Abstract
The fusion of TEL with platelet-derived growth factor receptor (PDGFR) β (TPβ) is found in a subset of patients with atypical myeloid neoplasms associated with eosinophilia and is the archetype of a larger group of hybrid receptors that are produced by rearrangements of PDGFR genes. TPβ is activated by oligomerization mediated by the pointed domain of TEL/ETV6, leading to constitutive activation of the PDGFRβ kinase domain. The receptor transmembrane (TM) domain is retained in TPβ and in most of the described PDGFRβ hybrids. Deletion of the TM domain (ΔTM-TPβ) strongly impaired the ability of TPβ to sustain growth factor-independent cell proliferation. We confirmed that TPβ resides in the cytosol, indicating that the PDGFRβ TM domain does not act as a transmembrane domain in the context of the hybrid receptor but has a completely different function. The ΔTM-TPβ protein was expressed at a lower level because of increased degradation. It could form oligomers, was phosphorylated at a slightly higher level, co-immunoprecipitated with the p85 adaptor protein, but showed a much reduced capacity to activate STAT5 and ERK1/2 in Ba/F3 cells, compared with TPβ. In an in vitro kinase assay, ΔTM-TPβ was more active than TPβ and less sensitive to imatinib, a PDGFR inhibitor. In conclusion, we show that the TM domain is required for TPβ-mediated signaling and proliferation, suggesting that the activation of the PDGFRβ kinase domain is not enough for cell transformation.
Keywords: Diseases/Cancer/Transformation, Oncogene, Receptors/Tyrosine Kinase, Leukemia, Signal Transduction, CMML, ETV6, PDGFRβ, Hydrophobic Sequences, Myeloproliferative Neoplasm
Introduction
PDGFRβ3 is a single-spanning transmembrane glycoprotein that binds to its dimeric ligand PDGF. It belongs to the type III receptor tyrosine kinase family, which also comprises PDGFRα, c-KIT, Flt3, and c-Fms (1). The canonical mechanism of activation of receptor tyrosine kinases requires ligand-induced dimerization, which brings two kinase domains close to each other and allows the phosphorylation of critical regulatory tyrosine residues in the activation loop of the catalytic core with a subsequent boost of the receptor kinase activity (2). Recent findings demonstrated that dimerization is not sufficient by itself to activate these receptors. The extracellular Ig-like domain D4 must reorganize and establish contacts between two neighbors receptors to provide optimal tyrosine kinase activation (3, 4). The intracellular juxtamembrane domain is devoted to the inhibition of the catalytic activity in the absence of the ligand by interacting with the kinase domain and inhibiting its activity (5–7). Mutations in the juxtamembrane region can alleviate this inhibition and activate the receptor in a ligand-independent manner (8, 9). The C-terminal tail of PDGFRβ is also blocking the receptor phosphorylation by allosteric inhibition (10). The phosphorylated receptor tyrosine residues bind SH2 domain-containing proteins, such as the p85 subunit of phosphatidylinositol 3-kinase, STAT5, or phospholipase Cγ. The cascade of events initiated by ligand binding will ultimately affect gene expression and modulate cell proliferation, differentiation, and motility (11–13).
PDGFR genes are found rearranged in a certain subset of chronic myeloid malignancies (14–16). The resulting hybrid genes encode constitutively active forms of receptors that contain the receptor intracellular kinase domain fused to the N-terminal part of a partner that can differ from one hybrid receptor to another. Among these, the hybrid between the ETV6 (ets variant gene 6)/TEL (translocation-ets-leukemia) transcription factor and PDGFRβ (TPβ) is the most recurrent one and is encountered in patients with chronic myelomonocytic leukemia (17). TPβ is activated in a ligand-independent manner by enforced dimerization mediated by the pointed (PNT, also called SAM or helix-loop-helix) domain of TEL (18). The activation of the transcription factor STAT5 has been demonstrated to be crucial for the transforming potential of the hybrid protein in cell lines and in mouse models (19, 20). Furthermore, the levels of both STAT5 and TPβ proteins are critical for cell transformation (21). In particular, TPβ is not efficiently degraded in cells, and we showed that its increased stability promotes cell proliferation (22). Recently, activation of ERK signaling proteins was indicated as a mediator of TPβ-induced stem cell differentiation (23).
In TPβ and in most of the described PDGFRβ hybrids, but not in hybrids derived from other receptor tyrosine kinases, the transmembrane sequence of the receptor is retained in the fusion protein. No particular function has been ascribed to that hydrophobic sequence in the context of hybrid receptors so far. Here, we show that the transmembrane (TM) domain is required for TPβ-mediated cell proliferation and STAT5 signaling but not for the activation of the kinase domain.
EXPERIMENTAL PROCEDURES
Antibodies and Reagents
Anti-PDGFRβ (958), anti-PDGFRα (951), anti-phosphotyrosine (pY99), and anti-FLAG (D8) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-phospho-STAT5 (tyrosine 694) and anti-ppERK (threonine 202 and tyrosine 204) antibodies conjugated to Alexa-Fluor 647 were purchased from BD Transduction Laboratories. Anti-p85 antibody was obtained from Millipore. Anti-rabbit IgG conjugated to Alexa-Fluor 594 was purchased from Molecular Probes. Anti-mouse and anti-rabbit IgG conjugated to phycoerythrin were obtained from Jackson Immunoresearch. Rat monoclonal anti-myelin basic protein was obtained from Millipore. Mouse monoclonal anti-HA tag antibody (clone 12CA5) was obtained from Roche Applied Science. Mouse monoclonal antibodies against β-actin (clone AC-15) and FLAG (M2) were purchased from Sigma. The antibody against phosphotyrosine 581 of human PDGFRβ was produced and validated as described previously (24).
Constructs and Mutagenesis
TPβ in pMSCV-eGFP vector was described elsewhere (18). All of the TPβ mutants were created by site-directed mutagenesis using QuikChange XL-II kit (Stratagene) according to the manufacturer's instructions. All of the constructs were checked by sequencing. FLAG and HA tag were cloned at the 5′ site of TPβ into a previously introduced Age1 site replacing the ATG codon of TPβ. Human HA-PDGFRβ was cloned in pEF-BOS-puro as described (25). The HA tag was inserted after the sequence encoding the signal peptide of PDGFRβ in the position predicted by the SignalP software (26).
Cell Culture, Transfection, Infection, Thymidine Incorporation, and Stability Assays
Ba/F3, BOSC, porcine aortic endothelial (PAE) (27), 32D (28, 29), and HEK-293T cells were maintained in Dulbecco's modified Eagle's medium (DMEM; Sigma) with 10% FBS. Ba/F3 cell culture was supplemented with interleukin 3 (IL3).
Transient transfections of BOSC, PAE, and HEK-293T cells were performed using the calcium phosphate method as described elsewhere (22). Ba/F3 and 32D cell lines stably expressing receptors were created as previously reported (30). Briefly, retroviral supernatants were generated by co-transfection of the BOSC packaging cell line with plasmid DNA encoding the receptor and the ecotropic envelope protein using the calcium phosphate method (22). After 48 h, the supernatants were harvested and used in a spin infection protocol. One million cells were centrifuged for 2 h at 37 °C and 300 × g in presence of viral supernatant (1 ml) and Polybrene (20 μg; Sigma). The cells were then resuspended in medium with 10% FBS and IL3. After 24 h, GFP-positive cells were isolated by fluorescence-activated cell sorting and maintained in DMEM with FBS and IL3. All of the cells used in the experiments showed an equal level of GFP expression.
