Abstract
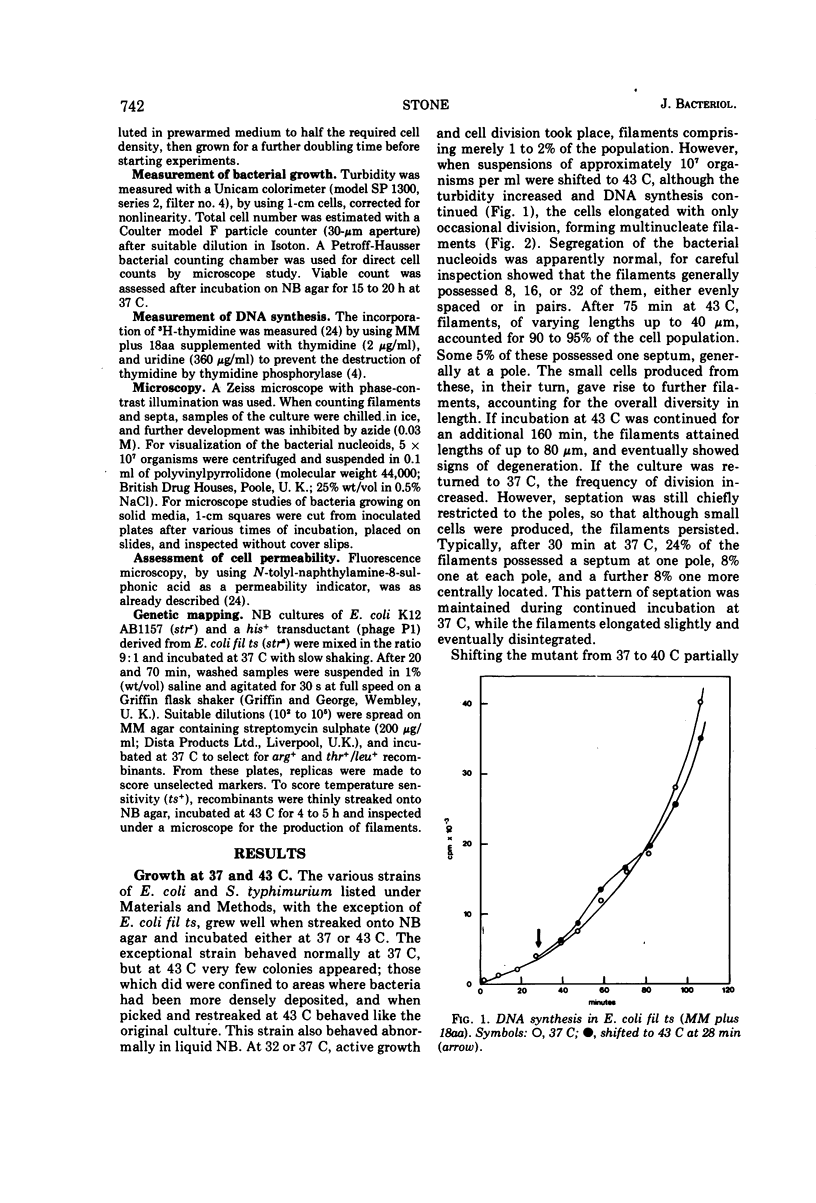
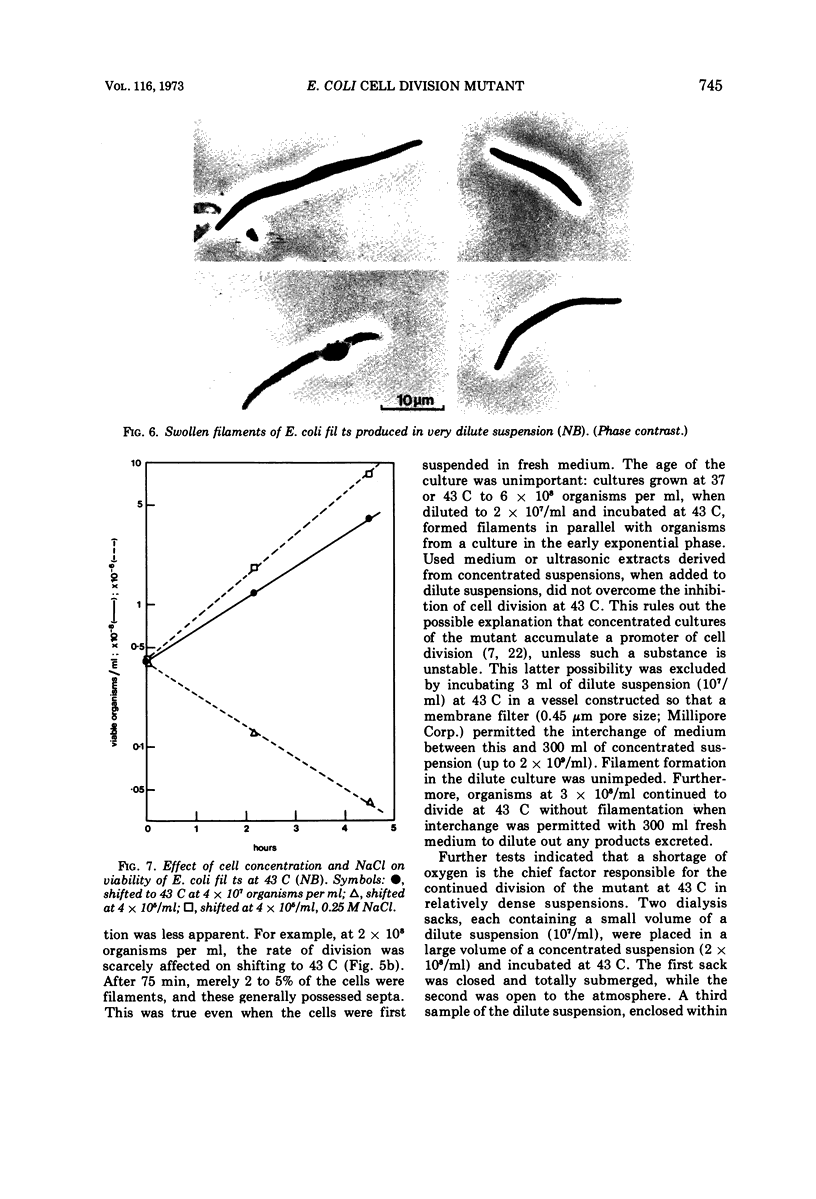
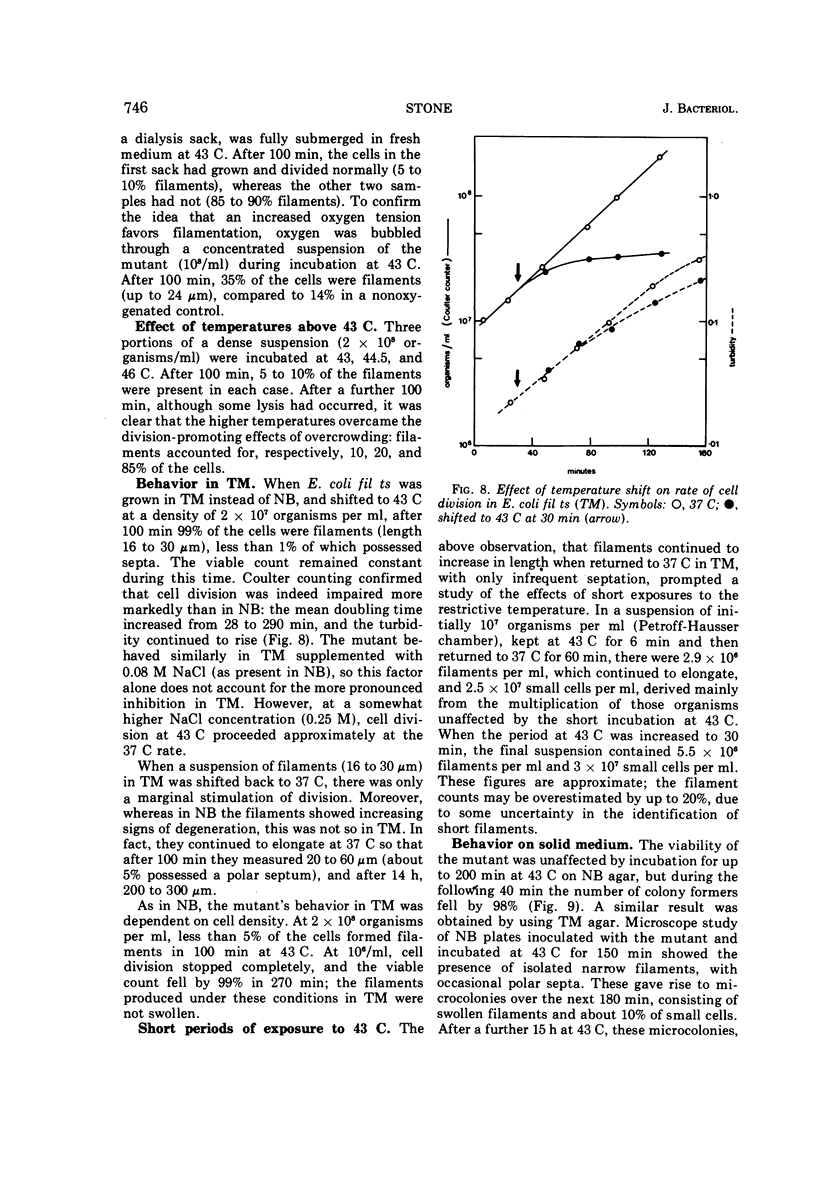
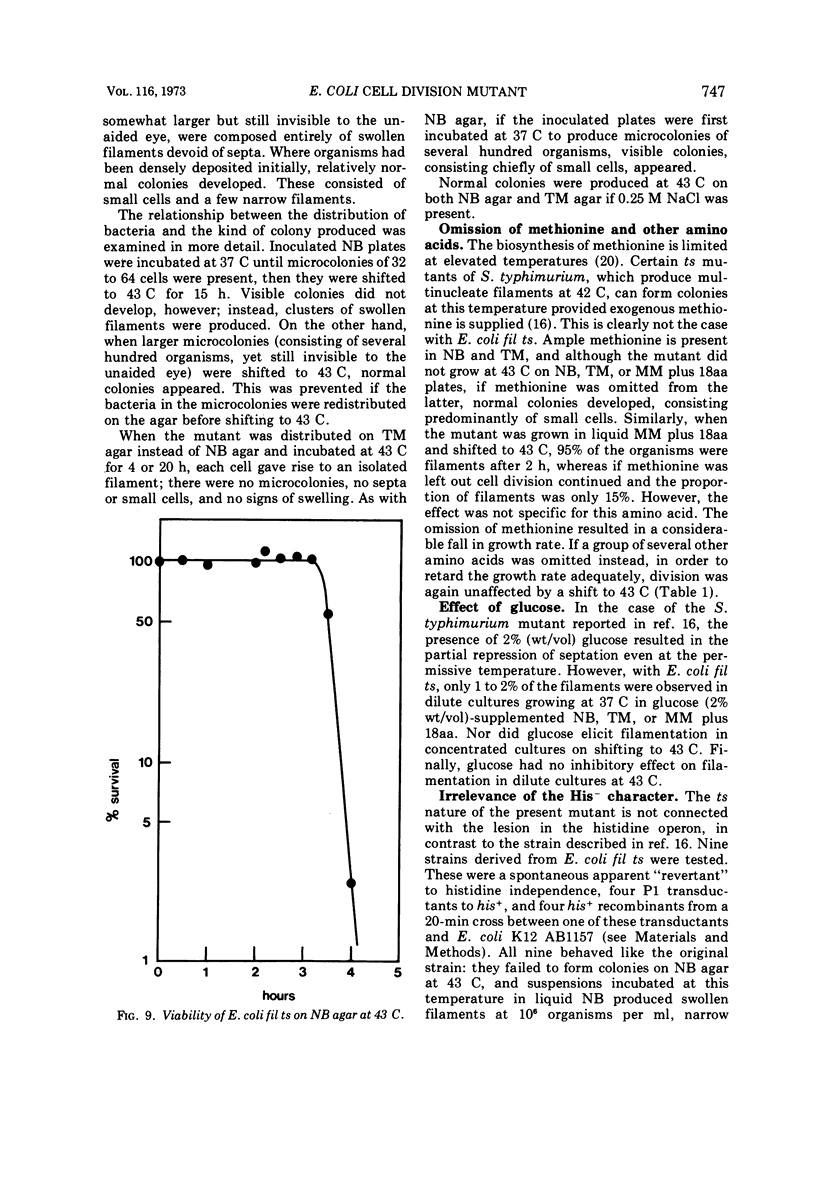
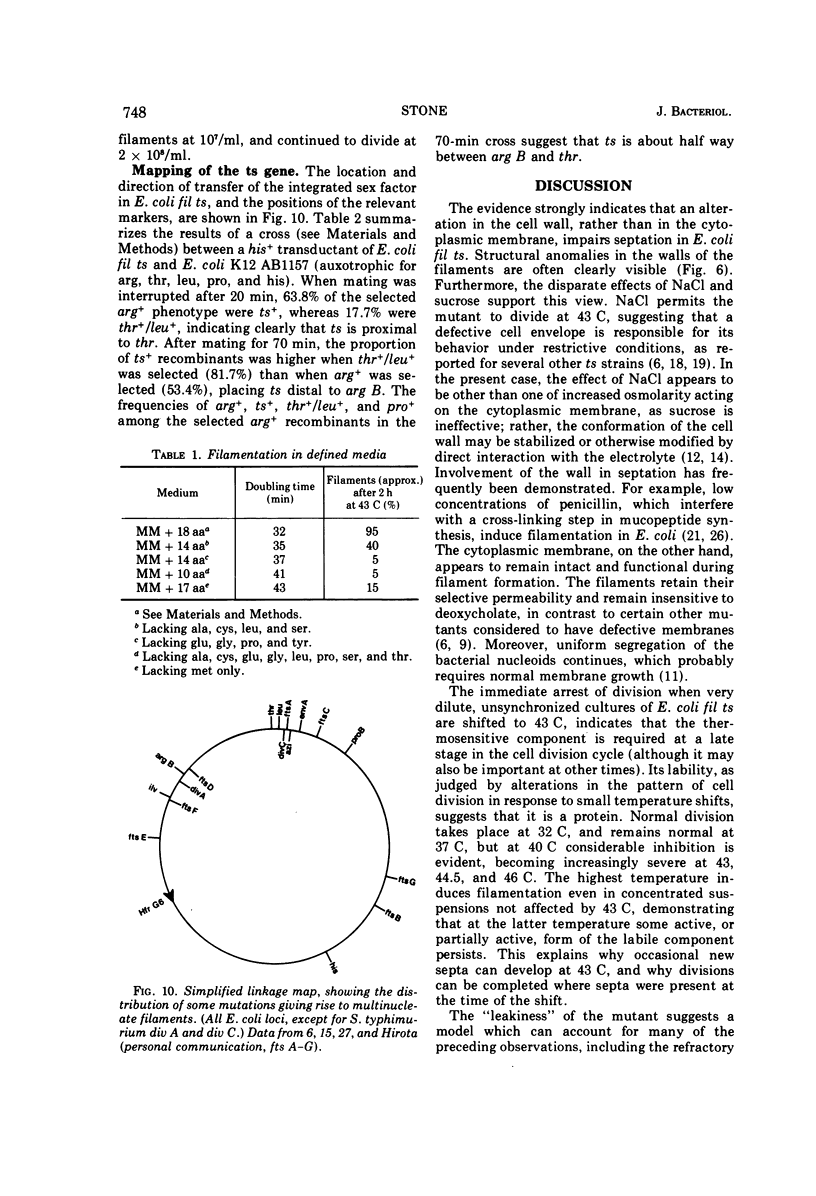
Escherichia coli fil ts forms multinucleate filaments when suspensions of about 107 organisms per ml are shifted from 37 to 43 C in rich medium. Occasional septation continues, chiefly at the poles, and immediately becomes more frequent when the filaments are returned to 37 C. The addition of chloramphenicol (200 μg/ml) at either temperature initially stimulates the formation of polar septa. When very dilute suspensions of the strain (<106 organisms per ml) are shifted to the restrictive temperature, the inhibition of septation is more complete and only seldom reversible. Conversely, cell division is little affected when suspensions of >108 organisms per ml, or microcolonies of several hundred organisms on agar, are incubated at 43 C; evidence is presented that this is a consequence of a slight reduction in the mutant's growth rate. In certain media, septation is blocked irreversibly by even brief exposure to 43 C, after which cell elongation without division proceeds at 37 C for some hours. Several findings, when considered together, suggest that the cytoplasmic membrane is normal