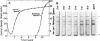Abstract
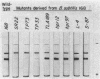
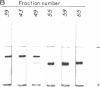
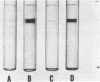
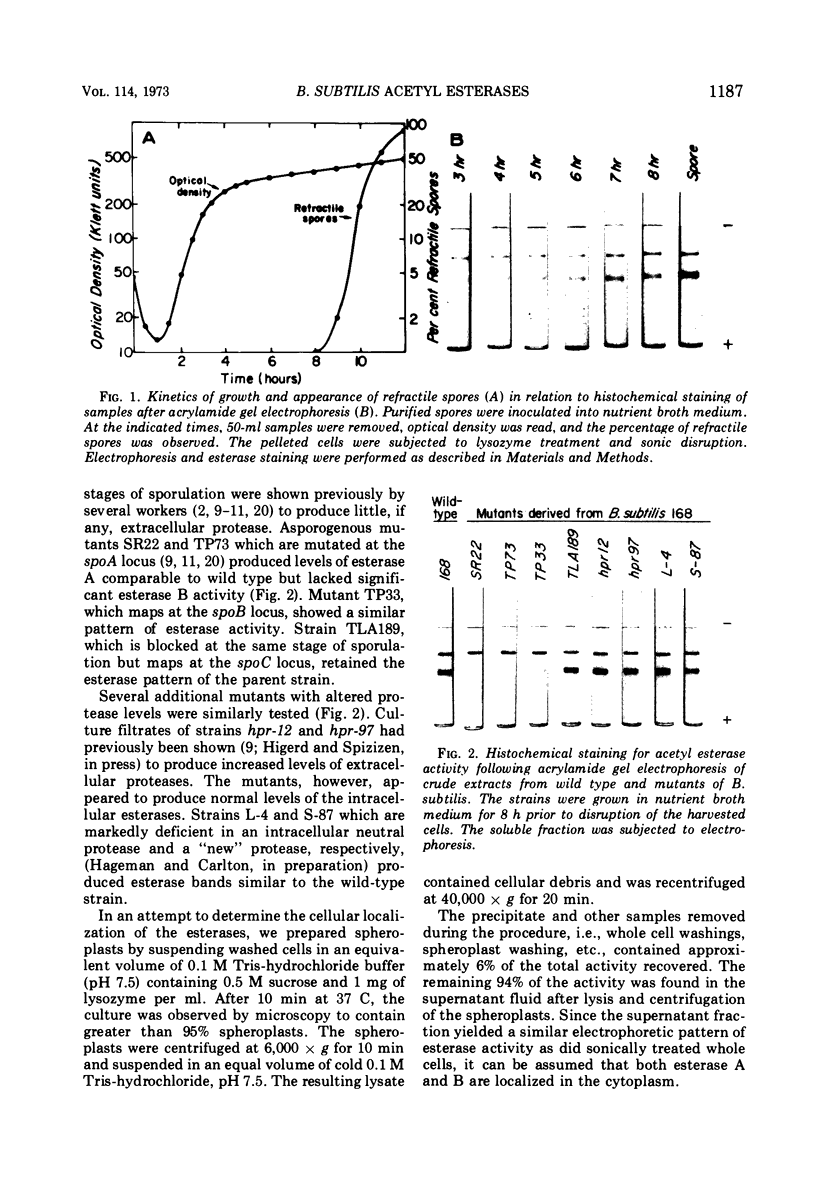
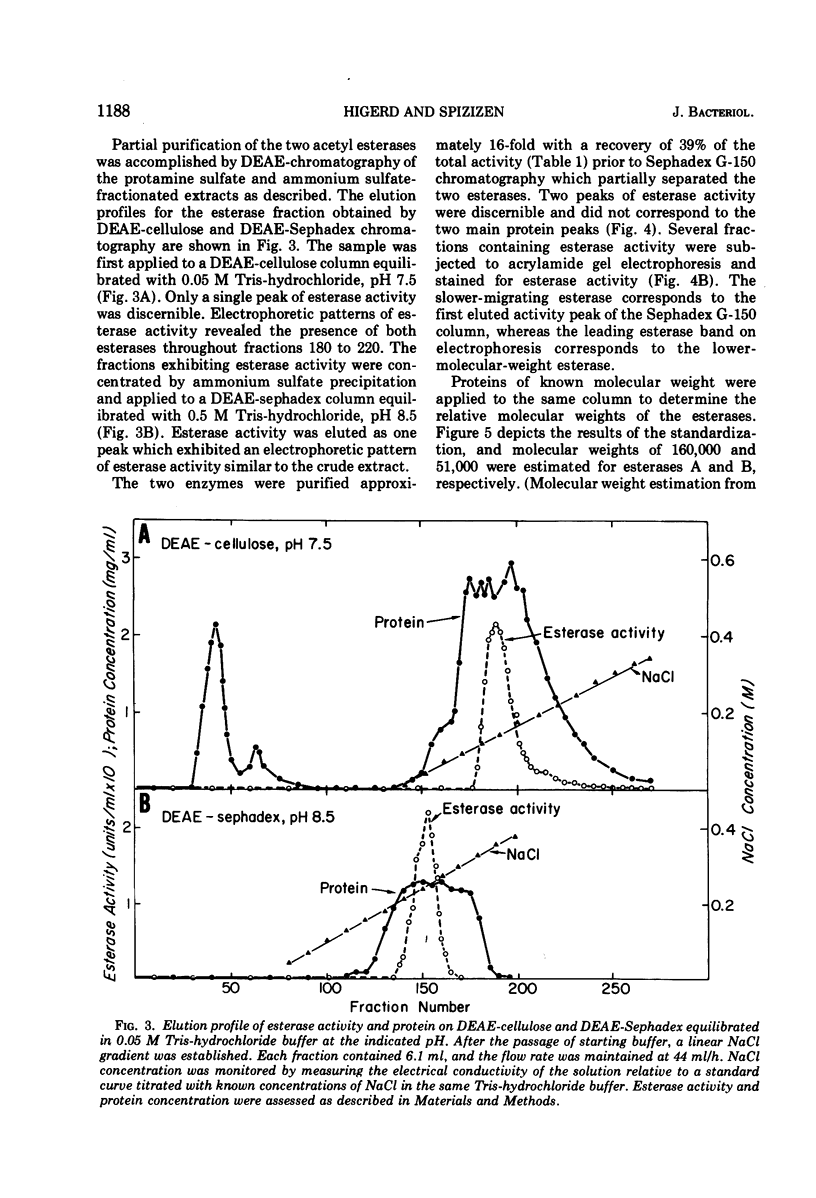
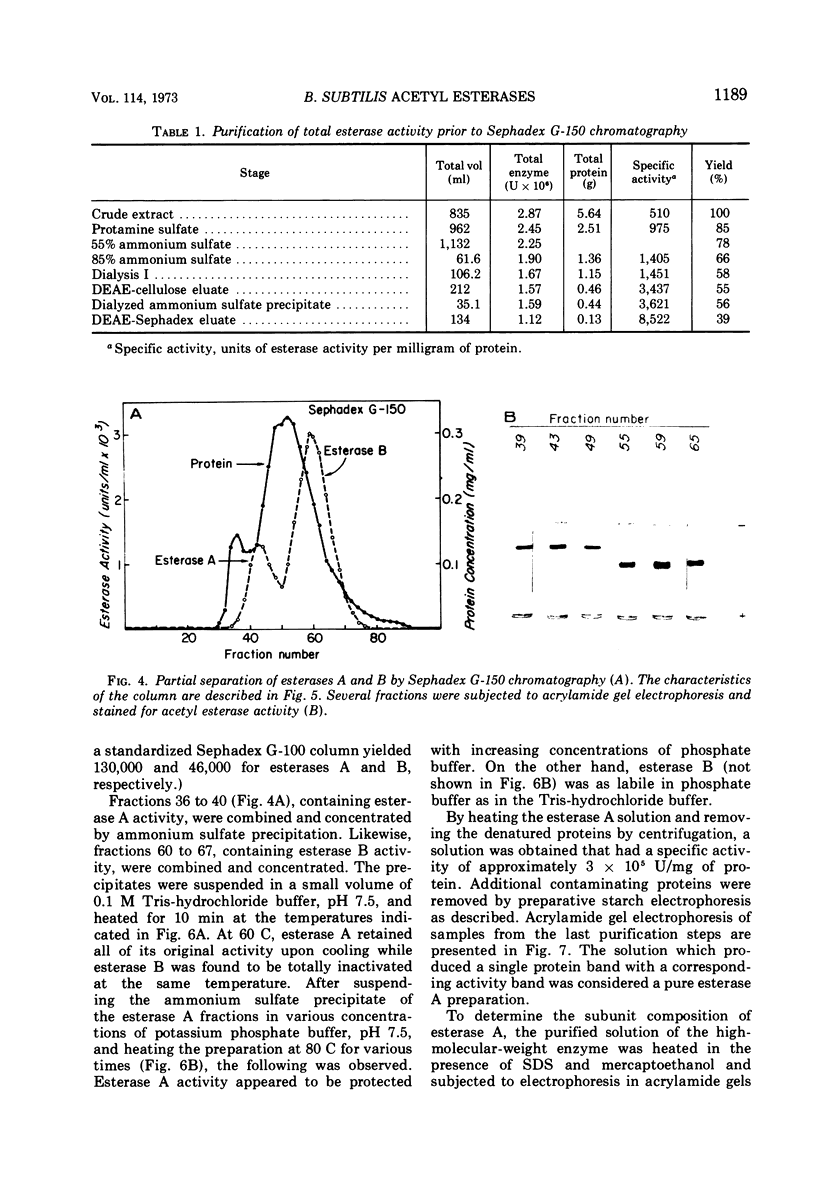
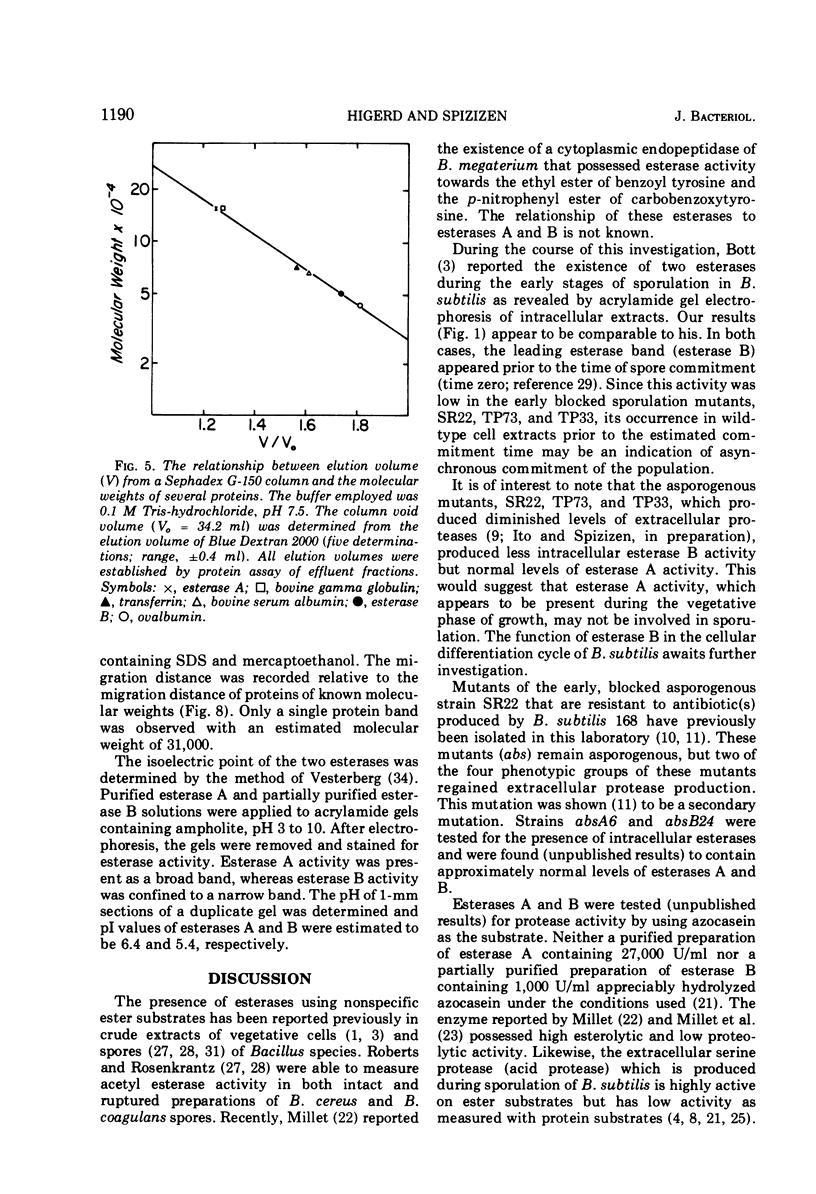
Acrylamide gel electrophoresis of crude cellular extracts of Bacillus subtilis revealed the presence of two acetyl esterases. Esterase A, the slower migrating enzyme, was found to be present in both vegetative and sporulating cells, whereas esterase B activity was more abundant after exponential growth ceased. Both esterases were present in the supernatant fraction of lysed spheroplasts and in a disrupted spore preparation. Of four pleiotropic asporogenous mutants tested, three exhibited decreased esterase B activity. Esterases A and B were partially purified by differential precipitation and co-chromatographed on diethylaminoethyl (DEAE)-cellulose (pH 7.5) and DEAE-Sephadex (pH 8.5). By employing gel filtration chromatography, the two esterases were separated, and molecular weights of 160,000 and 51,000 were estimated for esterases A and B, respectively. Esterase A was further purified to electrophoretic homogeneity by differential heating and preparative starch block electrophoresis. Sodium dodecyl sulfate-acrylamide gel electrophoresis of purified esterase A yielded a single protein band with a molecular weight of 31,000. The pI values of esterases A and B were determined to be 6.4 and 5.4, respectively.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balassa G. Biochemical genetics of bacterial sporulation. I. Unidirectional pleiotropic interactions among genes controlling sporulation in Bacillus subtilis. Mol Gen Genet. 1969;104(1):73–103. [PubMed] [Google Scholar]
- Boyer H. W., Carlton B. C. Production of two proteolytic enzymes by a transformable strain of Bacillus subtilis. Arch Biochem Biophys. 1968 Nov;128(2):442–455. doi: 10.1016/0003-9861(68)90050-7. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Feder J., Ladenburg K., Delente J., Wildi B. S. Intracellular proteases of Bacillus stearothermophilus. Appl Microbiol. 1971 Dec;22(6):1055–1057. doi: 10.1128/am.22.6.1055-1057.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUNTELBERG A. V., OTTESEN M. Purification of the proteolytic enzyme from Bacillus subtilis. C R Trav Lab Carlsberg Chim. 1954;29(3-4):36–48. [PubMed] [Google Scholar]
- Hageman J. H., Carlton B. C. An enzymatic and immunological comparison of two proteases from a transformable Bacillus subtilis with the "subtilisins". Arch Biochem Biophys. 1970 Jul;139(1):67–79. doi: 10.1016/0003-9861(70)90045-7. [DOI] [PubMed] [Google Scholar]
- Ito J., Mildner G., Spizizen J. Early blocked asporogenous mutants of Bacillus subtilis 168. I. Isolation and characterization of mutants resistant to antibiotic(s) produced by sporulating Bacillus subtilis 168. Mol Gen Genet. 1971;112(2):104–109. doi: 10.1007/BF00267488. [DOI] [PubMed] [Google Scholar]
- Keay L. Neutral proteases of the genus Bacillus. Biochem Biophys Res Commun. 1969 Jul 23;36(2):257–265. doi: 10.1016/0006-291x(69)90323-4. [DOI] [PubMed] [Google Scholar]
- Leighton T. J., Doi R. H. The stability of messenger ribonucleic acid during sporulation in Bacillus subtilis. J Biol Chem. 1971 May 25;246(10):3189–3195. [PubMed] [Google Scholar]
- Losick R., Shorenstein R. G., Sonenshein A. L. Structural alteration of RNA polymerase during sporulation. Nature. 1970 Aug 29;227(5261):910–913. doi: 10.1038/227910a0. [DOI] [PubMed] [Google Scholar]
- MCCONN J. D., TSURU D., YASUNOBU K. T. BACILLUS SUBTILIS NEUTRAL PROTEINASE. I. A ZINC ENZYME OF HIGH SPECIFIC ACTIVITY. J Biol Chem. 1964 Nov;239:3706–3715. [PubMed] [Google Scholar]
- Maia J. C.C., Kerjan P., Szulmajster J. DNA-dependent RNA polymerase from vegetative cells and from spores of Bacillus subtilis. IV. Subunit composition. FEBS Lett. 1971 Mar 22;13(5):269–274. doi: 10.1016/0014-5793(71)80238-7. [DOI] [PubMed] [Google Scholar]
