cytoplasm, or both. Thus, both proteins were co-localized in the same cellular compartment (Fig. 2E). In contrast, the H82A mutation significantly reduced the nuclear localization of Vpx, as observed in immunoblot analysis of subcellular fractions (Fig. 2E). These data indicate that Vpx and A3A are co-localized in the same subcellular compartments, supporting our findings that Vpx and A3A interact.
Because multimerization of viral proteins may be involved in the regulation of protein activity (27, 28), we analyzed Vpx and the mutant H82A for the capability of Vpx-Vpx interaction. FLAG-tagged and HA-tagged Vpx or H82A-Vpx were transiently co-expressed in 293T cells and immunoprecipitated via FLAG tag. As confirmed by anti-HA immunoblot analysis, Vpx was able to form homomeric protein complexes (Fig. 2F). In contrast, the H82A-Vpx was not co-precipitated, thus demonstrating that the H82A mutant is not capable of forming homomeric complexes. These results confirm that Vpx is required to overcome the monocyte-specific postentry restriction of HIV-1, whereas this capacity was reduced using the complex formation and A3A interacting-deficient H82A-Vpx.
Vpx Enhances Degradation of A3A
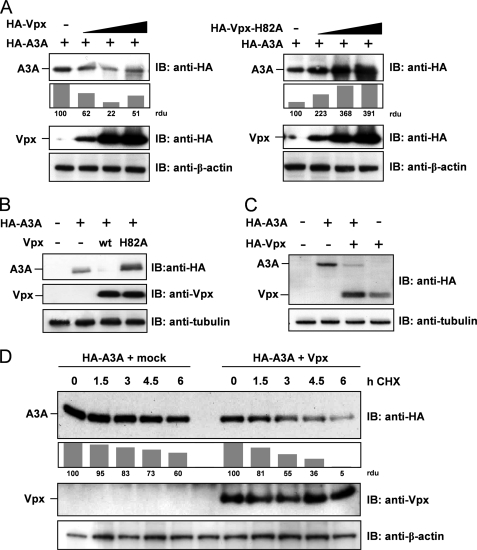
To gain further insights into Vpx-mediated molecular mechanisms, we investigated the influence of Vpx on the steady-state protein level of A3A. Therefore, increasing amounts of Vpx-encoding expression plasmids were co-transfected with a constant amount of A3A expression plasmids. The 293T cell lysates were analyzed in parallel for Vpx and A3A protein levels by immunoblot analysis. In the presence of increasing Vpx protein levels, we detected decreasing amounts of A3A protein levels (Fig. 3A, left) suggesting a Vpx-mediated degradation of A3A. In contrast, the H82A mutant of Vpx failed to reduce but increased A3A protein levels (Fig. 3A, right), potentially by interfering with cellular protein degradation pathways. In addition, we determined that a molar ratio of 1:6 of A3A and Vpx expression plasmids was most effective in terms of Vpx-mediated reduction of A3A (Fig. 3B). These findings were not limited to 293T cells because we confirmed A3A protein reduction upon Vpx co-expression in the monocytic U937 cell line (Fig. 3C), 293T, and HeLa cells (data not shown), suggesting a cell type-independent mechanism. In addition, endogenous A3A protein levels within U937 monocytes were affected by Vpx expression (supplemental Fig. 4).
FIGURE 3.
Vpx, but not H82A-Vpx, supports degradation of APOBEC3A. A: left, 293T cells were transfected with constant amounts of HA-tagged A3A and increasing amounts of HA-Vpx-encoding plasmid. Proteins were detected by immunoblot (IB) analysis with antibodies against HA tag and β-actin as loading control. Protein band intensity was quantified with densitometry, defining A3A protein levels in the absence of Vpx as 100 relative density units (rdu). Right, 293T cells were transfected and analyzed as the left panel using the H82A-Vpx mutant. B, 293T cells were transfected with HA-A3A and Vpx or H82A-Vpx in a A3A:Vpx DNA ratio of 1:6. Cell lysates were analyzed by immunoblotting using anti-HA and anti-Vpx antibodies. C, U937 monocytic cells were transfected with HA-A3A and HA-Vpx in a DNA ratio of 1:1. Cell lysates were immunoblotted and analyzed with an anti-HA antibody. D, 293T cells were transfected with plasmids encoding HA-A3A and Vpx or empty vector in a DNA ratio of 1:5. 42 h after transfection and 6 h before harvest, cells were treated with 100 μg/ml cycloheximide (CHX) for the indicated period. Western blot analysis was performed as described in B, and protein bands were quantified defining A3A protein levels of cycloheximide-untreated cells as 100 relative density units.
