Abstract
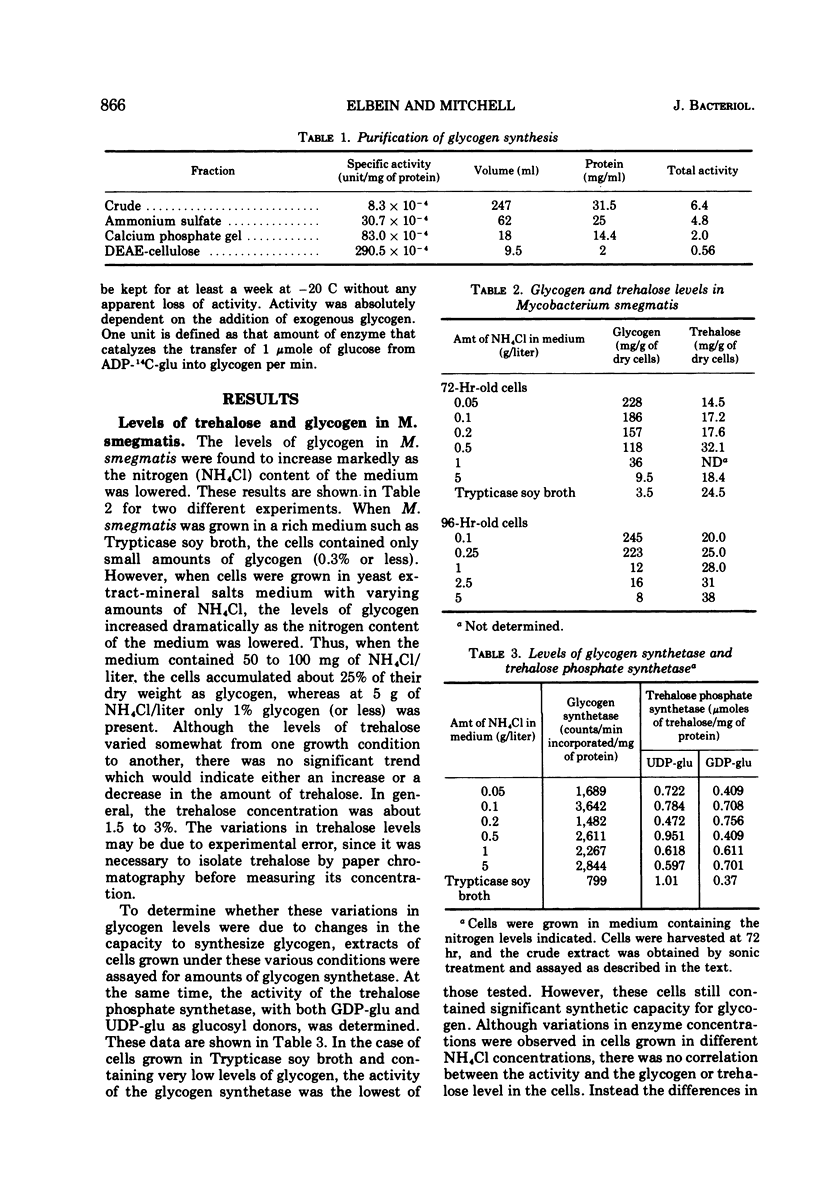
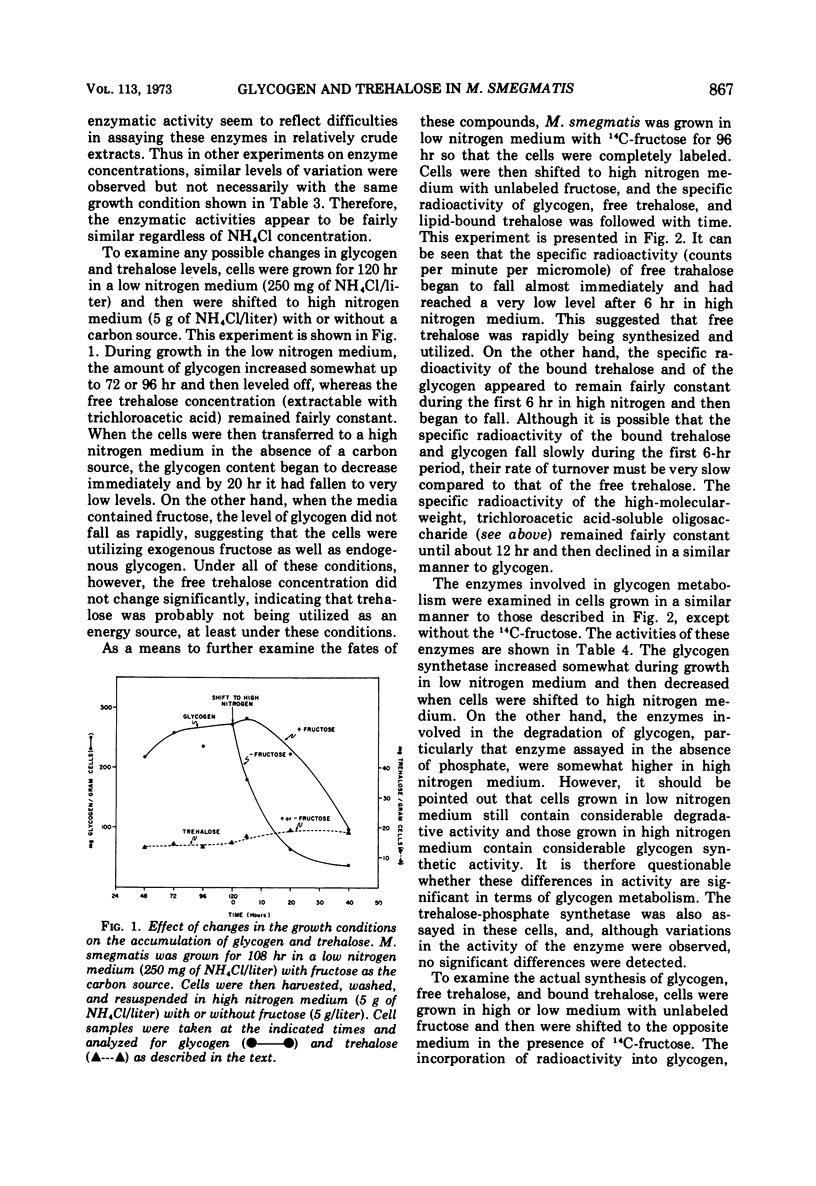
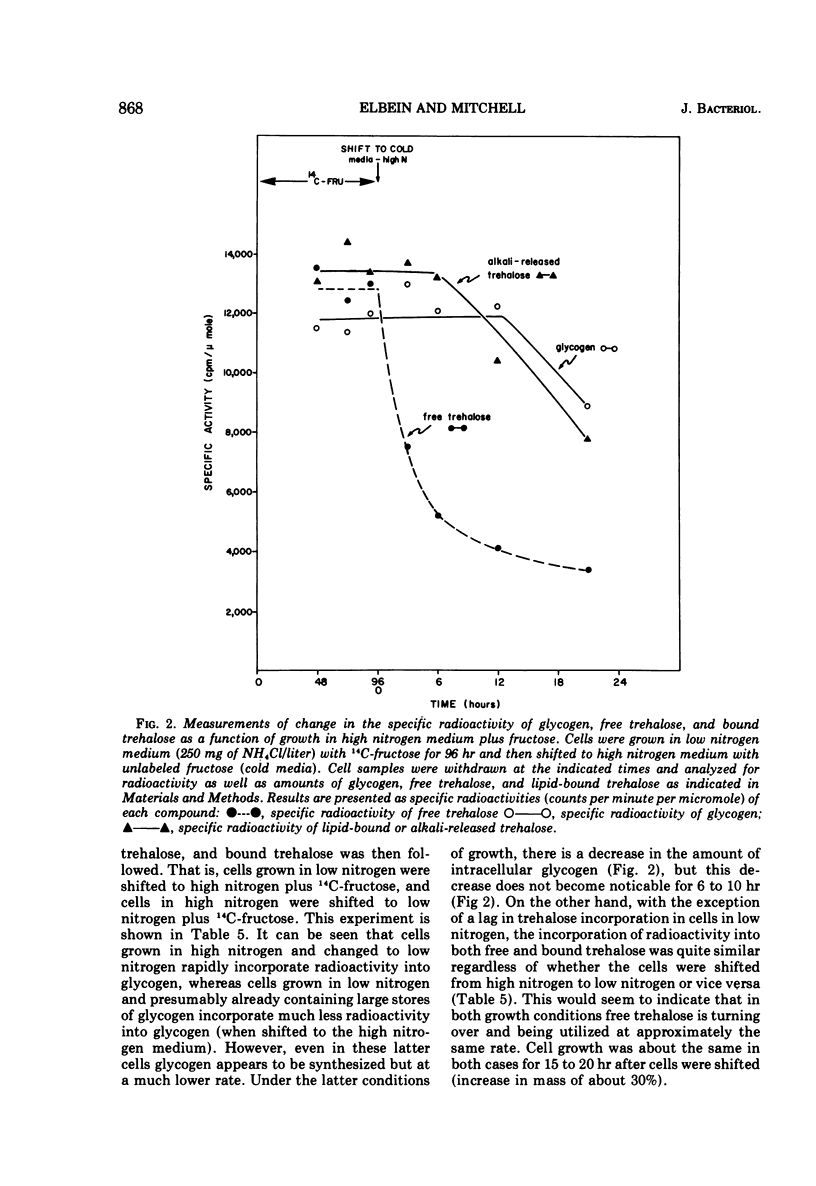
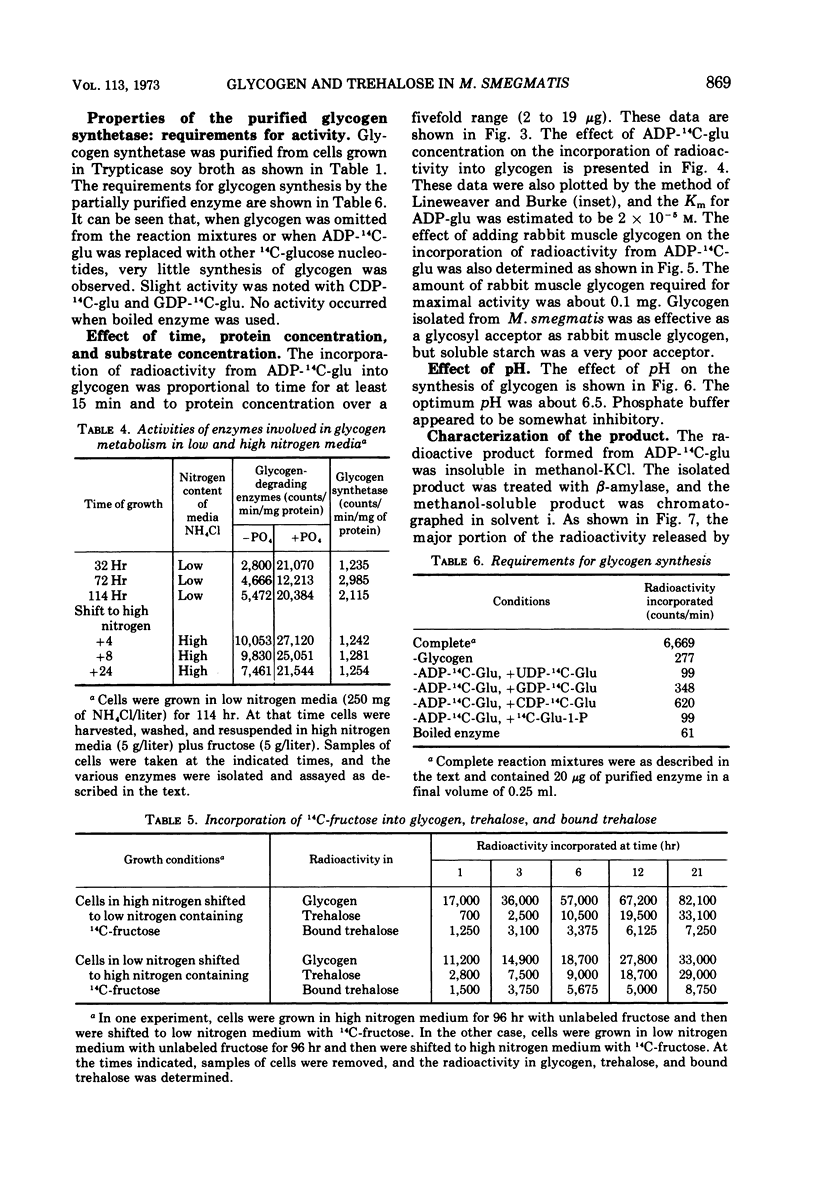
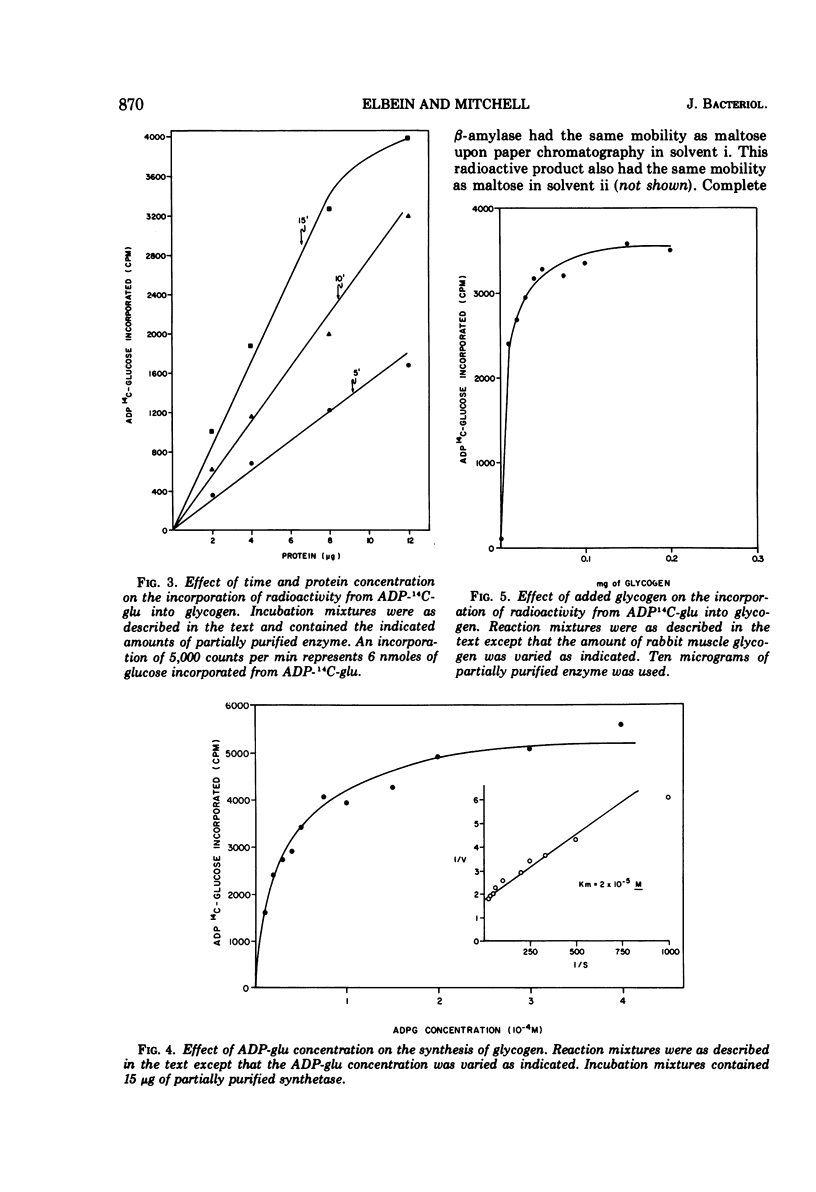
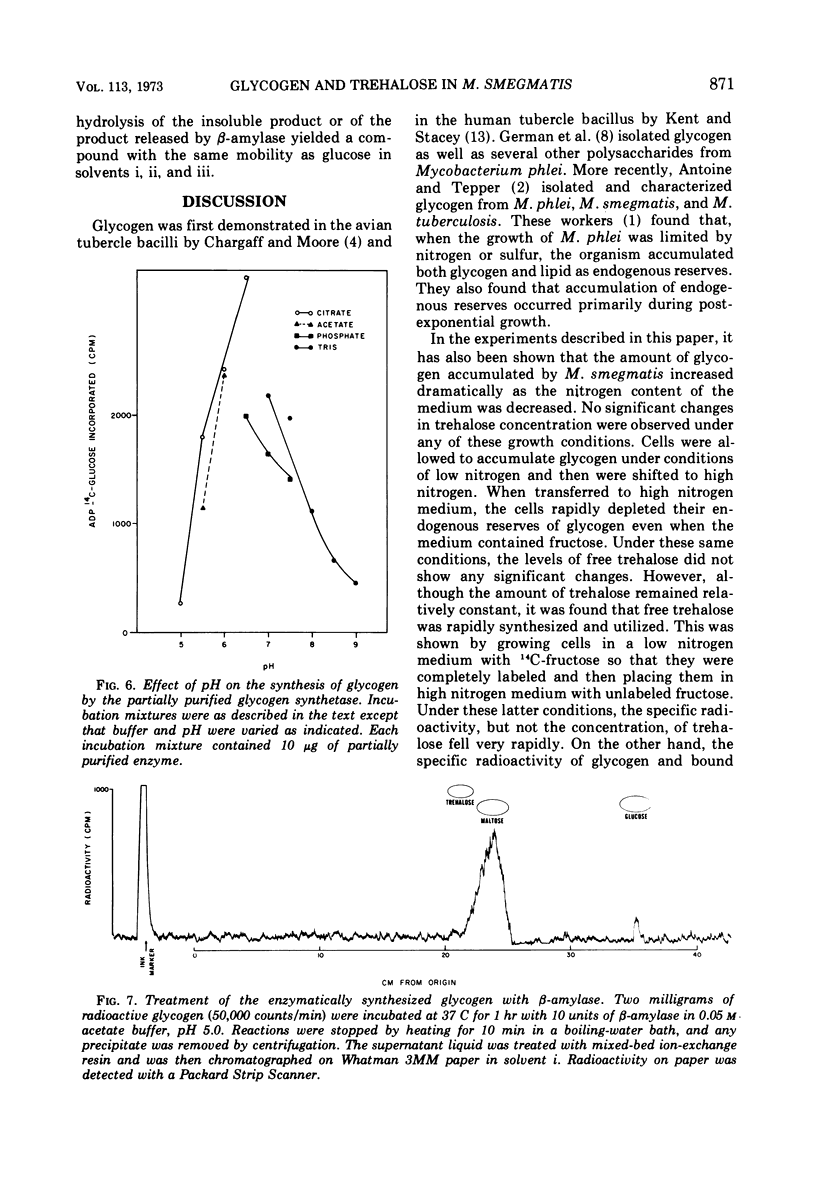
The levels of glycogen, free trehalose, and lipid-bound trehalose were compared in Mycobacterium smegmatis grown under various conditions of nitrogen limitation. In a mineral salts medium supplemented with yeast extract and containing fructose as the carbon source, the accumulation of glycogen increased dramatically as the NH4Cl content of the medium was lowered. However, levels of free trehalose remained relatively constant. Cells were grown in low nitrogen medium and were then shifted to medium containing high nitrogen. Under these conditions, there was a rapid accumulation of glycogen in low nitrogen, and this glycogen was