Alternatively, 107 32D cells were electroporated with 60 μg of DNA and diluted in 30 ml of DMEM with 10% FBS and IL3 as described earlier (28, 29). After 48 h, the cells were selected with 3 μg/ml puromycin for 14 days. Homogenous 32D cell lines expressing PDGFRβ were described previously (29).
In [3H]thymidine incorporation assays, Ba/F3 cells stably expressing receptors were washed extensively and seeded in triplicate in a 96-well plate in DMEM with 10% FBS in the presence of the indicated growth factor. After 20 h, [3H]thymidine (0.5 μCi/well; GE Healthcare) was added for 4 h. The cells were then harvested, and the incorporation of [3H]thymidine was quantified using a TopCount instrument (PerkinElmer Life Sciences).
Ba/F3 cells stability assays were performed as described previously (22). Briefly, 2 × 106 cells expressing the indicated hybrid receptors were incubated with 50 μg/ml cycloheximide for the indicated periods of time. The samples were collected at each time point and analyzed by Western blot.
Cross-linking Treatment, Immunoprecipitation, and Western Blot
Cross-linking assays were performed in the presence of bis(sulfosuccinimidyl)-suberate (BS3; Pierce). Briefly, 2 × 105 cells were washed once with ice-cold PBS and then lysed in 200 μl of 50 mm HEPES, pH 7.5, 150 mm NaCl, glycerol 10% (w/v), Triton 1% (w/v), EDTA 1 mm, 1 mm Pefabloc (Roche Applied Science), 1 μg/ml aprotinin, and 1 mm Na3VO4. After clearing by centrifugation, the lysates were incubated with 0.25, 0.5, or 1 mm BS3 for 1.5 h at 4 °C. The reactions were stopped by the addition of 50 mm Tris-HCl for 15 min at room temperature. The samples were analyzed by Western blot as described (22). Quantification of bands was performed after incubation with secondary fluorescent antibodies (IRDye) using the Odyssey system (Li-Cor) or using the ImageJ software (31) on scans of BioMax films (Kodak).
In immunoprecipitation experiments, the cells were lysed 24 h after transfection in 25 mm Tris-HCl, pH 7.4, 150 mm NaCl, 5 mm EDTA, 10% glycerol, 1% Triton X-100, 1 mm Pefabloc, 1 μg/ml aprotinin, and 1 mm Na3VO4. The cell lysates were centrifuged full speed for 20 min at +4 °C. In co-immunoprecipitation experiments with p85, the lysates were cleared with protein A/G for 1 h before being incubated overnight with the antibody. In double tag immunoprecipitations, the cell lysates were incubated overnight with 1 μg of antibody at +4 °C after the centrifugation. Antibody complexes were collected by adding protein A/G Ultralink (Pierce) for 1 h at +4 °C, washed extensively, and then analyzed by Western blot.
Flow Cytometry and Immunofluorescence Staining
For cell surface staining, about 5 × 105 cells were incubated for 1 h at 4 °C with primary antibody diluted in Hanks' buffer complemented with 3% FBS and 1% NaN3 (HAFA buffer). Conditions without antibody were included as a control. After one wash with HAFA, the cells were incubated with secondary antibody conjugated to phycoerythrin for 45 min at 4 °C in the dark. After one more wash, the cells were analyzed by flow cytometry.
In intracellular staining experiments, the cells were washed extensively and incubated for 4 h in absence of IL3 and, in some experiments, with 500 nm imatinib. As a positive control, some cells were restimulated with IL3 for 5 min after starvation. The cells were fixed with 2% formaldehyde in PBS for 10 min at 37 °C and then permeabilized with methanol on ice for 30 min. Following two washing steps with HAFA buffer, the cells were incubated with the antibody conjugated to Alexa-Fluor 647 (BD Transduction Laboratories) for 1 h at room temperature. The cells were washed and analyzed by flow cytometry. A condition without antibody was included as an additional control. The average of at least two independent experiments is shown with S.D., and a Student's t test was applied. In double staining experiments, the cells were at first incubated for 1 h with anti-PDGFRβ antibody and, after two washing steps, with anti-phospho-STAT5 Alexa-Fluor 647 and anti-rabbit phycoerythrin. Staining with the two antibodies separately was also included as a control. One representative experiment is shown (n = 4).
Immunofluorescence staining of transfected PAE cells was performed as follows: the cells grown on coverslips were fixed with 4% formaldehyde in PBS for 20 min and then washed three times with cold PBS. Permeabilization was performed with 5% FBS and 0.5% saponin in PBS for 1 h. The cells were then incubated with anti-PDGFRβ antibody overnight at +4 °C. The cells were washed three times with TBST (50 mm Tris-HCl, 150 mm NaCl, 0.1% Tween 20, pH 7.6) and then incubated for 1 h with anti-rabbit antibodies conjugated to phycoerythrin. The coverslips were washed, mounted on slides, and observed with a fluorescence microscope (630× magnification).
In Vitro Phosphorylation Assays
The receptors were immunoprecipitated from transiently transfected 293T cells as described above. To test receptor autophosphorylation, the cells were treated with 1 μm imatinib for 4 h prior to lysis. For in vitro kinase reactions, the immunoprecipitated receptors were incubated with 50 μm ATP (Fermentas) in 50 μl of 50 mm HEPES, pH 7.4, and 10 mm MgCl2. To test kinase activity toward an exogenous substrate, 10 μg of dephosphorylated myelin basic protein (MBP; Active Motif) were added to the reaction mixture. The reactions were incubated for 15 min at 30 °C, then stopped by the addition of Laemmli buffer, and analyzed by Western blot. Phosphorylated receptors and MBP were separated on 8 and 18% gels, respectively.
RESULTS
The PDGFRβ Transmembrane Domain Is Critical for TPβ-mediated Proliferation of Hematopoietic Cells
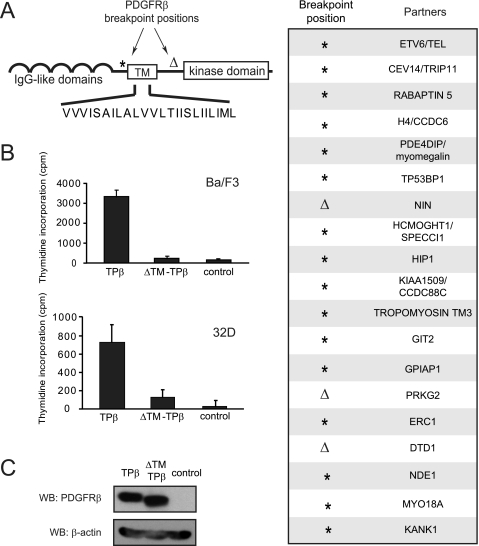
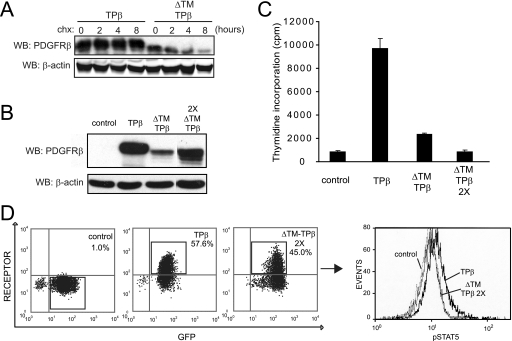
The PDGFRβ gene breakpoints that are associated with translocations and reported in hematological diseases are located either before or after the exon sequence encoding the receptor TM domain. Most of the translocation products reported so far retain the TM domain in the fusion protein (Fig. 1A, right panel). To understand the significance of this observation, we analyzed the role of the TM domain in TPβ, which is the most recurrent PDGFRβ hybrid form found in chronic myeloid malignancies. We created a mutant of TPβ devoid of TM sequence (ΔTM-TPβ) and examined its activity in the Ba/F3 and 32D hematopoietic cell lines. In line with previous reports, expression of TPβ in these cell lines supported short term proliferation in the absence of IL3 as detected in thymidine incorporation assays. TPβ-expressing cells could also be maintained in culture without IL3 for longer periods of time, becoming cytokine-independent cells. Expression of the ΔTM-TPβ mutant instead resulted in a dramatic reduction of IL3-independent cell growth in the short term proliferation assay (Fig. 1B). ΔTM-TPβ cells could not reproducibly generate cytokine-independent cell lines. Our results suggest that the TM sequence has an important role in TPβ transforming properties in hematopoietic cells.
