Abstract
Idiopathic pulmonary arterial hypertension (PAH) is a life-threatening condition characterized by pulmonary arteriolar remodeling. This investigation aimed to identify genes involved specifically in the pathogenesis of PAH and not other forms of pulmonary hypertension (PH). Using genomewide microarray analysis, we generated the largest data set to date of RNA expression profiles from lung tissue specimens from 1) 18 PAH subjects and 2) 8 subjects with PH secondary to idiopathic pulmonary fibrosis (IPF) and 3) 13 normal subjects. A molecular signature of 4,734 genes discriminated among these three cohorts. We identified significant novel biological changes that were likely to contribute to the pathogenesis of PAH, including regulation of actin-based motility, protein ubiquitination, and cAMP, transforming growth factor-β, MAPK, estrogen receptor, nitric oxide, and PDGF signaling. Bone morphogenic protein receptor type II expression was downregulated, even in subjects without a mutation in this gene. Women with PAH had higher expression levels of estrogen receptor 1 than normal women. Real-time quantitative PCR confirmed differential expression of the following genes in PAH relative to both normal controls and PH secondary to IPF: a disintegrin-like and metalloprotease with thrombospondin type 1 motif 9, cell adhesion molecule with homology to L1CAM, cytochrome b558 and β-polypeptide, coagulation factor II receptor-like 3, A-myb myeloblastosis viral oncogene homolog 1, nuclear receptor coactivator 2, purinergic receptor P2Y, platelet factor 4, phospholamban, and tropomodulin 3. This study shows that PAH and PH secondary to IPF are characterized by distinct gene expression signatures, implying distinct pathophysiological mechanisms.
Keywords: bone morphogenic protein receptor type II, estrogen, idiopathic pulmonary fibrosis, microarrays, mitogen-activated protein kinase, nitric oxide, platelet-derived growth factor
pulmonary arterial hypertension (PAH) is a disease characterized by elevated mean pulmonary arterial pressures (≥25 mmHg at rest or ≥30 mmHg during exercise) (56) and subsequent right ventricular hypertrophy and failure. Vascular remodeling, manifested by excessive proliferation of vascular endothelium, smooth muscle cells, and fibroblasts, resulting in thickening of the walls of the pulmonary arterioles and formation of plexiform lesions, is the underlying cause of the increased vascular resistance (65). The mean survival time without treatment is ∼2.8 yr, and the ratio of affected women to men is up to 3:1 (38, 46). PAH is termed idiopathic when sporadic, and familial in the 6% of cases with a positive family history. Both forms appear to share the same pathophysiological processes (16).
Bone morphogenic protein (BMP) receptor type II (BMPR2) mutations, including exon duplications and deletions and gene rearrangements, have been identified in ∼23% of all PAH cases and constitute the largest identified genetic risk for the disease (38). Mutations in this gene frequently produce a truncated protein, resulting in the downregulation of BMP signaling, which, in turn, increases proliferation of vascular smooth muscle cells via Smad1/5/8 (35, 76). BMPR1A and BMPR2 expression are reduced in PAH lung tissue (4, 13, 31), even in the absence of any identifiable mutation (35). More recently, protein expression changes in peripheral blood mononuclear cells were shown to distinguish familial PAH patients with BMPR2 mutations from obligate BMPR2 carriers without PAH (32). BMP-2 appears to inhibit pulmonary artery smooth muscle cell proliferation through BMPR2, peroxisome proliferator-activated receptor-γ, and apolipoprotein E (22). While these studies underscore the importance of the transforming growth factor-β (TGF-β)/BMP signaling pathway in the onset and development of PAH, most cases of PAH occur in the absence of BMPR2 mutations, and conversely BMPR2 mutations in carriers do not always lead to PAH. Furthermore, BMPR2 gene therapy failed to ameliorate monocrotaline PAH in one model (31). These observations suggest that additional factors, both genetic and environmental, may be required for the development of the clinical phenotype.
Besides germline mutations in BMPR2, somatic mutations in other genes, such as Bax, angiopoietin-1, vascular endothelial growth factor (VEGF), Smads, thromboxane, prostacyclin, plasminogen activator inhibitor, serotonin transporter, and Von Willebrand factor, have also been suggested to contribute to the development of the disease (9, 18, 23, 32 and references therein, 47, 58, 62, 69, 75). Functional and molecular studies have shown that PAH is associated with upregulation of the transient receptor potential channel calcium channels (77) and downregulation of the voltage-gated K+ 1.5 potassium channel (7), which lead to increases in intracellular K+ and Ca2+ concentrations and, in turn, lead to contraction, proliferation, and resistance to apoptosis of pulmonary artery smooth muscle cells (7, 77). These changes appear to be dependent, in part, on the antiapoptotic gene survivin (30), nuclear factor of activated T cells (7), and hypoxia-inducible factor-1α (6). Expression changes in interleukin-6 and NADPH oxidase 4 (NOX4) have been reported among other modifiers to result in vasoconstriction and remodeling in pulmonary artery smooth muscle cells (20, 21, 28, 33). Pulmonary artery endothelial cells derived from PAH lung tissue have shown increased expression of arginase II and signal transducer and activator of transcription 3 (STAT3) and decreased expression of nitric oxide (NO) (28, 74).
RNA expression profiling of lung tissue offers an unbiased, genomewide approach to identifying potential biological pathways responsible for the pathogenesis of PAH (53). This approach is free of any a priori assumptions as to which biological pathways are important. Geraci and colleagues (19) previously performed a genomewide RNA expression study using lung tissue specimens from six PAH subjects and six normal control subjects. Although this study was groundbreaking, since its completion, there has been an evolution in microarray technology, bioinformatics analytic tools, and availability of lung tissue specimens.
In this study, we performed genomewide RNA expression profiles in lung tissue specimens obtained from 18 human subjects with PAH and 8 subjects with pulmonary hypertension (PH) secondary to idiopathic pulmonary fibrosis (IPF) at the time of lung or heart-lung transplantation, along with 13 normal control subjects, to uncover novel clues to understanding the pathophysiology of this complex disease. The BMPR2 gene was analyzed in all PAH subjects by direct sequencing and multiplex ligation-dependent probe amplification to detect point mutations and gene rearrangements. Expression profiles of PAH lungs were compared with profiles of lungs from normal controls and PH secondary to IPF to identify genes uniquely differentially expressed in PAH. We used IPF subjects with secondary PH as a control cohort to enrich our data set for RNA expression changes that were specific to the arteriolar remodeling unique to PAH, rather than changes that were secondary to the hemodynamics of PH of any etiology. We report changes in several biological pathways and networks associated with PAH and identify novel potential targets for future therapeutic interventions.
MATERIALS AND METHODS
Tissue specimens.
Fresh frozen lung tissue specimens from PAH subjects (n = 18), IPF subjects with secondary PH (n = 8), and normal controls (n = 13) were obtained from the University of Pittsburgh Health Sciences Tissue Bank following approval by the University of Pittsburgh Institutional Review Board and informed consent by subjects contributing tissue specimens. Table 1 presents demographic and clinical data of all subjects donating lung tissue samples used in this study.
Table 1.
Demographic and clinical characteristics of subjects
| Cohort | n | Age, yr | Race | Sex (M/F) | PVRI, Wood units | MPAP, mmHg |
|---|---|---|---|---|---|---|
| PAH | 18 | 44 ± 10 | White 17; Native American 1 | 7/11 | 20 ± 9 | 55 ± 7 |
| IPF with PH | 8 | 60 ± 5 | White 7; African American 1 | 5/3 | 11 ± 5 | 45 ± 12 |
| Normal controls | 13 | 60 ± 11 | White 12; African American 1 | 5/8 |
Values are means ± SE; n, no. of subjects. PAH, pulmonary arterial hypertension; IPF, idiopathic pulmonary fibrosis; PH, pulmonary hypertension; M, male; F, female; PVRI, pulmonary vascular resistance index; MPAP, mean pulmonary arterial pressure.
BMPR2 mutation screening.
DNA was phenol-chloroform extracted from frozen tissue using standard protocols. Primer 3 software (http://frodo.wi.mit.edu/primer3/) was used to design primers for amplification of exons 1–13 by PCR (Supplemental Table 1; the online version of this article contains supplemental data). Following PCR, amplicons were purified with ExoSap (USB) and sequenced bidirectionally with ABI Big Dye terminator on an ABI 3100 Genetic Analyzer to detect mutations. Duplications or deletions of exons are not detectable by PCR and direct sequencing. Therefore, to screen for exon duplications or deletions, we performed multiplex ligation-dependent probe amplification using a commercially available kit (MRC-Holland, kit P093), according to the manufacturer's instructions (3).
Genomewide RNA expression profiling.
RNA was isolated from lung tissue using Trizol (Invitrogen) and purified using the RNeasy Mini kit (Qiagen). Microarray experiments were carried out as described elsewhere (48). Briefly, RNA quantity was determined on an Agilent Nanodrop and quality assessed on an Agilent 2100 Bioanalyzer, according to the manufacturer's instructions. Five hundred nanograms of total RNA meeting quality control criteria were Cy3 labeled using the Agilent Low RNA Input Linear Amplification Kit PLUS (One-Color). After purification and fragmentation, aliquots of each sample were hybridized to an Agilent Whole Human Genome 4 × 44 K microarray, sequentially washed, and scanned on an Agilent DNA microarray scanner. RNA expression data were analyzed with Agilent Feature Extraction Software.
Microarray data analysis.
