Abstract
Alcoholism is one of the most prevalent substance dependence disorders in the world. Advances in research in the neurobiological mechanisms underlying alcohol dependence have identified specific neurotransmitter targets for the development of pharmacological treatments. Acamprosate, marketed under the brand name Campral, is an orally administered drug available by prescription in the U.S. and throughout much of the world for treating alcohol dependence. Its safety and efficacy have been demonstrated in numerous clinical trials worldwide. Here we provide an overview of acamprosate in the context of the neurobiological underpinnings of alcohol dependence. We propose that unlike previously available pharmacotherapies, acamprosate represents a prototype of a neuromodulatory approach in the treatment of alcohol dependence. A neuromodulatory approach seeks to restore the disrupted changes in neurobiology resulting from chronic alcohol intake. It is our opinion that a neuromodulatory approach will provide a heuristic framework for developing more effective pharmacotherapies for alcohol dependence.
Keywords: acamprosate, alcohol dependence, alcoholism, Campral, treatment
Acamprosate is a safe and well-tolerated pharmacotherapy that has been studied in numerous clinical trials worldwide. It has been used to treat alcohol dependence in over 1.5 million patients since its introduction in Europe in 1989 and is currently available in most European and Latin American countries, Australia, parts of Asia and Africa [1], and more recently in the United States. Acamprosate, in combination with psychosocial support, was approved by the Food and Drug Administration (FDA) in July 2004 for the maintenance of abstinence from alcohol in detoxified alcohol-dependent patients. To date, its efficacy has been reported in 23 double-blind, placebo-controlled clinical trials conducted in 15 different countries. A recent survey found that acamprosate is now the most widely prescribed therapeutic agent for the treatment of alcoholism in the United States of America [2].
Alcohol-use disorders, which include both alcohol abuse and dependence, make up one of the most prevalent categories of substance use disorders in the US, affecting almost 18 million Americans. The Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition (DSM-IV)[3] characterizes alcohol dependence as a maladaptive pattern of drinking leading to clinically significant impairment, as manifested by a compulsion to drink, a lack of control over the amount of alcohol consumed and continued drinking, despite a realization of the problems associated with it. Physiological symptoms of tolerance and withdrawal may also be present. One of the most challenging aspects of recovering from alcohol dependence is maintaining abstinence after acute withdrawal and avoiding subsequent relapse to drinking [4].
Three medications are currently approved for the treatment of alcohol dependence—disulfiram, naltrexone, and acamprosate. Current research indicates that acamprosate has a unique mechanism of action, which may have implications for its therapeutic use [5]. In contrast to disulfiram, which causes aversive behavior through negative physical effects, or naltrexone, which tempers the pleasurable effects of alcohol, acamprosate acts to normalize dysregulation in neurochemical systems that have been implicated in the biological mechanisms of alcohol dependence.
In this review, we discuss the clinical efficacy and safety profile of acamprosate in the treatment of alcohol dependence. We provide a brief discussion of the neurobiological changes that occur to the central nervous system during chronic alcohol intake. This is provided to highlight the fact that the physiological response to initial alcohol exposure is different than that observed following chronic exposure (i.e., neuroadaptive changes occur during the transition from initial alcohol use to alcohol dependence). We propose that unlike previously available pharmacotherapies, acamprosate reprsesents a prototype of a neuromodulatory approach in the treatment of alcohol dependence. A neuromodulatory approach seeks to restore the disrupted changes in neurobiology resulting from chronic alcohol intake. It is our opinion that a neuromodulatory approach will provide a heuristic framework for developing more effective pharmacotherapies for alcohol dependence.
CHEMISTRY, FORMULATION, DOSAGE AND ADMINISTRATION
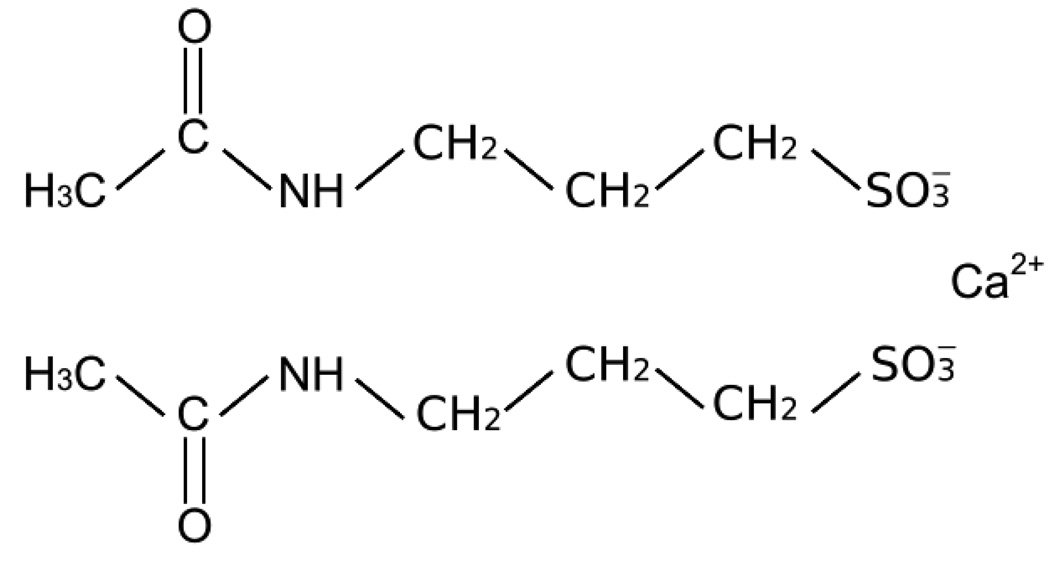
The chemical structure of acamprosate can be seen in Figure 1. Acamprosate, calcium acetylhomotaurinate, is a synthetic compound with a chemical structure similar to the amino acid neurotransmitter gamma-aminobutyric acid (GABA) and the amino acid neuromodulator taurine [6]. Acamprosate is available by prescription as a 333-mg tablet, and the recommended dosage regimen is two 333-mg tablets taken orally three times a day. Acamprosate may be prescribed in either a bottle or blistercard at equal expense. The blistercard indicates day and time of dose and may be superior for facilitating and monitoring medication compliance.
Figure 1.
The chemical structure of acamprosate.
PHARMACOKINETICS AND METABOLISM
Acamprosate is absorbed via the gastrointestinal tract, with pharmacokinetic linearity in terms of dose and time. The mean maximum plasma concentration (Cmax) of acamprosate was 180 ng/ml, following oral administration of a single 2 × 333 mg dose to healthy volunteers [7,8]. Steady-state peak plasma concentrations after 2 × 333 mg administered three times daily averaged 350 ng/ml and were reached within 3–8 hours following oral dosing [9]. Absolute bioavailability of acamprosate under fasting conditions is approximately 11% [7]; after food intake, bioavailability decreases by approximately 20%, but this decrease lacks clinical significance [10]. Steady-state plasma concentrations of acamprosate are reached within 5 days of dosing and the terminal half-life ranges from 20–33 hours following the standard 2 × 333 dosing regime. Plasma protein binding is negligible. Importantly, acamprosate is not metabolized in the liver [11] and is excreted unchanged in the urine [10].
Because acamprosate is not metabolized by the liver, the pharmacokinetics of acamprosate are not altered in patients with mild to moderate hepatic insufficiency (Groups A and B of the Child-Pugh classification), indicating that no dosage adjustments are necessary [9]. After a single dose administration (2 × 333 mg) to patients with severe renal impairment (creatinine clearance ≤30 mL/min), peak plasma concentrations were 4-fold higher, and plasma elimination half-life was 2.6-fold longer compared to healthy subjects [10]. Due to the risk of accumulation of acamprosate with prolonged administration of therapeutic doses in renally-impaired patients [10], the use of acamprosate is contraindicated in patients with severe renal impairment, and a dosage adjustment to one 333-mg tablet administered three times daily is recommended in patients with moderate renal impairment (creatinine clearance 30–50 mL/min [9,10]. No dose adjustment is required for patients with mild renal impairment (creatinine clearance >50 mL/min). Since acamprosate is excreted primarily by the kidney and elderly patients are more likely to have diminished renal function, dosage adjustments may be necessary.
The pharmacokinetics of acamprosate have not been evaluated in pediatric or geriatric populations. No significant pharmacokinetic differences are observed between male and female subjects [9]. Acamprosate is assigned a Category C labeling in pregnancy. The animal reproduction studies demonstrated some teratogenic effects in rat fetuses at doses comparable to the human dose on a mg/m2 basis and in rabbit fetuses at doses approximately three times the human dose. In the rat these malformations included hydronephrosis, malformed iris, retinal dysplasia, and retroesophageal sublclavian artery; hydronephrosis was also observed in the rabbit [12]. Hydronephrosis is a distension and dilation of the renal pelvis and calyces that can lead to progressive atrophy of the kidney. To date, there are no adequate well-controlled studies of acamprosate in pregnant women; therefore, acamprosate should only be used during pregnancy if the potential benefit justifies the potential risk to the fetus.
DRUG-DRUG INTERACTIONS
Acamprosate is not metabolized by the liver and therefore is unlikely to cause drug-drug interactions via cytochrome P450 inhibition. The pharmacokinetics of acamprosate are not altered by co-administration with diazepam, disulfiram, antidepressants, or alcohol—substances that are often taken by patients with alcohol dependence. In pharmacokinetic studies with human subjects, co-administration with naltrexone increased the rate and extent of acamprosate absorption [13.14]. These results suggest that combination therapy may improve the bioavailability of acamprosate without compromising its tolerability [13.14].
A BRIEF OVERVIEW OF THE NEUROBIOLOGY OF ALCOHOL DEPENDENCE
Alcohol dependence is a complex psychological and neurobiological disorder. It is important to recognize that as an individual moves from initial alcohol use to the other end of the spectrum (dependence), parallel dynamic changes are occurring throughout the nervous system (i.e., these systems are constantly moving targets). These perturbations and disruptions result in neuroadaptations that contribute to overall dysregulations in normal daily behaviors (e.g., work, social interactions) as well as the development of alcohol dependence. Several key receptors mediate the response to acute and chronic alcohol intake [4,5]. This section identifies only a few neural systems known to be affected by alcohol to highlight the changes that occur as one transitions from initial to chronic heavy alcohol intake.