rapidly depleted when cells were placed in high nitrogen medium. Again the concentration of free trehalose remained fairly constant. However, when cells were grown in low nitrogen medium with [14C]fructose and then transferred to high nitrogen medium with unlabeled fructose, the specific radioactivity (counts per minute per micromole) of the free trehalose fell immediately, indicating that it was being synthesized and turned over continually. On the other hand, the specific radioactivity of the glycogen and bound trehalose declined much more slowly, suggesting that these two compounds were not turning over as rapidly or were being synthesized at a much slower rate. Experiments on the incorporation of [14C]fructose into glycogen and trehalose indicated that cells in high nitrogen medium synthesized much less glycogen than those in low nitrogen. However, synthesis of both free trehalose and bound trehalose was the same in both cases. The specific enzymatic activities of the glycogen synthetase and the trehalose phosphate synthetase varied somewhat from one growth condition to another, but there was no correlation between enzymatic activity and the amount of glycogen or trehalose, suggesting that changes in glycogen levels were not due to increased synthetic capacity. The glycogen synthetase was purified about 35-fold and its properties were examined. This enzyme was specific for adenosine diphosphate glucose as the glucosyl donor.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoine A. D., Tepper B. S. Characterization of glycogens from mycobacteria. Arch Biochem Biophys. 1969 Oct;134(1):207–213. doi: 10.1016/0003-9861(69)90267-7. [DOI] [PubMed] [Google Scholar]