To exclude that Vpx-mediated reduction of A3A protein levels is due to a restricted expression, we analyzed the protein turnover of A3A as a function of Vpx. To inhibit new protein synthesis, the mRNA translation was retained by cycloheximide in a time-dependent manner in A3A- and Vpx-transfected cells. In the presence of constant Vpx protein levels, A3A was degraded to 5 relative density units within 6 h compared with 100 units of initial protein amounts, whereas A3A was degraded only to 60 density units in the absence of Vpx (Fig. 3D). We concluded that Vpx-mediated reduction of A3A levels is independent of protein synthesis and acts via significant acceleration of A3A degradation. Interestingly, H82A-Vpx critically affects A3A degradation by a yet unknown mechanism of protein stabilization.
DISCUSSION
Monocytes, and to a lesser extent, macrophages and dendritic cells, are poorly susceptible to HIV-1 infection (29, 30). In contrast, infection of monocytes is managed by viruses of the HIV-2/SIVmac/SIVsm lineage, thereby strictly depending on the presence of Vpx. Thus, Vpx-deficient HIV-2/SIVsm/SIVmac viruses are restricted in myeloid cells like HIV-1 (4, 5, 13). However, predelivery of Vpx to monocytes and monocyte-derived cells by VLPs strongly enhances infection of different lentiviruses by a yet unknown mechanism (4, 7, 10, 12, 13). In addition, γ-retroviruses were found to benefit from Vpx in these cells (31). Therefore, it was concluded that monocytes and macrophages harbor a cell-specific restriction factor that seems to be specifically counteracted by Vpx (4).
In monocytes and partly in macrophages, A3A was set into focus as putative postentry restriction factor, whose absence would eliminate resistance against HIV-1 (18). However, the restrictive capacity of A3A in target cells remains to be confirmed independently, as we and others (32) did not succeed in knocking down A3A in monocytes.
In the context of HIV restriction, incorporation of a Vpr.A3A fusion protein into particles was described as reducing their infectivity accompanied by editing of the viral genome (33), but it is noteworthy that A3A does not constitute a lentivirus restriction in target cells per se, as also cell lines with endogenous A3A expression (U937, A.301) remain permissive to Vpx-deficient lentiviruses such as HIV-1 (data not shown). Under the assumption that A3A might be restrictive to these viruses, we speculate that, e.g. cellular quiescence or the absence of other myeloid factors might be a further prerequisite for restriction in myeloid cells.
Nevertheless, A3A was reported to have a restrictive capacity against adeno-associated virus and retrotransposons reducing the number of produced particles or retrotransposition events independently of deaminase activity toward the respective transcripts (25, 34–36). However, in these experimental settings, the A3A mode of action took place in the producer cell and not in the target cell.
Very recently, a publication from Stenglein et al. (32) demonstrated that A3A destabilizes foreign double-stranded DNA, and coincidently, extensive foreign DNA editing was observed in monocytes. Whether this A3A activity contributes to restriction of incoming lentiviral transcripts prior integration remains to be proven.