Starting with dopamine; acute alcohol administration in intoxicating doses (20–40 mM) activates neurons in the ventral tegmental area both in vivo in anesthetized rats [15] and in vitro using extracellular recording in brain slice preparations [16]. Following dependence and during withdrawal from alcohol, there is a decrease in dopaminergic activity in the ventral tegmental area that has been linked to the dysphoria of acute and protracted withdrawal [17–19]. The decrease in dopaminergic activity in the ventral tegmental area is consistent with microdialysis studies showing decreases in dopamine release in the nucleus accumbens during alcohol withdrawal. Reduced dopaminergic neurotransmission is prolonged, outlasting the physical signs of withdrawal [18,20].
In the absence of alcohol, neuronal excitatory (glutamate receptors) and inhibitory (GABA receptors) activity is maintained in equilibrium [4,5]. Acute alcohol at very low doses blocks responses of the N-methyl-d-aspartate (NMDA) receptor, seen initially in vitro in electrophysiological studies [21–23] and subsequently in vivo [24]. Alcohol decreases the function of all three major classes of ionotropic glutamate receptor subtypes: NMDA, kainate, and alpha-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) [22,25]; resulting in a decrease in glutamatergic activity. Alcohol in intoxicating doses (20–40 mM) activates neurons in the ventral tegmental area both in vivo in anesthetized rats [15] and in vitro using extracellular recording in brain slice preparations [16]. Several behavioral, neurochemical, and electrophysiological findings have suggested that chronic heavy alcohol use or withdrawal may upregulate NMDA receptor function in brain [26–31]. These studies suggested a role for glutamate in the behavioral sensitization elicited by repeated alcohol withdrawal, suggesting that glutamate receptors play a major role in the hyperexcitability following withdrawal from alcohol, and perhaps in alcohol-seeking behavior associated with dependence [32].
Molecular studies have shown that GABA-induced chloride fluxes are increased by acute alcohol exposure in cultured neurons [33] and synaptoneurosomes [34,35]; resulting in enhanced GABA activity. Chronic alcohol exposure, in contrast to the effects of acute alcohol, generally downregulates GABAa receptor function as measured by chloride ion flux [36], without changes in receptor density or affinity. Moreover, decreases in GABA-mediated chloride ion flux have been reported shortly after withdrawal from chronic exposure in cortex and cerebellum [37]
Acute alcohol exposure also inhibits voltage-gated calcium channels [38,39]. In contrast, chronic exposure to ethanol results in an up-regulation in the density of voltage-dependent calcium channels [40,41].
These studies illustrate that these systems contribute to the acute behavioral effects of alcohol and that during chronic alcohol exposure, the body compensates with neuroadaptive adjustments within these systems. These neuroadaptions presumably are triggered in order to restore normal neuronal excitability in the presence of ethanol. Therefore, it important to note that in the absence of alcohol (i.e., during the course of abstinence and/or detoxification) these systems are in a state of disregulation.
MECHANISM OF ACTION OF ACAMPROSATE
Acamprosate is an analogue of amino acid neurotransmitters such as taurine and homocysteic acid [42]. Naassila et al. [43] and al Qatari et a [44] have demonstrated that acamprosate binds to a specific spermidine-sensitive site that modulates the NMDA receptor in a complex way [42]. Their work suggests that acamprosate acts as a “partial co-agonist” at the NMDA receptor, such that low concentrations enhance activation when receptor activity is low, and high concentrations inhibit activation when receptor activity is high. Earlier work had indicated that acamprosate might have inhibitory effects on NMDA receptors [45]. This may be particularly relevant to the success of acamprosate as a pharmacotherapy given that chronic exposure to ethanol results in an up-regulation of NMDA receptors [29,46] and an up-regulation in the density of voltage-dependent calcium channels [40,41]. Thus, sudden alcohol abstinence causes the excessive numbers of NMDA receptors to be more active than normal, and to produce the symptoms associated with acute alcohol withdrawal, such as delirium tremens and seizures and with the more persisting symptoms associated with early abstinence, such as craving and disturbances in sleep and mood [47]. Withdrawal from alcohol induces a surge of excitatory neurotransmitters like glutamate, which in turn activates the NMDA receptors [48]. Conversely, acamprosate promotes the release of taurine in the brain [42]. Taurine is a major inhibitory neuromodulator/neurotransmitter and an increase in taurine availability would also contribute to a decrease in hyperexcitability. Thus, each of these changes produced by acamprosate may contribute to the decreased neuronal hyperexcitability observed during early abstinence [49]. These changes may underly the symptoms associated with relapse, such as craving, anxiety and insomnia. Therefore, it has been hypothesized that acamprosate may promote abstinence by minimizing or negating some of the physiological changes produced by chronic heavy ethanol exposure [50]. Further support for this hypothesis is provided by a recent polysomnography study that found acamprosate reversed alcohol-related changes in sleep architecture in humans [51].
THE NEUROPROTECTIVE EFFECTS OF ACAMPROSATE
A growing body of work suggests that acamprosate may have neuroprotective effects [52–57]. For example, it has been reported that acamprosate inhibited the neurotoxicity caused by anoxia in an animal model of stroke [52]. Neuroprotection could be particularly important in the treatment of alcohol dependence given the potential impact of chronic ethanol exposure and withdrawal on neuronal survival [30,46,58]. For example, research with neuronal cultures has shown that an up-regulation in NMDA receptor function occurs after ethanol exposure [59,60]. At the onset of withdrawal, this up-regulation can lead to neurotoxicity (cell death) caused by NMDA receptor overactivation and a subsequent excess influx of calcium [60]. It should be noted that acamprosate does not appear to produce neurotoxicity at clinical dosing [54]. Acamprosate has been shown to significantly reduce medium change-induced toxicity [54], results consistent with those showing that acamprosate protects against neuronal damage produced by experimental induced ischemia [52]. Furthermore, acamprosate reduces spermidine-induced neurotoxicity, but not NMDA-induced toxicity [54]. From these results, it appears that acamprosate inhibits this aspect of withdrawal-induced toxicity in ways that are different than those of direct NMDA receptor antagonism. This suggests that the effects of acamprosate during withdrawal from alcohol are largely a consequence of its indirect (possibly via the spermidine site) inhibitory effects on NMDA receptors [57].
ANIMAL MODELS OF ALCOHOL SELF-ADMINISTRATION AND WITHDRAWAL
Acamprosate has been shown to reduce ethanol consumption in rodents that have an extended history of ethanol exposure or are ethanol-dependent [62–64]. In contrast, acamprosate appears to have less of an effect on alcohol consumption in alcohol naïve and non-dependent rats [63,65,66]. More specifically, Le Magnen et al. [63] reported a 50–70% reduction in ethanol intake in ethanol dependent animals after the administration of 200 mg/kg acamprosate, where only a dose of 450 mg/kg reduced drinking in alcohol-naïve animals. Boismare et al [62] also reported a decrease in ethanol intake in animals given chronic exposure to acamprosate (200 mg/kg). However, the rats used in this study were selected for the highest ethanol intake, and consequently only 24% of the total population of rats was tested. More recently, acamprosate was shown to reduce ethanol intake in C57BL/6J mice using the drinking-in-the-dark procedure (used to obtain higher intake of alcohol [67] and to show efficacy in an escalation model of self-administration [68]. Acamprosate has been shown to reduce the increased ethanol consumption associated with a period of enforced abstinence from ethanol (the alcohol deprivation effect) in rats [64,65]. In the study by Spanagel et al [64], acamprosate decreased the alcohol deprivation effect and also reduced ethanol intake below baseline levels, whereas Heyser et al [65] reported only an elimination of the alcohol deprivation effect, with no effect on baseline intake of alcohol. However, it is important to note that in the study by Spanagel et al [64] the rats had 24-h access to ethanol for 8 months prior to testing, while animals in the Heyser et al [65] study were trained for only 3 months in a limited access paradigm (30 min/day). Taken together, the action of acamprosate may be strongest in animals that prefer alcohol or have a history of alcohol dependence.
In addition to the direct effects on ethanol consumption, acamprosate has been reported to attenuate some of the behavioral and neurochemical events associated with ethanol withdrawal [69–72]. For example, acamprosate reduces the hyperactivity and elevated glutamate levels observed during the first 12 hours of ethanol withdrawal [69]. Acamprosate has also been shown to attenuate ethanol withdrawal anxiety-like behavior in the plus-maze test [70,71] and to attenuate handling induced convulsions during ethanol withdrawal [72]. In addition, acamprosate has been shown to inhibit cue-induced reinstatement of alcohol-seeking behavior in an operant conditioning model [73]. However, not all aspects of withdrawal are reduced by acamprosate, such as withdrawal-induced hypothermia [74]. Taken together, these findings further support the hypothesis that acamprosate may have differential effects on ethanol-related behaviors specific to the history of ethanol experience (dependent versus nondependent) and/or preference for alcohol. Thus, these results provide support for the use of acamprosate specifically as an anti-relapse medication.
CLINICAL SAFETY AND TOLERABILITY
The safety profile of acamprosate is quite favorable. It is important to note that given the complex nature of both alcohol dependence and the symptoms of early abstinence it is often difficult to make a complete distinction between alcohol-related symptoms and adverse side-effects of the drugs per se. However, given this caveat, the adverse events associated with acamprosate tend to be mild and transient. Additionally, Rosenthal et al [75] reported in a re-analysis of clinical safety data that new adverse events were unlikely to emerge after the first 4 weeks of treatment.
The only adverse event consistently reported across trials more frequently in acamprosate-treated patients (16%) with respect to placebo-treated patients (10%) was mild and transient diarrhea [75]. In a study by Paille et al., [76], the proportion of patients that experienced diarrhea appeared to be dose-dependent: 7.5% of the patients who received 1332 mg/day of acamprosate reported diarrhea versus 12% of patients who received 1998 mg/day (in this study the rate of diarrhea among placebo patients was 3.4%). However, no dose effect on adverse events was noted in a second dose-response study by Pelc et al. [77].