at the restrictive temperature, and that the block in septation is caused by a defect in the cell wall: it is largely overcome by NaCl, but not by sucrose; in some circumstances the filaments become swollen and develop localized bulges in the wall, yet the membrane remains intact and retains its selective permeability; lastly, the strain is insensitive to deoxycholate at both temperatures. The mutation has been mapped between arg B and thr, at a locus which appears to be distinct from others known primarily to influence cell division.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed N., Rowbury R. J. Antibiotics and cell division in a filament-forming mutant of Salmonella typhimurium. Microbios. 1971 Dec;4(15):181–191. [PubMed] [Google Scholar]
- Ahmed N., Rowbury R. J. Temperature-sensitive cell division component in a mutant of Salmonella typhimurium. J Gen Microbiol. 1971 Jul;67(1):107–115. doi: 10.1099/00221287-67-1-107. [DOI] [PubMed] [Google Scholar]
- Allen R. G., Smith J. A., Knudsen R. C., Walker J. R. Initial characterization of temperature-sensitive cell division mutants of Escherichia coli. Biochem Biophys Res Commun. 1972 Jun 9;47(5):1074–1079. doi: 10.1016/0006-291x(72)90943-6. [DOI] [PubMed] [Google Scholar]
- Budman D. R., Pardee A. B. Thymidine and thymine incorporation into deoxyribonucleic acid: inhibition and repression by uridine of thymidine phosphorylase of Escherichia coli. J Bacteriol. 1967 Nov;94(5):1546–1550. doi: 10.1128/jb.94.5.1546-1550.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieśla Z., Bagdasarian M., Szczurkiewicz W., Przygońska M., Klopotowski T. Defective cell division in thermosensitive mutants of Salmonella typhimurium. Mol Gen Genet. 1972;116(2):107–125. doi: 10.1007/BF00582221. [DOI] [PubMed] [Google Scholar]
- Cieśla Z., Bagdassarian M. Imparired DNA synthesis and envelope defect in a mutant of Salmonella typhimurium. Mol Gen Genet. 1972;116(2):126–138. doi: 10.1007/BF00582222. [DOI] [PubMed] [Google Scholar]
- Fisher W. D., Adler H. I., Shull F. W., Jr, Cohen A. Properties of a cell fraction that repairs damage to the cell division mechanism of Escherichia coli. J Bacteriol. 1969 Feb;97(2):500–505. doi: 10.1128/jb.97.2.500-505.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstetter C. E. DNA synthesis during the division cycle of rapidly growing Escherichia coli B/r. J Mol Biol. 1968 Feb 14;31(3):507–518. doi: 10.1016/0022-2836(68)90424-5. [DOI] [PubMed] [Google Scholar]
- Hirota Y., Mordoh J., Jacob F. On the process of cellular division in Escherichia coli. 3. Thermosensitive mutants of Escherichia coli altered in the process of DNA initiation. J Mol Biol. 1970 Nov 14;53(3):369–387. doi: 10.1016/0022-2836(70)90072-0. [DOI] [PubMed] [Google Scholar]
