Abstract
Background
The binge-drinking model in rodents using intragastric injections of ethanol (EtOH) for 4 days results in argyrophilic corticolimbic tissue classically interpreted as indicating irreversible neuronal degeneration. However, recent findings suggest that acquired argyrophilia can also identify injured neurons that have the potential to recover. The current in vivo magnetic resonance imaging and spectroscopy study was conducted to test the hypothesis that binge EtOH exposure would injure but not cause the death of neurons as previously ascertained postmortem.
Methods
After baseline MR scanning, 11 of 19 rats received a loading dose of 5g/kg EtOH via oral gavage, then a maximum of 3g/kg every 8 hours for 4 days, for a total average cumulative EtOH dose of 43±1.2g/kg and average blood alcohol levels of 258±12mg/dL. All animals were scanned after 4 days of gavage (post-gavage scan) with EtOH (EtOH group) or dextrose (Con group), and again after 7 days of abstinence from EtOH (recovery scan).
Results
Tissue shrinkage at the post-gavage scan was reflected by significantly increased lateral ventricular volume in the EtOH group compared with the Con group. At the post-gavage scan, the EtOH group had lower dorsal hippocampal N-acetyl aspartate and total creatine and higher choline-containing compounds than the Con group. At the recovery scan, neither ventricular volume nor metabolite levels differentiated the groups.
Conclusions
Rapid recovery of ventricular volume and metabolite levels with removal of the causative agent argues for transient rather than permanent effects of a single EtOH binge episode in rats.
Keywords: ethanol, imaging, rat, neurochemicals, ventricular system, recovery, liver
INTRODUCTION
Alcohol use disorders are characterized by the consumption of excessive quantities of alcohol and are associated with volume reductions in cortical and subcortical brain structures (for review 1) and deficits in specific cognitive and motor functions (for review 2, 3). Conversely, alcoholics who abstain can enjoy significant recovery of brain volume (4, 5) and improvements in neuropsychological functions (6). These consistent observations have been variously interpreted, but hypotheses from animal studies suggest that binge alcohol exposure produces cell death (7), and abstinence leads to cell proliferation and neurogenesis (8). Despite profound plasticity of the human brain, evidence supporting brain volume recovery due to new cell proliferation and formation of appropriate connections is scarce to nil (cf., 9, 10). A more feasible interpretation is that brain injury by binge alcohol exposure is not permanent.
The 4-day binge protocol has been used extensively to model alcoholism in rodents because a continuously sustained elevation of blood ethanol (EtOH) concentrations by intragastric administration has proven adequate for rapid induction of physical dependence on EtOH in the rat, evidenced by withdrawal signs after EtOH removal (e.g., 11, 12). Although signs of physical dependence are not essential for a DSM-IV-R diagnosis of alcohol dependence in humans, such signs provide objective evidence for alcohol dependence in animal models (cf., 13). In the adult rat, histological examination following a 4-day binge reveals argyrophilia in the olfactory bulbs, dentate gyrus, insular, piriform, perirhinal, and entorhinal cortices (14–16). Argyrophilia refers to the ability of tissue elements to form submicroscopic grains of metallic silver when incubated in a solution containing silver ions, with the assumption that silver-stained neurons are moribund or dead (e.g., 17, 18). However, neuropathogical evidence indicating necrosis in rodents as a consequence of binge drinking has only been described qualitatively (7).
Magnetic resonance (MR) methods provide a noninvasive means for identification, visualization, and quantification of brain pathologies, their evolution, and their potential resolution. MR imaging (MRI) of the brains of chronically dependent alcoholic human adults commonly reveals a robust phenotype of widespread tissue damage and complementary ventriculomegaly (e.g., 1, 19, 20). We have previously modeled ex vacuo ventriculomegaly in the wild-type Wistar rat by demonstrating 30% ventricular expansion with average blood alcohol levels (BALs) of 445mg/dL attained after 24wks of involuntary exposure via vaporized EtOH (21). To our knowledge, there are no reports on the acute effects of binge drinking on ventricular volume in vivo in either human alcoholics or animal models of alcoholism.
MR spectroscopy (MRS) enables assessment of the neurochemical status of discrete brain structures with the potential of identifying mechanisms underlying selective brain pathologies. Increases in total creatine (tCr), choline-containing compounds (Cho), and myo-Inositol (mI) have been reported in the parietal gray matter of heavy drinkers compared with light drinkers; even higher concentrations of tCr, Cho, and mI were observed in binge drinkers compared with heavy drinkers (22). In wild-type Wistar rats exposed to vaporized EtOH for 24wks, BALs of 445mg/dL were associated with increases in Cho in dorsal hippocampal and striatal regions and no significant effects on tCr or mI (23).
This experiment examined the reversibility of brain injury after EtOH-binge exposure. If EtOH causes injury but not death of neurons, evidence for brain structural (measured with MRI) and metabolic (measured with MRS) insult should recover following removal of EtOH. Specifically, the expectation was that acute, high doses of EtOH would result in ventricular enlargement, increases in Cho, and decreases in NAA in dorsal hippocampal tissue, as previously observed after 24wks of vaporized EtOH exposure (23), but that all three markers of brain insult would normalize following abstinence from EtOH. This pattern would contrast with the persistent decreases in NAA observed after neuronal loss, for example, in ischemia (24). The return of MR-detected abnormalities to baseline, especially NAA, would suggest transient rather than permanent damage as a consequence of a single binge EtOH exposure.
METHODS
Animals
The animals, 19 wild-type, male Wistar rats (Charles River Laboratories) were singly housed with free access to food and water; lights were on for 12hrs starting at 6:00hr. Rats weighed 264.46±4.07g at the baseline scan. The Institutional Animal Care and Use Committees at SRI International and Stanford University approved all procedures.
Treatment
After baseline scanning, 11 rats were assigned to the EtOH group and received an initial dose of 5g/kg 25% EtOH w/v via oral gavage, then a maximum of 3g/kg every 8hrs for 4 days (volume average: 3.06±.86). Control animals received equivalent volumes of 5% dextrose with treatments occurring at comparable times to the experimental animals: ~8:00, 16:00, and 24:00hr. On each of the 4 days, all animals were weighed and blood samples were taken from the tail vein 3 times per day to determine BALs in plasma assayed for alcohol content based on direct reaction with the enzyme alcohol oxidase (Analox Instruments Ltd., UK). EtOH was administered according to body weight, BALs, and behavioral intoxication state assessed using Majchrowicz’s (1975) 6-point scale (range 0–5). To maintain similar weights between the 2 groups, if an EtOH rat’s weight diminished to 10% or below that of the average of the Con groups’ weights, Critical Care (Oxproline, Murdock, NE) was administered intragastrically 2 times per day to that animal. At the end of 4 days of treatment, the EtOH animals had received daily doses of 8.5–11.5g/kg/animal, a total average cumulative dose of 43±1.2g/kg/animal, had average BALs of 258±12mg/dL, and peak BALs of 418±20.3mg/dL.
MR Scanning Procedures
Schedule
All animals were scanned at baseline (scan 1), after 4 days of binge EtOH treatment (post-gavage scan 2; within 10hrs of the last EtOH dose), and after 7–8 days of recovery (recovery scan 3).
Anesthesia and Monitoring
Animals were held in an MR-invisible structure providing support for a radio frequency (RF) coil and a nose cone for delivery of isoflurane anesthesia (1.5–3.5%) and oxygen (1.5L/min) (25, 26). For each rat, blood oxygen saturation, pulse rate, rectal temperature, and respiration were monitored throughout the ~2hr experiment.
MRI Acquisition
The experiments were conducted on a clinical 3T GE Signa MR scanner equipped with a high-strength insert gradient coil (peak strength=600mT/m, peak slew rate=3200T/m/s, 27). The gradient system was operated at maximum amplitude of 500mT/m with a slew rate of 1800mT/m/ms. A custom-made rat brain quadrature head coil (Ø=44mm) was used for both RF excitation and signal reception. A gradient-recalled echo localizer scan was used to position the animals in the scanner and for graphical prescription of the subsequent scans. High resolution, dual-echo, fast spin-echo (FSE) images were acquired in the coronal plane, transaxial to the magnet system bore (TE1/TE2/TR=12/60/5000ms, FOV=64×48mm2, 256×192 matrix, echo train length=8, 50 slices, .3mm thick, 0mm separation, 4 acquisitions, in-plane resolution=.25×.25mm2).
