Abstract
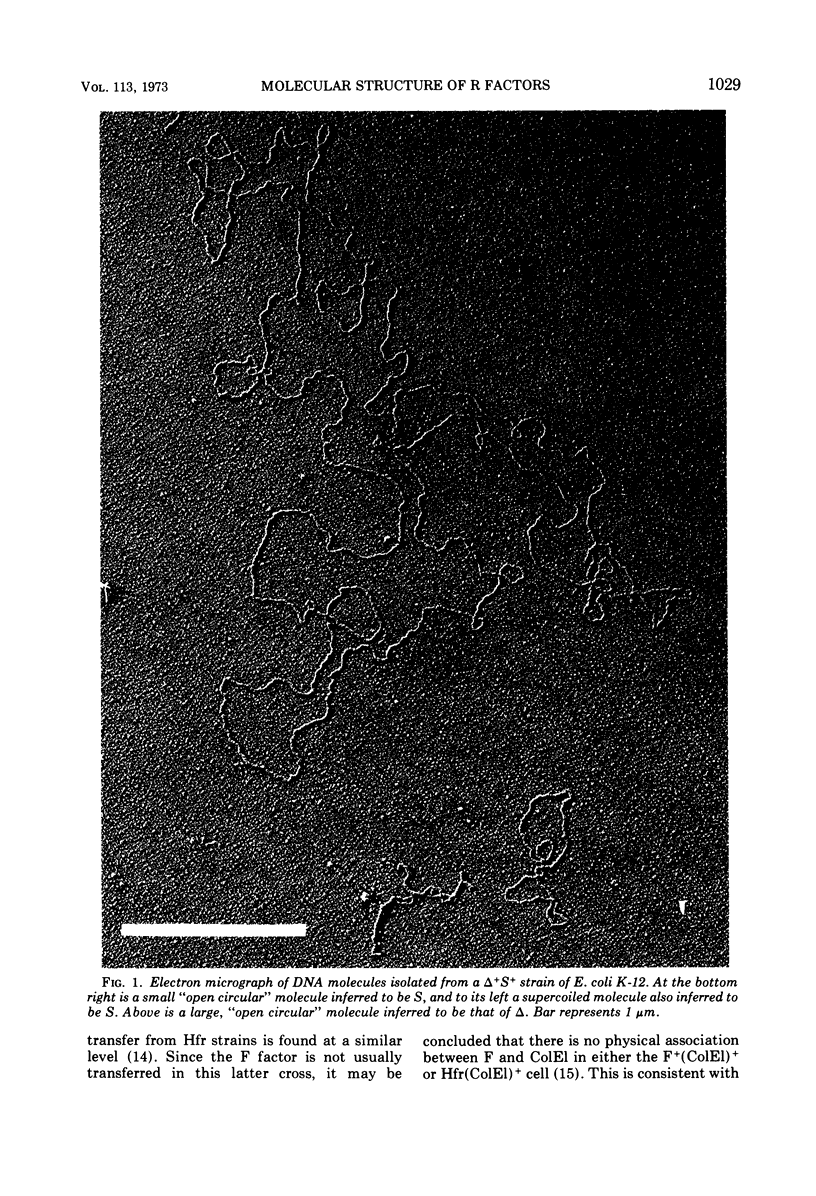
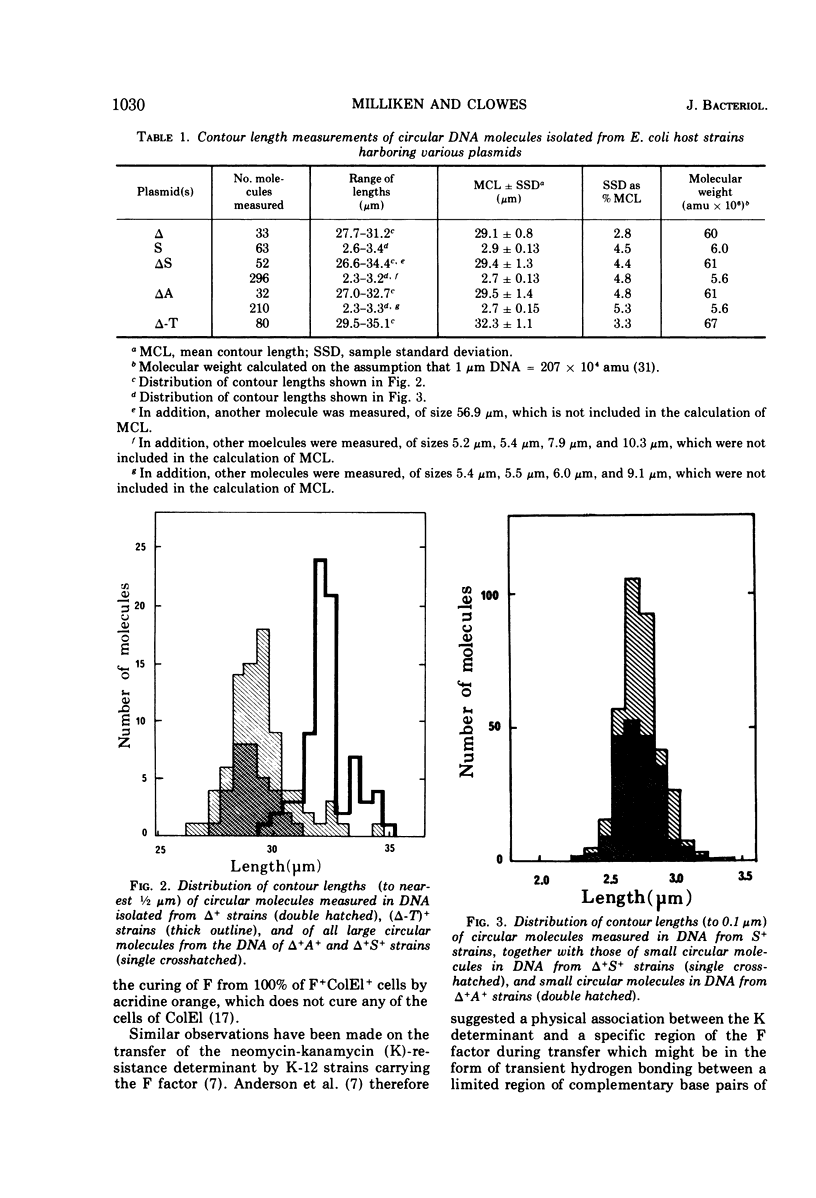
Plasmid DNA from Escherichia coli strains harboring drug resistance either of the infectious or noninfectious kind has been separated by CsCl centrifugation of crude cell lysates in the presence of ethidium bromide and examined by electron microscopy. Plasmid deoxyribonucleic acid (DNA) from an S+ strain (which has the property of noninfectious streptomycin-sulfonamide resistance) consists of a monomolecular covalently closed circular species of 2.7 μm in contour length (5.6 × 106 atomic mass units; amu). DNA from a strain carrying a transfer factor, termed Δ, but no determinant for drug resistance, is a monomolecular covalently closed circular species of 29.3 μm in contour length (61 × 106 amu). DNA from either Δ+A+ or Δ+S+ strains, (which are respectively ampicillin or streptomycin-sulfonamide resistant, and can transfer this drug resistance), shows a bimodal distribution of molecules of contour lengths 2.7 μm and 29.3 μm, whereas DNA from a (Δ-T)+ strain (showing infectious tetracycline resistance) contains only one species of molecule measuring 32.3 μm (67 × 106 amu). We conclude that ampicillin resistance is carried by a DNA molecule (the A determinant) of 2.7 μm, and streptomycin-sulfonamide resistance is carried by an independent molecule (the S determinant) of similar size. These molecules are not able to effect their own transfer, but can be transmitted to other cells due to the simultaneous presence of the transfer factor, Δ, which also constitutes an independent molecule, of size 29.3 μm. In general, there appears to be little recombination or integration of the A or S molecules into that of Δ, although a small proportion (5–10%) of recombinant molecules cannot be excluded. In contrast, the third drug-resistance determinant, that for tetracycline resistance (denoted as T), is integrated in the Δ molecule to form the composite structure Δ-T of size 32.3 μm, which determines infectious tetracycline resistance. The Δ+A+ and Δ+S+ strains are defined as harboring plasmid aggregates, and the (Δ-T)+ strain is defined as carrying a plasmid cointegrate; the properties of all three strains are characteristic of strains harboring R factors. These results are compatible with the previously published genetic data. The number of Δ molecules per cell appears to be equal to the chromosomal number irrespective of growth phase, and this plasmid can thus be defined as stringently regulated in DNA replication. In contrast, S and A exist as multiple copies, probably in at least a 10-fold excess of chromosomal copy number. S and A can thus be defined as relaxed in the regulation of their DNA replication.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson E. S. A rapid screening test for transfer factors in drug-sensitive Enterobacteriaceae. Nature. 1965 Dec 4;208(5014):1016–1017. doi: 10.1038/2081016a0. [DOI] [PubMed] [Google Scholar]
- Anderson E. S. Influence of the delta transfer factor on the phage sensitivity of salmonellae. Nature. 1966 Nov 19;212(5064):795–799. doi: 10.1038/212795a0. [DOI] [PubMed] [Google Scholar]
- Anderson E. S., Lewis M. J. Characterization of a transfer factor associated with drug resistance in Salmonella typhimurium. Nature. 1965 Nov 27;208(5013):843–849. doi: 10.1038/208843a0. [DOI] [PubMed] [Google Scholar]
- Anderson E. S., Lewis M. J. Drug resistance and its transfer in Salmonella typhimurium. Nature. 1965 May 8;206(984):579–583. doi: 10.1038/206579a0. [DOI] [PubMed] [Google Scholar]
- Anderson E. S., Mayhew J. N., Grindley N. D. Transfer of a neomycin-kanamycin resistance determinant by the F factor of Escherichia coli K12. Nature. 1969 Apr 26;222(5191):349–351. doi: 10.1038/222349a0. [DOI] [PubMed] [Google Scholar]
- Anderson E. S., Natkin E. Transduction of resistance determinants and R factors of the transfer systems by phage Plkc. Mol Gen Genet. 1972;114(3):261–265. doi: 10.1007/BF01788895. [DOI] [PubMed] [Google Scholar]
- Anderson E. S., Perret D. Mobilization of transduced tetracycline resistance by the delta transfer factor in Salmonella typhimurium and S. typhi. Nature. 1967 May 20;214(5090):810–811. doi: 10.1038/214810b0. [DOI] [PubMed] [Google Scholar]
- Anderson E. S. The ecology of transferable drug resistance in the enterobacteria. Annu Rev Microbiol. 1968;22:131–180. doi: 10.1146/annurev.mi.22.100168.001023. [DOI] [PubMed] [Google Scholar]
- Bazaral M., Helinski D. R. Circular DNA forms of colicinogenic factors E1, E2 and E3 from Escherichia coli. J Mol Biol. 1968 Sep 14;36(2):185–194. doi: 10.1016/0022-2836(68)90374-4. [DOI] [PubMed] [Google Scholar]
- Bazaral M., Helinski D. R. Replication of a bacterial plasmid and an episome in Escherichia coli. Biochemistry. 1970 Jan 20;9(2):399–406. doi: 10.1021/bi00804a029. [DOI] [PubMed] [Google Scholar]
- CLOWES R. C. TRANSFERT G'EN'ETIQUE DES FACTEURS COLICINOG'ENES. Ann Inst Pasteur (Paris) 1964 Nov;107:SUPPL–SUPPL:92. [PubMed] [Google Scholar]
- CLWES R. C., MOODY E. E., PRITCHARD R. H. THE ELIMINATION OF EXTRACHROMOSOMAL ELEMENTS IN THYMINELESS STRAINS OF ESCHERICHIA COLI K12. Genet Res. 1965 Feb;6:147–152. doi: 10.1017/s0016672300004018. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. E. Existence of the colicinogenic factor-sex factor ColI-b-P9 as a supercoiled circular DNA-protein relaxation complex. Biochem Biophys Res Commun. 1970 Oct 9;41(1):150–156. doi: 10.1016/0006-291x(70)90481-x. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Properties of a supercoiled deoxyribonucleic acid-protein relaxation complex and strand specificity of the relaxation event. Biochemistry. 1970 Oct 27;9(22):4428–4440. doi: 10.1021/bi00824a026. [DOI] [PubMed] [Google Scholar]
