Abstract
Microglia are resident CNS immune cells that are active sensors in healthy brain and versatile effectors under pathological conditions. Cerebral ischemia induces a robust neuroinflammatory response that includes marked changes in the gene-expression profile and phenotype of a variety of endogenous CNS cell types (astrocytes, neurons and microglia), as well as an influx of leukocytic cells (neutrophils, macrophages and T-cells) from the periphery. Many molecules and conditions can trigger a transformation of surveying microglia to microglia of an alerted or reactive state. Here we review recent developments in the literature that relate to microglial activation in the experimental setting of in vitro and in vivo ischemia. We also present new data from our own laboratory demonstrating the direct effects of in vitro ischemic conditions on the microglial phenotype and genomic profile. In particular, we focus on the role of specific molecular signaling systems, such as hypoxia inducible factor-1 and Toll-like receptor-4, in regulating the microglial response in this setting. We then review histological and novel radiological data that confirm a key role for microglial activation in the setting of ischemic stroke in humans. We also discuss recent progress in the pharmacologic and molecular targeting of microglia in acute ischemic stroke. Finally, we explore how recent studies on ischemic preconditioning have increased interest in pre-emptively targeting microglial activation in order to reduce stroke severity.
Keywords: hypoxia inducible factor, ischemia, ischemic preconditioning, microglia, Toll-like receptor
Stroke accounts for more than half of all patients hospitalized for acute neurological disease in the USA and is the second most common cause of death worldwide. Over 80% of strokes are of the ischemic variety and are due to acute vascular occlusion. Several pharmacotherapies for acute stroke are currently available, including thrombolytics and antiplatelet agents; however, their efficacy is modest and their use is limited, owing to temporal restrictions on administration and risks of adverse events [1]. This situation prevails despite intense research efforts and numerous clinical trials. To date, drug development efforts have targeted modulators of ion channels, scavengers of oxygen radicals, inhibitors of apoptosis and antagonists of excitotoxic neurotransmitters. However, trials with modulators of these targets have failed so far because of lack of efficacy, adverse side-effects or shortcomings in experimental design [2–4]. Recently, a new potential target, the neuroinflammatory response in ischemia, has attracted the attention of a number of stroke researchers [2,5]. An inflammatory response is initiated in the metabolically active but neurophysiologically silent region surrounding the infarct core known as the ischemic penumbra. This response is characterized by an increase in vascular permeability, influx of leukocytes, hyperthermia and activation of microglia [5].
The temporal sequence of inflammatory events that accompanies an ischemic event has been well characterized using animal models of focal cerebral ischemia. Early changes involve activation of astrocytes and microglia that migrate to the site of injury and produce proinflammatory cytokines, such as IL-1β and -6, as well as TNF-α [6]. In addition, there is release of chemokines, such as monocyte chemotactic protein-1 (MCP-1/CCL2) and macrophage inhibitory factor-1α (MIP-1α/CCL3), which recruit leukocytes from the periphery [6]. Invading leukocytes also release inflammatory cytotoxins such as oxygen radicals and proteases that may be destructive to the compromized ischemic tissue. Studies of inhibited leukocyte infiltration have shown a reduction in neuronal damage, confirming the role that inflammation plays in exacerbating stroke-related tissue injury [7,8]. Several studies have effectively used ex vivo flow cytometry to characterize the inflammatory infiltrate in the ischemic penumbra following stroke [9,10]. Microglial and macrophage infiltration is robust and reaches its peak 48–72 h following transient middle cerebral artery occlusion (MCAO) in mice [9,10]. Invasion of neutrophils is similarly strong, but occurs slightly later (peaks at 72 h) [9,10]. There is also infiltration of lympho cytes, dendritic cells and natural killer cells, albeit in substantially lesser numbers [9].
Microglia, the tissue macrophages of the CNS, are active sensors and versatile effector cells in normal and pathologic brain [11]. Microglia can shift activity states depending on surrounding conditions. Under normal conditions they are characterized by a small cell body with fine, ramified processes and low expression of surface antigens [12]. Although sometimes referred to as `resting', recent live-imaging studies have demonstrated that microglial cellular processes are constantly in motion, monitoring their environment for signals from surrounding neural cells [13,14]. During brain injury, microglia rapidly undergo a shift in their effector program by transforming their morphology, proliferating, releasing proinflammatory compounds and increasing expression of immunomodulatory surface antigens [11,15]. In selected settings, however, `activated' microglia can function in a neuroprotective manner and promote regeneration by releasing growth factors and modulating the immune response [15–17]. This neuroprotective phenotype may be the product of `alternative' activation triggered by IL-4, -10 and -13. These cytokines alter gene expression patterns away from toxic and proinflammatory mediators such as TNF-α and IL-1β towards protective and anti-inflammatory mediators such as IGF-1 and TGF-β [18].
Effects of experimental ischemia on microglia: in vitro studies
In vitro studies examining the direct effects of ischemia on microglia have focused primarily on cell viability and induction of classical activation parameters under conditions of near complete oxygen–glucose deprivation (OGD). Using an anaerobic chamber combined with exposure to an aglycemic medium containing nonmetabolizable glucose-derivative 2-deoxyglucose (2-DG), Lyons and Kettenmann exposed primary rat microglia to either severe hypoxia alone (≤0.3% oxygen), aglycemia alone or severe combined hypoxia/aglycemia for 6 h, and then switched to normoglycemic/normoxic culture medium for the duration of the experiment [19]. Using both ethidium bromide staining and assays for lactate dehydrogenase (LDH), they demonstrated that 88% of microglia died by the end of the 6 h combined severe hypoxia/aglycemia exposure and that this percentage increased slightly to 93% at 7 days following the insult. Microglial cell death immediately following the insult was less severe when mannitol was substituted for 2-DG, a difference the authors attributed to the free-radical scavenging capability of mannitol. Interestingly, they carried out parallel 6 h combined severe hypoxia/aglycemia exposure experiments on cultured rat astrocytes and oligodendrocytes. Astrocytes showed substantially less cell death under these conditions, although oligodendrocytes showed cell death rates similar to microglia. Under purely hypoxic conditions (normoglycemia), oligodendrocytes demonstrated a marked increase in cell death, whereas both microglial and astrocytic cultures were unaffected. Under purely aglycemic conditions (normoxia), all three glial cell types showed survival patterns indistinguishable from controls. Yenari and Giffard demonstrated that mouse microglia were also susceptible to prolonged severe hypoxic (≤0.2% oxygen)/aglycemic injury but resistant to purely hypoxic injury [20]. However, contrary to the Lyons and Kettenmann study, they demonstrated that mouse microglia were quite sensitive to glucose deprivation with 40 and 75% cell death at 24 and 30 h, respectively [20]. The increased sensitivity in the Yenari et al. study most likely reflects the longer time interval of aglycemia exposure; however, species differences between rat and mouse microglia may also play a role. Park et al. demonstrated that exposure of primary mouse microglia (pMG), as well as mouse microglial cell lines BV-2 and N9, to severe hypoxic conditions (≤0.2% oxygen/normoglycemia) for 8 h followed by 24 h incubation in noxmoxic/normoglycemic medium resulted in a significant release of nitric oxide (NO) [21]. The same conditions resulted in a small but quantifiable release of TNF-α in BV-2 cells, along with a 72% reduction in cell viability (compared with normoxic controls) [21]. A total of 6 h of severe hypoxia (without subsequent exposure to normoxia) was sufficient to increase expression of inducible NO synthase (iNOS) in BV-2 cells [21]. Brief periods of severe hypoxia resulted in phosphorylation of p38 MAPK, but not other closely related MAPKs ERK1/2 and c-Jun N-terminal kinase (JNK) in BV-2 cells. Pharmacologic inhibition of p38, but not ERK1/2, significantly attenuated the hypoxia-induced increases in NO, iNOS and TNF-α in BV-2 cells. In primary microglia derived from rat brain, exposure to 8 h of severe hypoxia (≤0.2% oxygen) resulted in increased expression of iNOS and TNF-α, as well as phosphorylation of all three of the abovementioned MAPKs [22]. The same study demonstrated that severe hypoxia potentiated lipopolysaccharide (LPS)-induced increases in both iNOS and TNF-α [22]. Another recent study demonstrated that exposure of pMG cells to more moderate levels of hypoxia (≤1% oxygen) combined with aglycemia for 6 h resulted in marked increases in matrix metalloproteinase (MMP)-9 mRNA expression. Interestingly, this effect was absent in microglia derived from mice that were genetically deficient for either tissue plasminogen activator (tPA) or low-density lipoprotein receptor-related protein (LRP)-1 – a known cleavage substrate for tPA. MMP-9, endogenous tPA and LRP1 have all been implicated in stroke pathophysiology and particularly with increases in permeability of the neurovascular unit [23].
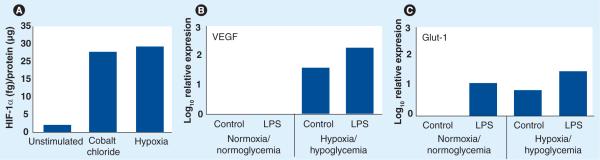
Although the number of studies examining the direct effects of in vitro hypoxic/ischemic conditions on microglia is relatively low, a larger number of studies have examined the effects of these conditions on peripheral myeloid cell function. Many of these studies have focused on activation of hypoxia inducible factor (HIF)-1 (Box 1). Most of these studies have used moderate to mild levels of hypoxia (1–8% oxygen), consistent with the hypoxic microenvironment found in peripheral tissue following a variety of proinflammatory injuries [24]. Exposure to 1% oxygen for 16 h markedly increased total cellular HIF-1α protein levels in both human [25] and mouse [26] macrophages. After 8 h, hypoxia induced 8-, 11- and 13-fold increases in mRNA levels for HIF-1 gene targets VEGF, PGK1 and Glut-1, respectively [27]. Conditional targeting of HIF-1α in myeloid cells using Cre/loxP technology has allowed researchers to investigate the effects of HIF-1α deficiency in the myeloid-cell lineage (LysMcre/HIF1αloxp mouse strain) using different assays of inflammation [24]. These important studies have demonstrated that, in macrophages, glycolysis depends on HIF-1α under both normoxic and hypoxic conditions and that the decrease in energy production caused by impaired glycolysis in these cells can markedly inhibit inflammatory responses [27,30,31]. In these studies, HIF-1α was indispensable for myeloid-cell infiltration and tissue destruction, as well as proinflammatory cytokine secretion. Furthermore, a number of studies have demonstrated that treatment of peripheral myeloid cells with pathogen-associated molecular patterns (PAMPs), such as LPS, leads to induction of HIF-1 activity [30,32–34]. This induction is dependent on the activation of TLR4 (Box 2) [30,35]. More recently, several groups have reported induction of HIF-1 activity in microglia. Exposure of N9 cells to 1% oxygen for 4 h resulted in a marked increase in binding of HIF-1 to hypoxia-response element (HRE) DNA sequences by electrophoretic mobility gel-shift assay (EMSA). The same study reported hypoxia-induced expression of HIF-1 target gene chemokine CXCR4 [36]. In BV-2 cells, exposure to 1% oxygen for 6 h induced a threefold increase in HRE luciferase reporter gene activity [41]. Interestingly, treatment with LPS also induced small but significant increases in HIF-1α mRNA and total protein levels in BV-2 cells. In our laboratory, we were interested in determining the effects of moderate hypoxia on HIF-1α activity in pMG. We demonstrate here that treatment with 1% oxygen for 6 h induces an approximately tenfold increase in steady-state levels of total HIF-1α protein in pMG (Figure 1A). A similar size induction of HIF-1α in pMG could also be seen following treatment with cobalt chloride – an inhibitor of prolylhydroxylase proteins (PHDs). When we exposed pMG to moderate hypoxia combined with hypoglycemia (250 μM glucose) for 24 h, we measured increases in expression of mRNAs for HIF-1 gene targets VEGF (Figure 1B) and Glut-1 (Figure 1C). Interestingly, as in peripheral myeloid cells, addition of LPS augmented the hypoxia/hypoglycemia-induced increases in both VEGF (Figure 1B) and Glut-1 (Figure 1C) mRNAs in pMG. Furthermore, as in peripheral immune cells, LPS was able to induce expression of at least one HIF-1 target (Glut-1), even in the absence of hypoxia/hypoglycemia (Figure 1C).
Figure 1. Induction of hypoxia-inducible factor-1 and its downstream gene targets in primary microglia.
For determination of total cellular (A), wild-type (WT) primary mouse microglia (pMG) were serum-starved and then either unstimulated, stimulated with cobalt chloride (200 μM) or treated under hypoxic conditions (pO2 = 1%) for 6 h. Lysates were prepared and total cellular HIF-1α ELISA carried out as per manufacturers' instructions (R&D Systems, MN, USA). In (B & C), serum-starved pMG were exposed to either normoxic (pO2 = 21%)/normoglycemic (5 mM glucose) conditions or hypoxic (pO2 = 1%)/hypoglycemic (250 μM glucose) conditions ± 100 EU/ml LPS (Associates of Cape Cod, Inc., MA, USA) for 24 h. Total RNA was extracted and samples were processed by qRT-PCR for steady-state levels of VEGF and Glut-1 mRNA as described [42]. In (A−C), each bar in the graph shows the result of a representative individual experiment (n = 2 for all conditions).
