Abstract
We have further examined the mechanisms for a severe mitochondrial energetic deficit, deenergization, and impaired respiration in complex I that develop in kidney proximal tubules during hypoxia-reoxygenation, and their prevention and reversal by supplementation with α-ketoglutarate (α-KG) + aspartate. The abnormalities preceded the mitochondrial permeability transition and cytochrome c loss. Anaerobic metabolism of α-KG + aspartate generated ATP and maintained mitochondrial membrane potential. Other citric-acid cycle intermediates that can promote anaerobic metabolism (malate and fumarate) were also effective singly or in combination with α-KG. Succinate, the end product of these anaerobic pathways that can bypass complex I, was not protective when provided only during hypoxia. However, during reoxygenation, succinate also rescued the tubules, and its benefit, like that of α-KG + malate, persisted after the extra substrate was withdrawn. Thus proximal tubules can be salvaged from hypoxia-reoxygenation mitochondrial injury by both anaerobic metabolism of citric-acid cycle intermediates and aerobic metabolism of succinate. These results bear on the understanding of a fundamental mode of mitochondrial dysfunction during tubule injury and on strategies to prevent and reverse it.
Keywords: rabbit, kidney, α-ketoglutarate, glycine, succinate, adenosine-5′-triphosphate
Mitochondrial dysfunction has long been considered to play a central role in the development of cell injury during ischemia-reperfusion and hypoxia-reoxygenation (12). However, the contributions of various biochemical alterations seen in this setting have not been fully defined, and it has been difficult to distinguish primary events from secondary processes associated with generalized cellular damage caused by ATP depletion. Several developments have changed this situation. A major advance has been recognition of the mitochondrial permeability transition (MPT), a porous defect of the inner mitochondrial membrane. Developing initially as a potential sensitive megachannel regulated by a mitochondrial matrix cyclophilin, the MPT evolves to become a proteinaceous membrane pore with a size exclusion limit of ~1,500 Da, and thereby compromises mitochondrial integrity following diverse stimuli (2, 12, 24, 32). Independently or in association with the MPT, mitochondrial outer membranes may also become permeable under specific injurious circumstances to proteins residing in the intermembrane space (17, 30, 49, 69). Mitochondrial release of one such protein, cytochrome c, has twofold effects. Because of its role as an electron shuttle, dislocation of cytochrome c compromises respiration (49, 58). As a cytosolic cofactor required to activate caspase 9, it can trigger apoptosis (49, 58, 69).
Both the MPT and mitochondrial release of cytochrome c have been invoked as factors involved in the death of cells during or following hypoxia and ischemia (12, 29, 32, 49). Cyclosporine A has been shown to bind mitochondrial cyclophilin and suppress development of the MPT (2, 68). Coordinate suppression of both the MPT and cell killing by cyclosporine has suggested that the MPT is a determinant of lethal outcome during hypoxia (34, 40) and post-hypoxic or post-ischemic reoxygenation (14, 25, 44, 57). Bax-mediated cytochrome c release in the absence of the MPT contributes to both necrosis and apoptosis of cultured kidney tubule cells during prolonged hypoxia and hypoxia-reoxygenation (48, 49).
Proximal tubules have relatively little or no glycolytic capacity, making them dependent on aerobic mitochondrial metabolism for ATP synthesis (1, 47, 67). Accordingly, ATP concentrations in freshly isolated proximal tubules decline steeply during hypoxia, in spite of the presence of glucose (62). We have found that the tubule cells develop a severe mitochondrial functional deficit that is expressed during reoxygenation following >30-min hypoxia, despite availability of substrates optimized for aerobic proximal tubule metabolism, and glycine to maintain plasma membrane integrity (62, 64). The abnormality is characterized by incomplete recovery of mitochondrial membrane potential (ΔΨm) and cellular ATP, impaired respiration utilizing substrates that donate electrons to respiratory complex I, and persistence of hypoxia-induced mitochondrial matrix condensation (64). Respiratory functions of complexes II, III, and IV remain largely intact (64). The lesion is partially ameliorated by chemical inhibitors of the MPT, including cyclosporine (62). In recent studies (64), we have shown that a metabolic strategy, that promotes anaerobic mitochondrial metabolism to generate ATP and maintain ΔΨm during hypoxia, can prevent development of the mitochondrial lesion and, importantly, can also reverse the mitochondrial defects and enable cellular recovery, even if it is introduced only during reoxygenation, after completion of the hypoxic period (64).
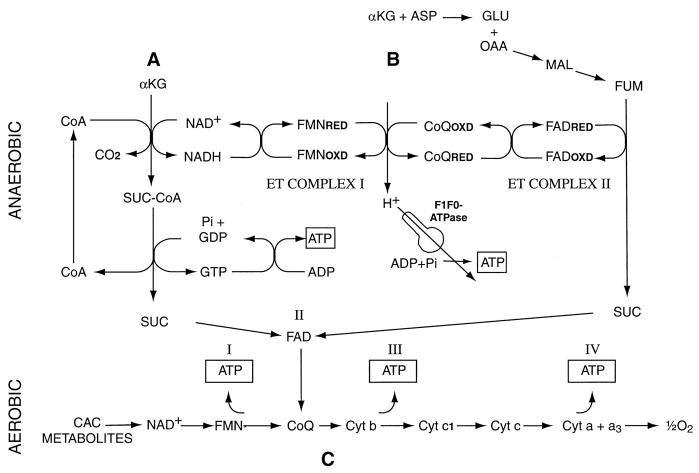
Anaerobic mitochondrial metabolism can generate ATP and maintain mitochondrial energization via two pathways (Fig. 1): A) substrate-level phosphorylation during the conversion of α-ketoglutarate to succinate by α-ketoglutarate dehydrogenase (23, 27, 28, 42); and B) electron transport in complexes I and II driven by reduction of fumarate to succinate coupled to the oxidation of reduced ubiquinone that is generated via NADH from citric acid cycle (CAC) reducing equivalents (23, 27, 42, 52). These reducing equivalents are shown in Fig. 1 as being provided by α-ketoglutarate dehydrogenase because that reaction will be favored with concomitant α-ketoglutarate supplementation, but any source of NADH can serve this purpose. In our recent studies, stimulation of these metabolic pathways by supplementation of the tubules with α-ketoglutarate + aspartate (α-KG/ASP) strikingly ameliorated the energetic deficit that developed during hypoxia-reoxygenation (64). The pathways shown in Fig. 1 indicate that other CAC intermediates and related compounds should be able to substitute for α-KG and aspartate. These include glutamate, which is relatively abundant in the kidney (61), and could, therefore, be a large source of α-KG, as well as malate or fumarate, which require less metabolism than aspartate to promote pathway B (Fig. 1).
Fig. 1.
Metabolic pathways for protective effects of citric acid cycle (CAC) metabolites. A: substrate level phosphorylation. During hypoxia or anaerobiosis, α-ketoglutarate (α-KG) is metabolized to succinate (SUC) with production of GTP that transphosphorylates ADP to ATP. B: anaerobic respiration in electron transport (ET) complexes I and II. Reduction of fumarate (FUM) to succinate coupled by the FAD-containing flavoprotein of succinate dehydrogenase (complex II) to oxidation of reduced ubiquinone (CoQRED) drives proton extrusion by complex I, which increases membrane potential (ΔΨm) and can generate an additional molecule of ATP using the mitochondrial F1F0-ATPase. In the process, NADH is anaerobically oxidized to NAD+, which can then promote further metabolism of α-KG. Transamination of aspartate (ASP) to oxalacetate (OAA) serves as a source of malate (MAL) and fumarate. Not shown is the conversion of OAA to MAL that also oxidizes NADH formed during the decarboxylation of α-KG and, thus, favors that reaction independently of anaerobic electron transport. C: aerobic bypass of complex I. When electron transport resumes during reoxygenation, succinate provided directly or produced via pathways A and B bypasses complex I of the electron transport chain, allowing ATP production in complexes III and IV.
These considerations and our recently reported findings (64) raise important questions. The strong protection against mitochondrial hypoxic injury afforded by provision of α-KG and aspartate during hypoxia is readily explained by postulating a single process. By anaerobically generating ATP and/or maintaining ΔΨm, they preempt the development of damage to respiratory complex I and other inner mitochondrial membrane components. However, there are at least two explanations for the beneficial effects afforded by provision of the substrates only during reoxygenation, and they are not mutually exclusive. One is that the same mechanisms of substrate-level phosphorylation and anaerobic respiration that prevent the lesion from developing during hypoxia are responsible. The other explanation is that, during reoxygenation, succinate, which is a product of both pathways A and B (Fig. 1), donates electrons to complex III via complex II (succinate dehydrogenase), bypassing the limitation of metabolism of complex I substrates (pathway C in Fig. 1). In this fashion, succinate-dependent aerobic respiration via normal electron transport can support ΔΨm and generate ATP, even in mitochondria with impaired function of complex I, and thereby repair and rescue cells. The absence of the terminal electron acceptor, oxygen, would preclude benefit from succinate by this mechanism during hypoxia.
In the present studies, we have systematically investigated whether one or more of a range of CAC intermediates and related compounds other than α-KG/ASP can modify the hypoxia-reoxygenation mitochondrial insult and the resulting cellular energetic deficit of proximal tubule cells. The ability of the metabolites to prevent or reverse injury was assessed by measuring the recovery of cell ATP concentration and ΔΨm during reoxygenation. These studies were designed not only to further delineate the mechanisms of mitochondrial damage and protection, but also to provide information bearing on the likelihood of their expression during proximal tubule injury in vivo, where both tissue and circulating levels of the full spectrum of available metabolites must be considered. Our results indicate that the protective effects of α-KG/ASP on both mitochondrial energization and recovery of cell ATP can be duplicated to various degrees by other CAC intermediates, either alone, or in combination. However, the protective effects are specific for subsets of metabolites depending on whether protection occurs during hypoxia or reoxygenation. Moreover, the data indicate that the aerobic pathway of protection provided by succinate is important and that the recovery process, once initiated by protective substrates, is maintained even if they are withdrawn. These observations provide new insights into a fundamental mode of mitochondrial dysfunction during a common form of cell injury in the kidney and other tissues, and suggest potentially powerful approaches for modifying the lesion and subsequent damaging processes.
METHODS
Isolation of tubules
Proximal tubules were prepared from kidney cortex of female New Zealand White rabbits (1.5–2.0 kg; Oakwood Farms, Oakwood, MI) by digestion with combinations of Worthington Type I (Worthington, Freehold, NJ) and Sigma Blend Type H or F collagenase and centrifugation on self-forming Percoll gradients as described (61, 62, 64).
Experimental procedure
Incubation conditions generally followed our published protocols (62, 64). Tubules were suspended at 3.0–5.0-mg tubule protein/ml in a 95% O2/5% CO2-gassed medium containing (in mM) 110 NaCl, 2.6 KCl, 25 NaHCO3, 2.4 KH2PO4, 1.25 CaCl2, 1.2 MgCl2, 1.2 MgSO4, 5 glucose, 4 sodium lactate, 0.3 alanine, 5.0 sodium butyrate, 3% dialyzed dextran (Pharmacia, T-40), and 2 mM glycine. The medium was also supplemented with 0.5 mg/ml bovine gelatin (75 bloom) to suppress aggregation of the isolated tubules during the prolonged experimental incubation periods. After 15-min preincubation at 37°C, tubules were resuspended in fresh medium with experimental agents and regassed with either 95% O2-5% CO2 (controls) or 95% N2-5% CO2 (hypoxia). Hypoxic tubules were kept at pH 6.9 to simulate tissue acidosis during ischemia in vivo (62). After 60 min, samples were taken for analysis. The remaining tubules were washed twice to remove any experimental substrates being tested for their efficacy only during hypoxia and were then resuspended in fresh 95% O2-5% CO2-gassed, pH 7.4 medium, with experimental agents as needed. In the reoxygenation medium, 2.0-mM sodium heptanoate replaced sodium butyrate, and, to insure availability of purine precursors for ATP resynthesis, 250 μM AMP or ATP was added (62) in most experiments. The supplemental medium, purine, eliminates any effect of hypoxia-induced decreases of the intracellular purine pool (59) to limit recovery of ATP and, thus, allows the cell ATP levels to be a better index of the functional state of the mitochondria. After 60 or 120 min of reoxygenation, samples were taken again for analysis. Cell ATP and lactate dehydrogenase (LDH) release were measured as previously described (62). Other parameters were assayed as in the following sections.
Staining with ΔΨm-sensitive dyes
For staining with tetramethylrhodamine methyl ester [(TMRM), Molecular Probes, Eugene, OR)] (36), at the end of the desired experimental period, a 0.5 ml aliquot of the tubule suspension was mixed with an equal volume of room temperature phosphate-buffered saline containing 1.0 μM TMRM. After 1 min, the tubules were pelleted, washed twice in an ice-cold solution containing (in mM) 110 NaCl, 25 Na-HEPES, pH 7.2, 1.25 CaCl2, 1.0 MgCl2, 1.0 KH2PO4, 3.5 KCl, 5.0 glycine, and 5% polyethylene glycol (average MW 8000), and then held in this solution in the dark at 4°C until they were examined by confocal microscopy. For staining with the carbocyanine dye, 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazocarbocyanine iodide (JC-1, Molecular Probes) (45, 54), an aliquot from a 1,000× stock solution in dimethyl sulfoxide was mixed with an equal volume of calf serum, dispersed as an intermediate 100× stock solution in phosphate-buffered saline, and then added at the end of the desired experimental period to a final concentration of 5 μg/ml in the tubule suspension. The suspension was regassed with O2/CO2 and incubated in the dark for an additional 15 min at 37°C, then tubules were pelleted, washed three times in the same solution as used for the TMRM studies, and held in that solution in the dark at 4°C until either viewing of individual tubules by confocal microscopy or measurements of fluorescence on samples of the whole suspension. In some studies, vital dye exclusion was concomitantly assessed by inclusion of 2 μg/ml propidium iodide along with the TMRM or for the last 1–2 min of the period of JC-1 exposure.
Laser-scanning confocal microscopy of TMRM and JC-1 stained tubules
Samples of the washed tubules were loaded into a Dvorak-Stotler chamber (Lucas-Highland, Chantilly, VA) and allowed to settle for 10–15 min in the cold, then rapidly viewed with a 100 × Plan Apochromat lens (NA 1.4) using a Nikon Diaphot microscope attached to a Bio-Rad MRC 600-laser scanning confocal system equipped with a krypton/argon mixed-gas laser at the wavelength settings described with the results and the Figs. Illustrations shown with the results are representative of changes that were uniformly seen in tubules from 3–5 separate experiments.
Measurement of JC-1 fluorescence in suspension (54)
Immediately after sampling and washing, a 300-μl aliquot of the tubules containing 1.2–1.5 mg protein was brought up to 2.5 ml with additional ice-cold wash solution and then scanned during continuous gentle stirring using a Photon Technology International (Monmouth Junction, New Jersey) Alphascan fluorometer at 488-nm excitation/500–625-nm emission collected in right angle mode of the fluorometer. Under these conditions, the peak of the green fluorescence of the monomeric form of the dye was at 530 nm and the red fluorescence of the J-aggregates peaked at 590 nm. This procedure allowed for collection of data for both forms of the dye from a single rapid scan so that there was no deterioration of the signal from photobleaching or continued mixing and warming in the chamber.
Measurement of cytochrome c release
At the end of the desired experimental period, tubules were pelleted and re-suspended in a solution containing (in mM) 250 sucrose, 10 KCl, 1.5 MgCl2, 1 EDTA, 1 EGTA, 10 K-HEPES, pH 7.1, 10 phenylmethylsulfonyl fluoride 0.25 mg/ml digitonin, 16 μg/ml benzamidine, 10 μg/ml phenanthroline, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 10 μg/ml pepstatin A at room temperature. After 5 min incubation at room temperature, which allowed release of all lactate dehydrogenase, the suspensions were centrifuged at 12,000 g for 2 min Pellets and supernatants were saved at −80°C for analysis of cytochrome c distribution by immunoblotting with a monoclonal antibody to cytochrome c (clone 7H8.2C12, Pharmingen, San Diego, CA) as previously described (48). The relative distribution of cytochrome c between the supernatants and pellets was quantitated by densitometry using Kodak 1D software version 2.0.2 (Kodak, Rochester, NY).
Determination of total amino-acid levels
Amino acids were measured on neutralized trichloroacetic acid extracts of the tubule suspension by a Varian-9012 high pressure liquid chromatography system equipped with Auto Sampler-9100. Precolumn derivatization with o-phthalaldehyde and fluorescence detection were employed as previously (61).
Assay of succinate
Succinate was assayed on neutralized trichloroacetic acid extracts of the tubule suspensions exactly as in (4) except for the use of fluorometric detection to monitor NADH consumption. Succinate was converted to succinyl-CoA by reaction with coenzyme A and inosine triphosphate in the presence of succinyl-CoA synthetase (Roche Molecular Bioproducts, Indianapolis, IN). The inosine diphosphate formed was used to convert phosphoenolpyruvate to pyruvate in the presence of pyruvate kinase. The pyruvate was then reduced by NADH to lactate in the presence of lactate dehydrogenase. NADH consumption was linear for succinate concentrations up to 30 μM.
Determination of 15N-labeled metabolites
For GC-MS analysis of 15N-labeled amino acids, a 50-μl aliquot of neutralized trichloracetic-acid extract of the whole tubule suspension processed as for determination of ATP (62) was applied to an AG-50 column (100–200 mesh; 0.5 × 2.5 cm). The column was washed with 3 ml of deionized H2O. Amino acids were eluted with 3 ml of NH4OH. For determination of 15N isotopic enrichment, amino acids were converted to t-butyldimethylsilyl derivatives (37). 15N enrichment in glutamine and glutamate was monitored using the following ions: m/z 432, 432, for glutamine and m/z 432, 433 for glutamate. Calculation of 15N atom% excess (APE) was carried out as described (38). The production of 15N-labeled amino acid was calculated as 15N nmol/mg protein = C × APE/100 where C is the total concentration measured by HPLC.
Reagents
Reagents were from Sigma (St. Louis, MO) unless otherwise indicated and were of the highest grade commercially available. Agents solubilized in ethanol or dimethyl sulfoxide were delivered from ≥1,000× stock solutions. All substrates tested were provided from ≥100×, pH-adjusted stocks of their Na+ salts, except for acetoacetate, which was the Li+ salt. [15N]aspartate was obtained from MSD Isotopes (Quebec, Canada). It behaved identically to unlabeled aspartate with respect to all experimental effects. Cyclosporine A was from Calbiochem (San Diego, CA).
Statistics
Paired and unpaired t-tests were used as appropriate. Where experiments consisted of multiple groups they were analyzed statistically by analysis of variance for repeated measure or independent-group designs as needed. Individual-group comparisons for the multi-group studies were then made by using the Newman-Keuls test for multiple comparisons (SigmaStat, SPSS, Chicago, IL). P < 0.05 was considered to be statistically significant. The Ns given represent the numbers of separate tubule preparations studied.
RESULTS
Prevention and reversal of hypoxia-reoxygenation-induced energy deficits by combinations of CAC metabolites
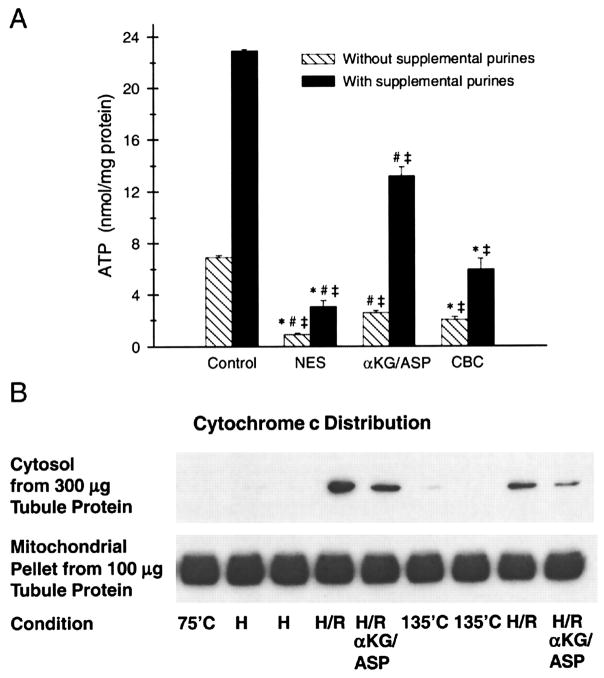
Tubules subjected to 60 min hypoxia and 60 min of reoxygenation with no further additions to the glucose, lactate, alanine, and fatty acid that normally serve as optimal substrates for the preparation (1, 47, 61, 62, 64) had severely impaired recovery of cell ATP levels [no extra substrate (NES) group in Fig. 2A]. This energetic deficit occurred despite the presence of glycine, which prevents plasma membrane damage, as measured by LDH release and vital dye exclusion under these conditions [(62, 64) and studies described below]. Addition to the medium of supplemental purine to provide precursors for resynthesis of ATP (62), increased ATP levels in both control and reoxygenated tubules, but did not eliminate the large difference between the two conditions (Fig. 2A). Cytochrome c was retained in the mitochondria throughout 60 min of hypoxia and was not detected in the cytosol (Fig. 2B). During reoxygenation, only negligible amounts of cytochrome c were seen in the cytosol, the vast bulk being retained intra-mitochondrially (Fig. 2B).
Fig. 2.
Energetic deficit after hypoxia-reoxygenation and its modification by mitochondrial permeability transition (MPT) inhibitors and α-KG/ASP. A: ATP levels of reoxygenated tubules. Tubules were subjected to 60 min hypoxia (2 mM glycine, pH 6.9) and 60 min reoxygenation (2 mM glycine, pH 7.4) with either no extra substrate (NES), or 4 mM α-ketoglutarate + 4 mM aspartate (α-KG/ASP) during reoxygenation alone, or cyclosporine A (5 μM) plus butacaine (30 μM) plus L-carnitine (2 mM) (CBC) during both hypoxia and reoxygenation. Reoxygenation was studied both without and with supplemental exogenous purine (250 μM ATP) to promote recovery of cell ATP. Control values shown are for parallel flasks from the same preparations incubated under oxygenated conditions for the same total duration as the experimental groups. The purine-supplemented control group had 250 μm ATP in its medium for the last 60 min of incubation. Cell ATP levels are means ± SE for N = 4–6, *significantly different from corresponding α-KG/ASP group, #significantly different from corresponding CBC group, and ‡significantly different from corresponding control group. B: immunoblot of cytochrome c in membrane pellets containing mitochondria and the corresponding cytosol supernatants. Tubules were incubated under oxygenated control conditions for either 75 min (75′C) or 135 min (135′C), for 60 min hypoxia (H), or for 60 min hypoxia followed by 60 min reoxygenation (H/R) with or without 4 mM α-KG/ASP. Repeated conditions are from separate flasks. To provide a visible signal, the amount of protein for the cytosolic fractions was increased 3 fold relative to its actual proportion between the 2 types of samples. The percentages of total cytochrome c in the supernatants were 0.50 ± 0.12 in the H/R group without supplemental substrates and 0.21 ± 0.07 in the α-KG/ASP-treated group (N = 3).
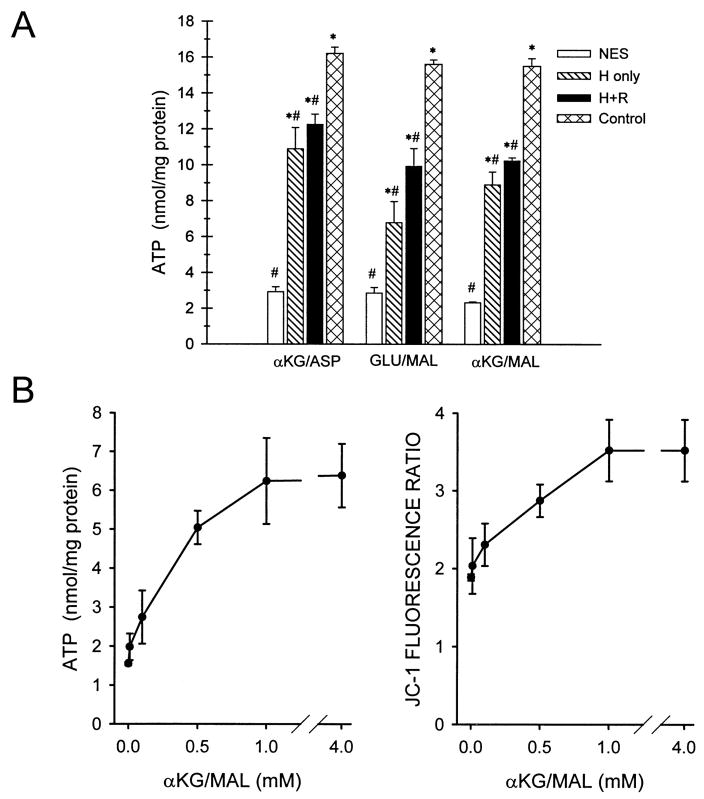
We have previously reported that both MPT inhibitors and promotion of anaerobic mitochondrial metabolism by α-KG/ASP can ameliorate the energetic deficits seen in reoxygenated tubules (62, 64). This behavior is demonstrated by the studies depicted in Fig. 2A that provide direct comparisons of the relative efficacy of the two maneuvers in paired experiments on the same tubule preparations. Supplementation with α-KG/ASP during reoxygenation strongly increased the recovery of cell ATP and was more effective than a combination of chemical inhibitors of the MPT, cyclosporine, butacaine, and carnitine. Benefit was seen both with and without supplemental purine in the medium (Fig. 2A). In additional experiments shown in Fig. 3A, supplementation with α-KG/ASP only during hypoxia was also effective, as was inclusion of the extra substrates during both hypoxia and reoxygenation.
Fig. 3.
Benefit of alternative combinations of CAC metabolites. A: the basic experimental design was similar to that of the Fig. 2A studies except that extra test substrates were provided during hypoxia (H) alone or during hypoxia plus reoxygenation (H+R), and supplemental exogenous purine (250 μM AMP) was used during reoxygenation of all flasks. Glutamate plus malate (GLU/MAL) and α-ketoglutarate plus malate (α-KG/MAL), like α-KG/ASP, were delivered at a final concentration of 4 mM of each substrate. Cell ATP levels are means ± SE for N = 3–5, *significantly different from corresponding no extra substance (NES) group and #significantly different from corresponding oxygenated control group. B: concentration dependence of protective effects of α-KG/MAL. Tubules were subjected to hypoxia plus reoxygenation as in Fig. 2A with 250 μM AMP as the supplemental exogenous purine. α-KG/MAL was added only during reoxygenation at the indicated concentrations followed by measurement at the end of reoxygenation of ATP and of the 590/530 nm JC-1 fluorescence ratio. Values are means ± SE for N = 4–8 at each concentration of α-KG/MAL. Every α-KG/MAL flask had a paired unsupplemented flask prepared from the same hypoxia sample. Values for these unsupplemented flasks did not differ among the groups and are pooled for clarity of presentation as the 0 mM point. All concentrations of α-KG/MAL except 0.01 mM significantly increased ATP and the JC-1 fluorescence ratio relative to the paired unsupplemented flasks.
As summarized in Fig. 1, other CAC metabolites and compounds feeding into the cycle could have effects similar to α-KG/ASP. Replacement of aspartate with malate in the combination (α-KG/MAL) did not diminish the protection observed (Fig. 3A). Glutamate plus malate (GLU/MAL) was slightly less effective than the other two combinations. Similar to our previous observations with α-KG/ASP (64), both α-KG/MAL and GLU/MAL maintained slightly, but significantly higher ATP levels during hypoxia than in tubules with no extra substrate. For the conditions in Fig. 3A, end hypoxia ATP levels (in nmol/mg protein) were: no extra substrate: 0.28 ± 0.02, α-KG/ASP: 0.44 ± 0.02, α-KG/MAL: 0.42 ± 0.01, and GLU/MAL: 0.40 ± 0.02. We also used the α-KG/MAL combination to test the concentration dependence of the beneficial effects of substrate addition during the reoxygenation period. Significant improvement of ATP recovery was seen at a concentration as low as 0.1 mM of α-KG/MAL and was maximal at 1.0 mM (Fig. 3B).
Increases of mitochondrial energization induced by protective substrates during reoxygenation
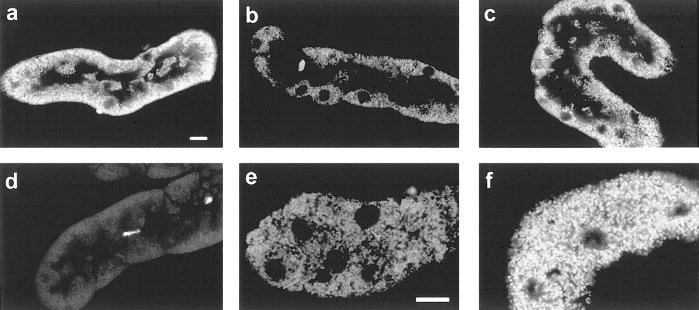
Mitochondrial membrane potential (ΔΨm) is both an important direct marker of integrity of the inner mitochondrial membrane and a regulator of the MPT pore (24, 32, 36, 53). It is, thus, a critical parameter in understanding the mechanism of the substrate effects, despite the limitations of available techniques for its measurement in intact cells (35). We investigated changes of ΔΨm in the tubules using two different membrane-permeant, cationic fluorophores, TMRM and JC-1 (36, 45, 54). In control tubules, TMRM stained the mitochondria brightly in their typical basolateral locations (Fig. 4a). TMRM uptake was entirely blocked by the mitochondrial uncoupler, FCCP (Fig. 4d). During reoxygenation without supplemental substrates, the majority of cells exposed to TMRM at the end of the experimental period displayed mitochondrial uptake (Fig. 4, b and e), but in most of them the signal was substantially weaker than that seen in the controls (Fig. 4a), consistent with a reduced but not absent level of energization. The majority of tubules treated with α-KG/ASP (Fig. 4c and f) had bright-basal punctate staining similar to the controls. These studies, utilizing TMRM, provide high-resolution confocal images for visualization and show clearly that mitochondria were not completely deenergized. However, self-quenching by TMRM (15) complicates comparison of the signals in the control and injured tubules because it tends to exaggerate the signals from the partially energized mitochondria in the reoxygenated tubules that have lower levels of TMRM uptake. To further assess differences between the various experimental conditions we used JC-1.
Fig. 4.
Mitochondrial tetramethylrhodamine methyl ester (TMRM) uptake. Tubules were stained with TMRM and propidium iodide after: oxygenated control incubation (a); 60 min hypoxia followed by 60 min reoxygenation with no extra substrates (b, e) or with 4 mM α-KG/ASP during reoxygenation (c, f); or 5 μM FCCP + 5 mM glycine for 15 min (d). Optical sections were viewed at 568 nm excitation, 585 nm emission. Panels a–d are cuts through the midplanes of the tubules showing the basally oriented mitochondria around the periphery of the tubule profile. The unstained areas in the center of each tubule are the apical portions of the cells, which are devoid of mitochondria, and the tubule lumens. Nuclei are seen as the circular areas devoid of staining. The full tubule profile in panel d occupies approximately the same area and is similarly oriented to those in the other panels, but is poorly seen because the mitochondria are unstained. The brightly stained circular structures are the rare propidium iodide-positive nuclei that were no more frequent than those seen in controls. Panels e and f are higher magnification cuts through the basal sections of the cells to show mitochondria at higher resolution. Bars in panels a and e are 10 μm. Results are representative of 8–10 tubules from 4–5 different preparations viewed under each experimental condition.
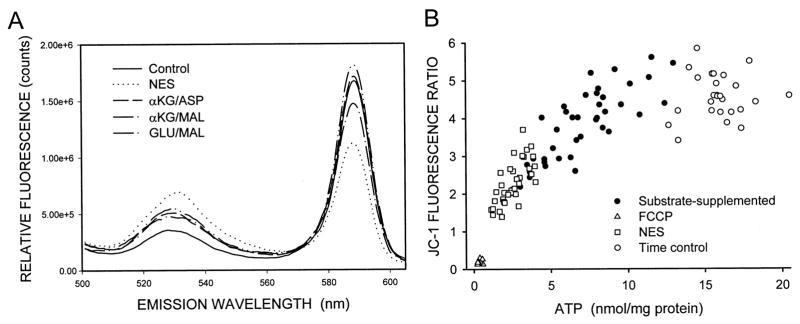
JC-1 has the unique property, among ΔΨm-sensitive fluorophores, of developing large, reversible shifts in its fluorescence signal at the levels of uptake induced by the high-membrane potentials characteristic of energized mitochondria due to the formation of red fluorescent J-aggregates of the molecule. At the levels of JC-1 uptake seen during lower ΔΨm, the fluorophore remains as a green-fluorescent monomer (45, 54). Figure 5A shows emission scans of tubules that were loaded with JC-1 at the end of hypoxia-reoxygenation with either no extra substrate or with the indicated substrate combinations provided only during the reoxygenation period. The green fluorescence of the monomeric form of the molecule (530 nm peak) that predominates at low ΔΨm, was least in the control, highest in the no extra substrate sample, and intermediate in the substrate supplemented samples. Conversely, the red fluorescence (590 nm peak) from the high ΔΨm-induced JC-1 aggregates was greatest in the control, lowest in the no extra substrate sample, and intermediate in the substrate-supplemented samples. Figure 6 shows the appearance of the red aggregates as viewed by confocal microscopy. The red signal from the high ΔΨm-dependent JC-1 aggregates in controls (Fig. 6a), like that of TMRM, was primarily in a punctate, basolateral distribution consistent with mitochondrial localization. FCCP treatment almost completely prevented formation of visible J-aggregates, and those that were present were seen in apical, nonmitochondrial compartments (Fig. 6b). During reoxygenation without supplemental substrates (Figs. 6c), staining was weaker than that of the control, but was still present in a majority of cells. With supplemental GLU/MAL (Fig. 6d) or α-KG/ASP or α-KG/MAL (not shown), strong staining for JC-1 aggregates was restored in most tubules.
Fig. 5.
JC-1 fluorescence in solution. A: tubules were incubated under oxygenated control conditions, or for 60 min hypoxia plus 60 min reoxygenation as in Fig. 3A with either no extra substrate (NES) or 4 mM of either α-KG/ASP, α-KG/MAL, or GLU/MAL during reoxygenation. These incubations were followed by staining with JC-1, washing, and scanning of suspensions of the intact tubules at 488 nm excitation and 500–625 nm emission. The tracings shown are representative of those obtained from 10–20 experiments under each of the conditions. B: JC-1 ratios (590/530 nm) for multiple individual experiments done under the same conditions as shown for Fig. 5A plotted against the ATP levels of the same samples. Each point is the result of a single experiment. Also shown are JC-1 ratios for tubules incubated with 5 μM FCCP in the presence of 5 mm glycine for 15 min.
Fig. 6.
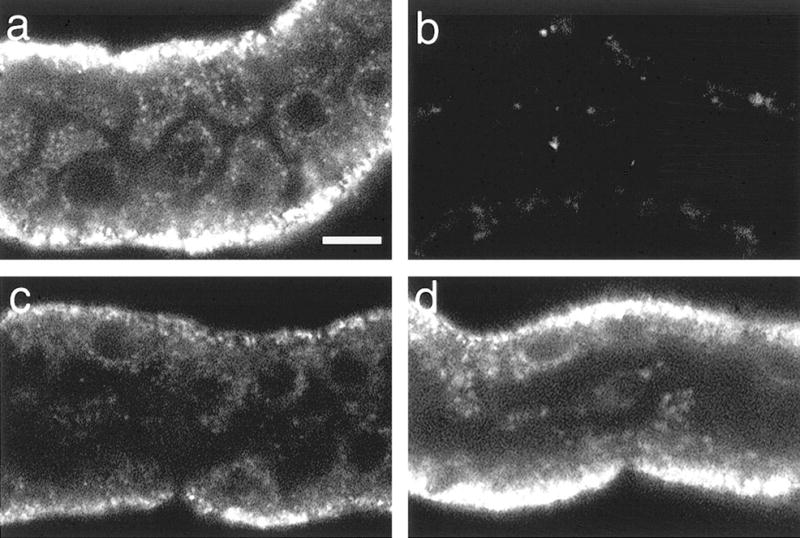
Confocal microscopy of red JC-1 aggregates after hypoxia plus reoxygenation. Tubules were stained with JC-1 after: oxygenated control incubation (a); 5 μM FCCP + 5 mM glycine for 15 min (b); or 60 min hypoxia followed by 60 min reoxygenation with no extra substrates (c) or with 4 mM glutamate + malate during reoxygenation (d), then viewed by confocal microscopy at 568-nm excitation, 585-nm emission. Magnifications are the same for all panels. Bar: 10 μm. Unavoidable photobleaching results in dropout of the signal from the most severely affected mitochondria in the no extra substrate group.
Only the multimeric, red form of JC-1 specifically measures ΔΨm -dependent mitochondrial uptake of the probe (3, 13, 35). However, we have found that effects of the experimental maneuvers on JC-1 uptake in the model are similarly indicated by both the 590 nm signal and the 590/530 nm ratio under all conditions studied, and the ratio helps normalize for unavoidable differences among samples in amounts of tubule protein and increases sensitivity for detecting small differences between conditions. For these reasons, we report the JC-1 data as 590/530 nm ratios. Ratios for the samples shown in Fig. 5A were: Control: 4.64; no extra substrate (NES): 1.62; α-KG/ASP: 3.33; α-KG/MAL: 3.29; GLU/MAL: 3.06. Figure 5B plots 590/530-nm ratios as a function of the ATP levels measured in multiple samples from a series of experiments using the same protective substrate combinations shown in Fig. 5A. Recoveries of ATP and of the JC-1 fluorescence ratios closely paralleled each other. A similar relationship was seen in the studies depicted in Fig. 3B that assessed the concentration dependence of protection by α-KG/MAL. Significant increases of ATP and of the 590/530-nm JC-1 fluorescence ratio relative to the paired, unsupplemented flasks, were measured at concentrations as low as 100 μM for each substrate, with a similar dose dependence for the effects on both parameters.
Substrate specificity of protection
The data from the present studies presented up to this point, along with our earlier work (64), have indicated that combinations of CAC substrates that support anaerobic mitochondrial metabolism powerfully modify the defects of mitochondrial energization and recovery of cell ATP seen during hypoxia-reoxygenation. To further test the hypothesis that both anaerobic and aerobic mechanisms contribute to these effects (Fig. 1) and to determine whether the substrate combinations initially assessed were, in fact, providing the strongest protection, it was necessary to systematically evaluate the full range of CAC intermediates and related metabolites under both aerobic and anaerobic conditions. Our initial studies of this type focused on efficacy of substrates provided only during reoxygenation (Fig. 7). Based on those data, we then selectively assessed the active metabolites for their protective effects during hypoxia (Fig. 8). For clarity of presentation and analysis, the results for the large number of compounds tested during reoxygenation are grouped according to whether they are intrinsic intermediates of the CAC (Fig. 7, A and B) or require additional metabolism before entry in the cycle (Fig. 7, C and D). For the most part, this classification was also predictive of the efficacy of the metabolites.
Fig. 7.
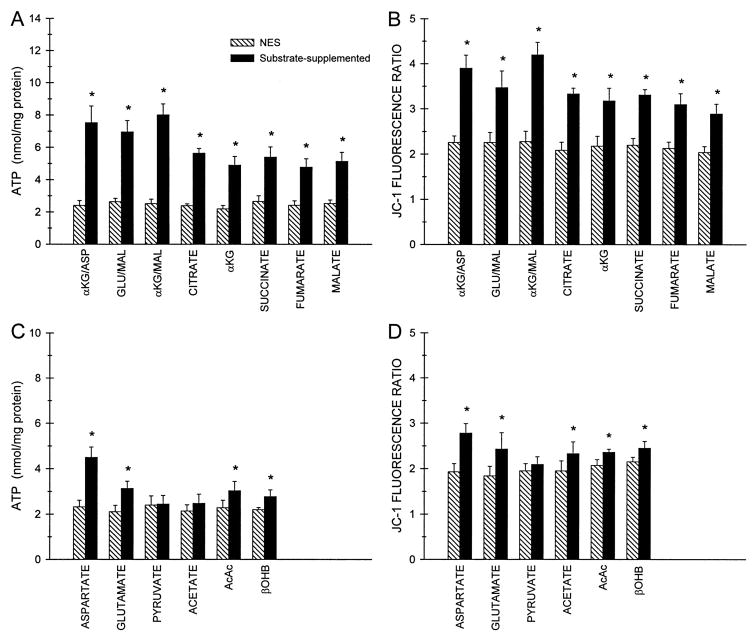
Protection by individual CAC intermediates and related metabolites delivered only during reoxygenation. At the end of 60 min hypoxia in the presence of 2 mM glycine at pH 6.9 tubules were resuspended in oxygenated medium at pH 7.4 with 2 mM glycine and 250 μM AMP. This suspension was divided in half so that one of the aliquots received no extra substrate (NES) and the other aliquot was supplemented with the indicated substrate or substrate combination (all additions 4 mM). ATP levels (panels A and C) and 590/530 nm JC-1 fluorescence ratios (panels B and D) measured after 60 min reoxygenation are means ± SE for N = 5–12, *significantly different from the corresponding paired NES group. Values for oxygenated control tubules incubated in parallel (not shown) were ATP, 20.0 ± 0.69 nmol/mg protein; 590/530 nm JC-1 fluorescence ratio, 4.38 ± 0.10. AcAc, acetoacetate; βOHB, β-hydroxybutyrate.
Fig. 8.
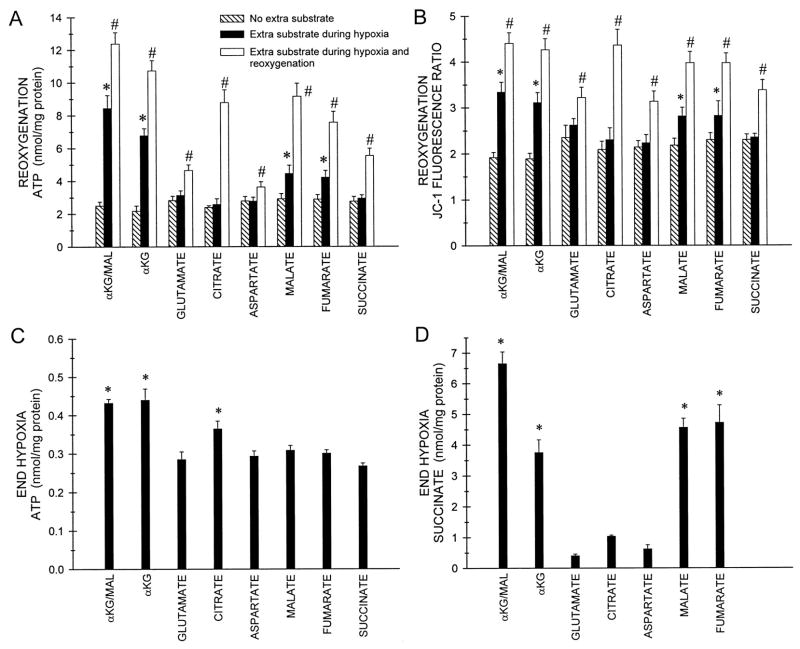
Protection by individual CAC intermediates and related metabolites delivered only during hypoxia or during hypoxia and reoxygenation. Tubules were incubated with either no extra substrate or with 4 mM α-ketoglutarate plus malate (α-KG/MAL), or the indicated individual substrates (each at 4 mM) during 60 min hypoxia in the presence of 2 mM glycine at pH 6.9, then were washed and incubated either with or without the same extra substrate during reoxygenation at pH 7.4 with 2 mM glycine and 250 μM AMP. A: cell ATP values at the end of reoxygenation. Oxygenated control (not graphed) is the same as the value given with Fig. 7. B: JC-1 fluorescence ratios at the end of reoxygenation. Oxygenated control (not graphed) is the same as the value given with Fig. 7. C: cell ATP values at the end of hypoxia. Oxygenated control (not graphed) was 7.10 ± 0.33 nmol/mg protein. This value is lower than the control ATP for the reoxygenation period because it precedes the addition of AMP to the medium. D: succinate levels at the end of hypoxia. Oxygenated control and end hypoxia with no extra substrate succinate levels (not graphed) were 0.51 ± 0.05 and 0.39 ± 0.10 nmol/mg protein, respectively. All values are means ± SE for N = 4–12, *significantly different from the corresponding no extra substrate group. #Significantly different from corresponding no extra substrate and extra substrate during only hypoxia groups.
When provided individually during reoxygenation, each of the components of the effective substrate combinations, α-ketoglutarate, aspartate, glutamate, and malate, improved both ATP levels and increased the JC-1 fluorescence ratio, but none had as strong an effect as the combinations (Fig. 7A–D). Among them, glutamate was distinctly less active than the others. Citrate, fumarate, and succinate were all beneficial, with similar degrees of efficacy to each other and to α-KG and malate. Acetate, which is present at mM levels in rabbit serum (9), acetoacetate, β-hydroxybutyrate, and pyruvate provided little or no benefit.
The effects of succinate are of particular interest. As a product of both pathways available for anaerobic ATP generation (Fig. 1), succinate could theoretically inhibit them and thereby worsen the insult. The data in Fig. 7 show that this clearly does not occur because succinate protected as well as the other CAC intermediates provided individually during reoxygenation. The protection by succinate, shown in Fig. 7, could derive from effects of succinate to promote ATP production because it bypasses the block in the electron transport chain at complex I that characterizes the lesion (64) and/or to stimulate forward operation of the CAC with generation of α-KG, which can then undergo substrate-level phosphorylation. In contrast to the anaerobic pathways available for protection by α-KG, aspartate, malate, and fumarate, bypass of complex I by succinate obligately requires aerobic electron transport (Fig. 1). Substantial conversion of succinate to other CAC intermediates also requires the high levels of CAC activity maintained under aerobic conditions. To confirm that aerobic electron transport is necessary for the benefits of succinate supplementation and to better define the pathways involved in protection by the other substrates, we next assessed the effects of succinate and the other active metabolites when provided individually only during hypoxia, and, in the same experiments, during hypoxia and reoxygenation (Fig. 8). The data obtained from these studies address both the mechanism of succinate’s effects and considerations that importantly bear on understanding the activity of the other intermediates.
Delivered only during hypoxia, succinate was not protective (Fig. 8, A and B). Moreover, when present during both hypoxia and reoxygenation (Fig. 8, A and B), succinate’s effects on ATP and JC-1 fluorescence were not greater than when it was used during reoxygenation alone (compare with reoxygenation alone values in Fig. 7A and B that are from paired experiments and are plotted on the same scale), clearly showing that aerobic electron transport is necessary for succinate to be beneficial.
α-Ketoglutarate, malate and fumarate, which directly promote the anaerobic metabolic pathways (Fig. 1), were all effective when provided only during hypoxia, although less so than the combination of α-KG plus malate (Fig. 8, A and B). All of these compounds were more effective when provided during hypoxia and reoxygenation than when provided during hypoxia alone (Fig. 8, A and B) or during reoxygenation alone (Fig. 7, A and B), further demonstrating that their mechanisms for protection were active during both anaerobic and aerobic conditions and that the effects during each period could be at least partially additive. α-Ketoglutarate, malate, and fumarate all promoted succinate accumulation during hypoxia (Fig. 8D), consistent with operation of pathways A and B in Fig. 1 that produce succinate as their end product. α-KG and malate together had additive effects on succinate accumulation (Fig. 8D). Among these substrates, only α-KG increased ATP during hypoxia (Fig. 8C).
Neither citrate, aspartate, or glutamate was protective when provided only during hypoxia (Fig. 8, A and B). Increases of succinate during hypoxia in this group of substrates were minimal or absent (Fig. 8D), suggesting that these compounds were not metabolized sufficiently to promote the anaerobic pathways of protection. Citrate did maintain higher levels of ATP during hypoxia, although not to the same extent as α-KG (Fig. 8C). The effect of citrate on ATP during hypoxia may explain why citrate during hypoxia and reoxygenation (Fig. 8, A and B) was significantly more protective than citrate during reoxygenation alone (Fig. 7, A and B). The failure of glutamate during hypoxia alone to protect via metabolism to α-KG likely reflects the inhibition of glutamate dehydrogenase, resulting from the large increase of the NADH/NAD+ ratio during hypoxia, because the conversion of glutamate to α-KG by glutamate dehydrogenase requires NAD+ (39). Glutamate during hypoxia did not increase either ATP or succinate (Fig. 8, C and D). Glutamate during hypoxia plus reoxygenation (Fig. 8, A and B) was not more effective than glutamate during reoxygenation alone (Figs. 7A and 8B).
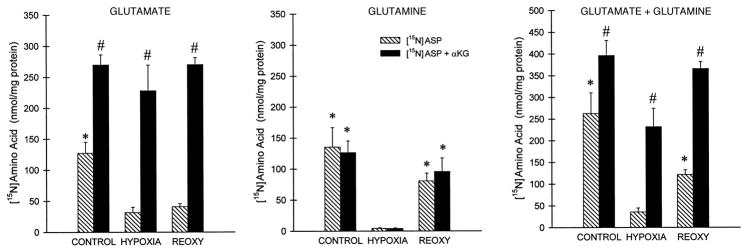
The lack of benefit from aspartate alone during hypoxia is best explained by the absence of an acceptor (i.e., α-KG) for transamination of the aspartate to oxalacetate, which is required for further metabolism of aspartate in the anaerobic protective pathway (Fig. 1). To test whether this was the case, we directly assessed transamination by following production of 15N-labeled glutamate and glutamine from 15N-labeled aspartate (Fig. 9). Appearance of the 15N label from aspartate in both glutamate and glutamine was very low during hypoxia compared with control and reoxygenated tubules. Addition of α-KG during control incubation as well as during hypoxia and reoxygenation substantially increased glutamate accumulation resulting from transfer of aspartate 15N, but had little effect on the accumulation of glutamine. This insensitivity of glutamine accumulation to α-KG-driven glutamate production is consistent with prior studies of rabbit tubules (9). The totals of [15N]glutamate plus [15N]glutamine production (Fig. 9C) show, most clearly, that net transamination was minimal only during hypoxia with aspartate alone, the condition under which aspartate was not protective at all. The increase of transamination with aspartate alone during reoxygenation presumably reflects the availability of endogenous α-KG from resumption of CAC activity, however, the amount of transamination under this condition was still relatively small (Fig. 9). This likely accounts for why aspartate during reoxygenation, although protective, tended to be less effective (Figs. 7A and 8A) than malate or fumarate.
Fig. 9.
Production of 15N-labeled amino acids from [15N]aspartate with and without α-KG. Tubules were supplemented with [15N]aspartate (4 mM) or [15N]aspartate+α-KG (4 mM each) during either 60 min of oxygenated control incubation, 60 min of hypoxia, or 60 min of reoxygenation (REOXY) following 60 min of hypoxia. All other conditions during hypoxia and reoxygenation are the same as for Fig. 8. Results are the product of 15N isotopic enrichment (atom% excess/100) times concentration (nmol/mg protein). Values are means ± SE for 3 experiments. *Significantly different from corresponding hypoxia value; #significantly different from corresponding aspartate alone condition.
Succinate can be produced from all of the other protective metabolites by either forward or reverse activity of the CAC depending on the compound and can support respiration and mitochondrial ATP production by bypassing the electron transport block in complex I (Fig. 1). This raises the question of whether the improvements of ATP production and energization during reoxygenation are just due to the continuing presence of high concentrations of succinate and its support for energization and ATP production via bypass of the lesion. To address this issue, we tested whether the benefit provided by the protective substrates was maintained after their withdrawal (Fig. 10). For this purpose, tubules were treated during 60 min of reoxygenation with either α-KG/MAL or succinate and then incubation was continued for an additional 60 min, either with or without the protective substrate. The wash procedure at 60 min left less than 0.02% of the supplemental substrate in the medium. As shown in Fig. 10, dramatic further recovery of ATP occurred between 60 and 120 min of reoxygenation irrespective of whether the protective substrates were continued. Energization as measured with JC-1 at the end of 120 min was also similarly improved irrespective of whether the protective substrates were present during the last 60 min These results indicate that continued presence of high concentrations of the protective substrates is not required after their initial effects to restore mitochondrial function. The large absolute and relative increases of ATP recovery during the second 60 min of reoxygenation also importantly emphasize the powerful effect of substrate supplementation to alleviate the underlying mitochondrial lesion with major resulting improvement of cellular energetics.
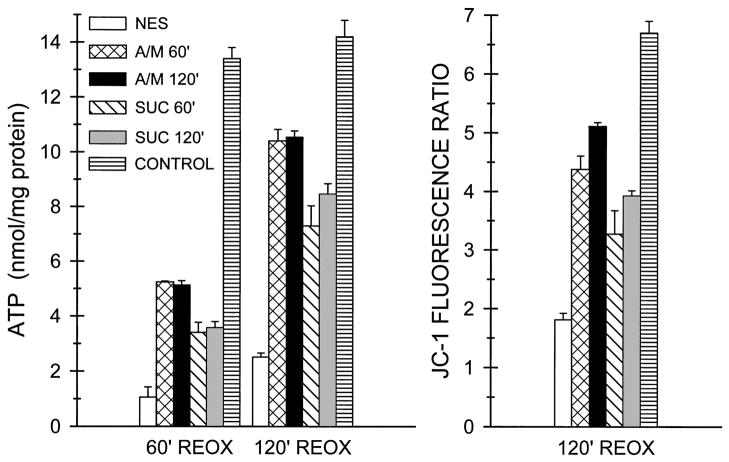
Fig. 10.
Maintenance of improved mitochondrial function after withdrawal of protective substrates. Tubules were subjected to hypoxia ± reoxygenation as for the Fig. 7 studies with either no extra substrate (NES) during reoxygenation or substrate supplementation during reoxygenation as follows: 4 mM α-KG/MAL for 60 min of reoxygenation (A/M 60′), 4 mM α-KG/MAL for 120 min of reoxygenation (A/M 120′), 4 mM succinate for 60 min of reoxygenation (SUC 60′), 4 mM succinate for 120 min of reoxygenation (SUC 120′). Tubules were sampled for ATP levels at 60 min reoxygenation (60′ REOX) and for ATP levels and JC-1 fluorescence at 120 min reoxygenation (120′ REOX). Values are means ± SE from three experiments, each with duplicate samples for every condition. All 120′ REOX values are significantly greater than the corresponding 60′ REOX values. All substrate-supplemented values are significantly greater than the corresponding NES group values. All α-KG/MAL values are significantly greater than the corresponding succinate values. Control values are for preparations incubated under oxygenated conditions for the same total durations as the experimental groups and are significantly greater than the corresponding values for the experimental groups.
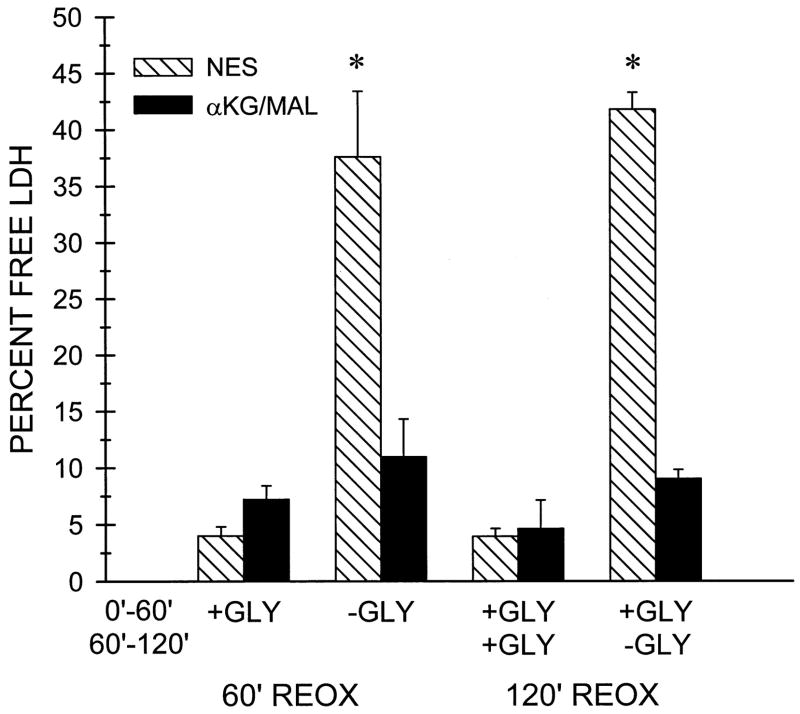
We have previously reported that withdrawal of glycine from tubules that are not supplemented with protective substrates results in rapid cell death with LDH release during the first 60 min of reoxygenation (62). Figure 11 shows that provision of α-KG/MAL at this time almost completely prevents the LDH release. Withdrawal of glycine at the end of 60-min reoxygenation in the absence of protective substrates led to a similar amount of LDH release between 60 and 120 min of reoxygenation, and this LDH release was also blocked by α-KG/MAL. The rapid LDH release in the unprotected tubules after withdrawal of glycine is consistent with a necrotic form of cell death. This was confirmed by light and electron microscopy (not shown).
Fig. 11.
Lactate dehydrogenase (LDH) release after glycine (GLY) withdrawal. Tubules were subjected to 60 min hypoxia followed by 120 min reoxygenation in the presence of either no extra substrate (NES) or with 4 mM α-KG/MAL during reoxygenation. In the indicated groups designated as -GLY for the 0–60 min and 60–120 min reoxygenation (REOX) periods, glycine was withdrawn either immediately at the start of reoxygenation or at 60 min of reoxygenation. LDH release was measured at 60 and 120 min of reoxygenation. Tubules for all groups were similarly washed and resuspended in fresh medium at both the start of reoxygenation and at 60 min of reoxygenation, so the values for LDH release shown are the amounts released during the 0–60 and 60–120 min reoxygenation periods. Data are means ± SE from four experiments. *P < 0.05 vs. corresponding α-KG/MAL group. LDH release by oxygenated control tubules that had not been subjected to hypoxia averaged 1.4 ± 0.1% during the 0 to 60-min period and 1.1 ± 0.05% during the 60- to 120-min period irrespective of the presence of glycine.
DISCUSSION
The studies in this paper provide new insights into: a) the basis for mitochondrial alterations during a ubiquitous form of tissue injury highly relevant to proximal tubule pathology during ischemic acute renal failure, b) their relationships to general mechanisms of mitochondrial dysfunction of widespread recent investigative interest, and c) a powerful effect of CAC metabolites to ameliorate the biochemical lesion.
The MPT and cytochrome c release have received considerable attention during the past several years as mechanisms by which mitochondria become damaged and contribute to cell death (12, 24, 30, 32, 49, 58, 69). Despite the severity of the insult studied, neither of these processes accounted for the energetic deficit in the proximal tubules. The MPT has been implicated in several models of hypoxia-reoxygenation injury (14, 21, 22, 34, 40, 44, 57) and might reasonably have been expected to occur. Relatively long-lived, solute-selective substates of the MPT have been described in isolated mitochondria (6), and could contribute to the deenergization observed in the tubules, as well as explain the amelioration of the lesion by chemical inhibitors of the MPT (62). The main respiratory abnormality in reoxygenated tubules is in complex I (64), which is similar to the behavior of mitochondria isolated from whole tissues after ischemia and ischemia-reperfusion (12, 20, 46). This damage to complex I might conceivably be a precursor lesion that eventually leads to the MPT, since recent work suggests that, in addition to previously implicated components of the pore such as the adenine nucleotide translocase (68), complex I proteins may form part of the MPT pore (18). However, the increase of inner membrane permeability to ions resulting from the MPT (2, 24, 32, 36) would have precluded the partial retention of ΔΨm observed in the reoxygenated tubules, making it unlikely that the MPT had developed over the time frame of our studies. The microfluorometric observations (Figs. 4 and 6) are important for this conclusion because they demonstrate that the changes of ΔΨm occur in individual cells rather than as shifts in relative proportions of cells that either remain fully energized or become fully deenergized. It is theoretically possible that individual mitochondria within cells could develop the MPT and consequently become fully deenergized, while others remain fully energized, thus decreasing the overall ΔΨm-dependent fluorescence without eliminating it entirely. However, the confocal observations with TMRM, which was relatively resistant to photobleaching, suggest that mitochondria in affected cells mostly developed uniform partial losses of ΔΨm (Fig. 4). This conclusion is further supported by our ultrastructural studies, which have shown that all mitochondria in the majority of unprotected, reoxygenated tubule cells have a condensed configuration rather than the swelling expected for the MPT (64).
Leakage of cytochrome c from mitochondria into the cytosol has been invoked as a damage mechanism that contributes to cell death (16, 17, 30, 49, 69). Dislocation of the cytochrome from its normal location in the space between the inner and outer mitochondrial membranes interrupts electron transport, thereby inhibiting oxidative phosphorylation, and its presence in the cytosol can trigger apoptosis. Loss of the cytochrome can occur via either selective permeabilization of outer mitochondrial membranes or the membrane damage that accompanies the MPT. However, the defects of ATP synthesis that were seen in the reoxygenated tubules were not associated with significant losses of mitochondrial cytochrome c (Fig. 2B). These results importantly complement our prior observation that respiratory function of complex IV, which depends on electrons donated by cytochrome c, remains intact during the cellular insult caused in proximal tubules by 60 min hypoxia and 60 min reoxygenation (64). We have detected little or no cytochrome c release from the isolated proximal tubules during up to 180 min of hypoxia (not shown), a period that is sufficient to induce substantial release of the protein and subsequent apoptosis in cultured proximal tubule cells (48). It will be of interest to determine why this mechanism of injury is so suppressed in fully differentiated proximal tubules. The mitochondrial lesion can certainly be lethal, but, as shown by the Fig. 11 studies, cell death is predominantly by the glycine-sensitive plasma membrane lesion that causes necrosis and rapid LDH release.
Mitochondrial anaerobic substrate-level phosphorylation and respiration are used for energetic support by diving mammals (27) and have been demonstrated to maintain low levels of functionally significant ATP synthesis in hypoxic heart and kidney (8, 23, 28, 31, 41–43, 55, 65), as well as to be beneficial for survival during hemorrhagic shock (10), although their precise mechanism of action in the injury settings has been controversial because of the small amounts of ATP produced anaerobically (65). We have shown that maintenance of ATP by the protective substrates during hypoxia is a result of substrate-level phosphorylation, while anaerobic respiration in complexes I and II supports ΔΨm (64). These effects and the data in the present studies provide an explanation for the benefits of the substrates on organ function despite the relatively small amounts of ATP produced. This ATP, because of its continued availability in the mitochondrial matrix, in combination with the concomitant increases of ΔΨm prevents and reverses a persistent, severe state of mitochondrial dysfunction involving damage to complex I that precedes the MPT and cytochrome c release. The selective importance of the substrate effects at the mitochondrial level is emphasized by observations that the substrates do not alter the plasma membrane damage measured by LDH release or failure to exclude vital dyes during hypoxia in the absence of glycine [(60) and additional data not shown] when oxidative phosphorylation is limited by oxygen deprivation. In contrast, during reoxygenation, improvement of mitochondrial function by the substrates under aerobic conditions that permit resumption of oxidative phosphorylation generates ATP to prevent the plasma membrane damage and LDH release that occurs if glycine is withdrawn from tubules without protective substrates that have not recovered mitochondrial function (Fig. 11).
Supplementation with protective substrates produced relatively large parallel increases of both cellular ATP levels and mitochondrial energization during reoxygenation, with the JC-1 fluorescence values in the best-protected groups reaching control levels. This indicates that the protective effects of the substrates can induce recovery of essentially normal ΔΨm. Although reportedly not an issue for JC-1 (51), cellular entry of potentiometric fluorophores, like their mitochondrial uptake, can be plasma membrane potential dependent so that decreases of the plasma membrane potential as expected during ATP depletion, due to inhibition of the Na+ pump, could reduce mitochondrial uptake of the fluorophores independently of changes of ΔΨm (35, 54). This consideration doesn’t affect our conclusion that mitochondria of the unprotected tubules are not completely deenergized because, despite any limitation of cellular uptake, mitochondrial energization is detected with both TMRM and JC-1. Decreased fluorophore uptake across the plasma membrane resulting from ATP depletion-induced plasma membrane depolarization could exaggerate the differences between the unprotected and substrate-protected tubules during reoxygenation. However, we have recently shown for oxygenated control tubules as well as for posthypoxic tubules, reoxygenated both with and without protective substrates, that JC-1 fluorescence after uptake of the fluorophore in digitonin-containing intracellular buffer is similar to that of tubules loaded with JC-1 in the usual fashion without permeabilization (63). There is evidence that the proton motive force across the inner mitochondrial membrane, most of which consists of ΔΨm, must be maintained at 80–90% of its maximal value for oxidative phosphorylation to occur (35). Thus the capacity of even moderately deenergized mitochondria for ATP synthesis by oxidative phosphorylation may be severely impaired or completely suppressed.
The substrate-induced increases of ATP and of the 590/530 nm JC-1 fluorescence ratio measured during reoxygenation (Figs. 2A, 3A and B, 5B, 7A and B, 8A and B, and 10) are much larger than the increments of ATP (Fig. 8C) and JC-1 fluorescence (64) produced by the substrates during hypoxia, and, as shown by the Fig. 10 studies, do not require continued presence of high concentrations of the supplemental protective substrates. Therefore, the improvement during reoxygenation results from a combination of primary effects of protective substrates on the ‘rescue’ pathways (Fig. 1) followed by global, self-perpetuating restoration of aerobic mitochondrial function. The large, progressive increases of ATP during the second hour of reoxygenation (Fig. 10) are particularly impressive in this regard.
Our measurements of 13C-labeled metabolites in tubules incubated with [13C]aspartate during the hypoxia-reoxygenation maneuvers have provided direct evidence for operation in the tubules of the anaerobic pathways of α-KG/ASP metabolism shown in Fig. 1 (64). Aspartate is theoretically advantageous in combination with α-KG because it provides oxalacetate that serves as a direct substrate acceptor for NADH formed during the oxidation of α-KG to succinyl-CoA and, thereby, promotes continuing anaerobic substrate-level phosphorylation (23, 64). Our studies in the present paper with the additional substrate combinations, however, show that malate in combination with α-KG is just as effective as aspartate, probably because pathway B in Fig. 1 can also utilize the NADH formed from oxidation of α-KG and, thus, maintain continued substrate level phosphorylation from anaerobic metabolism of α-KG.
The experiments testing individual substrates (Fig. 8) provide strong support for the involvement of both the anaerobic and aerobic protective mechanisms shown in Fig. 1. During hypoxia, interconversion of CAC intermediates by normal forward operation of the cycle is limited and electron transport is blocked except for the cycling between complexes I and II in pathway B. Under these conditions, only α-KG, which feeds directly into pathway A to promote substrate-level phosphorylation, and malate and fumarate, which feed into pathway B to promote anaerobic respiration, were unequivocally beneficial when provided individually. In this regard, it should be noted that Fig. 1 shows the interaction between pathways A and B to illustrate the synergistic effect of stimulating both of them simultaneously, but coupling between α-KG metabolism in pathway A and anaerobic respiration in pathway B is not obligate. NADH from any source will maintain anaerobic respiration in complexes I and II. The measurements of succinate production during hypoxia (Fig. 8D) indicate that anaerobic metabolism yielding succinate is required for protection. The substrates that were effective during hypoxia (α-KG, malate, and fumarate) all induced accumulation of succinate. The substrates that were ineffective during hypoxia (glutamate, citrate, and aspartate) did not. The data that ATP levels during hypoxia were increased by α-KG, but not by malate or fumarate, are consistent with the involvement of separate pathways in the protective effects of α-KG, as opposed to those of malate and fumarate (Fig. 8C), and provide additional evidence for the conclusion from our prior work (64) that anaerobic respiration in complexes I and II accounts for increments of ΔΨm during hypoxia, but does not contribute to increases of ATP. The effects of the two pathways were at least partly additive since α-KG, malate, and fumarate individually did not protect as strongly as the substrate combinations that stimulate both pathways.
During reoxygenation, all the CAC intermediates tested, and aspartate, were protective (Fig. 7). The efficacy of citrate and aspartate individually during reoxygenation suggests that under aerobic conditions there is enough forward operation of the CAC before correction of the lesion to provide sufficiently high levels of α-KG from the added citrate to drive the substrate-level phosphorylation rescue pathway, and enough α-KG from endogenous metabolites, to support transamination of aspartate to allow it to be utilized. The strong benefit of succinate during reoxygenation could be due to provision of α-KG from forward operation of the CAC, but much more likely derives from the ability of succinate to bypass the respiratory block for complex I-dependent substrates that fundamentally characterizes the lesion (64). This would generate ATP and increase ΔΨm, with resulting correction of the complex I dysfunction and other elements of the underlying lesion. The experiments in Fig. 10 clearly show that supplemental succinate, like α-KG/MAL, can be removed from the medium at 60 min reoxygenation without impairing further recovery, indicating sustained improvement of the underlying lesion.
It may be questioned how substrates, such as malate and fumarate, that act primarily through pathway B, can protect when provided individually if complex I, which is an essential component for pathway B, is damaged. During hypoxia, we presume that the presence of malate or fumarate, from the start of hypoxia, prevents the damage to complex I by maintaining ΔΨm and, thereby, allows continuing activity of pathway B. During reoxygenation, the provision of succinate by these substrates would bypass complex I, allowing recovery of ATP and ΔΨm to effect repair of the respiratory chain defect. This consideration lends further support to a major role for the aerobic-bypass pathway of succinate metabolism in the protective effects during reoxygenation.
Neither acetoacetate, which induces a highly oxidized state of mitochondrial pyridine nucleotides, nor β-hydroxybutyrate, which promotes their reduction (11), had substantial effects on the mitochondrial dysfunction of the tubules. Oxidant injury has been implicated in complex I dysfunction (20, 33), but we have not found strong or consistent effects of antioxidants on the tubule lesion (data not shown). Pyruvate is protective for ischemic myocardium (29) and anoxic hepatocytes (5), has important antioxidant effects during tissue injury (50), and could also feed into the CAC, but had no activity in our model by itself.
Glutamate alone had a surprisingly weak protective effect during reoxygenation and was ineffective during hypoxia. We also tested glutamine, which had no protective effect at all (data not shown). Rabbit proximal tubules, in contrast to those from the rat and the dog, produce glutamine from glutamate (9), which can shunt glutamate away from α-KG. Under oxygenated conditions, however, substantial conversion of glutamate to α-KG by both transamination and glutamate dehydrogenase occurs simultaneously with glutamine synthesis in rabbit tubules (9). During hypoxia, glutamate dehydrogenase activity will be limited by the high NADH/NAD+ ratio (39). We have been able to enhance the protection provided by glutamate during both hypoxia and reoxygenation by combining it with pyruvate, which will favor its conversion to α-KG by transamination (data not shown).
The behavior of glutamate is of relevance to the question of whether these substrate effects are already fully expressed during ischemia-reperfusion of the intact kidney due to the normal tissue content of metabolites. Glutamate is present at mM levels in renal cortex (61), but was relatively ineffective. In contrast, levels of α-KG in the circulation (10–30 μM), and renal cortex (100–300 μM) (7, 26), are below or at the low end of the effective concentration range shown in Fig. 3B. The already low cortical levels of α-KG drop sharply during ischemia of the rat kidney in vivo (26), and we have similar data for the rabbit kidney (not shown), suggesting that, as in the isolated tubules, glutamate and other endogenous substrates do not maintain sufficient α-KG availability for protection of mitochondrial function during injury conditions in vivo. Among the other protective metabolites, aspartate, malate, fumarate, and citrate all approximate 0.5 to 1.0 mM in vivo (66). Further dose-dependence studies will be necessary to assess whether these metabolites alone or in combination are of benefit at these concentrations. However, the persistent mitochondrial condensation during reoxygenation that characterizes the lesion in the isolated tubules (64) is seen in at least a subpopulation of proximal tubules during reperfusion after clamp ischemia in vivo, including tubules in the cortex where reperfusion is relatively complete (19). Cortical ATP levels are also slow to recover during reperfusion (56). This suggests that the benefits of CAC metabolites on mitochondrial function are not fully provided by the available endogenous substrates and that effective delivery of appropriate substrates could improve recovery of mitochondrial function during ischemia-reperfusion in vivo.
In summary, the present studies extend and complement our earlier work (62, 64) to provide evidence for a pivotal mitochondrial defect during hypoxia-reoxygenation injury to proximal tubules involving impaired function of complex I and mitochondrial deenergization. The mitochondrial dysfunction produces a severe cellular energy deficit that is highly subject to modulation as a function of the availability of specific CAC metabolites. Metabolism of α-KG to succinate by α-KG dehydrogenase, and cycling of electrons in complexes I and II driven by conversion of fumarate to succinate, generate ATP and support ΔΨm anaerobically during hypoxia to prevent the lesion. The same processes plus bypass of the complex I block by succinate can reverse the lesion during reoxygenation. The small direct effects on ATP production and ΔΨm from metabolism via the anaerobic pathways under the injury conditions are then amplified with large increases of ATP recovery and mitochondrial energization during continued reoxygenation, reflecting a sustained global improvement of mitochondrial function under aerobic conditions. These observations provide insight into a critical factor that likely regulates the resistance of tubules to ischemia-reperfusion injury and that may be subject to enhancement in vivo. They are also highly pertinent to the use of isolated tubules for studies of the mechanisms of sublethal structural changes during ATP depletion states insofar as they allow assessment in a controlled and highly manipulable fashion of more severe hypoxic insults followed by metabolic recovery, than would otherwise be possible.
Acknowledgments
We appreciate the technical assistance of Magaly Abarzua, Rebecca Anderer, Julie Davis, Yuan Hua Wen, and Ilana Nissim.
These studies were supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-34275 and DK-39255, and Office of Naval Research Grant N00014–95–1-584 to J. M. Weinberg; National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-37139 to M. A. Venkatachalam; and National Institute of Diabetes and Digestive and Kidney Diseases DK-53761 to I. Nissim.
Preliminary reports of some of the data appeared in abstract form as: J Am Soc Neprol 9: 591A, 1998.
References
- 1.Balaban RS, Mandel LJ. Metabolic substrate utilization by rabbit proximal tubule. An NADH fluorescence study. Am J Physiol Renal Fluid Electrolyte Physiol. 1988;254:F407–F416. doi: 10.1152/ajprenal.1988.254.3.F407. [DOI] [PubMed] [Google Scholar]
- 2.Bernardi P. The permeability transition pore. Control points of a cyclosporin A-sensitive mitochondrial channel involved in cell death. Biochim Biophys Acta. 1996;1275:5–9. doi: 10.1016/0005-2728(96)00041-2. [DOI] [PubMed] [Google Scholar]
- 3.Bernardi P, Scorrano L, Colonna R, Petronilli V, Di Lisa F. Mitochondria and cell death. Mechanistic aspects and methodological issues. Eur J Biochem. 1999;264:687–701. doi: 10.1046/j.1432-1327.1999.00725.x. [DOI] [PubMed] [Google Scholar]
- 4.Beutler HO. Succinate. In: Bergmeyer HU, Bergmeyer J, Grabl M, editors. Methods of Enzymatic Analysis. 3. VII. Weinheim: VCH Verlagsgesellschaft; 1985. pp. 25–33. [Google Scholar]
- 5.Borle AB, Stanko RT. Pyruvate reduces anoxic injury and free radical formation in perfused rat hepatocytes. Am J Physiol Gastrointest Liver Physiol. 1996;270:G535–G540. doi: 10.1152/ajpgi.1996.270.3.G535. [DOI] [PubMed] [Google Scholar]
- 6.Broekemeier KM, Klocek CK, Pfeiffer DR. Proton selective substate of the mitochondrial permeability transition pore: regulation by the redox state of the electron transport chain. Biochemistry. 1998;37:13059–13065. doi: 10.1021/bi980820c. [DOI] [PubMed] [Google Scholar]
- 7.Burlina A. 2-Oxoglutarate. In: Bergmeyer HU, Bergmeyer J, Grabl M, editors. Methods of Enzymatic Analysis. 3. VII. Weinheim: VCH Verlagsgesellschaft; 1985. pp. 20–24. [Google Scholar]
- 8.Cascarano J, Chick WL, Seidman I. Anaerobic rat heart: effect of glucose and Krebs cycle metabolites on rate of beating. Proc Soc Exp Biol Med. 1968;127:25–30. doi: 10.3181/00379727-127-32612. [DOI] [PubMed] [Google Scholar]
- 9.Chauvin M, Frederique M, Martin G, Mispelter J, Baverel G. The rabbit kidney tubule simultaneously degrades and synthesizes glutamate. A 14C NMR study. J Biol Chem. 1997;272:4705–4716. doi: 10.1074/jbc.272.8.4705. [DOI] [PubMed] [Google Scholar]
- 10.Chick WL, Weiner R, Cascareno J, Zweifach BW. Influence of Krebs-cycle intermediates on survival in hemorrhagic shock. Am J Physiol. 1968;215:1107–1110. doi: 10.1152/ajplegacy.1968.215.5.1107. [DOI] [PubMed] [Google Scholar]
- 11.Costantini P, Chernyak BV, Petronilli V, Bernardi P. Modulation of the mitochondrial permeability transition pore by pyridine nucleotides and dithiol oxidation at two separate sites. J Biol Chem. 1996;271:6746–6751. doi: 10.1074/jbc.271.12.6746. [DOI] [PubMed] [Google Scholar]
- 12.Di Lisa F, Bernardi P. Mitochondrial function as a determinant of recovery or death in cell response to injury. Mol Cell Biochem. 1998;184:379–391. [PubMed] [Google Scholar]
- 13.Di Lisa F, Blank PS, Colonna R, Gambassi G, Silverman HS, Stern MD, Hansford RG. Mitochondrial membrane potential in single living adult rat cardiac myocytes exposed to anoxia or metabolic inhibition. J Physiol (Lond) 1995;486:1–13. doi: 10.1113/jphysiol.1995.sp020786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duchen MR, McGuinness O, Brown LA, Crompton M. On the involvement of a cyclosporin A sensitive mitochondrial pore in myocardial reperfusion injury. Cardiovasc Res. 1993;27:1790–1794. doi: 10.1093/cvr/27.10.1790. [DOI] [PubMed] [Google Scholar]
- 15.Emaus RK, Grunwald R, Lemasters JJ. Rhodamine 123 as a probe of transmembrane potential in isolated rat-liver mitochondria: spectral and metabolic propterties. Biochim Biophys Acta. 1986:436–448. doi: 10.1016/0005-2728(86)90112-x. [DOI] [PubMed] [Google Scholar]
- 16.Eskes R, Antonsson B, Osen-Sand A, Montessuit S, Richter C, Sadoul R, Mazzei G, Nichols A, Martinou JC. Bax-induced cytochrome C release from mitochondria is independent of the permeability transition pore but highly dependent on Mg2+ ions. J Cell Biol. 1998;143:217–224. doi: 10.1083/jcb.143.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finucane DM, Waterhouse NJ, Amarante-Mendes GP, Cotter TG, Green DR. Collapse of the inner mitochondrial transmembrane potential is not required for apoptosis of HL60 cells. Exp Cell Res. 1999;251:166–174. doi: 10.1006/excr.1999.4527. [DOI] [PubMed] [Google Scholar]
- 18.Fontaine E, Eriksson O, Ichas F, Bernardi P. Regulation of the permeability transition pore in skeletal muscle mitochondria. Modulation by electron flow through the respiratory chain complex I. J Biol Chem. 1998;273:12662–12668. doi: 10.1074/jbc.273.20.12662. [DOI] [PubMed] [Google Scholar]
- 19.Glaumann B, Glaumann H, Berezesky IK, Trump BF. Studies on cellular recovery from injury: II. Ultrastructural studies of the recovery of the pars convoluta of the rat kidney from temporary ischemia. Virchows Arch. 1977;24:1–18. [PubMed] [Google Scholar]
- 20.Gonzalez-Flecha B, Boveris A. Mitochondrial sites of hydrogen peroxide production in reperfused rat kidney cortex. Biochim Biophys Acta. 1995;1243:361–366. doi: 10.1016/0304-4165(94)00160-y. [DOI] [PubMed] [Google Scholar]
- 21.Griffiths EJ, Halestrap AP. Protection by Cyclosporin A of ischemia/reperfusion-induced damage in isolated rat hearts. J Mol Cell Cardiol. 1993;25:1461–1469. doi: 10.1006/jmcc.1993.1162. [DOI] [PubMed] [Google Scholar]
- 22.Griffiths EJ, Halestrap AP. Mitochondrial non-specific pores remain closed during cardiac ischaemia, but open upon reperfusion. Biochem J. 1995;307:93–98. doi: 10.1042/bj3070093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gronow GHJ, Cohen JJ. Substrate support for renal functions during hypoxia in the perfused rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol. 1984;247:F618–F631. doi: 10.1152/ajprenal.1984.247.4.F618. [DOI] [PubMed] [Google Scholar]
- 24.Gunter TE, Pfeiffer DR. Mechanisms by which mitochondria transport calcium. Am J Physiol Cell Physiol. 1990;258:C755–C786. doi: 10.1152/ajpcell.1990.258.5.C755. [DOI] [PubMed] [Google Scholar]
- 25.Halestrap AP, Connern CP, Griffiths EJ, Kerr PM. Cyclosporin A binding to mitochondrial cyclophilin inhibits the permeability transition pore and protects hearts from ischaemia/reperfusion injury. Mol Cell Biochem. 1997;174:167–172. [PubMed] [Google Scholar]
- 26.Hems DA, Brosnan JT. Effects of ischaemia on content of metabolites in rat liver and kidney in vivo. Biochem J. 1970;120:105–111. doi: 10.1042/bj1200105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hochachka PW, Owen TG, Allen JF, Whittow GC. Multiple end products of anaerobiosis in diving vertebrates. Comp Biochem Physiol B Biochem Mol Biol. 1975;50:17–22. doi: 10.1016/0305-0491(75)90292-8. [DOI] [PubMed] [Google Scholar]
- 28.Hunter EF., Jr Anaerobic phosphorylation due to a coupled oxidation-reduction between ketoglutaric acid and oxalacetic acid. J Biol Chem. 1949;177:361–371. [PubMed] [Google Scholar]
- 29.Kerr PM, Suleiman MS, Halestrap AP. Reversal of permeability transition during recovery of hearts from ischemia and its enhancement by pyruvate. Am J Physiol Heart Circ Physiol. 1999;276:H496–H502. doi: 10.1152/ajpheart.1999.276.2.H496. [DOI] [PubMed] [Google Scholar]
- 30.Kroemer G, Dallaporta B, Resche-Rigon M. The mitochondrial death/life regulator in apoptosis and necrosis. Annu Rev Physiol. 1998;60:619–642. doi: 10.1146/annurev.physiol.60.1.619. [DOI] [PubMed] [Google Scholar]
- 31.Laplante A, Vincent G, Poirier M, Des Rosiers C. Effects and metabolism of fumarate in the perfused rat heart. A 13C mass isotopomer study. Am J Physiol Endocrinol Metab. 1997;272:E74–E82. doi: 10.1152/ajpendo.1997.272.1.E74. [DOI] [PubMed] [Google Scholar]
- 32.Lemasters JJ, Nieminen AL, Qian T, Trost LC, Elmore SP, Nishimura Y, Crowe RA, Cascio WE, Bradham CA, Brenner DA, Herman B. The mitochondrial permeability transition in cell death: a common mechanism in necrosis, apoptosis and autophagy. Biochim Biophys Acta. 1998;1366:177–196. doi: 10.1016/s0005-2728(98)00112-1. [DOI] [PubMed] [Google Scholar]
- 33.Malis CD, Bonventre JV. Mechanism of calcium potentiation of oxygen free radical injury to renal mitochondria. A model for post-ischemic and toxic mitochondrial damage. J Biol Chem. 1986;261:14201–14208. [PubMed] [Google Scholar]
- 34.Nazareth W, Yafei N, Crompton M. Inhibition of anoxia-induced injury in heart myocytes by cyclosporin A. J Mol Cell Cardiol. 1991;23:1351–1354. doi: 10.1016/0022-2828(91)90181-k. [DOI] [PubMed] [Google Scholar]
- 35.Nicholls DG, Ward MW. Mitochondrial membrane potential and neuronal glutamate excitotoxicity: mortality and millivolts. Trends Neurosci. 2000;23:166–174. doi: 10.1016/s0166-2236(99)01534-9. [DOI] [PubMed] [Google Scholar]
- 36.Nieminen AL, Saylor AK, Tesfai SA, Herman B, Lemasters JJ. Contribution of the mitochondrial permeability transition to lethal injury after exposure of hepatocytes to t-butylhydroperoxide. Biochem J. 1995;307:99–106. doi: 10.1042/bj3070099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nissim I, Brosnan ME, Yudkoff M, Nissim I, Brosnan JT. Studies of hepatic glutamine metabolism in the perfused rat liver with 15N-labeled glutamine. J Biol Chem. 1999;264:28958–28965. doi: 10.1074/jbc.274.41.28958. [DOI] [PubMed] [Google Scholar]
- 38.Nissim I, Starr SE, Sullivan KE, Campbell DE, Douglas SD, Diakhin Y, Nissim I, Yudkoff M. Rapid method for determining the rate of DNA synthesis and cellular proliferation. Anal Biochem. 2000;278:198–205. doi: 10.1006/abio.1999.4427. [DOI] [PubMed] [Google Scholar]
- 39.Nissim I, States B, Nissim I, Lin Z, Yudkoff M. Hormonal regulation of glutamine metabolism by OK cells. Kidney Int. 1996;47:96–105. doi: 10.1038/ki.1995.11. [DOI] [PubMed] [Google Scholar]
- 40.Pastorino JG, Snyder JW, Serroni A, Hoek JB, Farber JL. Cyclosporin and carnitine prevent the anoxic death of cultured hepatocytes by inhibiting the mitochondrial permeability transition. J Biol Chem. 1993;268:13791–13798. [PubMed] [Google Scholar]
- 41.Pearl JM, Hiramoto J, Laks H, Drinkwater DC, Jr, Chang PA. Fumarate-enriched blood cardioplegia results in complete functional recovery of immature myocardium. Ann Thorac Surg. 1994;57:1636–1641. doi: 10.1016/0003-4975(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 42.Penney DG, Cascarno J. Anaerobic rat heart. Effects of glucose and tricarboxylic acid-cycle metabolites on metabolism and physiological performance. Biochem J. 1970;118:221–227. doi: 10.1042/bj1180221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pisarenko OI. Mechanisms of myocardial protection by amino acids: facts and hypotheses. Clin Exp Pharmacol Physiol. 1996;23:627–633. doi: 10.1111/j.1440-1681.1996.tb01748.x. [DOI] [PubMed] [Google Scholar]
- 44.Qian T, Nieminen AL, Herman B, Lemasters JJ. Mitochondrial permeability transition in pH-dependent reperfusion injury to hepatocytes. Am J Physiol Cell Physiol. 1997;273:C1783–C1792. doi: 10.1152/ajpcell.1997.273.6.C1783. [DOI] [PubMed] [Google Scholar]
- 45.Reers M, Smith TW, Chen LB. J-Aggregate formation of a carbocyanine as a quantitative fluorescent indicator of membrane potential. Biochemistry. 1991;30:4480–4486. doi: 10.1021/bi00232a015. [DOI] [PubMed] [Google Scholar]
- 46.Rouslin W. Mitochondrial complexes I, II, III, IV, and V in myocardial ischemia and autolysis. Am J Physiol Heart Circ Physiol. 1983;244:H743–H748. doi: 10.1152/ajpheart.1983.244.6.H743. [DOI] [PubMed] [Google Scholar]
- 47.Ruegg CE, Mandel LJ. Bulk isolation of renal PCT and PST. I. Glucose-dependent metabolic differences. Am J Physiol Renal Fluid Electrolyte Physiol. 1990;259:F164–F175. doi: 10.1152/ajprenal.1990.259.1.F164. [DOI] [PubMed] [Google Scholar]
- 48.Saikumar P, Dong Z, Patel Y, Hall K, Hopfer U, Weinberg JM, Venkatachalam MA. Role of hypoxia-induced Bax translocation and cytochrome c release in reoxygenation injury. Oncogene. 1998;17:3401–3415. doi: 10.1038/sj.onc.1202590. [DOI] [PubMed] [Google Scholar]
- 49.Saikumar P, Dong Z, Weinberg JM, Venkatachalam MA. Mechanisms of cell death in hypoxia/reoxygenation injury. Oncogene. 1998;17:3341–3349. doi: 10.1038/sj.onc.1202579. [DOI] [PubMed] [Google Scholar]
- 50.Salahudeen AK, Clark EC, Nath KA. Hydrogen peroxide-induced renal injury. A protective role for pyruvate in vitro and in vivo. J Clin Invest. 1991;88:1886–1893. doi: 10.1172/JCI115511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salvioli S, Ardizzoni A, Franceschi C, Cossarizza A. JC-1, but not DiOC6(3) or rhodamine 123, is a reliable fluorescent probe to assess delta psi changes in intact cells: implications for studies on mitochondrial functionality during apoptosis. FEBS Lett. 1997;411:77–82. doi: 10.1016/s0014-5793(97)00669-8. [DOI] [PubMed] [Google Scholar]
- 52.Sanadi DR, Fluharty AL. On the mechanism of oxidative phosphorylation. VII. The energy-requiring reduction of pyridine-nucleotide by succinate and energy yielding oxidation of reduced pyridine nucleotide by fumarate. Biochemistry. 1963;2:523–528. doi: 10.1021/bi00903a023. [DOI] [PubMed] [Google Scholar]
- 53.Scorrano L, Petronilli V, Bernardi P. On the voltage dependence of the mitochondrial permeability transition pore: a critical appraisal. J Biol Chem. 1997;272:12295–12299. doi: 10.1074/jbc.272.19.12295. [DOI] [PubMed] [Google Scholar]
- 54.Smiley ST, Reers M, Mottola-Hartshorn C, Lin M, Chen A, Smith TW, Steele GD, Jr, Chen LB. Intracellular heterogeneity in mitochondrial membrane potentials revealed by a J-aggregate-forming lipophilic cation JC-1. Proc Natl Acad Sci USA. 1991;88:3671–3675. doi: 10.1073/pnas.88.9.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Snaith CD, Wright G, Lofkin M. The effects of aspartate and 2-oxoglutarate upon glycolytic energy metabolites and mechanical recovery following global ischaemia in isolated rat hearts. J Mol Cell Cardiol. 1992;24:305–315. doi: 10.1016/0022-2828(92)93167-i. [DOI] [PubMed] [Google Scholar]
- 56.Stromski ME, Cooper K, Thulin G, Gaudio KM, Siegel NJ, Shulman RG. Chemical and functional correlates of post-ischemic renal ATP levels. Proc Natl Acad Sci USA. 1986;83:6142–6145. doi: 10.1073/pnas.83.16.6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Uchino H, Elmér E, Uchino K, Li PA, He QP, Smith ML, Siesjö BK. Amelioration by cyclosporin A of brain damage in transient forebrain ischemia in the rat. Brain Res. 1998;812:216–226. doi: 10.1016/s0006-8993(98)00902-0. [DOI] [PubMed] [Google Scholar]
- 58.Varnes ME, Chiu SM, Xue LY, Oleinick NL. Photodynamic therapy-induced apoptosis in lymphoma cells: translocation of cytochrome c causes inhibition of respiration as well as caspase activation. Biochem Biophys Res Commun. 1999;255:673–679. doi: 10.1006/bbrc.1999.0261. [DOI] [PubMed] [Google Scholar]
- 59.Weinberg JM. Adenine nucleotide metabolism by isolated kidney tubules during oxygen deprivation. