Abstract
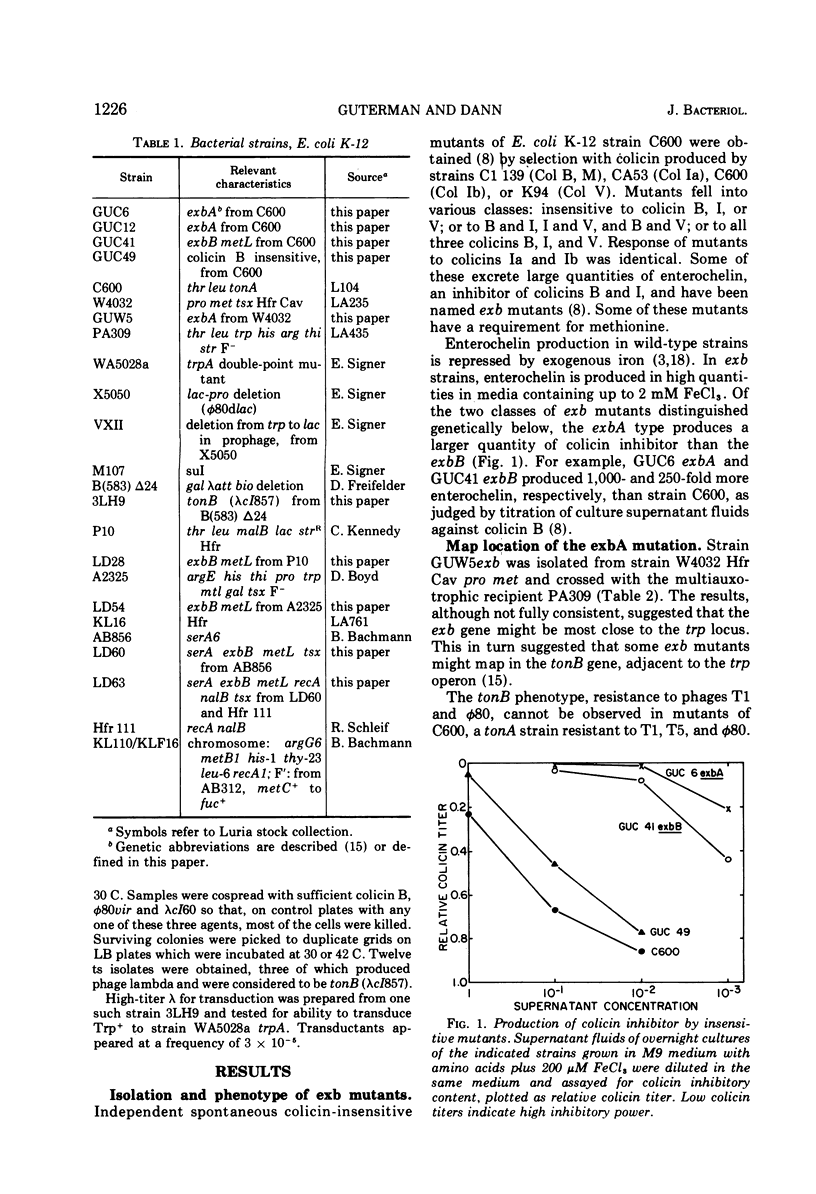
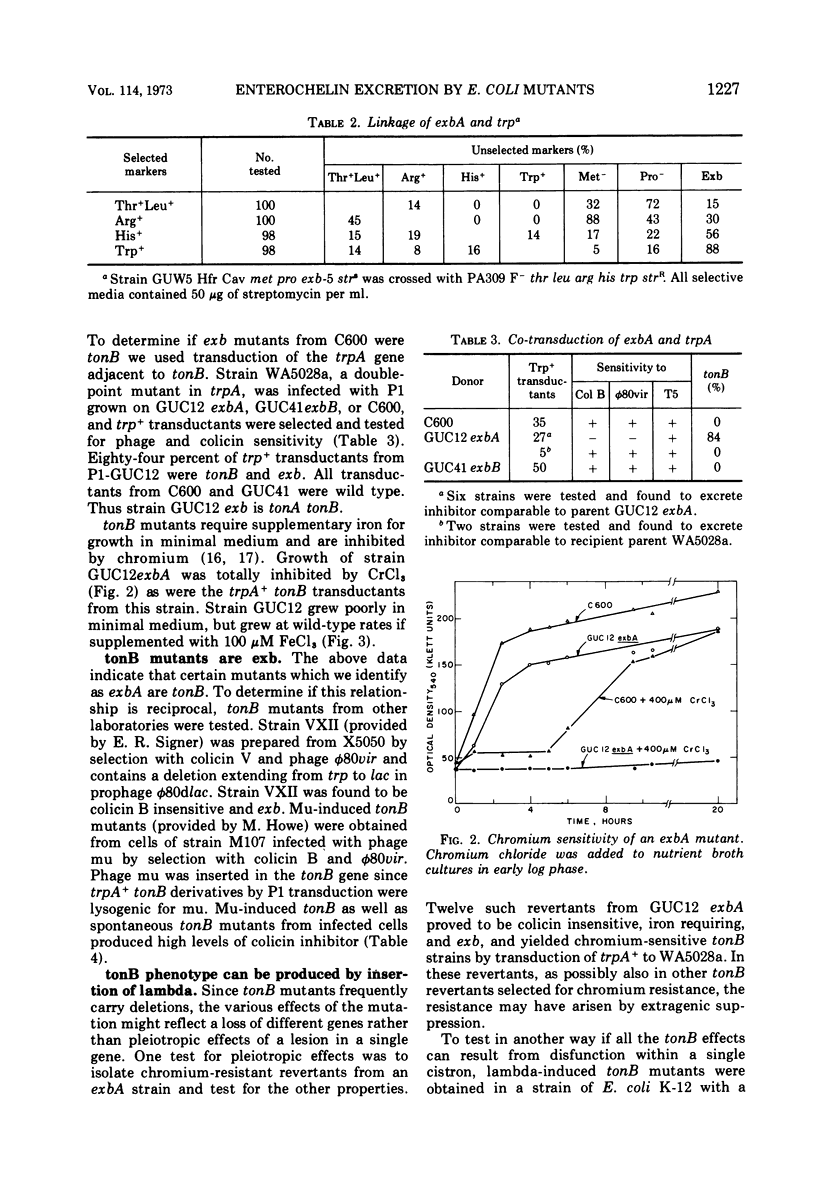
Escherichia coli mutants that are insensitive to colicins B and I hyperproduce and excrete the iron chelator enterochelin, which is an inhibitor of these colicins. These mutants are classified as exbA and exbB. The exbA mutants are chromium sensitive and require iron for growth, and the mutations are located in the tonB region at min 25 of the E. coli chromosome. tonB mutants in which the genome of phage lambda is inserted into the bacterial chromosome within the tonB gene also exhibit enterochelin excretion. The exbB mutants require methionine and probably result from deletions which are located between min 56 and 58. Colicin insensitivity, enterochelin excretion and methionine auxotrophy are recessive in exbB merodiploids. The methionine requirement of exbB strains is satisfied by cystathionine or homocysteine, and exbB mutants are sensitive to ethionine.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumenthal T. P1 transduction: formation of heterogenotes upon cotransduction of bacterial genes with a P2 prophage. Virology. 1972 Jan;47(1):76–93. doi: 10.1016/0042-6822(72)90241-3. [DOI] [PubMed] [Google Scholar]
- Brot N., Goodwin J., Fales H. In vivo and in vitro formation of 2,3-dihydroxybenzoylserine by Escherichia coli K12. Biochem Biophys Res Commun. 1966 Nov 22;25(4):454–461. doi: 10.1016/0006-291x(66)90227-0. [DOI] [PubMed] [Google Scholar]
- Bryce G. F., Brot N. Iron transport in Escherichia coli and its relation to the repression of 2,3-dihydroxy-N-benzoyl-L-serine synthetase. Arch Biochem Biophys. 1971 Feb;142(2):399–406. doi: 10.1016/0003-9861(71)90503-0. [DOI] [PubMed] [Google Scholar]
- Coukell M. B., Yanofsky C. Influence of chromosome structure on the frequency of tonB trp deletions in Escherichia coli. J Bacteriol. 1971 Mar;105(3):864–872. doi: 10.1128/jb.105.3.864-872.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. B., Gibson F., Luke R. K., Newton N. A., O'Brien I. G., Rosenberg H. Mutations affecting iron transport in Escherichia coli. J Bacteriol. 1970 Oct;104(1):219–226. doi: 10.1128/jb.104.1.219-226.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRATIA J. P. R'ESISTANCE 'A LA COLICINE B CHEZ E. COLI. RELATIONS DE SP'ECIFICIT'E ENTRE COLICINES B, I ET V ET PHAGE T-4. ETUDE G'EN'ETIQUE. Ann Inst Pasteur (Paris) 1964 Nov;107:SUPPL–SUPPL:151. [PubMed] [Google Scholar]
- Gratia J. P. Studies on defective lysogeny due to chromosomal deletion in Escherichia coli. I. Single lysogens. Biken J. 1966 Jun;9(2):77–87. [PubMed] [Google Scholar]
- Guterman S. K. Colicin B: mode of action and inhibition by enterochelin. J Bacteriol. 1973 Jun;114(3):1217–1224. doi: 10.1128/jb.114.3.1217-1224.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence D. A., Smith D. A., Rowbury R. J. Regulation of methionine synthesis in Salmonella typhimurium: mutants resistant to inhibition by analogues of methionine. Genetics. 1968 Apr;58(4):473–492. doi: 10.1093/genetics/58.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke R. K., Gibson F. Location of three genes concerned with the conversion of 2,3-dihydroxybenzoate into enterochelin in Escherichia coli K-12. J Bacteriol. 1971 Aug;107(2):557–562. doi: 10.1128/jb.107.2.557-562.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas W. K. Mapping of genes involved in the synthesis of spermidine in Escherichia coli. Mol Gen Genet. 1972;119(1):1–9. doi: 10.1007/BF00270439. [DOI] [PubMed] [Google Scholar]
