Abstract
Neutrophils are important in the host response against invading pathogens. One chemical defense mechanism employed by neutrophils involves the production of myeloperoxidase (MPO)-derived HOCl. 2-Chlorohexadecanal (2-ClHDA) is a naturally occurring lipid product of HOCl targeting the vinyl ether bond of plasmalogens. Previous studies have shown that exogenously-added 2-ClHDA is oxidized to 2-chlorohexadecanoic acid (2-ClHA) and reduced to 2-chlorohexadecanol (2-ClHOH) by endothelial cells. These studies show that both 2-ClHA and 2-ClHOH are produced in activated neutrophils in an MPO- and time-dependent manner and are released by neutrophils into media. 2-ClHDA levels peak following 30 min of phorbol 12-myristate-13-acetate stimulation. In contrast, 2-ClHA and 2-ClHOH levels steadily increased over 60 min, suggesting a precursor-product relationship between 2-ClHDA and both 2-ClHA and 2-ClHOH. Additional experiments using wild-type CHO.K1 and CHO.K1 cells deficient in fatty aldehyde dehydrogenase (FALDH), FAA.K1A, demonstrated that 2-ClHDA oxidation to 2-ClHA is dependent on FALDH activity. Furthermore, mice exposed to intranasal Sendai virus displayed lung neutrophil recruitment, as well as elevated 2-ClHA levels in plasma and bronchoalveolar lavage compared with control-treated mice. Taken together, these data demonstrate, for the first time, that metabolites of 2-ClHDA are produced both in vivo as well as in isolated human neutrophils.
Keywords: fatty aldehydes, plasmalogens, myeloperoxidase, reactive chlorinating species, hypochlorous acid, inflammation
Neutrophils are the most abundant of the innate immune system's cellular arsenal that combats invading pathogens (1–3). Upon activation, neutrophils undergo a respiratory burst leading to the production and release of hydrogen peroxide (H2O2) and concomitant release of myeloperoxidase (MPO) from the azurophilic granules. MPO amplifies the oxidant response of neutrophils by converting H2O2 to hypochlorous acid (4, 5). Hypochlorous acid is in equilibrium with chlorine gas (4). Collectively, hypochlorous acid, its conjugate anion, hypochlorite, and chlorine gas comprise the reactive chlorinating species (RCS) produced by activated neutrophils. In addition to targeting invading organisms, these RCS also attack host macromolecules, including proteins, nucleic acids, and lipids (1, 6–13). RCS attack of the vinyl ether bond of plasmalogens results in the release of α-chlorofatty aldehyde and unsaturated lysophosphatidylcholine production (14). One of the α-chlorofatty aldehyde molecular species, 2-chlorohexadecanal (2-ClHDA), is produced by activated neutrophils and monocytes and has been shown to accumulate in infarcted myocardium (8) and human atheromas (15).
Radiolabeled 2-ClHDA, 2-Cl-[3H]HDA, has been shown to be readily oxidized to 2-chlorohexadecanoic acid (2-ClHA) and reduced to 2-chlorohexadecanol (2-ClHOH) by endothelial cells (16). Both radiolabeled 2-ClHA and 2-ClHOH were released from endothelial cells. Assuming that α-chloro compounds are used by the fatty acid-fatty alcohol cycle, it seems likely that 2-ClHDA is an intermediate between 2-ClHA and 2-ClHOH in the fatty alcohol cycle (17). Thus, exogenously applied 2-ClHDA is metabolized by endothelial cells, which suggests that similar metabolic pathways are present for endogenously produced 2-ClHDA derived from RCS targeting of plasmalogens.
Because neutrophil derived 2-ClHDA levels peak within the first 30 min of stimulation by phorbol ester (18), it is likely that decreasing levels of 2-ClHDA following 30 min of phorbol ester stimulation is due to reduced 2-ClHDA production under conditions that 2-ClHDA is metabolized. Accordingly, it is predicted that stable 2-ClHDA metabolites may be detected in vivo under proinflammatory conditions. In the present study, both 2-ClHA and 2-ClHOH are shown to accumulate in activated human neutrophils following peak accumulation of 2-ClHDA. Furthermore, 2-ClHA is found in both the plasma and bronchoalveolar lavage of mice infected intranasally with Sendai virus (SeV). Additional studies support a role for the enzymatic pathways of the fatty alcohol cycle for the production of these additional members of a chlorinated lipidome that is produced by the initial targeting of plasmalogens by RCS.
MATERIALS AND METHODS
Synthesis and purification of 2-Cl-[d4]HDA, 2-Cl-[d4]HOH, 2-ClHOH, 2-Cl-[d4]HA, and 2-ClHA
2-Cl-[d4]HDA was synthesized and purified as previously described (18). 2-Cl-[d4]HOH, 2-ClHOH, and 2-Cl-[d4]HA were synthesized and purified as previously described (16).
2-ClHDA metabolism by long-chain fatty aldehyde dehydrogenase deficient cells
Wild-type Chinese hamster ovary (CHO.K1) cells and an isolated mutant cell line (FAA.K1A) having defective long-chain fatty aldehyde dehydrogenase (FALDH) activity (FAO; EC 1.1.1.192), specifically FALDH activity, were incubated at 37°C in 5% CO2/95% air and cultured in Ham's F-12 medium with 10% fetal bovine serum (2% when treating cells) and supplemented with 1 mM glutamine (19, 20). Both CHO.K1 and FAA.K1A cells were treated with 1 μM 2-ClHDA in DMSO for 30, 60, 120, or 240 min. At the end of the incubations, media were removed and centrifuged to remove any cells and cellular debris. Cells were scraped in methanol-saline (1:1). Media and cells were extracted by the method of Bligh and Dyer (21) in the presence of 2-Cl-[d4]HOH or 2-Cl-[d4]HA. Samples used to quantify 2-ClHOH were derivatized with 2,3,4,5,6-pentafluorobenzoyl (PFB) chloride, forming PFB esters, which were analyzed by GC-MS using selected ion monitoring (SIM) as previously described (16). Lastly, samples containing 2-ClHA were analyzed by LC-ESI/MS as previously described (16), except that a Supelco Discovery® HS 18 column was employed.
Neutrophil activation and chemotaxis
As previously described (18), whole blood (50 ml) was taken from healthy volunteers and anticoagulated with EDTA (final concentration 5.4 mM) prior to the isolation of neutrophils using a Ficoll-Hypaque gradient as previously described (22). These studies were approved and authorized by the Saint Louis University Institutional Review Board Protocol 9952. Informed consent was obtained from the human subjects. Pelleted neutrophils were resuspended in HBSS (pH 7.3) and immediately subjected to experimental protocols. In indicated experiments, neutrophils were resuspended in HBSS supplemented with 3-aminotriazole (ATZ; 10 mM). Neutrophils (0.5 × 106 cells/ml) were stimulated with phorbol 12-myristate-13-acetate (PMA; 200 nM) for indicated times at 37°C before terminating the reactions with the addition of methanol containing the appropriate deuterated internal standard. To quantitate 2-ClHA and 2-ClHOH released into the HBSS versus that which was associated with the neutrophils, cells were pelleted at the end of the reaction and the supernatant drawn off. Cells were resuspended in 2 ml HBSS and methanol containing either 2-Cl-[d4]HOH, 2-Cl-[d4]HA, or 2-Cl-[d4]HDA was added to both the supernatant containing released chlorinated lipids and the resuspended cell pellet. Following a modified Bligh and Dyer extraction of the lipids (21), samples were prepared for quantification. Samples used to quantify 2-ClHDA were derivatized with PFB hydroxylamine to form PFB oximes, which were analyzed by GC-MS using SIM as previously described (18). Samples used to quantitate 2-ClHOH and 2-ClHA were analyzed as described above.