- Antoine A. D., Tepper B. S. Environmental control of glycogen and lipid content of Mycobacterium phlei. J Gen Microbiol. 1969 Feb;55(2):217–226. doi: 10.1099/00221287-55-2-217. [DOI] [PubMed] [Google Scholar]
- BIRCH G. G. TREHALOSES. Adv Carbohydr Chem. 1963;18:201–225. doi: 10.1016/s0096-5332(08)60243-x. [DOI] [PubMed] [Google Scholar]
- DAWES E. A., RIBBONS D. W. SOME ASPECTS OF THE ENDOGENOUS METABOLISM OF BACTERIA. Bacteriol Rev. 1964 Jun;28:126–149. doi: 10.1128/br.28.2.126-149.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbein A. D. Carbohydrate metabolism in streptomycetes. II. Isolation and enzymatic synthesis of trehalose. J Bacteriol. 1967 Nov;94(5):1520–1524. doi: 10.1128/jb.94.5.1520-1524.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbein A. D. Trehalose phosphate synthesis in Streptomyces hygroscopicus: purification of guanosine diphosphate D-glucose: D-glucose-6-phosphate 1-glucosyl-transferase. J Bacteriol. 1968 Nov;96(5):1623–1631. doi: 10.1128/jb.96.5.1623-1631.