- Hirota Y., Ryter A., Jacob F. Thermosensitive mutants of E. coli affected in the processes of DNA synthesis and cellular division. Cold Spring Harb Symp Quant Biol. 1968;33:677–693. doi: 10.1101/sqb.1968.033.01.077. [DOI] [PubMed] [Google Scholar]
- Knowles C. J. Salt induces changes of turbidity and volume of E. coli. Nat New Biol. 1971 Feb 3;229(5):154–155. doi: 10.1038/newbio229154a0. [DOI] [PubMed] [Google Scholar]
- Marquis R. E. Salt-induced contraction of bacterial cell walls. J Bacteriol. 1968 Mar;95(3):775–781. doi: 10.1128/jb.95.3.775-781.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matney T. S., Goldschmidt E. P., Erwin N. S., Scroggs R. A. A preliminary map of genomic sites for F-attachment in Escherichia coli K12. Biochem Biophys Res Commun. 1964 Oct 14;17(3):278–281. doi: 10.1016/0006-291x(64)90397-3. [DOI] [PubMed] [Google Scholar]
- Murray M. L., Hartman P. E. Overproduction of hisH and hisF gene products leads to inhibition of cell cell division in Salmonella. Can J Microbiol. 1972 May;18(5):671–681. doi: 10.1139/m72-105. [DOI] [PubMed] [Google Scholar]