Image Postprocessing
Baseline data of one individual animal were used to create a template for inter- and intra- animal registration. Using an affine registration procedure that spatially aligned brains and made all brains the same size but preserved the proportional size of internal brain structures (28, 29), each animal's baseline data were registered to the template. Similarly, each animal's scan 2 and scan 3 data were registered to its baseline data. The two transformation matrices were concatenated for a single reformatting step to produce equally sized and positioned images for region of interest evaluation of the lateral ventricles. The original .25×.25×.30mm3 data were up-sampled to .125×.125×.150mm3 and a 12.0×8.875×1.05mm3 region encompassing the lateral ventricles was defined on the template brain. T2 images were created from the early- and late- echo data; ventricle volume was defined as all voxels with T2 greater than 130ms in this region.
MRS Acquisition
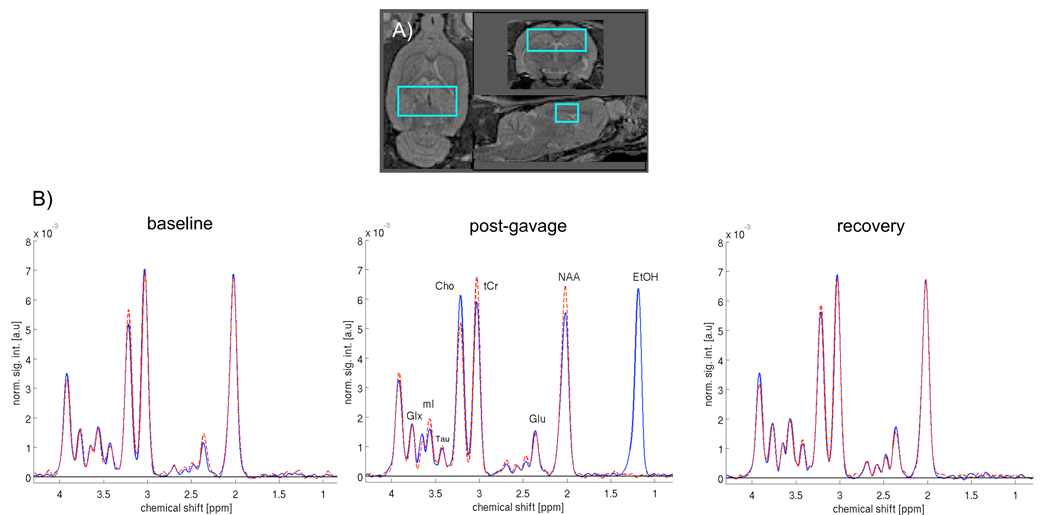
FSE images were used to prescribe a voxel (9.8×4×4mm3=156.8mm3) targeting the dorsal hippocampus and extending 2mm anterior and posterior to −4.00mm Bregma, 4.9mm to the right and left of midline, and 4mm inferior to −1.5mm Bregma, according to the atlas of Paxinos and Watson (30). Herein, dorsal hippocampus refers to the rat brain regions encompassed within this voxel, namely, the dorsal hippocampus, but also portions of the cingulum, corpus collosum, cortex, and thalamus. Spectroscopy was acquired with a constant time point-resolved spectroscopy (CT-PRESS) sequence (31–33) preceded by a 3-pulse chemical shift selective sequence for water suppression. Spectral acquisition without water suppression was preformed to measure tissue water content for normalization of metabolite signal intensities to the amount of tissue water in the voxel (for details, see 23). The quality of the spectra allowed evaluation of signals from N-acteyl aspartate (NAA, 2.02ppm), tCr (3.03ppm and 3.93ppm), Cho (3.24ppm), glutamate (Glu, 2.36ppm), and the combined resonances of glutamate and glutamine (Glx, 3.78ppm).
Behavioral Analysis
Neurological examination based on established guidelines (34, 35) was performed after 4 days of binge EtOH, and 3 times during the recovery week. Rats were rated (0=absent, 1=present) for neurological signs including autonomic, sensory, and motor functions.
Liver Analysis
At euthanasia (2.5–3.5% isoflurane, followed by transcardial perfusion), serum was collected and a liver chemistry assay was conducted. Aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase, gamma-glutamyl transpeptidase (GGT), total bilirubin, and direct bilirubin were measured. Left lateral lobe liver specimens from all rats were prepared with a standard hematoxylin and eosin (H&E) stain and a Masson’s Trichrome stain and evaluated (23).
Statistical Analysis
Group differences were analyzed using two group (Con vs. EtOH) by three time-point (baseline, post-gavage, and recovery) repeated-measures analysis of variance (ANOVA); where appropriate, Greenhouse-Geiser (GG) correction was applied to account for observations that do not obey the assumption of being uncorrelated with constant variance. Only group effects and interactions were of interest to this analysis. Follow-up between-group and within-group differences were determined by 2-tailed t-tests. Spearman Rank Order tests evaluated correlations.
RESULTS
Binge Ethanol Affects Weight and Modifies Behavior
Table 1 presents weights per group at each scan. Group differences in body weight were evident only at the recovery scan when EtOH animals were significantly lighter than Con animals (t(17)=2.6, p=.018). The EtOH group lost 16% body weight during binge EtOH exposure, but gained 24.7% during recovery.
Table 1.
Scan Schedule and Weights (in grams, mean ± SD)
| N | Scan 1 baseline |
Scan 2 post-gavage |
Scan 3 recovery |
|
|---|---|---|---|---|
| Control | 8 | 261.31 ± 21.11 | 241.61 ± 21.81 | 309.08 ± 27.66 |
| Ethanol | 11 | 266.76 ± 15.56 | 223.64 ± 19.74 | 278.42 ± 23.38* |
p≤.05 for between group unpaired t-tests
