Abstract
Gene array studies comparing cystic fibrosis (CF) and non-CF genotypes should reveal factors that explain variability in CF lung disease progression, yielding insights that lead to improved CF care. To date, studies have reached conflicting conclusions, perhaps due to experimental differences and divergent statistical approaches. This review aims: 1) to summarize the findings of four recent gene studies comparing CF and non-CF genotypes, and 2) to reanalyze original data using a recently developed statistical approach, with the aim of identifying genes and paths consistently regulated by the CF genotype. We identified four studies evaluating the effect of the ΔF508-CFTR mutation on human airway epithelial cell gene expression, restricting our investigation to human airway epithelial cell studies whose data were accessible in NCBI's Gene Expression Omnibus or the European Bioinformatic Institute's ArrayExpress. Gene expression patterns showed consistent repression of MHC class I antigen presentation genes in CF human airway epithelia, suggesting a novel mechanistic explanation for poor clearance of viral and bacterial infections by CF patients. We also examined proinflammatory and NF-κB genes, whose induction is widely accepted as a hallmark of the CF genotype, but found little evidence of induction, consistent with a recent review (Machen TE, Am J Physiol Cell Physiol 291: C218–C230, 2006.). In conclusion, our analysis suggests that the CF genotype may impair immune function in airway epithelial cells but may not increase inflammation. Additional studies are required to determine whether MHC class I gene repression in CF reduces antigen presentation at the protein level and whether repression impairs immune function.
Keywords: cystic fibrosis transmembrane conductance regulator, MHC class I, microarray, gene arrays, meta-analysis
cystic fibrosis (CF) is an autosomal recessive, inherited disease caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene (4, 16, 34). Although there are more than 1,600 known mutations in CFTR, ∼70% of individuals with CF carry a three base pair deletion resulting in the loss of a phenylalanine residue at position 508 of the protein (ΔF508). The ΔF508 CFTR protein misfolds in the endoplasmic reticulum and is subsequently degraded by the proteasome, preventing it from trafficking to the plasma membrane where it normally functions as a secretory chloride ion channel in a variety of tissues including the lung, pancreas, and intestine (34). Considerable evidence reveals that CFTR also transports bicarbonate and regulates a variety of other ion channels and transporters (16). Individuals with the ΔF508 CFTR mutation exhibit defects in pulmonary host defense mechanisms that lead to chronic bacterial infection and inflammation, resulting in a progressive decline in lung function and death before an average age of 38 (31).
The progression of CF lung disease varies enormously, even among patients sharing identical CF genotypes (7). Some patients who are homozygous for the ΔF508 CFTR mutation develop severe lung disease at an early age, whereas others reach adulthood with relatively normal respiratory function (13, 25, 51). Clearly, the CFTR genotype is not the only determinant of CF lung disease progression. Many factors have been identified that contribute to the variability in lung disease in CF including, but not limited to, infectious exposure to Pseudomonas aeruginosa and Burkolderia cepacia, nutritional and socioeconomic status, gene-environment interactions, and tobacco smoking (7). Recent evidence also suggests that other genes, notably proinflammatory genes, may play a significant role in determining the severity of lung disease in CF (7, 28).
In recent years, several groups have performed gene array studies to identify gene expression differences between CF and non-CF individuals that might account for some of the variability in CF lung disease (44, 45, 48, 50), hoping that an understanding of these differences would have major prognostic and therapeutic implications. Interestingly, with the exception of proinflammatory pathways, which were upregulated in some but not all array studies (44, 45, 48, 50), each study identified different genes or genetic pathways, and none were consistently and differentially expressed in CF (44, 45, 48, 50). The lack of similar findings among these studies may be due to experimental differences, including the type of tissue examined (i.e., nasal brushings, stable clones of airway epithelial cells, airway epithelial cells in primary culture, and stable bronchial epithelial cell lines), the genotype of the cells used (i.e., ΔF508/ΔF508 vs. ΔF508/W1282X), and because no two studies used the same statistical approach to identify differentially expressed genes.
The goal of this review is twofold. First, it reviews published gene array studies designed to identify genes modified by the CF genotype. Second, it reanalyzes four publically available gene array data sets using a recently developed approach (15, 26, 27, 29, 37). We hypothesized that a uniform analysis might identify genes and pathways common to the studies chosen for analysis that might shed light on the CF disease process, and that these observations would have major prognostic and therapeutic implications. To this end, we reviewed the literature and searched publicly available, archived gene array databases and identified four recent studies evaluating the effect of the ΔF508-CFTR mutation on human airway epithelial cell gene expression (44, 45, 48, 50). We restricted our investigation to studies that were conducted on human airway cells and whose data were accessible in the NCBI Gene Expression Omnibus (www.ncbi.nlm.nih.gov/geo) or the European Bioinformatic Institute's ArrayExpress (www.ebi.ac.uk/microarray-as/ae/). Some published gene array studies were not included because the raw data, necessary for our method of analysis, were not available (10, 21).
Our exploratory method of reanalyzing these studies, described below, found that genes involved in MHC class I antigen presentation were consistently downregulated in CF airway epithelial cells. This observation provides a novel mechanistic explanation for the observation that CF patients have an inability to clear viral and bacterial infections (46). Because it is generally accepted that proinflammatory genes and NF-κB genes are upregulated in CF, we also examined genes in these pathways in all four data sets. However, we did not find evidence that proinflammatory genes are upregulated in CF, consistent with the recent review by Machen (28). The review of the literature by Machen and our analysis of published gene array data do not support the dogma that mutations in CFTR per se upregulate proinflammatory genes. Our analysis suggests that the reduced ability of CF patients to clear bacterial and viral infection may result from the downregulation of genes involved in MHC class I antigen presentation. Additional studies are required to follow up on this analysis of gene array data to determine if downregulation of genes involved in MHC class I antigen presentation reduces the ability of CF patients to clear bacterial and viral infections.
OVERVIEW OF PUBLISHED CF GENE ARRAY DATA: THE CELLS
Table 1 presents a brief overview of the experimental approaches used in the four studies chosen for analysis. Virella-Lowell et al. (44) used immortalized bronchial epithelial cells expressing the ΔF508/W1282X mutation (IB3-1 cells isolated from a single individual) and IB3-1 cells rescued with wild-type (wt)-CFTR (S9). Zabner et al. (50) examined primary cultures of tracheal/bronchial epithelial cultures isolated from 10 CF patients (ΔF508/ΔF508) and 10 primary cultures of tracheal/bronchial epithelial cultures isolated from non-CF donor lungs. Wright et al. (47) isolated nasal epithelial cells from 12 non-CF and 11 CF patients (ΔF508/ΔF508). Finally, Verhaeghe et al. (43) compared an immortalized fetal tracheal CF cell line (CFT-2 cells: ΔF508/ΔF508) isolated from a single individual to a fetal tracheal cell line derived from one non-CF fetus (NT-1 cells). In summary, the four studies used CF and wt-CFTR cells that were obtained from very different sources.
Table 1.
Summary of the experimental designs used by the original authors
| CF Genotype | Tissue | N | Affymetrix Platform | Reference |
|---|---|---|---|---|
| ΔF508/W1282X | Isogenic bronchial cells (IB3-1 and S9) | 1/1 | U95Av2 | Virella-Lowell et al. (44) |
| ΔF508/ΔF508 | Tracheal and bronchial cells in primary culture | 10/10 | HGU133A | Zabner et al. (50) |
| ΔF508/ΔF508 | Nasal brushings | 12/11* | HGU-133A,B | Wright et al. (47) |
| ΔF508/ΔF508 | Fetal tracheal cells (CFT-2 and NT-1) | 1/1 | HGU-133Plus2 | Verhaeghe et al. (43) |
Most studies compared nonisogenic samples from cystic fibrosis (CF) and non-CF patients bearing a homozygous ΔF508 mutation. Virella-Lowell et al. (44) compared isogenic IB3-1 to S9 cells. No two studies used tissues of identical origin, and each study used a different Affymetrix array platform. N = the number of wild-type (WT)/CF samples measured.