FIGURE 1.
The PDGFRβ transmembrane domain is critical for TPβ-mediated proliferation of hematopoietic cells. A, the domain organization of PDGFRβ is shown with the two main breakpoint positions depicted with an asterisk and an open triangle. The position marked by an asterisk is usually in the intron preceding exon 11, which encodes the TM sequence, but breakpoints in introns before exon 9 or 10 were also reported. The sequence of the TM domain is shown in capital letters. In the right panel are listed the fusion partners of PDGFRβ with the corresponding breakpoint positions in PDGFRβ. B, Ba/F3 or 32D cell lines were transduced with TPβ or ΔTM-TPβ using a bicistronic retroviral vector encoding GFP and sorted according to GFP levels. The cells were incubated for 24 h in absence of IL3. Proliferation was measured by a [3H]thymidine incorporation assay as described under “Experimental Procedures.” All of the cell lines proliferated equally in the presence of IL3 (data not shown). S.D. were calculated from triplicate cultures in a representative experiment. Vector-transfected cells were used as a control. C, total cell lysates derived from the Ba/F3 cell lines used in B were analyzed by Western blot (WB) with anti-PDGFR and anti-β-actin antibodies. The total cell lysates derived from the 32D cell lines were analyzed similarly (shown in Fig. 2A).
TPβ Is Not a Membrane-spanning Protein
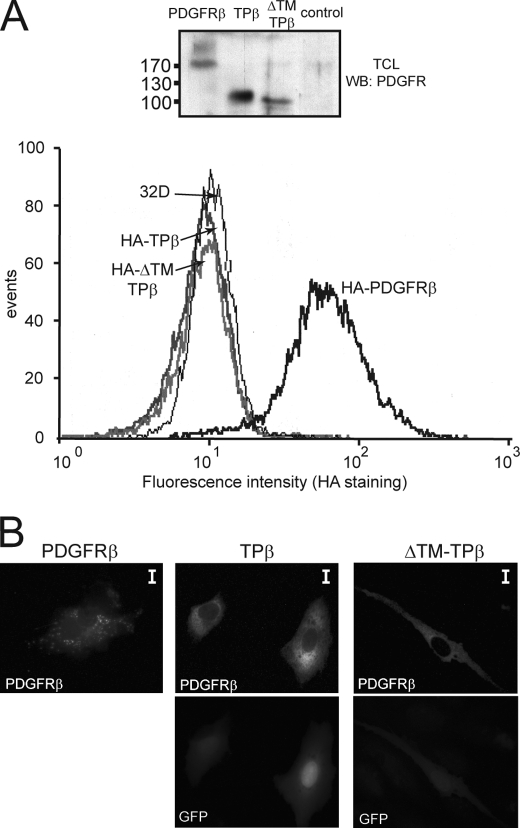
In TPβ, the sequence encoding the signal peptide and the extracellular domain of PDGFRβ is replaced by the sequence encoding the N-terminal part of TEL. Even though this makes the cell surface expression of the protein an unlikely event, the presence of the TM sequence could still allow the insertion into membranes. To check the plasma membrane localization of the fusion protein, we created tagged versions of TPβ and ΔTM-TPβ introducing an N-terminal HA tag and analyzed their expression by flow cytometry (Fig. 2A). HA-tagged wild-type PDGFRβ, which was used as positive control for the staining, showed a strong surface signal with the anti-HA antibody, whereas both forms of hybrid TPβ, with and without the TM domain, were negative for the anti-HA staining. Because the absence of cell surface expression of the hybrids did not exclude localization at the level of intracellular membranes, PAE cells were transfected with the receptors, stained with anti-PDGFRβ antibody, and analyzed by fluorescence microscopy (Fig. 2B). Activated wild-type PDGFRβ showed a typical punctuated pattern, which results from the internalized receptor complexes that are formed in the presence of the ligand (32). TPβ- and ΔTM-TPβ-expressing cells appeared with a diffuse staining in the cytoplasm and did not show accumulation of the proteins in any particular subcellular compartment like the endoplasmic reticulum or the Golgi. This was confirmed by confocal microscopy (data not shown) and was in line with a previous report (33). Altogether these observations confirmed that TPβ is not a membrane protein.
FIGURE 2.
TPβ is not a membrane spanning protein. A, intact 32D cells stably expressing the HA-tagged form of wild-type PDGFRβ, TPβ, or ΔTM-TPβ were stained with anti-HA antibodies and analyzed by flow cytometry. Untransfected 32D cells were used as control. Total cell lysates (TCL) from the same cell lines were analyzed by Western blot (WB) with anti-PDGFRβ antibodies. B, PAE cells were transfected with the indicated receptors and stained with anti-PDGFRβ antibodies and fluorescent secondary antibodies. The cells were analyzed by fluorescent microscopy. GFP is co-expressed with TPβ and ΔTM-TPβ from the bicistronic vector used for transfection. The scale bars correspond to 10 μm.
Deletion of the TM Domain Affects TPβ Cross-linking
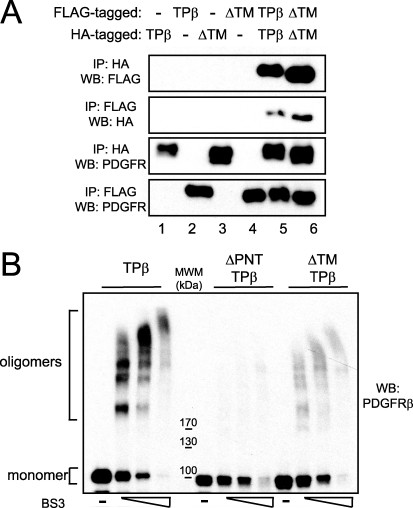
The pointed domain of TEL mediates oligomerization of TPβ, which is essential for the constitutive activation of the hybrid and transformation of hematopoietic cell lines (18). Recently, it has been shown that a purified peptide containing the TM domain of PDGFRβ is able to form dimers in vitro (34). We sought to determine whether the TM sequence could contribute to the oligomerization in the context of the hybrid receptor. We initially tested the ability of ΔTM-TPβ to self-associate by using a co-immunoprecipitation approach with two different tags. FLAG-tagged and HA-tagged versions of the hybrid were co-transfected in 293T cells, alone or in combination, as illustrated in Fig. 3A. After immunoprecipitation with anti-HA antibodies, the interaction between two differently tagged proteins was examined by Western blot against the FLAG tag and vice versa. As expected, HA- and FLAG-TPβ were co-immunoprecipitated (Fig. 3A, lane 5, two upper panels). Similar results were obtained with ΔTM-TPβ, indicating that TPβ self-association is retained in the ΔTM-TPβ mutant (lane 6, two upper panels). To further assess the oligomerization of ΔTM-TPβ, we performed cross-linking experiments in the presence of BS3, a cross-linker that has been previously used to study the oligomerization of TPβ (35). Cell lysates obtained from Ba/F3 cells expressing TPβ or ΔTM-TPβ were incubated with increasing doses of BS3, and oligomer formation was visualized by Western blot (Fig. 3B). As a negative control we used the ΔPNT-TPβ mutant, which lacks the domain required for oligomerization. As previously reported, high molecular species of TPβ protein were visible after treatment with the cross-linker, whereas only the monomeric form of ΔPNT-TPβ was visible, indicating that the TM domain alone does not induce aggregation in the absence of the PNT domain. In addition, ΔTM-TPβ could still form oligomers with a similar pattern of bands compared with TPβ but with decreased signal intensity. Altogether these experiments showed that the TM domain is not absolutely required for TPβ oligomerization but favors its cross-linking.