Neurological signs differentiated EtOH and Con groups during treatment and recovery. On day 4 of binge treatment, relative to Con rats, EtOH rats were more likely to sit motionless (t(17)=2.4, p=.03), stare straight ahead (t(17)=2.9, p=.009), exhibit gait disturbances (t(17)=2.7, p=.015), or splay legs when picked up (t(17)=2.4, p=.03). During the first 2 days of recovery, EtOH animals were more irritable (t(17)=2.4, p=.03) and likely to vocalize when handled (t(17)=3.1, p=.007) than Con animals. After 4 days of recovery, the two groups were behaviorally indistinguishable.
Binge Ethanol Reversibly Increases Ventricular Volume
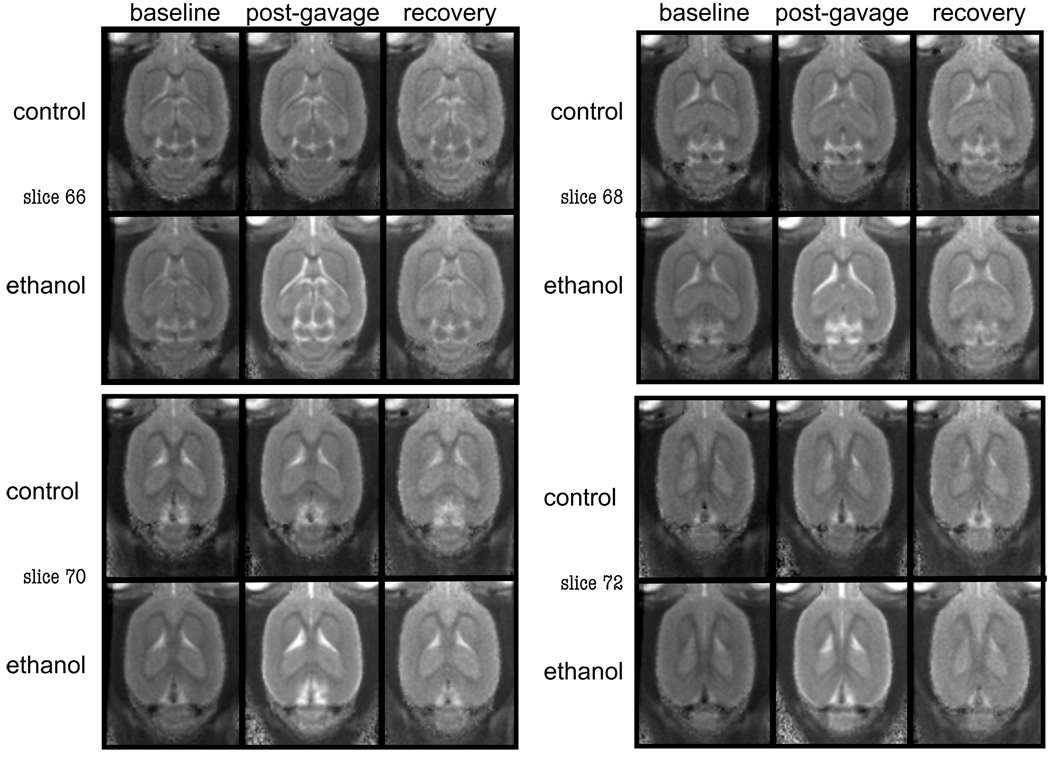
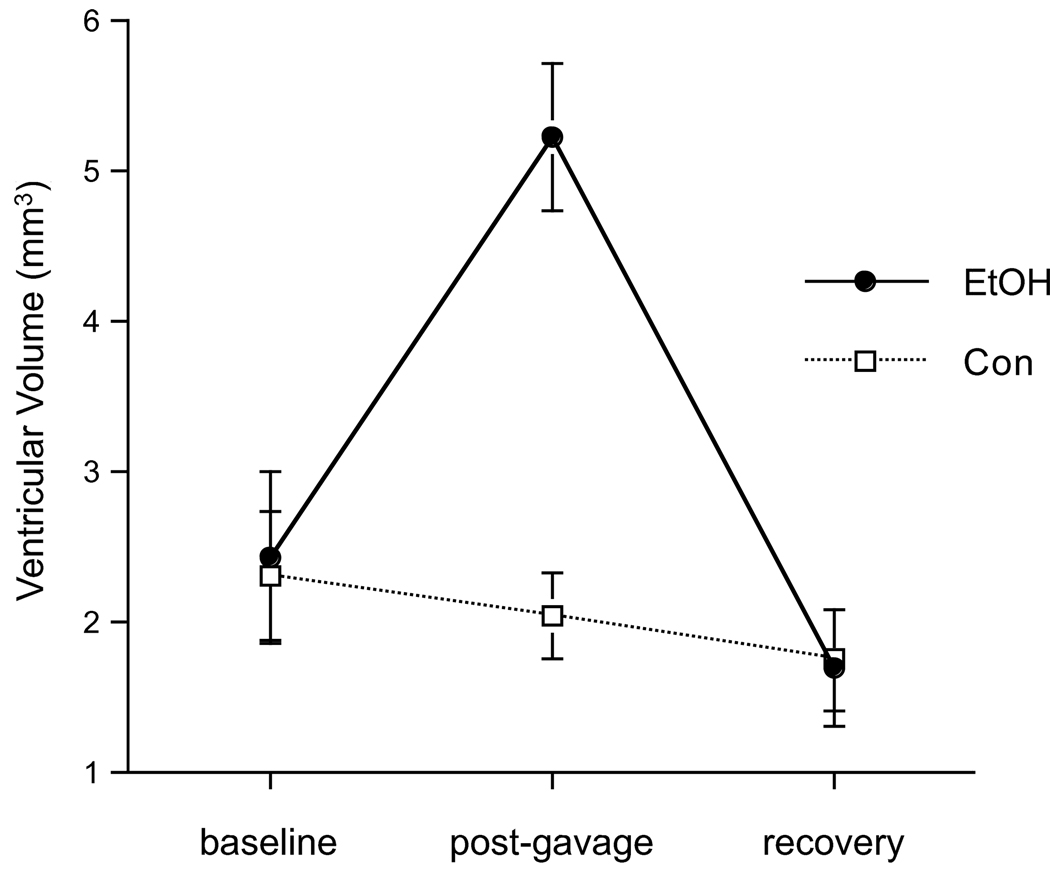
A 2-group, repeated-measures ANOVA yielded a group-by-time interaction (F(2,34)=10.79, p=.0002, GG=.0004), indicating that EtOH exposure modified ventricular volume. Post-gavage, ventricular volume in the EtOH group had increased from baseline (t(10)=3.9, p=.0029) and was greater than in the Con group (t(17)=5.1, p=.0001, Figure 1). At recovery, ventricular volume no longer differentiated the groups: the EtOH group evidenced a significant ventricular volume decrease between MRI 2 and MRI 3 (t(10)=5.5, p=.0003) and achieved full recovery (Table 2, Figure 2).
Figure 1.
Structural, group averaged computed T2-images showing example slices from Con and EtOH treated animals demonstrating increased ventricular volume in the EtOH animal at the post-gavage scan.
Table 2.
Ventricular Volume (in mm3, mean ± SD)
| baseline | post-gavage | recovery | |
|---|---|---|---|
| Control | 2.31 ± 1.23 | 2.05 ± .79 | 1.76 ± .94 |
| Ethanol | 2.44 ± 1.86 | 5.24 ± 1.63* | 1.70 ± 1.31 |
p≤.0005 for between group unpaired t-tests
Figure 2.
Quantification of ventricular volume (mean ± SE) at the 3 scan points (i.e., baseline, post-gavage, and recovery).
Binge Ethanol Reversibly Modifies Select Metabolite Levels
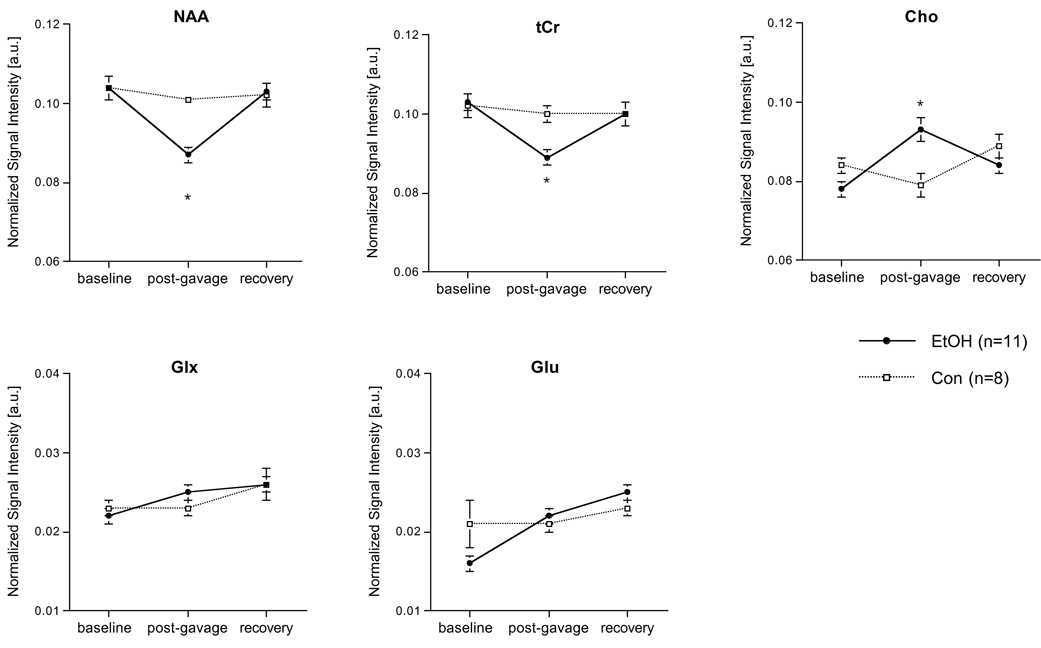
A 2-group, repeated-measures (3 scan sessions, 7 metabolite signals) ANOVA revealed a group-by-metabolite-by-time (F(12,204)=10.02, p=.0001, GG=.0001) interaction indicating a significant EtOH effect on metabolite levels. Four days of binge EtOH treatment resulted in lower NAA (t(17)=5.23, p=.0001) and tCr (t(17)=3.24, p=.0048) and higher Cho (t(17)=3.48, p=.0029) in the EtOH than the Con group (Figure 3 and Figure 4). Within the EtOH group, changes between baseline and post-gavage scans were significant for NAA (decreased; t(10)=4.38, p=.0014), tCr (decreased; t(10)=3.84, p=.0033) and Cho (increased; t(10)=6.78, p=.0001). A family-wise Bonferroni correction for 5 metabolites and 3 time points examined with pair-wise tests would require a 1-tailed significance of p≤.006, and a 2-tailed significance of p≤.003: even with a 2-tailed Bonferroni correction, the decrease in NAA and increase in Cho are significant between groups and within the EtOH group.
Figure 3.
a) Axial (left), coronal (top right), and sagittal (bottom right) views of a rat brain demonstrating voxel placement*. b) Averaged spectra of the Con group (n=8, dashed, red) and EtOH group (n=11, solid, blue) at the 3 scan points. Note the EtOH peak visible in the post-gavage spectra of the EtOH group. *see text for details on structures included in voxel.
Figure 4.
Water-referenced metabolite levels in the dorsal hippocampal voxel at baseline, post-gavage, and recovery scans. * p<.05. Error bars represent standard errors.
After 7 days of recovery, metabolite levels no longer distinguished the groups. Within the EtOH group, the changes between the post-gavage and recovery scan indicated a significant increase in NAA (t(10)=6.83, p=.0001) and tCr (t(10)=4.81, p=.0007), and a decrease in Cho (t(10)=2.64, p=.0246), such that levels of these metabolites at recovery were not different from Con animals.
Binge Ethanol Does Not Affect the Liver
Blood collected at euthanasia after a week of recovery did not reveal group differences in the levels of any liver enzymes monitored or in the levels of total or direct bilirubin. Postmortem histopathology provided no evidence for hepatic steatosis, alcoholic hepatitis, or alcoholic cirrhosis.
Relevance of Choline-Containing Compounds
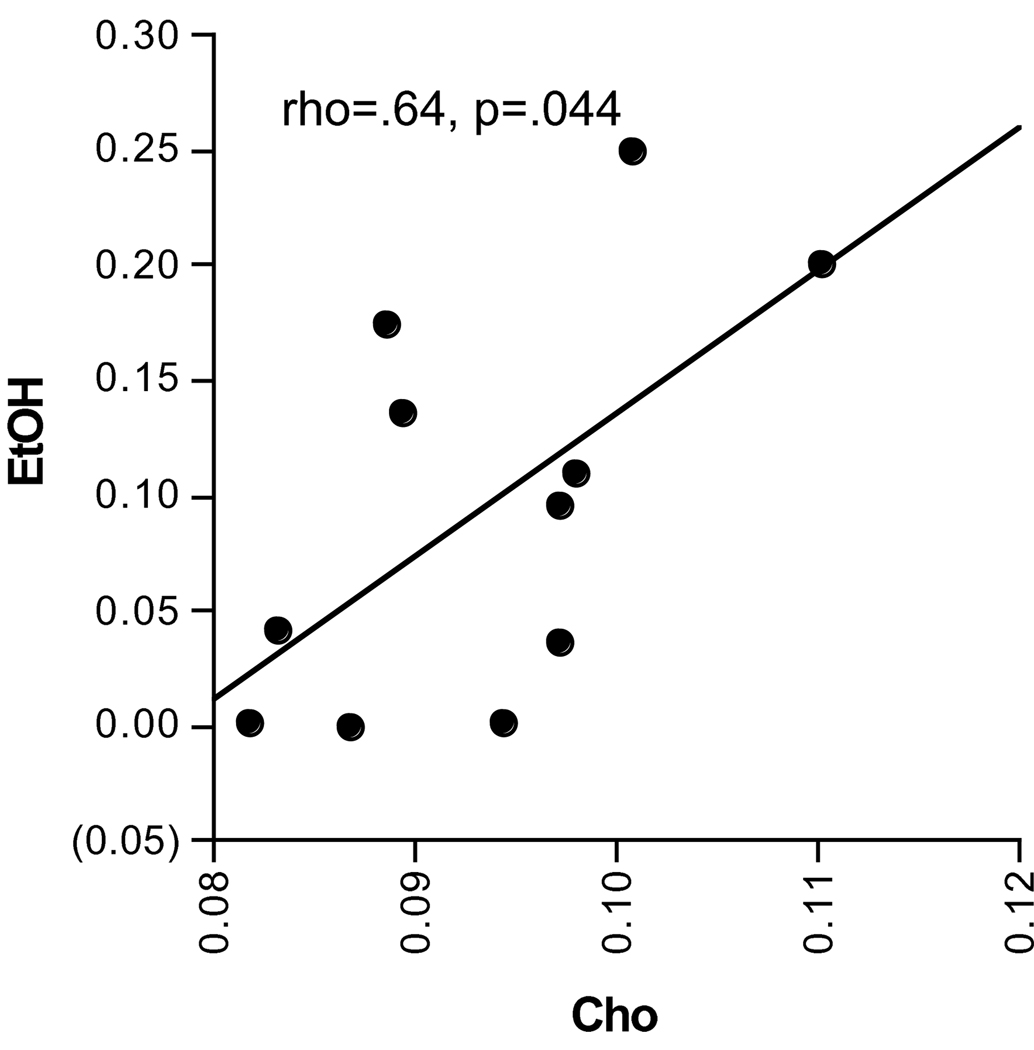
Simple regression analysis included only EtOH animals to test relations between metabolite levels and other quantified parameters. Of the 7 metabolites in the 3 scanning sessions, Cho at the post-gavage scan was the only significant metabolite correlate of in vivo EtOH levels at the post-gavage scan (rho=.64, p=.044; Figure 5).
Figure 5.
Correlation between Cho and EtOH levels at the post-gavage scan as quantified using MRS.
DISCUSSION
This study tested the hypothesis that specific brain regions of wild-type Wistar rats binge-exposed to EtOH would reveal reversible injury. Four days of binge EtOH exposure resulted in brain insult detected with MRI as an increase in ventricular volume and with MRS in the dorsal hippocampus as a decrease in the levels of NAA and tCr and an increase in Cho. In support of the hypothesis, examination of EtOH exposed animals after 7 days of recovery demonstrated complete reversibility of both ventricular dilation and changes in metabolite concentrations.
Binge Ethanol Reversibly Increases Ventricular Volume
Structural MRI of the brains of chronically dependent alcoholic human adults commonly reveals a robust phenotype of widespread tissue damage and complementary ventriculomegaly (e.g., 1, 19, 20). The current results demonstrate that high BALs are necessary to observe significant ventricular dilation in rodents and together with previous results (21, 36) suggest that the rate or pattern whereby high BALs are achieved is critical in determining extent of dilation. Specifically, ventricular enlargement is far more pronounced in rats achieving average BALs of 258mg/dL in just 4 days of involuntary intragastric administration of EtOH (115% increase) than in rats achieving average BALs of 222mg/dL over 24wks with involuntary vaporized EtOH exposure (30% increase, 21) or alcohol preferring P-rats achieving average BALs of 125mg/dL gradually with voluntarily EtOH consumption (36) where only modest ventricular enlargement was noted (cf., 37, 38). Another potential contributor to the more pronounced ventricular enlargement observed herein is reduced thiamine: acute alcohol exposure interferes with the absorption of thiamine from the gastrointestinal tract (39). Thiamine levels may be more compromised in acute EtOH intoxication than in models that expose rats to EtOH over longer periods.
Enlargement of the lateral ventricles is assumed to result from atrophy of the surrounding brain tissue. Postmortem studies have interpreted an increase in acquired argyrophilia in rodent corticolimbic regions following 4 days of binge EtOH exposure as reflecting irreversible neuronal degeneration (7, 15). However, recent findings suggest that not all silver-stained neurons die; indeed, argyrophilia is often seen in only temporarily injured and recovering neurons (40–42). Given the rapid reversibility of the considerable ventricular enlargement observed in this study, it is proposed that brain cells (neurons, astrocytes, oligodendrocytes, microglia) and their processes shrink rather than degenerate in response to binge EtOH exposure (43, 44). While another potential interpretation for brain volume recovery is neurogenesis (38), binge EtOH exposure actually reduces neurogenesis in the adult hippocampus (45). Indeed, peak rebound cell proliferation occurs at day 7 of abstinence (8) and could not explain the profound recovery in volume already evident at day 7.
Binge Ethanol Reversibly Modifies Selective Metabolite Levels
Herein, binge EtOH treatment resulted in significant decreases in NAA and tCr, and a significant increase in Cho in the dorsal hippocampus of wild-type Wistar rats; no other MRS detectable metabolites were significantly affected. In rodents, ad libitum exposure to 20% EtOH did not result in significant changes to NAA, whereas 24wks of vaporized EtOH exposure revealed a trend for reduced NAA in the EtOH compared with air-exposed groups (23). The reversibility of NAA declines following abstinence from alcohol in humans (e.g., 46, 47) contrasts with findings in epilepsy (e.g., 48) and ischemia (e.g., 49) in which neuronal loss incontrovertibly occurs. Together, these findings indicate that transient decreases in NAA likely reflect temporary dysfunction and reversible injury (50). Corroborating current findings, healthy human subjects acutely exposed to 300mL of a beverage containing .65–.75g/kg EtOH reveal significantly reduced tCr compared with baseline levels (51). Finally, although Cho alterations in response to EtOH intoxication are highly variable (e.g., 46, 47, 51, 52), actively drinking alcohol-dependent humans (22, 53) and rodents exposed to vaporized EtOH for 24wks (23) or as the sole fluid source for 16wks (54) demonstrate elevated levels of Cho.
Despite their presence at relatively high concentrations, the metabolic and neurochemical functions of MRS detectable metabolites are controversial, making definitive interpretations challenging. One potential interpretation for the post-gavage metabolic changes is that EtOH alters the osmotic balance of brain cells. EtOH is associated with increased intracranial pressure (e.g., 55), brain cell swelling (e.g., 56), and cerebral edema (e.g., 57) and both NAA (58) and tCr (59) have identified roles as osmolytes. In hyponatremia, decreased extracellular fluid osmolality can cause brain cell swelling (60). However, while hyponatremia presents with neurological symptoms similar to those seen following alcohol withdrawal (61), measurements in hyponatremic rats demonstrate reductions in the levels of tCr, glutamate, glutamine, glycerophosphorylcholine, and mI (62, 63) and rat cortical slices following acute hypo-osmotic shock reveal decreases in NAA, tCr, glutamate, glycerol-phosphorylcholine, phosphorylcholine, and choline (64). In the current study, binge-EtOH exposure resulted in an increase in Cho and no significant between-group differences in glutamate or Glx. Furthermore, cell swelling or edema would likely cause ventricular shrinkage rather than ventricular enlargement. Together, the Cho increase and ventricular dilation argue against osmotic imbalance as an interpretation for the changes observed.
Another potential interpretation is that the changes observed are due to EtOH effects on white matter (2, 65, 66). Changes to brain white matter occur in alcoholism (e.g., 67). One proposed function for NAA is a source of acetate for myelin lipid synthesis in oligodendrocytes (68). Given that NAA is reduced in acute multiple sclerosis lesions (e.g., 69), the reduction in NAA observed herein may reflect acute demyelination. The increase in Cho could likewise represent demyelination: the signal intensity of Cho increases in acute multiple sclerosis lesions during active myelin breakdown (69). However, findings regarding tCr in multiple sclerosis white matter lesions are equivocal, with the majority of studies reporting either an increase or no significant overall change in tCr (70), rather than a decrease as observed in the current study. Thus, with the exception of the directionality of tCr changes, white matter injury is a viable explanation for the current results.
Aspartoacylase, the enzyme that catalyzes the deacetylation of NAA is present in high concentrations in the liver (71) and both creatine (72) and choline (73) can be synthesized in the liver, suggesting that alterations to liver function may impact cerebral levels of these metabolites (59). However, this study did not reveal any effects of the binge EtOH exposure paradigm on liver enzymes or histopathology when assayed following 7 days of recovery. Furthermore, hepatic encephalopathy, an alcohol-related liver disease, is associated with a different cerebral metabolic profile from that observed here (74).
A final interpretation of the current findings is that binge EtOH intoxication compromises the brain’s normal energy utilization. A direct link between energy metabolism and NAA synthesis in mitochondria (75–79) suggests that NAA reductions may be due to energy depletion-induced impairments in mitochondrial NAA synthesis (cf., 68). As tCr reflects the substrates available for the brain’s high-energy phosphate metabolism (80), decreased tCr levels may also reflect brain energy depletion. Acute EtOH administration to rats results in phosphocreatine deficits (81) and rodents exposed to EtOH for 1 month reveal decreases in brain mitochondrial enzyme activities involved in oxidative phosphorylation (82, 83). A role for increased Cho as a marker of depressed brain energy utilization is also plausible. In rats, acute administration of choline chloride induces choline increases in arterial blood, cerebral spinal fluid, and brain homogenates suggesting that brain choline concentrations closely parallel fluctuations in plasma levels (84). After middle cerebral artery occlusion in rats, blood choline levels are significantly elevated (85). In hippocampal slices, a sharp increase in choline occurs when ATP values drop (86). Elevated brain choline may occur as a consequence of choline uptake from plasma (87), as occurs following hypoxic challenge (85, 88, 89). Indeed, a rich literature demonstrates a correlation between ATP deficiency and an increase in the release of free fatty acids (e.g., 90, 91, 92). Together, decreased NAA and tCr and increased Cho may reflect impaired energy in the brain in response to 4 days of binge EtOH intoxication (51, 93).
The reversibility in the levels of these metabolites with 7 days recovery provides evidence against neuronal loss or permanent damage as a result of this binge EtOH exposure protocol. Nonetheless, continuing EtOH exposure and the associated cycles of vulnerability could lead to permanent changes, including neuronal loss.
Binge Ethanol Does Not Significantly Affect the Liver
Neither the liver chemistry assay of serum nor histopathology of the left lateral lobe performed after 7 days of abstinence revealed liver damage as a consequence of binge EtOH exposure. Steatosis has been demonstrated in rats exposed to vaporized EtOH for a total 24wks (23) or rats exposed to a low fat diet and intragastric EtOH infusions for 15–85 days (94). Cirrhosis and fibrosis are rarely observed in rodents exposed to EtOH unless the diet is rich in polyunsaturated fatty acids (95).
Relevance of Choline-Containing Compounds
Elevated levels of Cho have been noted in non-abstinent chronic heavy drinkers (22), in social and moderate drinkers (53), and in rodents exposed to EtOH (23, 54). Together with the current finding of a significant correlation between Cho and EtOH levels as measured with MRS after binge EtOH intoxication, this converging evidence suggests that the presence of alcohol per se leads to an increase in Cho and the intriguing possibility of Cho as a surrogate in vivo marker for recent alcohol consumption.
Limitations
Every reasonable effort was made to treat the EtOH and Con groups equivalently; with respect to handling and volume of fluid administered, both groups endured an equivalent level of stress. We did not, however, account for differences related to the burning sensation potentially caused by EtOH compared with dextrose administration. Furthermore, the EtOH group was clearly intoxicated during the post-gavage scan (Figure 3). Thus, during imaging sessions, the EtOH group was concurrently exposed to EtOH and the anesthetic isoflurane, and the combination of EtOH with isoflurane may have affected cerebral metabolite levels. This possibility is difficult to test and is an inevitable consequence of the current in vivo experimental design. However, an in vitro rodent study investigating the effects of isoflurane demonstrated that while isoflurane increased brain glucose levels, it had no effect on the levels of other metabolites (96). Finally, brain tissue alterations due to osmotic effects may have changed either tissue water content, the relaxation rates of the metabolites, or both and thereby affected the signal intensities in the spectrum. However, if tissue water in the voxel increased, all metabolites would have displayed lower signals. Alternatively, if all metabolites showed a trend towards longer relaxation rates, all metabolites would have displayed higher signals. A generalized effect could not explain the observed signal decreases from NAA and tCr, and increase in Cho.
Concluding Remarks
A single episode of binge EtOH exposure to alcohol-naïve rats results in brain insult detected as ex vacuo ventriculomegaly. EtOH binge-induced declines in NAA and tCr and increases in Cho argue for depleted brain energy that may cause brain cells and their processes to retract (97) leading to widespread tissue shrinkage. That ventricular volume and metabolite levels return to baseline after 7 days of abstinence supports the brain’s ability to recover quickly from a single episode of binge EtOH exposure through healing of injured cells and not by neuronal regeneration.
ACKNOWLEDGEMENTS
Grant Sponsor: NIAAA
Grant Numbers: AA013521-INIA, AA005965, AA012388, AA017168
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES/CONFLICTS OF INTEREST
The authors report no biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.Sullivan EV, Pfefferbaum A. Neurocircuitry in alcoholism: A substrate of disruption and repair. Psychopharmacology (Berl) 2005;180:583–594. doi: 10.1007/s00213-005-2267-6. [DOI] [PubMed] [Google Scholar]
- 2.Sullivan EV. Compromised pontocerebellar and cerebellothalamocortical systems: speculations on their contributions to cognitive and motor impairment in nonamnesic alcoholism. Alcoholism, clinical and experimental research. 2003;27:1409–1419. doi: 10.1097/01.ALC.0000085586.91726.46. [DOI] [PubMed] [Google Scholar]
- 3.Oscar-Berman M, Marinkovic K. Alcohol: effects on neurobehavioral functions and the brain. Neuropsychol Rev. 2007;17:239–257. doi: 10.1007/s11065-007-9038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pfefferbaum A, Sullivan EV, Mathalon DH, Shear PK, Rosenbloom MJ, Lim KO. Longitudinal changes in magnetic resonance imaging brain volumes in abstinent and relapsed alcoholics. Alcoholism: Clinical and Experimental Research. 1995;19:1177–1191. doi: 10.1111/j.1530-0277.1995.tb01598.x. [DOI] [PubMed] [Google Scholar]
- 5.Cardenas VA, Studholme C, Gazdzinski S, Durazzo TC, Meyerhoff DJ. Deformation-based morphometry of brain changes in alcohol dependence and abstinence. Neuroimage. 2007;34:879–887. doi: 10.1016/j.neuroimage.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenbloom MJ, Rohlfing T, O'Reilly A, Sassoon SA, Pfefferbaum A, Sullivan EV. Improvement in memory and static balance with abstinence in alcoholic men and women: Selective relations with changes in regional ventricular volumes. Psychiatry Research: Neuroimaging. 2007;155:91–102. doi: 10.1016/j.pscychresns.2006.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Obernier JA, Bouldin TW, Crews FT. Binge ethanol exposure in adult rats causes necrotic cell death. Alcoholism: Clinical and Experimental Research. 2002;26:547–557. [PubMed] [Google Scholar]
- 8.Nixon K, Crews FT. Temporally specific burst in cell proliferation increases hippocampal neurogenesis in protracted abstinence from alcohol. J Neurosci. 2004;24:9714–9722. doi: 10.1523/JNEUROSCI.3063-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhardwaj RD, Curtis MA, Spalding KL, Buchholz BA, Fink D, Bjork-Eriksson T, et al. Neocortical neurogenesis in humans is restricted to development. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:12564–12568. doi: 10.1073/pnas.0605177103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rakic P. Neurogenesis in adult primates. Progress in brain research. 2002;138:3–14. doi: 10.1016/S0079-6123(02)38067-1. [DOI] [PubMed] [Google Scholar]
- 11.Majchrowicz E. Induction of physical dependence upon ethanol and the associated behavioral changes in rats. Psychopharmacologia. 1975;43:245–254. doi: 10.1007/BF00429258. [DOI] [PubMed] [Google Scholar]
- 12.Faingold CL, et al. The Majchrowicz binge alcohol protocol: an intubation technique to study alcohol dependence in rats. Current protocols in neuroscience / editorial board, Jacqueline N Crawley. 2008;Chapter 9(Unit 9):28. doi: 10.1002/0471142301.ns0928s44. [DOI] [PubMed] [Google Scholar]
- 13.McBride WJ, Li TK. Animal models of alcoholism: neurobiology of high alcohol-drinking behavior in rodents. Crit Rev Neurobiol. 1998;12:339–369. doi: 10.1615/critrevneurobiol.v12.i4.40. [DOI] [PubMed] [Google Scholar]
- 14.Collins MA, Corso TD, Neafsey EJ. Neuronal degeneration in rat cerebrocortical and olfactory regions during subchronic "binge" intoxication with ethanol: possible explanation for olfactory deficits in alcoholics. Alcoholism: Clinical and Experimental Research. 1996;20:284–292. doi: 10.1111/j.1530-0277.1996.tb01641.x. [DOI] [PubMed] [Google Scholar]
- 15.Crews FT, Braun CJ, Hoplight B, Switzer RC, 3rd, Knapp DJ. Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcoholism: Clinical and Experimental Research. 2000;24:1712–1723. [PubMed] [Google Scholar]
- 16.Zou JY, Martinez DB, Neafsey EJ, Collins MA. Binge ethanol-induced brain damage in rats: effect of inhibitors of nitric oxide synthase. Alcoholism: Clinical and Experimental Research. 1996;20:1406–1411. doi: 10.1111/j.1530-0277.1996.tb01141.x. [DOI] [PubMed] [Google Scholar]
- 17.Du F, Eid T, Schwarcz R. Neuronal damage after the injection of aminooxyacetic acid into the rat entorhinal cortex: a silver impregnation study. Neuroscience. 1998;82:1165–1178. doi: 10.1016/s0306-4522(97)00354-0. [DOI] [PubMed] [Google Scholar]
- 18.Lo Bianco C, Ridet JL, Schneider BL, Deglon N, Aebischer P. alpha -Synucleinopathy and selective dopaminergic neuron loss in a rat lentiviral-based model of Parkinson's disease. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:10813–10818. doi: 10.1073/pnas.152339799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hommer DW, Momenan R, Kaiser E, Rawlings RR. Evidence for a gender-related effect of alcoholism on brain volumes. Am J Psychiatry. 2001;158:198–204. doi: 10.1176/appi.ajp.158.2.198. [DOI] [PubMed] [Google Scholar]
- 20.Fein G, Di Sclafani V, Cardenas VA, Goldmann H, Tolou-Shams M, Meyerhoff DJ. Cortical gray matter loss in treatment-naive alcohol dependent individuals. Alcoholism: Clinical and Experimental Research. 2002;26:558–564. [PMC free article] [PubMed] [Google Scholar]
- 21.Pfefferbaum A, Zahr NM, Mayer D, Vinco S, Orduna J, Rohlfing v, et al. Ventricular expansion in wild-type Wistar rats after alcohol exposure by vapor chamber. Alcoholism, clinical and experimental research. 2008;32:1459–1467. doi: 10.1111/j.1530-0277.2008.00721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meyerhoff DJ, Blumenfeld R, Truran D, Lindgren J, Flenniken D, Cardenas V, et al. Effects of heavy drinking, binge drinking, and family history of alcoholism on regional brain metabolites. Alcoholism: Clinical and Experimental Research. 2004;28:650–661. doi: 10.1097/01.ALC.0000121805.12350.CA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zahr NM, Mayer D, Vinco S, Orduna J, Luong R, Sullivan EV, et al. In vivo evidence for alcohol-induced neurochemical changes in rat brain without protracted withdrawal, pronounced thiamine deficiency, or severe liver damage. Neuropsychopharmacology. 2009;34:1427–1442. doi: 10.1038/npp.2008.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sager TN, Hansen AJ, Laursen H. Correlation between N-acetylaspartate levels and histopathologic changes in cortical infarcts of mice after middle cerebral artery occlusion. J Cereb Blood Flow Metab. 2000;20:780–788. doi: 10.1097/00004647-200005000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Adalsteinsson E, Hurd RE, Mayer D, Sailasuta N, Sullivan EV, Pfefferbaum A. In vivo 2D J-resolved magnetic resonance spectroscopy of rat brain with a 3-T clinical human scanner. NeuroImage. 2004;22:381–386. doi: 10.1016/j.neuroimage.2003.12.046. [DOI] [PubMed] [Google Scholar]
- 26.Pfefferbaum A, Adalsteinsson E, Sullivan EV. In vivo structural imaging of the rat brain with a 3-T clinical human scanner. J Magn Reson Imaging. 2004;20:779–785. doi: 10.1002/jmri.20181. [DOI] [PubMed] [Google Scholar]
- 27.Chronik B, Alejski A, Rutt BK. Design and fabrication of a three-axis multilayer gradient coil for magnetic resonance microscopy of mice. MAGMA. 2000;10:131–146. doi: 10.1007/BF02601848. [DOI] [PubMed] [Google Scholar]
- 28.Studholme C, Hill DLG, Hawkes DJ. Automated three-dimensional registration of magnetic resonance and positron emission tomography brain images by multiresolution optimization of voxel similarity measures. Med Phys. 1997;24:25–35. doi: 10.1118/1.598130. [DOI] [PubMed] [Google Scholar]
- 29.Rohlfing T, West JB, Beier J, Liebig T, Taschner CA, Thomale UW. Registration of functional and anatomical MRI: accuracy assessment and application in navigated neurosurgery. Comput Aided Surg. 2000;5:414–425. doi: 10.1002/igs.1003. [DOI] [PubMed] [Google Scholar]
- 30.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. London: Elsevier Academic Press; 2005. [Google Scholar]
- 31.Mayer D, Spielman DM. Detection of glutamate in the human brain at 3 T using optimized constant time point resolved spectroscopy. Magn Reson Med. 2005;54:439–442. doi: 10.1002/mrm.20571. [DOI] [PubMed] [Google Scholar]
- 32.Mayer D, Zahr NM, Sullivan EV, Pfefferbaum A. In vivo metabolite differences between the basal ganglia and cerebellum of the rat brain detected with proton MRS at 3T. Psychiatry Res. 2007;154:267–273. doi: 10.1016/j.pscychresns.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dreher W, Leibfritz D. Detection of homonuclear decoupled in vivo proton NMR spectra using constant time chemical shift encoding: CT-PRESS. Magnetic resonance imaging. 1999;17:141–150. doi: 10.1016/s0730-725x(98)00156-8. [DOI] [PubMed] [Google Scholar]
- 34.Becker HC. Animal models of alcohol withdrawal. Alcohol Research Health. 2000;24:105–113. [PMC free article] [PubMed] [Google Scholar]