- Nagai K., Kaneko H., Tamura G. Thermosensitive mutant of Escherichia coli requiring new protein synthesis to recover cellular division ability. Biochem Biophys Res Commun. 1971 Feb 19;42(4):669–675. doi: 10.1016/0006-291x(71)90540-7. [DOI] [PubMed] [Google Scholar]
- Reeve J. N., Groves D. J., Clark D. J. Regulation of Cell Division in Escherichia coli: Characterization of Temperature-Sensitive Division Mutants. J Bacteriol. 1970 Dec;104(3):1052–1064. doi: 10.1128/jb.104.3.1052-1064.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricard M., Hirota Y. Effet des sels sur le processus de division cellulaire d'E. coli. C R Acad Sci Hebd Seances Acad Sci D. 1969 Mar 3;268(9):1335–1338. [PubMed] [Google Scholar]
- Ron E. Z., Davis B. D. Growth rate of Escherichia coli at elevated temperatures: limitation by methionine. J Bacteriol. 1971 Aug;107(2):391–396. doi: 10.1128/jb.107.2.391-396.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz U., Asmus A., Frank H. Autolytic enzymes and cell division of Escherichia coli. J Mol Biol. 1969 May 14;41(3):419–429. doi: 10.1016/0022-2836(69)90285-x. [DOI] [PubMed] [Google Scholar]
- Smith H. S., Pardee A. B. Accumulation of a protein required for division during the cell cycle of Escherichia coli. J Bacteriol. 1970 Mar;101(3):901–909. doi: 10.1128/jb.101.3.901-909.