FIGURE 3.
ΔTM-TPβ oligomerization. A, 293T cells were transfected with the HA- or FLAG-tagged forms of TPβ or ΔTM-TPβ as indicated. Hybrid receptors were immunoprecipitated (IP) with anti-HA or anti-FLAG antibodies and then immunoblotted with anti-HA, anti-FLAG, or anti-PDGFRβ antibodies as indicated. B, cell lysates obtained from Ba/F3 cell lines expressing TPβ, ΔTM-TPβ, or ΔPNT-TPβ were left untreated or treated with increasing concentrations of BS3 as described under “Experimental Procedures.” The cell lysates were analyzed for the presence of oligomers by immunoblotting with anti-PDGFRβ antibodies. MWM, molecular weight marker. WB, Western blot.
ΔTM-TPβ Signal Transduction Is Decreased
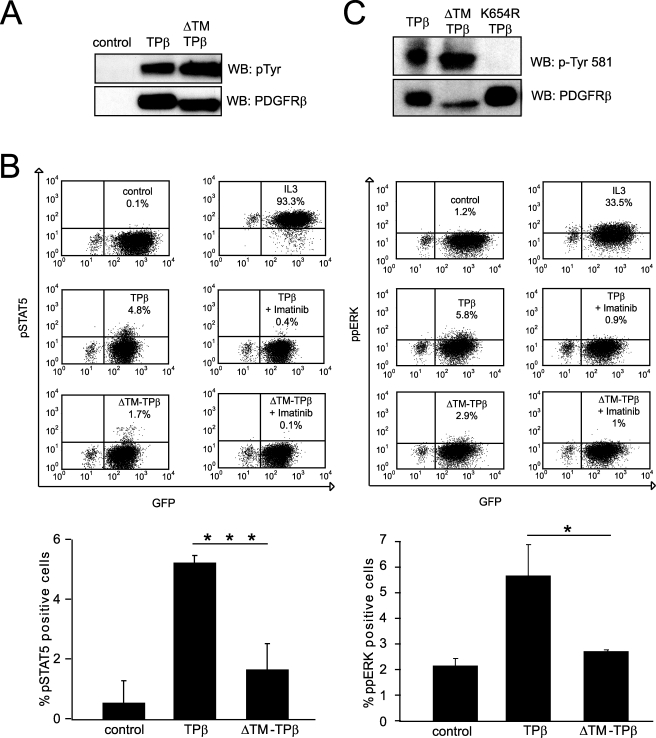
We next analyzed the phosphorylation of the ΔTM-TPβ mutant. Total cell lysates from Ba/F3 cell lines were immunoblotted with general anti-phosphotyrosine antibodies. Both TPβ and ΔTM-TPβ were strongly phosphorylated, as shown in Fig. 4A. To compare the signaling properties of the two proteins, we performed intracellular staining of phospho-STAT5 and phospho-ERK1/2 (Fig. 4B), which are two important signaling mediators of TPβ (19, 20, 23). As expected, TPβ induced the phosphorylation of both proteins in Ba/F3 cells. This effect was inhibited by treatment with imatinib (500 nm), which blocks selectively the PDGFRβ activity at that concentration (Fig. 4B). ΔTM-TPβ showed a much lower percentage of positive cells in comparison with TPβ in the staining for activated STAT5 and ERK. These data indicated that ΔTM-TPβ was phosphorylated but unable to signal effectively, in line with its inability to sustain cell growth.
FIGURE 4.
ΔTM-TPβ is phosphorylated but showed impaired STAT5 and ERK1/2 activation. A, total cell lysates from Ba/F3 cells expressing the indicated receptors were analyzed for receptor phosphorylation by Western blot (WB) with anti-phosphotyrosine antibodies and then reblotted with anti-PDGFRβ antibodies. Vector-transfected cells were used as control. B, Ba/F3 cells expressing the indicated hybrid receptors were starved for 4 h. The cells were then permeabilized and stained with anti-pSTAT5 or anti-ppERK antibodies and analyzed by flow cytometry as described under “Experimental Procedures.” As controls, the cells were treated with IL3 for 5 min or with imatinib for 4 h before the fixation step. The percentages of GFP and pSTAT5/ppERK-positive cells are indicated in the upper right quadrant of the dot plots shown. The averages of at least two independent experiments are shown in the histogram with S.D. *, p ≤ 0.05; ***, p ≤ 0.001. C, total cell lysates from TPβ or ΔTM-TPβ were analyzed by Western blot with anti-PDGFRβ phosphotyrosine 581 antibodies. The kinase inactive mutant K654R-TPβ was used as negative control for the specificity of the phosphotyrosine antibody. The filter was stripped and reblotted with anti-PDGFRβ antibodies.
Tyrosine 581, which is located within the juxtamembrane domain of PDGFRβ, has been described, together with tyrosine 579 and 775, as a docking site for STAT5 (36). Among the three sites, the sequence surrounding tyrosine 581 fits better the suggested consensus sequence for STAT5 recruitment (25), and its mutation in wild-type PDGFRβ has the strongest effect on STAT5 phosphorylation (36). Therefore, we assessed the phosphorylation of PDGFRβ tyrosine 581 using a phospho-specific antibody in Western blot experiments (24). Fig. 4C shows that tyrosine 581 was phosphorylated to a similar extent in ΔTM-TPβ and TPβ. As a control for the specificity of the antibody, we used a kinase-inactive mutant, K654R TPβ (Fig. 4C) (37). The observation that ΔTM-TPβ is strongly phosphorylated on tyrosines, including the most important STAT5 docking site, suggested that the inability of ΔTM-TPβ to signal was not due to a defect in phosphorylation.
Increased Degradation of ΔTM-TPβ
Because we observed that the level of expression of ΔTM-TPβ in Ba/F3 cells was lower than TPβ in some cell lines (Fig. 2, for instance), we analyzed the stability of the protein by inhibiting protein synthesis with cycloheximide. As shown in Fig. 5A, ΔTM-TPβ was degraded faster than TPβ, suggesting that the TM deletion destabilized the protein. To test whether the impaired oncogenic activity ΔTM-TPβ resulted from its lower level of expression, we transduced the ΔTM-TPβ cell line a second time and resorted the cells that had an increased GFP level. We obtained the ΔTM-TPβ2X cell line, which presents a higher level of expression as shown by Western blot and flow cytometry in Fig. 5. The protein level of ΔTM-TPβ2X was more comparable with the level of TPβ. Additional bands were observed on the ΔTM-TPβ2X Western blot, possibly because of proteolysis, alternative translation start, or post-translational modifications (38). We then analyzed the different Ba/F3 cell lines in IL3-independent proliferation assays (Fig. 5C). Both ΔTM-TPβ and ΔTM-TPβ2X had a much reduced level of proliferation in the absence of IL3 compared with TPβ. We next tested the signaling properties of these cells and performed intracellular staining for receptor and phospho-STAT5. We calculated the percentage of cells with activated STAT5 within the cell population positive for the receptor and GFP (Fig. 5D). The percentage of phospho-STAT5-positive cells was on average 3.6 ± 1.1 times higher in TPβ compared with ΔTM-TPβ 2X (n = 4). Altogether, these findings indicated that ΔTM-TPβ was expressed at a lower level, as a result of increased degradation. However, this may not be the only factor explaining the defect in ΔTM-TPβ signaling and proliferation.
FIGURE 5.
The ΔTM-TPβ protein is degraded faster. A, Ba/F3 cells expressing the indicated forms of hybrid receptors were used in stability assays with 50 μg/ml cycloheximide as described under “Experimental Procedures.” The samples were collected at different time points and analyzed by Western blot (WB) with anti-PDGFRβ and anti-β-actin antibodies. B, total cell lysates from Ba/F3 cells expressing TPβ, ΔTM-TPβ transduced only once with retroviruses (ΔTM-TPβ), or those transduced twice (ΔTM-TPβ2X) were analyzed with anti-PDGFRβ and anti-β-actin antibodies. Vector-transduced cells were used as a control. All of the samples were analyzed on the same Western blot, and some lanes were cut out of the final image for clarity. C, the same cell lines as in B were used in a proliferation assay as described for Fig. 1B. All of the cell lines proliferated equally in the presence of IL3 (data not shown). The cells transduced once or twice with TPβ proliferated at a similar level (data not shown). D, TPβ and ΔTM-TPβ2X cells were stained for phospho-STAT5 and PDGFRβ. The dot plots show the percentages of cells expressing GFP and PDGFRβ. The histogram shows the phospho-STAT5 staining in the population positive for GFP and for the PDGFRβ indicated by rectangles. TPβ is shown with a bold line, ΔTM-TPβ is shown with a thin line, and control is shown with a dashed line. One representative experiment is shown (n = 4). Vector-expressing cells were used as a control.
Deletion of the TM Domain Increases the in Vitro Kinase Activity of TPβ
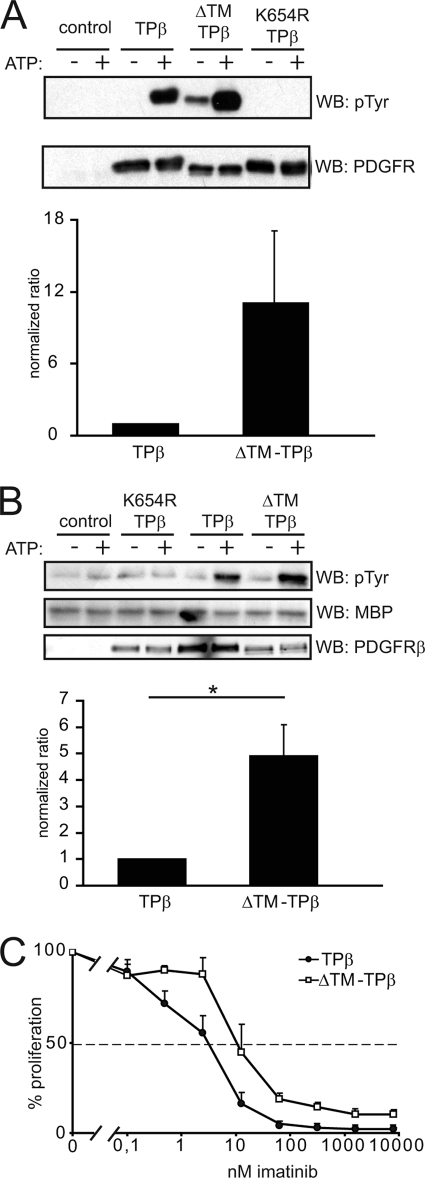
The experiments described above showed that TM deletion strongly impaired the ability of TPβ to activate signaling and transform hematopoietic cells but did not abolish receptor phosphorylation. We noticed that the general tyrosine phosphorylation of ΔTM-TPβ was slightly higher than TPβ. This observation prompted us to compare the activity of the kinase domain of the ΔTM-TPβ mutant versus TPβ in vitro. In a first set of experiments, we compared the ability of immunopurified receptors to autophosphorylate in the presence of ATP. Receptor-transfected cells were initially treated with imatinib, which prevented phosphorylation of tyrosine residues. The receptors were then immunoprecipitated, washed, and subjected to in vitro kinase assays in the absence or presence of ATP. As an additional negative control, we used the kinase-dead mutant K654R TPβ. The receptor phosphorylation was then quantified by immunoblotting with anti-phosphotyrosine antibodies. As shown in Fig. 6A, the autophosphorylation of the ΔTM-TPβ protein was increased compared with TPβ. Surprisingly, the treatment with imatinib did not completely dephosphorylate ΔTM-TPβ. This might be due to a difference in the sensitivity of ΔTM-TPβ to the drug in comparison with TPβ. In agreement with this hypothesis, when we exposed transduced Ba/F3 cells to increasing doses of imatinib, a higher concentration was required to inhibit the proliferation of cells expressing ΔTM-TPβ (Fig. 6C). This difference in imatinib IC50 might reflect a change in the protein conformation, which could bind imatinib less efficiently (39).
FIGURE 6.
Deletion of the TM domain increases TPβ kinase activity in vitro. A, 293T cells were transfected with the indicated forms of the receptors and treated with 1 μm imatinib for 4 h to prevent receptor phosphorylation. Hybrid receptors were then immunoprecipitated with anti-PDGFRβ antibodies, extensively washed to remove imatinib, and used in an in vitro phosphorylation assay in the presence or absence of ATP. The reaction products were analyzed by immunoblotting with anti-phosphotyrosine and anti-PDGFRβ antibodies. The blots were quantified using an Odyssey instrument. Phosphorylation data were normalized by dividing by the total amount of receptor after background substraction (values in the absence of ATP). The results from two independent experiments were expressed as average fold increase compared with TPβ. B, receptors were expressed in cells and treated as in A except that the imatinib treatment was not applied. The in vitro reactions were performed in the presence of 10 μg of MBP. The reaction products were analyzed by anti-phosphotyrosine and anti-MBP immunoblotting. MBP phosphorylation values were normalized by dividing by the total amount of receptor, after background subtraction. The results from two independent experiments were expressed as average fold increase compared with TPβ. C, Ba/F3 cells expressing TPβ or ΔTM-TPβ were used in [3H]thymidine incorporation assays in the presence of increasing doses of imatinib. Proliferation was expressed as a percentage of the condition without imatinib. WB, Western blot.
We next assessed the activity of hybrid receptors toward the exogenous substrate MBP in an in vitro kinase assay (Fig. 6B). In this experiment, as in the autophosphorylation assay, the ΔTM-TPβ mutant performed better than TPβ. This was particularly clear in the quantitative analysis of the results. In conclusion, the ΔTM-TPβ mutant harbored an increased in vitro kinase activity toward itself and toward exogenous substrates, in comparison with TPβ.
ΔTM-TPβ Can Recruit Signaling Proteins
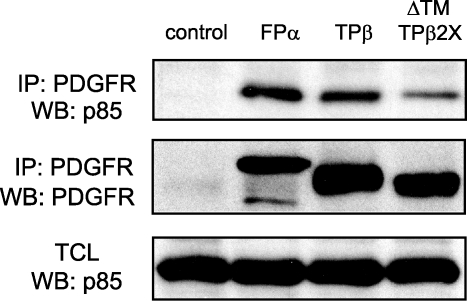
The strong in vitro catalytic activity of ΔTM-TPβ was surprising because the mutant was unable to signal efficiently in cells. This discrepancy could be explained by a defective recruitment of signaling proteins by ΔTM-TPβ. We could not detect the association with STAT5 with TPβ by co-immunoprecipitation (data not shown), most likely because phosphorylated STAT5 quickly dissociates from the receptor and migrates to the nucleus. Then we tested the association with another signaling protein, the phosphatidylinositol 3-kinase regulatory subunit p85, in Ba/F3 cell lines. As shown in Fig. 7, endogenous p85 was co-immunoprecipitated with TPβ as well as with ΔTM-TPβ. As an additional control, we used cells expressing the FIP1L1-PDGFRα hybrid protein, which also interacted with p85. This experiment shows that the ΔTM-TPβ mutant has the ability to recruit signaling proteins such as p85
FIGURE 7.
ΔTM-TPβ can associate with p85. The indicated receptors were immunoprecipitated (IP) from stably expressing Ba/F3 cell lines and immunoblotted with anti-p85 and anti-PDGFR antibodies. Total cell lysates were analyzed with anti-p85 antibodies. FIP1L1-PDGFRα- (FPα) and vector-expressing cells were used as a control. WB, Western blot.
TM Sequence Requirements for Transformation by TPβ
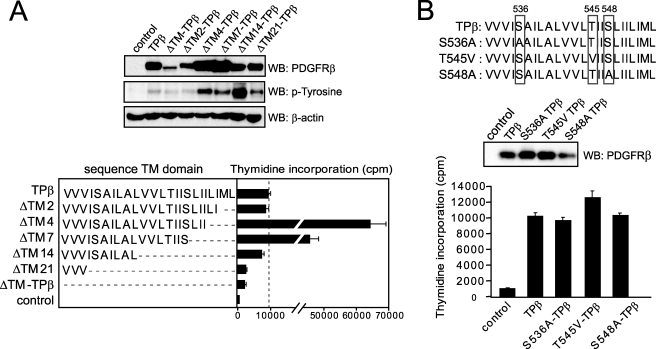
To determine which part of the TM sequence was required for TPβ activation, we performed sequential deletions and tested the mutants in Ba/F3 cell proliferation assays. As shown in Fig. 8A, deletion of 14 amino acid residues had no impact on cell growth, whereas deletion of 21 amino acids recapitulated the effect of the ΔTM mutation. These results suggested that a short hydrophobic sequence is enough to preserve TPβ activity. Deletion of 4 and 7 amino acids consistently resulted in much higher levels of protein expression. Thus, we could not make a conclusion regarding the effect of these deletions on cell proliferation.
FIGURE 8.
Transmembrane sequence requirements for optimal TPβ activation. A, the transmembrane sequence of each TPβ deletion mutant is shown. Mutant hybrids were transduced in Ba/F3 cells, and their ability to stimulate cell growth in the absence of cytokine was tested by thymidine incorporation as described for Fig. 1B. Vector-transfected cells were used as control. All of the cell lines proliferated equally in the presence of IL3 (data not shown). The total cell lysates were analyzed by Western blot (WB) with anti-phosphotyrosine, anti-PDGFRβ, and anti-β-actin antibodies. B, the same experiments were performed with the TPβ mutants S536A, T545V, and S548A.
The PDGFRβ TM sequence contains two serine and one threonine residue within the hydrophobic stretch (boxed residues in Fig. 8B). In particular, threonine 545 has been shown to be important for a productive interaction between PDGFRβ and the E5 protein of bovine papilloma virus in membranes (40), and serine 536 is one of the amino acids lost in ΔTM21 compared with ΔTM14. To analyze the role of these three amino acids in the activation of TPβ, we mutated the serine residues into alanine and the threonine into a valine and tested transduced Ba/F3 cell lines for IL3-independent proliferation. As shown in Fig. 8B, the cells expressing the mutants proliferated similarly compared with cells expressing TPβ, suggesting that these polar residues are dispensable for the activation of TPβ.
Interruption of the Juxtamembrane Domain Can Rescue the Activity of ΔTM-TPβ
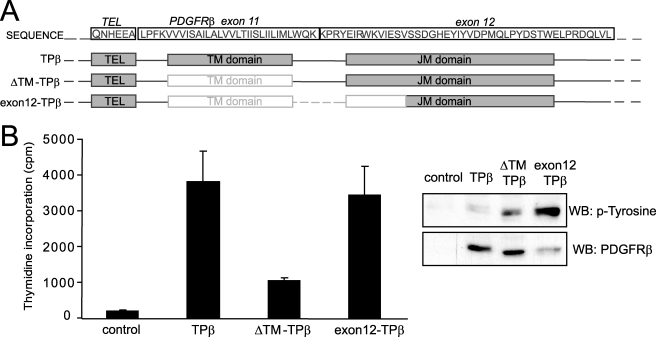
Breakpoints falling before the TM domain are generally located in the large intron between exons 10 and 11, which encodes the TM domain, as illustrated for TPβ in Fig. 9A. This particular position could either reflect the requirement of a TM domain for proper activation of the hybrids or a certain weakness in this chromosomal region. Breakpoints falling within the exon 12, which encodes the juxtamembrane region, have been reported in a few cases such as the PRKG2-PDGFRβ fusion, in which approximately one-third of the juxtamembrane (JM) domain is deleted (41).
FIGURE 9.
Interruption of the juxtamembrane domain can overcome the effect of the deletion of the TM domain in TPβ. A, the positions of the TM and JM domains are indicated in the gray boxes below the amino acid sequence of TPβ. The deletions in ΔTM-TPβ and in exon12-TPβ are indicated by faded boxes. B, Ba/F3 cell lines expressing the indicated forms of hybrid receptors were used in a proliferation assay as described for Fig. 1B. The total cell lysates derived from the same cells were analyzed by Western blot (WB) with anti-PDGFRβ and anti-phosphotyrosine antibodies. All of the cell lines proliferated equally in the presence of IL3 (data not shown).
We generated a deletion in TPβ removing the TM domain and a portion of the juxtamembrane domain (exon12-TPβ; Fig. 9A) and tested transduced Ba/F3 cells for cytokine-independent proliferation. We observed that the exon12-TPβ mutant sustained cell growth to an extent similar to TPβ (Fig. 9B), although it was expressed at a lower level. The phosphorylation of exon12-TPβ was stronger than TPβ, similar to what we observed for ΔTM-TPβ (Fig. 9B). Altogether our observations suggested that the presence of a TM domain in PDGFRβ hybrids is not an absolute requirement, provided that the JM domain is also deleted.
DISCUSSION
Here, we show that the transmembrane domain of PDGFRβ has a crucial role in the transformation of hematopoietic cells by TPβ. This was surprising because such a hydrophobic stretch can destabilize cytosolic proteins. Nevertheless, evidence from a number of receptor studies have shown that TM sequences are important for orientation and stabilization of active dimeric membrane receptors (34, 42–44).
Deletion of the TPβ TM sequence did not seem to change the subcellular localization of the protein, because cell surface and intracellular staining indicated that TPβ and ΔTM-TPβ reside in the cytosol, in line with previous reports (33). In addition, the ΔTM14-TPβ mutant, which retained only 10 residues of the TM sequence, was still active, although its TM domain is most likely too short to span a lipid bilayer.
The ΔTM-TPβ protein was expressed at a lower level, because of an increased degradation, which likely contributes to its lack of transforming activity, in line with published results (22). However, other mechanisms must be involved, because increasing the expression of ΔTM-TPβ did not augment cell signaling and proliferation.
The cross-linking of ΔTM-TPβ induced a pattern of bands that were similar in size to those observed with TPβ but was consistently less efficient. This might reflect a decreased oligomerization of ΔTM-TPβ. However, the co-immunoprecipitation experiment was unaffected by the TM deletion. In addition, ΔTM-TPβ phosphorylation and kinase activity were not reduced, as one would expect if oligomerization was impaired. For these reasons, we speculate that decreased cross-linking may reflect an altered disposition of the polypeptides, which exposes fewer residues to the cross-linker, decreasing the efficiency of the cross-linking reaction. This is further supported by recent data showing that the purified TM domain of PDGFRβ can be cross-linked (34). Thus, it is possible that the TM domains are clustered in the TPβ oligomer, even though they are not required for the oligomerization process itself, driven by the PNT domain.
The in vitro kinase assays showed that the ΔTM-TPβ kinase activity was enhanced, whereas the sensitivity to imatinib was reduced in comparison with TPβ. It is known that imatinib binds to the kinase ATP-binding pocket in its inactive state. The reduced sensitivity of ΔTM-TPβ to imatinib suggested that the conformation of its ATP-binding pocket is modified in a way that makes it unable to fit imatinib as efficiently as in TPβ. Noticeably, the fact that the removal of the hydrophobic sequence from TPβ reduced protein stability could also indicate that the ΔTM mutation alters TPβ folding. These data suggest a model in which the TM domain contributes to the activation of TPβ by imposing to the PDGFRβ kinase domain a conformation that is optimal for signaling. Further work on the PDGFR structure may indicate whether this is a valid hypothesis.
We observed a reduced activation of STAT5 and ERK1/2 in ΔTM-TPβ cells, which contrasted with its enhanced kinase activity and phosphorylation. ΔTM-TPβ protein was also able to associate with signaling proteins like p85, which is expected if the hybrid receptor is properly phosphorylated. It was also able to phosphorylate an exogenous substrate such as MBP, at least in vitro, but failed to induce the phosphorylation of STAT5 in cells. One possible explanation for this discrepancy could be that the hyperactivation of the ΔTM-TPβ kinase results in the recruitment of a negative regulator, such as a tyrosine phosphatase.
The TM sequence of PDGFRβ is predicted to adopt an α-helical conformation and to determine the relative orientation of the catalytic subunits in the dimeric receptor (34, 45, 46). The way in which two adjacent kinase domains face each other was shown to be critical for receptor activation in experiments where a dimerization motif was shifted in the PDGFRβ TM domain and caused periodic activation of the receptor (47). Deletion of two amino acids produces a rotation that abolishes the activity of PDGFRβ and other receptors such as Neu or the erythropoietin receptor (47, 48). In TPβ, the deletion of two amino acids was expected to cause a rotation of two adjacent kinase domains of half a turn, but we did not observe any inactivation of TPβ, which argues against a role for the TM domain in providing proper orientation to the kinase subunits in this case. This might be related to the fact that the pointed domain induces the polymerization of TPβ, whereas the wild-type receptor undergoes dimerization (34, 35, 49).
The constitutive activation of the kinase domain of receptor tyrosine kinases is generally believed to be sufficient for malignant transformation of cells. However, in the present report we showed that ΔTM-TPβ presented a high kinase activity in vitro and is phosphorylated in cells but is unable to activate STAT5 and ERK1/2 and to support cell proliferation. This indicates that the constitutive activation of the kinase domain is not enough to transform cells.
Our results with the exon12-TPβ mutant showed that a breakpoint in exon 12, with the consequent deletion of part of the inhibitory JM domain, is able to overcome the lack of the TM domain. This goes in line with previous reports describing the disruption of the JM domain as an alternative mechanism of activation for PDGFRβ, independently from ligand-induced dimerization and from fusion with oligomerization domains (50). Thus, the TM domain seemed to be required only in PDGFRβ hybrids that have an intact JM domain, which represent the majority of the cases described so far.
Most receptor tyrosine kinase fusion products include a dimerization domain in addition to the kinase domain. The present work suggests that the linker sequence between these two domains may also play an important role in the efficient activation of the oncoprotein. This is in line with recent reports pointing to key conformational changes in the region between the ligand-binding domain and the kinase domain of PDGFRβ and c-KIT upon receptor dimerization (3, 4). In conclusion, our work revealed a new role for the PDGFRβ TM domain in the context of the cytosolic TPβ protein and possibly of other hybrid oncoproteins derived from PDGFRβ.
Acknowledgments
We are grateful to A. Tonon for excellent technical assistance with cell sorting and to Dr. S. Constantinescu for very helpful discussions.
This work was supported by grants from the Bekales Foundation, from Action de Recherche Concertée (Communauté Française de Belgique), and from the Salus Sanguinis Foundation, Belgium.
- PDGFR
- platelet-derived growth factor receptor
- STAT5
- signal transducers and activator of transcription 5
- ERK
- extracellular signal-regulated kinase
- PNT
- pointed domain
- TM
- transmembrane domain
- JM
- juxtamembrane domain
- IL
- interleukin
- BS3
- bis(sulfosuccinimidyl)-suberate
- FBS
- fetal bovine serum
- HA
- hemagglutinin
- GFP
- green fluorescent protein
- PAE
- porcine aortic endothelial
- DMEM
- Dulbecco's modified Eagle's medium
- PBS
- phosphate-buffered saline
- MBP
- myelin basic protein.
REFERENCES
- 1.Andrae J., Gallini R., Betsholtz C. (2008) Genes Dev. 22, 1276–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schlessinger J. (2000) Cell 103, 211–225 [DOI] [PubMed] [Google Scholar]
- 3.Yang Y., Yuzawa S., Schlessinger J. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 7681–7686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yuzawa S., Opatowsky Y., Zhang Z., Mandiyan V., Lax I., Schlessinger J. (2007) Cell 130, 323–334 [DOI] [PubMed] [Google Scholar]
- 5.Chan P. M., Ilangumaran S., La Rose J., Chakrabartty A., Rottapel R. (2003) Mol. Cell. Biol. 23, 3067–3078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Griffith J., Black J., Faerman C., Swenson L., Wynn M., Lu F., Lippke J., Saxena K. (2004) Mol. Cell 13, 169–178 [DOI] [PubMed] [Google Scholar]
- 7.Irusta P. M., Luo Y., Bakht O., Lai C. C., Smith S. O., DiMaio D. (2002) J. Biol. Chem. 277, 38627–38634 [DOI] [PubMed] [Google Scholar]
- 8.Irusta P. M., DiMaio D. (1998) EMBO J. 17, 6912–6923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakao M., Yokota S., Iwai T., Kaneko H., Horiike S., Kashima K., Sonoda Y., Fujimoto T., Misawa S. (1996) Leukemia 10, 1911–1918 [PubMed] [Google Scholar]
- 10.Chiara F., Bishayee S., Heldin C. H., Demoulin J. B. (2004) J. Biol. Chem. 279, 19732–19738 [DOI] [PubMed] [Google Scholar]
- 11.Demoulin J. B., Enarsson M., Larsson J., Essaghir A., Heldin C. H., Forsberg-Nilsson K. (2006) Growth Factors 24, 184–196 [DOI] [PubMed] [Google Scholar]
- 12.Demoulin J. B., Ericsson J., Kallin A., Rorsman C., Rönnstrand L., Heldin C. H. (2004) J. Biol. Chem. 279, 35392–35402 [DOI] [PubMed] [Google Scholar]
- 13.Essaghir A., Dif N., Marbehant C. Y., Coffer P. J., Demoulin J. B. (2009) J. Biol. Chem. 284, 10334–10342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones A. V., Cross N. C. (2004) Cell Mol. Life Sci. 61, 2912–2923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gotlib J., Cools J. (2008) Leukemia 22, 1999–2010 [DOI] [PubMed] [Google Scholar]
- 16.Medves S., Duhoux F., Ferrant A., Toffalini F., Ameye G., Libouton J. M., Poirel H. A., Demoulin J. B. (2010) Leukemia, in press [DOI] [PubMed] [Google Scholar]
- 17.Golub T. R., Barker G. F., Lovett M., Gilliland D. G. (1994) Cell 77, 307–316 [DOI] [PubMed] [Google Scholar]
- 18.Carroll M., Tomasson M. H., Barker G. F., Golub T. R., Gilliland D. G. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 14845–14850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sternberg D. W., Tomasson M. H., Carroll M., Curley D. P., Barker G., Caprio M., Wilbanks A., Kazlauskas A., Gilliland D. G. (2001) Blood 98, 3390–3397 [DOI] [PubMed] [Google Scholar]
- 20.Tomasson M. H., Sternberg D. W., Williams I. R., Carroll M., Cain D., Aster J. C., Ilaria R. L., Jr., Van Etten R. A., Gilliland D. G. (2000) J. Clin. Invest. 105, 423–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cain J. A., Xiang Z., O'Neal J., Kreisel F., Colson A., Luo H., Hennighausen L., Tomasson M. H. (2007) Blood 109, 3906–3914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toffalini F., Kallin A., Vandenberghe P., Pierre P., Michaux L., Cools J., Demoulin J. B. (2009) Haematologica 94, 1085–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dobbin E., Graham C., Corrigan P. M., Thomas K. G., Freeburn R. W., Wheadon H. (2009) Exp. Hematol. 37, 111–121 [DOI] [PubMed] [Google Scholar]
- 24.Persson C., Sävenhed C., Bourdeau A., Tremblay M. L., Markova B., Böhmer F. D., Haj F. G., Neel B. G., Elson A., Heldin C. H., Rönnstrand L., Ostman A., Hellberg C. (2004) Mol. Cell. Biol. 24, 2190–2201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Demoulin J. B., Uyttenhove C., Van Roost E., DeLestré B., Donckers D., Van Snick J., Renauld J. C. (1996) Mol. Cell. Biol. 16, 4710–4716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Emanuelsson O., Brunak S., von Heijne G., Nielsen H. (2007) Nat. Protoc. 2, 953–971 [DOI] [PubMed] [Google Scholar]
- 27.Demoulin J. B., Seo J. K., Ekman S., Grapengiesser E., Hellman U., Rönnstrand L., Heldin C. H. (2003) Biochem. J. 376, 505–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Demoulin J. B., Grasso L., Atkins J. M., Stevens M., Louahed J., Levitt R. C., Nicolaides N. C., Renauld J. C. (2000) FEBS Lett. 482, 200–204 [DOI] [PubMed] [Google Scholar]
- 29.Classen J. F., Henrohn D., Rorsman F., Lennartsson J., Lauwerys B. R., Wikström G., Rorsman C., Lenglez S., Franck-Larsson K., Tomasi J. P., Kämpe O., Vanthuyne M., Houssiau F. A., Demoulin J. B. (2009) Arthritis Rheum. 60, 1137–1144 [DOI] [PubMed] [Google Scholar]
- 30.Staerk J., Kallin A., Demoulin J. B., Vainchenker W., Constantinescu S. N. (2005) J. Biol. Chem. 280, 41893–41899 [DOI] [PubMed] [Google Scholar]
- 31.Abramoff M. D., Magelhaes P. J., Ram S. J. (2004) Biophotonic Int. 11, 36–42 [Google Scholar]
- 32.Hellberg C., Schmees C., Karlsson S., Ahgren A., Heldin C. H. (2009) Mol. Biol. Cell 20, 2856–2863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jousset C., Carron C., Boureux A., Quang C. T., Oury C., Dusanter-Fourt I., Charon M., Levin J., Bernard O., Ghysdael J. (1997) EMBO J. 16, 69–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oates J., King G., Dixon A. M. (2009) Biochim. Biophys. Acta 1798, 605–615 [DOI] [PubMed] [Google Scholar]
- 35.Sjöblom T., Boureux A., Rönnstrand L., Heldin C. H., Ghysdael J., Ostman A. (1999) Oncogene 18, 7055–7062 [DOI] [PubMed] [Google Scholar]
- 36.Valgeirsdóttir S., Paukku K., Silvennoinen O., Heldin C. H., Claesson-Welsh L. (1998) Oncogene 16, 505–515 [DOI] [PubMed] [Google Scholar]
- 37.Sorkin A., Westermark B., Heldin C. H., Claesson-Welsh L. (1991) J. Cell Biol. 112, 469–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poirel H., Oury C., Carron C., Duprez E., Laabi Y., Tsapis A., Romana S. P., Mauchauffe M., Le Coniat M., Berger R., Ghysdael J., Bernard O. A. (1997) Oncogene 14, 349–357 [DOI] [PubMed] [Google Scholar]
- 39.Schindler T., Bornmann W., Pellicena P., Miller W. T., Clarkson B., Kuriyan J. (2000) Science 289, 1938–1942 [DOI] [PubMed] [Google Scholar]
- 40.Nappi V. M., Schaefer J. A., Petti L. M. (2002) J. Biol. Chem. 277, 47149–47159 [DOI] [PubMed] [Google Scholar]
- 41.Walz C., Metzgeroth G., Haferlach C., Schmitt-Graeff A., Fabarius A., Hagen V., Prümmer O., Rauh S., Hehlmann R., Hochhaus A., Cross N. C., Reiter A. (2007) Haematologica 92, 163–169 [DOI] [PubMed] [Google Scholar]
- 42.Lu X., Gross A. W., Lodish H. F. (2006) J. Biol. Chem. 281, 7002–7011 [DOI] [PubMed] [Google Scholar]
- 43.Mendrola J. M., Berger M. B., King M. C., Lemmon M. A. (2002) J. Biol. Chem. 277, 4704–4712 [DOI] [PubMed] [Google Scholar]
- 44.Roth L., Nasarre C., Dirrig-Grosch S., Aunis D., Crémel G., Hubert P., Bagnard D. (2008) Mol. Biol. Cell 19, 646–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gullick W. J., Bottomley A. C., Lofts F. J., Doak D. G., Mulvey D., Newman R., Crumpton M. J., Sternberg M. J., Campbell I. D. (1992) EMBO J. 11, 43–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petti L. M., Reddy V., Smith S. O., DiMaio D. (1997) J. Virol. 71, 7318–7327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bell C. A., Tynan J. A., Hart K. C., Meyer A. N., Robertson S. C., Donoghue D. J. (2000) Mol. Biol. Cell 11, 3589–3599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seubert N., Royer Y., Staerk J., Kubatzky K. F., Moucadel V., Krishnakumar S., Smith S. O., Constantinescu S. N. (2003) Mol. Cell 12, 1239–1250 [DOI] [PubMed] [Google Scholar]
- 49.Eriksson A., Rorsman C., Ernlund A., Claesson-Welsh L., Heldin C. H. (1992) Growth Factors 6, 1–14 [DOI] [PubMed] [Google Scholar]
- 50.Stover E. H., Chen J., Folens C., Lee B. H., Mentens N., Marynen P., Williams I. R., Gilliland D. G., Cools J. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 8078–8083 [DOI] [PMC free article] [PubMed] [Google Scholar]