Neutrophil chemotaxis was assessed as previously described (23). Selected concentrations of formyl-Met-Leu-Phe (fMLP), 2-ClHA, and 2-ClHOH in DMSO or DMSO alone (negative control) were diluted in chemotaxis buffer comprised of HBSS (pH 7.3), 1% BSA (w/v), and 10 mM HEPES and loaded into the lower wells of a Boyden chemotaxis chamber. A 3 μm pore-size cellulose nitrate filter was placed over the bottom compartments of the wells. Neutrophils were resuspended in modified HBSS at a concentration of 4 × 106 neutrophils/ml so that the top compartment of each well contained 2 × 105 neutrophils (50 μl). The Boyden chamber was incubated for 35 min at 37°C followed by staining and dehydrating the filters. Chemotaxis was assessed by the leading front method (23). Net neutrophil migration was reported in terms of micrometers.
Endogenous production of 2-ClHA in bronchoalveolar lavage fluid and plasma from SeV-infected mice
All animal procedures were conducted in accordance with guidelines published in the Guide for the Care and Use of Laboratory Animals (National Research Council, National Academy Press, Washington, DC, 1996) and were approved by the Animal Care Committee of Saint Louis University. Mice were infected with parainfluenza 1 virus strain Sendai/52 (SeV), which causes a neutrophilic bronchiolitis (24). Adult C57BL/6 mice 8–12 weeks of age were anesthetized by intraperitoneal injection of ketamine HCl (86.98 mg/kg) plus xylazine HCl (13.04 mg/kg). SeV was administered at a dose of 500 pfu/g body weight via intranasal inoculation. The virus was administered in a volume of 30 μl sterile PBS; control mice received 30 μl of sterile PBS only. Bronchoalveolar lavage was performed via tracheal cannulation with 1.0 ml of sterile saline. Cells were removed from the lavage by centrifugation, leaving the bronchoalveolar lavage fluid (BALF). Plasma was prepared from EDTA anticoagulated fresh blood taken from cardiac puncture. Both BALF and plasma were collected at the end of experimental intervals and stored at −80°C until extracted with an appropriate amount of 2-Cl-[d4]HA. Lipid extracts derived from plasma samples were derivatized with 2,3,4,5,6-pentafluorobenzyl bromide (PFB Br). PFB Br and N’,N’-diisopropylethylamine were dissolved in acetonitrile to yield a 10% and 5% solution, respectively. PFB Br and N’,N’-diisopropylethylamine were added to the dried lipid extracts and reacted for 30 min at 45°C to produce the pentafluorobenzyl ester of 2-ClHA. The samples are then dried under nitrogen and resuspended in hexanes for analysis by negative ion chemical ionization (NICI)-GC-MS using SIM for m/z 289 and m/z 293, which correspond to the natural and deuterated standard.
Total lung RNA was isolated using the RNeasy® Mini Kit (Qiagen). For these studies, lungs were snap frozen and pulverized at the temperature of liquid nitrogen in preparation for the isolation of RNA. cDNA was synthesized using the iScript™ cDNA Synthesis Kit (Bio-Rad) followed by real-time PCR using iQ™ SYBR® Green Supermix to amplify SeV-specific RNA (accession number X58886), forward primer nt 3909-3928, reverse primer nt 4049-4068; and published mouse 36B4 primers (25). Products of real-time PCR were run out on a 1% agarose gel to confirm product size of the amplicon and to quantify levels of SeV amplicon normalized to 36B4 using ImageJ software.
Lung MPO activity was measured from whole lung tissue homogenates as previously described (26) and normalized to tissue dry weight. One unit of MPO activity was defined as that degrading 1 μmol H2O2 in 1 min at 25°C.
Statistical analysis
Statistical analysis was performed between multiple groups using ANOVA. Experiments containing only two groups were subjected to a two-tailed Student's t-test.
RESULTS
Endogenous production of 2-ClHA and 2-ClHOH in phorbol ester-activated human neutrophils
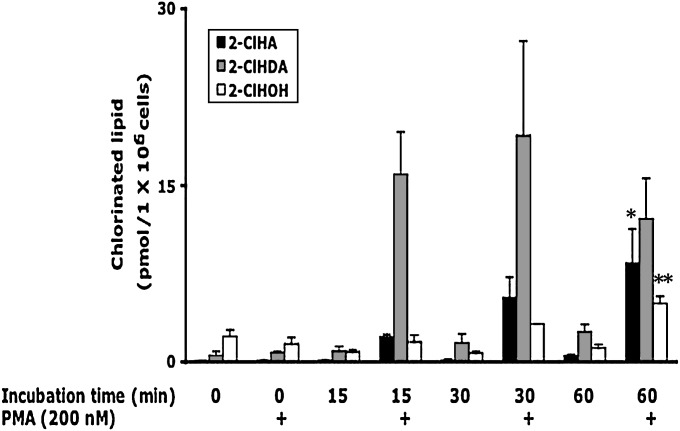
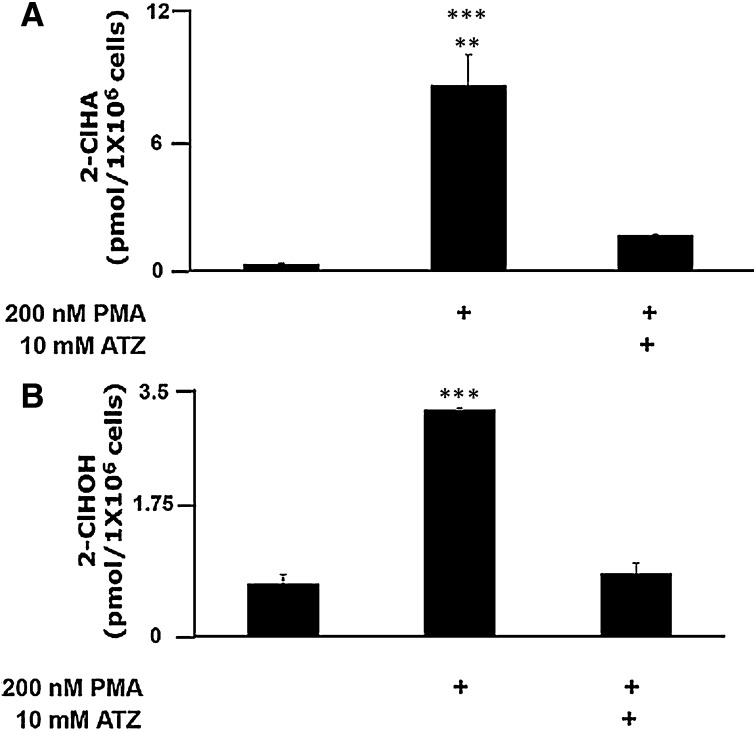
Because PMA-activated neutrophils have previously been shown to produce 2-ClHDA (18) prior to the finding that exogenously added 2-ClHDA is metabolized to 2-ClHA and 2-ClHOH (16), initial studies were devised to delineate the time course of the production of 2-ClHDA in comparison to its metabolites in activated human neutrophils. Data in Fig. 1 show that maximal 2-ClHDA accumulation occurs following 15–30 min of PMA stimulation. In contrast, a steady increase in 2-ClHA and 2-ClHOH is observed between 15–60 min of PMA stimulation. All three chlorinated species accumulated in human neutrophils only under conditions of PMA stimulation. To demonstrate MPO dependence for 2-ClHA and 2-ClHOH in activated neutrophils, cells were treated with PMA (200 nM) or were pretreated with the MPO inhibitor ATZ (10 mM) before addition of PMA (200 nM). The level of 2-ClHA (Fig. 2A) and 2-ClHOH (Fig. 2B) in PMA-stimulated cells in the presence of ATZ is significantly inhibited in comparison to cells incubated with PMA only. It should be noted that previous studies have shown that 2-ClHDA accumulation in PMA-stimulated neutrophils is similarly inhibited by ATZ (18).