- 35.Pitkin SR, Savage LM. Aging potentiates the acute and chronic neurological symptoms of pyrithiamine-induced thiamine deficiency in the rodent. Behavioiral Brain Research. 2001;119:167–177. doi: 10.1016/s0166-4328(00)00350-8. [DOI] [PubMed] [Google Scholar]
- 36.Pfefferbaum A, Adalsteinsson E, Sood R, Mayer D, Bell R, McBride WJ, et al. Longitudinal brain MRI study of the alcohol-preferring rat: Part II: Effects of voluntary chronic alcohol consumption. Alcoholism: Clinical and Experimental Research. 2006;30:1248–1261. doi: 10.1111/j.1530-0277.2006.00146.x. [DOI] [PubMed] [Google Scholar]
- 37.Fadda F, Rossetti ZL. Chronic ethanol consumption: From neuroadaptation to neurodegeneration. Prog Neurobiol. 1998;56:385–431. doi: 10.1016/s0301-0082(98)00032-x. [DOI] [PubMed] [Google Scholar]
- 38.Nixon K. Alcohol and adult neurogenesis: roles in neurodegeneration and recovery in chronic alcoholism. Hippocampus. 2006;16:287–295. doi: 10.1002/hipo.20162. [DOI] [PubMed] [Google Scholar]
- 39.Martin PR, Singleton CK, Hiller-Sturmhofel S. The role of thiamine deficiency in alcoholic brain disease. Alcohol Res Health. 2003;27:134–142. [PMC free article] [PubMed] [Google Scholar]
- 40.Zsombok A, Toth Z, Gallyas F. Basophilia, acidophilia and argyrophilia of "dark" (compacted) neurons during their formation, recovery or death in an otherwise undamaged environment. Journal of neuroscience methods. 2005;142:145–152. doi: 10.1016/j.jneumeth.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 41.Ishida K, Ungusparkorn C, Hida H, Aihara N, Ida K, Nishino H. Argyrophilic dark neurons distribute with a different pattern in the brain after over hours treadmill running and swimming in the rat. Neuroscience letters. 1999;277:149–152. doi: 10.1016/s0304-3940(99)00870-8. [DOI] [PubMed] [Google Scholar]
- 42.Baram TZ, Eghbal-Ahmadi M, Bender RA. Is neuronal death required for seizure-induced epileptogenesis in the immature brain? Progress in brain research. 2002;135:365–375. doi: 10.1016/S0079-6123(02)35033-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miguel-Hidalgo JJ, Wei J, Andrew M, Overholser JC, Jurjus G, Stockmeier CA, et al. Glia pathology in the prefrontal cortex in alcohol dependence with and without depressive symptoms. Biological psychiatry. 2002;52:1121–1133. doi: 10.1016/s0006-3223(02)01439-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cullen KM, Halliday GM. Chronic alcoholics have substantial glial pathology in the forebrain and diencephalon. Alcohol and Alcoholism Suppl. 1994;2:253–257. [PubMed] [Google Scholar]
- 45.Nixon K, Crews FT. Binge ethanol exposure decreases neurogenesis in adult rat hippocampus. J Neurochem. 2002;83:1087–1093. doi: 10.1046/j.1471-4159.2002.01214.x. [DOI] [PubMed] [Google Scholar]
- 46.Bendszus M, Weijers HG, Wiesbeck G, Warmuth-Metz M, Bartsch AJ, Engels S, et al. Sequential MR imaging and proton MR spectroscopy in patients who underwent recent detoxification for chronic alcoholism: correlation with clinical and neuropsychological data. American Journal of Neuroradiology. 2001;22:1926–1932. [PMC free article] [PubMed] [Google Scholar]
- 47.Parks MH, Dawant BM, Riddle WR, Hartmann SL, Dietrich MS, Nickel MK, et al. Longitudinal brain metabolic characterization of chronic alcoholics with proton magnetic resonance spectroscopy. Alcoholism: Clinical and Experimental Research. 2002;26:1368–1380. doi: 10.1097/01.ALC.0000029598.07833.2D. [DOI] [PubMed] [Google Scholar]
- 48.Bengzon J, Mohapel P, Ekdahl CT, Lindvall O. Neuronal apoptosis after brief and prolonged seizures. Progress in brain research. 2002;135:111–119. doi: 10.1016/S0079-6123(02)35011-8. [DOI] [PubMed] [Google Scholar]
- 49.Taoufik E, Probert L. Ischemic neuronal damage. Current pharmaceutical design. 2008;14:3565–3573. doi: 10.2174/138161208786848748. [DOI] [PubMed] [Google Scholar]
- 50.Demougeot C, Marie C, Giroud M, Beley A. N-acetylaspartate: a literature review of animal research on brain ischaemia. Journal of neurochemistry. 2004;90:776–783. doi: 10.1111/j.1471-4159.2004.02583.x. [DOI] [PubMed] [Google Scholar]
- 51.Biller A, Bartsch AJ, Homola G, Solymosi L, Bendszus M. The effect of ethanol on human brain metabolites longitudinally characterized by proton MR spectroscopy. J Cereb Blood Flow Metab. 2009 doi: 10.1038/jcbfm.2009.12. [DOI] [PubMed] [Google Scholar]
- 52.Durazzo TC, Gazdzinski S, Banys P, Meyerhoff DJ. Cigarette smoking exacerbates chronic alcohol-induced brain damage: a preliminary metabolite imaging study. Alcoholism: Clinical and Experimental Research. 2004;28:1849–1860. doi: 10.1097/01.alc.0000148112.92525.ac. [DOI] [PubMed] [Google Scholar]
- 53.Ende G, Walter S, Welzel H, Demirakca T, Wokrina T, Ruf M, et al. Alcohol consumption significantly influences the MR signal of frontal choline-containing compounds. NeuroImage. 2006;32:740–746. doi: 10.1016/j.neuroimage.2006.03.049. [DOI] [PubMed] [Google Scholar]
- 54.Lee H, Holburn GH, Price RR. Proton MR spectroscopic studies of chronic alcohol exposure on the rat brain. J Magn Reson Imaging. 2003;18:147–151. doi: 10.1002/jmri.10335. [DOI] [PubMed] [Google Scholar]
- 55.McQueen JD, Posey JB. Changes in intracranial pressure and brain hydration during acute ethanolism. Surgical neurology. 1975;4:375–379. [PubMed] [Google Scholar]
- 56.Aschner M, Allen JW, Mutkus LA, Cao C. Ethanol-induced swelling in neonatal rat primary astrocyte cultures. Brain research. 2001;900:219–226. doi: 10.1016/s0006-8993(01)02314-9. [DOI] [PubMed] [Google Scholar]
- 57.Collins MA, Zou JY, Neafsey EJ. Brain damage due to episodic alcohol exposure in vivo and in vitro: furosemide neuroprotection implicates edema-based mechanism. The FASEB Journal. 1998;12:221–230. doi: 10.1096/fasebj.12.2.221. [DOI] [PubMed] [Google Scholar]
- 58.Taylor DL, Davies SE, Obrenovitch TP, Doheny MH, Patsalos PN, Clark JB, et al. Investigation into the role of N-acetylaspartate in cerebral osmoregulation. Journal of neurochemistry. 1995;65:275–281. doi: 10.1046/j.1471-4159.1995.65010275.x. [DOI] [PubMed] [Google Scholar]
- 59.Ross B, Bluml S. Magnetic resonance spectroscopy of the human brain. The Anatomical record. 2001;265:54–84. doi: 10.1002/ar.1058. [DOI] [PubMed] [Google Scholar]
- 60.Farrell C, Del Rio M. Hyponatremia. Pediatrics in review / American Academy of Pediatrics. 2007;28:426–428. doi: 10.1542/pir.28-11-426. [DOI] [PubMed] [Google Scholar]
- 61.Lambie DG. Alcoholic brain damage and neurological symptoms of alcohol withdrawal--manifestations of overhydration. Medical hypotheses. 1985;16:377–388. doi: 10.1016/0306-9877(85)90058-1. [DOI] [PubMed] [Google Scholar]
- 62.Lien YH, Shapiro JI, Chan L. Study of brain electrolytes and organic osmolytes during correction of chronic hyponatremia. Implications for the pathogenesis of central pontine myelinolysis. The Journal of clinical investigation. 1991;88:303–309. doi: 10.1172/JCI115292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Verbalis JG, Gullans SR. Hyponatremia causes large sustained reductions in brain content of multiple organic osmolytes in rats. Brain research. 1991;567:274–282. doi: 10.1016/0006-8993(91)90806-7. [DOI] [PubMed] [Google Scholar]
- 64.Bothwell JH, Rae C, Dixon RM, Styles P, Bhakoo KK. Hypo-osmotic swelling-activated release of organic osmolytes in brain slices: implications for brain oedema in vivo. Journal of neurochemistry. 2001;77:1632–1640. doi: 10.1046/j.1471-4159.2001.00403.x. [DOI] [PubMed] [Google Scholar]
- 65.De la Monte SM. Disproportionate atrophy of cerebral white matter in chronic alcoholics. Arch Neurol. 1988;45:990–992. doi: 10.1001/archneur.1988.00520330076013. [DOI] [PubMed] [Google Scholar]
- 66.Pfefferbaum A, Rosenbloom M, Rohlfing T, Sullivan EV. Degradation of association and projection white matter systems in alcoholism detected with quantitative fiber tracking. Biological psychiatry. 2009;65:680–690. doi: 10.1016/j.biopsych.2008.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Alling C, Bostrom K. Demyelination of the mamillary bodies in alcoholism. A combined morphological and biochemical study. Acta Neuropathologica (Berl) 1980;50:77–80. doi: 10.1007/BF00688539. [DOI] [PubMed] [Google Scholar]
- 68.Moffett JR, Ross B, Arun P, Madhavarao CN, Namboodiri AM. N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Progress in neurobiology. 2007;81:89–131. doi: 10.1016/j.pneurobio.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mader I, Rauer S, Gall P, Klose U. (1)H MR spectroscopy of inflammation, infection and ischemia of the brain. European journal of radiology. 2008 doi: 10.1016/j.ejrad.2008.02.033. [DOI] [PubMed] [Google Scholar]
- 70.Caramanos Z, Narayanan S, Arnold DL. 1H-MRS quantification of tNA and tCr in patients with multiple sclerosis: a meta-analytic review. Brain. 2005;128:2483–2506. doi: 10.1093/brain/awh640. [DOI] [PubMed] [Google Scholar]
- 71.D'Adamo AF, Crispin Smith J, Woiler C. The occurrence of N-acetlyaspartate amidohydrolase (Aminoacyclase II) in the developing rat. J Neurochem. 1973;20:1275–1278. doi: 10.1111/j.1471-4159.1973.tb00097.x. [DOI] [PubMed] [Google Scholar]
- 72.Wyss M, Wallimann T. Creatine metabolism and the consequences of creatine depletion in muscle. Molecular and cellular biochemistry. 1994:133–134. 51–66. doi: 10.1007/BF01267947. [DOI] [PubMed] [Google Scholar]
- 73.Li Z, Vance DE. Phosphatidylcholine and choline homeostasis. Journal of lipid research. 2008;49:1187–1194. doi: 10.1194/jlr.R700019-JLR200. [DOI] [PubMed] [Google Scholar]
- 74.Cordoba J, Sanpedro F, Alonso J, Rovira A. 1H magnetic resonance in the study of hepatic encephalopathy in humans. Metabolic brain disease. 2002;17:415–429. doi: 10.1023/a:1021926405944. [DOI] [PubMed] [Google Scholar]
- 75.Bates TE, Strangward M, Keelan J, Davey GP, Munro PMG, Clark JB. Inhibition of N-acetylaspartate production: Implications for H-1 MRS studies in vivo. Neuroreport. 1996;7:1397–1400. [PubMed] [Google Scholar]
- 76.Patel TB, Clark JB. Synthesis of N-acetyl-L-aspartate by rat brain mitochondria and its involvement in mitochondrial/cytosolic carbon transport. The Biochemical journal. 1979;184:539–546. doi: 10.1042/bj1840539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Heales SJ, Davies SE, Bates TE, Clark JB. Depletion of brain glutathione is accompanied by impaired mitochondrial function and decreased N-acetyl aspartate concentration. Neurochemical research. 1995;20:31–38. doi: 10.1007/BF00995149. [DOI] [PubMed] [Google Scholar]
- 78.Dautry C, Vaufrey F, Brouillet E, Bizat N, Henry PG, Conde F, et al. Early N-acetylaspartate depletion is a marker of neuronal dysfunction in rats and primates chronically treated with the mitochondrial toxin 3-nitropropionic acid. J Cereb Blood Flow Metab. 2000;20:789–799. doi: 10.1097/00004647-200005000-00005. [DOI] [PubMed] [Google Scholar]
- 79.Demougeot C, Garnier P, Mossiat C, Bertrand N, Giroud M, Beley A, et al. N-Acetylaspartate, a marker of both cellular dysfunction and neuronal loss: its relevance to studies of acute brain injury. Journal of neurochemistry. 2001;77:408–415. doi: 10.1046/j.1471-4159.2001.00285.x. [DOI] [PubMed] [Google Scholar]
- 80.Sartorius A, Lugenbiel P, Mahlstedt MM, Ende G, Schloss P, Vollmayr B. Proton magnetic resonance spectroscopic creatine correlates with creatine transporter protein density in rat brain. Journal of neuroscience methods. 2008;172:215–219. doi: 10.1016/j.jneumeth.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 81.Altura BM, Altura BT. Role of magnesium and calcium in alcohol-induced hypertension and strokes as probed by in vivo television microscopy, digital image microscopy, optical spectroscopy, 31P-NMR, spectroscopy and a unique magnesium ion-selective electrode. Alcoholism, clinical and experimental research. 1994;18:1057–1068. doi: 10.1111/j.1530-0277.1994.tb00082.x. [DOI] [PubMed] [Google Scholar]
- 82.Marin-Garcia J, Ananthakrishnan R, Goldenthal MJ. Heart mitochondria response to alcohol is different than brain and liver. Alcoholism, clinical and experimental research. 1995;19:1463–1466. doi: 10.1111/j.1530-0277.1995.tb01008.x. [DOI] [PubMed] [Google Scholar]
- 83.Carter AJ, Muller RE, Pschorn U, Stransky W. Preincubation with creatine enhances levels of creatine phosphate and prevents anoxic damage in rat hippocampal slices. Journal of neurochemistry. 1995;64:2691–2699. doi: 10.1046/j.1471-4159.1995.64062691.x. [DOI] [PubMed] [Google Scholar]
- 84.Klein J, Koppen A, Loffelholz K, Schmitthenner J. Uptake and metabolism of choline by rat brain after acute choline administration. Journal of neurochemistry. 1992;58:870–876. doi: 10.1111/j.1471-4159.1992.tb09337.x. [DOI] [PubMed] [Google Scholar]
- 85.Scremin OU, Jenden DJ. Focal ischemia enhances choline output and decreases acetylcholine output from rat cerebral cortex. Stroke; a journal of cerebral circulation. 1989;20:92–95. doi: 10.1161/01.str.20.1.92. [DOI] [PubMed] [Google Scholar]
- 86.Djuricic B, Olson SR, Assaf HM, Whittingham TS, Lust WD, Drewes LR. Formation of free choline in brain tissue during in vitro energy deprivation. J Cereb Blood Flow Metab. 1991;11:308–313. doi: 10.1038/jcbfm.1991.63. [DOI] [PubMed] [Google Scholar]
- 87.Allen DD, Smith QR. Characterization of the blood-brain barrier choline transporter using the in situ rat brain perfusion technique. Journal of neurochemistry. 2001;76:1032–1041. doi: 10.1046/j.1471-4159.2001.00093.x. [DOI] [PubMed] [Google Scholar]
- 88.Jenden DJ, Scremin OU. Effects of hypoxia and hypercapnia on whole body release and clearance of choline. The American journal of physiology. 1995;268:R1520–R1525. doi: 10.1152/ajpregu.1995.268.6.R1520. [DOI] [PubMed] [Google Scholar]
- 89.Jope RS, Jenden DJ. Choline and phospholipid metabolism and the synthesis of acetylcholine in rat brain. Journal of neuroscience research. 1979;4:69–82. doi: 10.1002/jnr.490040110. [DOI] [PubMed] [Google Scholar]
- 90.Bazan NG., Jr Effects of ischemia and electroconvulsive shock on free fatty acid pool in the brain. Biochimica et biophysica acta. 1970;218:1–10. doi: 10.1016/0005-2760(70)90086-x. [DOI] [PubMed] [Google Scholar]
- 91.Trovarelli G, De Medio GE, Montanini I. The influence of CDP-choline on brain lipid metabolism during ischemia. Il Farmaco; edizione scientifica. 1982;37:663–668. [PubMed] [Google Scholar]
- 92.Katsura K, Rodriguez de Turco EB, Folbergrova J, Bazan NG, Siesjo BK. Coupling among energy failure, loss of ion homeostasis, and phospholipase A2 and C activation during ischemia. Journal of neurochemistry. 1993;61:1677–1684. doi: 10.1111/j.1471-4159.1993.tb09803.x. [DOI] [PubMed] [Google Scholar]
- 93.Jaatinen P, Rintala J. Mechanisms of ethanol-induced degeneration in the developing, mature, and aging cerebellum. Cerebellum (London, England) 2008;7:332–347. doi: 10.1007/s12311-008-0034-z. [DOI] [PubMed] [Google Scholar]
- 94.Tsukamoto H, French SW, Benson N, Delgado G, Rao GA, Larkin EC, et al. Severe and progressive steatosis and focal necrosis in rat liver induced by continuous intragastric infusion of ethanol and low fat diet. Hepatology (Baltimore, Md. 1985;5:224–232. doi: 10.1002/hep.1840050212. [DOI] [PubMed] [Google Scholar]
- 95.French SW, Nash J, Shitabata P, Kachi K, Hara C, Chedid A, et al. Pathology of alcoholic liver disease. VA Cooperative Study Group 119. Seminars in liver disease. 1993;13:154–169. doi: 10.1055/s-2007-1007346. [DOI] [PubMed] [Google Scholar]
- 96.Kofke WA, Hawkins RA, Davis DW, Biebuyck JF. Comparison of the effects of volatile anesthetics on brain glucose metabolism in rats. Anesthesiology. 1987;66:810–813. doi: 10.1097/00000542-198706000-00016. [DOI] [PubMed] [Google Scholar]
- 97.Esiri MM. Ageing and the brain. The Journal of pathology. 2007;211:181–187. doi: 10.1002/path.2089. [DOI] [PubMed] [Google Scholar]