HIF: Hypoxia inducible factor; LPS: Lipopolysaccharide.
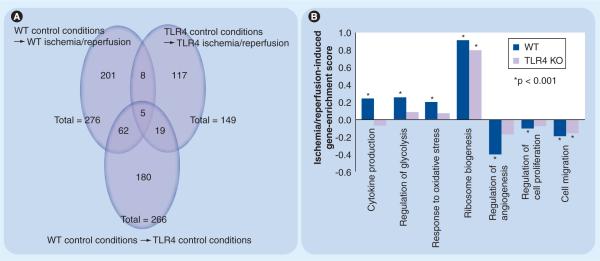
In Figure 2, we present additional new experimental data from our own laboratory; genomic microarray analyses on pMG derived from either wild-type (WT) or TLR4−/− mice following exposure to either `ischemia–reperfusion' or `control' conditions. These data have provided further molecular insights into both the effect of ischemia on the microglial phenotype and the role of microglial TLR4 in ischemia-induced neuroinflammation. Sequential 24 h intervals of hypoxia/hypoglycemia (ischemia) and then normoxia/normoglycemia (reperfusion) induced significant differential regulation of 276 and 149 individual genes in WT and TLR4−/−pMG, respectively (Figure 2A). Of these, only 13 (eight plus five) individual genes were regulated in common. Of the 136 (149 minus 13) genes disparately regulated in the two strains following ischemia/reperfusion, only 19 were disparately regulated between the two strains under control conditions. In Figure 2B, we provide selected examples of seven specific biological processes (BP) with relevance to either microglial function or stroke pathophysiology that were significantly regulated in WT pMG. Note that only two of the seven BPs were similarly regulated in TLR4−/−pMG. These data demonstrate several points: first, exposure to ischemia–reperfusion induces numerous and robust changes in the transcriptome of WTpMG. These changes are apparent globally at both the individual gene and BP gene set levels. Second, the genomic responses of WT and TLR4−/−pMG to in vitro ischemia–reperfusion are markedly disparate. These differences suggest a fundamental role for TLR4 in determining the microglial phenotype in this setting. Third, most of the differences in the genomic response of WT and TLR4−/−pMG to ischemia–reperfusion are independent of baseline differences between pMG from each strain under control conditions. This suggests that the sequential exposure to hypoxia/hypoglycemia and normoxia/normoglycemia result in a unique mechanism that specifically differentiates pMG with intact or deficient TLR4 signaling. One possible explanation is that ischemia/reperfusion induces release of endogenous TLR4 agonists or danger-associated molecular patterns (DAMPs) from dying microglia that function in an autocrine/paracrine manner to activate TLR4 signaling in WT, but not TLR4−/−, surviving pMG (Figure 3). Fourth, the significant regulation by ischemia–reperfusion in WT, but not TLR4−/− pMG of certain BP gene sets (e.g., cytokine production and response to oxidative stress) suggests specific functions for microglial TLR4 in ischemia. For example, it suggests that TLR4 signaling mediates an ischemia-induced upregulation of multiple specific proinflammatory cytokines in microglia. Data mining in the cytokine production BP reveals genes for a host of factors involved in proinflammatory pathways, including multiple TLRs, TLR-associated signaling proteins and interleukins, as well as TNF-ligand and TNF-receptor superfamily members. Investigating individual gene expression, we identified significant upregulation of TNF-ligand superfamily member 10, IL-18 and NF-κB inhibitor interacting RAS-like protein-1 (NKiras1) in the WT, but not the TLR4−/−, pMG exposed to ischemia. From the same BP, TNF-receptor superfamily member 13 was significantly downregulated in the WT, but not in the TLR4−/−, pMG exposed to ischemia. This pattern indicates that ischemia-induced expression of specific proinflammatory cytokines from microglia in a TLR4-dependent manner, while at the same time, desensitizing them to further activation by decreasing expression of a key cytokine receptor and increasing expression of an inhibitor of a proinflammatory transcription factor. Other research groups have focused on examining the indirect effects of in vitro ischemia on microglial activation. These experiments have characterized the effects of conditioned medium (CM) transferred from OGD-stressed neuronal or glial cell populations to naive microglial cultures. Kaushal and Schlichter demonstrated that microglia exposed to OGD-stressed neuronal CM resulted in a selective classical activation profile: specifically, release of TNF-α and activation of NF-κB, but no induction of p38 MAPK or NO production. Using a Transwell™ chamber system, the authors then transferred these `activated' microglia into coincubation with naive neurons and assessed apoptotic neuronal cell death. Exposure to the `activated' microglia resulted in significant increases in apoptotic neuronal cell death as determined by both TUNEL/DAPI staining and assays for caspases 3 and 8. The authors went on to demonstrate that exposing the microglia to metabatropic glutamate receptor II (mGluRII) agonist DCG-IV (in place of exposure to CM from OGD-stressed neurons) also resulted in release of TNF-α, but no induction of NO production. Transfer of the DCG-IV-stimulated microglia into coculture with naive neurons resulted in a significant increase in TUNEL staining compared with neurons exposed to untreated microglia. This increase in toxicity could be inhibited by coexposure of the microglia to mGluRII antagonists (EGLU and LY341495), or coexposure of the neurons to excess soluble TNF-α receptor (sTNFR)1. OGD-stressed neurons produced sufficient glutamate to activate microglial mGluRII. Neuronal cell apoptotic cell death induced by coculturing with activated microglia that had been exposed to OGD-stressed neuronal CM could again be inhibited by coexposure of the microglia to mGluRII antagonists, or coexposure of the neurons to excess sTNFR1. Finally, exposing the microglia to glutamate itself (in place of DCG-IV or CM from OGD-stressed neurons) and then coculturing with naive neurons resulted in a significant increase in TUNEL staining compared with neurons exposed to untreated microglia. This increased neurotoxicity could be inhibited by coexposure of the microglia to mGluRII antagonists or to an NFκB pathway inhibitor. The authors concluded that these results point to involvement of glutamate from OGD-stressed neurons in activating microglial mGluRIIs and increasing their neurotoxic functions through activation of NFκB and production of TNF-α (Figure 4).
Figure 2. Microarray analyses on wild type and TLR4−/− primary mouse microglia following exposure to experimental or control conditions.
WT and TLR4−/− primary mouse microglia (pMG) were exposed to hypoxic/hypoglycemic (`ischemic') or normoxic/normoglycemic (`control') conditions for 24 h in triplicate. All cells were then exposed to 24 h of normoxic/normoglycemic (`reperfusion') conditions. RNA was extracted, analyzed and processed for cDNA synthesis and cDNAs were hybridized to a Mouse Gene 1.0 ST Array and raw data processed, normalized and analyzed as described [43,44]. Individual genes with evidence for significant differential expression between strains (WT vs TLR4−/−) and/or conditions (ischemia–reperfusion vs control) were identified as described [45]. Comparisons showing distinct and overlapping subsets of regulated individual genes are presented as Venn diagrams (A). Individual genes were sorted into biological processes (BP) using gene-set enrichment analyses (GSEA), as described [46,47]. Differential ischemia-induced regulation of selected BP categories between strains is presented here as a bar graph (B). All microarray data have been deposited in the Gene Expression Omnibus Database under accession number GSE18602.
KO: Knockout; TLR: Toll-like receptor; WT: Wild-type.
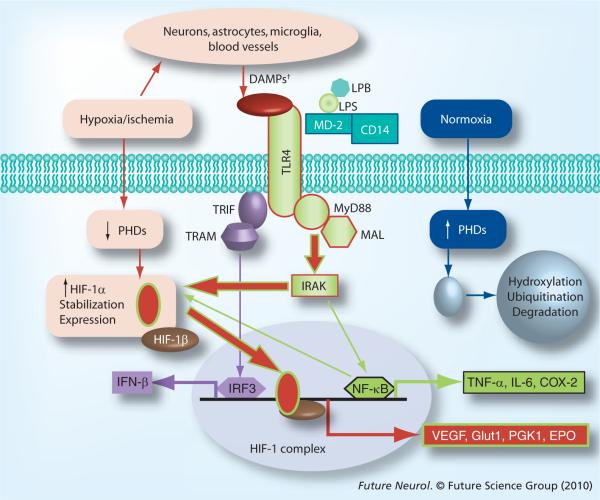
Figure 3. Select TLR4 and HIF-1α signaling pathways in microglia.
Pathways associated with normoxia, hypoxia/ischemia and TLR4 activation by LPS are outlined in blue, red or green, respectively. †DAMPS include HSP, HMGB1 and other endogenous ligands for TLR4.
DAMP: Danger-associated molecular pattern; HIF: Hypoxia inducible factor; LPB: LPS-binding protein; LPS: Lipopolysaccharide; PHD: Prolylhydroxylase protein; TLR: Toll-like receptor; TRAM: TRIF-related adaptor molecule; TRIF: Toll–IL-1 receptor domain-containing adaptor factor.
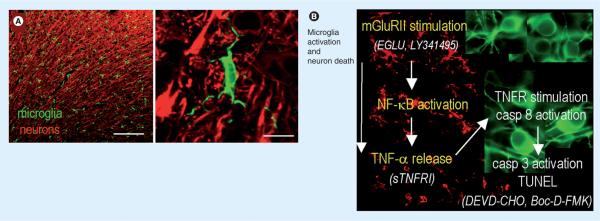
Figure 4. Summary of findings and model of the ischemic penumbra.
(A) Representative confocal micrographs of the healthy rat cortex, showing the dense network of ramified microglia whose processes are in close apposition to the neurons. Scale bars: left, 200 μm; right, 10 μm. A higher-magnification image shows a typical microglial cell with processes abutting the neuronal cell body (near top) and wrapping around the axonal processes. Microglia are labeled with a rabbit polyclonal antibody against a membrane protein, IBA1, and an Alexa 488-conjugated goat anti-rabbit secondary antibody (green). Neurons are labeled with a mouse monoclonal MAP2 antibody and Cy3-conjugated goat antimouse secondary antibody (red). (B) After a stroke, an inflammatory response develops over time, microglia become activated (their processes retract and they migrate to the damage site), and neuronal damage propagates into the surrounding tissue, the ischemic `penumbra'. Note that the colors are reversed from (A); microglia (red) are labeled with IBA1 antibody and a Cy3-conjugated secondary antibody, whereas rat cortical neurons (green) are labeled with microtubule-associated protein-2 and a FITC-conjugated secondary antibody. Author's interpretation [49]: “In these representative confocal micrographs from the rat brain after a stroke, microglia (green) are ramified and densely distributed in the uninjured contralateral hemisphere (top image), but retract their processes and eventually become rounded up as they progressively activate on the damaged ipsilateral side (bottom two images). In this study, using an in vitro model of the stroke penumbra, we identified several events: glutamate, released by the oxygen–glucose deprivation-stressed neuron–astrocyte cultures, reached sufficient concentrations to stimulate microglial mGluRIIs; this stimulation was blocked by the selective mGluRII antagonists, EGLU or LY341495; microglial activation was accompanied by activation of NF-κB, not p38 MAPK; activated microglia produced and released TNF-α; TNF-α interacted with the target neurons, activating their p55/TNF1 receptors, as judged by activation of caspase-8. Neurotoxicity was reduced by scavenging TNF-α with sTNFR1; the target neurons died by apoptosis, as judged by caspase-3 activation and TUNEL; caspase 3 activation was required for excess neuron killing, because it was prevented by the caspase-3 inhibitor, DEVD-CHO, and by the broad-spectrum caspase inhibitor, Boc-D-FMK.”
mGluR: Metabatropic glutamate receptor II; sTNFR1: Soluble TNF-α receptor 1; TNFR: TNF receptor.
Adapted with permission from [49].
The experimental paradigm described by Kaushal and Schlicter is novel, and in many ways conforms to their description of it as an in vitro model of the ischemic penumbra. However, one limitation of their model system is that the microglia themselves are never exposed to hypoxia/hypoglycemia. This, of course, is not the case during stroke in vivo and it is clear from earlier published work [19,20,48], as well as the data presented here in Figures 1 & 2, that ischemia markedly influences microglial phenotype and gene expression. This limitation is partially addressed in the work of another study.