- Nagel de Zwaig R., Luria S. E. Genetics and physiology of colicin-tolerant mutants of Escherichia coli. J Bacteriol. 1967 Oct;94(4):1112–1123. doi: 10.1128/jb.94.4.1112-1123.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada K., Weisberg R. A., Gottesman M. E. Prophage lambda at unusual chromosomal locations. I. Location of the secondary attachment sites and the properties of the lysogens. J Mol Biol. 1972 Feb 14;63(3):483–503. doi: 10.1016/0022-2836(72)90443-3. [DOI] [PubMed] [Google Scholar]
- Smith D. A., Childs J. D. Methionine genes and enzymes of Salmonella typhimurium. Heredity (Edinb) 1966 May;21(2):265–286. doi: 10.1038/hdy.1966.22. [DOI] [PubMed] [Google Scholar]
- Taylor A. L. Current linkage map of Escherichia coli. Bacteriol Rev. 1970 Jun;34(2):155–175. doi: 10.1128/br.34.2.155-175.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. C., Newton A. An additional step in the transport of iron defined by the tonB locus of Escherichia coli. J Biol Chem. 1971 Apr 10;246(7):2147–2151. [PubMed] [Google Scholar]
- Wang C. C., Newton A. Iron transport in Escherichia coli: relationship between chromium sensitivity and high iron requirement in mutants of Escherichia coli. J Bacteriol. 1969 Jun;98(3):1135–1141. doi: 10.1128/jb.98.3.1135-1141.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young I. G., Gibson F. Regulation of the enzymes involved in the biosynthesis of 2,3-dihydroxybenzoic acid in Aerobacter aerogenes and Escherichia coli. Biochim Biophys Acta. 1969 May 6;177(3):401–411. doi: 10.1016/0304-4165(69)90302-x. [DOI] [PubMed] [Google Scholar]