- Cohen A., Fisher W. D., Curtiss R., 3rd, Adler H. I. The properties of DNA transferred to minicells during conjugation. Cold Spring Harb Symp Quant Biol. 1968;33:635–641. doi: 10.1101/sqb.1968.033.01.071. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Miller C. A. Multiple molecular species of circular R-factor DNA isolated from Escherichia coli. Nature. 1969 Dec 27;224(5226):1273–1277. doi: 10.1038/2241273a0. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Miller C. A. Non-chromosomal antibiotic resistance in bacteria. II. Molecular nature of R-factors isolated from Proteus mirabilis and Escherichia coli. J Mol Biol. 1970 Jun 28;50(3):671–687. doi: 10.1016/0022-2836(70)90092-6. [DOI] [PubMed] [Google Scholar]
- Cooper S., Helmstetter C. E. Chromosome replication and the division cycle of Escherichia coli B/r. J Mol Biol. 1968 Feb 14;31(3):519–540. doi: 10.1016/0022-2836(68)90425-7. [DOI] [PubMed] [Google Scholar]
- Davies J., Brzezinska M., Benveniste R. The problems of drug-resistant pathogenic bacteria. R factors: biochemical mechanisms of resistance to aminoglycoside antibiotics. Ann N Y Acad Sci. 1971 Jun 11;182:226–233. doi: 10.1111/j.1749-6632.1971.tb30659.x. [DOI] [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freifelder D., Folkmanis A., Kirschner I. Studies on Escherichia coli sex factors: evidence that covalent circles exist within cells and the general problem of isolation of covalent circles. J Bacteriol. 1971 Mar;105(3):722–727. doi: 10.1128/jb.105.3.722-727.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grindley J. N., Anderson E. S. I-like resistance factors with the fi+ character. Genet Res. 1971 Jun;17(3):267–271. doi: 10.1017/s0016672300012295. [DOI] [PubMed] [Google Scholar]
- Hickson F. T., Roth T. F., Helinski D. R. Circular DNA forms of a bacterial sex factor. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1731–1738. doi: 10.1073/pnas.58.4.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H., Tomizawa J. Prophage P1, and extrachromosomal replication unit. Cold Spring Harb Symp Quant Biol. 1968;33:791–798. doi: 10.1101/sqb.1968.033.01.091. [DOI] [PubMed] [Google Scholar]
- Inselburg J. R factor deoxyribonucleic acid in chromosomeless progeny of Escherichia coli. J Bacteriol. 1971 Feb;105(2):620–628. doi: 10.1128/jb.105.2.620-628.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontomichalou P., Mitani M., Clowes R. C. Circular R-factor molecules controlling penicillinase synthesis, replicating in Escherichia coli under either relaxed or stringent control. J Bacteriol. 1970 Oct;104(1):34–44. doi: 10.1128/jb.104.1.34-44.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang D. Molecular weights of coliphages and coliphage DNA. 3. Contour length and molecular weight of DNA from bacteriophages T4, T5 and T7, and from bovine papilloma virus. J Mol Biol. 1970 Dec 28;54(3):557–565. doi: 10.1016/0022-2836(70)90126-9. [DOI] [PubMed] [Google Scholar]