Processed microarray data were log2-transformed and cyclic loess normalized (72), and then expressed as the difference of log of gProcessed Signal (Agilent Feature Extraction) and log of geometric mean of controls. A twofold difference in normalized expression value was used to estimate differential expression. Hierarchical clustering analysis was carried out using Scoregene statistical package (available at http://compbio.cs.huji.ac.il/scoregenes/) and visualized using the TreeView program (50). The q-value, computed using the SAM (significance analysis of microarrays) package (66) in the R statistical environment (44), was used to identify differentially expressed genes. The q-value represents the minimal false discovery rate at which an individual hypothesis test may be called significant. A gene was declared to be differentially expressed if the gene had a q-value < 5% in a three-way SAM analysis (normal controls vs. IPF with PH vs. PAH) and also had a q-value < 1% in at least one of the three pairwise SAM analyses. Ingenuity Pathway Analysis software was applied to identify differentially expressed gene clusters in biological pathways and networks. The complete microarray data set generated in this study is available in the Gene Expression Omnibus (GEO) database (www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE15197, accession number GSE15197).
Real-time quantitative PCR.
Quantitative PCR (QPCR) quantification was performed as described (1) for selected genes using RNA extracted from lung tissue specimens from subjects with PAH, subjects with PH secondary to IPF, and normal subjects, to confirm findings from our microarray studies: a disintegrin-like and metalloprotease with thrombospondin type 1 motif 9 (ADAMTS9); BMPR1A; BMPR2; cell adhesion molecule with homology to L1CAM (CHL1); cytochrome b558, β-polypeptide (CYBB); E2F transcription factor-1 (E2F1); estrogen receptor-α (ESR1); coagulation factor II (thrombin) receptor-like 3 (F2RL3); A-myb myeloblastosis viral oncogene homolog 1 (MYBL1); nuclear receptor coactivator 2 (NCOA2); purinergic receptor P2Y (P2RY1); platelet factor 4 (PF4); phospholamban (PLN); protein phosphatase (PP) 2, catalytic subunit, α-isoform (PPP2CA); prostaglandin-endoperoxide synthase 2 (PTGS2); tropomodulin 3 (TMOD3); and VEGF A (VEGFA). cDNA was synthesized using the SuperScript III First-Strand System (Invitrogen). The cDNA was then used as template for QPCR with gene-specific primers. Gene specific primers were designed using Genscript software (www.genscript.com/ssl-bin/app/primer), avoiding regions of homology between multiple genes (Supplemental Table 2). QPCR was carried out on an ABI Prism 7000 Sequence Detection System with the Express SYBR GreenER kit with premixed ROX (Invitrogen). QPCR results were analyzed using ABI Prism 7000 SDS software (Applied Biosystems). Threshold cycle values were normalized to gylceraldehyde-3-phosphate dehydrogenase. ΔΔThreshold cycle values were estimated, and statistical power computed using Student's t-test.
Protein analysis.
Frozen lung tissue was homogenized in 20 volumes of protein extraction buffer [in mM, 50 Tris at pH 8.0, 200 NaCl, 20 NaF, 20 β-glycerolphosphate, 1 DTT, with 0.5% Nonidet P-40, 1 protease inhibitor tablet/7 ml buffer (Roche)]. The homogenate was allowed to settle on ice for 10 min and centrifuged at 10,000 g for 10 min, and the supernatant was used for protein studies. Protein quantities were measured by the Bradford method (Bio-Rad). Immunoblots were performed as described (5) using equal amounts (30–60 μg) of total protein in each lane of an appropriate concentration protein gel (Pierce). Samples were transferred from the gel to a polyvinylidene difluoride membrane. The membrane was blocked and incubated at 4°C overnight with primary antibody at a titer recommended by the supplier, followed by the appropriate horseradish peroxidase-conjugated secondary antibody (Santa Cruz) (1:2,000 dilution) at room temperature for 1 h. Proteins were visualized and quantified by enhanced chemiluminescence (Pierce). The following primary antibodies were used: BMPR2 (R&D Systems, AF811), P2RY1 (Sigma-Aldrich, P6487), PTGS2 (Santa Cruz Biotechnology, SC1747), and VEGFA (Abcam, ab46154). Signal intensity was normalized to protein loading using a second immunoblot with primary antibody specific for gylceraldehyde-3-phosphate dehydrogenase.
Statistical analysis.
Statistical analysis of genomewide RNA expression data from microarrays is described above. Unpaired Student's t-tests were performed to compare data obtained from experimental and control cohorts. One-way ANOVA, followed by the Bonferroni correction post hoc test, was used for multiple comparisons. A value of P ≤ 0.05 was considered significant.
RESULTS AND DISCUSSION
This study represents the largest gene expression data set from human PAH lung tissue samples and contrasts PAH RNA expression patterns with not only normal control subjects, but also subjects with secondary PH. Histological studies have demonstrated that vascular remodeling in interstitial pulmonary disorders such as IPF is characterized by absence of endothelial proliferation compared with PAH, which is driven by smooth muscle and endothelial proliferation (63). Inclusion of PH secondary to IPF as a comparison cohort enriched our data set for RNA expression changes that were specific to the arteriolar remodeling unique to PAH, rather than changes that were secondary to the hemodynamics of PH of any etiology. We identified major changes in the expression of many genes and biological pathways that appeared to be unique to PAH. The expression profiles for the three cohorts clearly distinguished PAH from PH secondary to IPF and from normal controls. Because of the limited availability of tissue samples, there was some heterogeneity in age and sex among the three cohorts, which could potentially lead to some spurious differences in gene expression between cohorts. However, analysis of the expression of selected individual genes showed little intracohort variation on the basis of sex and age.
BMPR2 mutation screening.
All 18 PAH subjects were screened for point mutations and exon deletions and duplications in BMPR2. A mutation was identified in a single subject: a deletion of nucleotides 187G–188G, resulting in the premature truncation of the protein at residue 63. The prevalence of mutations, particularly exon deletions and duplications, appeared to be somewhat lower in this cohort than reported in other cohorts (3, 11). Unlike previous reports, this study focused exclusively on PAH subjects who were undergoing lung and heart-lung transplants.
Genomewide RNA expression profiles.
Whole genome RNA expression profiles of lung tissue specimens from PAH subjects (n = 18) were compared with those from normal controls (n = 13) and subjects with PH secondary to IPF (n = 8) (Supplemental Fig. 1). Two different analyses of the expression data were performed. First, we identified a total of 13,889 genes differentially expressed at q ≤ 5% in the PAH cohort relative to normal controls, with 8,678 and 5,211 genes being upregulated and downregulated in PAH, respectively. The biological function of 6,149 of these genes was identified using Ingenuity Pathway Analysis software.
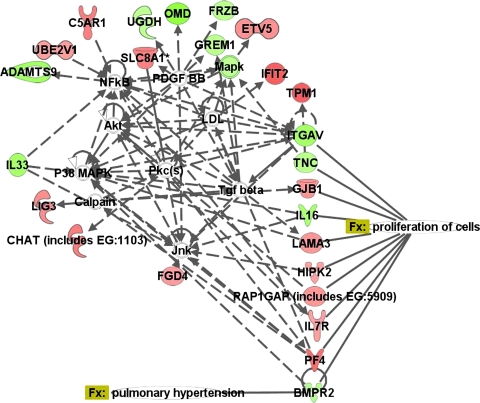
A second analysis was carried out to identify genes with changes in expression unique to PAH compared with both normal controls and PH secondary to IPF at q ≤ 5% (Fig. 1) and q ≤ 1%. A total of 4,734 and 1,604 genes were differentially expressed in PAH relative to both normal controls and PH secondary to IPF at q ≤ 5% and q ≤ 1%, respectively. A large number of these genes were involved in regulation of gene expression, cell signaling, RNA posttranscriptional changes, and cellular assembly and organization (Supplemental Tables 3 and 4). Table 2 presents a short list of 43 genes selected on the basis of their functional role in cell signaling, vascular function and disease, cellular assembly, organization, development, and proliferation. These genes were highly up- and downregulated in PAH (q ≤ 1%) relative to the other two cohorts and may play an important role in the pathogenesis of the disease. Since BMPR2 has been implicated in PAH, a network of genes functionally associated with BMPR2 was generated using Ingenuity (Fig. 2). The expression of a total of 25 BMPR2-associated genes was changed in PAH relative to normal controls and PH secondary to IPF, of which at least nine genes were involved in cell proliferation.
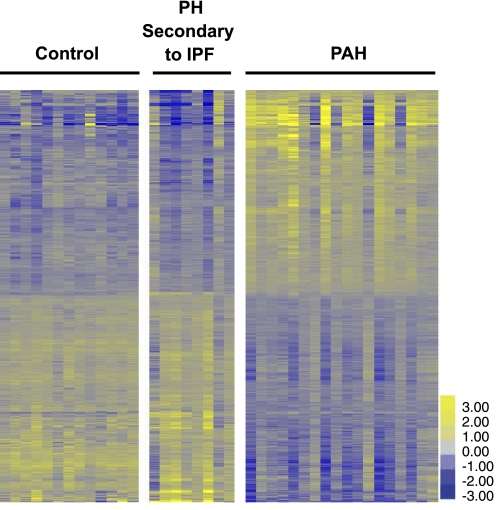
Fig. 1.
Heat map representing genes (q ≤ 5%) with expression changes unique to the pulmonary arterial hypertension (PAH) cohort. 4,734 genes were differentially expressed in PAH relative to both normal controls and pulmonary hypertension (PH) secondary to idiopathic pulmonary fibrosis (IPF), generating a signature discriminating among all three cohorts. 2,377 genes were concomitantly upregulated in PAH compared with both normal controls and PH secondary to IPF; 2,357 genes were concomitantly downregulated. Each row represents a gene, and each column represents a sample. Yellow indicates upregulation, and blue indicates downregulation.
Table 2.
List of genes highly differentially expressed (q ≤ %) in PAH lungs relative to both normal controls and PH secondary to IPF
| Gene Symbol | Description | Ingenuity Canonical Pathway | Log2 Fold Change (PAH vs. Control, PAH vs. PH Secondary to IPF) |
|---|---|---|---|
| Cell-to-cell signaling and interaction | |||
| IL16 | Interleukin 16 | −1.9, −2.4 | |
| PF4 | Platelet factor 4 | 2.1, 4.01 | |
| CEACAM3 | Carcinoembryonic antigen-related cell adhesion molecule 3 | −1.6, −1.7 | |
| GP1BA | Glycoprotein Ib (platelet), α-polypeptide | 1.5, 3.6 | |
| ITGAV | Integrin, αV | Integrin signaling | −2.01, −1.5 |
| C5AR1 | Complement component 5a receptor 1 | Complement system | 1.8, 2.4 |
| F2RL3 | Coagulation factor II (thrombin) receptor-like 3 | 2.1, 3.2 | |
| CYBB | Cytochrome b558, β-polypeptide | Leukocyte extravasation signaling | 2.5, 2.8 |
| GREM1 | Gremlin 1 | −1.5, −2.1 | |
| TNC | Tenascin C | −2, −2 | |
| LAMA3 | Laminin, α3 | 1.6, 1.5 | |
| GJB1 | Gap junction protein, β1 | 1.6, 1.9 | |
| MSN | Moesin | Leukocyte extravasation signaling | 1.9, 2.5 |
| RAP1GAP | RAP1 GTPase activating protein | cAMP-mediated signaling | 1.6, 1.8 |
| EPHA3 | EPH receptor A3 | Ephrin Receptor signaling | −1.6, −2.1 |
| CD46 | CD46 molecule, complement regulatory protein | Complement system | −2.2, −2.8 |
| CHAT | Choline acetyltransferase | Glycerophospholipid metabolism | 1.9, 1.3 |
| Cardiovascular system development and function | |||
| CASQ2 | Calsequestrin 2 | Calcium signaling | −1.8, −2.1 |
| SFRS1 | Splicing factor, arginine/serine-rich 1 | −1.6, −1.5 | |
| PLN | Phospholamban | Nitric oxide signaling in the cardiovascular system | −1.7, −2.9 |
| PPP2CA | Protein phosphatase 2 (formerly 2A), catalytic subunit, α-isoform | β-Adrenergic pathway | −1.9, −1.1 |
| SLC8A1 | Solute carrier family 8, member 1 | Calcium signaling | 1.8, 1.7 |
| TPM1 | Tropomyosin 1 | Calcium signaling | 2.2, 1.9 |
| PDE4D | Phosphodiesterase 4D | cAMP-mediated signaling | 1.8, 1.7 |
| Cardiovascular disease | |||
| CA1 | Carbonic anhydrase I | Nitrogen metabolism | 1.7, 2.1 |
| KCNMB3 | Potassium large-conductance calcium-activated channel, subfamily M β-member 3 | −1.6, −1.4 | |
| P2RY1 | Purinergic receptor P2Y | G protein-coupled receptor signaling | 2.1, 2.8 |
| Cellular assembly and organization | |||
| NRTN | Neurturin | Ephrin receptor signaling | 2.3, 1.3 |
| KALRN | Kalirin | −1.7, −1.3 | |
| CHL1 | Cell adhesion molecule with homology to L1CAM | −3.0, −2.9 | |
| HK2 | Hexokinase 2 | Aminosugars metabolism | 2.6, 2.4 |
| HIPK2 | Homeodomain interacting protein kinase 2 | p53 signaling | 1.5, 1.1 |
| FGD4 | FYVE, RhoGEF, and PH domain containing 4 | 1.5, 2.0 | |
| MPP7 | Membrane protein, palmitoylated 7 | 2.1, 2.6 | |
| GABBR1 | γ-Aminobutyric acid (GABA) B receptor, 1 | GABA receptor signaling | −1.6, −1.3 |
| NCOA2 | Nuclear receptor coactivator 2 | Estrogen receptor signaling | −1.9, −1.2 |
| Cellular growth and proliferation | |||
| SLC1A2 | Solute carrier family 1, member 2 | Glutamate receptor signaling | 1.7, 1.8 |
| IL7R | Interleukin 7 receptor | 1.5, 1.7 | |
| Cell cycle | |||
| CCNG2 | Interleukin 7 receptor | −1.8, −2.0 | |
| UBA3 | Ubiquitin-like modifier activating enzyme 3 | −1.7, −1.4 | |
| Cellular compromise and cellular development | |||
| SLC20A1 | Solute carrier family 20, member 1 | −1.7, −1.4 | |
| RAP1GAP | RAP1 GTPase activating protein | cAMP-mediated signaling | 1.6, 1.1 |
| IL33 | Interleukin 33 | −1.9, −1.3 | |
Fig. 2.
Network of genes unique to PAH associated with bone morphogenic protein receptor, type II (BMPR2), with an overlay of associated diseases and functions. Green indicates downregulation, red indicates upregulation, and color intensity is proportional to fold change. ADAMTS9, ADAM metallopeptidase with thrombospondin type 1 motif 9; C5AR1, complement component 5a receptor 1; CHAT, choline acetyltransferase; ETV5, ets variant 5; FGD4, FYVE, RhoGEF, and PH domain containing 4; FRZB, frizzled-related protein; GJB1, gap junction protein, β1; GREM1, gremlin 1; HIPK2, homeodomain interacting protein kinase 2; IFIT2, interferon-induced protein with tetratricopeptide repeats 2; IL16, interleukin 16; IL33, interleukin 33; IL7R, interleukin 7 receptor; ITGAV, integrin, αV; LAMA3, laminin, α3; LIG3, ligase III, DNA, ATP dependent; Mapk, mitogen-activated protein kinase; OMD, osteomodulin; PF4, platelet factor 4; RAP1GAP, RAP1 GTPase activating protein; SLC8A1, solute carrier family 8 (sodium/calcium exchanger), member 1; TGF-β, transforming growth factor-β; TNC, tenascin C; TPM1, tropomyosin 1 (α); UBE2V1, ubiquitin-conjugating enzyme E2 variant 1; UGDH, UDP-glucose dehydrogenase. In this and subsequent figures, relationships between molecules are represented as follows: single lines, binding; line with bar at the end, inhibition; line with filled arrowhead, activation; line with bar and filled arrowhead, activation and inhibition; line with circular head, result of action; line with closed arrowhead, translocation; arrow, reaction; arrow with diamond, enzyme catalyzed reaction; bold line, direct interaction; and dotted line, indirect interaction.
Validation of microarray data.
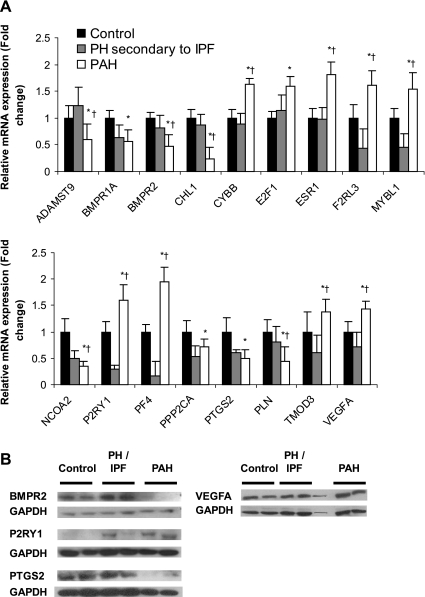
Differential expression of 17 genes in PAH relative to normal controls and PH secondary to IPF was confirmed by QPCR (Fig. 3A), in part to document the technical validity of our microarray assays. However, as detailed individually below, each gene was selected on the basis of its respective biological role that may be relevant in the pathophysiology of PAH. Although some genes, such as BMPR1A, BMPR2, PTGS2, and VEGFA (4, 13), have been previously implicated in PAH, we also examined novel genes that may participate in its pathophysiology, including ADAMTS9, CHL1, CYBB/NOX2, E2F1, ESR1, F2RL3, MYBL1, NCOA2, P2RY1, PF4, PLN, PPP2CA, and TMOD3.
Fig. 3.
Validation of microarray data. A: quantitative PCR (QPCR) validation of changes in RNA expression of selected genes suggested by microarray data. For each gene the leftmost (solid) bar represents the normal control cohort (n = 5), the middle (shaded) bar represents PH secondary to IPF (n = 4), and the rightmost (open) bar represents the PAH cohort (n = 5). Standard errors are indicated for each bar. *P < 0.05 for PAH relative to normal control; †P < 0.05 for PAH relative to PH secondary to IPF. B: immunoblot validation of corresponding expression changes at the protein level of selected genes. See materials and methods for definitions of acronyms.
The enzyme PPP2CA is one of four major PPs that are responsible for the dephosphorylation of serine and threonine residues in proteins, and its overexpression leads to cell cycle arrest and apoptosis (39). In contrast, MYBL1 is a member of the MYB transcription family that increases the rate of transcription in smooth muscle cells (57). QPCR confirmed the downregulation of PPP2CA in PAH relative to normal controls and the upregulation of MYBL1 in PAH, compared with both normal control and PH secondary to IPF. Hence, differential expression of PPP2CA and MYBL1 in PAH may be some of the factors mediating smooth muscle cell and other cell proliferation in this disease. E2F1 is a member of the E2F family of transcription factors that control cell cycle, and overexpression of the E2F1 forces vascular smooth muscle cells to enter the S phase and, subsequently, to undergo apoptosis (54). E2F1 was overexpressed in PAH. NCOA2 belongs to the nuclear hormone receptor family that are conditional transcription factors, vital to cell growth and development. Its overexpression has been detected in both proliferating and confluent myoblasts (OMIM: 601993). Reduced expression of NCOA2 was observed in PAH relative to both control and PH secondary to IPH. Overexpression of E2F1 and underexpression of NCOA2 in PAH may represent a late-stage development.
Nonphagocytic NADPH oxidases (NOX1, NOX2, and NOX4, and their subunits) have been suggested to play an important role in the regulation of hypertrophy, remodeling, and angiogenesis in the systemic circulation. While one study reported a 2.5-fold upregulation of NOX4 in idiopathic PAH lungs relative to normal controls and increased NOX4 expression in association with development of PH in mice exposed to chronic hypoxia (33), another study demonstrated that increased NOX2/CYBB expression is essential for chronic hypoxia-induced PH (26). Our study demonstrated a 3.5-fold upregulation of NOX2/CYBB in human lung PAH tissue specimens relative to normal controls and a similar upregulation relative to PH secondary to IPF, supporting the role of NOX2/CYBB in vascular remodeling.
P2RY1 belongs to the family of G protein-coupled receptors and functions as a receptor for extracellular ATP and ADP. It is essential for ADP-induced platelet activation, aggregation, and thrombosis (25). Coagulation F2RL3 participates in the coagulation system and is necessary for platelet activation by thrombin (51). A major physiological role of PF4 appears to be neutralization of heparin-like molecules on the endothelial surface of blood vessels, thereby promoting coagulation. It is also suggested to play a role in inflammation (15) and fibroblast proliferation (40). P2RY1, F2RL3, and PF4 were upregulated in PAH tissues relative to both normal controls and PH secondary to IPF.
Cell migration is vital for many cellular functions, including vascular proliferation and vascular repair. In vitro studies in human microvascular endothelial cells-1 suggest that TMOD3 overexpression results in cell depolarization that is associated with decreased cell motility (17). Since TMOD3 is expressed in smooth muscle cells (12) and plexiform lesions in PAH are formed in part by migrating smooth muscle cells (67), we suggest that decreased expression of TMOD3 might have a role in regulating migration of smooth muscle cells in PAH. CHL1 encodes neural cell adhesion molecules required for migration and differentiation of neuronal cells. More recently, decreased expression of CHL1 in a highly invasive melanoma cell line has highlighted its potential role as a tumor suppressor (61). Similarly, downregulation of ADAMTS9 in tumor cell lines has provided functional evidence of its tumorigenic suppressive ability. ADAMTS9 is a member of the ADAM metalloproteinases that have been implicated in the cleavage of proteoglycans, organ development, and the inhibition of angiogenesis (27). Although CHL1 is reported to be highly expressed in normal lung tissues (68), expression of CHL1 and ADAMTS9 was downregulated in PAH tissues relative to both normal controls and PH secondary to IPF. We hypothesize that reduced expression of CHL1 may affect cellular proliferation, facilitating the progression of PAH.
ESR1 expression was selected for validation by QPCR because of a predilection of PAH for females. Phosphorylated PLN enhances Ca2+ sequestration into the sarcoplasmic reticulum, mediating vasodilatory action in vascular smooth muscle (24). The potential functional roles in PAH of both genes are discussed in greater detail below. Expression changes at the protein level were confirmed by immunoblot for four of these genes discussed above: BMPR2, VEGFA, PTGS2, and P2RY1 (Fig. 3B).
Biological pathways.
Using Ingenuity Pathway Analysis software, we interrogated our microarray data to gain novel insights into the complex pathophysiology of PAH. Fold changes of all genes in PAH relative to normal controls were computed for the entire expression data set and overlaid onto different pathways. Next, similar analyses were carried out for genes exhibiting changes in PAH relative to normal controls at q ≤ 5% and in PAH relative to both normal controls and PH secondary to IPF at q ≤ 5%. Among the most significant biological pathways perturbed in PAH relative to both normal controls and PH secondary to IPF, the top three differentially expressed pathways were regulation of actin-based motility by Rho, cyclic adenosine monophosphate (cAMP)-mediated signaling, and the protein ubiquitination pathway. An in-depth analysis of these top pathways and several other pathways selected on the basis of earlier studies and our present analysis is presented below. All expression changes are presented as log2 fold change. Descriptions of individual genes in each pathway are listed in Supplemental Table 5.
Regulation of actin-based motility by Rho.
Cell migration is vital for cellular and tissue remodeling, and improper regulation of cell migration can result in many abnormal processes. The actin cytoskeleton is essential for cell migration and is regulated by small GTPases of the Rho family (70). In brief, GTP-bound activated cell division cycle 42, Rac, and Ras homolog (Rho) specifically bind to activate their downstream effectors, which are either kinases or scaffolding proteins. These downstream proteins, in turn, activate diverse signaling pathways that affect the actin cytoskeleton and cellular morphology (2). In lungs, Rho GTPases control vascular remodeling, and their sustained activation can result in development of PH. For example, in smooth muscle cells, RhoA activates Rho kinase, which helps maintain sustained contraction via increased phosphorylation of myosin light chains, and, in endothelial cells, RhoA stimulates the formation of stress fibers that results in increased permeability (71). Although Rho kinase was downregulated in our analysis, downstream effector protein kinases, such as p21 protein (cell division cycle 42/Rac)-activated kinase 1, p21 protein (cell division cycle 42/Rac)-activated kinase 2, phosphatidylinositol-5-phosphate 4-kinase, type II, α and β were upregulated in PAH tissues (Supplemental Table 5). These data taken together suggest that overexpression of protein kinases in PAH lung tissues may be one of the factors leading to tissue remodeling associated with the disease.
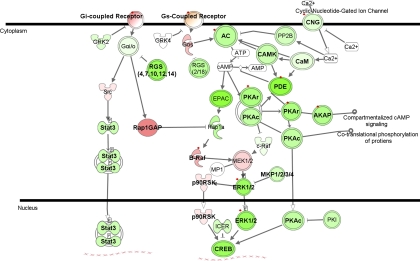
cAMP-mediated signaling pathway.
cAMP is an intracellular second messenger that is important for various biological processes, including regulation of vascular tone in PAH. Cyclic nucleotide phosphodiesterases (PDEs) catalyze the hydrolysis of cAMP and cyclic guanosine monophosphate. Increased expression of PDE1A, PDE1C, PDE3B, and PDE5A in PAH pulmonary artery smooth muscle cells have been observed in association with decreased cAMP levels and increased proliferation of pulmonary artery smooth muscle cells in PAH patients (36). Inhibition of PDE isoforms results in relaxation of smooth muscle cells (37, 43). Our data confirmed increased expression of PDE3B and also show increased expression of PDE4A, PDE4C, and PDE4D. However, we observed decreased expression of PDE1A and PDE1C, perhaps suggesting differences in expression between cellular constituents of lung tissue. Comparison of our data with previous reports suggests that the isoform PDE3B may be the most important in the pathogenesis of PAH (Fig. 4, Supplemental Table 5) (36). Although the PDE5 inhibitors sildenafil and tadalafil are commonly used to treat PAH, this isoform did not appear to be significantly differentially expressed in PAH subjects. None of the PAH subjects in this study were receiving this treatment immediately before lung explantation.
Fig. 4.
Differentially expressed genes in the cAMP signaling pathway. Green indicates downregulation, red indicates upregulation, and color intensity is proportional to fold change. Bold typeface represents genes differentially expressed in PAH samples relative to normal controls (q ≤ 5%). Red diamonds indicate genes differentially expressed in PAH samples relative to both normal controls and PH secondary to IPF (q ≤ 5%). AC, 3′,5′-cyclic AMP synthetase; AKAP, A kinase (PRKA) anchor protein; B-raf, v-raf murine sarcoma viral oncogene homolog B1; CAMK, Ca2+/calmodulin-dependent protein kinase; CNG, cyclic nucleotide gated channel β1; CREB, creb protein; EPAC (synonym RAPGEF3), Rap guanine nucleotide exchange factor 3; GRK2 (synonym ADRBK1), adrenergic, β, receptor kinase 1; GRK4 (synonym ADRBK4), adrenergic, β, receptor kinase 4; ICER, cAMP-responsive element modulator; MKP, member of dual specificity phosphatase; MP1, MAPK scaffold protein 1; PDE, phosphodiesterase; PKA, protein kinase, cAMP dependent, catalytic subunit; RGS, regulator of G protein signaling; STAT3, signal transducer and activator of transcription 3.
Protein ubiquitination pathway.
Proteolysis of cellular protein is vital for regulation of many basic cellular processes, such as cell cycle and division, response to stress and extracellular modulators, modulation of cell surface receptors, ion channels and the secretory pathway, DNA repair, regulation of the immune and inflammatory responses, and apoptosis. Ubiquitination involves activation of ubiquitin by an E1 ubiquitin-activating enzyme, followed by transfer of ubiquitin to E3 ubiquitin-protein ligase via E2 ubiquitin-conjugating enzymes (10). In our data set, 32 of the 48 differentially expressed molecules in this pathway were downregulated in PAH relative to both normal controls and PH secondary to IPF at q ≤ 5%, among which anaphase promoting complex subunit 1 (ANAPC1); ANAPC5; proteasome 26S subunit, ATPase, 5; proteasome 26S subunit, ATPase, 10; ubiquitin-conjugating enzyme E2E 1 (UBE2E1), UBE2E3, UBE2G1, ubiquitin-specific peptidase 28 (USP28), UBE2 variant 1, and USP13 were significant at q ≤ 1% (Supplemental Table 5). ANAPC is an E3 ubiquitin ligase that targets specific proteins for proteasomal degradation, thereby allowing progression of the cell cycle (42). Overexpression of UBE2 variant proteins results in an increase in cell growth rate and protects cells against stress-induced apoptosis (52, 59). Similarly, overexpression of certain USPs has been associated with enhanced cell proliferation (41). Interestingly, all of the ANAPC subunits and a majority of the UBE2 and USP subunits were downregulated in PAH, suggesting a compensatory mechanism to counteract excessive cell growth in PAH. Thus the crucial regulatory role played by this pathway in cellular growth, along with downregulation of the pathway in our data set, suggests that a detailed study of ubiquitination in PAH is warranted.
TGF-β signaling pathway.
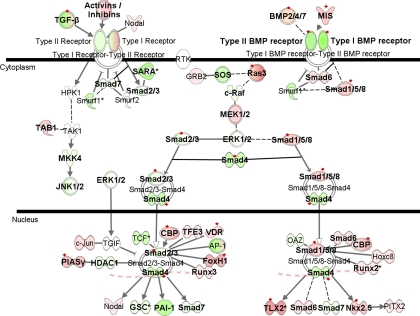
The TGF-β family comprises a large number of structurally related polypeptide growth factors, each regulating a array of cellular processes, including cell proliferation, lineage determination, differentiation, motility, adhesion, and death (29). It has also been implicated in the development of PAH (78), and pulmonary artery smooth muscle cells isolated from PAH subjects have shown resistance to anti-proliferative effects of BMPs, even in the absence of BMPR2 mutations (35). Validation experiments performed for BMPR2 expression changes in PAH subjects excluded the single PAH subject with BMPR2 mutation (Fig. 3). Hence, our data confirmed that downregulation of BMPR2 occurs even in the absence of any identifiable mutation, including exonic deletions and duplications. Furthermore, our data demonstrated differential expression of 49 genes in the TGF-β signaling pathway in PAH subjects relative to normal controls, and the expression of 19 of these genes was perturbed in PAH subjects relative to both normal controls and PH secondary to IPF (Fig. 5, Supplemental Table 5). Novel genes that had not been previously associated with PAH and were highly overexpressed compared with other genes in the same pathway include muscle RAS oncogene homolog, forkhead box H1, and T-cell leukemia homeobox 2. These changes may reflect downstream effects resulting from expression changes in TGF-β and BMP receptors.
Fig. 5.
Differentially expressed genes in the TGF-β signaling pathway. Green indicates downregulation, red indicates upregulation, and color intensity is proportional to fold change. Bold typeface represents genes differentially expressed in PAH samples relative to normal controls (q ≤ 5%). Red diamonds indicate genes differentially expressed in PAH samples relative to both normal controls and PH secondary to IPF (q ≤ 5%). BMP2/4/7, bone morphogenic protein 2/4/7; c-Raf, v-raf-1 murine leukemia viral oncogene homolog 1; CBP, CBP/P300; FoxH1, forkhead box H1; GRB2, growth factor receptor-bound protein 2; GSC, goosecoid homeobox; HDAC1, histone deacetylase 1; Hoxc8, homeobox C8; HPK1, mitogen-activated protein kinase kinase kinase kinase 1; MEK1/2, MAP 2 kinase 1; MIS, anti-Mullerian hormone; MKK4, mitogen-activated protein kinase kinase 4; Nkx2.5, NK2 transcription factor related, locus 5; OAZ (synonym ZNF423), zinc finger protein 423; PAI-1, serpin peptidase inhibitor, clade E; PIASγ, protein inhibitor of activated STAT, 4; PITX2, paired-like homeodomain 2; Runx2, runt-related transcription factor 2; Runx3, runt-related transcription factor 3; SARA, zinc finger, FYVE domain containing 9; Smad1/5/8, SMAD family member 1/5/8; Smad2/3, SMAD family member 2/3; Smad4, SMAD family member 4; Smad6, SMAD family member 6; Smad7, SMAD family member 7; Smurf, SMAD-specific E3 ubiquitin protein ligase; SOS, member of growth factor receptor-bound protein 2; TAB1, mitogen-activated protein kinase kinase kinase 7 interacting, protein 1; TAK1 (synonym MAP3K7), mitogen-activated protein kinase kinase kinase 7; TFE3, transcription factor binding to IGHM enhancer 3; TGIF, TGF-B-induced factor homeobox; TLX2, T-cell leukemia homeobox 2; VDR, vitamin D (1,25-dihydroxyvitamin D3) receptor.
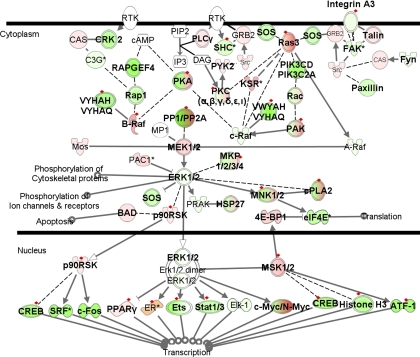
ERK/MAPK signaling pathway.
The mitogen-activated protein kinase (MAPK)-mediated pathway is a complex signal transduction pathway essential to numerous cell functions, such as adaptation to environmental stress, proliferation, differentiation, and apoptosis (34). Figure 6 presents an overlay of our expression data on the MAPK pathway. Forty-three genes were either upregulated or downregulated exclusively in PAH relative to normal controls and PH secondary to IPF. Interestingly, genes encoding phospholipase A2 (PLA2), such as, PLA2G1B (group IB), PLA2G2D (group IID), PLA2G4B (group IVB), PLA2G12A (group XIIA), and PLA2G12B (group XIIB), were all upregulated. On the other hand, almost all serine/threonine PP encoding genes, such as PPP1CB (PP1, catalytic subunit, β-isoform), PPP1CC (PP1, catalytic subunit, γ-isoform), PPP1R12A [PP1, regulatory (inhibitor) subunit 12A], and PPP2CA (PP2, catalytic subunit, α-isoform) were downregulated, with the exception of PPP1R3B/D [PP1, regulatory (inhibitor) subunit 3D], which was upregulated (Supplemental Table 5). Recent studies have suggested that PPs, particularly PP2CAα, dephosphorylate Smads 2 and 4, inhibit TGF-β and BMP receptors, and result in rapid inhibition of these signaling pathways (34). Therefore, these changes may represent a secondary compensatory response to decreased TGF-β and BMP signaling in PAH. Furthermore, PLA2 is involved in apoptosis (60), again suggesting a compensatory mechanism to counteract excessive cell growth in PAH. Interplay between phospholipases and PPs in the MAPK pathway may play a vital role in the development of PAH, and detailed studies focused on these genes may provide new insights into the pathogenesis of the disease.
Fig. 6.
Differentially expressed genes in the ERK/MAPK signaling pathway. Green indicates downregulation, red indicates upregulation, and color intensity is proportional to fold change. Bold typeface represents genes differentially expressed in PAH samples relative to normal controls (q ≤ 5%). Red diamonds indicate genes differentially expressed in PAH samples relative to both normal controls and PH secondary to IPF (q ≤ 5%). 4E-BP1, eukaryotic translation initiation factor 4E binding protein 1; ATF-1, activating transcription factor 1; B-Raf, v-raf murine sarcoma viral oncogene homolog B1; BAD, BCL2-associated agonist of cell death; CAS, member of breast cancer anti-estrogen resistance 1; c-Fos, v-fos FBJ murine osteosarcoma viral oncogene homolog; c-Raf, v-raf-1 murine leukemia viral oncogene homolog 1; CRK2, v-crk sarcoma virus CT10 oncogene homolog (avian); cPLA2, phospholipase A2; eIF4E, eukaryotic translation initiation factor 4E; Elk-1, member of ETS oncogene family; ER, estrogen receptor 1; Ets, E74-like factor 1 (ets domain transcription factor); FAK, PTK2 protein tyrosine kinase 2; Fyn, FYN oncogene related to SRC, FGR, YES; KSR, kinase suppressor of ras 1; HSP27, heat shock protein 27; MNK1/2, MAP kinase interacting serine/threonine kinase 1; PAC1 (synonym DUSP2), dual-specificity phosphatase 2; PAK, p21 activated protein kinase; PIP2, 1-phosphatidyl-D-myo-inositol 4,5-bisphosphate; PKA, p21 activated protein kinase; PLCγ, phospholipase Cγ; PP1/PP2A, protein phosphatase 1/2A; PPARγ, peroxisome proliferator-activated receptor-γ; PRAK (synonym MAPK5), mitogen-activated protein kinase-activated protein kinase 5; p90RSK, ribosomal protein S6 kinase, 90 kDa, polypeptide 1; PYK2, PTK2B protein tyrosine kinase 2β; RAPGEF4, cAMP-Gef; RPS6KA1, ribosomal protein S6 kinase, 90 kDa, polypeptide 1; SHC, Src homology 2 domain containing transforming protein 1; Src, v-src sarcoma (Schmidt-Ruppin A-2) viral oncogene homolog (avian); SRF, serum response factor (c-fos serum response element-binding transcription factor); Stat1/3, signal transducer and activator of transcription 1/3 (acute-phase response factor); YWHAQ, tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, θ-polypeptide; YWHAZ, tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, ζ-polypeptide.
Platelet-derived growth factor signaling pathway.
Platelet-derived growth factor (PDGF) and its receptors are known to increase proliferation and migration of smooth muscle cells and fibroblasts. PDGFs B and C (PDGFB and PDGFC) were upregulated in PAH, while both the PDGF-α and the PDGF-β receptors were downregulated in PAH subjects relative to both normal controls and PH secondary to IPF. Among other downstream molecules in the same pathway, phosphatidylinositol 3-kinase catalytic α-polypeptide, phosphatidylinositol 3-kinase catalytic δ-polypeptide, and RASA1 were ∼1.5-fold downregulated, while muscle RAS oncogene homolog and Janus kinase 1 were almost twofold upregulated relative to both normal controls and PH secondary to IPF. STAT3 increases proliferation of airway smooth muscle cells (55). However, STAT3 expression was decreased in PAH lung tissue relative to normal controls and PH secondary to IPF (Supplemental Fig. 2 and Supplemental Table 5). Thus, although some expression changes are consistent with smooth muscle proliferation, other changes, such as downregulation of PDGF-α and -β receptors and a corresponding decrease in expression of STAT3 may represent a secondary response characteristic of end-stage PAH, in which an attempt is made to limit proliferation of smooth muscle cells.
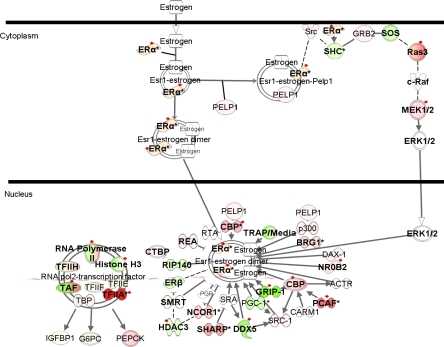
Estrogen signaling pathway.
Gene expression data for the estrogen signaling pathway receptors demonstrated upregulation of ESR1 in PAH subjects relative to normal controls and PH secondary to IPF (Fig. 7, Supplemental Table 5), also confirmed by QPCR (Fig. 3A). PAH has a predilection for females, prompting us to carry out a sex-specific analysis based on the individual values obtained from the processed microarray data. Segregation of PAH and normal control samples into females and males showed that female PAH subjects (n = 11) had a higher mean expression of ESR1 than female controls (n = 5) (0.477 ± 0.177 vs. −0.408 ± 0.41, P ≤ 0.01). However, the same pattern held true for male PAH subjects (n = 7) relative to male controls (n = 4) (0.22 ± 0.19 vs. −0.40 ± 0.08, P ’ 0.01). It has been suggested that 17β-estradiol attenuates hypoxia-induced PH by stimulating endothelial NO synthase (14, 45). The increased ESR1 expression observed in our study is consistent with either a role in the pathogenesis of PAH, or a secondary protective effect.
Fig. 7.
Differentially expressed genes in the estrogen receptor signaling pathway. Green indicates downregulation, red indicates upregulation, and color intensity is proportional to fold change. Bold typeface represents genes differentially expressed in PAH samples relative to normal controls (q ≤ 5%). Red diamonds indicate genes differentially expressed in PAH samples relative to both normal controls and PH secondary to IPF (q ≤ 5%). ACTR (synonym NCOA3), nuclear receptor coactivator 3; BRG1, SWI/SNF related, matrix associated, actin-dependent regulator of chromatin, subfamily a, member 4; CARM1, coactivator-associated arginine methyltransferase 1; CTBP, COOH-terminal binding protein; DAX1 (synonym NR0B1), nuclear receptor subfamily 0, group B, member 1; DDX5, DEAD (Asp-Glu-Ala-Asp) box polypeptide 5; Erα, estrogen receptor 1; Erβ, estrogen receptor 2 (ER-β); G6PC, glucose-6-phosphatase, catalytic subunit; GRIP-1, nuclear receptor coactivator 2; HDAC3, histone deacetylase 3; IGFBP1, insulin-like growth factor binding protein 1; NCOR1, nuclear receptor corepressor 1; NR0B2, nuclear receptor subfamily 0, group B, member 2; PCAF, K (lysine) acetyltransferase 2B; PELP1, proline, glutamate, and leucine rich protein 1; PEPCK, phosphoenolpyruvate carboxykinase; PGC1, peroxisome proliferator-activated receptor-γ, coactivator 1α; REA, prohibitin 2; RIP140, nuclear receptor interacting protein 1; RTA (synonym RBM9), RNA binding motif protein 9; SHARP, spen homolog, transcriptional regulator (Drosophila); SMRT, nuclear receptor corepressor 2; SRA, steroid receptor RNA activator 1; PGR, progesterone receptor; TAF, TAF2 RNA polymerase II, TATA box binding protein-associated factor; TFIIA, general transcription factor IIA; TFIIH, general transcription factor IIA; TRAP/Media, transformation/transcription domain-associated protein.
Furthermore, Wu and colleagues (73) demonstrated that ESR1 target genes are differentially expressed in rat lungs in response to intermittent hypoxia, suggesting that ESR1 plays a key regulatory role during intermittent hypoxia response. We examined the expression of ESR1 target genes (8) in our data set (Supplemental Table 6). A total of 258 ESR1 targets (147 upregulated and 111 downregulated) were differentially expressed in PAH relative to normal controls and PH secondary to IPF (q ≤ 1%). A few target genes included BMPR2, VEGF, and PLN (discussed in detail under TGF-β signaling and NO signaling pathways), providing further evidence implicating the estrogen signaling pathway in the pathogenesis of this disease.
NO signaling.
NO is synthesized in endothelial cells by NO synthase (endothelial NO synthase) and signals the surrounding vascular smooth muscle cells to relax, thus resulting in vasodilation. It is also an inhibitor of smooth muscle cell proliferation (49). Among the genes differentially expressed in this pathway, PDE3B (see under cAMP-mediated signaling pathway) and VEGFA were upregulated, and PLN was downregulated in PAH relative to both normal controls and PH secondary to IPF (Supplemental Table 5). While VEGFA has been implicated in the pathogenesis of PAH (see Introduction), our study for the first time demonstrated its overexpression in PAH relative to PH secondary to IPF (Fig. 3A), suggesting that VEGF upregulation is specific to PAH rather than all forms of PH. Inhibition of PLN by phosphorylation may be an important mechanism by which endogenous vasodilators, such as NO, prostacyclin, and adenosine receptor, control vascular smooth muscle tone (24). Therefore, the trend to decreased PLN may represent an endogenous adaptive response to PAH.
Arachidonic acid metabolism.
Arachidonic acid is a precursor to prostacyclin, thromboxane, and leukotrienes. Downregulation of prostaglandin I2 (prostacyclin) synthase, which converts prostaglandin to prostacyclin, has been reported in lung tissue from patients with PAH and secondary PH (64). Our study showed decreased expression of PTGS2 (Fig. 3), which converts arachidonic acid to prostaglandin, in PAH lungs relative to normal controls and PH secondary to IPF. Prostanoids are a commonly used treatment for PAH, and these data suggest that they may act by attenuating a deficiency in endogenous prostacyclin. Although seven of the PAH subjects were being treated with prostanoids, and two were on a combination of prostanoids and endothelin receptor antagonists before transplant, there was no significant difference in the expression of PTGS2 between prostanoid-treated and untreated patients (data not shown).
Conclusion.
The present study provides a detailed analysis of genomewide RNA expression in PAH lung tissue and establishes a molecular signature differentiating between isolated PAH and PH secondary to IPF, thereby identifying potential differences in pathophysiology between PAH and secondary PH. Differentially expressed genes were participants in different biological functions, such as cell morphology, cell signaling, cell proliferation, tissue development and morphology, and immune response. Overall, these data are consistent with the paradigm that remodeling of several cellular components of the pulmonary vasculature is central to the pathogenesis of PAH, as opposed to secondary PH. Downregulation of the BMPR2 gene even in the absence of mutations in this gene confirms that impairment of this signaling pathway is a common mechanism of PAH, regardless of genetic etiology. We also demonstrated that higher expression of ESR1 in both female and male PAH subjects compared with sex-matched normal controls. Further studies searching for mutations in these genes in PAH subjects and detailed in vitro and in vivo experiments examining downstream targets of these different genes will undoubtedly further elucidate the pathophysiology of this complex disease. This, in turn, will result in the development of better therapeutic agents and biomarkers for PAH.
GRANTS
This study was supported by a Doris Duke Charitable Foundation Clinical Scientist Development Grant (F. Ahmad); the University of Pittsburgh Competitive Medical Research Fund (F. Ahmad); Grant 5UL1 RR024153-02 (F. Ahmad) from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH); NIH Roadmap for Medical Research, NIH Grants HL095401 (F. Ahmad), HL073745 (N. Kaminski), and HL095397 (N. Kaminski); the Dorothy P. and Richard P. Simmons Endowed Chair for Pulmonary Research fund (N. Kaminski); a Postdoctoral Fellowship from the Pulmonary Hypertension Association (R. Rajkumar); and the University of Pittsburg Heart Failure/Transplantation Program (D. McNamara, Director).
DISCLAIMER
The contents of this report are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at //www.ncrr.nih.gov/. Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearchloverview-translational.asp.
DISCLOSURES
No conflicts of interest are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Dr. Samuel Yousem for generously sharing control lung tissue sections, and to Dr. Rajiv Dhir and Michelle Bisceglia for assistance in retrieving tissue specimens from the University of Pittsburgh Health Sciences Tissue Bank.
REFERENCES
- 1.Ahmad F, Banerjee SK, Lage ML, Huang XN, Smith SH, Saba S, Rager J, Conner DA, Janczewski AM, Tobita K, Tinney JP, Moskowitz IP, Perez-Atayde AR, Keller BB, Mathier MA, Shroff SG, Seidman CE, Seidman JG. The role of cardiac troponin T quantity and function in cardiac development and dilated cardiomyopathy. PLoS ONE 3: e2642, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmadian MR, Wittinghofer A, Schmidt G. The actin filament architecture: tightly regulated by the cells, manipulated by pathogens. International Titisee Conference on the actin cytoskeleton: from signalling to bacterial pathogenesis. EMBO Rep 3: 214–218, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aldred MA, Vijayakrishnan J, James V, Soubrier F, Gomez-Sanchez MA, Martensson G, Galie N, Manes A, Corris P, Simonneau G, Humbert M, Morrell NW, Trembath RC. BMPR2 gene rearrangements account for a significant proportion of mutations in familial and idiopathic pulmonary arterial hypertension. Hum Mutat 27: 212–213, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Atkinson C, Stewart S, Upton PD, Machado R, Thomson JR, Trembath RC, Morrell NW. Primary pulmonary hypertension is associated with reduced pulmonary vascular expression of type II bone morphogenetic protein receptor. Circulation 105: 1672–1678, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Banerjee SK, Ramani R, Saba S, Rager J, Tian R, Mathier MA, Ahmad F. A PRKAG2 mutation causes biphasic changes in myocardial AMPK activity and does not protect against ischemia. Biochem Biophys Res Commun 360: 381–387, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Bonnet S, Michelakis ED, Porter CJ, Andrade-Navarro MA, Thebaud B, Haromy A, Harry G, Moudgil R, McMurtry MS, Weir EK, Archer SL. An abnormal mitochondrial-hypoxia inducible factor-1 alpha-Kv channel pathway disrupts oxygen sensing and triggers pulmonary arterial hypertension in fawn hooded rats: similarities to human pulmonary arterial hypertension. Circulation 113: 2630–2641, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Bonnet S, Rochefort G, Sutendra G, Archer SL, Haromy A, Webster L, Hashimoto K, Bonnet SN, Michelakis ED. The nuclear factor of activated T cells in pulmonary arterial hypertension can be therapeutically targeted. Proc Natl Acad Sci U S A 104: 11418–11423, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bourdeau V, Deschenes J, Metivier R, Nagai Y, Nguyen D, Bretschneider N, Gannon F, White JH, Mader S. Genome-wide identification of high-affinity estrogen response elements in human and mouse. Mol Endocrinol 18: 1411–1427, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Christman BW, McPherson CD, Newman JH, King GA, Bernard GR, Groves BM, Loyd JE. An imbalance between the excretion of thromboxane and prostacyclin metabolites in pulmonary hypertension. N Engl J Med 327: 70–75, 1992 [DOI] [PubMed] [Google Scholar]
- 10.Ciechanover A. The ubiquitin-proteasome pathway: on protein death and cell life. EMBO J 17: 7151–7160, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cogan JD, Vnencak-Jones CL, Phillips JA, Lane KB, 3rd, Wheeler LA, Robbins IM, Garrison G, Hedges LK, Loyd JE. Gross BMPR2 gene rearrangements constitute a new cause for primary pulmonary hypertension. Genet Med 7: 169–174, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Conley CA. Leiomodin and tropomodulin in smooth muscle. Am J Physiol Cell Physiol 280: C1645–C1656, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Du L, Sullivan CC, Chu D, Cho AJ, Kido M, Wolf PL, Yuan JX, Deutsch R, Jamieson SW, Thistlethwaite PA. Signaling molecules in nonfamilial pulmonary hypertension. N Engl J Med 348: 500–509, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Earley S, Resta TC. Estradiol attenuates hypoxia-induced pulmonary endothelin-1 gene expression. Am J Physiol Lung Cell Mol Physiol 283: L86–L93, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Eisman R, Surrey S, Ramachandran B, Schwartz E, Poncz M. Structural and functional comparison of the genes for human platelet factor 4 and PF4alt. Blood 76: 336–344, 1990 [PubMed] [Google Scholar]
- 16.Elliott CG. Genetics of pulmonary arterial hypertension: current and future implications. Semin Respir Crit Care Med 26: 365–371, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Fischer RS, Fritz-Six KL, Fowler VM. Pointed-end capping by tropomodulin3 negatively regulates endothelial cell motility. J Cell Biol 161: 371–380, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geggel RL, Carvalho AC, Hoyer LW, Reid LM. von Willebrand factor abnormalities in primary pulmonary hypertension. Am Rev Respir Dis 135: 294–299, 1987 [DOI] [PubMed] [Google Scholar]
- 19.Geraci MW, Moore M, Gesell T, Yeager ME, Alger L, Golpon H, Gao B, Loyd JE, Tuder RM, Voelkel NF. Gene expression patterns in the lungs of patients with primary pulmonary hypertension: a gene microarray analysis. Circ Res 88: 555–562, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Guibert C, Marthan R, Savineau JP. Modulation of ion channels in pulmonary arterial hypertension. Curr Pharm Des 13: 2443–2455, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Hagen M, Fagan K, Steudel W, Carr M, Lane K, Rodman DM, West J. Interaction of interleukin-6 and the BMP pathway in pulmonary smooth muscle. Am J Physiol Lung Cell Mol Physiol 292: L1473–L1479, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Hansmann G, de Jesus Perez VA, Alastalo TP, Alvira CM, Guignabert C, Bekker JM, Schellong S, Urashima T, Wang L, Morrell NW, Rabinovitch M. An antiproliferative BMP-2/PPAR gamma/apoE axis in human and murine SMCs and its role in pulmonary hypertension. J Clin Invest 118: 1846–1857, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones PL, Cowan KN, Rabinovitch M. Tenascin-C, proliferation and subendothelial fibronectin in progressive pulmonary vascular disease. Am J Pathol 150: 1349–1360, 1997 [PMC free article] [PubMed] [Google Scholar]
- 24.Karczewski P, Hendrischke T, Wolf WP, Morano I, Bartel S, Schrader J. Phosphorylation of phospholamban correlates with relaxation of coronary artery induced by nitric oxide, adenosine, and prostacyclin in the pig. J Cell Biochem 70: 49–59, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Leon C, Hechler B, Freund M, Eckly A, Vial C, Ohlmann P, Dierich A, LeMeur M, Cazenave JP, Gachet C. Defective platelet aggregation and increased resistance to thrombosis in purinergic P2Y(1) receptor-null mice. J Clin Invest 104: 1731–1737, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu JQ, Zelko IN, Erbynn EM, Sham JS, Folz RJ. Hypoxic pulmonary hypertension: role of superoxide and NADPH oxidase (gp91phox). Am J Physiol Lung Cell Mol Physiol 290: L2–L10, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Lo PH, Leung AC, Kwok CY, Cheung WS, Ko JM, Yang LC, Law S, Wang LD, Li J, Stanbridge EJ, Srivastava G, Tang JC, Tsao SW, Lung ML. Identification of a tumor suppressive critical region mapping to 3p14.2 in esophageal squamous cell carcinoma and studies of a candidate tumor suppressor gene, ADAMTS9. Oncogene 26: 148–157, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Masri FA, Xu W, Comhair SA, Asosingh K, Koo M, Vasanji A, Drazba J, Anand-Apte B, Erzurum SC. Hyperproliferative apoptosis-resistant endothelial cells in idiopathic pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 293: L548–L554, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Massague J. TGF-beta signal transduction. Annu Rev Biochem 67: 753–791, 1998 [DOI] [PubMed] [Google Scholar]
- 30.McMurtry MS, Archer SL, Altieri DC, Bonnet S, Haromy A, Harry G, Puttagunta L, Michelakis ED. Gene therapy targeting survivin selectively induces pulmonary vascular apoptosis and reverses pulmonary arterial hypertension. J Clin Invest 115: 1479–1491, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McMurtry MS, Moudgil R, Hashimoto K, Bonnet S, Michelakis ED, Archer SL. Overexpression of human bone morphogenetic protein receptor 2 does not ameliorate monocrotaline pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 292: L872–L878, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Meyrick BO, Friedman DB, Billheimer DD, Cogan JD, Prince MA, Phillips JA, 3rd, Loyd JE. Proteomics of transformed lymphocytes from a family with familial pulmonary arterial hypertension. Am J Respir Crit Care Med 177: 99–107, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mittal M, Roth M, Konig P, Hofmann S, Dony E, Goyal P, Selbitz AC, Schermuly RT, Ghofrani HA, Kwapiszewska G, Kummer W, Klepetko W, Hoda MA, Fink L, Hanze J, Seeger W, Grimminger F, Schmidt HH, Weissmann N. Hypoxia-dependent regulation of nonphagocytic NADPH oxidase subunit NOX4 in the pulmonary vasculature. Circ Res 101: 258–267, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Moorhead GB, Trinkle-Mulcahy L, Ulke-Lemee A. Emerging roles of nuclear protein phosphatases. Nat Rev Mol Cell Biol 8: 234–244, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Morrell NW, Yang X, Upton PD, Jourdan KB, Morgan N, Sheares KK, Trembath RC. Altered growth responses of pulmonary artery smooth muscle cells from patients with primary pulmonary hypertension to transforming growth factor-beta(1) and bone morphogenetic proteins. Circulation 104: 790–795, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Murray F, Patel HH, Suda RY, Zhang S, Thistlethwaite PA, Yuan JX, Insel PA. Expression and activity of cAMP phosphodiesterase isoforms in pulmonary artery smooth muscle cells from patients with pulmonary hypertension: role for PDE1. Am J Physiol Lung Cell Mol Physiol 292: L294–L303, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Netherton SJ, Maurice DH. Vascular endothelial cell cyclic nucleotide phosphodiesterases and regulated cell migration: implications in angiogenesis. Mol Pharmacol 67: 263–272, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Newman JH, Phillips JA, 3rd, Loyd JE. Narrative review: the enigma of pulmonary arterial hypertension: new insights from genetic studies. Ann Intern Med 148: 278–283, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Ofek P, Ben-Meir D, Kariv-Inbal Z, Oren M, Lavi S. Cell cycle regulation and p53 activation by protein phosphatase 2C alpha. J Biol Chem 278: 14299–14305, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Oppenheim JJ, Zachariae CO, Mukaida N, Matsushima K. Properties of the novel proinflammatory supergene “intercrine” cytokine family. Annu Rev Immunol 9: 617–648, 1991 [DOI] [PubMed] [Google Scholar]
- 41.Ovaa H, Kessler BM, Rolen U, Galardy PJ, Ploegh HL, Masucci MG. Activity-based ubiquitin-specific protease (USP) profiling of virus-infected and malignant human cells. Proc Natl Acad Sci U S A 101: 2253–2258, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peters JM. The anaphase-promoting complex: proteolysis in mitosis and beyond. Mol Cell 9: 931–943, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Polson JB, Strada SJ. Cyclic nucleotide phosphodiesterases and vascular smooth muscle. Annu Rev Pharmacol Toxicol 36: 403–427, 1996 [DOI] [PubMed] [Google Scholar]
- 44.R Development Core Team R: A Language and Environment for Statistical Computing Vienna: R Foundation for Statistical Computing, 2006 [Google Scholar]
- 45.Resta TC, Kanagy NL, Walker BR. Estradiol-induced attenuation of pulmonary hypertension is not associated with altered eNOS expression. Am J Physiol Lung Cell Mol Physiol 280: L88–L97, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Rich S, Dantzker DR, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Koerner SK, et al. Primary pulmonary hypertension. A national prospective study. Ann Intern Med 107: 216–223, 1987 [DOI] [PubMed] [Google Scholar]
- 47.Richter A, Yeager ME, Zaiman A, Cool CD, Voelkel NF, Tuder RM. Impaired transforming growth factor-beta signaling in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 170: 1340–1348, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Rosas IO, Richards TJ, Konishi K, Zhang Y, Gibson K, Lokshin AE, Lindell KO, Cisneros J, Macdonald SD, Pardo A, Sciurba F, Dauber J, Selman M, Gochuico BR, Kaminski N. MMP1 and MMP7 as potential peripheral blood biomarkers in idiopathic pulmonary fibrosis. PLoS Med 5: e93, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Said SI. Mediators and modulators of pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 291: L547–L558, 2006 [DOI] [PubMed] [Google Scholar]
- 50.Saldanha AJ. Java Treeview–extensible visualization of microarray data. Bioinformatics 20: 3246–3248, 2004 [DOI] [PubMed] [Google Scholar]
- 51.Sambrano GR, Weiss EJ, Zheng YW, Huang W, Coughlin SR. Role of thrombin signalling in platelets in haemostasis and thrombosis. Nature 413: 74–78, 2001 [DOI] [PubMed] [Google Scholar]
- 52.Sancho E, Vila MR, Sanchez-Pulido L, Lozano JJ, Paciucci R, Nadal M, Fox M, Harvey C, Bercovich B, Loukili N, Ciechanover A, Lin SL, Sanz F, Estivill X, Valencia A, Thomson TM. Role of UEV-1, an inactive variant of the E2 ubiquitin-conjugating enzymes, in in vitro differentiation and cell cycle behavior of HT-29-M6 intestinal mucosecretory cells. Mol Cell Biol 18: 576–589, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Selman M, Pardo A, Kaminski N. Idiopathic pulmonary fibrosis: aberrant recapitulation of developmental programs? PLoS Med 5: e62, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shelat HS, Liu TJ, Hickman-Bick DL, Barnhart MK, Vida T, Dillard PM, Willerson JT, Zoldhelyi P. Growth suppression of human coronary vascular smooth muscle cells by gene transfer of the transcription factor E2F-1. Circulation 103: 407–414, 2001 [DOI] [PubMed] [Google Scholar]
- 55.Simeone-Penney MC, Severgnini M, Rozo L, Takahashi S, Cochran BH, Simon AR. PDGF-induced human airway smooth muscle cell proliferation requires STAT3 and the small GTPase Rac1. Am J Physiol Lung Cell Mol Physiol 294: L698–L704, 2008 [DOI] [PubMed] [Google Scholar]
- 56.Simonneau G, Galie N, Rubin LJ, Langleben D, Seeger W, Domenighetti G, Gibbs S, Lebrec D, Speich R, Beghetti M, Rich S, Fishman A. Clinical classification of pulmonary hypertension. J Am Coll Cardiol 43: 5S–12S, 2004 [DOI] [PubMed] [Google Scholar]
- 57.Sonenshein GE. Down-modulation of c-myc expression induces apoptosis of B lymphocyte models of tolerance via clonal deletion. J Immunol 158: 1994–1997, 1997 [PubMed] [Google Scholar]
- 58.Sullivan CC, Du L, Chu D, Cho AJ, Kido M, Wolf PL, Jamieson SW, Thistlethwaite PA. Induction of pulmonary hypertension by an angiopoietin 1/TIE2/serotonin pathway. Proc Natl Acad Sci U S A 100: 12331–12336, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Syed NA, Andersen PL, Warrington RC, Xiao W. Uev1A, a ubiquitin conjugating enzyme variant, inhibits stress-induced apoptosis through NF-kappaB activation. Apoptosis 11: 2147–2157, 2006 [DOI] [PubMed] [Google Scholar]
- 60.Taketo MM, Sonoshita M. Phospolipase A2 and apoptosis. Biochim Biophys Acta 1585: 72–76, 2002 [DOI] [PubMed] [Google Scholar]
- 61.Tschentscher F, Husing J, Holter T, Kruse E, Dresen IG, Jockel KH, Anastassiou G, Schilling H, Bornfeld N, Horsthemke B, Lohmann DR, Zeschnigk M. Tumor classification based on gene expression profiling shows that uveal melanomas with and without monosomy 3 represent two distinct entities. Cancer Res 63: 2578–2584, 2003 [PubMed] [Google Scholar]
- 62.Tuder RM, Chacon M, Alger L, Wang J, Taraseviciene-Stewart L, Kasahara Y, Cool CD, Bishop AE, Geraci M, Semenza GL, Yacoub M, Polak JM, Voelkel NF. Expression of angiogenesis-related molecules in plexiform lesions in severe pulmonary hypertension: evidence for a process of disordered angiogenesis. J Pathol 195: 367–374, 2001 [DOI] [PubMed] [Google Scholar]
- 63.Tuder RM, Cool C, Jennings C, Voelkel NF. Pulmonary vascular involvement in interstitial lung disease (ILD). In: Interstitial Lung Disease, edited by Schwarz MI, King T. Ontario: Decker, 1998, p. 251–264 [Google Scholar]
- 64.Tuder RM, Cool CD, Geraci MW, Wang J, Abman SH, Wright L, Badesch D, Voelkel NF. Prostacyclin synthase expression is decreased in lungs from patients with severe pulmonary hypertension. Am J Respir Crit Care Med 159: 1925–1932, 1999 [DOI] [PubMed] [Google Scholar]
- 65.Tuder RM, Marecki JC, Richter A, Fijalkowska I, Flores S. Pathology of pulmonary hypertension. Clin Chest Med 28: 23–42, vii, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A 98: 5116–5121, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Veyssier-Belot C, Cacoub P. Role of endothelial and smooth muscle cells in the physiopathology and treatment management of pulmonary hypertension. Cardiovasc Res 44: 274–282, 1999 [DOI] [PubMed] [Google Scholar]
- 68.Wei MH, Karavanova I, Ivanov SV, Popescu NC, Keck CL, Pack S, Eisen JA, Lerman MI. In silico-initiated cloning and molecular characterization of a novel human member of the L1 gene family of neural cell adhesion molecules. Hum Genet 103: 355–364, 1998 [DOI] [PubMed] [Google Scholar]
- 69.Welsh CH, Hassell KL, Badesch DB, Kressin DC, Marlar RA. Coagulation and fibrinolytic profiles in patients with severe pulmonary hypertension. Chest 110: 710–717, 1996 [DOI] [PubMed] [Google Scholar]
- 70.Wittmann T, Waterman-Storer CM. Cell motility: can Rho GTPases and microtubules point the way? J Cell Sci 114: 3795–3803, 2001 [DOI] [PubMed] [Google Scholar]
- 71.Wojciak-Stothard B, Leiper J. Rho GTPases and hypoxia in pulmonary vascular endothelial cells. Methods Enzymol 439: 267–283, 2008 [DOI] [PubMed] [Google Scholar]
- 72.Wu W, Dave N, Tseng GC, Richards T, Xing EP, Kaminski N. Comparison of normalization methods for CodeLink Bioarray data. BMC Bioinformatics 6: 309, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu W, Dave NB, Yu G, Strollo PJ, Kovkarova-Naumovski E, Ryter SW, Reeves SR, Dayyat E, Wang Y, Choi AM, Gozal D, Kaminski N. Network analysis of temporal effects of intermittent and sustained hypoxia on rat lungs. Physiol Genomics 36: 24–34, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xu W, Kaneko FT, Zheng S, Comhair SA, Janocha AJ, Goggans T, Thunnissen FB, Farver C, Hazen SL, Jennings C, Dweik RA, Arroliga AC, Erzurum SC. Increased arginase II and decreased NO synthesis in endothelial cells of patients with pulmonary arterial hypertension. FASEB J 18: 1746–1748, 2004 [DOI] [PubMed] [Google Scholar]
- 75.Yeager ME, Halley GR, Golpon HA, Voelkel NF, Tuder RM. Microsatellite instability of endothelial cell growth and apoptosis genes within plexiform lesions in primary pulmonary hypertension. Circ Res 88: E2–E11, 2001 [DOI] [PubMed] [Google Scholar]
- 76.Yu PB, Deng DY, Beppu H, Hong CC, Lai C, Hoyng SA, Kawai N, Bloch KD. Bone morphogenetic protein (BMP) type II receptor is required for BMP-mediated growth arrest and differentiation in pulmonary artery smooth muscle cells. J Biol Chem 283: 3877–3888, 2008 [DOI] [PubMed] [Google Scholar]
- 77.Yu Y, Fantozzi I, Remillard CV, Landsberg JW, Kunichika N, Platoshyn O, Tigno DD, Thistlethwaite PA, Rubin LJ, Yuan JX. Enhanced expression of transient receptor potential channels in idiopathic pulmonary arterial hypertension. Proc Natl Acad Sci U S A 101: 13861–13866, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zakrzewicz A, Kouri FM, Nejman B, Kwapiszewska G, Hecker M, Sandu R, Dony E, Seeger W, Schermuly RT, Eickelberg O, Morty RE. The transforming growth factor-beta/Smad2,3 signalling axis is impaired in experimental pulmonary hypertension. Eur Respir J 29: 1094–1104, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.