Lai and Todd have used a similar experimental paradigm to characterize the effects of CM collected from microglia exposed to moderate hypoxia (≤1% oxygen) and normoglycemia for 4 h followed by 24 h normoxia on naive neuronal cultures that are subsequently exposed to moderate hypoxia for 2 h followed by 24 h normoxia [48]. The CM from hypoxic microglia induced 32% neuronal cell death whereas CM from nonhypoxic control microglia had no effect. Pretreatment of the microglia with tetracyclines (either minocycline or doxycycline), prior to the hypoxic pulse attenuated the increase in neuronal cell death induced by CM from the hypoxic microglia. Tetracyclines have been demonstrated, both in vivo and in vitro, to inhibit classical proinflammatory microglial activation, including cytokine release, NO production and prostaglandin release [50,51]. Moderate hypoxia also induced microglial expression of iNOS, as well as release of NO, IL-1β and TNF-α [48]. These effects were also all attenuated by minocycline. Interestingly, hypoxia also induced microglial production of neuroprotective factors including BDNF and GDNF [48]. These effects were unaffected by pretreatment of the microglia with either of the tetracyclines. These complex but well-controlled experiments suggest that hypoxic microglia increase their expression of both proinflammatory and prosurvival factors and that some of these effects can be modulated pharmacologically. One limitation of the Lai and Todd paradigm, however, is that the hypoxic microglia were not also exposed to hypoglycemia (as they would be in the ischemic penumbra in vivo [52]). As noted above, microglia respond differently to the combination of hypoxia/hypoglycemia than to either insult alone [19,20]. A limitation of all the above in vitro ischemia paradigms, including our own, is that they do not take into account changes in pH levels or the concentration of lactic acid, both of which are known to be markedly altered in the ischemic penumbra [52].
In vivo, cerebral ischemia is accompanied by breakdown of the BBB and extravasation of plasma-derived proteins and bioactive phospholipids. The effects of a number of these factors on microglia in vitro have been well described, including thrombin [53], albumin [54], immunoglobulins [55], complement [56] and lysophosphatidic acid (LPA) [57]. Similarly, in the ischemic penumbra, there is endogenous release of cytokines and chemokines, as well as prostaglandins, cannabinoids and nucleotides (particularly ATP), which induce a variety of responses in microglia [58]. It is likely that all these factors substantially modulate the microglial response to ischemia [11]. Moreover, microglia in vivo are in direct contact and constant communication with neurons at baseline, which helps maintain their non-activated state. Lack of the neuronal membrane glycoprotein CD200, for example, leads to increased microglial activation in various injury models [59]. Another mediator, the chemokine fractalkine (CX3CL1), is tonically released by neurons. Absence of its receptor (CX3CR1) on microglia exacerbates microglial activation and neurotoxicity in neurodegenerative diseases, including Parkinson's disease and amyotrophic lateral sclerosis [60]. In addition, microglia express receptors for most neurotransmitters. While activation of some specific neurotransmitter receptors can lead to microglial activation, in general, neurotransmitters suppress microglial activation. Adenosine, for example, can stimulate resting microglia, but more strikingly attenuates cytokine release following LPS exposure [61]. Similarly, activation of microglial GABA [62] or adrenergic [63] receptors both suppress LPS-induced interleukin release. Glutamate has a more complex role: while activation of mGluR2 causes a neurotoxic microglial response [49], as previously described, activation of group III mGluR is neuroprotective [64].
In summary, despite inherent limitations to the reductionist approach, in vitro studies have contributed a sizable amount of information and numerous key insights into the function of microglia under ischemic conditions. Microglia are clearly susceptible to cell death induced by severe hypoxia, particularly when combined with aglycemia. However, under more moderate ischemic conditions (comparable with those observed in the ischemic penumbra), microglia survive and undergo broad and profound changes in both their genomic profile and phenotype. In this setting, HIF-1 and its downstream gene targets are induced and contribute to the ischemia-altered microglial physiologic state. Innovative coculture and medium-transfer experiments have clearly demonstrated that microglia respond robustly to soluble signals released from ischemic neuronal and glial cell populations, and, once activated in this manner, can markedly influence neuronal cell viability.
Effects of experimental ischemia on microglia: in vivo studies
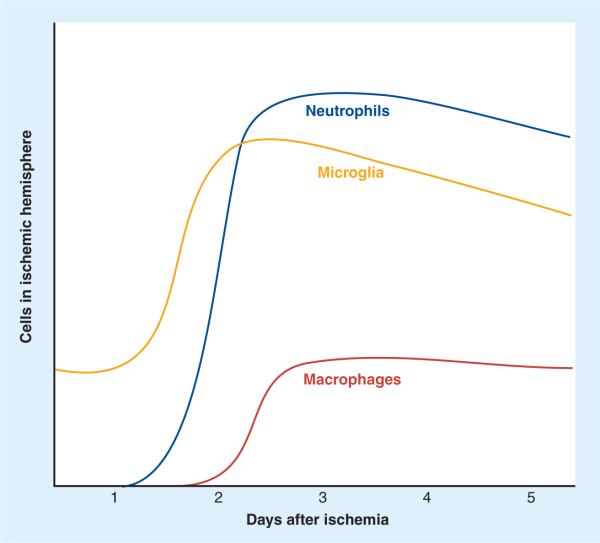
Ischemic injury in rodent models reliably leads to activation and proliferation of microglia. Studies using immunohistochemical techniques to describe this activation process can be confusing as a result of the array of different antibodies used. Some of these antibodies recognize proteins that are expressed by activated cells only, and none are entirely specific for resident microglia. Significant effort has thus been put into developing better methods to follow microglia after ischemia. A first step was to identify phenotypic changes associated with microglial activation in vivo. Ramified `resting' (or more accurately `surveying') microglia are readily detected throughout the adult brain, using markers such as lectin or antibodies against ionized calcium-binding adaptor molecule (IBA)-1. At 12–24 h following permanent middle cerebral artery occlusion (MCAO) in rats, microglia become activated, they acquire a more amoeboid phenotype with an enlarged cell body and stout processes. These cells appear in the infarct core, but also in the surrounding tissue, and are followed by the infiltration of neutrophils into the infarct core, starting at approximately 24 h after MCAO [65]. A common finding of studies regarding permanent MCAO in rats and mice is that activated microglia consistently appear in the penumbral tissue at the edge of the infarct before obvious infarction (as identified by loss of MAP2 reactivity or cresyl violet staining) or neuronal apoptosis are present [66]. Activated microglia appear not only in the ipsilateral cortex but also spread to the thalamus and to the contralateral side 7 days after MCAO [67]. The number of activated microglia continues to increase throughout the first week after MCAO, at which time they cannot be microscopically or immunohistochemically differentiated from invading macrophages [68]. Schroeter and colleagues designed an elegant experiment to help differentiate activated, amoeboid microglia from infiltrating blood-borne macrophages. They depleted peripheral macrophages in rats by using dichloromethylenediphosphonate and found that, for the first 3 days following photothrombosis, there was no difference between rats with depleted macrophages and control animals in the intensity of staining for activated microglia at the edge of the resulting peripheral cortical infarct. Significant numbers of infiltrating macrophages did not appear in the infarcted tissue until 6 days after MCAO [69]. The concept that the innate microglia, rather than blood-borne immune cells, are the predominant immune cells in the brain for the first days after ischemia (Figure 5) was further verified by Schilling and coworkers. They transplanted mice with bone marrow from mice expressing green fluorescent protein (GFP) 3 months before subjecting them to 30 min of transient MCAO [70]. Microglia were activated and proliferated as early as day 1 after ischemia, whereas GFP-positive neutrophils did not start infiltrating the ischemic hemisphere until day 2, peaking at day 4. Macrophages entered the picture late, starting to infiltrate only on day 4 and peaking at day 10 after ischemia. Using the same model, this group also found that resident microglia were almost exclusively responsible for phagocytosis of dead and dying neurons during the first 3 days after ischemia [71].
Figure 5. Temporal course of infiltration of inflammatory cells in experimental models of stroke.
Activated microglia appear in the ipsilateral hemisphere early after focal ischemia, followed by an influx of neutrophils into the parenchyma. Macrophage infiltration after ischemia is a later event in most experimental paradigms.
Flow cytometry is increasingly used to characterize the cellular inflammation that takes place after cerebral ischemia. Microglia and infiltrating macrophages can be separated based on a differential degree of myeloid surface marker CD45 expression. While both cell types express both CD11b and CD45, CD45 levels are high in macrophages and low in resting microglia [72,73]. The number of CD45high cells in the ischemic hemisphere increases, starting at 18 h after transient MCAO and continues to increase until 48 h post-MCAO. At 24 h, 40% of CD45-positive cells are CD45high, in other words activated microglia or macrophages. Neutrophils invade only after microglial activation, starting at 48 h after MCAO. T cells invade only in small numbers and late after MCAO; T-cell counts increase twofold at 72 h [10]. A follow-up study by the same group using a similar paradigm confirmed that, while the percentage of CD45-positive cells that are microglia remains stable in the ischemic compared with the nonischemic hemisphere 48 h after MCAO (~60%), the percentage of activated microglia as defined by CD11bhigh compared with CD11blow expression increases from 25 to 57% [74].
A recent study provided a more in-depth look at the temporal and spatial dynamics of immune cell infiltration in the ischemic hemisphere after transient MCAO in mice. A combination of immunohistochemistry and flow cytometry experiments demonstrated that microglia and macrophages are the most abundant cell types present in the ischemic hemisphere during the first 72 h following MCAO. In sham-operated animals there were 27,000 microglia/hemisphere (80% of all immune cells). This number increased to 60,000 microglia/ischemic hemisphere on day 1 after MCAO. Invasion of neutrophils started on day 1 but did not reach large numbers (65,000/hemisphere) until day 3. Microglia and macrophages were differentiated more thoroughly in this study based on their level of CD45 expression: CD45low cells indicated resting microglia, CD45intermediate cells indicated activated microglia, and CD45high cells indicated macrophages. Based on that definition, macrophages were increased on day 1 (20,000/ischemic hemisphere) and day 3 (25,000/ischemic hemisphere) [9].
Positron emission tomography (PET) enables imaging of immune cell aggregation in the brains of living animals by using specific ligands. Cells of the mononuclear phagocyte lineage, including macrophages and reactive microglia, express the peripheral-type benzodiazepine receptor (PBR), whereas PBR expression in the brain is very low under physiological conditions. The isoquinolinecarboxamide derivative1-(2-chlorophenyl)-N-methyl-N-(1-methyl-propyl)-3-isoquinolinecarboxamide (PK11195) selectively binds to PBR, and [3H]PK11195 has been used extensively to detect microglial activation in ischemic rodent brain tissue by autoradiography [75]. With the aid of PET, PK11195 is increasingly utilized for the same purpose in living animals [75]. After 60 min transient MCAO in rats, a PK11195 signal appears at 4 days, and is even more pronounced at 7 days [75]. Immunohistochemical analysis of animals in the same study corroborated microglial activation starting at day 1 and increasing until day 7 in the ischemic core. mRNA expression of PBR in brain tissue was increased at 3 days, preceding the PET signal by 24 h. A caveat of this study is that PBR expression was heterogeneous, largely owing to high expression within microhemorrhages that were frequent within the infarct core. In addition, reactive astrocytes in the periphery of the infarct also expressed PBR, rendering the signal somewhat unspecific [76].
Using a newer, more sensitive radioligand for PBR, [18F]DPA (N,N-diethyl-2-(2-(4-(2-fluoroethoxy)phenyl)5,7-dimethylpyrazolo[1,5-a]pyrimidin-3-yl)acetamide)-714, Martin and coworkers reached similar conclusions. After 2 h MCAO in rats, the PET signal increased at 7 days, peaked at 11, and was still elevated at 30 days. Most signal colocalized with immunohistochemically CD11b-positive amoeboid cells that conglomerated in the infarct core starting at 4 and peaking at 11 days. In parallel, PBR expression increased in astrocytes that form a glial scar around the infarct core. The number of PBR-expressing astrocytes increased until 30 days; however, the PET signal followed the microglia/macrophage profile, peaking at 11 days – a finding that suggests that unspecific detection of astrocytes may not be a major problem of this technique [77].
Using a rat model of permanent ischemia by macrosphere-embolization and simultaneous imaging with PK11195 and 18F-2-fluoro-2-deoxy-D-glucose (FDG) to assess tissue viability, Schroeter and colleagues recently found that the PBR signal was localized not to the infarct core, but rather to the perimeter of the infarct, which was still perfused (intact perfusion and increased metabolism) [75]. According to immunohistochemical analysis, activated microglia and macrophages were localized in this area and formed a thick rim around the pan-necrotic infarct core. The authors conclude that the increased energy demand in this area (detected by FDG-PET) puts this area at risk after permanent ischemia. They postulate that this penumbral region is amenable to protective intervention [75].
PK11195-PET, which attempts to image the activation of innate microglia after ischemia, is nicely complemented by novel MRI techniques that use systemically applied ultra-small particles of iron oxide (USPIO) to label and image infiltrating blood-borne macrophages. USPIO are preferentially phagocytosed by monocytes before clearance within the reticuloendothelial system of the liver, spleen and lymph nodes. Upon acute migration into the diseased nervous system, these iron oxide-laden macrophages become visible on MRI because the superparamagnetic effects of iron oxide result in a signal loss on T2-weighted and/or bright contrast on T1-weighted MRI. Proof-of-concept for stroke was established by Rausch and coworkers [78]. They injected rats intravenously with USPIO 5 h after permanent MCAO, followed by repeated MRI on days 1, 2, 4, and 7. On T2-weighted images, areas of signal loss were found around the lesion on day 1 and inside the lesion until day 4, when they started to dissipate. These areas of signal loss were in full spatial agreement with activated microglia/macrophages, as detected by immunohistology using ED1. Several investigators using mouse and rat models of permanent and transient ischemia have since been able to confirm that macrophage infiltration only begins after a significant delay of several days. Most macrophages, and the correlating loss of T2 MRI signal, are found at the edge of the infarct and in or around the meningeal vessels [79].
There is some ongoing controversy as to what extent USPIO diffuses passively into the brain after disruption of the BBB, which could falsely suggest macrophage invasion. Mice injected with USPIO at 5 h after permanent MCAO and assessed by MRI and immunohistochemistry at 6 and 24 h demonstrated consistent USPIO-related signal changes on MRI but no evidence of iron-laden macrophages in the ischemic area, suggesting that most of the MRI signal is caused by leakage of unbound USPIO through an injured BBB [80,81]. This appears to be partly related to the type of USPIO used, however, since other groups using USPIO from a different manufacturer did not confirm significant leakage through the BBB.
Denes and colleagues found no signal loss after repeated USPIO injections up to day 3 after 30 min MCAO in mice and concluded that no significant macrophage invasion occurred at this early stage of infarct development. They did find infiltration of neutrophils beginning at 48 h using immunohistochemistry. Of note, the authors found that microglia phagocytose invading neutrophils, which they speculate may help to control and limit the inflammatory response after ischemia [82].
Further evidence that microglia may be policing the post-ischemic inflammatory response comes from a recent study using transgenic mice that express HSV1-thymidine kinase under control of the CD11b promoter (CD11b-TKmt30 mice). Proliferating microglia/macrophages were selectively ablated in these animals by ganciclovir treatment starting 48 h before 1 h of transient MCAO and continuing until 72 h after ischemia [83]. This regimen ablated proliferating microglia, but did not affect the resting microglia population in CD11b-TKmt30 mice. Interestingly, NF-κB activation was increased at 72 h, although not at 24 h after ischemia in transgenic animals only. Similarly, mRNA for the cytokines IL-6, IL-1β and TNF-α, as well as protein expression of IL-6 and TNF-α were significantly increased in transgenic animals at 72 h after MCAO. In WT animals, cytokine expression was already decreased at this time point. Furthermore, infarct size at 72 h was increased by 31% in transgenic mice compared with WT. At the same time, expression of IGF-1 was reduced 1.8-fold when microglia were ablated. In line with this, injection of macrophage-colony stimulating factor (M-CSF) increased the number of proliferating Mac-2 positive microglia and increased IGF-1 expression. The authors concluded that proliferating microglia produce neurotrophic factors such as IGF-1 and help reduce inflammation; thus, they are neuroprotective after ischemic brain injury. Interestingly, infarct size was only measured at 72 h in this study. Others have reported that infarct size does not change extensively beyond 24 h after transient MCAO in the mouse. Microglial ablation demonstrated no effect on cytokine expression at 24 h in this study, posing the question of whether infarct size at 24 h would have been similar between groups and only increased later in the transgenic animals. One possible interpretation of these findings is that some microglia undergo alternative activation after ischemia and produce anti-inflammatory cytokines and IGF-1 and, thus, limit ongoing inflammation that would otherwise lead to infarct size expansion beyond 24 h. Alternatively, this could also be accomplished by microglia phagocytosing or otherwise limiting the function of invading neutrophils.
A series of studies using transplantation of cultured microglia to limit ischemic injury provide further evidence that microglia may have beneficial effects after ischemia. Kitamura and colleagues injected 4000–50,000 microglia cultured from neonatal rats into the lateral ventricles of adult rats 1 h into a 2 h transient MCAO procedure. They found that transplanted microglia were present in the infarcted striatum at 8 days after injury [84] and reduced infarct size at 3 days, as well as improved behavioral outcome (longer times on the rotor rod) at 8 days after MCAO [85]. Similarly, neuronal death and functional deficit after global ischemia induced by two-vessel occlusion could be reduced in gerbils by intra-arterial injection of 106 cultured microglia immediately before ischemia or after reperfusion [86]. The mechanism by which transplantation of microglia reduces ischemic injury is unclear. While it is possible that a higher number of microglia are more efficient at limiting invading neutrophils, the constitutively mildly activated cultured microglia may also elicit an additional immune response in the brain, which then leads to secondary protection. A balance between injury and regeneration determines the final extent of tissue damage after an ischemic insult. While microglia may participate in limiting injury, evidence suggests that they may also support regeneration after ischemia. Cultured microglia that are activated by IFN-γ or IL-4 can promote neuronal differentiation of embryonic neurospheres in culture, partly through IGF-1 signaling [87]. Similarly, microglia proliferate in the subventricular zone (SVC) and remain there for up to 16 weeks after transient MCAO in rats, during which time neurogenesis is active in this region. At the same time, IGF-1 production is increased in the SVC. By contrast, microglial activation and proliferation in the infarct and penumbra peak at 2 weeks after MCAO and decline thereafter. These findings have led to some speculation that proliferating microglia in the SVC may provide long-term support for neurogenesis in that region after ischemia [88]. However, this is a controversial topic since there is also evidence that microglia may hinder neurogenesis. In a recent study, rats treated with minocycline for 4 weeks after transient MCAO had improved functional outcome after 6 weeks. Although infarct size was not affected by minocycline, fewer activated microglia were present in the dentate gyrus; and this was accompanied by a marked increase in neurogenesis, as assessed by 5-bromo-2-deoxyuridine (BrdU) and NeuN double-labeled cells [89].
Much of the evidence supporting a detrimental role of microglia after brain ischemia in vivo is indirect and derived from observations of negative effects of signaling pathways attributed to microglia. A prime example is TLR4, which is expressed in high levels at the cell surface of microglia and is activated by LPS, as well as DAMPs such as HSP and HMGB1 (Box 2 & Figure 3). Some strains of mice either have a deletion of the tlr4 gene (C57BL/10ScNJ) or possess a tlr4 gene with a point mutation that renders cells that express the TLR4 protein hyporesponsive to LPS (C3H/HeJ). Neuronal death and infarct size after global, as well as focal, ischemia is reduced in both of these mouse strains, as is NF-κB activation [90–93]. Of note, since cerebral blood flow was not measured in these studies, it is unknown whether insult severity was similar between experimental groups. It should also be noted that neither neurological outcomes nor infarct volume following MCAO has been reported to date in the genetically engineered TLR4−/− mice used for the genomic studies on pMG shown in Figure 2. TLR4 protein expression was upregulated in WT mice after global [93], as well as focal [91], ischemia, and was detected in astrocytes and in lectin-positive microglia [91]. TLR4 is also expressed at high levels by peripheral macrophages and to a lesser extent by neurons [94,95]; therefore, microglia may not account exclusively for the beneficial effect of TLR4 abrogation. Additional support for the notion that microglia are mediators of ischemic damage comes from experiments investigating the induction of ischemic tolerance by LPS preconditioning (PC) (Box 3). LPS PC, which likely involves signaling via TLR4, not only reduces infarct size after MCAO, but also blocks microglial activation and infiltration of neutrophils 48 h after MCAO [74]. Furthermore, TLR4 (and by implication, microglia) appears to be instrumental in inducing ischemic tolerance by ischemic PC (IPC) (Box 3). While TLR4-deficient mice generally are advantaged after permanent MCAO and sustain smaller infarcts compared with WT mice (28 vs 35 mm3), they are resistant to IPC, which leads to only 18% reduction of infarct size after 6 min PC ischemia compared with a 60% reduction seen in WT animals [96].
In summary, the role of microglia in cerebral ischemic injury has been examined using a great variety of in vivo experimental paradigms. It is noteworthy that there are some significant discrepancies between how microglia behave in vivo compared with in vitro. For example, in vivo microglia tend to proliferate in the ischemic penumbra following stroke whereas in vitro they exhibit significant cell death under severe hypoxic/hypoglycemic conditions. This may be due in part to secretion of growth factors by neighboring astrocytes and neurons in the in vivo setting. Alternatively, this difference may be due to the greater availability of potentially metabolizable sources of energy (most notably lactate) in vivo, but not in vitro. More broadly however, the in vivo findings clearly demonstrate that microglia function as a central cell type in the pathophysiology of cerebral ischemia. However, the heterogeneity of these experimental paradigms, the diversity of findings reported and the limitations in the specificity of microglial markers combine to make it difficult to extract an exact role specifically for microglia in the ischemic cascade. What is clear, however, is that microglia are rather complex actors within the interplay of injury and regeneration after cerebral ischemia in vivo. It is also clear that microglia act in concert with peripheral immune cells, as well as other CNS-intrinsic glial cells (particularly astrocytes), to orchestrate both neuroinflammatory and neuroprotective responses.
Role of microglia in stroke in humans
Similar to the in vivo animal model studies previously discussed, a number of histological studies have documented the presence of activated microglia in the ischemic penumbra following stroke in humans. Abundant reactive microglia/macrophages (as determined by cellular morphology, as well as by immunostaining for a variety of surface antigen and cytokine markers) have been found in the ischemic core within 1–2 days following ischemic infarction [105,106]. These cells persist in the ischemic hemisphere for a number of weeks following stroke. Microglia/macrophages were found predominantly at the periphery of the ischemic core with significant extension into the peri-infarct zones. Interestingly, there have been several case reports from autopsies of patients who died shortly after a cerebral ischemic insult who also had an underlying medical condition resulting in near-complete depletion of bone marrow reserve. In one case, robust microglial/macrophage staining was observed in the infarcted cortex 10 days following the event [107]. The authors argued that this finding supported the concept of an intrinsic brain-derived origin for these macrophage-like cells. In another case, macrophage-like cells in the infarcted lesion were scarce [108]. Correspondingly, the authors suggested a predominantly hematogenous origin for macrophage-like cells in stroked human brain in patients without underlying bone marrow depletion (i.e., the vast majority of stroke patients). Despite technical advances in microscopy, the question of origin of the macrophage-like cells in stroked human brain has been difficult to fully resolve using traditional staining and immunohistochemistry approaches. However, novel radiologic methods have provided data that has led to important insights into the temporal and spatial dynamics of inflammatory cell infiltration into the ischemic brain in humans.
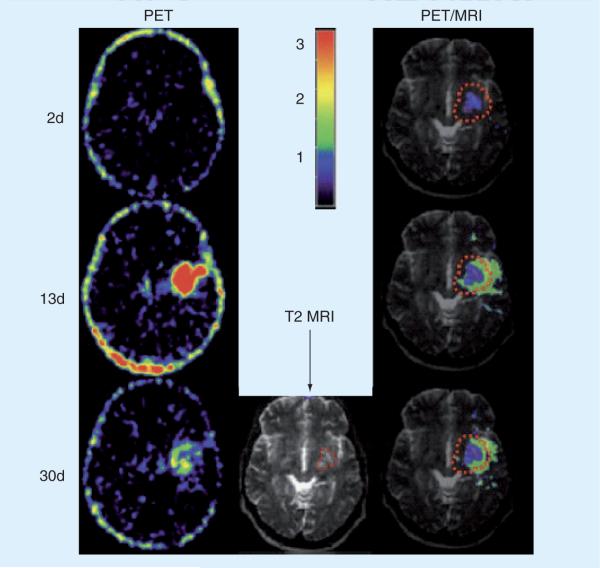
PET imaging of radiolabeled ligand11C-(R)-PK11195 has been used effectively to map the presence of activated microglia/macrophages in a variety of neurological diseases, including stroke [109]. Using this method, combined with structural brain imaging by MRI, Price et al. sequentially and quantitatively demonstrated that intrinsic activated microglia map to the peri-infarct zone in the subacute phase of ischemic stroke (Figure 6) [110]. Importantly, these findings were corrected for changes in cerebral blood volume (CBV) and referenced to non-involved ipsilateral cerebellar tissue [110]. The temporal characteristics of the response showed minimal binding within the first 72 h after stroke but rose significantly over the first week with some reduction by weeks 3 to 4. Spatially, binding was high within the ischemic core within 48 h but did not extend to the peri-infarct zones until 7–10 days after stroke onset. The authors commented that the extended time period for maximal 11C-(R)-PK11195 signal (and presumptive microglial activation/macrophage infiltration) may present a target for novel therapeutic agents designed to limit late neuronal damage and improve outcome [110]. Another PET study in human stroke patients reported long-lasting and significant increases in 11C-(R)-PK11195 binding in the thalamus ipsilateral to the affected hemisphere [111]. This increase was again observed 7 days after the infarct and persisted for a period of more than 4 weeks. The authors suggested that these findings indicated pathologic changes secondary to retrograde or anterograde degeneration of connecting fibers. A MRI-based study using USPIO particles suggested hematogenous uptake of macrophages within brain parenchyma within a similar time frame but independent of BBB function [112]. Another recent study of neutrophil migration in ischemic stroke suggests that there is a heterogeneous response across ischemic strokes of similar types, of which microglia form only a part [113]. These latter two studies in human stroke [112,113], along with similar data in rodent models [9,10], are important reminders that the role of microglial activation in ischemic stroke pathophysiology needs to be viewed in the context of a broader post-stroke immune response.
Figure 6. 11C-(R)-PK11195 radiolabeled activated microglia map to the peri-infarct zone in the sub-acute phase of stroke in humans.
Case shown depicts PET and MRI imaging findings at 2, 13 and 30 days postischemic infarction in the left basal ganglia of a 43-year-old woman. A color binding potential scale referring to the left hand column is provided. The right hand column demonstrates significant binding potential within the ischemic core (purple) and peri-infarct penumbra (green).
Adapted with permission from [110].
To summarize the above histological and radiological data from humans, it is likely that both intrinsic brain-derived microglia and invading peripheral macrophages enter the ischemic penumbra in large numbers following stroke in humans. The overall temporal and spatial dynamics of this response in human stroke resembles that described in the various in vivo animal models discussed in the previous section, although the time intervals involved for both infiltration and persistence of inflammatory cells in the ischemic human brain are significantly longer.
Therapeutic approaches targeting microglial function in stroke
Several means of modulating microglial responses are currently under investigation as potential therapeutic interventions in a variety of neurological diseases [58]. There is some in vitro evidence to suggest that specific viral vectors can be used to carry out effective targeted gene delivery to microglial cells [114]. The ability of viral vectors to carry out similar functions in vivo awaits further investigation. Work by several groups have demonstrated that modulating the systemic immune system by active or passive immunization with myelin-derived proteins can markedly reduce infarct volume and attenuate the neuroinflammatory response following acute ischemic injury [115,116]. Interestingly, administration of LPS at the time of reperfusion in these tolerization models results in sensitization to CNS antigens (rather than tolerance), increases in classical proinflammatory responses in the brain and worsening of neurological outcome [117]. While the mechanism of this LPS-triggered transformation from tolerance to sensitization in not completely understood, it likely involves modulation of both the innate and adaptive immune responses in both the brain and the periphery. Finally, pharmacologic agents that modulate microglial function (either by attenuation of classical proinflammatory pathways or by stimulation of alternative immunomodulatory programs) are of particular interest. As mentioned above, minocycline has anti-inflammatory properties related to the downregulation of microglial activation. In one small randomized, controlled, clinical trial in which minocycline (or placebo) was administered orally for 5 days starting within 6–24 h of stroke onset, neurological outcomes at 90 days were significantly better in the minocycline-treated patients [118]. Larger randomized clinical trials will be required to determine if minocycline is an effective treatment for acute ischemic stroke. In addition, since minocycline has multiple mechanisms of action, additional basic science mechanistic studies will be required to determine the contribution of microglial inhibition to any potential therapeutic effect. As previously described, multiple specific molecular targets that are expressed extensively, albeit not exclusively, on microglia are actively being investigated as potential therapeutic targets in acute ischemic stroke in humans. These include TLRs, thrombin receptor (proteolytically activated receptor [PAR]1) and prostaglandin receptor antagonists. Much of the focus to date has been on the ability of these antagonists to attenuate deleterious inflammatory responses in the penumbra following stroke (and thus be used as treatments in the immediate aftermath of stroke). However, data from PC studies suggests that pharmacologic agonists for these same signaling systems could be useful as pre-emptive protective agents [38,119]. A major theme in these PC studies focuses on the ability of the PC stimulus to attenuate and reprogram the CNS inflammatory response to subsequent injurious ischemia [74,99]. Although this strategy, at first glance, might seem impractical, it is important to keep in mind that robust clinical epidemiological data indicate high rates of ischemic stroke in the immediate aftermath of a transient ischemic attack [120] or in the perioperative period following particular surgeries such as carotid endarterectomy [121] and coronary artery bypass [122]. Thus, with the ready identification of large numbers of immediate high stroke-risk patients, pre-emptive/protective approaches, including ones that target microglia/macrophages, become more plausible and attractive.
Future perspective
Therapeutic strategies that specifically target microglia are not yet available; however, the use of viral vectors, immune tolerance strategies and specific pharmacological agonists/antagonists all represent viable approaches to future therapies for acute ischemic stroke. All of these approaches could plausibly modulate microglial function to a large extent following ischemic injury. Given the complex interplay between intrinsic brain-derived microglial responses and peripheral immune cell responses following stroke; and the fact that microglia and peripheral macrophages share extensive overlap in their surface antigen repertoire and cellular receptor profile, it may be neither feasible nor desirable to fashion future therapeutic approaches that target microglia to the exclusion of macrophages. A more promising approach might be to pursue strategies that result in favorable modulation of, and complementary shifts in, the genomic profile and phenotypic state of both of these critical immune cell types in the setting of ischemia. As described above, recent in vitro and in vivo studies on microglia have identified a number of molecular targets and signaling systems, modification of which could result in these desired effects. In characterizing the efficacy of these various therapeutic approaches in a nimal model systems, it may be helpful to use a c ombination of immunohistochemical, ex vivo flow cytometric and radiological methods to assess cell-type specific effects on microglia and invading macrophages in addition to monitoring stroke outcome measures. A comprehensive dataset of this nature can then be used to o ptimize and refine therapeutic approaches in an iterative manner and to add considerably to our mechanistic understanding of ischemic stroke pathophysiology.
Box 1. Hypoxia inducible factor.
-
■
Hypoxia inducible factor (HIF)-1 is a heterodimeric transcription factor that consists of the constitutively expressed HIF-1β subunit and the oxygen-tension regulated HIF-1α subunit. In the presence of oxygen, hydroxylation of the proline residues in the oxygen-dependent degradation domain of HIF-1α is catalyzed by the prolylhydroxylase proteins prolylhydroxylase protein (PHD)-1 and -3 [28].
-
■
Prolyl-hydroxylated HIF-1α is subsequently targeted for ubiquitin-dependent degradation. Thus, HIF-1α is present only at very low levels under normoxic conditions.
-
■
However, in hypoxic conditions, PHD-1 and -3 are themselves targeted for ubiquitin-dependent degradation leading to stabilization of HIF-1α. HIF-1α enters the nucleus, dimerizes with HIF-1β (to form the HIF-1 complex) and promotes transcription of genes with hypoxia response elements (HRE) in their promoter regions (Figure 3) [28]. These genes encode proteins involved in glucose transport/metabolism (glucose transporter-1 and phosphoglyceratekinase-1), vascular tone (adrenomedullin and endothelin-1), proliferation (PDGF and TGF-β), angiogenesis/neuroprotection (erythropoietin and VEGF) [28].
-
■
Many of these HIF-1-induced genes enhance hypoxic resistance and some researchers have hypothesized that HIF-1 plays an important role in neuroprotective phenomena, such as ischemic preconditioning (Box 3) [29].
Box 2. Toll-like receptors.
-
■
Toll-like receptors (TLRs) are a family of pattern-recognition receptors involved in the identification of, and response to, foreign pathogens.
-
■
To date, 11 TLRs have been identified in mammals, and each recognizes different pathogen-associated molecular patterns. TLRs are expressed on antigen-presenting immune cells and are critical in the innate immune response.
-
■
The TLR family includes receptors for bacterial cell wall/membrane components, such as lipotechoic acid (TLR2), peptidoglycan (TLR2) and lipopolysaccharide (LPS; TLR4). Other TLRs specifically recognize viral RNA (TLR3) and DNA (TLR9) molecules as well as bacterial cellular structures such as flagellin (TLR5) [37,38].
-
■
TLR4 induces signaling through recruitment of two competing sets of intracellular adaptor proteins: first, myeloid differentiation factor 88 (MyD88) and MyD88 adaptor-like protein (Mal) and, second, Toll–IL-1 receptor domain-containing adaptor factor (TRIF) and TRIF-related adaptor molecule (TRAM) (Figure 3).
-
■
Recruitment of the MyD88/Mal adaptor protein set leads to formation of the IL-1 receptor-associated kinase (IRAK) complex and downstream signaling cascades resulting in activation of the nuclear transcription factor NF-κB.
-
■
Activation of NF-κB in turn initiates a robust proinflammatory genomic program that includes induction of expression of cytokines such as TNF-α and IL-6. By contrast, recruitment of the TRIF/TRAM adaptor proteins results in downstream signaling that activates IRF3.
-
■
Activation of IRF3 in turn induces cell protective and anti-inflammatory changes in gene expression, including induction of immunomodulatory cytokine IFN-β. Activation of TLRs by endogenous ligands released from ischemia-injured cerebral vasculature and parenchyma has been proposed as a possible mechanism for initiation of proinflammatory responses in stroke (Figure 3) [37]. These endogenous ligands are sometimes referred to as danger-associated molecular patterns.
-
■
Proposed endogenous activators of TLRs include components released from necrotic cells (heat shock proteins [HSP], high mobility group box 1), extracellular matrix (fibronectin, hyaluronic acid and heparan sulfate), damaged blood vessels (fibrinogen/fibrin) and immune cells (defensin and elastase).
-
■
Many of these studies are limited by the possibility of LPS contamination in commercial-grade tissue/plasma-derived reagent preparations [39]. However, a recent carefully controlled investigation using highly purified, low endotoxin reagents has confirmed HSP60 as a legitimate endogenous activator of TLR4 [40]. In this study, HSP60 triggered neuronal injury in a mixed cortical cell culture model specifically by activating microglia in a TLR4- and MyD88-dependent manner.
Box 3. Preconditioning induces tolerance to subsequent ischemic insults by a brief exposure to ischemia or other non-lethal stress.
-
■
Ischemic preconditioning (IPC) imposes a brief episode of ischemia to induce a delayed window of transient tolerance. The protective effect of IPC requires 24−48 h to develop but is extremely robust and has been confirmed in numerous animal models. Whether the human brain can be preconditioned to tolerate ischemia is unknown. A number of recent clinical studies suggest that stroke patients who suffered a recent prior transient ischemic attack (TIA) have better outcomes than those who did not, implying that a human correlate to experimental IPC exists [97,98].
-
■
A number of additional stimuli can induce similar cross-tolerance towards ischemia, including short episodes of hypoxia, cortical spreading depression, hyperthermia, certain drugs and lipopolysaccharide (LPS).
-
■
The mechanism of LPS-induced preconditioning (PC) in the brain is an active area of investigation [74,99]. The specific cell type(s) that mediate the effect are unknown; however, LPS-induced PC strongly attenuates the robust microglial cell activation seen in the ischemic penumbra following prolonged ischemia [74].
-
■
In addition, the proinflammatory cytokine TNF-α, which is made predominantly by microglia in the brain [100], appears to play a critical role in LPS-induced PC as the phenomenon is completely absent in TNF-α-knockout mice [99].
-
■
PC genetically reprograms the brain's response to ischemia, which in turn forms the basis of ischemic tolerance [101]. Novel genomic approaches have recently been introduced and have demonstrated a striking array of gene expression changes in whole brain following a PC stimulus [101].
-
■
Interestingly, genetic reprogramming induced by PC using the antibiotic erythromycin attenuates the expression of inflammatory mediators after ischemia [102]. This PC-induced reprogramming may involve alternative activation of microglia, which is characterized by preferential expression of anti-inflammatory mediators and downregulation of proinflammatory cytokines [18].
-
■
A recent study indeed found that the inflammatory response to stroke after LPS-induced PC was shifted in this way to a type I interferon response, mediated by signal transduction through IRF3 [103].
-
■
The relevance of this phenomenon for ischemia-induced PC is unclear, however, since recent observations suggest that the innate immune response can differentiate between pathogen-associated molecular patterns such as LPS, and danger-associated molecular patterns such as HSP60 or HMGB1, which are released after ischemia [104].
-
■
The cell type-specific contribution of microglia to these genomic changes and the preconditioning phenomenon is currently under investigation.
Executive summary.
Microglia & neuroinflammation in stroke
-
■
A robust inflammatory response occurs in the ischemic penumbra following stroke. This response begins immediately but evolves over hours and days.
-
■
The response is complex and multifaceted but is characterized in part by an influx of leukocytes and a marked alteration in the microglial phenotype.
-
■
Responding to soluble signals from ischemic neurons and astrocytes, as well as plasma-derived factors, microglia proliferate and undergo a shift in their effector program, transforming their morphology and releasing both proinflammatory and immunomodulatory compounds.
-
■
The microglial response is modulated directly by ischemia itself and through exposure to danger-associated molecular patterns (DAMPs) released in the injured brain.
Effects of experimental ischemia on microglia: in vitro studies
-
■
In culture, severe ischemic conditions induce significant reductions in microglial viability.
-
■
Microglia are considerably more sensitive than astrocytes to ischemic injury.
-
■
While direct effects of ischemia on classical proinflammatory parameters in microglia are modest, combined hypoxia/hypoglycemia induces significant and broad-spectrum changes in the microglial genomic profile.
-
■
Ischemia induces robust activation of hypoxia inducible factor (HIF)-1 in microglia and expression of downstream HIF-1 gene targets (VEGF and Glut-1).
-
■
HIF-1 responses in microglia are augmented by DAMP-induced activation of surface receptors, including Toll-like receptors (TLRs).
-
■
Coculture and medium transfer experiments demonstrate that microglia respond to soluble signals released from ischemic neurons and glia.
Effects of experimental ischemia on microglia: in vivo studies
-
■
Immunohistochemical and ex vivo flow cytometric techniques have defined the temporal and spatial dynamics of immune cell infiltration in the ischemic penumbra.
-
■
Microglia are the first responders, followed closely by neutrophils and macrophages, and later by lymphocytes.
-
■
Positron emission tomography (PET) tracing microglia/macrophage selective radioligands and novel MRI techniques using ultra-small particles of iron oxide (USPIO), have confirmed a robust microglial/macrophage presence in the ischemic penumbra following stroke.
-
■
A transgenic mouse model that allows for selective ablation of proliferating microglia/macrophages has hinted at a neuroprotective function for myeloid cells in the acute phase of ischemic stroke.
-
■
Microglia also participate over longer time intervals in postischemic regeneration and neurogenesis.
-
■
A number of in vivo studies have implicated microglia as a critical cell-type in both the lipopolysaccharide preconditioning and ischemic preconditioning (IPC) phenomena.
Role of microglia in stroke in humans
-
■
PET and MRI techniques have confirmed the presence of activated microglia and macrophages in the peri-infarct zone during the subacute phase of stroke in humans.
-
■
The temporal and spatial dynamics of the inflammatory response in human stroke is similar to that seen in animal models; however, the time intervals for infiltration and persistence of microglia/macrophages are longer in humans.
Therapeutic approaches targeting microglial function in stroke
-
■
Strategies employing myeloid selective viral vectors, as well as both passive and active immunization approaches are ongoing areas of research in the field.
-
■
Numerous pharmacologic agents that modulate microglial function are also under investigation.
-
■
Some of these pharmacologic agents function as receptor antagonists that dampen the inflammatory response following stroke and are thus envisioned as treatments in the immediate aftermath of stroke.
-
■
Other agents function as either receptor agonists or activators of protective signaling pathways that could be used clinically in a pre-emptive manner in selective populations of patients at high and immediate risk for stroke.
Acknowledgements
The authors would like thank Richard V Lee, Theo K Bammler, Richard P Beyer and Frederico Farin for technical support.
Financial & competing interests disclosure This work was supported by NIH/NINDS grants NS44337 (Thomas Möller) and NS047309 (Jonathan R Weinstein), as well as American Heart Association grant 0750078Z (Thomas Möller). Additional funding support (for Ines P Koerner) was provided by the Medical Research Foundation of Oregon.
Footnotes
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
■of interest
■■of considerable interest
- 1.Mohr JP, Choi DW, Grotta JC, Weir B, Wolf PA. Stroke: Pathophysiology, diagnosis, and management. Fourth Edition Churchill Livingstone (an Imprint of Elsevier, Inc.); 2004. [Google Scholar]
- 2.Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22(9):391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 3.Green RA, Odergren T, Ashwood T. Animal models of stroke: do they have value for discovering neuroprotective agents? Trends Pharmacol. Sci. 2003;24(8):402–408. doi: 10.1016/S0165-6147(03)00192-5. [DOI] [PubMed] [Google Scholar]
- 4.Stroke Therapy Academic Industry Roundtable (STAIR) Preclinical Recommendations. Writing Committee composed of the following members: Finklestein SP, Fisher M (Chairman), Furlan AJ, Goldstein LB, Gorelick PB, Kaste M, Lees KR, Traystman RJ Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke. 1999;30(12):2752–2758. doi: 10.1161/01.str.30.12.2752.
- 5.Becker KJ. Targeting the central nervous system inflammatory response in ischemic stroke. Curr. Opin. Neurol. 2001;14(3):349–353. doi: 10.1097/00019052-200106000-00014. [DOI] [PubMed] [Google Scholar]
- 6.Lai AY, Todd KG. Microglia in cerebral ischemia: molecular actions and interactions. Can. J. Physiol. Pharmacol. 2006;84(1):49–59. doi: 10.1139/Y05-143. [DOI] [PubMed] [Google Scholar]
- 7.Becker K, Kindrick D, Relton J, Harlan J, Winn R. Antibody to the α4 integrin decreases infarct size in transient focal cerebral ischemia in rats. Stroke. 2001;32(1):206–211. doi: 10.1161/01.str.32.1.206. [DOI] [PubMed] [Google Scholar]
- 8.Goussev AV, Zhang Z, Anderson DC, Chopp M. P-selectin antibody reduces hemorrhage and infarct volume resulting from mca occlusion in the rat. J. Neurol. Sci. 1998;161(1):16–22. doi: 10.1016/s0022-510x(98)00262-7. [DOI] [PubMed] [Google Scholar]
- 9.Gelderblom M, Leypoldt F, Steinbach K, et al. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke. 2009;40(5):1849–1857. doi: 10.1161/STROKEAHA.108.534503. [DOI] [PubMed] [Google Scholar]; ■■ Most detailed and comprehensive analysis of the temporal dynamics of immune cell accumulation in the rodent stroke models available to date.
- 10.Stevens SL, Bao J, Hollis J, Lessov NS, Clark WM, Stenzel-Poore MP. The use of flow cytometry to evaluate temporal changes in inflammatory cells following focal cerebral ischemia in mice. Brain Res. 2002;932(1–2):110–119. doi: 10.1016/s0006-8993(02)02292-8. [DOI] [PubMed] [Google Scholar]
- 11.Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 2007;10(11):1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- 12.Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19(8):312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 13.Davalos D, Grutzendler J, Yang G, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 2005;8(6):752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 14.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308(5726):1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 15.Melchior B, Puntambekar SS, Carson MJ. Microglia and the control of autoreactive T cell responses. Neurochem. Int. 2006;49(2):145–153. doi: 10.1016/j.neuint.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartz M, Butovsky O, Bruck W, Hanisch UK. Microglial phenotype: is the commitment reversible? Trends Neurosci. 2006;29(2):68–74. doi: 10.1016/j.tins.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Streit WJ. Microglia as neuroprotective, immunocompetent cells of the CNS. Glia. 2002;40(2):133–139. doi: 10.1002/glia.10154. [DOI] [PubMed] [Google Scholar]
- 18.Colton CA. Heterogeneity of microglial activation in the innate immune response in the brain. J. Neuroimmune Pharmacol. 2009;4(4):399–418. doi: 10.1007/s11481-009-9164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lyons SA, Kettenmann H. Oligodendrocytes and microglia are selectively vulnerable to combined hypoxia and hypoglycemia injury in vitro. J. Cereb. Blood Flow Metab. 1998;18(5):521–530. doi: 10.1097/00004647-199805000-00007. [DOI] [PubMed] [Google Scholar]; ■ One of the earliest and most comprehensive in vitro analyses of the direct effects of severe ischemic conditions on microglia and other glial cell types.
- 20.Yenari MA, Giffard RG. Ischemic vulnerability of primary murine microglial cultures. Neurosci. Lett. 2001;298(1):5–8. doi: 10.1016/s0304-3940(00)01724-9. [DOI] [PubMed] [Google Scholar]
- 21.Park SY, Lee H, Hur J, et al. Hypoxia induces nitric oxide production in mouse microglia via p38 mitogen-activated protein kinase pathway. Brain. Res. Mol. Brain Res. 2002;107(1):9–16. doi: 10.1016/s0169-328x(02)00421-7. [DOI] [PubMed] [Google Scholar]
- 22.Guiwen G, Narayan RB. Hypoxia/reoxygenation differentially modulates NF-κB activation and inos expression in astrocytes and microglia. Antioxidants & Redox Signaling. 2006;8(5–6):911–918. doi: 10.1089/ars.2006.8.911. [DOI] [PubMed] [Google Scholar]
- 23.Sheehan JJ, Tsirka SE. Fibrin-modifying serine proteases thrombin, tpa, and plasmin in ischemic stroke: a review. Glia. 2005;50(4):340–350. doi: 10.1002/glia.20150. [DOI] [PubMed] [Google Scholar]
- 24.Sitkovsky M, Lukashev D. Regulation of immune cells by local-tissue oxygen tension: HIF1 α and adenosine receptors. Nat. Rev. Immunol. 2005;5(9):712–721. doi: 10.1038/nri1685. [DOI] [PubMed] [Google Scholar]
- 25.Burke B, Tang N, Corke KP, et al. Expression of HIF-1α by human macrophages: Implications for the use of macrophages in hypoxia-regulated cancer gene therapy. J. Pathol. 2002;196(2):204–212. doi: 10.1002/path.1029. [DOI] [PubMed] [Google Scholar]
- 26.Acosta-Iborra B, Elorza A, Olazabal IM, et al. Macrophage oxygen sensing modulates antigen presentation and phagocytic functions involving IFN-γ production through the HIF-1α transcription factor. J. Immunol. 2009;182(5):3155–3164. doi: 10.4049/jimmunol.0801710. [DOI] [PubMed] [Google Scholar]
- 27.Cramer T, Yamanishi Y, Clausen Be, et al. Hif-1α is essential for myeloid cell-mediated inflammation. Cell. 2003;112(5):645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■ Describes generation and characterization of the LysMcre/HIF1αloxp mouse strain. Demonstrates that myeloid cell HIF-1 is required for optimal inflammatory responses in peripheral immune cells.
- 28.Bernhardt WM, Warnecke C, Willam C, Tanaka T, Wiesener MS, Eckardt KU. Organ protection by hypoxia and hypoxiainducible factors. Methods Enzymol. 2007;435:221–245. doi: 10.1016/S0076-6879(07)35012-X. [DOI] [PubMed] [Google Scholar]
- 29.Gidday JM. Cerebral preconditioning and ischaemic tolerance. Nat. Rev. Neurosci. 2006;7(6):437–448. doi: 10.1038/nrn1927. [DOI] [PubMed] [Google Scholar]
- 30.Peyssonnaux C, Cejudo-Martin P, Doedens A, Zinkernagel AS, Johnson RS, Nizet V. Cutting edge: essential role of hypoxia inducible factor-1α in development of lipopolysaccharide-induced sepsis. J. Immunol. 2007;178(12):7516–7519. doi: 10.4049/jimmunol.178.12.7516. [DOI] [PubMed] [Google Scholar]
- 31.Peyssonnaux C, Datta V, Cramer T, et al. HIF-1α expression regulates the bactericidal capacity of phagocytes. J. Clin. Invest. 2005;115(7):1806–1815. doi: 10.1172/JCI23865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jantsch J, Chakravortty D, Turza N, et al. Hypoxia and hypoxia-inducible factor-1α modulate lipopolysaccharide-induced dendritic cell activation and function. J. Immunol. 2008;180(7):4697–4705. doi: 10.4049/jimmunol.180.7.4697. [DOI] [PubMed] [Google Scholar]
- 33.Leibovich SJ, Chen JF, Pinhal-Enfield G, et al. Synergistic up-regulation of vascular endothelial growth factor expression in murine macrophages by adenosine A2a receptor agonists and endotoxin. Am. J. Pathol. 2002;160(6):2231–2244. doi: 10.1016/S0002-9440(10)61170-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramanathan M, Pinhal-Enfield G, Hao I, Leibovich SJ. Synergistic up-regulation of vascular endothelial growth factor (VEGF) expression in macrophages by adenosine A2a receptor agonists and endotoxin involves transcriptional regulation via the hypoxia response element in the VEGF promoter. Mol. Biol. Cell. 2007;18(1):14–23. doi: 10.1091/mbc.E06-07-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leung PY, Packard AEB, Stenzel-Poore MP. It's all in the family: multiple toll-like receptors offer promise as novel therapeutic targets for stroke neuroprotection. Future Neurol. 2009;4(2):201–208. doi: 10.2217/14796708.4.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang X, Li C, Chen Y, et al. Hypoxia enhances cxcr4 expression favoring microglia migration via HIF-1α activation. Biochem. Biophys. Res. Commun. 2008;371(2):283–288. doi: 10.1016/j.bbrc.2008.04.055. [DOI] [PubMed] [Google Scholar]
- 37.Kariko K, Weissman D, Welsh FA. Inhibition of toll-like receptor and cytokine signaling – a unifying theme in ischemic tolerance. J. Cereb. Blood Flow Metab. 2004;24(11):1288–1304. doi: 10.1097/01.WCB.0000145666.68576.71. [DOI] [PubMed] [Google Scholar]
- 38.Marsh BJ, Stenzel-Poore MP. Toll-like receptors: novel pharmacological targets for the treatment of neurological diseases. Curr. Opin. Pharmacol. 2008;8(1):8–13. doi: 10.1016/j.coph.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weinstein Jr, Swarts S, Bishop C, Hanisch UK, Moller T. Lipopolysaccharide is a frequent and significant contaminant in microglia-activating factors. Glia. 2008;56(1):16–26. doi: 10.1002/glia.20585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lehnardt S, Schott E, Trimbuch T, et al. A vicious cycle involving release of heat shock protein 60 from injured cells and activation of toll-like receptor 4 mediates neurodegeneration in the CNS. J. Neurosci. 2008;28(10):2320–2331. doi: 10.1523/JNEUROSCI.4760-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■ Careful demonstration that HSP60-induced neuronal injury in vitro depends on microglial activation via Toll-like receptor (TLR)4.
- 41.Oh YT, Lee JY, Yoon H, et al. Lipopolysaccharide induces hypoxia-inducible factor-1 α mRNA expression and activation via NADPH oxidase and SP1-dependent pathway in BV2 murine microglial cells. Neurosci. Lett. 2008;431(2):155–160. doi: 10.1016/j.neulet.2007.11.033. [DOI] [PubMed] [Google Scholar]
- 42.Weinstein JR, Zhang M, Kutlubaev M, et al. Thrombin-induced regulation of cd95(FAS) expression in the n9 microglial cell line: evidence for involvement of proteinase-activated receptor(1) and extracellular signal-regulated kinase 1/2. Neurochem. Res. 2009;34(3):445–452. doi: 10.1007/s11064-008-9803-9. [DOI] [PubMed] [Google Scholar]
- 43.Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 2004;3(3) doi: 10.2202/1544-6115.1027. [DOI] [PubMed] [Google Scholar]
- 44.Wu Z, Irizarry R, Gentleman RC, Murillo FM, Spencer F. A model based background adjustment for oligonucleotide expression arrays. J. Am. Stat. Assoc. 2004;99(468):909–917. [Google Scholar]
- 45.Tusher VG, Tibshirani R, Chu G. Significance ana lysis of microarrays applied to the ionizing radiation response. Proc. Natl Acad. Sci. USA. 2001;98(9):5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment ana lysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA. 2005;102(43):15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weydt P, Pineda VV, Torrence AE, et al. Thermoregulatory and metabolic defects in huntington's disease transgenic mice implicate PGC-1α in huntington's disease neurodegeneration. Cell Metab. 2006;4(5):349–362. doi: 10.1016/j.cmet.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 48.Lai AY, Todd KG. Hypoxia-activated microglial mediators of neuronal survival are differentially regulated by tetracyclines. Glia. 2006;53(8):809–816. doi: 10.1002/glia.20335. [DOI] [PubMed] [Google Scholar]; ■ Demonstrates that microglia exacerbate hypoxia-induced neuronal death and established a useful in vitro experimental paradigm that included the transfer of conditioned medium from hypoxic microglia to neuronal cultures.
- 49.Kaushal V, Schlichter LC. Mechanisms of microglia-mediated neurotoxicity in a new model of the stroke penumbra. J. Neurosci. 2008;28(9):2221–2230. doi: 10.1523/JNEUROSCI.5643-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■■ Demonstrates that ischemic neurons activate microglia through release of glutamate and subsequent activation of glutamate receptor mGluR II. Establishes useful in vitro experimental model of ischemic penumbra using co-culture system for neurons and microglia.
- 50.Yrjanheikki J, Keinanen R, Pellikka M, Hokfelt T, Koistinaho J. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc. Natl Acad. Sci. USA. 1998;95(26):15769–15774. doi: 10.1073/pnas.95.26.15769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yrjanheikki J, Tikka T, Keinanen R, Goldsteins G, Chan PH, Koistinaho J. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc. Natl Acad. Sci. USA. 1999;96(23):13496–13500. doi: 10.1073/pnas.96.23.13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Frykholm P, Hillered L, Langstrom B, Persson L, Valtysson J, Enblad P. Relationship between cerebral blood flow and oxygen metabolism, and extracellular glucose and lactate concentrations during middle cerebral artery occlusion and reperfusion: a microdialysis and positron emission tomography study in nonhuman primates. J. Neurosurg. 2005;102(6):1076–1084. doi: 10.3171/jns.2005.102.6.1076. [DOI] [PubMed] [Google Scholar]
- 53.Weinstein JR, Hong S, Kulman JD, et al. Unraveling thrombin's true microglia-activating potential: markedly disparate profiles of pharmaceutical-grade and commercial-grade thrombin preparations. J. Neurochem. 2005;95(4):1177–1187. doi: 10.1111/j.1471-4159.2005.03499.x. [DOI] [PubMed] [Google Scholar]
- 54.Hooper C, Taylor DL, Pocock JM. Pure albumin is a potent trigger of calcium signalling and proliferation in microglia but not macrophages or astrocytes. J. Neurochem. 2005;92(6):1363–1376. doi: 10.1111/j.1471-4159.2005.02982.x. [DOI] [PubMed] [Google Scholar]
- 55.Quan Y, Moller T, Weinstein JR. Regulation of fcγ receptors and immunoglobulin γ-mediated phagocytosis in mouse microglia. Neurosci. Lett. 2009;464(1):29–33. doi: 10.1016/j.neulet.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Möller T, Nolte C, Burger R, Verkhratsky A, Kettenmann H. Mechanisms of c5a and c3a complement fragment-induced Ca2+i signaling in mouse microglia. J. Neurosci. 1997;17(2):615–624. doi: 10.1523/JNEUROSCI.17-02-00615.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Möller T, Contos JJ, Musante DB, Chun J, Ransom BR. Expression and function of lysophosphatidic acid receptors in cultured rodent microglial cells. J. Biol. Chem. 2001;276(28):25946–25952. doi: 10.1074/jbc.M102691200. [DOI] [PubMed] [Google Scholar]
- 58.Garden GA, Moller T. Microglia biology in health and disease. J. Neuroimmune Pharmacol. 2006;1(2):127–137. doi: 10.1007/s11481-006-9015-5. [DOI] [PubMed] [Google Scholar]
- 59.Hoek RM, Ruuls SR, Murphy CA, et al. Down-regulation of the macrophage lineage through interaction with ox2 (cd200) Science. 2000;290(5497):1768–1771. doi: 10.1126/science.290.5497.1768. [DOI] [PubMed] [Google Scholar]
- 60.Cardona AE, Pioro EP, Sasse ME, et al. Control of microglial neurotoxicity by the fractalkine receptor. Nat. Neurosci. 2006;9(7):917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- 61.Van Der Putten C, Zuiderwijk-Sick EA, Van Straalen L, et al. Differential expression of adenosine A3 receptors controls adenosine A2a receptor-mediated inhibition of TLR responses in microglia. J. Immunol. 2009;182(12):7603–7612. doi: 10.4049/jimmunol.0803383. [DOI] [PubMed] [Google Scholar]
- 62.Kuhn SA, Van Landeghem FK, Zacharias R, et al. Microglia express GABA(B) receptors to modulate interleukin release. Mol. Cell Neurosci. 2004;25(2):312–322. doi: 10.1016/j.mcn.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 63.Dello Russo C, Boullerne AI, Gavrilyuk V, Feinstein DL. Inhibition of microglial inflammatory responses by norepinephrine: effects on nitric oxide and interleukin-1β production. J. Neuroinflammation. 2004;1(1):9. doi: 10.1186/1742-2094-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pinteaux-Jones F, Sevastou IG, Fry VA, Heales S, Baker D, Pocock JM. Myelin-induced microglial neurotoxicity can be controlled by microglial metabotropic glutamate receptors. J. Neurochem. 2008;106(1):442–454. doi: 10.1111/j.1471-4159.2008.05426.x. [DOI] [PubMed] [Google Scholar]
- 65.Mabuchi T, Kitagawa K, Ohtsuki T, et al. Contribution of microglia/macrophages to expansion of infarction and response of oligodendrocytes after focal cerebral ischemia in rats. Stroke. 2000;31(7):1735–1743. doi: 10.1161/01.str.31.7.1735. [DOI] [PubMed] [Google Scholar]
- 66.Rupalla K, Allegrini PR, Sauer D, Wiessner C. Time course of microglia activation and apoptosis in various brain regions after permanent focal cerebral ischemia in mice. Acta Neuropathol. 1998;96(2):172–178. doi: 10.1007/s004010050878. [DOI] [PubMed] [Google Scholar]
- 67.Morioka T, Kalehua AN, Streit WJ. Characterization of microglial reaction after middle cerebral artery occlusion in rat brain. J. Comp. Neurol. 1993;327(1):123–132. doi: 10.1002/cne.903270110. [DOI] [PubMed] [Google Scholar]
- 68.Jander S, Schroeter M, D'urso D, Gillen C, Witte OW, Stoll G. Focal ischaemia of the rat brain elicits an unusual inflammatory response: Early appearance of CD8+ macrophages/microglia. Eur J. Neurosci. 1998;10(2):680–688. doi: 10.1046/j.1460-9568.1998.00078.x. [DOI] [PubMed] [Google Scholar]
- 69.Schroeter M, Jander S, Huitinga I, Witte OW, Stoll G. Phagocytic response in photochemically induced infarction of rat cerebral cortex. The role of resident microglia. Stroke. 1997;28(2):382–386. doi: 10.1161/01.str.28.2.382. [DOI] [PubMed] [Google Scholar]; ■ First demonstration that macrophage infiltration after stroke is delayed.
- 70.Schilling M, Besselmann M, Leonhard C, Mueller M, Ringelstein EB, Kiefer R. Microglial activation precedes and predominates over macrophage infiltration in transient focal cerebral ischemia: a study in green fluorescent protein transgenic bone marrow chimeric mice. Exp. Neurol. 2003;183(1):25–33. doi: 10.1016/s0014-4886(03)00082-7. [DOI] [PubMed] [Google Scholar]; ■ Elegant study using bone marrow chimeric mice to distinguish resident microglia from infiltrating macrophages after stroke.
- 71.Schilling M, Besselmann M, Muller M, Strecker JK, Ringelstein EB, Kiefer R. Predominant phagocytic activity of resident microglia over hematogenous macrophages following transient focal cerebral ischemia: an investigation using green fluorescent protein transgenic bone marrow chimeric mice. Exp. Neurol. 2005;196(2):290–297. doi: 10.1016/j.expneurol.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 72.Carson MJ, Reilly CR, Sutcliffe JG, Lo D. Mature microglia resemble immature antigen-presenting cells. Glia. 1998;22(1):72–85. doi: 10.1002/(sici)1098-1136(199801)22:1<72::aid-glia7>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 73.Ford AL, Goodsall AL, Hickey WF, Sedgwick JD. Normal adult ramified microglia separated from other central nervous system macrophages by flow cytometric sorting. Phenotypic differences defined and direct ex vivo antigen presentation to myelin basic protein-reactive CD4+ T cells compared. J. Immunol. 1995;154(9):4309–4321. [PubMed] [Google Scholar]
- 74.Rosenzweig HL, Lessov NS, Henshall DC, Minami M, Simon RP, Stenzel-Poore MP. Endotoxin preconditioning prevents cellular inflammatory response during ischemic neuroprotection in mice. Stroke. 2004;35(11):2576–2581. doi: 10.1161/01.STR.0000143450.04438.ae. [DOI] [PubMed] [Google Scholar]
- 75.Schroeter M, Dennin MA, Walberer M, et al. Neuroinflammation extends brain tissue at risk to vital peri-infarct tissue: a double tracer 11c-pk11195- and 18F-FDGPET study. J. Cereb. Blood Flow Metab. 2009;29(6):1216–1225. doi: 10.1038/jcbfm.2009.36. [DOI] [PubMed] [Google Scholar]
- 76.Rojas S, Martin A, Arranz MJ, et al. Imaging brain inflammation with 11cpk11195 by PET and induction of the peripheral-type benzodiazepine receptor after transient focal ischemia in rats. J. Cereb. Blood Flow Metab. 2007;27(12):1975–1986. doi: 10.1038/sj.jcbfm.9600500. [DOI] [PubMed] [Google Scholar]
- 77.Martin A, Boisgard R, Theze B, et al. Evaluation of the PBR/TSPO radioligand 8F-DPA-714 in a rat model of focal cerebral ischemia. J. Cereb. Blood Flow Metab. 2009 doi: 10.1038/jcbfm.2009.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rausch M, Sauter A, Frohlich J, Neubacher U, Radu EW, Rudin M. Dynamic patterns of uspio enhancement can be observed in macrophages after ischemic brain damage. Magn. Reson. Med. 2001;46(5):1018–1022. doi: 10.1002/mrm.1290. [DOI] [PubMed] [Google Scholar]
- 79.Saleh A, Wiedermann D, Schroeter M, Jonkmanns C, Jander S, Hoehn M. Central nervous system inflammatory response after cerebral infarction as detected by magnetic resonance imaging. NMR Biomed. 2004;17(4):163–169. doi: 10.1002/nbm.881. [DOI] [PubMed] [Google Scholar]
- 80.Desestret V, Brisset JC, Moucharrafie S, et al. Early-stage investigations of ultrasmall superparamagnetic iron oxide-induced signal change after permanent middle cerebral artery occlusion in mice. Stroke. 2009;40(5):1834–1841. doi: 10.1161/STROKEAHA.108.531269. [DOI] [PubMed] [Google Scholar]
- 81.Wiart M, Davoust N, Pialat JB, et al. MRI monitoring of neuroinflammation in mouse focal ischemia. Stroke. 2007;38(1):131–137. doi: 10.1161/01.STR.0000252159.05702.00. [DOI] [PubMed] [Google Scholar]
- 82.Denes A, Vidyasagar R, Feng J, et al. Proliferating resident microglia after focal cerebral ischaemia in mice. J. Cereb. Blood Flow Metab. 2007;27(12):1941–1953. doi: 10.1038/sj.jcbfm.9600495. [DOI] [PubMed] [Google Scholar]
- 83.Lalancette-Hebert M, Gowing G, Simard A, Weng YC, Kriz J. Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J. Neurosci. 2007;27(10):2596–2605. doi: 10.1523/JNEUROSCI.5360-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■■ Demonstrates the detrimental consequences of microglial ablation, supporting a neuroprotective role for microglia after stroke.
