Abstract
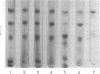
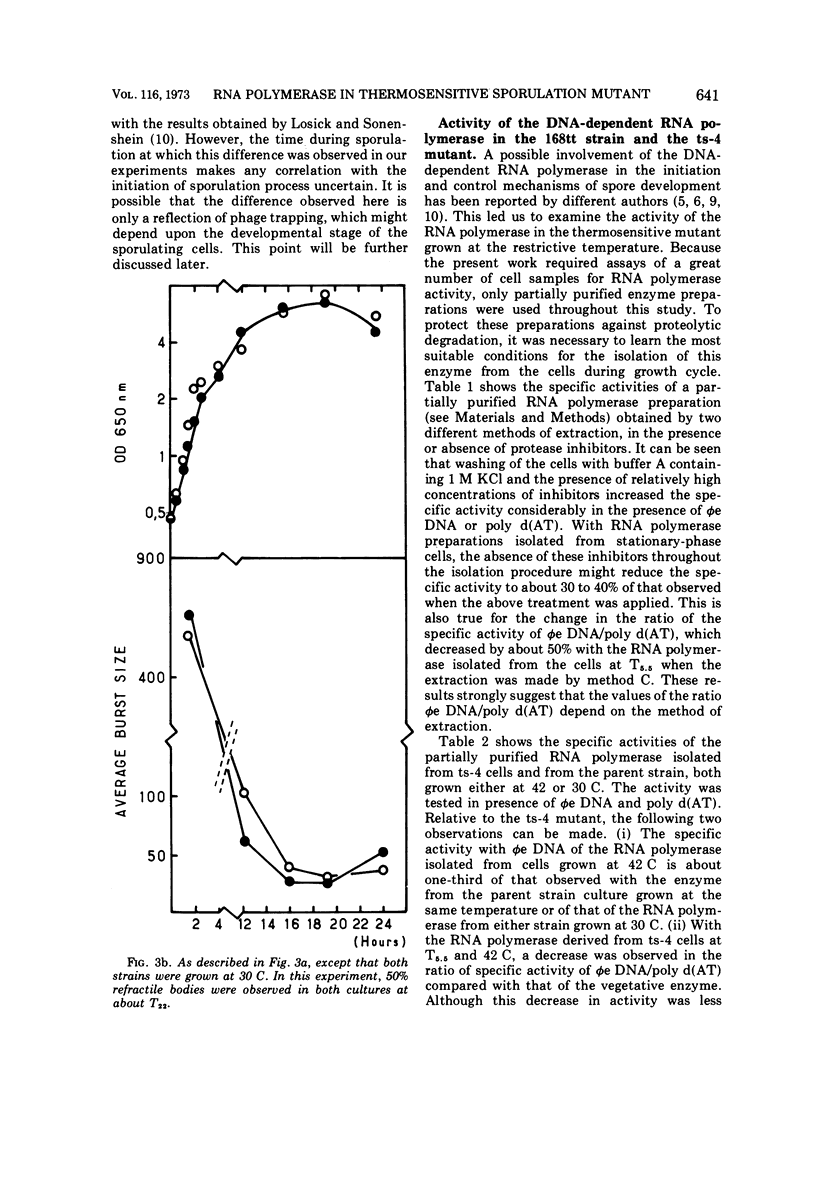
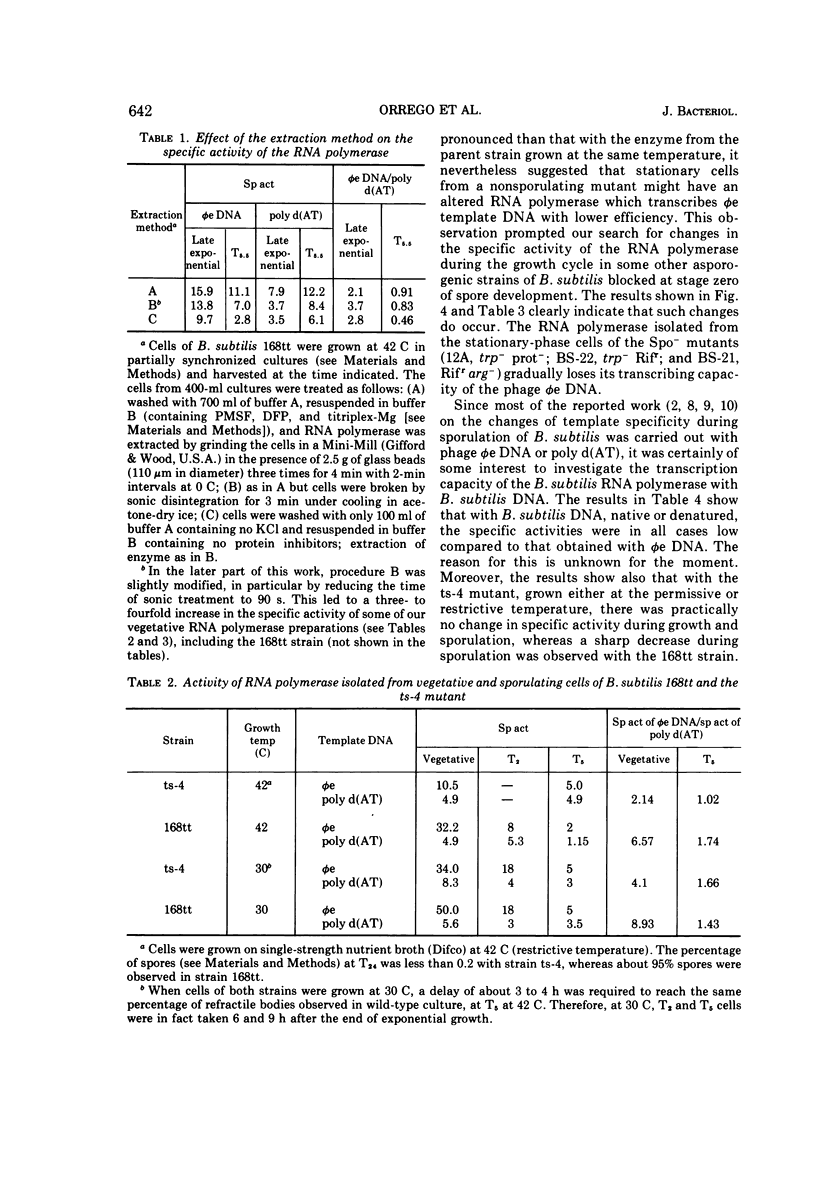
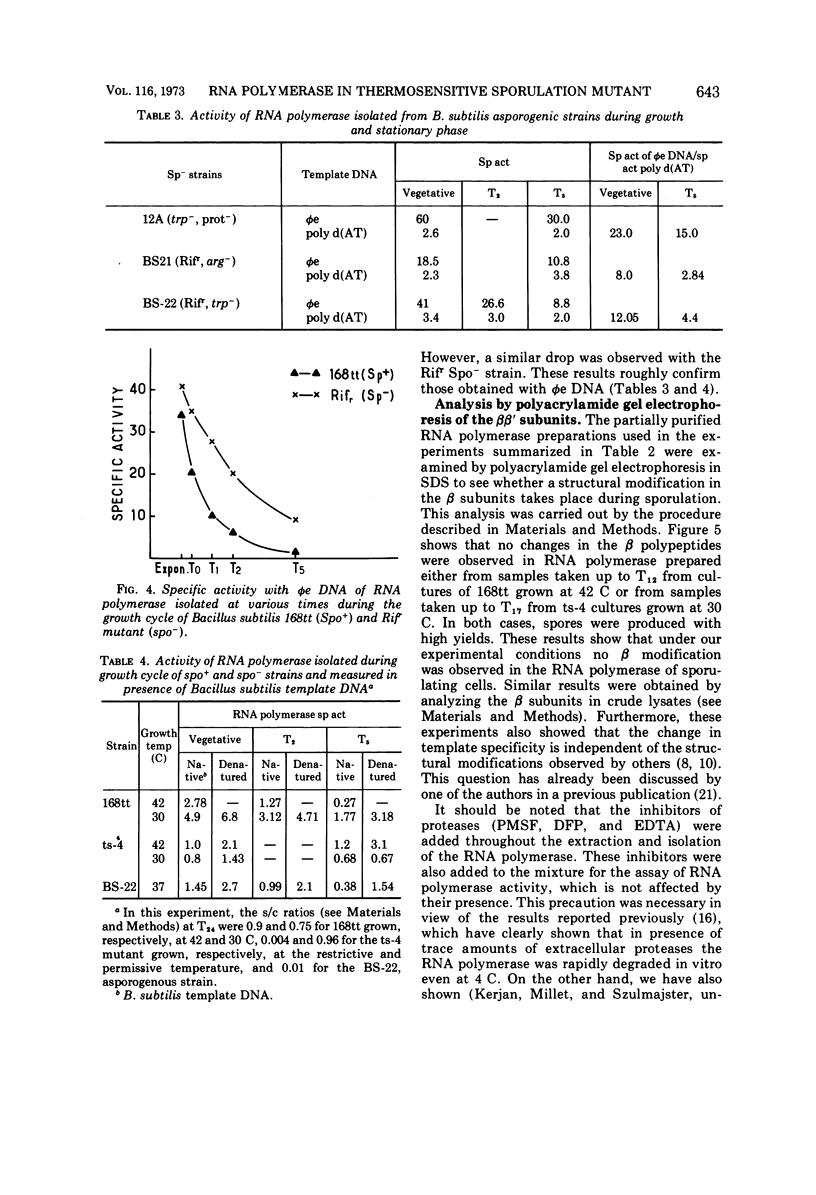
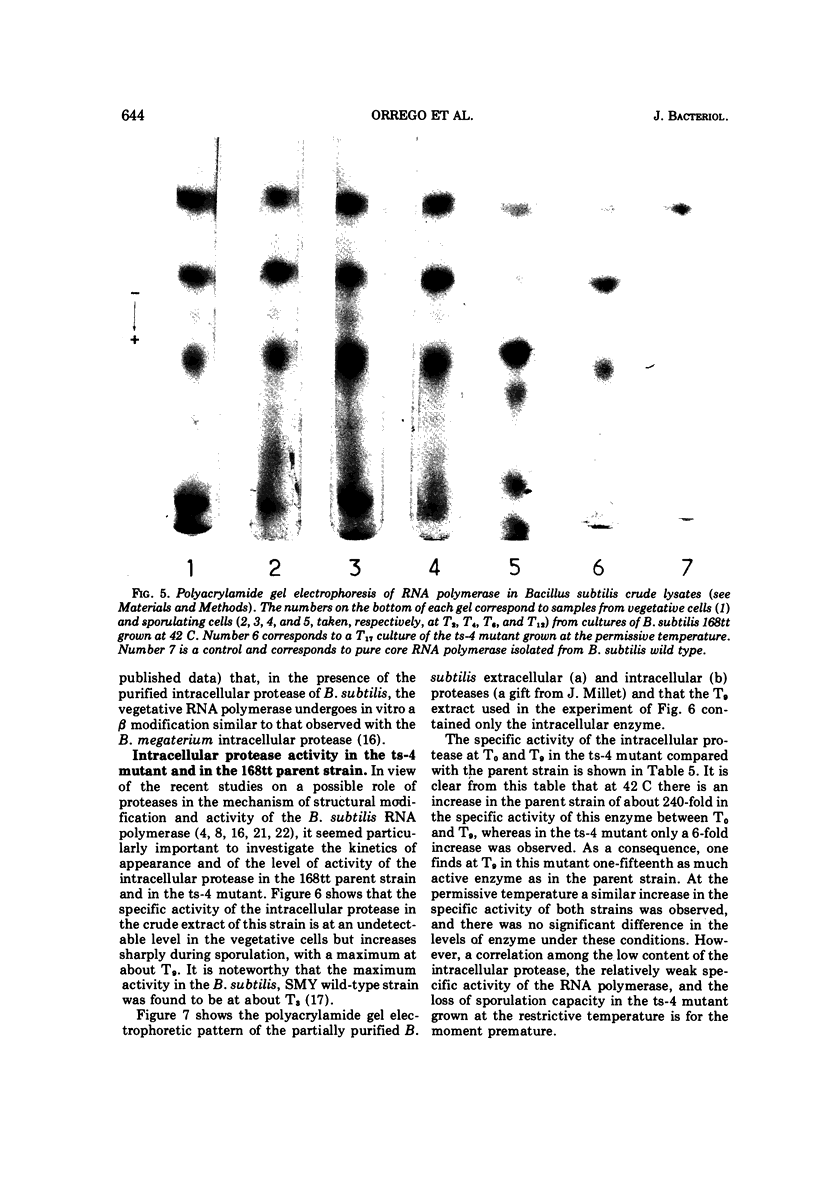
Partially synchronized cultures of a Bacillus subtilis thermosensitive sporulation mutant (ts-4) and the 168 try−try− (168tt) parental strain were infected with the virulent phage φe at various times during their growth cycle at 30 and 42 C (permissive and restrictive temperatures, respectively). It was shown that at the restrictive temperature the burst size in the parental strain was two- to threefold lower than in the ts-4 mutant. No such difference was observed at the permissive temperature. However, the time at which this difference was observed excludes a correlation between the burst size and initiation of the sporulation process. It was further found that the capacity to transcribe in vitro phage φe deoxyribonucleic acid by partially purified ribonucleic acid (RNA) polymerase from both strains decreased sharply if the source of enzyme was sporulating cells instead of vegetative ones. However, a similar decrease, although to a lesser extent, was observed with the RNA polymerase isolated from stationary-phase cells of the ts-4 mutant grown at the nonpermissive temperature, or with the enzyme derived from several other zero-stage sporulation mutants. At no time was a structural modification in the β subunits of