- Mandelstam J., Waites W. M. Sporulation in Bacillus subtilis. The role of exoprotease. Biochem J. 1968 Oct;109(5):793–801. doi: 10.1042/bj1090793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel J. F., Millet J. Physiological studies on early-blocked sporulation mutants of Bacillus subtilis. J Appl Bacteriol. 1970 Mar;33(1):220–227. doi: 10.1111/j.1365-2672.1970.tb05246.x. [DOI] [PubMed] [Google Scholar]
- Millet J. Caractérisation d'une endopeptidase cytoplasmique chez Bacillus megaterium en voie de sporulation. C R Acad Sci Hebd Seances Acad Sci D. 1971 Mar 29;272(13):1806–1809. [PubMed] [Google Scholar]
- Millet J. Characterization of proteinases excreted by Bacillus subtilis Marburg strain during sporulation. J Appl Bacteriol. 1970 Mar;33(1):207–219. doi: 10.1111/j.1365-2672.1970.tb05245.x. [DOI] [PubMed] [Google Scholar]
- Millet J., Kerjan P., Aubert J. P., Szulmajster J. Proteolytic conversion in vitro of B. subtilis vegetative RNA polymerase into the homologous spore enzyme. FEBS Lett. 1972 Jun 1;23(1):47–50. doi: 10.1016/0014-5793(72)80281-3. [DOI] [PubMed] [Google Scholar]
- Prestidge L., Gage V., Spizizen J. Protease activities during the course of sporulation on Bacillus subtilis. J Bacteriol. 1971 Sep;107(3):815–823. doi: 10.1128/jb.107.3.815-823.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAPPAPORT H. P., RIGGSBY W. S., HOLDEN D. A. A BACILLUS SUBTILIS PROTEINASE. I. PRODUCTION, PURIFICATION, AND CHARACTERIZATION OF A PROTEINASE FROM A TRANSFORMABLE STRAIN OF BACILLUS SUBTILIS. J Biol Chem. 1965 Jan;240:78–86. [PubMed] [Google Scholar]
- Roberts T. L., Rosenkrantz H. Acetyl esterase in Bacillus cereus spores. Can J Biochem. 1966 Jun;44(6):671–675. doi: 10.1139/o66-084. [DOI] [PubMed] [Google Scholar]
- Roberts T. L., Rosenkrantz H. Lipase and acetyl esterase activities in intact Bacillus coagulans spores. Can J Biochem. 1966 Jun;44(6):677–685. doi: 10.1139/o66-085. [DOI] [PubMed] [Google Scholar]
- SELIGMAN A. M., NACHLAS M. M. The colorimetric determination of lipase and esterase in human serum. J Clin Invest. 1950 Jan;29(1):31–36. doi: 10.1172/JCI102231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITHIES O. Zone electrophoresis in starch gels: group variations in the serum proteins of normal human adults. Biochem J. 1955 Dec;61(4):629–641. doi: 10.1042/bj0610629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer P. Sporulation and the production of antibiotics, exoenzymes, and exotonins. Bacteriol Rev. 1969 Mar;33(1):48–71. doi: 10.1128/br.33.1.48-71.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamir H., Gilvarg C. Density gradient centrifugation for the separation of sporulating forms of bacteria. J Biol Chem. 1966 Mar 10;241(5):1085–1090. [PubMed] [Google Scholar]
- ZAK B., COHEN J. Automatic analysis of tissue culture proteins with stable Folin reagents. Clin Chim Acta. 1961 Sep;6:665–670. doi: 10.1016/0009-8981(61)90112-7. [DOI] [PubMed] [Google Scholar]