- 84.Kitamura Y, Yanagisawa D, Inden M, et al. Recovery of focal brain ischemia-induced behavioral dysfunction by intracerebroventricular injection of microglia. J. Pharmacol. Sci. 2005;97(2):289–293. doi: 10.1254/jphs.sc0040129. [DOI] [PubMed] [Google Scholar]; ■ A study of microglia transplantation supporting a protective role of microglia in stroke.
- 85.Kitamura Y, Takata K, Inden M, et al. Intracerebroventricular injection of microglia protects against focal brain ischemia. J. Pharmacol. Sci. 2004;94(2):203–206. doi: 10.1254/jphs.94.203. [DOI] [PubMed] [Google Scholar]
- 86.Imai F, Suzuki H, Oda J, et al. Neuroprotective effect of exogenous microglia in global brain ischemia. J. Cereb. Blood Flow Metab. 2007;27(3):488–500. doi: 10.1038/sj.jcbfm.9600362. [DOI] [PubMed] [Google Scholar]
- 87.Butovsky O, Ziv Y, Schwartz A, et al. Microglia activated by IL-4 or IFN-γ differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol. Cell. Neurosci. 2006;31(1):149–160. doi: 10.1016/j.mcn.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 88.Thored P, Heldmann U, Gomes-Leal W, et al. Long-term accumulation of microglia with proneurogenic phenotype concomitant with persistent neurogenesis in adult subventricular zone after stroke. Glia. 2009;57(8):835–849. doi: 10.1002/glia.20810. [DOI] [PubMed] [Google Scholar]
- 89.Liu Z, Fan Y, Won SJ, et al. Chronic treatment with minocycline preserves adult new neurons and reduces functional impairment after focal cerebral ischemia. Stroke. 2007;38(1):146–152. doi: 10.1161/01.STR.0000251791.64910.cd. [DOI] [PubMed] [Google Scholar]
- 90.Cao CX, Yang QW, Lv FL, Cui J, Fu HB, Wang JZ. Reduced cerebral ischemia-reperfusion injury in toll-like receptor 4 deficient mice. Biochem. Biophys. Res. Commun. 2007;353(2):509–514. doi: 10.1016/j.bbrc.2006.12.057. [DOI] [PubMed] [Google Scholar]
- 91.Caso JR, Pradillo JM, Hurtado O, Lorenzo P, Moro MA, Lizasoain I. Toll-like receptor 4 is involved in brain damage and inflammation after experimental stroke. Circulation. 2007;115(12):1599–1608. doi: 10.1161/CIRCULATIONAHA.106.603431. [DOI] [PubMed] [Google Scholar]; ■ The strength of this study linking TLR4 to stroke injury is that it uses two different strains of TLR-4 deficient mice.
- 92.Hua F, Ma J, Ha T, et al. Differential roles of TLR2 and TLR4 in acute focal cerebral ischemia/reperfusion injury in mice. Brain Res. 2009;1262:100–108. doi: 10.1016/j.brainres.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hua F, Ma J, Ha T, et al. Activation of toll-like receptor 4 signaling contributes to hippocampal neuronal death following global cerebral ischemia/reperfusion. J. Neuroimmunol. 2007;190(1–2):101–111. doi: 10.1016/j.jneuroim.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tang SC, Lathia JD, Selvaraj PK, et al. Toll-like receptor-4 mediates neuronal apoptosis induced by amyloid β-peptide and the membrane lipid peroxidation product 4-hydroxynonenal. Exp. Neurol. 2008;213(1):114–121. doi: 10.1016/j.expneurol.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Qiu J, Nishimura M, Wang Y, et al. Early release of HMGB-1 from neurons after the onset of brain ischemia. J. Cereb. Blood Flow Metab. 2008;28(5):927–938. doi: 10.1038/sj.jcbfm.9600582. [DOI] [PubMed] [Google Scholar]
- 96.Pradillo JM, Fernandez-Lopez D, Garcia-Yebenes I, et al. Toll-like receptor 4 is involved in neuroprotection afforded by ischemic preconditioning. J. Neurochem. 2009;109(1):287–294. doi: 10.1111/j.1471-4159.2009.05972.x. [DOI] [PubMed] [Google Scholar]
- 97.Sitzer M, Foerch C, Neumann-Haefelin T, et al. Transient ischaemic attack preceding anterior circulation infarction is independently associated with favourable outcome. J. Neurol. Neurosurg. Psychiatry. 2004;75(4):659–660. doi: 10.1136/jnnp.2003.015875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wegener S, Gottschalk B, Jovanovic V, et al. Transient ischemic attacks before ischemic stroke: preconditioning the human brain? A multicenter magnetic resonance imaging study. Stroke. 2004;35(3):616–621. doi: 10.1161/01.STR.0000115767.17923.6A. [DOI] [PubMed] [Google Scholar]; ■ An early clinical study demonstrating that history of transient ischemic attack is associated with reduced deficit after subsequent stroke in same vascular territory.
- 99.Rosenzweig HL, Minami M, Lessov NS, et al. Endotoxin preconditioning protects against the cytotoxic effects of TNFα after stroke: a novel role for TNFα in LPS-ischemic tolerance. J. Cereb. Blood Flow Metab. 2007;27(10):1663–1674. doi: 10.1038/sj.jcbfm.9600464. [DOI] [PubMed] [Google Scholar]
- 100.Hanisch UK. Microglia as a source and target of cytokines. Glia. 2002;40(2):140–155. doi: 10.1002/glia.10161. [DOI] [PubMed] [Google Scholar]
- 101.Stenzel-Poore MP, Stevens SL, Xiong Z, et al. Effect of ischaemic preconditioning on genomic response to cerebral ischaemia: similarity to neuroprotective strategies in hibernation and hypoxia-tolerant states. Lancet. 2003;362(9389):1028–1037. doi: 10.1016/S0140-6736(03)14412-1. [DOI] [PubMed] [Google Scholar]; ■■ Early study using gene microarray technology to demonstrate that preconditioning reprograms the brain's transcriptional response to subsequent injury.
- 102.Koerner IP, Brambrink AM. Brain protection by anesthetic agents. Curr. Opin. Anaesthesiol. 2006;19(5):481–486. doi: 10.1097/01.aco.0000245271.84539.4c. [DOI] [PubMed] [Google Scholar]
- 103.Marsh B, Stevens SL, Packard AE, et al. Systemic lipopolysaccharide protects the brain from ischemic injury by reprogramming the response of the brain to stroke: a critical role for IRF3. J. Neurosci. 2009;29(31):9839–9849. doi: 10.1523/JNEUROSCI.2496-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen GY, Tang J, Zheng P, Liu Y. CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science. 2009;323(5922):1722–1725. doi: 10.1126/science.1168988. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■■ First study to demonstrate that immune cells differentiate stimulation by pathogen-associated molecular patterns versus danger-associated molecular patterns.
- 105.Tomimoto H, Akiguchi I, Wakita H, et al. Glial expression of cytokines in the brains of cerebrovascular disease patients. Acta Neuropathol. 1996;92(3):281–287. doi: 10.1007/s004010050519. [DOI] [PubMed] [Google Scholar]
- 106.Krupinski J, Kaluza J, Kumar P, Kumar S. Immunocytochemical studies of cellular reaction in human ischemic brain stroke. MAB anti-CD68 stains macrophages, astrocytes and microglial cells in infarcted area. Folia Neuropathol. 1996;34(1):17–24. [PubMed] [Google Scholar]
- 107.Neuwelt EA, Garcia JH, Mena H. Diffuse microglial proliferation after global ischemia in a patient with aplastic bone marrow. Acta Neuropathol. 1978;43(3):259–262. doi: 10.1007/BF00691588. [DOI] [PubMed] [Google Scholar]
- 108.Casanova MF, Troncoso JC, Price Dl. Hematogenous origin of brain macrophages: a case report. Neurology. 1986;36(6):844–847. doi: 10.1212/wnl.36.6.844. [DOI] [PubMed] [Google Scholar]
- 109.Banati RB. Visualising microglial activation in vivo. Glia. 2002;40(2):206–217. doi: 10.1002/glia.10144. [DOI] [PubMed] [Google Scholar]; ■ Reviews use of the peripheral benzodiazepine receptor ligand PK11195 to visualize microglial/macrophage activation in vivo.
- 110.Price CJ, Wang D, Menon DK, et al. Intrinsic activated microglia map to the peri-infarct zone in the subacute phase of ischemic stroke. Stroke. 2006;37(7):1749–1753. doi: 10.1161/01.STR.0000226980.95389.0b. [DOI] [PubMed] [Google Scholar]; ■ Demonstrates spatial and temporal dynamics of microglial/macrophage activation in the peri-infarct zone following stroke in humans.
- 111.Pappata S, Levasseur M, Gunn Rn, et al. Thalamic microglial activation in ischemic stroke detected in vivo by PET and11c 1195. Neurology. 2000;55(7):1052–1054. doi: 10.1212/wnl.55.7.1052. [DOI] [PubMed] [Google Scholar]
- 112.Saleh A, Schroeter M, Jonkmanns C, Hartung HP, Modder U, Jander S. In vivo MRI of brain inflammation in human ischaemic stroke. Brain. 2004;127(Pt 7):1670–1677. doi: 10.1093/brain/awh191. [DOI] [PubMed] [Google Scholar]
- 113.Price CJ, Menon DK, Peters Am, et al. Cerebral neutrophil recruitment, histology, and outcome in acute ischemic stroke: an imaging-based study. Stroke. 2004;35(7):1659–1664. doi: 10.1161/01.STR.0000130592.71028.92. [DOI] [PubMed] [Google Scholar]
- 114.Balcaitis S, Weinstein JR, Li S, Chamberlain JS, Moller T. Lentiviral transduction of microglial cells. Glia. 2005;50(1):48–55. doi: 10.1002/glia.20146. [DOI] [PubMed] [Google Scholar]
- 115.Becker KJ, Mccarron RM, Ruetzler C, et al. Immunologic tolerance to myelin basic protein decreases stroke size after transient focal cerebral ischemia. Proc. Natl Acad. Sci. USA. 1997;94(20):10873–10878. doi: 10.1073/pnas.94.20.10873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Frenkel D, Huang Z, Maron R, et al. Nasal vaccination with myelin oligodendrocyte glycoprotein reduces stroke size by inducing IL-10-producing CD4+ T cells. J. Immunol. 2003;171(12):6549–6555. doi: 10.4049/jimmunol.171.12.6549. [DOI] [PubMed] [Google Scholar]
- 117.Becker KJ, Kindrick DL, Lester MP, Shea C, Ye ZC. Sensitization to brain antigens after stroke is augmented by lipopolysaccharide. J. Cereb. Blood Flow Metab. 2005;25(12):1634–1644. doi: 10.1038/sj.jcbfm.9600160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lampl Y, Boaz M, Gilad R, et al. Minocycline treatment in acute stroke: an open-label, evaluator-blinded study. Neurology. 2007;69(14):1404–1410. doi: 10.1212/01.wnl.0000277487.04281.db. [DOI] [PubMed] [Google Scholar]
- 119.Stenzel-Poore MP, Stevens SL, King JS, Simon RP. Preconditioning reprograms the response to ischemic injury and primes the emergence of unique endogenous neuroprotective phenotypes: a speculative synthesis. Stroke. 2007;38(2):680–685. doi: 10.1161/01.STR.0000251444.56487.4c. [DOI] [PubMed] [Google Scholar]
- 120.Johnston SC, Gress DR, Browner WS, Sidney S. Short-term prognosis after emergency department diagnosis of TIA. JAMA. 2000;284(22):2901–2906. doi: 10.1001/jama.284.22.2901. [DOI] [PubMed] [Google Scholar]
- 121.Rothwell PM, Eliasziw M, Gutnikov SA, et al. Analysis of pooled data from the randomised controlled trials of endarterectomy for symptomatic carotid stenosis. Lancet. 2003;361(9352):107–116. doi: 10.1016/s0140-6736(03)12228-3. [DOI] [PubMed] [Google Scholar]
- 122.Nalysnyk L, Fahrbach K, Reynolds MW, Zhao SZ, Ross S. Adverse events in coronary artery bypass graft (CABG) trials: a systematic review and ana lysis. Heart. 2003;89(7):767–772. doi: 10.1136/heart.89.7.767. [DOI] [PMC free article] [PubMed] [Google Scholar]