Independently of whether A3A acts as potent lentiviral restriction factor in target cells, we observed an A3A decrease in monocytes infected with Vpx-encoding SIV accompanied by successful propagation. Furthermore, we have shown that Vpx interacts with A3A and enhances its degradation. The specificity of this process has been confirmed by using the Vpx mutant H82A, whose interaction with Vpx is abrogated due to a single-point mutation within the third helix of the protein. We assume that the H82A mutation does not affect Vpx integrity due to the Gag interaction capability that is crucial for virion incorporation (1). Interestingly, infection of monocytes with replication-incompetent HIV-1 cannot be rescued efficiently in the presence of H82A-Vpx compared with wt Vpx, although HIV-1 replication upon H82A Vpx delivery was apparent in a dose- and donor-dependent manner. This coherence has already been described for SIVsm wt virus, whereas viruses encoding for H82S-Vpx failed to replicate in macrophages (8). Based on these findings, we hypothesize that the interaction of Vpx with A3A in the target cell is a prerequisite for overcoming the monocyte restriction. Moreover, we detected that Vpx is able to form homomers, which recently was also found for the related protein Vpr (27). Vpx·Vpx and Vpx·A3A complex formation was abrogated in the H82A-Vpx mutant that also lost the ability to induce A3A protein degradation. Thus, our results indicate that homomer formation of Vpx might be important to mediate A3A degradation.
Reminiscent of the Vpx-enhanced degradation of A3A, the lentiviral accessory proteins Vif, Vpu, and Vpr have been described to promote degradation of host cell proteins for optimal virus propagation (37–39). Recently, other groups demonstrated that Vpx function is dependent on association with a CUL4·DDB1·DCAF1 ubiquitin ligase complex, which was described to be important for macrophage infection (4, 12, 13). In ongoing experiments we observed that Vpx, H82A-Vpx and A3A are poly ubiquitinated in cells and that A3A ubiquitination is affected by Vpx (data not shown), suggesting that Vpx might be able to accelerate the recruitment of the cellular ubiquitin machinery to A3A, thereby forcing its enhanced degradation.
We show that H82A-Vpx is unable to mediate crucial postentry processes to provide HIV-1 integration in monocytes that correlates with its incapability of A3A interaction, Vpx complex formation, or nuclear localization. Notably, a higher dose of predelivered H82A-Vpx to monocytes resulted in an increased susceptibility toward single-round infection with HIV-1. During multiple rounds of infection, the mutant Vpx was only impaired in certain donors, altogether indicating a residual function of H82A-Vpx, which is recovered upon increased protein levels. We hypothesize that Vpx function may be separated in at least two independent activities, and the H82A mutation impairs one of these functions such as binding to A3A. Initial experiments, examining whether Vpx might be capable of removing interferon α-induced infection barriers, remained negative (supplemental Fig. 5). However, because in addition to A3A, approximately 200 genes are known to be up-regulated by interferon α, we assume that this barrier is based on various potent antiviral factors.
Considering Vpx crucial for accumulation of viral reverse transcripts (10, 40), one could speculate on the involvement of A3A in affecting this process as A3G and A3F proteins were described to lower HIV-1 reverse transcript amounts in target cells (41, 42). However, unless the restrictive capacity of A3A is not confirmed, A3A might also be degraded as side reaction of Vpx function for yet unknown reasons.
Nevertheless, our findings that in addition to foamy virus Bet and lentiviral Vif, Vpx also counteracts cellular A3C proteins (43) highlight the outstanding role for virus evolution of these intrinsic defense proteins. Finally, the reason why some primate lentiviruses evolved a tropism for resting monocytes is still unclear, and further elucidation of Vpx function remains an important subject for future studies.
Supplementary Material
Acknowledgments
We thank Sylvia Panitz for excellent technical assistance, Daniela Marino for microscopy support, and Bryan Cullen for the A3A expression plasmid. SIVsmPBj1.9- and SIVsmPBj1.9 X2-encoding plasmids were a gift from Mark Sharkey and Mario Stevenson. The following reagents were obtained through the National Institutes of Health AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, National Institutes of Health: HIV-2 Vpx monoclonal antibody (6D2.6) from Dr. John C. Kappes and SIVmac p27 monoclonal antibody (55–2F12) from Dr. Niels Pedersen.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–4.
- HIV-2
- human immunodeficiency virus type 2
- HIV-1
- HIV type 1
- SIV
- simian immunodeficiency virus
- VLP
- virus-like particle
- A3G
- apolipoprotein B mRNA-editing catalytic polypeptide 3 family member G (APOBEC3G)
- A3A
- APOBEC3A
- HA
- hemagglutinin
- GST
- glutathione S-transferase
- EGFP
- enhanced green fluorescent protein
- m.o.i.
- multiplicity of infection
- wt
- wild type
- CUL4A
- cullin4A
- DDB1
- DNA damage-binding protein 1
- DCAF
- DDB1- and CUL4-associated factor 1
- TRIM
- tripartite motif-containing protein.
REFERENCES
- 1.Horton R., Spearman P., Ratner L. (1994) Virology 199, 453–457 [DOI] [PubMed] [Google Scholar]
- 2.Cheng X., Belshan M., Ratner L. (2008) J. Virol. 82, 1229–1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fletcher T. M., III, Brichacek B., Sharova N., Newman M. A., Stivahtis G., Sharp P. M., Emerman M., Hahn B. H., Stevenson M. (1996) EMBO J. 15, 6155–6165 [PMC free article] [PubMed] [Google Scholar]
- 4.Sharova N., Wu Y., Zhu X., Stranska R., Kaushik R., Sharkey M., Stevenson M. (2008) PLoS Pathog. 4, e1000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolfrum N., Mühlebach M. D., Schüle S., Kaiser J. K., Kloke B. P., Cichutek K., Schweizer M. (2007) Virology 364, 330–341 [DOI] [PubMed] [Google Scholar]
- 6.Hirsch V. M., Sharkey M. E., Brown C. R., Brichacek B., Goldstein S., Wakefield J., Byrum R., Elkins W. R., Hahn B. H., Lifson J. D., Stevenson M. (1998) Nat. Med. 4, 1401–1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schüle S., Kloke B. P., Kaiser J. K., Heidmeier S., Panitz S., Wolfrum N., Cichutek K., Schweizer M. (2009) PLoS One 4, e7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rajendra Kumar P., Singhal P. K., Subba Rao M. R., Mahalingam S. (2005) J. Biol. Chem. 280, 8553–8563 [DOI] [PubMed] [Google Scholar]
- 9.Belshan M., Ratner L. (2003) Virology 311, 7–15 [DOI] [PubMed] [Google Scholar]
- 10.Goujon C., Arfi V., Pertel T., Luban J., Lienard J., Rigal D., Darlix J. L., Cimarelli A. (2008) J. Virol. 82, 12335–12345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pancio H. A., Vander Heyden N., Ratner L. (2000) J. Virol. 74, 6162–6167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bergamaschi A., Ayinde D., David A., Le Rouzic E., Morel M., Collin G., Descamps D., Damond F., Brun-Vezinet F., Nisole S., Margottin-Goguet F., Pancino G., Transy C. (2009) J. Virol. 83, 4854–4860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Srivastava S., Swanson S. K., Manel N., Florens L., Washburn M. P., Skowronski J. (2008) PLoS Pathog. 4, e1000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nisole S., Stoye J. P., Saïb A. (2005) Nat. Rev. Microbiol. 3, 799–808 [DOI] [PubMed] [Google Scholar]
- 15.Chiu Y. L., Soros V. B., Kreisberg J. F., Stopak K., Yonemoto W., Greene W. C. (2005) Nature 435, 108–114 [DOI] [PubMed] [Google Scholar]
- 16.Kamata M., Nagaoka Y., Chen I. S. (2009) PLoS Pathog. 5, e1000342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santoni de Sio F. R., Trono D. (2009) PLoS One 4, e6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peng G., Greenwell-Wild T., Nares S., Jin W., Lei K. J., Rangel Z. G., Munson P. J., Wahl S. M. (2007) Blood 110, 393–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dewhurst S., Embretson J. E., Anderson D. C., Mullins J. I., Fultz P. N. (1990) Nature 345, 636–640 [DOI] [PubMed] [Google Scholar]
- 20.Bogerd H. P., Wiegand H. L., Doehle B. P., Lueders K. K., Cullen B. R. (2006) Nucleic Acids Res. 34, 89–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mühlebach M. D., Wolfrum N., Schüle S., Tschulena U., Sanzenbacher R., Flory E., Cichutek K., Schweizer M. (2005) Mol. Ther. 12, 1206–1216 [DOI] [PubMed] [Google Scholar]