We used 11 WT and 4 CF mild disease samples in our reanalysis of these data. Not all samples were available for download, and the 5 severe disease samples in these data were not included because they measure the effect of CF disease more than the CF genotype itself.
OVERVIEW OF PUBLISHED CF GENE ARRAY DATA: THE EXPERIMENTAL APPROACH
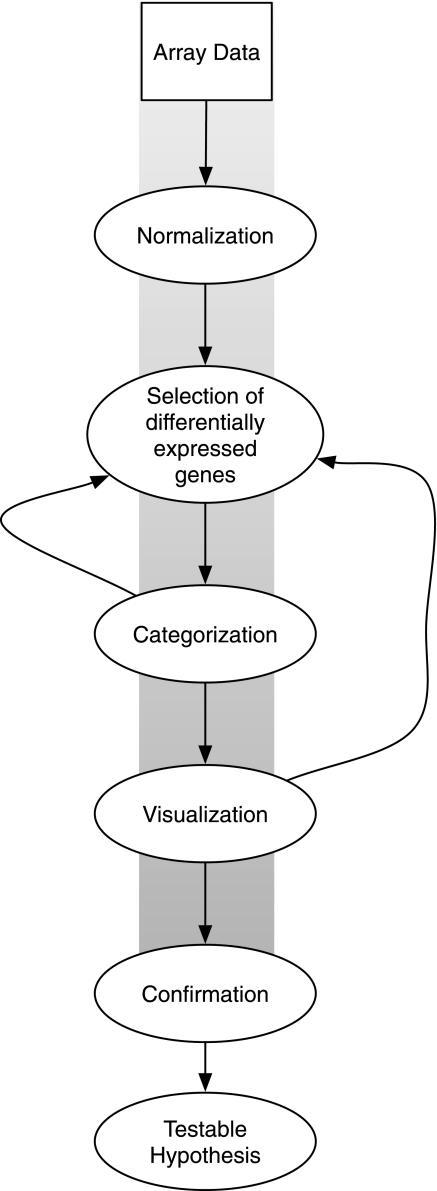
Figure 1 presents an overview of the data analysis approach used by all four CF studies, which includes in sequence: data normalization, selection of differentially expressed genes, categorization, visualization, and confirmation, which leads to a testable hypothesis requiring additional studies.
Fig. 1.
Steps used in gene array analysis: data normalization, selection of differentially expressed genes, categorization, visualization, confirmation, and formation of a testable hypothesis. The exploratory process, discussed in the text, involves iterations, as shown by the lines leading back to previous steps.
All four studies used single-channel microarrays and normalization techniques appropriate to the design. In single-channel experiments, each slide measures one sample. Since hybridization conditions vary slightly from slide to slide, a sample measured on one slide may produce values that are uniformly higher than the same sample measured on another. One can adjust for these differences by normalizing all slides, regardless of treatment conditions, so that each slide has the same median value, an approach used by Wright et al. (47). This practice assumes that gene regulation is essentially a zero sum game (the same number are regulated up as down) and that total gene expression is constant. The Affymetrix normalization used by the other three studies assumes that a predefined set of housekeeping genes is always expressed at the same level and adjusts intensities across all slides accordingly.
Selection of differentially expressed genes, the next step in Fig. 1, may involve simply choosing genes whose average expression differs by more than twofold between experimental conditions. For example, Verhaeghe et al. (43) used this approach to identify 2,424 out of 54,675 transcripts (4.5%) as differentially regulated, which equates to the 1,192 of 19,887 unique genes shown in Table 2. Selecting genes using a static fold change criterion raises two questions. First, can one safely ignore genes regulated less than the cutoff without missing important biological effects? Second, since a single outlier observation, not uncommon in microarray experiments, can substantially drive an average, can one safely ignore variability in the component measurements that make up an average? Virella-Lowell et al. (44) selected genes based on significance, not fold change, to address these issues, as did Wright et al. (47), whereas Zabner et al. (50) used both fold change and significance. Settling on a definition of significance, such as P < 0.05, is not trivial because of the number of genes measured simultaneously. Virella Lowell et al. chose instead to define significance as a false discovery rate (FDR) less than 0.05. While this measure sounds much like the familiar P < 0.05 often encountered in the literature, the P values of the individual genes Virella-Lowell et al. selected at an FDR of 0.05 were actually thousands of times smaller. They chose a more conservative cutoff in deference to the multiple hypothesis testing burden based on the following logic. One can argue statistically that 450 of 9,000 genes could reach a significance of 0.05 by chance alone (5), so on an array of 9,000 genes, reaching an effective P < 0.05 requires a much smaller starting value, such as P < 6 × 10−6. Wright, Zabner, Virella-Lowell, and their coauthors adjusted P values or target levels of significance to varying degrees based on this kind of thinking. For example, Zabner et al. chose to consider only genes with P < 0.00001 as significantly regulated. Since very small P values are rare in microarray studies involving biological replicates, correcting P values for multiple hypothesis testing may severely limit the number of genes available for further statistical and biological analysis, an issue we will address in more detail.
Table 2.
Summary of results reported by published studies including methods used to identify differentially regulated genes and pathways noted by the original authors
| Significant Gene Ratio | Significance Criteria | Regulated Genes or Pathways | Reference |
|---|---|---|---|
| 53/9,047 | SAM FDR; P (adjusted) <0.05 | Upregulation of proteasome subunits and ubiquitin-conjugating enzymes, downregulation of glycosylation enzymes and protease inhibitors. | Virella-Lowell et al. (44) |
| 24/13,077 | t-test, P < 0.00001, Fold change >2 | Upregulation of KCC4, and a novel interleukin receptor. The authors concluded that few genes change due to CF genotype alone. | Zabner et al. (50) |
| 32/18,229 | GeneSpring Error Model, P (adjusted) <0.05 | Upregulation of genes involved in protein ubiquitination*, mitochondrial oxidoreductase activity*, lipid metabolism*. Downregulation of airway defense, antigen presentation, lymphocyte differentiation, protein metabolism. | Wright et al. (47)* |
| 1,192/19,887 | Fold change >2 | Upregulation of proinflammatory genes, fibrosis, and matrix remodeling genes. Upregulation of NF-κB and AP-1 pathways. | Verhaeghe et al. (43) |
No gene was reported significant by more than 1 manuscript. DUOX2, which is involved in H2O2 production in the lung (32) and inflammation (NF-κB), was the only pathway cited as having a previously identified CF association. Significant gene ratio = number of genes selected divided by the number of genes on the array. SAM (43) false discovery rate and GeneSpring Cross Gene Error Model yield P values adjusted for multiple hypothesis testing based on a mechanism similar to Benjamini-Hochberg.
Authors identified differential expression between mild and severe disease, but not CF compared with non-CF in this path.