Since its approval in Europe in 1989, pharmacovigilance data has identified no serious health risk of acamprosate use in >1.5 million patients [78]. There were no differences between treatment groups in the rate of discontinuations due to adverse events (8% of acamprosate versus 6% of placebo patients had discontinued from trials of less than or equal to 6 months duration; 7% of patients in both groups had discontinued from trials longer than 6 months) [12]. In addition, concomitant use of alcohol has no effect on the pharmacokinetics of acamprosate, suggesting patients can safely continue acamprosate through a period of relapse [11]. Clinical investigations show no evidence of tolerance, dependence or the emergence of a withdrawal syndrome or rebound drinking when treatment is ceased [1]. Furthermore, a reanalysis of three efficacy trials submitted for FDA approval demonstrated that acamprosate had an advantage over placebo on efficacy outcomes for subjects with either normal or abnormal (greater than twice the upper limit of normal value) baseline liver function status [75]. This finding, along with the lack of hepatic metabolism, suggests that acamprosate can be safely and effectively used by patients with mild and moderate liver dysfunction. And finally, it is important to note that alcohol-dependent patients are higher at risk for severe depression and suicide compared to control populations [75]. In a detailed analysis of the reported data, Rosenthal [75] reported that the suicide rate (in terms of completed attempts) in the placebo group (0.10%) was similar to that observed in acamprosate-treated patients (0.13%).
Several studies have examined the neuropsychological effects of acamprosate over time and with repeat testing in both healthy and alcohol-dependent subjects [14,79,80]. The assessed cognitive domains include attention, concentration, learning, working memory, and long-term memory. Acamprosate showed no clinically relevant effects on cognition or reaction time. Most of these studies used a within-subject design, which provides power to detect very subtle changes, such as differences in performance of 1 word or a few milliseconds that may achieve statistical significance but lack clinical relevance [14,79,80].
ACAMPROSATE EFFECTS ON SLEEP ARCHITECTURE
Several recent studies suggest that acamprosate may help to normalize the underlying neurobiological mechanisms of alcohol-related disturbances in alertness and sleep [51]. Sleep disturbances are another challenge often faced by recently detoxified patients as it has been shown that sleep disturbances can persist for up to 4 years of complete abstinence [81–84]. More specifically, these initial studies show that acamprosate improved sleep continuity, restored stage III sleep, and increased rapid eye movement (REM) sleep latency, all of which are classically described as important determinants of relapses [85].
NEUROIMAGING STUDIES
Insight into the neurocircuitry changes in the human brain associated with the development and maintenance of addiction have come from neuroimaging techniques (eg, positron emission tomography [PET], functional magnetic resonance imaging [fMRI], and magnetic resonance spectroscopy [MRS]) [86]. These imaging studies provide evidence for the involvement of frontal cortical structures in the various stages of the addiction cycle (i.e., reinforcing responses to drugs during intoxication, activation during craving, and deactivation during withdrawal) [87] Several neuroimaging studies have been conducted to investigate the effects of acamprosate on neural activity in humans. In one randomized, double-blind, placebo-controlled, cross-over study (designed to ensure that each of the 8 healthy subjects acted as his own control) the results of dynamic MRS showed decreases in the regions for which N-acetylaspartate and glutamate are the main signal contributors [88]. The results suggest a central glutamatergic effect of acamprosate that is consistent with glutamate measurements in alcoholized rats treated with acamprosate [49]. The neuroimaging results with acamprosate are further supported by a recent double-blind, randomized, placebo-controlled study with a parallel group design using magnetoencephalography (MEG), a technology similar to electroencephalography (EEG) [89] In this experiment, 24 subjects meeting DSM-IV criteria for alcohol dependence received acamprosate or placebo treatment during the 8 days prior to withdrawal from alcohol and during the following 15 days of mandatory abstinence. The authors reported lower alpha slow-wave activity in the frontoparietal regions in patients receiving acamprosate, compared to placebo Thus, acamprosate appeared to have a sustained effect on the withdrawal-related hyperexcitatory state which allows alcohol-dependent patients to return to a normal level of neural activity more rapidly than placebo [89]. These findings support earlier preclinical studies that suggest a neuroprotective effect of acamprosate [55,61].
CLINICAL EFFICACY
Study Methodology
FDA approval of acamprosate was based primarily on efficacy data from three published European studies of 13-week [77] 48-week [90] and 52-week [76] duration, involving 998 alcohol-dependent patients. These pivotal clinical trials demonstrated that, in combination with psychosocial support, acamprosate was superior to placebo in maintaining abstinence, as indicated by a greater percentage of patients who remained completely abstinent throughout the treatment period, a longer time to first drink, and an increase in the percent days abstinent in those patients who experienced a relapse [76,77,90,91]. Overall, the safety and efficacy of acamprosate have been described in 23 published controlled clinical trials, involving approximately 6,500 patients [76,77,90–110].
Study treatment durations ranged from two months to one year. Several studies also included post-treatment follow-up periods of up to one year. All of the studies were double-blind, placebo-controlled, parallel-group comparisons with randomized assignment of patients to treatments. Although one trial involved alcohol-dependent adolescents [106], most of the trials involved patients 18–65 years of age who met DSM-III-R or DSM-IV criteria for alcohol dependence, had elevated baseline gamma-glutamyltransferase (GGT) levels (a biological marker of alcohol intake), were detoxified and abstinent for some period of time (typically for at least 5 days) prior to randomization, and were also participating in some form of psychosocial therapy. Exclusion criteria included serious medical or psychiatric disorders, pregnancy, and concomitant medications that could influence study outcome.
While the adolescent study and one early study used acamprosate at a lower dose of 1332 mg/day [92,106], others studied acamprosate at doses of either 1332 mg/day or 1998 mg/day, with dose adjusted by body weight (lower dose for patients whose weight was < 60 kg, higher dose for those whose weight was > 60 kg) [90,91,94–100, 105]. More recent studies used a fixed dose of 1998 mg/day [77,102–104,107], which was shown in an earlier French dose-ranging study to be more efficacious than the lower, 1332 mg/day dose, regardless of body weight [77]. Outcome measures were analyzed in the intent-to-treat population (ITT), i.e. those patients who had received at least one dose of study medication.
The principle efficacy parameters assessed in most studies of acamprosate included: percentage of patients completing the trial without taking one drink (rate of complete abstinence); length of time to any relapse of drinking (often referred to as Time to First Drink); sum of all abstinent periods throughout a trial called Cumulative Abstinence Duration (CAD), relapse rate at each study visit; rate of study completion; and change in biomarker levels (GGT, carbohydrate-deficient transferrin [CDT], mean corpuscular volume [MCV]). In many trials, CAD was transformed into a variable termed Percent Days Abstinent (PDA), which was essentially a CAD expressed as a fraction of the total duration of a trial [PDA = (CAD / total trial duration) × 100%]. This transformation allows for comparison of data between studies of different lengths [111].
Treatment compliance was determined by pill count at each study visit, and patients were considered abstinent if at each study interval they did not report taking a single drink since their last visit, did not miss a study visit, and, in most studies, had a self-report that was corroborated by biological marker data and collateral report. If any available information indicated drinking, the patient was considered non-abstinent.
Clinical Results
The duration of treatment, country of the trial, and outcomes are listed in Table 1. For the discussion of the clinical results in this paper, the trials are subdivided into short (treatment lasting ≤ 4 months), intermediate (6 months duration) and long-term (12 month) treatment efficacy. Of the nine short-term trials [77,91,95,104–108,110], five of these studies demonstrated significantly higher rates of complete abstinence in the acamprosate groups compared to placebo, including the trial involving adolescents [77,91,104,106,107]. One study used GGT levels as the primary outcome measure, which was significantly lower in the acamprosate group compared to placebo after three months of treatment [92]. A total of eight randomized, double-blind, placebo-controlled trials of six month duration have been conducted. Seven of these studies support the findings of the three-month studies showing that acamprosate improved abstinence rates and treatment retention in the treatment of alcohol dependence [93,99,98,102,103,109]. Most importantly, a clear positive effect was attributed to the use of acamprosate in the prevention of relapse during the six-month post-treatment follow-up period in two of these studies [93,98]. A total of five studies have evaluated the effect of acamprosate on long-term abstinence after a 12-month treatment period [76,90,96,97,100] that included a post-treatment follow-up period of either 6-month [76,97] or 12-month duration [90,96,100]. The first published long-term efficacy study reported higher percentages of patients completely abstinent with 1998 mg/day compared to 1332 mg/day acamprosate, and dose-dependent effects were also observed for time to first drink and mean CAD [76]. Acamprosate treatment was associated with significantly more patients remaining abstinent in all of the long-term trials [76,90,96,97,100]. Furthermore, these effects were maintained throughout the post-treatment follow-up phase. Significantly more acamprosate patients never had a relapse throughout the 12-month follow-up period (39.9% vs. 17.3%) compared to placebo [90,96,97].
Table 1.
Significant Clinical Outcomes for Acamprosate vs. Placebo in Published Randomized, Double-Blind, Controlled Trials [references]
| Duration of Study / Followup (Months) |
Number of Patients (N) |
Acamprosate Dosage |
Rate of Complete Abstinence |
Percent Days Abstinenta |
Time to First Drink |
||
|---|---|---|---|---|---|---|---|
| International Trials | Country | ||||||
| Namkoong et al, 2003 [105] | South Korea | 2 | 142 | 1332 or 1998 mg/dayb | − | − | − |
| Lhuintre et al, 1985 [91] | France | 3 | 85 | 1000–2250 mg/dayb | + | NM | NM |
| Lhuintre et al, 1990* [92] | France | 3 | 569 | 1332 mg/day | NM | NM | NM |
| Roussaux et al, 1996 [95] | Belgium | 3 | 127 | 1332 or 1998 mg/dayb | − | NM | NM |
| Pelc et al, 1997 [77] | Belgium, France | 3 | 188 | 1332 or 1998 mg/dayb | ++ | ++ | ++ |
| Baltieri et al., 2004 [107] | Brazil | 3/3 | 75 | 1998 mg/day | + | NM | NM |
| Kiefer et al, 2003†[104] | Germany | 3/3 | 160 | 1998 mg/day | ++ | NM | + |
| Morley et al, 2006†[110] | Australia | 3/3 | 169 | 1998 mg/day | NM | − | − |
| Niederhofer, et al. 2003 [106] | Austria | 3 | 26 | 1332 mg/day | ++ | ++ | NM |
| Pelc et al, 1992 [93] | Belgium, France | 6 | 102 | 1998 mg/day | ++ | ++ | NM |
| Ladewig et al, 1993 [94] | Switzerland | 6/6 | 61 | 1332 or 1998 mg/dayb | + | + | NM |
| Geerlings et al, 1997 [98] | Netherlands, Belgium, Luxembourg | 6/6 | 262 | 1332 or 1998 mg/dayb | + | + | + |
| Poldrugo, 1997 [99] | Italy | 6/6 | 246 | 1332 or 1998 mg/dayb | ++ | ++ | ++ |
| Chick et al, 2000 [101] | United Kingdom | 6/1 | 581 | 1998 mg/day | − | − | NM |
| Tempesta et al., 2000 [102] | Italy | 6/3 | 330 | 1998 mg/day | ++ | + | ++ |
| Gual, et al.2001 [103] | Spain | 6 | 288 | 1998 mg/day | + | ++ | NM |
| Paille et al, 1995 [76] | France | 12/6 | 538 | 1332 or 1998 mg/dayc | − | ++ | ++ |
| Sass et al, 1996 [90] | Germany | 12/12 | 272 | 1332 or 1998 mg/dayb | ++ | ++ | + |
| Whitworth et al, 1996 [96] | Austria | 12/12 | 448 | 1332 or 1998 mg/dayb | ++ | + | ++ |
| Barrias et al, 1997 [97] | Portugal | 12/6 | 302 | 1332 or 1998 mg/dayb | + | ++ | ++ |
| Besson et al, 1998‡ [100] | Switzerland | 12/12 | 110 | 1332 or 1998 mg/dayb | + | + | NM |
| U. S. Trials | |||||||
| Anton et al, 2006† [108] | 4/12 | 1383 | 3000 mg/day | NM | − | NM | |
| Mason et al, 2006 [109] | 6/2 | 601 | 2000 or 3000 mg/dayc | NM | +d | NM | |
γ-Glutamyltransferase level was the primary outcome measure in this study and was significantly lower in acamprosate patients than in placebo patients after 3 months of treatment (1.4 ± 1.56 vs. 2.0 ± 3.19 ¥ upper limit of normal, p=0.016).
Comparative and/or combination trial: patients randomized to receive acamprosate, naltrexone, acamprosate plus naltrexone, or placebo.
Patients randomized to receive acamprosate or placebo, and stratified for voluntary concomitant treatment with disulfiram.
Or the analogous measure of cumulative abstinent duration.
Based on patient’s body weight.
Assigned by randomization.
In patients demonstrating a baseline goal of abstinence.
Outcome Key: ++ (p<0.01); + (p>0.01 and p<0.05); − (p>0.05); NM (not measured).
Reprinted with permission from Focus, (Copyright 2006). American Psychiatric Association.
This table is updated from: Mason BJ, Crean R. Acamprosate in the treatment of alcohol dependence: clinical and economic considerations. Expert Rev Neurother 2007; 7(11): 1465–77.
There are studies that have failed to show a significant effect of acamprosate [95,101,105,108,110]. It is interesting to note that all but one of these studies involved a treatment period of ≤ 4 months [9,105,108,110]. Additionally, a 3-month study conducted in Belgium [95], included a number of patients meeting criteria for alcohol abuse rather than alcohol dependence and used a dose of acamprosate (1332 mg/day), which has been shown to be relatively less effective than the 1998 mg/day dose [77]. In an Australian study that failed to detect any differences between acamprosate and placebo, the authors reported that patients in the study had substantially high levels of emotional distress and moderate levels of disability in mental function (110). The results from a two-month clinical trial showed that acamprosate was not superior to placebo in reducing drinking in South Korean patients with severe alcohol dependence [105]. The observed lack of efficacy in this study may be a function of the unusually brief treatment duration and the high rate (68%) of non-abstinence within the previous two days of starting medication [1]. Importantly, acamprosate is not indicated for the induction of abstinence but rather for the maintenance of abstinence in alcohol-dependent patients who have been withdrawn from alcohol. More recently, the US COMBINE trial was conducted to evaluate the efficacy of acamprosate (3 g/day), naltrexone (100 mg/day) and behavioral therapies, alone and in combinations, for the treatment of alcohol dependence [108]. In factorial analyses comparing an amalgam of all groups receiving acamprosate to all those that did not, no additional benefit was found for acamprosate. Notably, improvements in percentage days abstinent in the COMBINE trial were observed in all groups receiving pills, both active and placebo, suggesting the beneficial role of pill taking. More specifically the percent days abstinent for the placebo group with Medical Management with and without Cognitive Behavior Intervention (CBI) was 79.8% and 73.8% respectively, whereas 66.6% was reported in the No Pills group [108]. It is not clear why there was such a strong placebo effect in this trial, however this effect may have made it difficult to detect any additional benefit of acamprosate.
The only intermediate duration treatment trial that did not demonstrate statistical superiority of acamprosate over placebo was conducted in the U.K. [101]. This study did not require a minimum of 5 days abstinence prior to randomization, and also allowed for a longer than usual period of time between detoxification and treatment (up to 56 days). As a result, a large percentage of randomized patients (32%) had relapsed prior to receiving their first dose of double-blind medication, likely contributing to a high dropout rate as well as to the absence of an acamprosate effect.
Several meta-analyses of these clinical studies have been published and in all cases the results of these meta-analyses show that abstinence rates were significantly higher in acamprosate-treated patients than in placebo-treated patients [111–115]. The effect sizes for acamprosate on percent abstinence have been reported to range from 1.5 to 1.58 (111,115). Although in a more detailed analysis of the factors that might affect this outcome, it is interesting to note that Mann [111] reported an increasing effect size across trial duration (1.33 at 3 months, 1.50 at 6 months and 1.95 at 12 months). As noted above, a larger percentage of trials lasting 6 or more months showed significant effects of acamprosate than those trials lasting 4 or fewer months. Another way to view these results is by determining the number of people needed to treat to obtain a particular outcome (number needed to treat [NNT]). The estimates of NNT range from 7.7 [113] to 10 [114]. All 5 meta-analyses conclude that acamprosate is particularly useful in a therapeutic approach targeted at abstinence. This conclusion is unchanged in a recent analysis [115] that included the negative results of the COMBINE trial [108].
Predicting the effects of acamprosate in clinical populations
Despite the beneficial effects of acamprosate in the treatment of alcohol dependence, acamprosate is not a panacea and there is much work to be done in the development of pharmacotherapies for drug addiction. Moreover, it is important to remember that alcoholism is a complex disorder and there is unlikely to be a “one-therapy fits all” solution. It is therefore critical to not only develop novel therapies, but to identify the conditions under which optimal efficacy is achieved. A post hoc analysis of the US study data identified variables significantly associated with treatment outcome, including the goal of total abstinence, stage of readiness to changes, significant psychiatric and substance use histories, and medication compliance [109]. The most robust treatment effects were observed in the subgroup of 214 patients having a baseline goal of total abstinence (70.0%, 2 g/day acamprosate; 72.5%, 3 g/day acamprosate; 58.1% placebo), suggesting that motivation to be abstinent may be an important determinant of treatment success with acamprosate. Using a pharmacogenomics analysis, the efficacy of acamprosate was enhanced depending on the C-allele frequency of the GABARA6 gene and the T-allele homozygotes for the C+1412T polymorphism of the GABARB2 gene [116]. By developing such predictors it may be possible to improve patient treatment matching and the overall success rate of acamprosate and to that end, any pharmacotherapy used in the treatment of alcohol dependence.
CONCLUSION
The major therapeutic challenge to successful management of alcohol dependence is the maintenance of abstinence and prevention of relapse. Prevention of relapse has the potential to reduce hospitalization and rehabilitation costs, as well as alcohol-related loss of productivity in the workplace. Over the past two decades, numerous well-controlled clinical trials have found that acamprosate, in combination with psychosocial support, is a safe and well-accepted therapy that, although not a panacea, prolongs periods of complete abstinence for many individuals and reduces the rate of relapse to drinking among recently abstinent alcohol-dependent patients. Given its excellent safety record and the result of clinical trials it is not surprising that it is now the most widely prescribed drug therapy in the treatment of alcoholism. Pharmacoeconomic studies both in Europe and the U.S. have demonstrated the potential cost-savings benefit of prescribing acamprosate as an adjunct to psychosocial support compared with nonpharmacological techniques alone [117–121]. Based on these findings, it appears that acamprosate will be an important advancement in the treatment of alcohol dependence in the U.S.
Acamprosate appears to work by normalizing the dysregulation of NMDA-mediated glutamatergic neurotransmission that occurs during chronic alcohol consumption and withdrawal, and thus attenuates one of the physiological mechanisms that may prompt relapse. Acamprosate requires around a week to reach steady-state levels in the nervous system and its effects on drinking behavior typically persist after the treatment is completed. Acamprosate can be viewed as a prototype of a neuromodulatory approach to the treatment of alcoholism. More specifically, earlier drugs such as disulfiram targeted alcohol metabolism and naltrexone acts as an antagonist on opioid receptors, whereas by its actions acamprosate works to ameliorate the underlying changes in neurochemistry caused by chronic alcohol intake and to restore homeostasis to those systems. Futhermore, it is a novel prototype in that it doesn’t use a punishment model (as in the case of antabuse) or agonist/antagonist approach to decrease the reinforcing value of alcohol. Rather, acamprosate appears to work following detoxification as an anti-relapse drug. It is interesting to note, this neuromodulatory (indirect) approach produces less side-effects and promotes superior compliance rates among patients [114]. However it is important to note that acamprosate is not a panacea and there is much work to be done in the development of pharmacotherapies for drug addiction. A number of other therapeutic agents are under investigation; these include serotonergic agents, anticonvulsants, GABA receptor indirect or partial agonists, and neurokinin-1 (NK1) antagonists [122]. It is our opinion that a neuromodulatory approach will provide a heuristic framework for developing efficient and effective pharmacotherapies for alcoholism.
Acamprosate is well suited for treating a broad population of alcohol-dependent patients given its excellent safety profile observed in clinical trials, along with several pharmacokinetic and pharmacodynamic characteristics. More specifically, it does not appear to interact with alcohol or compounds commonly prescribed for treating alcoholism (e.g., disulfiram, antidepressants, anxiolytics, neuroleptics, or hypnotics) nor does it appear to interact adversely with naltrexone. Acamprosate can be administered to patients with liver dysfunction, since it does not undergo significant hepatic metabolism (though it should not be used in patients with renal insufficiency). Acamprosate may be useful for methadone-maintained patients dependent on alcohol and narcotics as it does not cause acute opioid withdrawal syndrome in patients using opioids. And finally, it does not have any abuse potential and appears to have minimal pharmacological effects apart from those involved in reducing the rate of drinking. This evidence base suggests acamprosate should be routinely considered by medical professionals for patients entering alcoholism treatment, taking into account the patient’s treatment goals and preferences as well as the safety considerations outlined above.
Acknowledgements
We are grateful to Karyn Coveney for her editorial assistance in the preparation of this manuscript.
Work supported by grant number R01AA012602 from NIAAA.
List of Abbreviations
- AMPA
alpha-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate
- CAD
Cumulative Abstinence Duration
- CBI
Cognitive Behavior Intervention
- CDT
carbohydrate-deficient transferrin
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition
- EEG
electroencephalography
- FDA
Food and Drug Administration
- fMRI
functional magnetic resonance imaging
- GABA
gamma-aminobutyric acid
- GGT
gamma-glutamyltransferase
- ITT
intent-to-treat population
- MCV
mean corpuscular volume
- MRS
magnetic resonance spectroscopy
- MEG
magnetoencephalography
- NK1
neurokinin-1
- NMDA
N-methyl-d-aspartate
- NTT
number needed to treat
- PDA
Percent Days Abstinent
- PET
positron emission tomography
- REM
rapid eye movement
Footnotes
COI: Dr. Mason has served as a consultant to Forest Laboratories, Inc. and Merck Sante.
References
- 1.Mason BJ. Treatment of alcohol-dependent outpatients with acamprosate: a clinical review. J Clin Psychiatry. 2001;62 Suppl 20:42–48. [PubMed] [Google Scholar]
- 2.Mark TL, Kassed CA, Vandivort-Warren R, Levit KR, Kranzler HR. Alcohol and opioid dependence medications: prescription trends, overall and by physician specialty. Drug Alcohol Depend. 2009;99:345–349. doi: 10.1016/j.drugalcdep.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington DC: American Psychiatric Press; 1994. [Google Scholar]
- 4.Koob GF, Le Moal M. Neurobiology of Addiction. London: Academic Press; 2006. [Google Scholar]
- 5.De Witte P, Littleton J, Parot P, Koob G. Neuroprotective and abstinence-promoting effects of acamprosate: elucidating the mechanism of action. CNS Drugs. 2005;19:517–537. doi: 10.2165/00023210-200519060-00004. [DOI] [PubMed] [Google Scholar]
- 6.Littleton J. Acamprosate in alcohol dependence: how does it work? Addiction. 1995;90:1179–1188. doi: 10.1046/j.1360-0443.1995.90911793.x. [DOI] [PubMed] [Google Scholar]
- 7.Chabenat C, Ladure P, Moore N, Boucly P, Boismare F. Application of an analytical method to calcium acetylhomotaurinate determination in urine. Arzneimittelforschung. 1989;39:1413–1414. [PubMed] [Google Scholar]
- 8.Girault J, Gobin P, Fourtillan JB. Determination of calcium acetylhomotaurinate in human plasma and urine by combined gas chromatography-negative-ion chemical ionization mass spectrometry. J Chromatogr. 1990;530:295–305. doi: 10.1016/s0378-4347(00)82333-6. [DOI] [PubMed] [Google Scholar]
- 9.Saivin S, Hulot T, Chabac S, Potgieter A, Durbin P, Houin G. Clinical pharmacokinetics of acamprosate. Clin Pharmacokinet. 1998;35:331–345. doi: 10.2165/00003088-199835050-00001. [DOI] [PubMed] [Google Scholar]
- 10.Wilde MI, Wagstaff AJ. Acamprosate. A review of its pharmacology and clinical potential in the management of alcohol dependence after detoxification. Drugs. 1997;53:1038–1053. doi: 10.2165/00003495-199753060-00008. [DOI] [PubMed] [Google Scholar]
- 11.Durbin P, Hulot T, Chabac S. Pharmacodynamics and pharmacokinetics of acamprosate: An overview. In: Soyka M, editor. Acamprosate in Relapse Prevention of Alcoholism. Proceedings of the 1st Campral Symposium; Springer; Stuttgart, Germany. 1995. pp. 47–64. [Google Scholar]
- 12.Campral [package insert] Saint Louis, MO: Forest Pharmaceuticals, Inc.; 2005. [Google Scholar]
- 13.Mason BJ. Rationale for combining acamprosate and naltrexone for treating alcohol dependence. J Stud Alcohol Suppl. 2005;15:148–156. doi: 10.15288/jsas.2005.s15.148. [DOI] [PubMed] [Google Scholar]
- 14.Johnson BA, O'Malley SS, Ciraulo DA, Roache JD, Chambers RA, Sarid-Segal O, Couper D. Dose-ranging kinetics and behavioral pharmacology of naltrexone and acamprosate, both alone and combined, in alcohol-dependent subjects. J Clin Psychopharmacol. 2003;23:281–293. doi: 10.1097/01.jcp.0000084029.22282.bb. [DOI] [PubMed] [Google Scholar]
- 15.Gessa GL, Muntoni F, Collu M, Vargiu L, Mereu G. Low doses of ethanol activate dopaminergic neurons in the ventral tegmental area. Brain Res. 1985;348:201–203. doi: 10.1016/0006-8993(85)90381-6. [DOI] [PubMed] [Google Scholar]
- 16.Brodie MS, Shefner SA, Dunwiddie TV. Ethanol increases the firing rate of dopamine neurons of the rat ventral tegmental area in vitro. Brain Res. 1990;508:65–69. doi: 10.1016/0006-8993(90)91118-z. [DOI] [PubMed] [Google Scholar]
- 17.Diana M, Pistis M, Muntoni A, Rossetti ZL, Gessa G. Marked decrease of A10 dopamine neuronal firing during ethanol withdrawal syndrome in rats. Eur J Pharmacol. 1992;221:403–404. doi: 10.1016/0014-2999(92)90734-l. [DOI] [PubMed] [Google Scholar]
- 18.Diana M, Pistis M, Muntoni A, Gessa G. Mesolimbic dopaminergic reduction outlasts ethanol withdrawal syndrome: evidence of protracted abstinence. Neuroscience. 1996;71:411–415. doi: 10.1016/0306-4522(95)00482-3. [DOI] [PubMed] [Google Scholar]
- 19.Shen RY, Chiodo LA. Acute withdrawal after repeated ethanol treatment reduces the number of spontaneously active dopaminergic neurons in the ventral tegmental area. Brain Res. 1993;622:289–293. doi: 10.1016/0006-8993(93)90831-7. [DOI] [PubMed] [Google Scholar]
- 20.Bailey CP, Manley SJ, Watson WP, Wonnacott S, Molleman A, Little HJ. Chronic ethanol administration alters activity in ventral tegmental area neurons after cessation of withdrawal hyperexcitability. Brain Res. 1998;803:144–152. doi: 10.1016/s0006-8993(98)00654-4. [DOI] [PubMed] [Google Scholar]
- 21.Lima-Landman MT, Albuquerque EX. Ethanol potentiates and blocks NMDA-activated single-channel currents in rat hippocampal pyramidal cells. FEBS Lett. 1989;247:61–67. doi: 10.1016/0014-5793(89)81241-4. [DOI] [PubMed] [Google Scholar]
- 22.Lovinger DM, White G, Weight FF. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science. 1989;243:1721–1724. doi: 10.1126/science.2467382. [DOI] [PubMed] [Google Scholar]
- 23.Nie Z, Madamba SG, Siggins GR. Ethanol inhibits glutamatergic neurotransmission in nucleus accumbens neurons by multiple mechanisms. J Pharmacol Exp Ther. 1994;271:1566–1573. [PubMed] [Google Scholar]
- 24.Simson PE, Criswell HE, Johnson KB, Hicks RE, Breese GR. Ethanol inhibits NMDA-evoked electrophysiological activity in vivo. J Pharmacol Exp Ther. 1991;257:225–231. [PubMed] [Google Scholar]
- 25.Carta M, Ariwodola OJ, Weiner JL, Valenzuela CF. Alcohol potently inhibits the kainate receptor-dependent excitatory drive of hippocampal interneurons. Proc Natl Acad Sci U S A. 2003;100:6813–6818. doi: 10.1073/pnas.1137276100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Engberg G, Hajós M. Alcohol withdrawal reaction as a result of adaptive changes of excitatory amino acid receptors. Naunyn Schmiedebergs Arch Pharmacol. 1992;346:437–441. doi: 10.1007/BF00171087. [DOI] [PubMed] [Google Scholar]
- 27.Chandler LJ, Newsom H, Sumners C, Crews F. Chronic ethanol exposure potentiates NMDA excitotoxicity in cerebral cortical neurons. J Neurochem. 1993;60:1578–1581. doi: 10.1111/j.1471-4159.1993.tb03326.x. [DOI] [PubMed] [Google Scholar]
- 28.Snell LD, Tabakoff B, Hoffman PL. Radioligand binding to the N-methyl-D-aspartate receptor/ionophore complex: alterations by ethanol in vitro and by chronic in vivo ethanol ingestion. Brain Res. 1993;602:91–98. doi: 10.1016/0006-8993(93)90246-j. [DOI] [PubMed] [Google Scholar]
- 29.Hoffman PL, Tabakoff B. The role of the NMDA receptor in ethanol withdrawal. EXS. 1994;71:61–70. doi: 10.1007/978-3-0348-7330-7_7. [DOI] [PubMed] [Google Scholar]
- 30.Davidson M, Shanley B, Wilce P. Increased NMDA-induced excitability during ethanol withdrawal: a behavioural and histological study. Brain Res. 1995;674:91–96. doi: 10.1016/0006-8993(94)01440-s. [DOI] [PubMed] [Google Scholar]
- 31.Ripley TL, Little HJ. Ethanol withdrawal hyperexcitability in vitro is selectively decreased by a competitive NMDA receptor antagonist. Brain Res. 1995;699:1–11. doi: 10.1016/0006-8993(95)00445-v. [DOI] [PubMed] [Google Scholar]
- 32.Stephens DN. A glutamatergic hypothesis of drug dependence: extrapolations from benzodiazepine receptor ligands. Behav Pharmacol. 1995;6:425–446. [PubMed] [Google Scholar]
- 33.Mehta AK, Ticku MK. Ethanol potentiation of GABAergic transmission in cultured spinal cord neurons involves gamma-aminobutyric acidA-gated chloride channels. J Pharmacol Exp Ther. 1988;246:558–564. [PubMed] [Google Scholar]
- 34.Suzdak PD, Schwartz RD, Skolnick P, Paul SM. Alcohols stimulate gamma-aminobutyric acid receptor-mediated chloride uptake in brain vesicles: correlation with intoxication potency. Brain Res. 1988;444:340–345. doi: 10.1016/0006-8993(88)90943-2. [DOI] [PubMed] [Google Scholar]
- 35.Allan AM, Burnett D, Harris RA. Ethanol-induced changes in chloride flux are mediated by both GABA(A) and GABA(B) receptors. Alcohol Clin Exp Res. 1991;15:233–237. doi: 10.1111/j.1530-0277.1991.tb01862.x. [DOI] [PubMed] [Google Scholar]
- 36.Mhatre MC, Pena G, Sieghart W, Ticku MK. Antibodies specific for GABAA receptor alpha subunits reveal that chronic alcohol treatment down-regulates alpha-subunit expression in rat brain regions. J Neurochem. 1993;61:1620–1625. doi: 10.1111/j.1471-4159.1993.tb09795.x. [DOI] [PubMed] [Google Scholar]
- 37.Morrow AL, Suzdak PD, Karanian JW, Paul SM. Chronic ethanol administration alters gamma-aminobutyric acid, pentobarbital and ethanol-mediated 36Cl- uptake in cerebral cortical synaptoneurosomes. J Pharmacol Exp Ther. 1988;246:158–164. [PubMed] [Google Scholar]
- 38.Leslie SW, Barr E, Chandler J, Farrar RP. Inhibition of fast- and slow-phase depolarization-dependent synaptosomal calcium uptake by ethanol. J Pharmacol Exp Ther. 1983;225:571–575. [PubMed] [Google Scholar]
- 39.Dildy-Mayfield JE, Machu T, Leslie SW. Ethanol and voltage- or receptor-mediated increases in cytosolic Ca2+ in brain cells. Alcohol. 1992;9:63–69. doi: 10.1016/0741-8329(92)90011-x. [DOI] [PubMed] [Google Scholar]
- 40.Messing RO, Carpenter CL, Diamond I, Greenberg DA. Ethanol regulates calcium channels in clonal neural cells. Proc Natl Acad Sci U S A. 1986;83:6213–6215. doi: 10.1073/pnas.83.16.6213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dolin S, Little H, Hudspith M, Pagonis C, Littleton J. Increased dihydropyridine-sensitive calcium channels in rat brain may underlie ethanol physical dependence. Neuropharmacology. 1987;26:275–279. doi: 10.1016/0028-3908(87)90220-6. [DOI] [PubMed] [Google Scholar]
- 42.Dahchour A, De Witte P. Ethanol and amino acids in the central nervous system: assessment of the pharmacological actions of acamprosate. Prog Neurobiol. 2000;60:343–362. doi: 10.1016/s0301-0082(99)00031-3. [DOI] [PubMed] [Google Scholar]
- 43.Naassila M, Hammoumi S, Legrand E, Durbin P, Daoust M. Mechanism of action of acamprosate. Part I. Characterization of spermidine-sensitive acamprosate binding site in rat brain. Alcohol Clin Exp Res. 1998;22:802–809. [PubMed] [Google Scholar]
- 44.al Qatari M, Bouchenafa O, Littleton J. Mechanism of action of acamprosate. Part II. Ethanol dependence modifies effects of acamprosate on NMDA receptor binding in membranes from rat cerebral cortex. Alcohol Clin Exp Res. 1998;22:810–814. [PubMed] [Google Scholar]
- 45.Zeise ML, Kasparov S, Capogna M, Zieglgänsberger W. Acamprosate (calciumacetylhomotaurinate) decreases postsynaptic potentials in the rat neocortex: possible involvement of excitatory amino acid receptors. Eur J Pharmacol. 1993;231:47–52. doi: 10.1016/0014-2999(93)90682-8. [DOI] [PubMed] [Google Scholar]
- 46.Grant KA, Valverius P, Hudspith M, Tabakoff B. Ethanol withdrawal seizures and the NMDA receptor complex. Eur J Pharmacol. 1990;176:289–296. doi: 10.1016/0014-2999(90)90022-x. [DOI] [PubMed] [Google Scholar]
- 47.Tsai G, Coyle JT. The role of glutamatergic neurotransmission in the pathophysiology of alcoholism. Annu Rev Med. 1998;49:173–184. doi: 10.1146/annurev.med.49.1.173. [DOI] [PubMed] [Google Scholar]
- 48.Tsai GE, Ragan P, Chang R, Chen S, Linnoila VM, Coyle JT. Increased glutamatergic neurotransmission and oxidative stress after alcohol withdrawal. Am J Psychiatry. 1998;155:726–732. doi: 10.1176/ajp.155.6.726. [DOI] [PubMed] [Google Scholar]
- 49.Dahchour A, De Witte P, Bolo N, Nédélec JF, Muzet M, Durbin P, Macher JP. Central effects of acamprosate: part 1. Acamprosate blocks the glutamate increase in the nucleus accumbens microdialysate in ethanol withdrawn rats. Psychiatry Res. 1998;82:107–114. doi: 10.1016/s0925-4927(98)00016-x. [DOI] [PubMed] [Google Scholar]
- 50.Popp RL, Lovinger DM. Interaction of acamprosate with ethanol and spermine on NMDA receptors in primary cultured neurons. Eur J Pharmacol. 2000;394:221–231. doi: 10.1016/s0014-2999(00)00195-3. [DOI] [PubMed] [Google Scholar]
- 51.Staner L, Boeijinga P, Danel T, Gendre I, Muzet M, Landron F, Luthringer R. Effects of acamprosate on sleep during alcohol withdrawal: A double-blind placebo-controlled polysomnographic study in alcohol-dependent subjects. Alcohol Clin Exp Res. 2006;30:1492–1499. doi: 10.1111/j.1530-0277.2006.00180.x. [DOI] [PubMed] [Google Scholar]
- 52.Engelhard K, Werner C, Lu H, Möllenberg O, Zieglgänsberger W, Kochs E. The neuroprotective effect of the glutamate antagonist acamprosate following experimental cerebral ischemia. A study with the lipid peroxidase inhibitor u-101033e. Anaesthesist. 2000;49:816–821. doi: 10.1007/s001010070054. [DOI] [PubMed] [Google Scholar]
- 53.al Qatari M, Khan S, Harris B, Littleton J. Acamprosate is neuroprotective against glutamate-induced excitotoxicity when enhanced by ethanol withdrawal in neocortical cultures of fetal rat brain. Alcohol Clin Exp Res. 2001;25:1276–1283. doi: 10.1097/00000374-200109000-00006. [DOI] [PubMed] [Google Scholar]
- 54.Mayer S, Harris B, Gibson DA, Blanchard J, Prendergast MA, Holley RC, Littleton J. Acamprosate has no effect on NMDA-induced toxicity but reduces toxicity induced by spermidine or by changing the medium in organotypic hippocampal slice cultures from rat. Alcohol Clin Exp Res. 2002;26:655–662. [PubMed] [Google Scholar]
- 55.Koob GF, Mason BJ, De Witte P, Littleton J, Siggins GR. Potential neuroprotective effects of acamprosate. Alcohol Clin Exp Res. 2002;26:586–592. [PubMed] [Google Scholar]
- 56.Adde-Michel C, Hennebert O, Laudenbach V, Marret S, Leroux P. Effect of acamprosate on neonatal excitotoxic cortical lesions in in utero alcohol-exposed hamsters. Neurosci Lett. 2005;374:109–112. doi: 10.1016/j.neulet.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 57.Littleton JM. Acamprosate in alcohol dependence: implications of a unique mechanism of action. J Addict Med. 2007;1:115–125. doi: 10.1097/ADM.0b013e318156c26f. [DOI] [PubMed] [Google Scholar]
- 58.Davidson M, Wilce P. Chronic ethanol treatment leads to increased ornithine decarboxylase activity: implications for a role of polyamines in ethanol dependence and withdrawal. Alcohol Clin Exp Res. 1998;22:1205–1211. [PubMed] [Google Scholar]
