Abstract
Postsynaptic receptor desensitization has been observed to contribute to depression in immature synapses. However, it is not clear whether desensitization persists and causes depression in mature synapses. We investigate this issue at the endbulb of Held, the synapse made by auditory nerve (AN) fibers onto bushy cells (BCs) of the anteroventral cochlear nucleus, where depression could influence the processing of sound information. Experiments using cyclothiazide (CTZ) have implicated desensitization in endbulbs from postnatal day 16 (P16) to P21 mice, but application of γ-d-glutamylglycine (DGG) did not reveal desensitization in endbulbs >P22. To reconcile these findings, we have studied the effects of both CTZ and DGG on endbulbs from P5 to P40 CBA/CaJ mice. In paired-pulse protocols, both CTZ and DGG reduced depression in all ages at intervals <10 ms, consistent with their effects preventing desensitization. However, DGG increased depression at intervals >20 ms, consistent with DGG's use to prevent saturation. DGG application revealed receptor saturation even under conditions of very low release probability. Preventing desensitization by CTZ occluded the effects of DGG on desensitization and revealed the effects of saturation at short intervals. We developed an approach to separate DGG's effect on saturation from its effect on desensitization, which showed that desensitization has an impact during bursts of auditory nerve activity. Dynamic-clamp experiments indicated that desensitization can reduce BC spike probability and increase latency and jitter. Thus desensitization may affect sound processing in the mature auditory system.
INTRODUCTION
Information processing by the brain is influenced by diverse activity-dependent processes (Zucker and Regehr 2002). These processes include two postsynaptic mechanisms: receptor desensitization and saturation. Desensitization of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs) has been implicated in causing a fast component of depression (Chen et al. 2002; Rozov et al. 2001; Trussell et al. 1993; Wall 2005; Xu-Friedman and Regehr 2003; Yang and Xu-Friedman 2008). Some studies have suggested that the effects of desensitization decrease as synapses mature (Renden et al. 2005; Taschenberger et al. 2002, 2005). This has important implications for evaluating whether desensitization has any function in information processing in mature synapses.
One complication is that the two principal tools for studying AMPAR desensitization, i.e., cyclothiazide (CTZ) and γ-d-glutamylglycine (DGG), can also have nonspecific effects. CTZ prevents the conformational changes of AMPARs that lead to desensitization (Partin et al. 1993; Yamada and Tang 1993), but it can also influence channel kinetics and presynaptic release (Bellingham and Walmsley 1999; Diamond and Jahr 1995; Ishikawa and Takahashi 2001). CTZ is frequently used at low concentrations to avoid these nonspecific effects, which may lead to an underestimation of the importance of desensitization in mature synapses.
DGG, as a low-affinity antagonist, has very different properties from conventional high-affinity antagonists such as 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione (NBQX), which is normally used to block a fixed fraction of receptors. However, DGG, because it can rapidly dissociate, effectively protects a pool of AMPARs from desensitization (Crowley et al. 2007; Wong et al. 2003) and also prevents saturation (Clements et al. 1992; Foster et al. 2002; Wadiche and Jahr 2001). These two effects of DGG would be expected to have opposite effects on depression. In a normally depressing synapse, when pairs of stimuli are used to study plasticity, the paired-pulse ratio (PPR) would increase (i.e., less depression) when desensitization is prevented. Saturation is a sublinear response by AMPARs to glutamate and would affect larger excitatory postsynaptic currents (EPSCs) more than small ones. Thus when saturation is prevented, the PPR would decrease (i.e., more apparent depression) as the first EPSC (EPSC1) would be blocked relatively less than the second (EPSC2). A mixture of these effects is potentially ambiguous; thus when DGG is used for studying synaptic plasticity, it is important to separate the effects on desensitization from its effects on saturation.
To resolve these issues, we studied the endbulb of Held, which is a large, glutamatergic synapse made by auditory nerve (AN) fibers onto bushy cells (BCs) in the anteroventral cochlear nucleus (AVCN) (Brawer and Morest 1975; Fekete et al. 1984; Lorente de Nó 1981; Ostapoff and Morest 1991; Ryugo and Fekete 1982; Ryugo and Sento 1991; Ryugo et al. 1991). The characteristics of depression at the endbulb influence how auditory information is relayed to higher centers for further processing (Yang and Xu-Friedman 2009). A number of studies at the endbulb have reached potentially conflicting conclusions as to whether desensitization occurs (Isaacson and Walmsley 1995; Oleskevich et al. 2000; Yang and Xu-Friedman 2008) or whether it does not occur (Bellingham and Walmsley 1999; Wang and Manis 2008).
To reconcile these results, we used voltage-clamp recordings at near-physiological temperature in brain slices taken from mice aged postnatal day 5 (P5) to P40, during which period the endbulb reaches mature structure (Limb and Ryugo 2000). Experiments using both DGG and CTZ indicated that desensitization was present at all ages for short interpulse intervals. Experiments using DGG revealed saturation during paired stimulation at long intervals. We untangled the multiple effects of DGG by comparing the effects of DGG on depressed and nondepressed EPSCs, revealing a prolonged impact of desensitization. Dynamic-clamp experiments indicated that desensitization could reduce spike probability and increase the latency and jitter of BC spikes.
METHODS
All experiments were carried out with the approval of the University at Buffalo's Institutional Animal Care and Use Committee (protocol BIO05084N). Parasagittal slices (140 μm) of AVCN were cut into ice-cold solution containing (in mM): 76 NaCl, 75 sucrose, 25 NaHCO3, 25 glucose, 2.5 KCl, 1.25 NaH2PO4, 7 MgCl2, 0.5 CaCl2, from CBA/CaJ mice of three different age groups: P5–P10, P16–P20, and P35–P40. Slices were incubated at 34°C for 30 min in standard recording solution containing (in mM): 125 NaCl, 26 NaHCO3, 20 glucose, 2.5 KCl, 1.25 NaH2PO4, 1.5 MgCl2, 1.5 CaCl2, 4 Na l-lactate, 2 Na-pyruvate, 0.4 Na l-ascorbate, bubbled with 95% O2-5% CO2. This external calcium (Cae) is based on calcium levels measured in vivo (Hansen 1985; Manthei et al. 1973). Slices were maintained at room temperature until recording. BCs were patched under an Olympus BX51WI microscope with a Multiclamp 700B (Molecular Devices) controlled by an ITC-18 interface (Instrutech), driven by custom-written software (mafPC) running in Igor (WaveMetrics). The bath was perfused at 3–4 ml/min using a pump (403U/VM2; Watson-Marlow, Wilmington, MA), with saline running through an in-line heater to maintain the temperature at 34°C (SH-27B with TC-324B controller; Warner Instruments, Hamden, CT).
Pipettes were pulled from borosilicate glass (OD: 1.5 mm, ID: 0.86 mm; Sutter Instrument, Novato, CA) to a resistance of 1–2 MΩ and were filled with internal solution containing (in mM): (for voltage-clamp) 35 CsF, 100 CsCl, 10 EGTA, 10 HEPES, and 1 QX-314 or (for dynamic-clamp) 130 KMeSO3, 10 NaCl, 10 HEPES, 2 MgCl2, 0.5 EGTA, 0.16 CaCl2, 4 Na2ATP, 0.4 NaGTP, and 14 Tris-creatine phosphate (pH adjusted to 7.3, 310 mOsm). In some experiments, 1 mM Alexa-594 (Invitrogen) was added to the recording pipette and cell structure was examined during experiments using a 150-W xenon light source (Optiquip 770), with excitation filter 560/55, dichroic 595LP, and emission filter 645/75 (Chroma). For Fig. 1, A and B, after loading each cell with Alexa-594, the recording electrode was retracted; the slice was fixed overnight in 4% buffered paraformaldehyde, mounted on a slide, and coverslipped under Fluoromount (Southern Biotech). Images were acquired using a Zeiss Meta LSM 510 confocal microscope. All recordings were made in the presence of 10 μM strychnine to block glycine receptors. Some experiments used CTZ (50 μM), DGG (5 mM, except for the dose–response experiment of Fig. 2), NBQX (250 nM), or kynurenate (1 mM), where specified. Experiments were conducted in different Cae by replacing 1.5:1.5 CaCl2:MgCl2 with 0.75:2.25, 1:2, 2:1, and 3:0 mM CaCl2:MgCl2. For 3 Cae recordings, 5 μM 3(2-carboxypiperazin-4-yl)propyl-1-phosphonic acid (CPP) was added to block N-methyl-d-aspartate (NMDA) receptors. Presynaptic AN fibers were stimulated using a glass micropipette placed 30–50 μm away from the cell being recorded, with currents of 5–16 μA through a stimulus isolator (WPI A360). Activation of single presynaptic AN fibers was verified in all the experiments by slightly decreasing the stimulus amplitude or moving the pipette slightly from a different position on the slice, which resulted in failure. Cells were held at −70 mV with access resistance 3–7 MΩ, compensated to 70%.
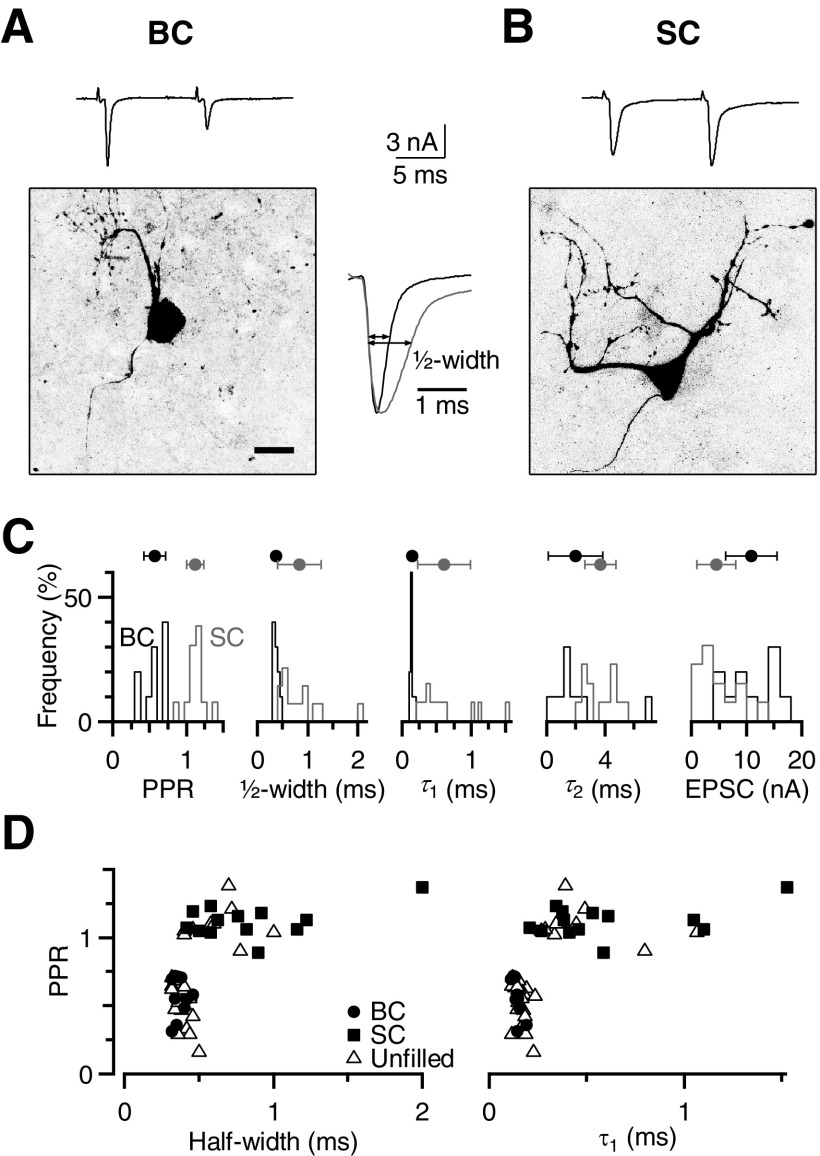
Fig. 1.
Distinguishing bushy cells (BCs) from stellate cells (SCs) in voltage-clamp recordings in the anteroventral cochlear nucleus (AVCN). A and B: identification of cell type using anatomical criteria. Confocal images of a representative BC (A) and SC (B), with excitatory postsynaptic currents (EPSCs) recorded in response to paired stimulation of a single excitatory input (Δt = 10 ms). Cells were patched using a recording pipette containing Alexa-594, which was then pulled off the cell before confocal imaging. The BC has a single dendritic arbor and shows paired-pulse depression, whereas the SC has multiple dendrites and shows paired-pulse facilitation. The scale bar is 20 μm. The inset between the images shows the first EPSC for both cells on an expanded timescale. For the example BC, τ1 = 0.21 ms, τ2 = 5.12 ms, and half-width = 0.46 ms. For the example SC, τ1 = 0.39, τ2 = 5.8, and half-width = 0.92 ms. C: histograms of EPSC properties for anatomically identified BCs and SCs. Paired-pulse ratio (PPR), half-width, and τ1 can distinguish between these 2 cell populations, but EPSC amplitude and τ2 are less favorable. Average values for the identified population are indicated by markers above the histograms (error bars indicate SD of the population in this figure only). D: the PPR plotted against half-width and τ1 from a larger population of cells. Closed symbols are those identified on the basis of anatomical structure, whereas open symbols are unidentified. All cells clearly fall into 2 distinct groups.
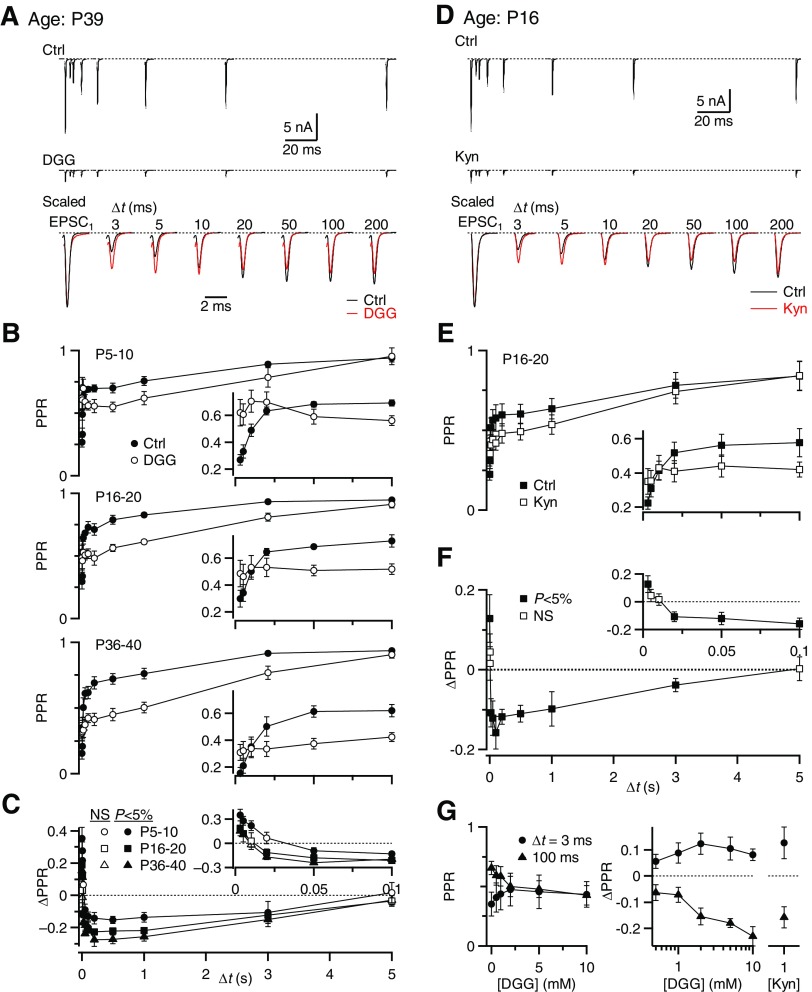
Fig. 2.
γ-d-Glutamylglycine (DGG) has multiple effects on paired-pulse plasticity at all ages. Auditory nerve (AN) inputs were stimulated with pairs of pulses at different time intervals (Δt) in 3 mM external calcium (3 Cae). Data are shown together for simplicity, but were collected in different trials in this and following figures. A: example traces recorded from a P39 BC in control conditions (top) and in 5 mM DGG (middle). The bottom panel shows control and DGG traces scaled by the EPSC1 amplitude. B: average results for 5 BCs each from P5–P10 (top), P16–P20 (middle), and P36–P40 animals (bottom). The insets expand the intervals Δt = 3 to 100 ms. C: the change in PPR (ΔPPR) is shown for 3 different age groups. Significant differences from 0 are indicated with filled symbols (P < 0.05). D: example recordings from a P16 BC in control (top) and in 1 mM kynurenate (middle). In the bottom panel, control and kynurenate traces have been scaled by their corresponding EPSC1 amplitude. E: average results for 6 BCs from P16–P20. F: the ΔPPR by kynurenate application for the cells in E. G: dose–response curve for PPR at Δt = 3 and 100 ms over different DGG concentrations (left). The ΔPPRs with respect to control conditions are shown for DGG (middle) and 1 mM kynurenate (right). All points are significantly different from 0 (P < 0.05), except for 0.5 mM DGG. Over the concentration range from 2 to 10 mM DGG, the effects on PPR were not statistically different (P > 0.1 for all pairwise comparisons, 2-tailed paired t-test, n = 5 cells). The effects of 1 mM kynurenate and 2 to 10 mM DGG were not statistically different (P > 0.1 for all pairwise comparisons, unpaired t-test; n = 7 cells in kynurenate).
Dynamic-clamp experiments were carried out at −60 mV using the ITC18 interface, which runs at 50 kHz. The conductances of native endbulbs could not be measured when cells were patched using the dynamic-clamp solution, so we based conductance amplitudes on population averages. Endbulb AMPAR EPSCs had peak amplitudes of 2 to 20 nA in voltage-clamp experiments (average 8.1 ± 3.6 nA, equivalent to 120 ± 50 nS, n = 75). We measured the conductance threshold as described previously (Xu-Friedman and Regehr 2005a,b), yielding values of 14 to 35 nS for different BCs (average 24 ± 1 nS, n = 17 cells). After measuring the threshold of an individual BC, we scaled the initial amplitude for synaptic conductance trains to 5 times threshold and scaled subsequent conductances according to the data in Fig. 8C. These amplitudes were then convolved with a representative unit AMPAR EPSC conductance from voltage-clamp experiments.
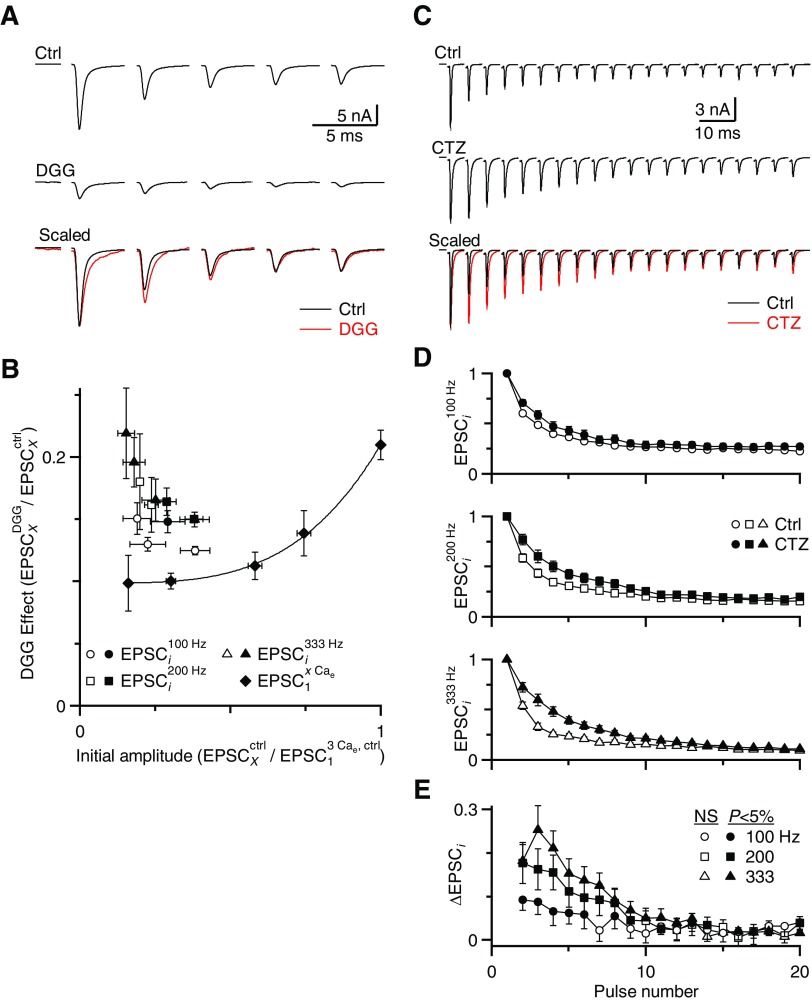
Fig. 8.
Desensitization persists during longer periods of activity. The presynaptic AN fiber was stimulated with trains of pulses in 1.5 Cae and the effects of DGG and CTZ were studied. A: EPSCs during 200-Hz trains for a representative experiment in control (top) and in DGG (middle). In the bottom panel, these traces are shown with EPSC1 amplitudes scaled to the same size. B: average data from 4 experiments are plotted similarly to Fig. 7. The line and solid symbols indicate the effects of DGG on EPSCs recorded in different Cae conditions (the saturation curve). Open symbols indicate the effect of DGG on EPSCs during 100-, 200-, and 333-Hz trains. The initial EPSC amplitude for the ith pulse in a train is given by EPSCixCae/EPSC13Cae and the effect of DGG is given by EPSCiDGG,xCae/EPSCiCtrl,xCae. The data for EPSC1 of the trains lie on the saturation curve, with later pulses extending toward the top left. Pulses that differed significantly from the saturation curve (P < 0.05) are plotted as filled symbols, indicating they showed significant desensitization. C: EPSCs during 200-Hz trains for a representative experiment in control (top) and in CTZ (middle). In the bottom panel, these traces are shown with EPSC1 amplitudes scaled to the same size. D: average changes in relative EPSC amplitude after CTZ application are shown over the course of 100-, 200-, and 333-Hz trains. This is calculated after normalizing to the first pulse in the train (analogous to ΔPPR in Figs. 2, 4, and 6). Points are averages of 11 or 12 cells from animals P16–P40. Filled symbols represent a significant increase in EPSC amplitude by CTZ at P < 0.05 (t-test).
Most chemicals were purchased from Sigma (St. Louis, MO); CTZ, DGG, NBQX, and CPP were purchased from Tocris Bioscience (Ellisville, MO). PPR was measured after first averaging multiple trials (typically three to five), according to the method of Kim and Alger (2001). Average data are presented as means ± SE, except in Fig. 1C, which presents the SD. Statistical tests are paired, one-tail, Student's t-test, except in Figs. 7 and 8B, which are based on 95% confidence intervals as described in results.
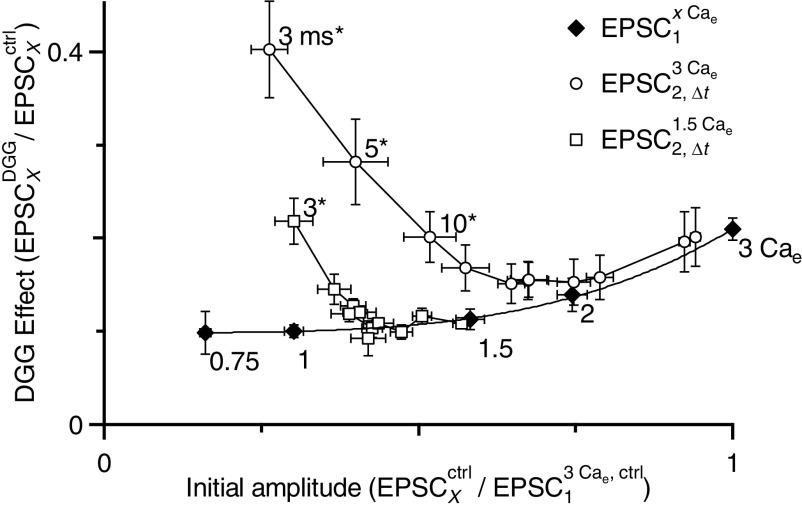
Fig. 7.
Distinguishing the effects of DGG on desensitization and saturation. The initial EPSC amplitude for all conditions is normalized to the resting EPSC in 3 Cae and the effect of DGG is calculated as the relative EPSC amplitude after DGG application. Closed symbols are the results of experiments in which Cae was varied. The initial EPSC amplitude is given by EPSC1xCae/EPSC13Cae and the effect of DGG is given by EPSC1DGG,xCae/EPSC1Ctrl,xCae. The line is a fit to these data using a power law (see results). The effect of DGG under these conditions depends on receptor saturation, so the fit is termed the “saturation curve.” Open symbols are the results of paired-pulse experiments in 1.5 (squares) or 3 (circles) Cae. The initial EPSC amplitude is given by EPSC2,ΔtxCae/EPSC13Cae and the effect of DGG is given by EPSC2,ΔtDGG,xCae/EPSC2,ΔtCtrl,xCae. The shortest intervals are at the top left and move down and to the right at longer intervals. Intervals that differ significantly from the saturation curve are labeled and marked by asterisks (see results). The EPSCs at short intervals differ from the saturation curve, most likely because DGG prevented desensitization. Data were pooled from experiments on endbulbs from animals P16–P40.
RESULTS
Bushy cell identification
Our first step was to establish a clear physiological criterion for identifying BCs in voltage-clamp recordings in mouse brain slices. Besides BCs, AN fibers also make synaptic contacts with stellate cells (SCs) in the AVCN. Because desensitization properties are highly dependent on synaptic structure, it was important to study a uniform population of cells. BCs and SCs can be clearly differentiated in current-clamp recordings based on their spike properties (Wu and Oertel 1984). In voltage-clamp recordings, Cs+ and QX-314 disrupt normal spiking, so spike properties are not available as a criterion. EPSC amplitude can be used instead, but in the course of our recordings, we realized that this criterion was yielding a mixed population of cells.
To address this, we made voltage-clamp recordings from AVCN neurons in 1.5 Cae while stimulating isolated, single AN fiber inputs with pairs of pulses with interpulse interval Δt = 10 ms. To identify the cell type, we included Alexa-594 in the recording pipette and examined cell structure under fluorescence. We identified BCs anatomically by a single primary dendrite and axon, whereas SCs had three or more processes (Fig. 1, A and B) (Brawer et al. 1974; Webster and Trune 1982; Wu and Oertel 1984). BC EPSCs typically decayed very rapidly (e.g., Fig. 1A) and showed paired-pulse depression as seen in previous studies (Isaacson and Walmsley 1995; Yang and Xu-Friedman 2008) (Fig. 1A). By contrast, SCs usually showed facilitation and the EPSCs decayed more slowly (e.g., Fig. 1B). We quantified EPSC decay by measuring the EPSC width at 50% of the peak amplitude (“half-width”; Fig. 1), as well as by fitting EPSC decay with a double exponential. The SC EPSC was slower by both criteria.
We made recordings from a number of identified BCs (n = 10 cells) and SCs (n = 13 cells). The PPR, half-width, and τ1 were quite distinct, whereas τ2 values and EPSC amplitudes were less effective at distinguishing the two cell types (Fig. 1C). To determine whether these physiological criteria were sufficient to unequivocally categorize unlabeled cells, we made similar measurements from a population of unidentified cells (n = 30 cells) and found that the PPR, half-width, and τ1 separated them into two distinct groups (Fig. 1D). Therefore we used PPR ≤0.71, half-width ≤0.5 ms, and τ1 ≤0.24 ms as criteria to identify BCs for our subsequent studies.
DGG has multiple effects on PPR
We first tested whether the effects of DGG on synaptic plasticity might change with age. To do this we stimulated a single AN input with pairs of pulses at different intervals (Δt) in 3 Cae. EPSC1 did show changes with age. In P5–P10 endbulbs, the average EPSC1 was 6.8 ± 2.1 nA, which increased to 13.3 ± 3.0 nA for P16–P20 and 12.4 ± 2.2 nA for P36–P40 (n = 5 in each age group). DGG application decreased the amplitude of EPSC1 to 21 ± 1.2% but did not affect the kinetics (Table 1, n = 32 cells, P > 0.5).
Table 1.
Measures of BC EPSC kinetics under different conditions
| Condition | τ1, ms | τ2, ms | Half-Width, ms |
|---|---|---|---|
| Control | 0.16 ± 0.01 | 4.04 ± 1.90 | 0.38 ± 0.01 |
| DGG (5 mM) | 0.18 ± 0.01 | 1.51 ± 0.16 | 0.39 ± 0.01 |
| CTZ (50 μM) | 0.40 ± 0.04 | 2.03 ± 0.23 | 0.61 ± 0.02 |
| DGG + CTZ | 0.46 ± 0.03 | 2.61 ± 0.50 | 0.65 ± 0.04 |
| NBQX (250 nM) | 0.21 ± 0.02 | 4.28 ± 0.68 | 0.40 ± 0.01 |
| DGG + NBQX | 0.19 ± 0.03 | 2.05 ± 0.65 | 0.41 ± 0.02 |
| CTZ + NBQX | 0.42 ± 0.04 | 2.10 ± 0.25 | 0.62 ± 0.03 |
| KYN (1 mM) | 0.17 ± 0.01 | 2.67 ± 0.11 | 0.42 ± 0.06 |
Values for τ1 and τ2 are from double-exponential fits. “Half-width” is the EPSC duration at half the peak amplitude (see Fig. 1).
The effects of DGG on EPSC2 depended on the interpulse interval. For Δt = 3 ms, the PPR increased from 0.27 ± 0.04 in control to 0.62 ± 0.09 in DGG for P5–P10 endbulbs, from 0.3 ± 0.06 to 0.49 ± 0.09 for P16–P20 endbulbs and from 0.15 ± 0.04 to 0.31 ± 0.06 for P36–P40 endbulbs (P < 0.05, n = 5 for all age groups; Fig. 2B). The increase in PPR (ΔPPR = PPRDGG − PPRCtrl) was 0.35 ± 0.07 for P5–P10, 0.19 ± 0.09 for P16–P20, and 0.15 ± 0.02 for P36–P40 endbulbs. At the intermediate Δt of 10 ms there was no significant change in PPR (Fig. 2C, bottom), consistent with Wang and Manis (2008). However, at longer intervals, we found that DGG application had the opposite effect, decreasing the PPR significantly for all ages tested (Fig. 2, A and B). The ΔPPR at Δt = 100 ms decreased by −0.13 ± 0.03 for P5–P10 endbulbs, −0.21 ± 0.02 for P16–P20, and −0.20 ± 0.03 for P36–P40 (Fig. 2C). The effect on PPR was maximal between Δt = 0.05 to 1 s and decreased as the synapse recovered back to resting conditions.
Our observation that DGG increased PPR at Δt = 3 ms suggests that receptor desensitization does occur. However, at longer intervals, PPR decreases, which is consistent with an effect of DGG on saturation. At intermediate intervals, it is possible that these two processes interact, making it appear that there is no desensitization or saturation.
To determine whether these observations result from a nonspecific effect of DGG, we tested another low-affinity AMPAR antagonist, kynurenate. Similar to DGG, kynurenate at 1 mM did not significantly affect the EPSC kinetics (Fig. 2D, n = 7 cells, P > 0.05, Table 1). Also similar to DGG, the PPR increased in kynurenate for Δt = 3 ms, but decreased for Δt ≥20 ms (Fig. 2, D and E). This suggests that the effects of both DGG and kynurenate on the PPR result specifically from their actions as low-affinity AMPAR antagonists.
DGG has been used at concentrations that vary from 1 to 10 mM in different studies (Crowley et al. 2007; Wadel et al. 2007; Wang and Manis 2008; Wong et al. 2003). To verify that our selection of 5 mM DGG did not qualitatively affect our results, we measured the PPR in different concentrations of DGG (Fig. 2G). As the concentration of DGG increased, the PPR increased for Δt = 3 ms and decreased for Δt = 100 ms (Fig. 2G, left). Changes at both intervals appeared to require ≥2 mM DGG and did not differ significantly at higher concentrations (P > 0.1 for all pairwise comparisons between data from 2 to 10 mM; Fig. 2G, middle, n = 5 cells for each point). The effects on PPR were statistically identical for 1 mM kynurenate and 5 mM DGG (P > 0.1, Fig. 2G, right). Therefore 5 mM DGG appeared to be an appropriate concentration to study both desensitization and saturation.
We used the model of Wadiche and Jahr (2001) to confirm that the effects of DGG on saturation and desensitization could not be separated by simple changes in experimental conditions. At all modeled concentrations of DGG and glutamate, there were effects on both saturation and desensitization and elimination of both had similar DGG dependence (Supplemental Fig. S1).1 Thus relief of both desensitization and saturation appears to result from the blocking of a large fraction of AMPARs.
DGG indicates AMPAR saturation
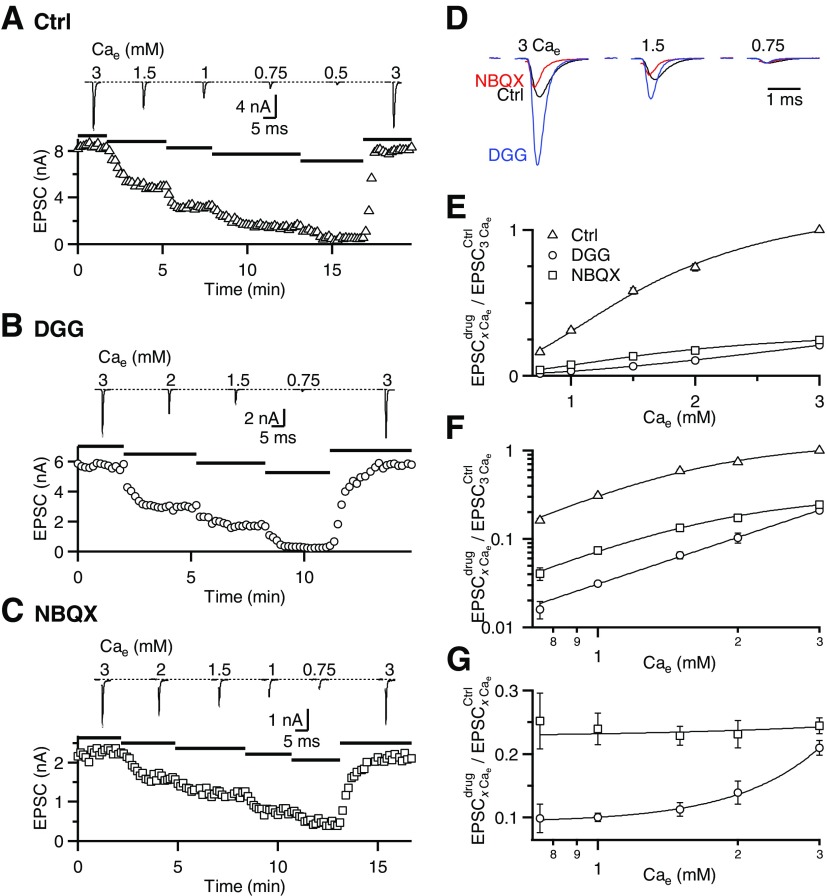
To clarify whether receptor saturation plays a role at the endbulb, we measured EPSC amplitudes in different Cae in endbulbs from P16–P40 animals. In a representative experiment, when Cae was changed from 3 to 1.5, 1, 0.75, and 0.5 mM, the EPSC amplitude decreased to 55, 32, 13, and 5% of control, respectively (Fig. 3A). We fit the relationship between Cae and EPSC using a Hill equation: EPSC = EPSCmax/[1 + (K1/2/Cae)n], with EPSCmax = 1.22 ± 0.09, n = 2.35 ± 0.25, and K1/2 = 1.60 ± 0.13 (Fig. 3, E and F).
Fig. 3.
DGG reveals saturation at the endbulb of Held. EPSC amplitudes measured in different Cae for 3 representative cells, measured in control conditions (A), in 5 mM DGG (B), and in 250 nM 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione (NBQX, C). Bottom panels show the peak EPSC amplitude over the course of the experiment. Top traces are average EPSCs when wash-in of each Cae had stabilized. D: average EPSCs for the experiments shown in A–C overlaid and scaled by the EPSC peak in 0.75 Cae, where saturation is presumably minimal. This indicates that DGG block is lower in high Cae, probably because of saturation. E and F: average EPSC amplitudes in control (8 to 23 cells), DGG (6 to 39 cells), and NBQX (7 to 25 cells) in different Cae, normalized to 3 Cae to allow averaging across experiments. Data are pooled for endbulbs from animals P16–P40. Data are fit using a Hill equation for control and NBQX and to a power law for DGG (see results). Data are plotted on a linear scale (E) or log-log (F). G: block by DGG (circles) and NBQX (squares) for each Cae. The greater block by DGG at low Cae indicates saturation. Constant block by NBQX indicates that the effects of DGG do not result from improved voltage clamp. The NBQX and DGG data are fitted with a straight line and power-law, respectively.
Next, we tested the effect of DGG on this relationship. DGG reduced the EPSC amplitude to 51, 32, and 4.7% when Cae was decreased to 2, 1.5, and 0.75 mM, respectively, in the example cell (Fig. 3B). Fitting the DGG data using a Hill equation gave us: EPSCmax = 0.90 ± 1.25, n = 1.95 ± 0.32, and K1/2 = 5.54 ± 5.59 (Fig. 3E). Although this approach yielded a nominally higher K1/2, the Hill parameters were not well constrained in DGG. Therefore we also fit these data using a power-law equation: EPSC = K(Cae)n, with K = 0.031 ± 0.001 and n = 1.7 ± 0.1 (Fig. 3F). The close fit with this simple relationship further suggests that AMPAR saturation is largely absent in the presence of DGG.
To confirm that the effect of DGG on EPSC amplitude results from receptor saturation and not from improved voltage clamp in DGG, we used 250 nM NBQX to block the EPSC amplitude to a similar extent as 5 mM DGG in 3 Cae. NBQX is a high-affinity AMPAR antagonist and thus has a much slower off-rate, so it does not prevent receptor saturation, although it would still improve the quality of the clamp (Diamond and Jahr 1997; Foster et al. 2005; Wadiche and Jahr 2001). For the representative experiment of Fig. 3C, NBQX reduced the EPSC amplitude to 76, 57, 36, and 21% when Cae was decreased from 3 to 2, 1.5, 1, and 0.75 mM, respectively. Similar results were found on average (Fig. 3, E and F). Fitting the data to a Hill equation yielded EPSCmax = 0.32 ± 0.05, n = 2.19 ± 0.35, and K1/2 = 1.75 ± 0.30, similar to the control condition (P > 0.05).
Another sign of saturation would be that block by DGG is greater in low-release conditions. To test this, we quantified the effect of DGG and NBQX in different Cae by calculating the EPSC amplitudes in drug relative to control (Fig. 3G). NBQX decreased the EPSC amplitude similarly across all conditions, but DGG decreased the EPSC amplitude more at lower Cae. These results are consistent with DGG, but not NBQX, relieving saturation at the endbulb. This suggests that part of the effects of DGG we observed in Fig. 2 are likely to result from saturation. The existence of saturation complicates the study of desensitization using DGG at the endbulb because these two postsynaptic processes have opposite effects on the PPR. Therefore separating the effect of DGG on saturation would be an important step to better understand whether there is desensitization at the endbulb.
CTZ prevents desensitization in older endbulbs
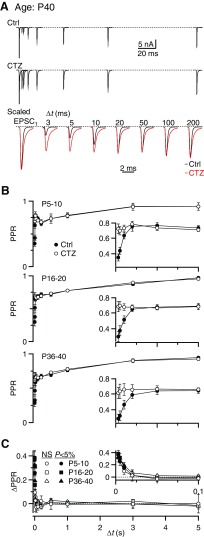
First, we wanted to test whether desensitization could account for the effects of DGG on PPR at short Δt values. To do this, we applied 50 μM CTZ in 3 Cae. In this series of experiments, the starting EPSC1 amplitude was very similar to the experiments using DGG in Fig. 2 (5.2 ± 2.3 nA for P5–P10, 14.0 ± 1.1 nA for P16–P20, and 10.2 ± 1.7 nA for P36–P40). Application of CTZ significantly prolonged EPSC decay in endbulbs at all ages (P < 0.001, n = 7, Fig. 4A, Table 1).
Fig. 4.
Cyclothiazide (CTZ) reduces depression at short intervals similarly at all ages. A: example traces from a P40 BC in control conditions (top) and in 50 μM CTZ (middle). The bottom panel shows control and CTZ traces scaled by the EPSC1 amplitude. B: average results for 5 BCs from P5–P10 (top), 6 BCs from P16–P20 (middle), and 7 BCs from P36–P40 animals (bottom). The insets expand the intervals Δt = 3 to 100 ms. C: the ΔPPR is shown for both age groups. Filled symbols indicate points that differ significantly from 0 for both groups (P < 0.05).
We compared the effects of CTZ on PPR in P5–P40 endbulbs. EPSC1 amplitude did not change significantly (106 ± 4%, n = 13, P > 0.5). By contrast, at short Δt values, the EPSC2 amplitude increased after CTZ application, leading to an increase in PPR in endbulbs up to P20, as previously observed (Fig. 4B, top) (Bellingham and Walmsley 1999; Isaacson and Walmsley 1996; Yang and Xu-Friedman 2008). For Δt = 3 ms, the PPR increased from 0.35 ± 0.04 to 0.72 ± 0.06 (P < 0.005, n = 5, Fig. 4B, top) for P5–P10 and from 0.3 ± 0.06 to 0.68 ± 0.06 (P < 0.005, n = 6, Fig. 4B, middle) for P16–P20. Similarly, in mice aged P36–P40, CTZ increased PPR at Δt = 3 ms from 0.29 ± 0.05 to 0.61 ± 0.07 (P < 0.005, n = 7, Fig. 4B, bottom). This effect on PPR was significant at intervals ≤10 ms (P < 0.05). The ΔPPR resulting from CTZ was greatest at Δt = 3 ms and decreased to about 0 at 20 ms and did not change significantly at longer intervals (Fig. 4C, bottom). The ΔPPR value was identical at all ages tested (0.37 ± 0.07 for P5–P10, 0.38 ± 0.06 for P16–P20, and 0.32 ± 0.07 for P36–P40). We previously found no detectable nonspecific presynaptic effects of CTZ at mouse endbulbs (Yang and Xu-Friedman 2008). This suggests that AMPAR desensitization affects depression at short intervals throughout the developmental period examined (P5–P40).
We further tested whether effects of CTZ and DGG on PPR resulted from poor voltage clamp by conducting similar experiments in the presence of 250 nM NBQX to reduce EPSC amplitudes. CTZ still significantly relieved depression at short intervals (ΔPPR at Δt of 3 ms = 0.21 ± 0.03, n = 9 cells; P < 0.05), but not at intervals >20 ms (Supplemental Fig. S2). Similarly for endbulbs already in NBQX, further addition of DGG increased PPR for intervals <10 ms (ΔPPR at Δt of 3 ms = +0.17 ± 0.03, n = 7 cells; P < 0.05) and decreased at intervals >10 ms (ΔPPR at Δt of 100 ms = −0.13 ± 0.03; P < 0.05; Supplemental Fig. S3).
CTZ and DGG do not increase PPR by increasing saturation
It is possible that CTZ could increase PPR by increasing the affinity of AMPARs for glutamate and increasing saturation. We previously showed that this does not occur in <P21 endbulbs (Yang and Xu-Friedman 2008). We confirmed this in our entire sample, by examining how CTZ individually affected the amplitudes of EPSC1 and EPSC2. Our prediction is that if CTZ primarily affects desensitization, it would increase EPSC2 most in highly desensitizing endbulbs, whereas the effects of CTZ on EPSC1 would be fairly uniform. However, if CTZ primarily increases saturation, then EPSC2, which is less saturated, should increase fairly uniformly across preparations, whereas EPSC1 would increase least for highly saturated endbulbs (Xu-Friedman and Regehr 2003).
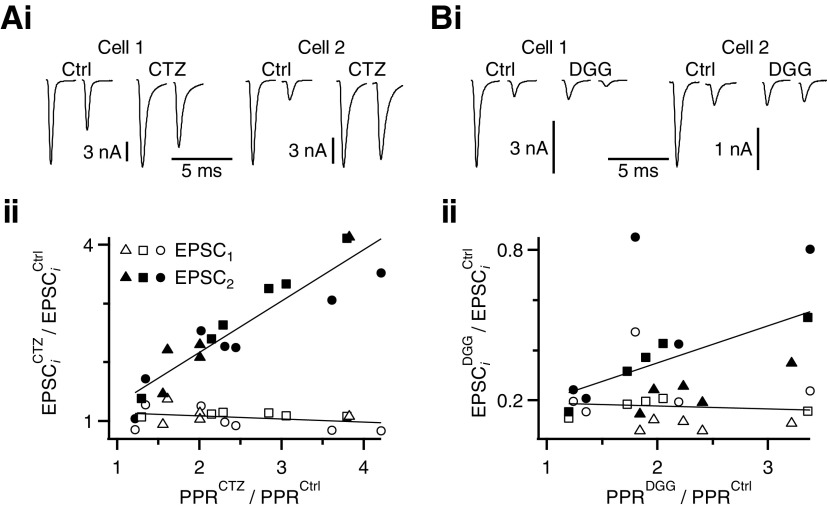
We tested this hypothesis by plotting the change in EPSC1 and EPSC2 for Δt = 3 ms against the effectiveness of CTZ at different endbulbs. CTZ application increased the PPR mainly by increasing EPSC2, but did not affect EPSC1 amplitude (Fig. 5A). The increase in EPSC2 amplitude was highly correlated with change in PPR (correlation coefficient = 0.93, Fig. 5A). The effects were similar at all ages.
Fig. 5.
The increase in PPR in CTZ and DGG for an interpulse interval of 3 ms is consistent with desensitization, not saturation, at all ages. Ai and Bi: example cells showing that application of CTZ and DGG have similar effects on EPSC1, but differential effects on EPSC2. Aii and Bii: relative changes in EPSC1 and EPSC2 amplitude are plotted with respect to relative changes in PPR for each individual endbulb. Data from different ages are indicated by triangles (P5–P10), squares (P16–P20), and circles (P36–P40). Data are fit using straight lines. EPSC1 is affected uniformly by CTZ (Aii) and DGG (Bii), but the effects on EPSC2 depend on the change in PPR. This is consistent with the idea that CTZ and DGG relieve desensitization (see results).
DGG had similar effects. Application of DGG decreased EPSC1 to a fairly uniform degree (Fig. 5B), with a correlation coefficient of −0.09. EPSC2 showed more variable effects. When the PPR did not change much, EPSC1 and EPSC2 changed similarly, but when PPR increased a lot, EPSC2 was blocked much less than EPSC1. The correlation between change in EPSC2 amplitude and change in PPR in DGG was also significant at 0.48. This value for DGG was smaller than what we observed in CTZ, probably because DGG also blocks saturation. Indeed, it appeared that there was a somewhat larger block by DGG for P5–P10 (triangles in Fig. 5Bii), although the trend was similar for all ages. These data are consistent with both CTZ and DGG preventing receptor desensitization, but not with their causing an increase in PPR by increasing saturation.
CTZ occludes the effect of DGG at short intervals
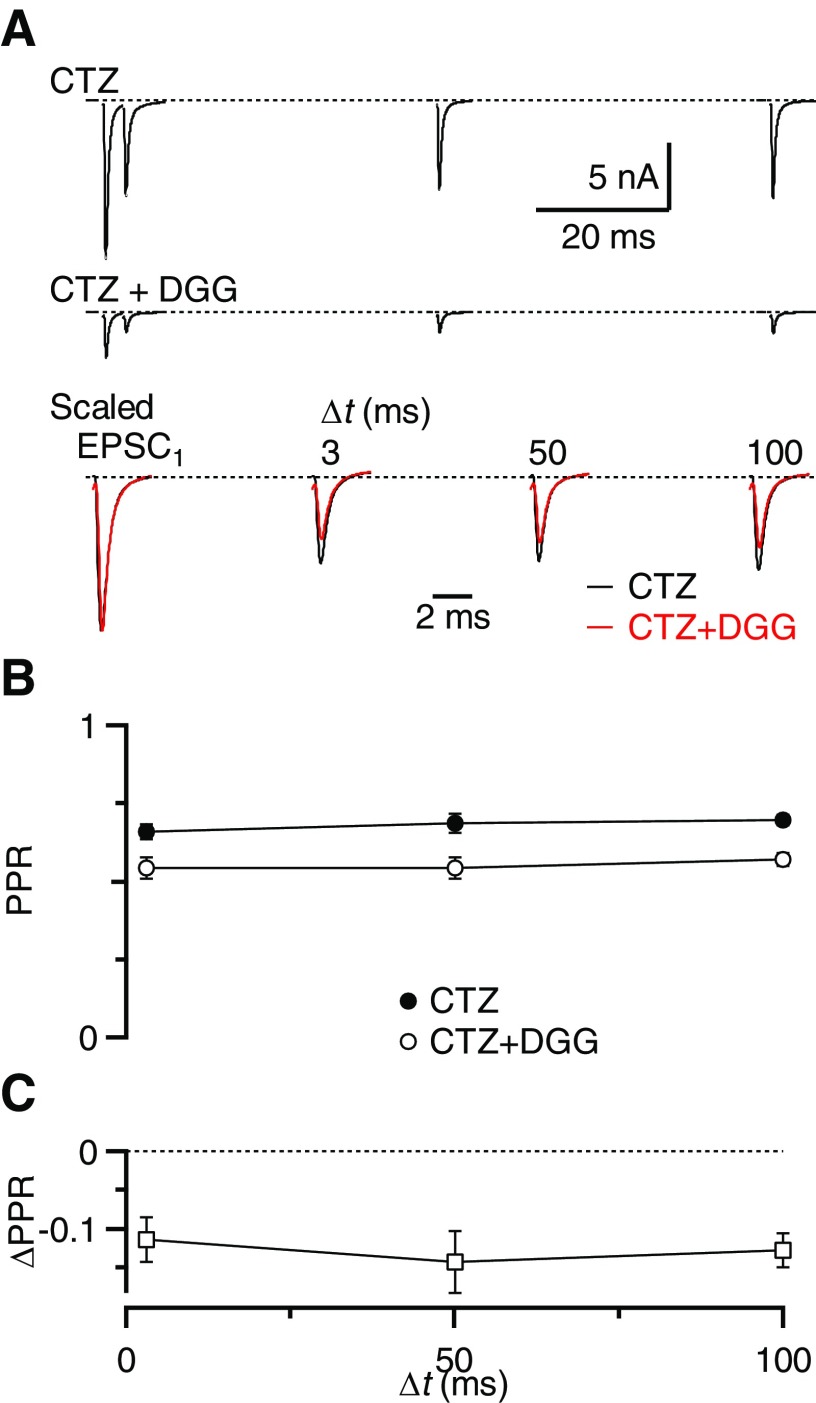
The experiments described so far suggest that DGG, CTZ, and kynurenate all prevent receptor desensitization at short intervals (≤3 ms). If this is true, then prior application of CTZ should prevent any further increase in PPR by DGG. In addition, the experiments using DGG and kynurenate indicate the presence of saturation for Δt ≥20 ms, although saturation is not visible at shorter intervals. We attributed the lack of evidence of saturation to a dominant effect of DGG and kynurenate on desensitization, whereby the PPR increases at short intervals. If this is true, then when desensitization is blocked beforehand by CTZ, DGG should cause a reduction in PPR at all values of Δt.
We tested these predictions by measuring PPR in CTZ before applying DGG for animals P16–P40. Application of DGG in the presence of CTZ did not change the EPSC kinetics compared with CTZ alone (Fig. 6A, Table 1, n = 8, P > 0.5). Unlike the experiments of Fig. 2, addition of DGG did not produce an increase in PPR. On the contrary, the PPR at Δt = 3 ms decreased from 0.65 ± 0.02 in CTZ alone to 0.54 ± 0.03 when DGG was added (n = 8, Fig. 6, A and B). The decrease in PPR was significant and similar for Δt = 3, 50, and 100 ms (P < 0.001, Fig. 6C).
Fig. 6.
CTZ occludes the effect of DGG on desensitization. A: example traces in the presence of CTZ (top) and CTZ + DGG (middle), for paired-pulse stimulation at Δt = 3, 50, and 100 ms. The bottom panel shows CTZ and CTZ + DGG traces scaled by the EPSC1 amplitude. B: average results for 8 BCs from animals P16–P40. PPR decreases uniformly at all intervals, indicating that CTZ abolished desensitization, so subsequent DGG reveals only saturation. C: the ΔPPR for the experiments in B is similar for all interpulse intervals and all are significantly below 0 (P < 0.05).
Thus the increase in PPR at short intervals by DGG was occluded by CTZ. This indicates that CTZ and DGG increase the PPR through the same pathway, most likely by relieving AMPAR desensitization. This experiment also indicates that receptor saturation influences PPR at short intervals (3 ms) as much as at longer intervals (e.g., 50 and 100 ms), but that DGG application in the absence of CTZ does not reveal it because of the greater impact of desensitization.
Isolating saturation reveals desensitization
We wanted to isolate the effect of DGG on receptor desensitization from its effect on saturation. To do that, we compared the effects of DGG on depressed versus nondepressed EPSCs of similar amplitude. For nondepressed EPSCs, we used our measurements in different Cae from Fig. 3G. These measurements were made on rested synapses, so they report the amount of block by DGG that resulted entirely from changes in the amount of presynaptic neurotransmitter release. We plotted the effect of DGG on EPSCs measured in different Cae (EPSC1DGG,xCae/EPSC1xCae) versus the initial EPSC amplitude (before DGG block), which we normalized to the 3 Cae condition to allow averaging across experiments (i.e., EPSC1xCae/EPSC13Cae) (filled symbols in Fig. 7). We fit these data to a simple power law (y = y0 + Axn) using least-squares, which yielded parameters: y0 = 0.1 ± 0.01, A = 0.11 ± 0.014, and n = 3.66 ± 1.36. We refer to this as the “saturation curve” because its shape depends on the amount of receptor saturation in different Cae. The saturation curve describes the block by DGG of an endbulb EPSC when the only parameter changing is the amount of neurotransmitter release.
We wanted to compare depressed EPSCs against the saturation curve. Our prediction was that any changes in PPR that resulted solely from changes in the amount of neurotransmitter release should lie along the same line. However, if there were changes in PPR that resulted from desensitization, they should show up as deviations from that line. For our depressed EPSCs, we used the results of paired-pulse experiments in 1.5 Cae and 3 Cae, where the starting EPSC amplitude on the abscissa was normalized to the resting EPSC in 3 Cae (i.e., EPSC2,ΔtxCae/EPSC13Cae). On the ordinate, the effect of DGG at each interval was then calculated (i.e., EPSC2,ΔtDGG,xCae/EPSC2,ΔtCtrl,xCae). These are shown as the open symbols in Fig. 7.
At short intervals, the block by DGG was much less than that predicted by the saturation curve for an EPSC of identical size. This difference most likely results from the effects of DGG in preventing desensitization. In other words, EPSC2 values at short interpulse intervals depressed in part because of desensitization, and not solely because of a decrease in neurotransmitter release. At longer intervals, the PPR data approached the saturation curve, indicating that the endbulb gradually recovered from desensitization, so depression at these longer intervals resulted primarily from changes in presynaptic release. To determine at which point the two curves merged, statistically, we compared the 95% confidence interval of the saturation curve against the 95% confidence intervals of each point (i.e., 1.96 × SE). Points that showed no overlap are significantly different at the P < 0.05 level and are indicated by asterisks (Fig. 7). This shows that desensitization persists at the endbulb at intervals as long as 10 ms in 3 Cae and 3 ms in 1.5 Cae. This differs from the simpler approach in Fig. 2, in which there was no visible effect at Δt = 10 ms on PPR because it did not take saturation into account.
Desensitization contributes to depression during trains
We tested whether AMPAR desensitization is significant during longer periods of activity. We stimulated the presynaptic AN fiber with 100-, 200-, and 333-Hz trains in 1.5 Cae and applied DGG to prevent desensitization. During these trains, as a result of depression and block by DGG, the resulting EPSC could be as small as 1–2% of control amplitudes and difficult to quantify accurately for average-sized endbulbs. Therefore to avoid potential bias in studying extremely large synapses, we restricted our attention to the first five pulses of a train. In the presence of DGG, EPSC2 to EPSC5 were blocked less than EPSC1 (see example in Fig. 8A). We analyzed these data using the same approach as that in Fig. 7, plotting the effect of DGG on the ith pulse in the train (EPSCiDGG/EPSCiCtrl) against the initial EPSC size (normalized to the resting EPSC in 3 Cae: EPSCiCtrl/EPSC13Cae). EPSC1 of the trains (open symbols, Fig. 8B) lay on the saturation curve (line and closed diamonds). However, many of the later EPSCs in the trains lay significantly above the line (filled symbols in Fig. 8B, P < 0.05, using the 95% confidence interval approach described earlier). This indicates that the later EPSCs were depressed not solely as a result of changes in neurotransmitter release, but also because of postsynaptic changes, most likely desensitization.
To test how long desensitization influenced depression during longer trains, we turned to CTZ. CTZ increased the EPSC amplitudes with respect to control (Fig. 8, C and D) for the first five pulses in 100-Hz trains and considerably longer in 200- and 333-Hz trains (filled symbols, Fig. 8E, n = 11). This indicates that desensitization is significant early in high-frequency trains and decreases thereafter.
Effect of desensitization on BC spiking
Finally, we tested whether the effects of desensitization on synaptic plasticity could play a role in modulating BC action potential firing. To study this issue, it is not possible to use fiber stimulation in combination with drug application because DGG and kynurenate also affect EPSC amplitude and CTZ also affects EPSC kinetics. Instead, we turned to the dynamic-clamp technique, using the measurements of EPSC amplitudes during trains before and after CTZ application (Fig. 8), convolved with a normalized EPSC. The starting conductance amplitudes were the same in the two conditions, but depressed at different rates, according to the results of Fig. 8.
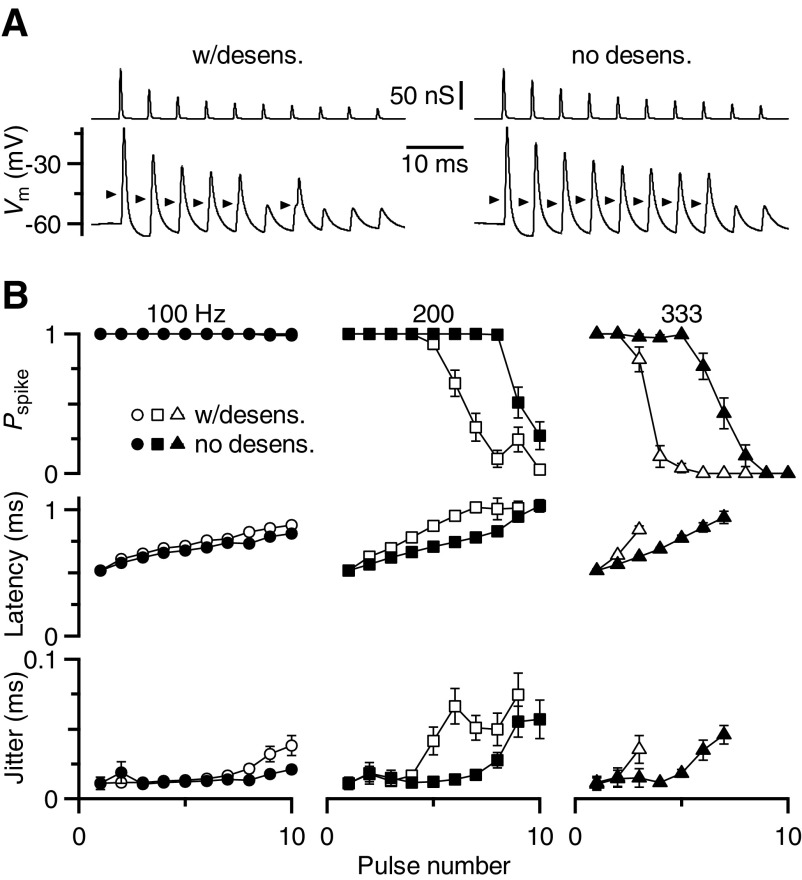
An example response is shown in Fig. 9A. When the BC was driven by normally depressing synaptic conductances in a 200-Hz train (“w/desens”), it showed reliable spiking early in the train. However, spiking failed later as the conductance depressed below threshold. When a 200-Hz train was presented based on EPSC amplitudes in the presence of CTZ (“0 desens”), spiking was preserved later in the train.
Fig. 9.
Effect of desensitization on BC firing properties. A: example dynamic-clamp traces showing conductance waveforms (top) and BC response (bottom). Conductance waveforms were based on peak EPSC amplitudes during 200-Hz trains in control (left) or CTZ (right) conditions. Arrows mark spike threshold, as estimated using the first or second derivative of the trace. B: average results from 17 cells, showing probability of spiking, latency, and jitter during dynamic-clamp experiments of 100-, 200-, and 333-Hz trains, with and without desensitization. Latency and jitter were undefined when the probability of spiking was low; points are shown only in which measurable values could be obtained in ≥5 experiments.
Similar results were found in 17 cells. For 100-Hz trains, depression did not bring the synapse below threshold (Fig. 9B, left). At higher frequencies, 200 and 333 Hz, synaptic conductances based on desensitizing synapses showed considerable spike failures for trains. When synaptic conductances were based on EPSC amplitudes without desensitization, spike probability was significantly elevated later in the train, leading to the transmission of a considerable number of additional spikes (Fig. 9B, middle and right).
DISCUSSION
We show here that desensitization contributes to depression in endbulbs of ages up to P40. We have shown this using two very different pharmacological approaches: CTZ, which interferes with the conformational changes that AMPARs undergo, and DGG, which protects a population of AMPARs from exposure to glutamate and thus desensitization. We also showed that these two drugs enhance depressed EPSCs through the same pathway because CTZ occludes the enhancement by DGG. Our results indicate that endbulbs are in this respect effectively mature by P16, which is shortly after the onset of hearing (Ehret 1976). DGG application also indicates that endbulbs are significantly saturated, even in low Cae conditions. Relief of desensitization and of saturation by DGG has opposite effects on plasticity and we describe an approach for separating these effects. Integrating those results with CTZ indicates that desensitization persists during the early parts of trains of activity and decreases afterward. Desensitization has significant effects on the likelihood of BC firing during trains and could influence auditory perception.
Desensitization and maturation
The CTZ experiments described here are consistent with our earlier study and suggest that desensitization affects PPR for as long as 20 ms after EPSC1 (Yang and Xu-Friedman 2008). This is consistent with the rate of recovery from desensitization observed not only at other synapses (Chen et al. 2002; Rozov et al. 2001; Trussell et al. 1993; Xu-Friedman and Regehr 2003) but also in expression systems (Partin et al. 1993; Robert and Howe 2003).
Our results indicate that desensitization contributes to plasticity as early as P5 and persists into mature endbulbs (P40). Therefore desensitization is a not merely a transitory feature of immature endbulbs and may play a role in endbulb function. This is not generally observed because several synapses show an early role of desensitization in plasticity that decreases as synapses mature. For example, at the calyx of Held, several studies have documented a contribution of desensitization to depression in young but not mature animals (Joshi and Wang 2002; Koike-Tani et al. 2008; Renden et al. 2005; Scheuss et al. 2002; Taschenberger et al. 2002, 2005; Wong et al. 2003). It is not clear what factor is critical for this decrease at the calyx nor why the endbulb differs. Desensitization may persist at the endbulb because of continued expression of AMPARs with rapid EPSC decay kinetics (Trussell 1999) and the effects on plasticity could be a secondary effect. Recent evidence in cultured neurons has suggested that there are mechanisms to remove desensitized AMPARs from the postsynaptic membrane (Heine et al. 2008). Our results suggest that at mature endbulbs such a mechanism either is not present or is not capable of replacing all desensitized receptors on such rapid timescales.
BC AMPARs are composed mainly of GluR3 and GluR4 subunits (Wang et al. 1998), which are rapidly desensitizing (Mosbacher et al. 1994). Outside-out patch experiments from BCs suggest that the flop isoforms of these subunits dominate (Gardner et al. 2001). CTZ is somewhat less effective at preventing desensitization in the flop isoforms relative to the flip isoforms of GluR3/4 (Partin et al. 1993). However, our data suggest that even this smaller relative effect is sufficient for cyclothiazide to prevent desensitization at endbulbs.
Saturation and DGG
We studied saturation at the endbulb by applying DGG and varying Cae conditions. The presence of saturation is revealed when the amount of block by DGG differs between different Cae. We saw that DGG had its greatest blocking effect at 0.75 Cae, which differed even from that at 1 Cae (Fig. 3). This difference could arise only if there was significantly more neurotransmitter release at each release site in 1 Cae, such as by having significant multivesicular release.
To evaluate whether there is likely to be multivesicular release, it is useful to know the probability of vesicle release (P). This value can be estimated using mean-variance analysis (Silver 2003). This approach has been used at the endbulb to study release sites (Oleskevich et al. 2000; Wang and Manis 2005). When saturation is removed by DGG, mean-variance data can be used to measure P for individual vesicles (Foster and Regehr 2004).
Since our DGG experiments were not designed for this specifically, we can only make a first estimate of P using mean-variance analysis (Supplemental Fig. S4). Experiments in our highest Cae showed no decrease in the EPSC variance for any of the nine cells we studied in this set of experiments, indicating that P in 3 Cae (P3Cae) ≤ 0.5. To estimate P in other Cae conditions, we scaled that upper bound by the relative EPSC amplitudes in DGG compared with 3 Cae [i.e., PxCae = (EPSCxCaeDGG/EPSC3CaeDGG)P3Cae], yielding estimates of P2Cae ≤ 0.24, P1.5Cae ≤ 0.16, P1Cae ≤ 0.09, and P0.75Cae ≤ 0.04.
The values of P in low Cae may appear to be small, but a significant amount of multivesicular release is still predicted by a simple binomial model of release. This is true even when the number of releasable vesicles is as low as five (see Supplemental Fig. S5), which is in the range of the mean number of docked vesicles measured in serial electron microscopy reconstructions of rat endbulbs (Nicol and Walmsley 2002). Furthermore, the amount of multivesicular release predicted by the binomial model changes significantly between our estimates of P0.75Cae and P1Cae. Thus DGG should block EPSCs to different extents, even at very low Cae, consistent with our observations in Fig. 3. A second prediction of this analysis is that release of as few as two vesicles per release site may cause significant saturation at the endbulb, which is consistent with some modeling studies (Franks et al. 2002; Raghavachari and Lisman 2004; Xu-Friedman and Regehr 2004).
Some aspects of endbulb structure somewhat undermine the binomial model. Most endbulb release sites (i.e., those opposite well-defined postsynaptic densities) appear conventional, with limited size and number of vesicles (Nicol and Walmsley 2002). However, there are also a number of highly extended release sites, with dozens of docked vesicles, which renders a strict interpretation of multivesicular release less appropriate. The close spacing of smaller release sites does not contribute a spillover component to the EPSC, such as that observed at the cerebellar mossy fiber (DiGregorio et al. 2002), although effects on desensitization are possible.
Using DGG to study depression
The presence of saturation complicates the use of DGG to study desensitization. This is demonstrated by our finding that DGG increased PPR for Δt <10 ms and decreased PPR for Δt >20 ms. We described an approach for separating the multiple effects of DGG. DGG's effects are first calibrated on EPSCs of different amplitudes (obtained by changing Cae) and then compared against the effects of DGG on depressed EPSCs. If depression results from a decrease in neurotransmitter release, then DGG should affect depressed EPSCs the same as those decreased by changing Cae. However, if depression results from other mechanisms, then DGG should have a different effect. We found that the effect of DGG on depressed EPSCs could not be explained solely as a decrease in neurotransmitter release, indicating that desensitization does play a role.
This is an important refinement for the method of using DGG to study desensitization. This approach should allow the detection of desensitization even when no changes in PPR are obvious. This could explain the difference in conclusions between this study and that by Wang and Manis (2008), which did not compare against the predictions of the saturation curve. Our results were fully consistent with those obtained more simply using CTZ, both with pairs of pulses and during trains.
Functional relevance
The functional implications of desensitization have been pointed out in earlier studies (Trussell 1999), including a study at P15–P21 endbulbs (Yang and Xu-Friedman 2008). Our results here extend the relevance of desensitization to older ages. One additional factor that we investigated here was the role of desensitization during trains. Earlier modeling results by Yang and Xu-Friedman (2008) predicted that desensitization would become less important during extended trains, given that glutamate release decreased with presynaptic vesicle depletion. However, direct tests of this using DGG and CTZ indicate that desensitization can cause a significant decrease in synaptic strength that persists for several pulses in high-frequency trains (Fig. 8).
It is a question whether desensitization would have behavioral consequences. This has been considered at length for chick endbulbs, which appear to trigger action potentials highly reliably even at very high frequencies in mature synapses (Brenowitz and Trussell 2001). However, the mammalian endbulb appears to differ in important respects. Endbulbs typically have a high safety factor, in that peak conductances for rested endbulbs average around 100 nS (range: 30–300 nS) and spike thresholds in BCs are typically <30 nS (Xu-Friedman and Regehr 2005a,b; Yang and Xu-Friedman 2009). Our dynamic-clamp experiments suggest that desensitization can contribute to bringing the synapse below threshold during high-frequency trains (Fig. 9). Thus desensitization could curtail the responses of mature BCs during brief, intense sounds, which could influence auditory perception. This would also be influenced by additional factors, including NMDA receptor activation (Pliss et al. 2009), delayed release, and spontaneous firing in AN fibers (Hermann et al. 2007; Taberner and Liberman 2005).
GRANTS
This work was supported by National Institute on Deafness and Other Communication Disorders Grant R01-DC-008125 to M. A. Xu-Friedman.
Supplementary Material
ACKNOWLEDGMENTS
We thank L. Pliss, G. Tao, J. Trimper, and H. Yang for help with experiments and for comments on the manuscript.
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Bellingham MC, Walmsley B. A novel presynaptic inhibitory mechanism underlies paired pulse depression at a fast central synapse. Neuron 23: 159–170, 1999. [DOI] [PubMed] [Google Scholar]
- Brawer JR, Morest DK. Relations between auditory nerve endings and cell types in the cat's anteroventral cochlear nucleus seen with the Golgi method and Nomarski optics. J Comp Neurol 160: 491–506, 1975. [DOI] [PubMed] [Google Scholar]
- Brawer JR, Morest DK, Kane EC. The neuronal architecture of the cochlear nucleus of the cat. J Comp Neurol 155: 251–300, 1974. [DOI] [PubMed] [Google Scholar]
- Brenowitz S, Trussell LO. Maturation of synaptic transmission at end-bulb synapses of the cochlear nucleus. J Neurosci 21: 9487–9498, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Blitz DM, Regehr WG. Contributions of receptor desensitization and saturation to plasticity at the retinogeniculate synapse. Neuron 33: 779–788, 2002. [DOI] [PubMed] [Google Scholar]
- Clements JD, Lester RA, Tong G, Jahr CE, Westbrook GL. The time course of glutamate in the synaptic cleft. Science 258: 1498–1501, 1992. [DOI] [PubMed] [Google Scholar]
- Crowley JJ, Carter AG, Regehr WG. Fast vesicle replenishment and rapid recovery from desensitization at a single synaptic release site. J Neurosci 27: 5448–5460, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond JS, Jahr CE. Asynchronous release of synaptic vesicles determines the time course of the AMPA receptor-mediated EPSC. Neuron 15: 1097–1107, 1995. [DOI] [PubMed] [Google Scholar]
- Diamond JS, Jahr CE. Transporters buffer synaptically released glutamate on a submillisecond time scale. J Neurosci 17: 4672–4687, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGregorio DA, Nusser Z, Silver RA. Spillover of glutamate onto synaptic AMPA receptors enhances fast transmission at a cerebellar synapse. Neuron 35: 521–533, 2002. [DOI] [PubMed] [Google Scholar]
- Ehret G. Development of absolute auditory thresholds in the house mouse (Mus musculus). J Am Audiol Soc 1: 179–184, 1976. [PubMed] [Google Scholar]
- Fekete DM, Rouiller EM, Liberman MC, Ryugo DK. The central projections of intracellularly labeled auditory nerve fibers in cats. J Comp Neurol 229: 432–450, 1984. [DOI] [PubMed] [Google Scholar]
- Foster KA, Crowley JJ, Regehr WG. The influence of multivesicular release and postsynaptic receptor saturation on transmission at granule cell to Purkinje cell synapses. J Neurosci 25: 11655–11665, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster KA, Kreitzer AC, Regehr WG. Interaction of postsynaptic receptor saturation with presynaptic mechanisms produces a reliable synapse. Neuron 36: 1115–1126, 2002. [DOI] [PubMed] [Google Scholar]
- Foster KA, Regehr WG. Variance-mean analysis in the presence of a rapid antagonist indicates vesicle depletion underlies depression at the climbing fiber synapse. Neuron 43: 119–131, 2004. [DOI] [PubMed] [Google Scholar]
- Franks KM, Bartol TM, Jr, Sejnowski TJ. A Monte Carlo model reveals independent signaling at central glutamatergic synapses. Biophys J 83: 2333–2348, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner SM, Trussell LO, Oertel D. Correlation of AMPA receptor subunit composition with synaptic input in the mammalian cochlear nuclei. J Neurosci 21: 7428–7437, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen AJ. Effect of anoxia on ion distribution in the brain. Physiol Rev 65: 101–148, 1985. [DOI] [PubMed] [Google Scholar]
- Heine M, Groc L, Frischknecht R, Beique JC, Lounis B, Rumbaugh G, Huganir RL, Cognet L, Choquet D. Surface mobility of postsynaptic AMPARs tunes synaptic transmission. Science 320: 201–205, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann J, Pecka M, von Gersdorff H, Grothe B, Klug A. Synaptic transmission at the calyx of Held under in vivo like activity levels. J Neurophysiol 98: 807–820, 2007. [DOI] [PubMed] [Google Scholar]
- Isaacson JS, Walmsley B. Receptors underlying excitatory synaptic transmission in slices of the rat anteroventral cochlear nucleus. J Neurophysiol 73: 964–973, 1995. [DOI] [PubMed] [Google Scholar]
- Isaacson JS, Walmsley B. Amplitude and time course of spontaneous and evoked excitatory postsynaptic currents in bushy cells of the anteroventral cochlear nucleus. J Neurophysiol 76: 1566–1571, 1996. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Takahashi T. Mechanisms underlying presynaptic facilitatory effect of cyclothiazide at the calyx of Held of juvenile rats. J Physiol 533: 423–431, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi I, Wang LY. Developmental profiles of glutamate receptors and synaptic transmission at a single synapse in the mouse auditory brainstem. J Physiol 540: 861–873, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Alger BE. Random response fluctuations lead to spurious paired-pulse facilitation. J Neurosci 21: 9608–9618, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike-Tani M, Kanda T, Saitoh N, Yamashita T, Takahashi T. Involvement of AMPA receptor desensitization in short-term synaptic depression at the calyx of Held in developing rats. J Physiol 586: 2263–2275, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limb CJ, Ryugo DK. Development of primary axosomatic endings in the anteroventral cochlear nucleus of mice. J Assoc Res Otolaryngol 1: 103–119, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorente de Nó R. The Primary Acoustic Nuclei. New York: Raven Press, 1981, p. 176. [Google Scholar]
- Manthei RC, Wright DC, Kenny AD. Altered CSF constituents and retrograde amnesia in rats: a biochemical approach. Physiol Behav 10: 517–521, 1973. [DOI] [PubMed] [Google Scholar]
- Mosbacher J, Schoepfer R, Monyer H, Burnashev N, Seeburg PH, Ruppersberg JP. A molecular determinant for submillisecond desensitization in glutamate receptors. Science 266: 1059–1062, 1994. [DOI] [PubMed] [Google Scholar]
- Nicol MJ, Walmsley B. Ultrastructural basis of synaptic transmission between endbulbs of Held and bushy cells in the rat cochlear nucleus. J Physiol 539: 713–723, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleskevich S, Clements J, Walmsley B. Release probability modulates short-term plasticity at a rat giant terminal. J Physiol 524: 513–523, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostapoff EM, Morest DK. Synaptic organization of globular bushy cells in the ventral cochlear nucleus of the cat: a quantitative study. J Comp Neurol 314: 598–613, 1991. [DOI] [PubMed] [Google Scholar]
- Partin KM, Patneau DK, Winters CA, Mayer ML, Buonanno A. Selective modulation of desensitization at AMPA versus kainate receptors by cyclothiazide and concanavalin A. Neuron 11: 1069–1082, 1993. [DOI] [PubMed] [Google Scholar]
- Pliss L, Yang H, Xu-Friedman MA. Context-dependent effects of NMDA receptors on precise timing information at the endbulb of Held in the cochlear nucleus. J Neurophysiol 102: 2627–2637, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavachari S, Lisman JE. Properties of quantal transmission at CA1 synapses. J Neurophysiol 92: 2456–2467, 2004. [DOI] [PubMed] [Google Scholar]
- Renden R, Taschenberger H, Puente N, Rusakov DA, Duvoisin R, Wang LY, Lehre KP, von Gersdorff H. Glutamate transporter studies reveal the pruning of metabotropic glutamate receptors and absence of AMPA receptor desensitization at mature calyx of Held synapses. J Neurosci 25: 8482–8497, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert A, Howe JR. How AMPA receptor desensitization depends on receptor occupancy. J Neurosci 23: 847–858, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozov A, Jerecic J, Sakmann B, Burnashev N. AMPA receptor channels with long-lasting desensitization in bipolar interneurons contribute to synaptic depression in a novel feedback circuit in layer 2/3 of rat neocortex. J Neurosci 21: 8062–8071, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryugo DK, Dodds LW, Benson TE, Kiang NY. Unmyelinated axons of the auditory nerve in cats. J Comp Neurol 308: 209–223, 1991. [DOI] [PubMed] [Google Scholar]
- Ryugo DK, Fekete DM. Morphology of primary axosomatic endings in the anteroventral cochlear nucleus of the cat: a study of the endbulbs of Held. J Comp Neurol 210: 239–257, 1982. [DOI] [PubMed] [Google Scholar]
- Ryugo DK, Sento S. Synaptic connections of the auditory nerve in cats: relationship between endbulbs of held and spherical bushy cells. J Comp Neurol 305: 35–48, 1991. [DOI] [PubMed] [Google Scholar]
- Scheuss V, Schneggenburger R, Neher E. Separation of presynaptic and postsynaptic contributions to depression by covariance analysis of successive EPSCs at the calyx of Held synapse. J Neurosci 22: 728–739, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver RA. Estimation of nonuniform quantal parameters with multiple-probability fluctuation analysis: theory, application and limitations. J Neurosci Methods 130: 127–141, 2003. [DOI] [PubMed] [Google Scholar]
- Taberner AM, Liberman MC. Response properties of single auditory nerve fibers in the mouse. J Neurophysiol 93: 557–569, 2005. [DOI] [PubMed] [Google Scholar]
- Taschenberger H, Leao RM, Rowland KC, Spirou GA, von Gersdorff H. Optimizing synaptic architecture and efficiency for high-frequency transmission. Neuron 36: 1127–1143, 2002. [DOI] [PubMed] [Google Scholar]
- Taschenberger H, Scheuss V, Neher E. Release kinetics, quantal parameters and their modulation during short-term depression at a developing synapse in the rat CNS. J Physiol 568: 513–537, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trussell LO. Synaptic mechanisms for coding timing in auditory neurons. Annu Rev Physiol 61: 477–496, 1999. [DOI] [PubMed] [Google Scholar]
- Trussell LO, Zhang S, Raman IM. Desensitization of AMPA receptors upon multiquantal neurotransmitter release. Neuron 10: 1185–1196, 1993. [DOI] [PubMed] [Google Scholar]
- Wadel K, Neher E, Sakaba T. The coupling between synaptic vesicles and Ca2+ channels determines fast neurotransmitter release. Neuron 53: 563–575, 2007. [DOI] [PubMed] [Google Scholar]
- Wadiche JI, Jahr CE. Multivesicular release at climbing fiber–Purkinje cell synapses. Neuron 32: 301–313, 2001. [DOI] [PubMed] [Google Scholar]
- Wall MJ. Short-term synaptic plasticity during development of rat mossy fibre to granule cell synapses. Eur J Neurosci 21: 2149–2158, 2005. [DOI] [PubMed] [Google Scholar]
- Wang Y, Manis PB. Synaptic transmission at the cochlear nucleus endbulb synapse during age-related hearing loss in mice. J Neurophysiol 94: 1814–1824, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Manis PB. Short-term synaptic depression and recovery at the mature mammalian endbulb of Held synapse in mice. J Neurophysiol 100: 1255–1264, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YX, Wenthold RJ, Ottersen OP, Petralia RS. Endbulb synapses in the anteroventral cochlear nucleus express a specific subset of AMPA-type glutamate receptor subunits. J Neurosci 18: 1148–1160, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster DB, Trune DR. Cochlear nuclear complex of mice. Am J Anat 163: 103–130, 1982. [DOI] [PubMed] [Google Scholar]
- Wong AY, Graham BP, Billups B, Forsythe ID. Distinguishing between presynaptic and postsynaptic mechanisms of short-term depression during action potential trains. J Neurosci 23: 4868–4877, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SH, Oertel D. Intracellular injection with horseradish peroxidase of physiologically characterized stellate and bushy cells in slices of mouse anteroventral cochlear nucleus. J Neurosci 4: 1577–1588, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu-Friedman MA, Regehr WG. Ultrastructural contributions to desensitization at cerebellar mossy fiber to granule cell synapses. J Neurosci 23: 2182–2192, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu-Friedman MA, Regehr WG. Structural contributions to short-term synaptic plasticity. Physiol Rev 84: 69–85, 2004. [DOI] [PubMed] [Google Scholar]
- Xu-Friedman MA, Regehr WG. Dynamic-clamp analysis of the effects of convergence on spike timing. I. Many synaptic inputs. J Neurophysiol 94: 2512–2525, 2005a. [DOI] [PubMed] [Google Scholar]
- Xu-Friedman MA, Regehr WG. Dynamic-clamp analysis of the effects of convergence on spike timing. II. Few synaptic inputs. J Neurophysiol 94: 2526–2534, 2005b. [DOI] [PubMed] [Google Scholar]
- Yamada KA, Tang CM. Benzothiadiazides inhibit rapid glutamate receptor desensitization and enhance glutamatergic synaptic currents. J Neurosci 13: 3904–3915, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Xu-Friedman MA. Relative roles of different mechanisms of depression at the mouse endbulb of Held. J Neurophysiol 99: 2510–2521, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Xu-Friedman MA. Impact of synaptic depression on spike timing at the endbulb of Held. J Neurophysiol 102: 1699–1710, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol 64: 355–405, 2002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.