- 22.Muckenfuss H., Kaiser J. K., Krebil E., Battenberg M., Schwer C., Cichutek K., Münk C., Flory E. (2007) Nucleic Acids Res. 35, 3784–3796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kappes J. C., Parkin J. S., Conway J. A., Kim J., Brouillette C. G., Shaw G. M., Hahn B. H. (1993) Virology 193, 222–233 [DOI] [PubMed] [Google Scholar]
- 24.Higgins J. R., Sutjipto S., Marx P. A., Pedersen N. C. (1992) J. Med. Primatol. 21, 265–269 [PubMed] [Google Scholar]
- 25.Muckenfuss H., Hamdorf M., Held U., Perkovic M., Löwer J., Cichutek K., Flory E., Schumann G. G., Münk C. (2006) J. Biol. Chem. 281, 22161–22172 [DOI] [PubMed] [Google Scholar]
- 26.Sleigh R., Sharkey M., Newman M. A., Hahn B., Stevenson M. (1998) Virology 245, 338–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fritz J. V., Didier P., Clamme J. P., Schaub E., Muriaux D., Cabanne C., Morellet N., Bouaziz S., Darlix J. L., Mély Y., de Rocquigny H. (2008) Retrovirology 5, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurg R., Uusen P., Sepp T., Sepp M., Abroi A., Ustav M. (2009) Virology 386, 353–359 [DOI] [PubMed] [Google Scholar]
- 29.Sonza S., Maerz A., Deacon N., Meanger J., Mills J., Crowe S. (1996) J. Virol. 70, 3863–3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neil S., Martin F., Ikeda Y., Collins M. (2001) J. Virol. 75, 5448–5456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaushik R., Zhu X., Stranska R., Wu Y., Stevenson M. (2009) Cell Host Microbe 6, 68–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stenglein M. D., Burns M. B., Li M., Lengyel J., Harris R. S. (2010) Nat. Struct. Mol. Biol. 17, 222–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aguiar R. S., Lovsin N., Tanuri A., Peterlin B. M. (2008) J. Biol. Chem. 283, 2518–2525 [DOI] [PubMed] [Google Scholar]
- 34.Chen H., Lilley C. E., Yu Q., Lee D. V., Chou J., Narvaiza I., Landau N. R., Weitzman M. D. (2006) Curr. Biol. 16, 480–485 [DOI] [PubMed] [Google Scholar]
- 35.Narvaiza I., Linfesty D. C., Greener B. N., Hakata Y., Pintel D. J., Logue E., Landau N. R., Weitzman M. D. (2009) PLoS Pathog. 5, e1000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vartanian J. P., Guétard D., Henry M., Wain-Hobson S. (2008) Science 320, 230–233 [DOI] [PubMed] [Google Scholar]
- 37.Wen X., Duus K. M., Friedrich T. D., de Noronha C. M. (2007) J. Biol. Chem. 282, 27046–27057 [DOI] [PubMed] [Google Scholar]
- 38.Luo K., Xiao Z., Ehrlich E., Yu Y., Liu B., Zheng S., Yu X. F. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 11444–11449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goffinet C., Allespach I., Homann S., Tervo H. M., Habermann A., Rupp D., Oberbremer L., Kern C., Tibroni N., Welsch S., Krijnse-Locker J., Banting G., Kräusslich H. G., Fackler O. T., Keppler O. T. (2009) Cell Host Microbe 5, 285–297 [DOI] [PubMed] [Google Scholar]
- 40.Goujon C., Rivière L., Jarrosson-Wuilleme L., Bernaud J., Rigal D., Darlix J. L., Cimarelli A. (2007) Retrovirology 4, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holmes R. K., Koning F. A., Bishop K. N., Malim M. H. (2007) J. Biol. Chem. 282, 2587–2595 [DOI] [PubMed] [Google Scholar]
- 42.Bishop K. N., Holmes R. K., Malim M. H. (2006) J. Virol. 80, 8450–8458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perkovic M., Schmidt S., Marino D., Russell R. A., Stauch B., Hofmann H., Kopietz F., Kloke B. P., Zielonka J., Ströver H., Hermle J., Lindemann D., Pathak V. K., Schneider G., Löchelt M., Cichutek K., Münk C. (2009) J. Biol. Chem. 284, 5819–5826 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.