- 59.Hu XJ, Ticku MK. Chronic ethanol treatment upregulates the NMDA receptor function and binding in mammalian cortical neurons. Brain Res Mol Brain Res. 1995;30:347–356. doi: 10.1016/0169-328x(95)00019-o. [DOI] [PubMed] [Google Scholar]
- 60.Hu XJ, Follesa P, Ticku MK. Chronic ethanol treatment produces a selective upregulation of the NMDA receptor subunit gene expression in mammalian cultured cortical neurons. Brain Res Mol Brain Res. 1996;36:211–218. doi: 10.1016/0169-328x(95)00223-f. [DOI] [PubMed] [Google Scholar]
- 61.Harris BR, Gibson DA, Prendergast MA, Blanchard JA, Holley RC, Hart SR, Scotland RL, Foster TC, Pedigo NW, Littleton JM. The neurotoxicity induced by ethanol withdrawal in mature organotypic hippocampal slices might involve cross-talk between metabotropic glutamate type 5 receptors and N-methyl-D-aspartate receptors. Alcohol Clin Exp Res. 2003;27:1724–1735. doi: 10.1097/01.ALC.0000093601.33119.E3. [DOI] [PubMed] [Google Scholar]
- 62.Boismare F, Daoust M, Moore N, Saligaut C, Lhuintre JP, Chretien P, Durlach J. A homotaurine derivative reduces the voluntary intake of ethanol by rats: are cerebral GABA receptors involved? Pharmacol Biochem Behav. 1984;21:787–789. doi: 10.1016/s0091-3057(84)80020-9. [DOI] [PubMed] [Google Scholar]
- 63.Le Magnen J, Tran G, Durlach J, Martin C. Dose-dependent suppression of the high alcohol intake of chronically intoxicated rats by Ca-acetyl homotaurinate. Alcohol. 1987;4:97–102. doi: 10.1016/0741-8329(87)90005-x. [DOI] [PubMed] [Google Scholar]
- 64.Spanagel R, Hölter SM, Allingham K, Landgraf R, Zieglgänsberger W. Acamprosate and alcohol: I. Effects on alcohol intake following alcohol deprivation in the rat. Eur J Pharmacol. 1996;305:39–44. doi: 10.1016/0014-2999(96)00174-4. [DOI] [PubMed] [Google Scholar]
- 65.Heyser CJ, Schulteis G, Durbin P, Koob GF. Chronic acamprosate eliminates the alcohol deprivation effect while having limited effects on baseline responding for ethanol in rats. Neuropsychopharmacology. 1998;18:125–133. doi: 10.1016/S0893-133X(97)00130-9. [DOI] [PubMed] [Google Scholar]
- 66.Stromberg MF, Mackler SA, Volpicelli JR, O#x00027;Brien CP. Effect of acamprosate and naltrexone, alone or in combination, on ethanol consumption. Alcohol. 2001;23:109–116. doi: 10.1016/s0741-8329(00)00137-3. [DOI] [PubMed] [Google Scholar]
- 67.Gupta T, Syed YM, Revis AA, Miller SA, Martinez M, Cohn KA, Demeyer MR, Patel KY, Brzezinska WJ, Rhodes JS. Acute effects of acamprosate and MPEP on ethanol Drinking-in-the-Dark in male C57BL/6J mice. Alcohol Clin Exp Res. 2008;32:1992–1998. doi: 10.1111/j.1530-0277.2008.00787.x. [DOI] [PubMed] [Google Scholar]
- 68.Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE. Intermittent access to 20#x00025; ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dahchour A, De Witte P. Acamprosate decreases the hypermotility during repeated ethanol withdrawal. Alcohol. 1999;18:77–81. doi: 10.1016/s0741-8329(98)00071-8. [DOI] [PubMed] [Google Scholar]
- 70.Cole JC, Littleton JM, Little HJ. Acamprosate, but not naltrexone, inhibits conditioned abstinence behaviour associated with repeated ethanol administration and exposure to a plus-maze. Psychopharmacology (Berl) 2000;147:403–411. doi: 10.1007/s002130050009. [DOI] [PubMed] [Google Scholar]
- 71.Farook JM, Krazem A, Lewis B, Morrell DJ, Littleton JM, Barron S. Acamprosate attenuates the handling induced convulsions during alcohol withdrawal in Swiss Webster mice. Physiol Behav. 2008;95:267–270. doi: 10.1016/j.physbeh.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kotlinska J, Bochenski M. The influence of various glutamate receptors antagonists on anxiety-like effect of ethanol withdrawal in a plus-maze test in rats. Eur J Pharmacol. 2008;598:57–63. doi: 10.1016/j.ejphar.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 73.Bachteler D, Economidou D, Danysz W, Ciccocioppo R, Spanagel R. The effects of acamprosate and neramexane on cue-induced reinstatement of ethanol-seeking behavior in rat. Neuropsychopharmacology. 2005;30:1104–1110. doi: 10.1038/sj.npp.1300657. [DOI] [PubMed] [Google Scholar]
- 74.Spanagel R, Putzke J, Stefferl A, Schöbitz B, Zieglgänsberger W. Acamprosate and alcohol: II. Effects on alcohol withdrawal in the rat. Eur J Pharmacol. 1996;305:45–50. doi: 10.1016/0014-2999(96)00175-6. [DOI] [PubMed] [Google Scholar]
- 75.Rosenthal RN, Gage A, Perhach JL, Goodman AM. Acamprosate: safety and tolerability in the treatment of alcohol dependence. J Addict Med. 2008;2:40–50. doi: 10.1097/ADM.0b013e31816319fd. [DOI] [PubMed] [Google Scholar]
- 76.Paille FM, Guelfi JD, Perkins AC, Royer RJ, Steru L, Parot P. Double-blind randomized multicentre trial of acamprosate in maintaining abstinence from alcohol. Alcohol Alcohol. 1995;30:239–247. [PubMed] [Google Scholar]
- 77.Pelc I, Verbanck P, Le Bon O, Gavrilovic M, Lion K, Lehert P. Efficacy and safety of acamprosate in the treatment of detoxified alcohol-dependent patients. A 90-day placebo-controlled dose-finding study. Br J Psychiatry. 1997;171:73–77. doi: 10.1192/bjp.171.1.73. [DOI] [PubMed] [Google Scholar]
- 78.Mason BJ. Acamprosate. Recent Dev Alcohol. 2003;16:203–215. [PubMed] [Google Scholar]
- 79.Schneider U, Wohlfarth K, Schulze-Bonhage A, Haacker T, Müller-Vahl KR, Zedler M, Becker H, Dengler R, Emrich HM. Effects of acamprosate on memory in healthy young subjects. J Stud Alcohol. 1999;60:172–175. doi: 10.15288/jsa.1999.60.172. [DOI] [PubMed] [Google Scholar]
- 80.Mason BJ, Goodman AM, Dixon RM, Hameed MH, Hulot T, Wesnes K, Hunter JA, Boyeson MG. A pharmacokinetic and pharmacodynamic drug interaction study of acamprosate and naltrexone. Neuropsychopharmacology. 2002;27:596–606. doi: 10.1016/S0893-133X(02)00368-8. [DOI] [PubMed] [Google Scholar]
- 81.Gross MM, Goodenough DR, Hastey J, Lewis E. Experimental study of sleep in chronic alcoholics before, during, and after four days of heavy drinking with a nondrinking comparison. Ann N Y Acad Sci. 1973;215:254–265. doi: 10.1111/j.1749-6632.1973.tb28281.x. [DOI] [PubMed] [Google Scholar]
- 82.Gross MM, Hastey JM. Slow wave sleep and carry-over of functional tolerance and physical dependence in alcoholics. Adv Exp Med Biol. 1975;59:477–493. doi: 10.1007/978-1-4757-0632-1_34. [DOI] [PubMed] [Google Scholar]
- 83.Williams HL, Rundell OH., Jr Altered sleep physiology in chronic alcoholics: reversal with abstinence. Alcohol Clin Exp Res. 1981;5:318–325. doi: 10.1111/j.1530-0277.1981.tb04905.x. [DOI] [PubMed] [Google Scholar]
- 84.Adamson J, Burdick JA. Sleep of dry alcoholics. Arch Gen Psychiatry. 1973;28:146–149. doi: 10.1001/archpsyc.1973.01750310116019. [DOI] [PubMed] [Google Scholar]
- 85.Brower KJ, Aldrich MS, Robinson EA, Zucker RA, Greden JF. Insomnia, self-medication, and relapse to alcoholism. Am J Psychiatry. 2001;158:399–404. doi: 10.1176/appi.ajp.158.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Koob GF. The neurobiology of addiction: a neuroadaptational view relevant for diagnosis. Addiction. 2006;101 Suppl 1:23–30. doi: 10.1111/j.1360-0443.2006.01586.x. [DOI] [PubMed] [Google Scholar]
- 87.Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bolo N, Nédélec JF, Muzet M, De Witte P, Dahchour A, Durbin P, Macher JP. Central effects of acamprosate: part 2. Acamprosate modifies the brain in-vivo proton magnetic resonance spectrum in healthy young male volunteers. Psychiatry Res. 1998;82:115–127. doi: 10.1016/s0925-4927(98)00017-1. [DOI] [PubMed] [Google Scholar]
- 89.Boeijinga PH, Parot P, Soufflet L, Landron F, Danel T, Gendre I, Muzet M, Demazières A, Luthringer R. Pharmacodynamic effects of acamprosate on markers of cerebral function in alcohol-dependent subjects administered as pretreatment and during alcohol abstinence. Neuropsychobiology. 2004;50:71–77. doi: 10.1159/000077944. [DOI] [PubMed] [Google Scholar]
- 90.Sass H, Soyka M, Mann K, Zieglgänsberger W. Relapse prevention by acamprosate. Results from a placebo-controlled study on alcohol dependence. Arch Gen Psychiatry. 1996;53:673–680. doi: 10.1001/archpsyc.1996.01830080023006. [DOI] [PubMed] [Google Scholar]
- 91.Lhuintre JP, Daoust M, Moore ND, Chretien P, Saligaut C, Tran G, Bosimare F, Hillemand B. Ability of calcium bis acetyl homotaurine, a GABA agonist, to prevent relapse in weaned alcoholics. Lancet. 1985;1:1014–1016. doi: 10.1016/s0140-6736(85)91615-0. [DOI] [PubMed] [Google Scholar]