Categorization assigns genes to groups based on responses to experimental conditions and biological function. The simplest response grouping involves segregating genes that went up from those that went down, but “up” and “down” can be subdivided further into more groups based on strength of regulation, making categorization a tedious task for experiments with multiple conditions or time points. Virella-Lowell et al. (44) performed hierarchical clustering, specifically K means analysis, to organize genes into six essential groups showing broadly similar responses to experimental conditions. Assigning genes to biological functions involves answering the question, what does this gene do? At some level, all genes nominally promote survival, but informative categorizations involve fairly specific functions. For example, Virella-Lowell et al. noted that several of the genes upregulated by the CF genotype were genes involved in protein turnover (ubiquitination). Virella-Lowell et al. devised their own functional categories, whereas Wright et al. (47) used categories defined by the Gene Ontology (GO) (1) Consortium (www.geneontology.org). The GO system begins with very general categories, e.g., “Biological Process” (∼18,000 genes), and breaks them down into very specific ones [e.g., “entrainment of circadian rhythm by photoperiod” (33 genes)]. The process of placing genes into categories can itself result in statistically significant associations as follows. One can ask whether a set of differentially regulated genes has more genes belonging to certain categories than one would expect a random selection of genes to contain. Using this kind of logic, Wright et al. noted that genes associated with airway defense in CF were overrepresented among the 30 genes identified downregulated by the CF genotype in their data set, citing a P value of P < 0.047 for this association. Verhaeghe et al. (43) used DAVID (9), a publicly available system (http://david.abcc.ncifcrf.gov/) to categorize differentially expressed genes and assess the significance of gene set enrichment.
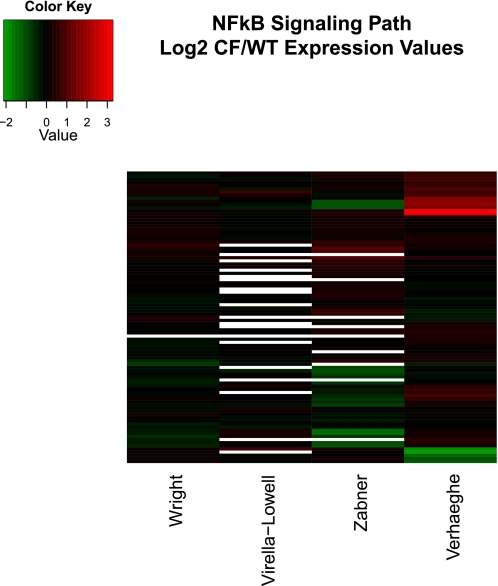
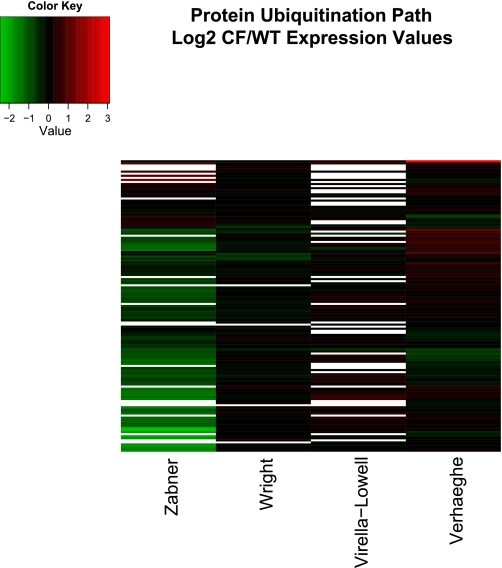
Data visualization of the four studies included familiar elements such as bar charts, scatterplots, and boxplots. Three of the four studies also used heatmaps, a less familiar representational method. Heatmaps (see Figs. 4 and 5) allow the simultaneous representation of many genes under many experimental conditions by using color to associate a numeric quantity such as fold change with each gene. Hierarchical clustering of the genes places genes with similar values (colors) next to each other, making patterns easier to grasp (11).
Fig. 4.
Hierarchically clustered heatmap of genes in IPA NF-κB path in the 4 studies discussed in this review. Fold change presented in log base 2 units (color key shown at top left). Genes (top to bottom): IKKβ, PRKCQ, BCL3, NFKBIE (includes EG:4794), JNK1, RelB, IL18, MAP4K4, IL1A, NFKBIA, IL1R1, A20, IL1B, CAMK4, BR3, IL1R2, TNFα, TLR6, LTA, MAP2K6, BMP2, MAP3K14, NF-κB2 p52, IL1F6, MAP2K7, Zap70, IL1F10, BIMP1, CALML5, CD40L, TRAF2, IRAK4, RANKL, IL1F5, TLR9, IKKβ, TAB1, TLR7, IL1F7, β-TrCP, PRKCB, TRAF6, TLR8, LCK, AKT2, NOS3, IL1F8, PKCζ, IL1F9, TIRAP, CD40, EGF, GH, TLR1, IRAK-M, RIP, NFKBIB, TRD@, MAP3K3, LTBR, TRA@, CALM3, NAK, TGFα, Bcl-10, GSK-3β, UBE2V1, MYD88, REL, TLR2, NAP1, NF-κB1 p50, TRAF3, IRAK1, IL1RN, MALT1, IKKα, p65/RelA, PLCγ2, TLR5, TLR3, AKT1, TTRAP, UBE2N, TAK1, BAFF, PKR, NCK1, BMP4, TLR4, Cot, AKT3, TRAF5.
Fig. 5.
Hierarchically clustered heatmap of genes in IPA protein ubiquitination path in the 4 studies discussed in this review. Fold change presented in log base 2 units. Genes (top to bottom): UCHL1, PSMD4, UBE2F, USP54, USP31, USP19, UBE2E2, UBE2D4, USP38, UBE3A, UBE2J2, THIMET, USP53, NEDD4, USP32, USP36, USP12, USP20, ZBTB12, PAN2 (includes EG:9924), USP29, USP18, UBE2C, BAP1, USP6, CDC34 (includes EG:997), SMURF1, USP2, USP5, USP39, USP27X, IFNG, TAP2, NEDD4L, HLA-C, HLA-A, USP40, SMURF2, PSMD2, USP3, UBE2I, UBE2B, B2M, USP4, UBE3B, PSMB9, PSMB8, TAP1, UBE2L6, UBE2D1, USP15, UBE2G2, USP13 (includes EG:8975), UBE2J1, USP10, BAG1, USP49, USP8, USP25, USP46, USP9Y, PSMD11, PSMD3, PSMC5, PSMD7, PSMB3, USP7, PSMC1, PSMD1, UBE2R2 (includes EG:54926), PSMD12, PSMD5, PSMB10, USO1, UBE2V2, USP14, PSME1, MED20, USP45, PSMC4, USP21 (includes EG:27005), USP24, E3 U box, UCHL5, UBE2H (includes EG:7328), USP34 (includes EG:9736), UBE2M, PSMC3, PSMD9, USP11, PSMD13, HLA-B, USP9X, USP47, PSMB6, UBE2G1, PSMC2, USP1, USP16, USP43, USP48, PSMA7, UBE2Q1, USP22, PSME2, PSMB4, PSMA5, UBE2D2, PSMD6, USP28, UBE2E3, PSMD14, UBE2N, UBE2L3, PSMB7, USP33, USP30, USP42, UBE2V1, UBE2A, PSMD8, E1, PSMC6, USP37 (includes EG:57695), PSMB2, PSMA2, PSMD10, PSMB5, UCHL3, UBE2D3, HSP90AA1, PSMB1, BIRC6, HSPA5, UBE2E1, USP26, UBE2S, PSMA4, HSPA8, PSMA1, PSMA3.
All four groups confirmed a small number of regulated genes by PCR. Verhaeghe et al. (43) additionally used ELISA and EMSA.
Using the experimental approach outlined above, the four studies reached a variety of different conclusions regarding what, if anything, the CF genotype does to gene expression. No gene was reported differentially expressed by more than one study. DUOX2, which is involved in H2O2 production in the lung (12), was the only gene cited as having a previously identified CF association (32), and the NF-κB inflammatory pathway was the only pathway cited as having a previously identified CF association (43). In short, these studies did not provide a unified set of conclusions regarding the genes or pathways up- or downregulated in CF.
A NEW, LESS RESTRICTIVE APPROACH TO ANALYZE GENE ARRAY DATA: EXPLORATORY GENE ARRAY ANALYSIS
Starting with the assumption that the ΔF508 mutation causes consistent gene expression differences unobserved by the four studies, we reanalyzed the data using a methodology recently developed by our group (15, 26, 27, 29). The exploratory gene array approach identifies biological patterns using the process shown in Fig. 1, but in addition utilizes iterations, as shown by the lines leading back to previous steps, as explained later in more detail. We call it an “exploratory” approach because of its roots in exploratory data analysis (42) and to highlight its nondeterministic character. For example, the exploratory approach uses gene regulation to identify paths of interest, but it can also use pathways to identify genes of interest. Exploratory data analysis has been previously described in detail (42) and has a different aim from the statistics of inference (8) common to most laboratory experiments, which are used to test an existing hypothesis. The design of the microarray, which typically measures an entire transcriptome in a single experiment, favors hypothesis generation, whereas less expensive, low-throughput experiments (i.e., PCR) provide more reliable measurements of individual genes, favoring hypothesis testing. For these reasons, we chose the exploratory approach shown in Fig. 1 and the less restrictive gene selection technique graphically shown in Fig. 2, which has been reported to improve agreement between microarray experiments performed in different laboratories (17, 38, 39, 40).
Fig. 2.
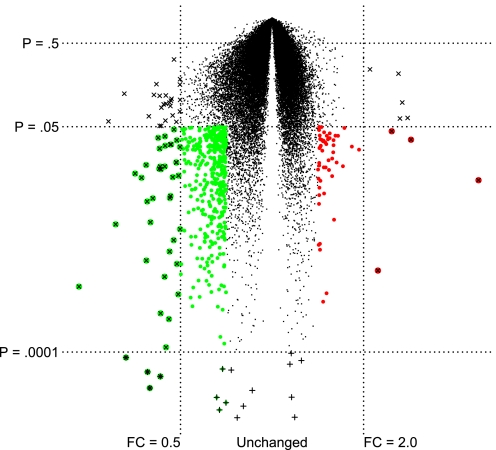
Volcano plot showing the average fold change of individual genes (x-axis) compared with P value in a t-test (y-axis) from cystic fibrosis (CF) compared with non-CF nasal brushings (48). Genes marked with × were strongly regulated, with a fold change (FC) greater than 2.0 or less than 0.5. Genes marked with + were highly significant (P < 0.0001), but the fold change was relatively small. Genes marked in color were significant (P < 0.05) and changed by a factor of at least 1.4 or 0.7. Larger colored circles denote greater regulation upward (red) or downward (green).
Figure 2 is a volcano plot of mild CF compared with healthy controls in the Wright et al. (47) study. It shows the P value and fold change of each gene, illustrating our gene selection method and contrasting it with the methods used by four original authors. The fold change criterion used by Verhaeghe et al. (43) selects all genes that increase by 2-fold or decrease by 0.5-fold, that is, genes marked with “×”. This strategy would include genes with marginal P values. At the other extreme, the high-significance criterion used by Virella-Lowell et al. (44) and Wright et al. selects genes marked with “+” as differentially expressed and sometimes includes genes with very small expression differences (e.g., less than a 2-fold increase and between a 0.5- and 1-fold decrease). Zabner et al. (50) used both a high-significance criterion (P < 0.00001) and a 2-fold cutoff to avoid the problem of selecting genes with small P values that had very small expression differences. This strategy corresponds to the genes marked with both + and × in Fig. 2 and identifies too few genes to analyze from a pathway perspective. By contrast, in our exploratory gene array approach, we adopted the hybrid approach recommended by Shi et al. (38, 39, 40) and Guo et al. (17), which filters out genes with P values less than 0.05, orders the remaining genes based on fold change, and selects genes that meet a target fold change. In Fig. 2, for example, 2,790 (6%) genes reached a significance of 0.05, most of which changed by less than 20% between CF and non-CF samples. Using P < 0.05 and a fold change of at least 1.4 up or 0.7 down, we identified the 473 genes marked in color in Fig. 2 for pathway analysis. In our reanalysis, we tried a variety of fold change cutoffs for each data set and used the cutoff that yielded the most statistically significant pathway results. In practice, the best fold change cutoff was the one that generated a list of ∼300 genes.
ANALYSIS OF PUBLISHED CF GENE ARRAY DATA USING THE EXPLORATORY GENE ARRAY ANALYSIS METHOD
The first step in the reanalysis of the published data considered what the original experiments measured and what they found. We chose the four studies because their data were available in a form that could be reanalyzed and because they were similar enough in the general protocol to enable meaningful comparisons between them. Even so, they differ enough to explain divergent results: immortalized cells are not the same as primary cultures, nasal epithelia are not the same as bronchial epithelia, and studies involving true biological replicates often lead to variable results. Moreover, as noted above, the statistical approach was different in each study.
ORIGINAL CONDITIONS AND RESULTS
Collectively, the published results of the four studies suggest that the CFTR genotype exerts unique effects contingent on experimental conditions, as previously noted. Estimates of the absolute number of genes affected by CF varies over two orders of magnitude, as shown in Table 2, and no common threads emerge in terms of pathways or individual genes.
We were interested in the Virella-Lowell (44) data set because it contains CF (IB3-1 cells) and non-CF IB3-1 cells complemented with wt-CFTR (S9 cells), an isogenic comparison differing only in the presence of a functional CFTR gene. Their design also involved a third genotype (CF cells overexpressing IL-10) and studies examining the effects of Pseudomonas on each genotype. Rather than using t-tests to compare each of their 66 possible contrasts individually, they began with Tusher's Significance of Microarray (SAM) (43) analysis, which offers a multiclass option similar to ANOVA. Using this multiclass option, they identified genes that were significantly different between any two conditions. They then used K means clustering to identify six clusters of genes that showed similar response patterns across various treatments. Visually inspecting these groups, they found that the CF genotype regulated 53 genes. The CF regulation pattern included upregulation of proteasome subunits and ubiquitin-conjugating enzymes and downregulation of protease inhibitors and protein glycosylation enzymes, as shown in Table 2. They speculated that upregulation of proteasomal subunits might relate to ΔF508 CFTR protein misfolding and the induction of an endoplasmic reticulum stress response. They also speculated that downregulation of glycosylation enzymes might reduce binding of Pseudomonas to the epithelial cell surface. Although PCR verification of some genes was not successful (i.e., phosphomannose isomerase, a glycosylating enzyme), PCR did confirm a decrease in the mRNA levels of SERPINB3, an antiprotease, which they thought might predispose the CF lung to enhanced neutrophil damage. PCR also confirmed an increase in IL-6, UBE21 (a ubiquitin-conjugating enzyme), and PSMB7, a proteasomal subunit.
The Zabner et al. (50) study compared gene expression profiles from 10 CF patients homozygous for the ΔF508 mutation to 10 non-CF patients by creating primary lung epithelial cell cultures from samples of tracheal or bronchial origin. T-tests identified 24 of 13,077 genes significant at P < 0.00001 with a fold change greater than 2. They performed PCR on 21 genes with suitable annotations, of which 12 confirmed. Among these genes, they noted that SLC12A7 (KCC4), a potassium/chloride cotransporter, showed increased expression in CF, and suggested that additional studies on SLC12A7 were warranted. They generally concluded that the ΔF508 mutation had a minimal effect on the gene expression of human airway epithelia.