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERMAN R. J., JONES A. S., NADARAJAH M. Polysaccharides of Mycobacterium phlei. Nature. 1961 Mar 25;189:1008–1009. doi: 10.1038/1891008a0. [DOI] [PubMed] [Google Scholar]
- Goren M. B. Sulfolipid I of Mycobacterium tuberculosis, strain H37Rv. I. Purification and properties. Biochim Biophys Acta. 1970 Jun 9;210(1):116–126. doi: 10.1016/0005-2760(70)90067-6. [DOI] [PubMed] [Google Scholar]
- Hey-Ferguson A., Elbein A. D. Purification of a D-mannose isomerase from Mycobacterium smegmatis. J Bacteriol. 1970 Mar;101(3):777–780. doi: 10.1128/jb.101.3.777-780.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hey A. E., Elbein A. D. Partial prufication and properties of a trehalase from Streptomyces hygroscopicus. J Bacteriol. 1968 Jul;96(1):105–110. doi: 10.1128/jb.96.1.105-110.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lapp D., Elbein A. D. Purification and properties of the adenosine diphosphate-glucose and uridine diphosphate-glucose pyrophosphorylases of Mycobacterium smegmatis: inhibition and activation of the adenosine diphosphate-glucose pyrophosphorylase. J Bacteriol. 1972 Oct;112(1):327–336. doi: 10.1128/jb.112.1.327-336.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- Winder F. G., Brennan P. J., McDonnell I. Effects of isoniazid on the composition of mycobacteria, with particular reference to soluble carbohydrates and related substances. Biochem J. 1967 Aug;104(2):385–393. doi: 10.1042/bj1040385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winder F. G., Collins P. B. Inhibition by isoniazid of synthesis of mycolic acids in Mycobacterium tuberculosis. J Gen Microbiol. 1970 Sep;63(1):41–48. doi: 10.1099/00221287-63-1-41. [DOI] [PubMed] [Google Scholar]