- 92.Lhuintre JP, Moore N, Tran G, Steru L, Langrenon S, Daoust M, Parot P, Ladure P, Libert C, Boismare F, Hillemand B. Acamprosate appears to decrease alcohol intake in weaned alcoholics. Alcohol Alcohol. 1990;25:613–622. doi: 10.1093/oxfordjournals.alcalc.a045057. [DOI] [PubMed] [Google Scholar]
- 93.Pelc I, Le Bon O, Verbanck P, Lehert PH, Opsomer L. Calcium acetyl homotaurinate for maintaining abstinence in weaned alcoholic patients: a placebo-controlled double-blind multi-centre study. In: Naranjo CA, Sellers EM, editors. Novel Pharmacological Interventions for Alcoholism. New York: Springer Verlag; 1992. pp. 348–352. [Google Scholar]
- 94.Ladewig D, Knecht T, Lehert P, Fendl A. Acamprosate--a stabilizing factor in long-term withdrawal of alcoholic patients. Ther Umsch. 1993;50:182–188. [PubMed] [Google Scholar]
- 95.Roussaux JP, Hers D, Ferauge M. Does acamprosate diminish the appetite for alcohol in weaned alcoholics? J Pharm Belg. 1996;51:65–68. [PubMed] [Google Scholar]
- 96.Whitworth AB, Fischer F, Lesch OM, Nimmerrichter A, Oberbauer H, Platz T, Potgieter A, Walter H, Fleischhacker WW. Comparison of acamprosate and placebo in long-term treatment of alcohol dependence. Lancet. 1996;347:1438–1442. doi: 10.1016/s0140-6736(96)91682-7. [DOI] [PubMed] [Google Scholar]
- 97.Barrias JA, Chabac S, Ferreira L, Fonte A, Potgieter AS, Teixeira de Sousa E. Acamprosate: multicenter Portuguese efficacy and tolerance evaluation study. Psiquiatria Clin. 1997;18:149–160. [Google Scholar]
- 98.Geerlings PJ, Ansoms C, van den Brink W. Acamprosate and prevention of relapse in alcoholics. Eur Addict Res. 1997;3:129–137. [Google Scholar]
- 99.Poldrugo F. Acamprosate treatment in a long-term community-based alcohol rehabilitation programme. Addiction. 1997;92:1537–1546. [PubMed] [Google Scholar]
- 100.Besson J, Aeby F, Kasas A, Lehert P, Potgieter A. Combined efficacy of acamprosate and disulfiram in the treatment of alcoholism: a controlled study. Alcohol Clin Exp Res. 1998;22:573–579. doi: 10.1111/j.1530-0277.1998.tb04295.x. [DOI] [PubMed] [Google Scholar]
- 101.Chick J, Howlett H, Morgan MY, Ritson B. United Kingdom Multicentre Acamprosate Study (UKMAS): a 6-month prospective study of acamprosate versus placebo in preventing relapse after withdrawal from alcohol. Alcohol Alcohol. 2000;35:176–187. doi: 10.1093/alcalc/35.2.176. [DOI] [PubMed] [Google Scholar]
- 102.Tempesta E, Janiri L, Bignamini A, Chabac S, Potgieter A. Acamprosate and relapse prevention in the treatment of alcohol dependence: a placebo-controlled study. Alcohol Alcohol. 2000;35:202–209. doi: 10.1093/alcalc/35.2.202. [DOI] [PubMed] [Google Scholar]
- 103.Gual A, Lehert P. Acamprosate during and after acute alcohol withdrawal: a double-blind placebo-controlled study in Spain. Alcohol Alcohol. 2001;36:413–418. doi: 10.1093/alcalc/36.5.413. [DOI] [PubMed] [Google Scholar]
- 104.Kiefer F, Jahn H, Tarnaske T, Helwig H, Briken P, Holzbach R, Kämpf P, Stracke R, Baehr M, Naber D, Wiedemann K. Comparing and combining naltrexone and acamprosate in relapse prevention of alcoholism: a double-blind, placebo-controlled study. Arch Gen Psychiatry. 2003;60:92–99. doi: 10.1001/archpsyc.60.1.92. [DOI] [PubMed] [Google Scholar]
- 105.Namkoong K, Lee BO, Lee PG, Choi MJ, Lee E. Acamprosate in Korean alcohol-dependent patients: a multi-centre, randomized, double-blind, placebo-controlled study. Alcohol Alcohol. 2003;38:135–141. doi: 10.1093/alcalc/agg038. [DOI] [PubMed] [Google Scholar]
- 106.Niederhofer H, Staffen W. Acamprosate and its efficacy in treating alcohol dependent adolescents. Eur Child Adolesc Psychiatry. 2003;12:144–148. doi: 10.1007/s00787-003-0327-1. [DOI] [PubMed] [Google Scholar]
- 107.Baltieri DA, De Andrade AG. Acamprosate in alcohol dependence: a randomized controlled efficacy study in a standard clinical setting. J Stud Alcohol. 2004;65:136–139. doi: 10.15288/jsa.2004.65.136. [DOI] [PubMed] [Google Scholar]
- 108.Anton RF, O'Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, LoCastro JS, Longabaugh R, Mason BJ, Mattson ME, Miller WR, Pettinati HM, Randall CL, Swift R, Weiss RD, Williams LD, Zweben A COMBINE Study Research Group. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- 109.Mason BJ, Goodman AM, Chabac S, Lehert P. Effect of oral acamprosate on abstinence in patients with alcohol dependence in a double-blind, placebo-controlled trial: the role of patient motivation. J Psychiatr Res. 2006;40:383–393. doi: 10.1016/j.jpsychires.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 110.Morley KC, Teesson M, Reid SC, Sannibale C, Thomson C, Phung N, Weltman M, Bell JR, Richardson K, Haber PS. Naltrexone versus acamprosate in the treatment of alcohol dependence: A multi-centre, randomized, double-blind, placebo-controlled trial. Addiction. 2006;101:1451–1462. doi: 10.1111/j.1360-0443.2006.01555.x. [DOI] [PubMed] [Google Scholar]
- 111.Mann K, Lehert P, Morgan MY. The efficacy of acamprosate in the maintenance of abstinence in alcohol-dependent individuals: results of a meta-analysis. Alcohol Clin Exp Res. 2004;28:51–63. doi: 10.1097/01.ALC.0000108656.81563.05. [DOI] [PubMed] [Google Scholar]
- 112.Kranzler HR, Van Kirk J. Efficacy of naltrexone and acamprosate for alcoholism treatment: a meta-analysis. Alcohol Clin Exp Res. 2001;25:1335–1341. [PubMed] [Google Scholar]
- 113.Rösner S, Leucht S, Lehert P, Soyka M. Acamprosate supports abstinence, naltrexone prevents excessive drinking: evidence from a meta-analysis with unreported outcomes. J Psychopharmacol. 2008;22:11–23. doi: 10.1177/0269881107078308. [DOI] [PubMed] [Google Scholar]
- 114.Bouza C, Angeles M, Munoz A, Amate JM. Efficacy and safety of naltrexone and acamprosate in the treatment of alcohol dependence: a systematic review. Addiction. 2004;99:811–828. doi: 10.1111/j.1360-0443.2004.00763.x. [DOI] [PubMed] [Google Scholar]
- 115.Dranitsaris G, Selby P, Negrete JC. Meta-analyses of placebo-controlled trials of acamprosate for the treatment of alcohol dependence: impact of the Combined Pharmacotherapies and Behavior Interventions Study. J Addict Med. 2009;3:74–82. doi: 10.1097/ADM.0b013e318182d890. [DOI] [PubMed] [Google Scholar]
- 116.Ooteman W, Naassila M, Koeter MW, Verheul R, Schippers GM, Houchi H, Daoust M, van den Brink W. Predicting the effect of naltrexone and acamprosate in alcohol-dependent patients using genetic indicators. Addict Biol. 2009;14:328–337. doi: 10.1111/j.1369-1600.2009.00159.x. [DOI] [PubMed] [Google Scholar]
- 117.Mason BJ, Crean R. Acamprosate in the treatment of alcohol dependence: clinical and economic considerations. Expert Rev Neurother. 2007;7:1465–1477. doi: 10.1586/14737175.7.11.1465. [DOI] [PubMed] [Google Scholar]
- 118.Schädlich PK, Brecht JG. The cost effectiveness of acamprosate in the treatment of alcoholism in Germany. Economic evaluation of the Prevention of Relapse with Acamprosate in the Management of Alcoholism (PRAMA) Study. Pharmacoeconomics. 1998;13:719–730. doi: 10.2165/00019053-199813060-00008. [DOI] [PubMed] [Google Scholar]
- 119.Palmer AJ, Neeser K, Weiss C, Brandt A, Comte S, Fox M. The long-term cost-effectiveness of improving alcohol abstinence with adjuvant acamprosate. Alcohol Alcohol. 2000;35:478–492. doi: 10.1093/alcalc/35.5.478. [DOI] [PubMed] [Google Scholar]
- 120.Annemans L, Vanoverbeke N, Tecco J, D’Hooghe D. Economic evaluation of Campral (Acamprosate) compared to placebo in maintaining abstinence in alcohol-dependent patients. Eur Addict Res. 2000;6:71–78. doi: 10.1159/000019013. [DOI] [PubMed] [Google Scholar]
- 121.Rychlik R, Siedentop H, Pfeil T, Daniel D. Cost-effectiveness of adjuvant treatment with acamprosate in maintaining abstinence in alcohol dependent patients. Eur Addict Res. 2003;9:59–64. doi: 10.1159/000068810. [DOI] [PubMed] [Google Scholar]
- 122.Koob GF, Lloyd GK, Mason BJ. Development of pharmacotherapies for drug addiction: a Rosetta stone approach. Nat Rev Drug Discov. 2009;8:500–515. doi: 10.1038/nrd2828. [DOI] [PMC free article] [PubMed] [Google Scholar]