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G., Rowbury R. J. Cell division in a mutant of Salmonella typhimurium which is temperature-sensitive for DNA synthesis. J Gen Microbiol. 1971 Mar;65(3):305–314. doi: 10.1099/00221287-65-3-305. [DOI] [PubMed] [Google Scholar]
- Stone A. B. General inhibition of Escherichia coli macromolecular synthesis by high multiplicites of bacteriophage phiX174. J Mol Biol. 1970 Jan 28;47(2):215–229. doi: 10.1016/0022-2836(70)90341-4. [DOI] [PubMed] [Google Scholar]
- Stone A. B. Histidine starvation and completion of the bacterial deoxyribonucleic acid replication cycle. Mol Gen Genet. 1972;119(3):267–276. doi: 10.1007/BF00333864. [DOI] [PubMed] [Google Scholar]
- Strominger J. L., Blumberg P. M., Suginaka H., Umbreit J., Wickus G. G. How penicillin kills bacteria: progress and problems. Proc R Soc Lond B Biol Sci. 1971 Dec 31;179(1057):369–383. doi: 10.1098/rspb.1971.0103. [DOI] [PubMed] [Google Scholar]
- Taylor A. L. Current linkage map of Escherichia coli. Bacteriol Rev. 1970 Jun;34(2):155–175. doi: 10.1128/br.34.2.155-175.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zusman D. R., Inouye M., Pardee A. B. Cell division in Escherichia coli: evidence for regulation of septation by effector molecules. J Mol Biol. 1972 Aug 14;69(1):119–136. doi: 10.1016/0022-2836(72)90027-7. [DOI] [PubMed] [Google Scholar]