the RNA polymerase observed during growth of the sporulating bacteria. We have also shown that, in addition to the relatively low specific activity of the RNA polymerase, the level of the intracellular protease activity is about 15-fold lower in the ts-4 mutant grown at the restrictive temperature than that of the parental strain grown at the same temperature. At the permissive temperature no such difference was observed between these two strains. However, the present data do not allow us to establish a correlation among the low content of intracellular protease, the weak specific activity of the RNA polymerase, and the loss of the sporulation capacity in the ts-4 mutant grown at the restrictive temperature.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonamy C., Hirschbein L., Szulmajster J. Synthesis of ribosomal ribonucleic acid during sporulation of Bacillus subtilis. J Bacteriol. 1973 Mar;113(3):1296–1306. doi: 10.1128/jb.113.3.1296-1306.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brevet J., Sonenshein A. L. Template specificity of ribonucleic acid polymerase in asporogenous mutants of Bacillus subtilis. J Bacteriol. 1972 Dec;112(3):1270–1274. doi: 10.1128/jb.112.3.1270-1274.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjan P., Szulmajster J. DNA dependent RNA polymerase from vegetative cells and from spores of B.Subtilis: III. Isolation of a stimulating factor. FEBS Lett. 1969 Nov 29;5(4):288–290. doi: 10.1016/0014-5793(69)80370-4. [DOI] [PubMed] [Google Scholar]