Fig. 1.
Temporal course of 2-ClHDA, 2-ClHOH, and 2-ClHA production in PMA-stimulated human neutrophils. Isolated human neutrophils (1 × 106) were incubated for indicated times with or without 200 nM PMA. At the end of incubations, lipids were extracted by a modified Bligh and Dyer technique (21), and 2-ClHDA and 2-ClHOH were converted to their PFB oxime and PFB ester, respectively, followed by GC-MS quantification employing NICI and SIM as described in Experimental Procedures. 2-ClHA was quantified by LC-MS employing SRM in the negative ion mode as described in Experimental Procedures. Error bars represent SEM, n = 3. *P < 0.05 versus 2-ClHA time 0, **P < 0.01 versus 2-ClHOH time 0 and 15 min.
Fig. 2.
MPO-dependent production of 2-ClHA and 2-ClHOH in isolated neutrophils. Isolated human neutrophils (1 × 106) were either pretreated with 10 mM ATZ or vehicle (ethanol, < 0.1%) alone for 5 min preceding a 30 min incubation with or without 200 nM PMA. At the end of incubations, lipids were extracted by a modified Bligh and Dyer technique (21), and 2-ClHA was quantified by LC-MS employing SRM in the negative ion mode (A), and 2-ClHOH was converted to its PFB ester and quantified by GC-MS employing NICI and SIM (B) as described in Experimental Procedures. Error bars represent SEM, n = 3. A: **P < 0.01 versus +PMA +ATZ, ***P < 0.001 versus negative control. B: ***P < 0.001 versus negative control and + PMA + ATZ.
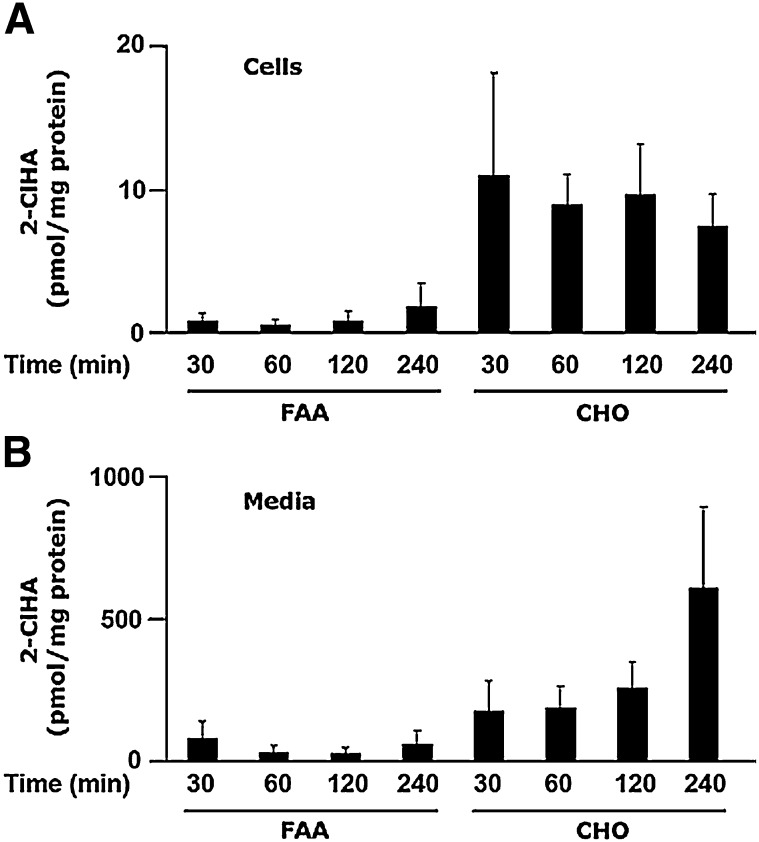
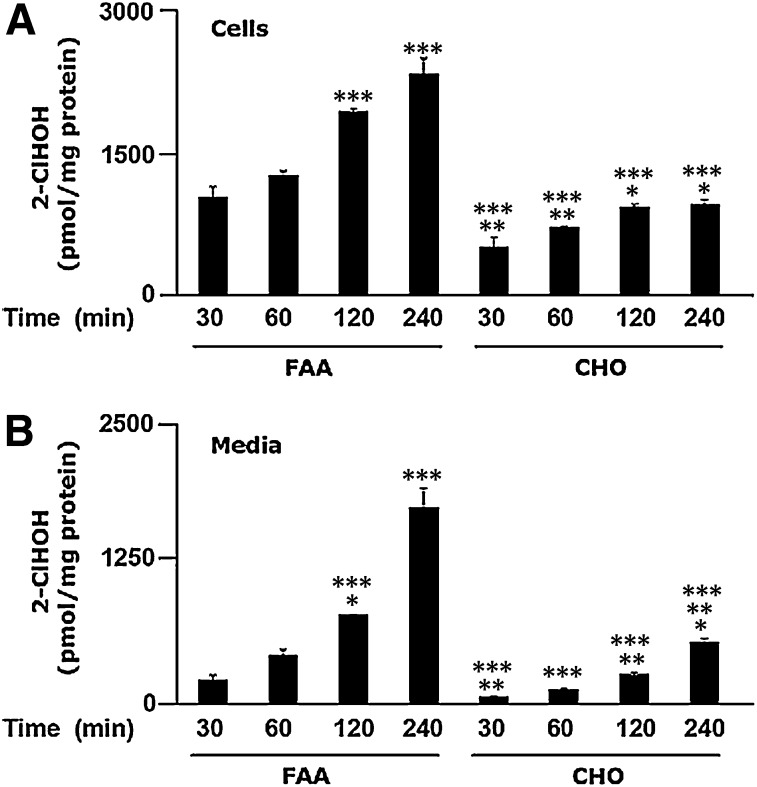
MPO activation mediates the production of 2-ClHDA; however, the mechanism responsible for 2-ClHDA metabolism to 2-ClHA and 2-ClHOH is not clear. Interconversions of fatty alcohol, fatty aldehyde, and fatty acid are dependent on enzymes involved in the fatty alcohol cycle (17). To determine the mechanism of 2-ClHDA oxidation, both CHO.K1 and FAA.K1A (a cell line defective in long-chain FALDH activity) were incubated in the presence of 2-ClHDA (1 μM). Metabolism of 2-ClHDA to 2-ClHA is much greater in the control CHO.K1 cells than in the FALDH-deficient FAA.K1A cells (Fig. 3A). Additionally, this disparate metabolism is also reflected by the large increases in 2-ClHA released into the media of CHO.K1 cells compared to FAA.K1A cells (Fig. 3B). In contrast, 2-ClFOH levels are increased in both the cells and the cell culture media in FAA.K1A cells that are treated with 1 μM 2-ClHDA, which contrasts with that observed in the CHO.K1 cells (Figs. 4A, B). Notably, cellular 2-ClHOH levels are 300-fold greater than cellular levels of 2-ClHA in the FAA.K1A cells. Levels of 2-ClHOH released into the media are approximately 3-fold greater than released 2-ClHA. A trend of increasing concentration of cellular and media 2-ClHOH with increasing incubations exists. Thus, as hypothesized, cells deficient in FALDH activity have greater levels of 2-ClHOH than the control CHO.K1 cells. It should also be noted that media lactate dehydrogenase levels were not increased with 2-ClHDA treatments with both FAA.K1A and CHO.K1 cells (data not shown). These results with the CHO.K1 and FAA.K1A cells support the role of FALDH in the metabolism of 2-ClHDA.
Fig. 3.
2-ClHDA metabolism to 2-ClHA is dependent on long-chain fatty alcohol:NAD+ oxidoreductase. CHO.K1 and FAA.K1A (deficient in long-chain fatty alcohol:NAD+ oxidoreductase) cell lines were treated with 2-ClHDA (1 μM) for indicated time intervals. At the end of each time point, cellular (A) and media (B) lipids were extracted in the presence of 2-Cl-[d4]HA as an internal standard and processed for analyses as described in Experimental Procedures. Error bars represent SEM, n = 3.
Fig. 4.
Long-chain fatty alcohol:NAD+ oxidoreductase defective cells produce more 2-ClHOH from 2-ClHDA than control cells. CHO.K1 (CHO) and FAA.K1A (FAA) cell lines were treated with 2-ClHDA (1 μM) between 30 min and 4 h. At the end of each time point, cellular (A) and media (B) lipids were extracted in the presence of 2-Cl-[d4]HOH as an internal standard and processed for analyses as described in Experimental Procedures. Error bars represent SEM, n = 3. Cells: *P < 0.05 versus CHO at time 30 min, **P < 0.01 (CHO at time 30 and 60 min) versus FAA at time 30 and 60 min, respectively, ***P < 0.001 (FAA at time 120 and 240 min vs. FAA at time 30 and 60 min, CHO at time 30 min vs. FAA at time 60 min, CHO at 30 to 240 min vs. FAA at 120 and 240 min). Media: *P < 0.05 (FAA at 120 min and CHO at 30 min vs. FAA 60 min, CHO at 240 min vs. FAA at 30 min), **P < 0.01 (CHO at 120 min vs. FAA at 120 min and CHO at 240 min vs. CHO at 30 and 60 min), ***P < 0.001 (FAA at 120 and 240 min vs. FAA at 30 min, FAA at 240 min vs. FAA at 60 min, FAA at 240 min and CHO at 30 and 60 min vs. FAA at 120 min, CHO at 30 to 240 min vs. FAA at 240 min).
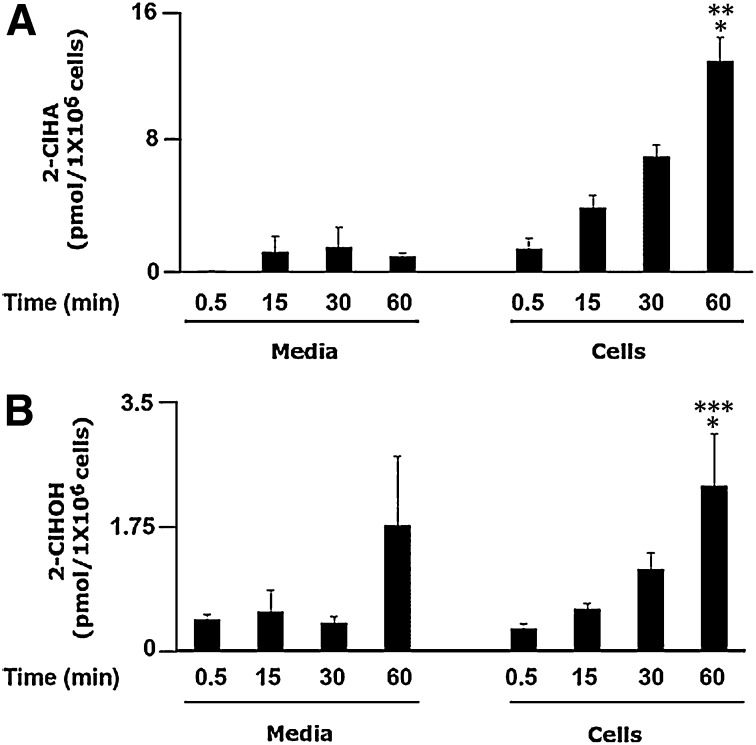
Based on these data showing differences in cell-associated 2-ClHA and 2-ClHOH in comparison to the levels in the cell culture media, additional studies were performed on activated neutrophils to delineate the relative amounts of chlorinated lipid species associated with the activated neutrophil compared with that released from the neutrophils. The levels of 2-ClHA released into the media under these conditions were less than those which were cell associated (approximately 7-fold difference between cells and media after 60 min of PMA stimulation); however, the levels of 2-ClHOH released into the media were similar to the cell-associated levels (Fig. 5A, B). In contrast to the CHO.K1 and FAA.K1A cells, activated neutrophils preferentially accumulate greater levels of 2-ClHA (Fig. 5A) than 2-ClHOH (Fig. 5B). Furthermore, neutrophils release a smaller proportion of endogenously produced 2-ClHA in comparison to that released by CHO.K1 cells as a result of the metabolism of exogenously added 2-ClHDA. This may demonstrate differences between cell types and/or endogenous production of 2-ClHDA versus treating cells with supraphysiological concentrations of 2-ClHDA.
Fig. 5.
Release of 2-ClHA and 2-ClHOH into media from PMA-stimulated isolated human neutrophils. Isolated human neutrophils (1 × 106) were incubated for indicated times with or without 200 nM PMA. At the end of each time point, cells were pelleted as described in Experimental Procedures. Both cells and media were extracted by a modified method of Bligh and Dyer (25) in the presence of deuterated internal standards before being subjected to quantification of 2-ClHA by LC-MS employing SRM in the negative ion mode (A) or converted to the PFB ester and subjected to quantitation of 2-ClHOH by GC-MS employing NICI and SIM (B) as described in Experimental Procedures. Error bars represent standard error, n = 3. A: *P < 0.05 versus cell associated at 15 min, **P < 0.01 versus cell associated at 0.5 min. B: **P < 0.01 versus cell associated at 30 min, ***P < 0.001 versus cell associated at 0.5 and 15 min.
Biological effect of 2-ClHA and 2-ClHOH on neutrophil function
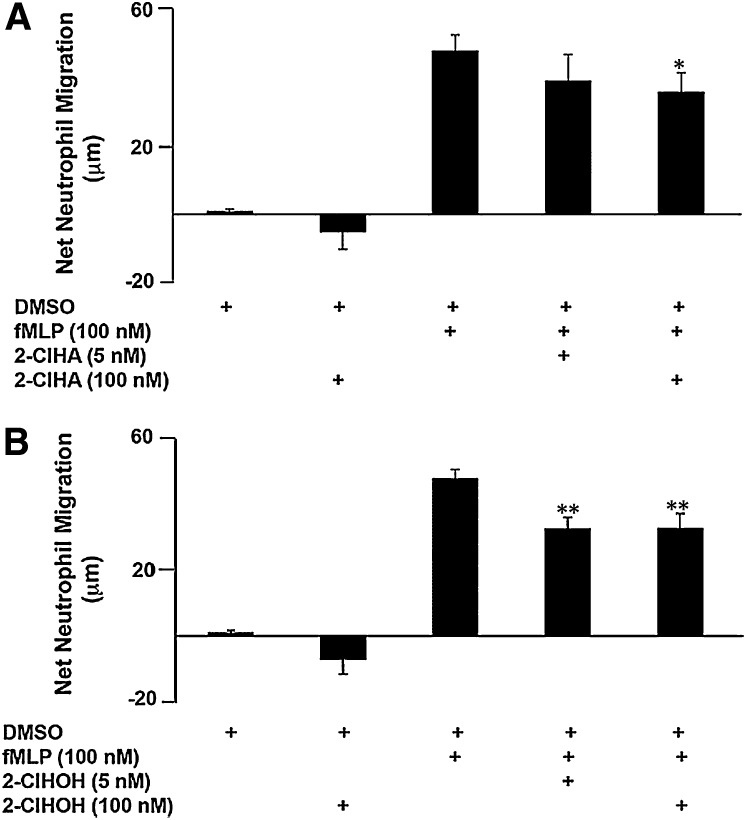
The presence of 2-ClHA and 2-ClHOH in the media of PMA-stimulated neutrophils raises the question of whether these metabolites act as chemotactic agents for leukocytes, including other neutrophils. It has been demonstrated that fatty aldehydes derived from lipid peroxidation (27, 28), including 2-ClHDA, are neutrophil chemoattractants (18). This study found that low to high nanomolar concentrations of 2-ClHA and 2-ClHOH are not chemoattractants (Fig. 6). Furthermore, when added to fMLP, a known neutrophil chemoattractant, 2-ClHA decreased net neutrophil migration (Fig. 6A) similar to 2-ClHOH (Fig. 6B).
Fig. 6.
2-ClHDA metabolites, 2-ClHA and 2-ClHOH, do not induce neutrophil chemotaxis. The lower wells of a multiwell Boyden chamber contained either chemotaxis buffer alone, chemotaxis buffer with DMSO, 100 nM fMLP (positive control), 100 nM 2-ClHA or 2-ClHOH, or a combination of 100 nM fMLP with 10 or 100 nM 2-ClHA or 2-ClHOH. 2 × 105 neutrophils were loaded into each upper well. Error bars represent SEM, n = 3. A: *P < 0.05 or (B) **P < 0.01 versus 100 nM fMLP.
Production of 2-ClHA in an animal model of a lower respiratory tract infection
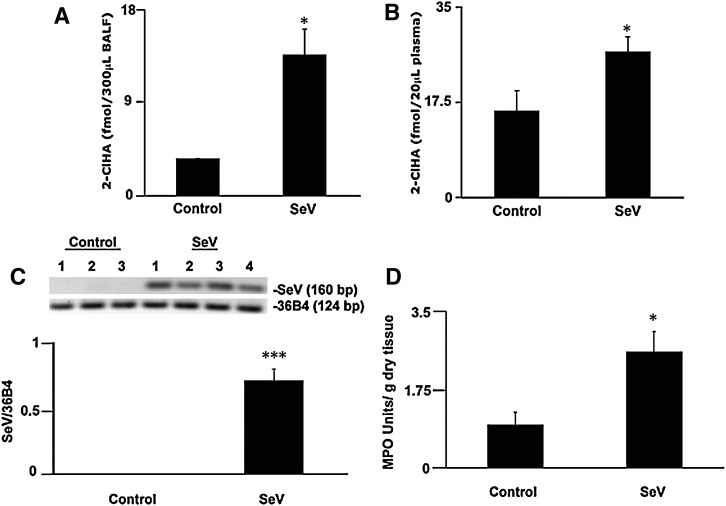
To determine if chlorinated lipid species are produced in vivo, we utilized a mouse model of respiratory viral infection. SeV is a natural mouse pathogen that causes a neutrophilic bronchiolitis. During SeV infection, neutrophil infiltration in the lung peaks on day 3 postinfection, prior to the peak of both macrophage and lymphocyte influx (24). Therefore, experiments were terminated on day 3 when both BALF and serum were collected. Neutrophil activation in the lungs on day 3 was confirmed by increased MPO activity in the lungs of infected mice compared with controls (Fig. 7D). Real-time PCR for SeV transcript was used to confirm the presence of virus in the lungs of infected mice; no viral transcript was detected in the uninfected controls (Fig. 7C). BALF from infected mice contained approximately 3-fold greater quantities of 2-ClHA than control uninfected mice (Fig. 7A). In addition to the localized 2-ClHA in the lungs, systemic 2-ClHA levels were increased. Plasma from infected mice had a greater abundance of 2-ClHA than in control counterparts (Fig. 7B). Neither 2-ClHDA nor 2-ClHOH were detected in BALF or in plasma from control and SeV-infected mice. Taken together, this provides evidence that 2-ClHA can be measured systemically, in addition to specific sites of inflammation, and these data suggest that 2-ClHA is a stable metabolite produced as a result of RCS attack of plasmalogens in vivo.
Fig. 7.
Plasma and BALF levels of 2-ClHA increase in mice exposed to SeV. Adult C57BL/6 mice were infected with SeV (14,000 pfu) intranasally; controls received sterile PBS. 2-ClHA was converted to its PFB ester and quantified by GC-MS employing NICI and SIM. Error bars represent SEM; control mouse BALF (n = 3), virus infected mouse BALF (n = 4) (A), control mouse plasma (n = 4), and virus infected mouse plasma (n = 7) (B). Total RNA was isolated from lungs of control (n = 3) and SeV-infected (n = 4) mice, and the level of SeV-specific RNA was quantified by densitometry of real-time PCR products run on agarose gel. Data were normalized to 36B4 mRNA levels (C). The presence of inflammatory cells in the lungs of control and SeV-infected mice was examined using an MPO activity assay (D). *P < 0.05 (A,B,D) and ***P < 0.005.
DISCUSSION
Although radiolabeled and stable isotope labeled 2-ClHDA has been shown to be metabolized to 2-ClHA and 2-ClHOH in isolated human neutrophils and in human coronary artery endothelial cells (16), their endogenous biosynthesis had not been elucidated previously in human neutrophils as well as in vivo systems. The present study demonstrates for the first time that both 2-ClHA and 2-ClHOH are endogenously produced in activated human neutrophils. The accumulation of these chlorinated lipid species is both MPO and time dependent. The temporal course of the accumulation of 2-ClHDA, 2-ClHA, and 2-ClHOH in conjunction with the previous studies with exogenously added 2-ClHDA suggests that these chlorinated lipid species are derived from RCS attack of plasmalogens leading to the release of 2-ClHDA that is subsequently metabolized to 2-ClHA and 2-ClHOH. 2-ClHA and 2-ClHOH levels are less than that of 2-ClHDA in phorbol ester-stimulated neutrophils (e.g., results shown in Fig. 1), and 2-ClHA and 2-ClHOH concentrations steadily increased over the time intervals examined in this study. In the present study, the metabolism of endogenously produced 2-ClHDA was predominantly directed toward 2-ClHA production. Also, previous studies showed that some radiolabeled 2-ClHDA is incorporated into complex lipid pools, including triglycerides and cholesterol esters (16), which suggests that 2-ClHA may be incorporated into complex lipids following neutrophil activation. Endogenous complex lipids containing chlorinated aliphatic residues remain to be demonstrated. However, complex lipid pools containing esterified 2-ClHA, if present, may represent a releasable storage depot for chlorinated fatty acids.
It is interesting that the present results show that chlorinated lipids remain predominantly cell associated when produced endogenously in neutrophils but are released into the media when provided exogenously to CHO.K1 and FAA.K1A cells. This difference may be due to disparate metabolism and export in these cells or, alternatively, differences in the metabolism of exogenously applied 2-ClHDA compared with that produced endogenously. Also, our previous studies using radiolabeled 2-ClHDA showed that 2-ClHDA is preferentially reduced to 2-ClHOH compared with 2-ClHA in both resting and PMA-stimulated neutrophils (16). It should be appreciated that endogenous production of 2-ClHDA likely occurs in membrane domains containing plasmalogens that are targeted by RCS. Membrane-associated 2-ClHDA may not be accessible to the same enzymic machinery that metabolizes exogenously applied 2-ClHDA. For example, it is possible that exogenously added 2-ClHDA may be targeted to, and metabolized in, the lysosome.
Patients affected by disorders of fatty alcohol metabolism such as Sjogren-Larson syndrome (SLS) or the autosomal recessive form of rhizomelic chondrodysplasia punctata have increased cellular fatty alcohol accumulation due to the inability to either oxidize the fatty alcohol or to incorporate the alcohol in ether-linked lipids such as plasmalogens, respectively (29–31). The metabolism of 2-ClHDA to 2-ClHA and 2-ClHOH was analyzed using a FALDH-defective cell line. It was determined that less 2-ClHA was produced and released from the FAA.K1A cell line in comparison to the control CHO.K1 cell line. In addition to cellular fatty alcohol accumulation, fatty alcohol is present in the plasma of SLS and autosomal recessive form of rhizomelic chondrodysplasia punctata patients and can act as a biomarker of enzymatic defects (29–31). Likewise, 2-ClHDA metabolism to 2-ClHOH was also significantly increased in the FAA.K1A cells in comparison to the control CHO.K1 cell line. Taken together, these data suggest that the oxidation/reduction of 2-ClHDA is mediated by enzymes of the fatty alcohol cycle (17). This is supported by the evidence that 2-ClHDA does not become reduced/oxidized without the presence of cells (16). Also, both FAA.K1A and CHO.K1 were not damaged by treatments with 1 μM 2-ClHDA. Previous studies using octadecanal have shown that 60 μM was preferentially cytotoxic to FAA.K1A cells (19). It will be interesting in the future to compare these cell lines for the relative cytotoxicity to 2-ClHDA. It should also be considered that SLS patients may have enhanced susceptibility to 2-ClHDA produced at sites of inflammation. We have previously shown that 2-ClHDA can form Schiff-base adducts (32) and the inability to convert 2-ClHDA to 2-ClHA in SLS patients may lead to accelerated injury at sites of inflammation mediated by localized concentrations of 2-ClHDA.
Given that 2-ClHA and 2-ClHOH were released from isolated activated neutrophils, the effect on neutrophil function, specifically neutrophil chemotaxis, of the two metabolites exogenously added was examined. It has been shown that pathophysiologic concentrations of palmitic acid are neutrophil chemorepellents (33) and decrease fMLP-stimulated superoxide release (34). However, it has also been demonstrated that palmitic acid does not negatively affect in vitro neutrophil chemotaxis and reactive oxygenating species production (35). The present results show that 100 nM of 2-ClHA or 2-ClHOH is not a neutrophil chemoattractant. In fact, the net neutrophil migration was similar to that of exposure to the DMSO vehicle alone. In contrast, 2-ClHA and 2-ClHOH decreased neutrophil migration by the neutrophil chemoattractant fMLP. Studies have shown the chemoattractive property of aldehydes such as hydroxynonenal (27, 28, 36, 37) and 2-ClHDA (18). This study suggests that 2-ClHDA metabolites 2-ClHA and 2-ClHOH are not chemoattractants. Thus, these data suggest that infiltration of neutrophils to sites of inflammation is tempered over time by the metabolism of 2-ClHDA to 2-ClHA and 2-ClHOH, which serves as a potential switch to reduce neutrophil chemoattraction.
In addition to demonstrating that activated human neutrophils produce a family of chlorinated lipids (i.e., 2-ClHDA, 2-ClHA, and 2-ClHOH), it was envisioned that in vivo production of these lipids would likely lead to the accumulation of the stable metabolites 2-ClHA and/or 2-ClHOH. Thus, experiments were performed to assess the accumulation of these lipids in a mouse model of respiratory viral infection that results in the recruitment and activation of neutrophils to the lung (24). SeV- specific RNA was present only in the lungs of SeV-infected mice, demonstrating virus replication. Resident macrophages of the lung also contain MPO, as was evident by MPO activity assessed in control-untreated mice. The 2-fold increase in MPO activity seen in SeV-infected mice indicates an infiltration of MPO containing leukocytes to the lungs of infected mice. It has been previously shown that neutrophils infiltrate the lungs of SeV-infected mice. Neutrophils are the predominant cell present in the BALF until day 5, when macrophages and lymphocytes increase in number (24). The involvement of neutrophil activation and MPO-derived products in this model was verified by showing that 2-ClHA accumulated in BALF collected from SeV-infected mice compared with control-treated mice. Moreover, 2-ClHA, which we assume is locally produced at the site of infection in the lung, is also increased in the plasma of these mice. The finding that only 2-ClHA is present suggests that activated neutrophils rapidly convert 2-ClHDA to 2-ClHA and release this lipid. This is supported by our findings using human neutrophils that show that 2-ClHDA levels peak 30 min after activation and then decrease due to metabolism to 2-ClHA and 2-ClHOH. The absence of 2-ClHOH in BALF and plasma suggests that 2-ClHA is the preferential metabolite that is released from neutrophils in comparison to 2-ClHOH. It is assumed that intracellular 2-ClHOH may be oxidized to 2-ClHA. Additionally, it should be appreciated that plasma fatty acid levels are at least 2–3 orders of magnitude greater than that of plasma fatty alcohol levels [e.g., plasma palmitoyl alcohol levels have been shown to be ∼200 nM (38)]. Taken together, these data suggest that plasma levels of the 2-ClHDA oxidation product, 2-ClHA, may serve as a stable biomarker of inflammation.
Taken together, the present study demonstrates a new group of chlorinated lipids that are produced by activated neutrophils. These lipids are also produced in vivo at sites of inflammation as well as increased in the circulation of mice with a respiratory infection. Although these studies focus on the role of neutrophils in the accumulation of these chlorinated lipid species, it is likely that they are also produced by activated monocytes and MPO-containing macrophages. The likelihood that monocytes and MPO-containing macrophages produce these chlorinated lipids is based on previous findings showing that human monocytes produce 2-ClHDA and other 2-chlorofatty aldehydes (39). These lipids may have utility as biomarkers of inflammation and may have important roles as small molecule mediators of the sequelae of inflammatory reactions.
Acknowledgments
The authors acknowledge Dr. Rita Heuertz for helpful discussion of the neutrophil chemotaxis data.
Footnotes
Abbreviations:
- ATZ
- 3-aminotriazole
- BALF
- bronchoalveolar lavage fluid
- 2-ClHA
- 2-chlorohexadecanoic acid
- 2-ClHDA
- 2-Chlorohexadecanal
- 2-ClHOH
- 2-chlorohexadecanol
- FALDH
- fatty aldehyde dehydrogenase
- MPO
- Myeloperoxidase
- NICI
- negative ion chemical ionization
- PFB
- 2,3,4,5,6-pentafluorobenzoyl
- PFB Br
- 2,3,4,5,6-pentafluorobenzyl bromide
- PMA
- phorbol 12-myristate-13-acetate
- RCS
- reactive chlorinating species
- SeV
- Sendai virus
- SIM
- selected ion monitoring
- SLS
- Sjogren-Larson syndrome
This work was supported in part by the National Institutes of Health grants HL088072 and HL074214 (DAF), and Pre-doctoral fellowship grant 0710163Z (DSA) from the American Heart Association. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or other granting agencies.
REFERENCES
- 1.Daugherty A., Dunn J. L., Rateri D. L., Heineke J. W. 1994. Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesion. J. Clin. Invest. 94: 437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klebanoff S. J. 1980. Oxygen metabolism and the toxic properties of phagocytes. Ann. Intern. Med. 93: 480–489. [DOI] [PubMed] [Google Scholar]
- 3.Weiss J., Victor M., Stendhal O., Elsbach P. 1982. Killing of gram-negative bacteria by polymophonuclear leukocytes: role of an O2-independent bacterial system. J. Clin. Invest. 69: 959–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harrison J. E., Schultz J. 1976. Studies on the chlorinating activity of myeloperoxidase. J. Biol. Chem. 251: 1371–1374. [PubMed] [Google Scholar]
- 5.Klebanoff S. J., Waltersdorph A. M., Rosen H. 1984. Antimicrobial activity of myeloperoxidase. Methods Enzymol. 105: 399–403. [DOI] [PubMed] [Google Scholar]
- 6.Albrich J. M., McCarthy C. A., Hurst J. K. 1981. Biological reactivity of hypochlorous acid: implications for microbicidal mechanisms of leukocyte myeloperoxidase. Proc. Natl. Acad. Sci. USA. 78: 210–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiss S. J., Klein R., Slivka A., Wei M. 1982. Chlorination of taurine by human neutrophils: evidence for hypochlorous acid generation. J. Clin. Invest. 70: 598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas E. L., Jefferson M.M., Grisham M.B. 1982. Myeloperoxidase-catalyzed incorporation of amines into proteins: role of hypochlorous acid and dichloramines. Biochemistry. 21: 6299–6308. [DOI] [PubMed] [Google Scholar]
- 9.Winterbourn C. C., van den Berg J. J., Roitman E., Kuypers F. A. 1992. Chlorohydrin formation from unsaturated fatty acids reacted with hypochlorous acid. Arch. Biochem. Biophys. 296: 547–555. [DOI] [PubMed] [Google Scholar]
- 10.Rakita R. M., Michel B. R., Rosen H. 1990. Differential inactivation of escherichia coli membrane dehydrogenases by a myeloperoxidase-mediated antimicrobial system. Biochemistry. 29: 1075–1080. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y., Rosen H., Madtes D. K., Shao B., Martin T. R., Heinecke J. W., Fu X. 2007. Myeloperoxidase inactivates TIMP-1 by oxidizing its N-terminal cysteine residue: an oxidative mechanism for regulating proteolysis during inflammation. J. Biol. Chem. 282: 31826–31834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carlin G., Djursäter R. 1988. Peroxidation of phospholipids promoted by myeloperoxidase. Free Radic. Res. Commun. 4: 251–257. [DOI] [PubMed] [Google Scholar]
- 13.Hazen S. L., Hsu F. F., Gaut J. P., Crowley J. R., Heinecke J. W. 1999. Modification of proteins and lipids by myeloperoxidase. Methods Enzymol. 300: 88–105. [DOI] [PubMed] [Google Scholar]
- 14.Albert C. J., Crowley J. R., Hsu F. F., Thukkani A. K., Ford D. A. 2001. Reactive chlorinating species produced by myeloperoxidase target the vinyl ether bond of plasmalogens: identification of 2-chlorohexadecanal. J. Biol. Chem. 276: 23733–23741. [DOI] [PubMed] [Google Scholar]
- 15.Thukkani A. K., McHowat J., Hsu F. F., Brennan M. L., Hazen S. L., Ford D. A. 2003. Identification of alpha-chloro fatty aldehydes and unsaturated lysophosphatidylcholine molecular species in human atherosclerotic lesions. Circulation. 108: 3128–3133. [DOI] [PubMed] [Google Scholar]
- 16.Wildsmith K. R., Albert C. J., Anbukumar D. S., Ford D. A. 2006. Metabolism of myeloperoxidase-derived 2-chlorohexadecanal. J. Biol. Chem. 281: 16849–16860. [DOI] [PubMed] [Google Scholar]
- 17.Rizzo W. B., Craft D. A., Dammann A. L., Phillips M. W. 1987. Fatty alcohol metabolism in cultured human fibroblasts. Evidence for a fatty alcohol cycle. J. Biol. Chem. 262: 17412–17419. [PubMed] [Google Scholar]
- 18.Thukkani A. K., Hsu F. F., Crowley J. R., Wysolmerski R. B., Albert C. J., Ford D. A. 2002. Reactive chlorinating species produced during neutrophil activation target tissue plasmalogens: production of the chemoattractant, 2-chlorohexadecanal. J. Biol. Chem. 277: 3842–3849. [DOI] [PubMed] [Google Scholar]
- 19.James P. F., Zoeller R. A. 1997. Isolation of animal cell mutants defective in long-chain fatty aldehyde dehydrogenase. Sensitivity to fatty aldehydes and Schiff's base modification of phospholipids: implications for Sjogren-Larsson syndrome. J. Biol. Chem. 272: 23532–23539. [DOI] [PubMed] [Google Scholar]
- 20.James P. F., Rizzo W. B., Lee J., Zoeller R. A. 1990. Isolation and characterization of a chinese hamster ovary cell line deficient in fatty alcohol:NAD+ oxidoreductase activity. Proc. Natl. Acad. Sci. USA. 87: 6102–6106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bligh E. G., Dyer W. J. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37: 911–917. [DOI] [PubMed] [Google Scholar]
- 22.Ferrante A., Thong Y. H. 1980. Simultaneous preparation of mononuclear and polymorphonuclear leucocytes from horse blood on ficoll-hypaque medium. J. Immunol. Methods. 34: 279–285. [DOI] [PubMed] [Google Scholar]
- 23.Zigmond S. H., Hirsch J. G. 1973. Leukocyte locomotion and chemotaxis. New methods for evaluation, and demonstration of a cell-derived chemotactic factor. J. Exp. Med. 137: 387–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shornick L. P., Wells A. G., Zhang Y., Patel A. C., Huang G., Takami K., Sosa M., Shukla N. A., Agapov E., Holtzman M. J. 2008. Airway epithelial versus immune cell stat1 function for innate defense against respiratory viral infection. J. Immunol. 180: 3319–3328. [DOI] [PubMed] [Google Scholar]
- 25.Suganami T., Nishida J., Ogawa Y. 2005. A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: role of free fatty acids and tumor necrosis factor alpha. Arterioscler. Thromb. Vasc. Biol. 25: 2062–2068. [DOI] [PubMed] [Google Scholar]
- 26.Bradley P. P., Priebat D. A., Christensen R. D., Rothstein G. 1982. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J. Invest. Dermatol. 78: 206–209. [DOI] [PubMed] [Google Scholar]
- 27.Curzio M., Esterbauer H., Di Mauro C., Cecchini G., Dianzani M. U. 1986. Chemotactic activity of the lipid peroxidation product 4-hydroxynonenal and homologous hydroxyalkenals. Biol. Chem. Hoppe Seyler. 367: 321–329. [DOI] [PubMed] [Google Scholar]
- 28.Curzio M., Di Mauro C., Esterbauer H., Dianzani M. U. 1987. Chemotactic activity of aldehydes. Structural requirements. Role in inflammatory process. Biomed. Pharmacother. 41: 304–314. [PubMed] [Google Scholar]
- 29.Rizzo W. B., Heinz E., Simon M., Craft D. A. 2000. Microsomal fatty aldehyde dehydrogenase catalyzes the oxidation of aliphatic aldehyde derived from ether glycerolipid catabolism: implications for Sjogren-Larsson syndrome. Biochim. Biophys. Acta. 1535: 1–9. [DOI] [PubMed] [Google Scholar]
- 30.Rizzo W. B. 1993. Sjogren-Larsson syndrome. Semin. Dermatol. 12: 210–218. [PubMed] [Google Scholar]
- 31.Rizzo W. B., Craft D. A. 1991. Sjogren-Larsson syndrome. Deficient activity of the fatty aldehyde dehydrogenase component of fatty alcohol:NAD+ oxidoreductase in cultured fibroblasts. J. Clin. Invest. 88: 1643–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wildsmith K. R., Albert C. J., Hsu F. F., Kao J. L., Ford D. A. 2006. Myeloperoxidase-derived 2-chlorohexadecanal forms schiff bases with primary amines of ethanolamine glycerophospholipids and lysine. Chem. Phys. Lipids. 139: 157–170. [DOI] [PubMed] [Google Scholar]
- 33.Hawley H. P., Gordon G. B. 1976. The effects of long chain free fatty acids on human neutrophil function and structure. Lab. Invest. 34: 216–222. [PubMed] [Google Scholar]
- 34.Bellavite P., Guarani P., Biasi D., Carletto A., Trevisan M. T., Caramaschi P., Bambara L. M., Corrocher R. 1995. Correlations between the intensity of fMLP-dependent respiratory burst and cellular fatty acid composition in human neutrophils. Br. J. Haematol. 89: 271–276. [DOI] [PubMed] [Google Scholar]
- 35.Akamatsu H., Niwa Y., Matsunaga K. 2001. Effect of palmitic acid on neutrophil functions in vitro. Int. J. Dermatol. 40: 640–643. [DOI] [PubMed] [Google Scholar]
- 36.Curzio M., Torrielli M. V., Giroud J. P., Esterbauer H., Dianzani M. U. 1982. Neutrophil chemotactic responses to aldehydes. Res. Commun. Chem. Pathol. Pharmacol. 36: 463–476. [PubMed] [Google Scholar]
- 37.Curzio M., Esterbauer H., Dianzani M. U. 1985. Chemotactic activity of hydroxyalkenals on rat neutrophils. Int. J. Tissue React. 7: 137–142. [PubMed] [Google Scholar]
- 38.Rizzo W. B., Craft D. A. 2000. Sjörgren-Larsson syndrome: accumulation of free fatty alcohols in cultured fibroblasts and plasma. J. Lipid Res. 41: 1077–1081. [PubMed] [Google Scholar]
- 39.Thukkani A. K., Albert C. J., Wildsmith K. R., Messner M. C., Martinson B. D., Hsu F. F., Ford D. A. 2003. Myeloperoxidase-derived reactive chlorinating species from human monocytes target plasmalogens in low density lipoprotein. J. Biol. Chem. 278: 36365–36372. [DOI] [PubMed] [Google Scholar]