- NAKAYA R., NAKAMURA A., MURATA Y. Resistance transfer agents in Shigella. Biochem Biophys Res Commun. 1960 Dec;3:654–659. doi: 10.1016/0006-291x(60)90081-4. [DOI] [PubMed] [Google Scholar]
- Nisioka T., Mitani M., Clowes R. C. Molecular recombination between R-factor deoxyribonucleic acid molecules in Escherichia coli host cells. J Bacteriol. 1970 Jul;103(1):166–177. doi: 10.1128/jb.103.1.166-177.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisioka T., Mitani M., Clowes R. Composite circular forms of R factor deoxyribonucleic acid molecules. J Bacteriol. 1969 Jan;97(1):376–385. doi: 10.1128/jb.97.1.376-385.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoades M., MacHattie L. A., Thomas C. A., Jr The P22 bacteriophage DNA molecule. I. The mature form. J Mol Biol. 1968 Oct 14;37(1):21–40. doi: 10.1016/0022-2836(68)90071-5. [DOI] [PubMed] [Google Scholar]
- Rownd R. Replication of a bacterial episome under relaxed control. J Mol Biol. 1969 Sep 28;44(3):387–402. doi: 10.1016/0022-2836(69)90368-4. [DOI] [PubMed] [Google Scholar]
- Silver R. P., Falkow S. Specific labeling and physical characterization of R-factor deoxyribonucleic acid in Escherichia coli. J Bacteriol. 1970 Oct;104(1):331–339. doi: 10.1128/jb.104.1.331-339.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. W., Gyles C. L. The relationship between different transmissible plasmids introduced by F into the same strain of Escherichia coli K12. J Gen Microbiol. 1970 Aug;62(3):227–285. doi: 10.1099/00221287-62-3-277. [DOI] [PubMed] [Google Scholar]
- Smith H. W., Linggood M. A. Transfer factors in Escherichia coli with particular regard to their incidence in enteropathogenic strains. J Gen Microbiol. 1970 Aug;62(3):287–299. doi: 10.1099/00221287-62-3-287. [DOI] [PubMed] [Google Scholar]
- Vapnek D., Lipman M. B., Rupp W. D. Physical properties and mechanism of transfer of R factors in Escherichia coli. J Bacteriol. 1971 Oct;108(1):508–514. doi: 10.1128/jb.108.1.508-514.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATANABE T., FUKASAWA T. Episome-mediated transfer of drug resistance in Enterobacteriaceae. I. Transfer of resistance factors by conjugation. J Bacteriol. 1961 May;81:669–678. doi: 10.1128/jb.81.5.669-678.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATANABE T., FUKASAWA T. Episome-mediated transfer of drug resistance in Enterobacteriaceae. III. Transduotion of resistance factors. J Bacteriol. 1961 Aug;82:202–209. doi: 10.1128/jb.82.2.202-209.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]