- Kerjean P., Marchetti J., Szulmajster J. RNA polymérase, DNA dépendante de spores de B. subtilis. II. Purification partielle et propriétés. Bull Soc Chim Biol (Paris) 1967;49(8):1139–1158. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee-Huang S., Warner R. C. The preparation and properties of ribonucleic acid polymerase from Azotobacter vinelandii. J Biol Chem. 1969 Jul 25;244(14):3793–3802. [PubMed] [Google Scholar]
- Linn T. G., Greenleaf A. L., Shorenstein R. G., Losick R. Loss of the sigma activity of RNA polymerase of Bacillus subtilis during sporulation. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1865–1869. doi: 10.1073/pnas.70.6.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losick R., Shorenstein R. G., Sonenshein A. L. Structural alteration of RNA polymerase during sporulation. Nature. 1970 Aug 29;227(5261):910–913. doi: 10.1038/227910a0. [DOI] [PubMed] [Google Scholar]
- Losick R., Sonenshein A. L. Change in the template specificity of RNA polymerase during sporulation of Bacillus subtilis. Nature. 1969 Oct 4;224(5214):35–37. doi: 10.1038/224035a0. [DOI] [PubMed] [Google Scholar]
- Maia J. C.C., Kerjan P., Szulmajster J. DNA-dependent RNA polymerase from vegetative cells and from spores of Bacillus subtilis. IV. Subunit composition. FEBS Lett. 1971 Mar 22;13(5):269–274. doi: 10.1016/0014-5793(71)80238-7. [DOI] [PubMed] [Google Scholar]