Wright et al. (47) used nasal brushings from 12 healthy volunteers and 11 CF patients homozygous for the ΔF508 mutation, chosen by virtue of very mild or very severe lung disease as measured by forced expiratory volume (FEV1). They limited qualifying CF patients to those in either the top or bottom 20th percentiles, facilitating gene expression comparisons between mild and severe CF disease. They noted that 9 out of 11 CF patients were infected with Pseudomonas. A cytopathological analysis of the nasal brushings showed them to consist primarily of airway epithelial cells, although squamous cells and inflammatory cells contributed ∼5% and 8%, respectively. They saw no significant difference between non-CF, mild CF, and severe CF in terms of cell composition, but the severe group showed a marginally higher percentage of inflammatory cells. The authors normalized their data and eliminated ∼25% of genes by virtue of low overall expression. Based on GeneSpring's modified t-test, they concluded that severe disease (low FEV1) regulates 569 genes at a corrected significance of P < 0.05. Only 32 genes met a significance threshold of P < 0.05 when comparing CF to non-CF. While one could use this as a validation of Zabner's conclusion that the CF genotype by itself regulates few genes, the small number of genes identified derives in part from the rather conservative correction applied to P values by GeneSpring. The least conservative correction strategy offered by GeneSpring would in practice require a starting P < 0.00003 to achieve a corrected value of P < 0.05 on an array with 18,000 spots. In any case, clustering revealed that 32 of the significant genes were associated with the CF genotype alone, of which 30 were downregulated by CF. They categorized these 30 downregulated genes by GO (1) and found that there were more genes associated with such categories as airway defense, antigen presentation, lymphocyte differentiation, and protein metabolism than one would expect a random selection of genes to contain. They used PCR to confirm reduced expression of DUOX2, an airway defense gene involved in pulmonary H2O2 production (12). In their discussion, they mention two specific antigen presentation genes, HLA-F and HLA-G, which were downregulated and play a role in nonclassical major histocompatibility complex Ib antigen presentation. These proteins bind to receptors on natural killer cells and have been implicated in immunoregulation (35). The authors noted decreased expression of CD2 and CD74, which are cell surface adhesion molecules expressed on mucosal T cells, suggesting that some of the gene regulation observed in this experiment derives from contributions made by cells of immune origin. CD2 mediates STAT involvement in the regulation of IFNγ, proposed to play a role in CF pathophysiology (30). Finally, the authors highlighted six genes downregulated by CF that are involved in lipid metabolism, a topic that has been reviewed recently (47). As noted in Table 2, differences in lipid metabolism and certain other paths were limited to comparisons between mild and moderate disease and were not strongly associated with the CF genotype in general.
Verhaeghe et al. (43) compared gene expression between two human immortalized fetal tracheal cell lines, one from a fetus bearing a homozygous ΔF508 CFTR mutation (CFT-2) and a control line (NT-1), but this was a small part of their analysis. They first demonstrated increased DNA binding of NF-κB in CFT-2 compared with NT-1 cells by EMSA, found increased transcriptional activity using a reporter gene, decreased half-life of IκB, and increased IκBα phosphorylation, all consistent with their starting hypothesis that the ΔF508 CFTR genotype leads to increased inflammatory gene expression through a classic NF-κB signaling cascade. In a similar set of experiments, they demonstrated increased AP-1 activation, which also regulates inflammatory gene expression. They found evidence of increased NF-κB and AP-1 activation by a reporter gene assay in HeLa cells transfected with ΔF508 CFTR compared with wild type. They also found evidence of inflammatory gene regulation in the CFT-2 cell lines as measured by gene arrays. They normalized their gene array data and selected 1,192 unique genes with a fold change greater than 2. They categorized these genes using DAVID (9) and identified “inflammation, fibrosis and matrix remodeling” and “cytokine-cytokine receptor interaction” paths as upregulated in CF. Based on these paths, they selected 11 genes including chemokines, cytokines, the basic fibroblast growth factor (bFGF), and the matrix metalloproteinase MMP-1 for further analysis. They confirmed most of these genes in a variety of ways, including independent gene expression measurements in ΔF508 HeLa and 16HBE14o- CF cell lines. Finally, they demonstrated that upstream regulators of NF-κB and AP-1, including ERK, IL-1β, and bFGF, were differentially expressed in ΔF508 cells compared with control, concluding that the absence of a functional CFTR at the plasma membrane leads to intrinsic AP-1 and NF-κB activity and a proinflammatory state mediated by IL-1β, bFGF, IKK, and ERK.
In summary, the four groups of authors reported quite different results in terms of what paths and genes are regulated by the CF genotype and how these changes influence CF lung disease. We wondered whether a reanalysis of the original data, using a single statistical approach, might identify genes or paths similarly regulated in multiple experiments and thereby provide a novel and consistent insight into CF.
REANALYSIS OF GENE ARRAY DATA
We acquired gene array data from the GEO database, a publicly available archive of 300,000 individual microarrays with 16 billion expression measurements (2) or from the original authors, as summarized in Table 1. We used robust multi-array average RMA (22) to normalize measurements and selected genes using the dual criterion method of Guo et al. (17) as described above and shown in Fig. 2. We adjusted the fold change parameter as needed, consistent with our exploratory methodology (15) to optimize pathway analysis results. We used Ingenuity (www.ingenuity.com) to identify pathway enrichment and visualized genes associated with enriched or suspected paths.
We began by looking for highly regulated genes in common between the four studies. We defined genes as “in common” when they were examined in all four studies and regulated in the same direction by two or more studies. In practice, genes present on the earliest generation arrays were also present on the later generation arrays, but the earlier arrays were substantially smaller, limiting this aspect of our analysis to 8,858 unique genes.
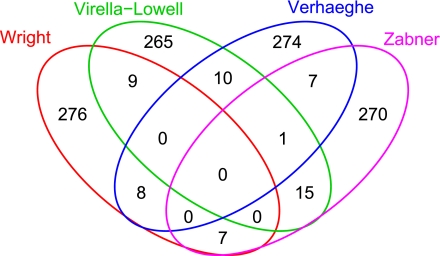
Figure 3 is a Venn diagram showing genes in common among the 300 most induced (upregulated) genes selected from each of the four studies. Each experiment identified different sets of genes as the most highly induced, with an average of 252 genes (83%) unique to a single study. No genes were among the 300 most highly induced in all four experiments. A similar survey of the 300 most repressed (downregulated) genes yielded similar results (93% unique), as did the 300 genes from each study with the smallest P values (89% unique) and a random selection of 300 genes (90% unique). These comparisons indicate that the genes that are reported to change in CF are unique to that study. If there are common gene patterns in the four studies, they do not appear among the most highly regulated or highly significant genes in each study. Thus, we decided to explore whether the studies showed similarity at the pathway level.
Fig. 3.
Four-way Venn diagram showing genes in common among the 300 most induced (upregulated) genes selected from each of the 4 studies. Note that no genes were strongly induced in all 4 studies. For example, in the red circle [Virella-Lowell et al. (44) study], 265 refers to the number of genes upregulated in the Virella-Lowell data only, 10 refers to the number of genes that are upregulated in both the Virella-Lowell and Verhaeghe et al. (43) study (green circle), and 1 refers to the number of genes that are upregulated in both the Virella-Lowell and Wright et al. (47) study (blue circle). The number 0 in the center that is encircled by all colored circles indicates the number of genes that are upregulated in all 4 studies.
PATHWAY REANALYSIS
As noted above, we use Ingenuity IPA to categorize gene array data. The IPA system performs best with lists of roughly 300 “network eligible genes”, that is, genes about which IPA has enough information to relate them to other genes or biological functions. To optimize IPA performance and make various experiments comparable in terms of list length meant adjusting the fold change criterion as follows. We used a fold change of 1.4 (up or down) in the case of Virella-Lowell et al. (44) to yield 263 network eligible genes, a fold change of 2.8 (up or down) to select 366 network eligible genes from the Zabner et al. (50) data, a fold change of 1.4 (up or down) to select 331 network eligible genes from the Wright et al. (47) data, and a fold change of 4 (up or down) to select 309 network eligible genes from the Verhaeghe et al. (43) data. Using the gene lists described above as input, IPA identified the antigen presentation path in both the Wright et al. data (P < 10−15) and Virella-Lowell et al. data (P < 10−4), providing our first evidence of pathway concordance. Ingenuity identified “Hepatic fibrosis/hepatic stellate cell activation” (P < 10−10) and “Airway pathology in chronic pulmonary disease” (P < 10−5) in the Verhaeghe et al. data, paths that include several of the genes Verhaeghe et al. chose for intensive study.
Observing that antigen presentation is significantly regulated in two studies raises an interesting question: how did genes from this pathway, regardless of fold change or significance, behave in all four studies? More generally, if a pathway was regulated in any single study, how did it behave in the others? To explore this question, we matched IPA pathway genes to average expression ratios and created hierarchically clustered heatmaps using the heatmap.2 function available from the R Foundation for Statistical Computing (http://www.R-project.org), Vienna, Austria. We clustered in two dimensions by Euclidian distance, placing similar genes and similar experiments next to each other on the heatmaps. Upregulated genes appear in shades of red, whereas downregulated genes appear in shades of green. Genes for which no data are available (because the array platform did not include a probe matching the gene) appear white. The color key in the legends of Figs. 45–6 specifies the amount of regulation in log base 2 units, so −1 corresponds to a fold change of 0.5 and +1 corresponds to a fold change of 2. Genes available in fewer than three experiments were excluded. The results are described below and presented in heatmaps in Figs. 4, 5, and 6. Our heatmaps present fold changes measured in different experiments side by side, preserving the scale of each independently controlled experiment. We chose this approach over normalization options such as Z scores because the ratios shared similar distributions and because we believe that a fold change of 2 is more biologically meaningful than the same quantity expressed in units based on standard deviation.
Fig. 6.
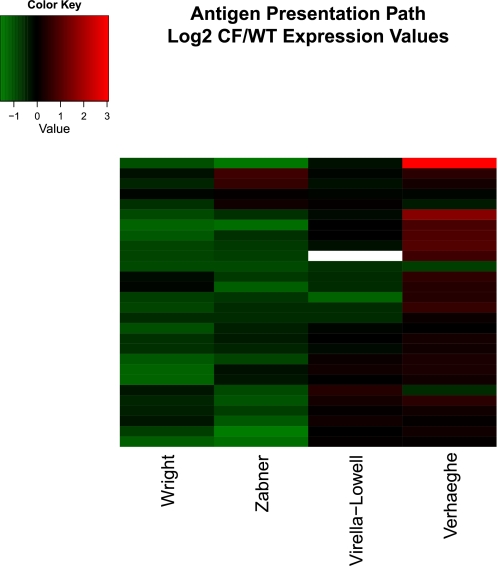
Hierarchically clustered heatmap of genes in IPA antigen presentation path in the 4 studies discussed in this review. Fold change presented in log base 2 units. Genes (top to bottom): HLA-DPA1, MR1, HLA-DOA, HLA-DOB, TAP2, HLA-DPB1, HLA-DMA, HLA-DRB1, HLA-E, HLA-C, HLA-B, HLA-A, MHC I-β. HLA-F, HLA-G, CALR, TAP1, HLA-DMB, CLIP, LMP7, LMP2, HLA-DQA1, LMPY, TPN, HLA-DRB4, LMPX, CNX, HLA-DRA.
NF-κB PATH
As mentioned earlier, many reports indicate that lack of functional CFTR enhances inflammation through a classic NF-κB signaling cascade. Figure 4 graphically represents the CF to non-CF expression ratios for this path. The figure shows strong regulation of many NF-κB genes in the Verhaeghe et al. (43) study, consistent with their results. The other studies reveal little NF-κB activation. This result is consistent with Machen's (28) conclusion that the apparent exaggerated innate immune response of CF airway epithelial cells may result from infectious processes that were present, but not measurable, or from downstream effects of anion channel loss, such as airway surface liquid acidification, oxidation, and increased Ca2+ signaling, rather than changes in gene expression, since cell culture models might not show these downstream effects.
PROTEIN UBIQUITINATION PATH
Both Virella-Lowell et al. (44) and Wright et al. (47) specifically noted that protein ubiquitination was regulated in CF. Figure 5 graphically represents the CF to non-CF expression ratios for this path. Virella-Lowell et al. noted upregulation of some genes in this pathway (red), which was confirmed here. Although the downregulation noted by Wright et al. is difficult to see in this representation, some downregulated genes are evident (green). The strongest evidence for upregulation of this path (red) is observed in the Verhaeghe et al. study, an effect not mentioned by the original authors. In summary, Fig. 5 does not show consistent regulation of the protein ubiquitination path in the four experiments.
ANTIGEN PRESENTATION PATH
While none of the original authors mentioned downregulation of the antigen presentation path directly, Wright et al. (47) noted downregulation of HLA-F and HLA-G. Using IPA and our exploratory gene analysis, we found this path significantly downregulated in three of the four gene array data sets, most notably in the studies by Zabner et al. (50), Wright et al., and Virella-Lowell et al (44). A visual inspection of Fig. 6 suggests substantially similar repression of antigen presentation genes in three of the four studies and substantial antigen presentation gene induction in the Verhaeghe et al. data, consistent with the proinflammatory response seen in that study.
DISCUSSION
Three key results from our analysis lay the foundation for further discussion and additional studies. First, we saw that if there are similarities in gene expression shared among the four studies, they do not manifest themselves in the most highly regulated or most significant genes. For example, only 10% of the most highly regulated genes in any study were strongly regulated in the same direction in another experiment (about what one would expect by chance), and no gene was strongly regulated in the same direction in any of the four experiments (Fig. 3). This explains why the authors, who understandably based their conclusions on highly regulated or highly significant genes, reached unique conclusions. Second, we observed NF-κB signaling changes in one study, but not the other three, suggesting that the CF genotype does not always regulate this path, consistent with a review by Machen (28). Third, we did detect evidence of repression of the antigen presentation path in three out of four studies (Fig. 6). Interestingly, we observed this effect, not by looking at highly significant genes, but by looking at all genes in a particular path. The core idea that gene expression analysis should focus less on the behavior of a few highly regulated genes and more on the behavior of biologically coordinated gene sets is well established (41, 49). We recommend that any meta-analysis of gene array data consider not just individual genes but also pathways, as described in our exploratory gene array method (Fig. 1).
To the extent that we can explain the progression of CF lung disease on the basis of consistent changes in gene expression, the evidence points more in the direction of impaired function of the immune system than toward intrinsic (i.e., not induced by pathogens) NF-κB-mediated inflammation. Additional studies are required to follow up on this analysis of gene array data to determine if downregulation of genes involved in MHC class I antigen presentation is causally linked to a reduced ability of CF patients to clear bacterial and viral infections. If gene expression changes in antigen presentation reflect biologically relevant changes in protein levels, reduced expression of antigen presentation genes might have interesting effects.
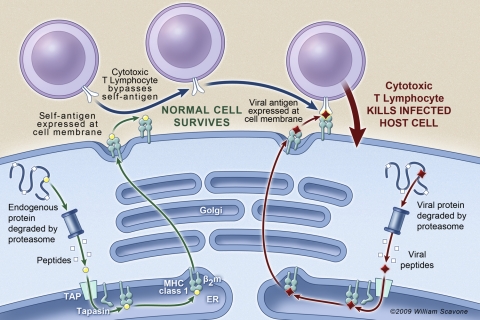
MHC class I antigen presentation alerts the immune system to viral infection, the presence of bacteria, and cellular transformation (3). For example, when an infected epithelial cell presents viral antigen to an appropriate cytotoxic T lymphocyte, the T cell triggers the death of the host cell, as shown in Fig. 7. Virally infected epithelial cells with reduced antigen presentation are more likely to escape immune surveillance and thereby increase the viral load and duration of infection. It is interesting to note that many viruses downregulate MHC class I expression in host cells as a mechanism to evade the immune response (18). Decreased viral antigen presentation may therefore make the CF lung more prone to viral infection.
Fig. 7.
Role of antigen presentation in the destruction of virally infected cells by cytotoxic T lymphocytes. Cells that fail to present viral antigen in sufficient quantity may escape immune surveillance. Reproduced with artist's permission: William Scavone.
Clinical and biological evidence suggest that decreased antigen expression might be relevant to CF lung disease. A rare genetic disorder, TAP (transporter associated with antigen presentation) deficiency, abrogates MHC class I antigen presentation and causes rhinosinusitis, chronic lung infection, and bronchiectasis (6), reminiscent of CF. TAP-deficient patients also experience opportunistic infection by Pseudomonas (52). Macrophages, present in the lungs and isolated by lavage, but not monocytes circulating in the bloodstream, also have defective antigen presentation in late-stage CF patients (24), perhaps accounting for the observation that CF patients generally mount a normal immune response except in the lungs. Finally, although CF patients do not have a higher incidence of viral infections of the lung compared with non-CF individuals (19, 33, 36), viral infections in CF are more severe and prolonged and are associated with pulmonary exacerbations and the progression of CF lung disease (46). Both observations are consistent with our gene analysis and highlight the role that the MHC class I antigen presentation system plays in the immune surveillance of viral infection.
Reduced antigen presentation by epithelial or immune cells may also disrupt other important aspects of immune regulation, leading to adverse effects in the absence of infection. Antigen presentation plays an important role in regulating lung inflammation (20) and in immune modulation in general (35). Natural killer cells attack cells that fail to present adequate levels of MHC class I proteins at their cell surface (23), and alveolar epithelial cells modulate immune activity through antigen presentation (14). Antigen presentation dysfunction may therefore mediate some of the lung tissue destruction seen in CF lung disease.
In conclusion, our exploratory method to analyze gene arrays made the new observation that genes involved in MHC class I antigen presentation were consistently downregulated in CF human airway epithelia. This finding is consistent with the clinical observation that CF patients have a reduced ability to clear bacterial and viral infections. Additional studies are required to follow up on this analysis of gene array data to determine if downregulation of genes and protein abundance in MHC I antigen presentation genes reduces the ability of CF patients to clear bacterial and viral infections.
GRANTS
This work was supported by the Cystic Fibrosis Research Development Program (STANTO7R0), a COBRE grant from the NCRR (P20 RR-018787), the Superfund Research Program (P42-ES-007373), and National Institutes of Health Grants RO1-DK/HL-45881 and RO1-HL-074175.
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
We are grateful to Terry Flotte and Chris Mueller for providing gene expression data, Jerry Wright for comments about his data set, and Thomas Girke for a four-way Venn diagram routine in R. Special thanks to Drs. Brent Berwin, Jen Bomberger, Sophie Moreau-Marquis, and Courtney Kozul for advice.
REFERENCES
- 1.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet 25: 25–29, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrett T, Troup DB, Wilhite SE, Ledoux P, Rudnev D, Evangelista C, Kim IF, Soboleva A, Tomashevsky M, Marshall KA, Phillippy KH, Sherman PM, Muertter RN, Edgar R. NCBI GEO: archive for high-throughput functional genomic data. Nucleic Acids Res 37: D885–D890, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartoszewski R, Rab A, Jurkuvenaite A, Mazur M, Wakefield J, Collawn JF, Bebok Z. Activation of the unfolded protein response by DeltaF508 CFTR. Am J Respir Cell Mol Biol 39: 448–457, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boucher RC. Evidence for airway surface dehydration as the initiating event in CF airway disease. J Intern Med 261: 5–16, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Breitling R. Biological microarray interpretation: the rules of engagement. Biochim Biophys Acta 1759: 319–327, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Caversaccio M, Bonél HM, Carter R, Williams AP, Gadola SD. TAP deficiency syndrome: chronic rhinosinusitis and conductive hearing loss. Eur Arch Otorhinolaryngol 265: 1289–1292, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Collaco JM, Cutting GR. Update on gene modifiers in cystic fibrosis. Curr Opin Pulm Med 14: 559–566, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis RH. Strong inference: rationale or inspiration? Perspect Biol Med 49: 238–250, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Dennis G, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, Lempicki RA. DAVID: Database for annotation, visualization, and integrated discovery. Genome Biol 4: P3, 2003 [PubMed] [Google Scholar]
- 10.Eidelman O, Srivastava M, Zhang J, Leighton X, Murtie J, Jozwik C, Jacobson K, Weinstein DL, Metcalf EL, Pollard HB. Control of the proinflammatory state in cystic fibrosis lung epithelial cells by genes from the TNF-alphaR/NFkappaB pathway. Mol Med 7: 523–534, 2001 [PMC free article] [PubMed] [Google Scholar]
- 11.Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA 95: 14863–14868, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fischer H. Mechanism and function of DUOX in epithelia of the lung. Antioxid Redox Signal 11: 2453–2465, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gallati S. Genetics of cystic fibrosis. Semin Respir Crit Care Med 24: 629–638, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Gereke M, Jung S, Buer J, Bruder D. Alveolar type II epithelial cells present antigen to CD4(+) T cells and induce Foxp3(+) regulatory T cells. Am J Respir Crit Care Med 179: 344–355, 2009. [DOI] [PubMed] [Google Scholar]
- 15.Gosse JA, Hampton TH, Davey JC, Hamilton JW. A new approach to analysis and interpretation of toxicogenomic gene expression data and its importance in examining biological response to low, environmentally relevant doses of toxicants. In: Toxicogenomics: A Powerful Tool For Toxicity Assessment, edited by Sahu SC. Wiley & Sons, 2008, p. 27–57 [Google Scholar]
- 16.Guggino WB, Stanton BA. New insights into cystic fibrosis: molecular switches that regulate CFTR. Nat Rev Mol Cell Biol 7: 426–436, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Guo L, Lobenhofer EK, Wang C, Shippy R, Harris SC, Zhang L, Mei N, Chen T, Herman D, Goodsaid FM, Hurban P, Phillips KL, Xu J, Deng X, Sun YA, Tong W, Dragan YP, Shi L. Rat toxicogenomic study reveals analytical consistency across microarray platforms. Nat Biotechnol 24: 1162–1169, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Hansen TH, Bouvier M. MHC class I antigen presentation: learning from viral evasion strategies. Nat Rev Immunol 9: 503–513, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Hiatt PW, Grace SC, Kozinetz CA, Raboudi SH, Treece DG, Taber LH, Piedra PA. Effects of viral lower respiratory tract infection on lung function in infants with cystic fibrosis. Pediatrics 103: 619–626, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Holt PG. Antigen presentation in the lung. Am J Respir Crit Care Med 162: S151–S156, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Ichikawa JK, Norris A, Bangera MG, Geiss GK, van't Wout AB, Bumgarner RE, Lory S. Interaction of Pseudomonas aeruginosa with epithelial cells: identification of differentially regulated genes by expression microarray analysis of human cDNAs. Proc Natl Acad Sci USA 97: 9659–9664, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4: 249–264, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Jonsson AH, Yokoyama WM. Natural killer cell tolerance licensing and other mechanisms. Adv Immunol 101: 27–79, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Knight RA, Kollnberger S, Madden B, Yacoub M, Hodson ME. Defective antigen presentation by lavage cells from terminal patients with cystic fibrosis. Clin Exp Immunol 107: 542–547, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Konstan MW, Morgan WJ, Butler SM, Pasta DJ, Craib ML, Silva SJ, Stokes DC, Wohl MEB, Wagener JS, Regelmann WE, Johnson CA, Scientific Advisory Group and the Investigators and Coordinators of the Epidemiologic Study of Cystic Fibrosis Risk factors for rate of decline in forced expiratory volume in one second in children and adolescents with cystic fibrosis. J Pediatr 151: 134–139, e131, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Kozul CD, Hampton TH, Davey JC, Gosse JA, Nomikos AP, Eisenhauer PL, Weiss DJ, Thorpe JE, Ihnat MA, Hamilton JW. Chronic exposure to arsenic in the drinking water alters the expression of immune response genes in mouse lung. Environ Health Perspect 117: 1108–1115, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kozul CD, Nomikos AP, Hampton TH, Warnke LA, Gosse JA, Davey JC, Thorpe JE, Jackson BP, Ihnat MA, Hamilton JW. Laboratory diet profoundly alters gene expression and confounds genomic analysis in mouse liver and lung. Chem Biol Interact 173: 129–140, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Machen TE. Innate immune response in CF airway epithelia: hyperinflammatory? Am J Physiol Cell Physiol 291: C218–C230, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Mattingly CJ, Hampton TH, Brothers KM, Griffin NE, Planchart A. Perturbation of defense pathways by low-dose arsenic exposure in zebrafish embryos. Environ Health Perspect 117: 981–987, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nichols D, Chmiel J, Berger M. Chronic inflammation in the cystic fibrosis lung: alterations in inter- and intracellular signaling. Clin Rev Allergy Immunol 34: 146–162, 2008 [DOI] [PubMed] [Google Scholar]
- 31.O'Sullivan BP, Freedman SD. Cystic fibrosis. Lancet 373: 1891–1904, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Pongnimitprasert N, El-Benna J, Foglietti MJ, Gougerot-Pocidalo MA, Bernard M, Braut-Boucher F. Potential role of the “NADPH oxidases” (NOX/DUOX) family in cystic fibrosis. Ann Biol Clin 66: 621–629, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Ramsey BW, Gore EJ, Smith AL, Cooney MK, Redding GJ, Foy H. The effect of respiratory viral infections on patients with cystic fibrosis. Am J Dis Child 143: 662–668, 1989 [DOI] [PubMed] [Google Scholar]
- 34.Riordan JR. CFTR function and prospects for therapy. Annu Rev Biochem 77: 701–726, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Rodgers JR, Cook RG. MHC class Ib molecules bridge innate and acquired immunity. Nat Rev Immunol 5: 459–471, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Saiman L, Siegel J; Cystic Fibrosis Foundation Consensus Conference on Infection Control Participants Infection control recommendations for patients with cystic fibrosis: microbiology, important pathogens, and infection control practices to prevent patient-to-patient transmission. Am J Infect Control 31: S1–S62, 2003 [PubMed] [Google Scholar]
- 37.Shaw JR, Colbourne JK, Davey JC, Glaholt SP, Hampton TH, Chen CY, Folt CL, Hamilton JW. Gene response profiles for Daphnia pulex exposed to the environmental stressor cadmium reveals novel crustacean metallothioneins. BMC Genomics 8: 477, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi L, Jones WD, Jensen RV, Harris SC, Perkins RG, Goodsaid FM, Guo L, Croner LJ, Boysen C, Fang H, Qian F, Amur S, Bao W, Barbacioru CC, Bertholet V, Cao XM, Chu TM, Collins PJ, Fan XH, Frueh FW, Fuscoe JC, Guo X, Han J, Herman D, Hong H, Kawasaki ES, Li QZ, Luo Y, Ma Y, Mei N, Peterson RL, Puri RK, Shippy R, Su Z, Sun YA, Sun H, Thorn B, Turpaz Y, Wang C, Wang SJ, Warrington JA, Willey JC, Wu J, Xie Q, Zhang L, Zhang L, Zhong S, Wolfinger RD, Tong W. The balance of reproducibility, sensitivity, and specificity of lists of differentially expressed genes in microarray studies. BMC Bioinformatics 9, Suppl 9: S10, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi L, Perkins RG, Fang H, Tong W. Reproducible and reliable microarray results through quality control: good laboratory proficiency and appropriate data analysis practices are essential. Curr Opin Biotechnol 19: 10–18, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Shi L, Tong W, Fang H, Scherf U, Han J, Puri RK, Frueh FW, Goodsaid FM, Guo L, Su Z, Han T, Fuscoe JC, Xu ZA, Patterson TA, Hong H, Xie Q, Perkins RG, Chen JJ, Casciano DA. Cross-platform comparability of microarray technology: intra-platform consistency and appropriate data analysis procedures are essential. BMC Bioinformatics 6, Suppl 2: S12, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 102: 15545–15550, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA 98: 5116–5121, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verhaeghe C, Remouchamps C, Hennuy B, Vanderplasschen A, Chariot A, Tabruyn SP, Oury C, Bours V. Role of IKK and ERK pathways in intrinsic inflammation of cystic fibrosis airways. Biochem Pharmacol 73: 1982–1994, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Virella-Lowell I, Herlihy JD, Liu B, Lopez C, Cruz P, Muller C, Baker HV, Flotte TR. Effects of CFTR, interleukin-10, and Pseudomonas aeruginosa on gene expression profiles in a CF bronchial epithelial cell Line. Mol Ther 10: 562–573, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Wat D, Gelder C, Hibbitts S, Cafferty F, Bowler I, Pierrepoint M, Evans R, Doull I. The role of respiratory viruses in cystic fibrosis. J Cyst Fibros 7: 320–328, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Worgall TS. Lipid metabolism in cystic fibrosis. Curr Opin Clin Nutr Metab Care 12: 105–109, 2009 [DOI] [PubMed] [Google Scholar]
- 47.Wright JM, Merlo CA, Reynolds JB, Zeitlin PL, Garcia JGN, Guggino WB, Boyle MP. Respiratory epithelial gene expression in patients with mild and severe cystic fibrosis lung disease. Am J Respir Cell Mol Biol 35: 327–336, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu T, Gu J, Zhou Y, Du L. Improving detection of differentially expressed gene sets by applying cluster enrichment analysis to Gene Ontology. BMC Bioinformatics 10: 240, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu Y, Tertilt C, Krause A, Quadri LE, Crystal RG, Worgall S. Influence of the cystic fibrosis transmembrane conductance regulator on expression of lipid metabolism-related genes in dendritic cells. Respir Res 10: 26, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zabner J, Scheetz TE, Almabrazi HG, Casavant TL, Huang J, Keshavjee S, McCray PB. CFTR DeltaF508 mutation has minimal effect on the gene expression profile of differentiated human airway epithelia. Am J Physiol Lung Cell Mol Physiol 289: L545–L553, 2005 [DOI] [PubMed] [Google Scholar]
- 51.Zielenski J. Genotype and phenotype in cystic fibrosis. Respiration; Internatl Rev Thoracic Dis 67: 117–133, 2000 [DOI] [PubMed] [Google Scholar]
- 52.Zimmer J, Andrès E, Donato L, Hanau D, Hentges F, de la Salle H. Clinical and immunological aspects of HLA class I deficiency. QJM 98: 719–727, 2005. [DOI] [PubMed] [Google Scholar]