- Matzura H., Molin S., Maaloe O. Sequential biosynthesis of the and ' subunits of the DNA-dependent RNA polymerase from Escherichia coli. J Mol Biol. 1971 Jul 14;59(1):17–25. doi: 10.1016/0022-2836(71)90410-4. [DOI] [PubMed] [Google Scholar]
- Millet J. Caractérisation d'une endopeptidase cytoplasmique chez Bacillus megaterium en voie de sporulation. C R Acad Sci Hebd Seances Acad Sci D. 1971 Mar 29;272(13):1806–1809. [PubMed] [Google Scholar]
- Millet J., Kerjan P., Aubert J. P., Szulmajster J. Proteolytic conversion in vitro of B. subtilis vegetative RNA polymerase into the homologous spore enzyme. FEBS Lett. 1972 Jun 1;23(1):47–50. doi: 10.1016/0014-5793(72)80281-3. [DOI] [PubMed] [Google Scholar]
- Reysset G., Millet J. Characterization of an intracellular protease in B. subtillus during sporulation. Biochem Biophys Res Commun. 1972 Oct 17;49(2):328–334. doi: 10.1016/0006-291x(72)90414-7. [DOI] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Sonenshein A. L., Roscoe D. H. The course of phage phi-e infection in sporulating cells of Bacillus subtilis strain 3610. Virology. 1969 Oct;39(2):265–275. doi: 10.1016/0042-6822(69)90047-6. [DOI] [PubMed] [Google Scholar]
- Szulmajster J., Bonamy C., Laporte J. Isolation and properties of a temperature-sensitive sporulation mutant of Bacillus subtilis. J Bacteriol. 1970 Mar;101(3):1027–1037. doi: 10.1128/jb.101.3.1027-1037.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]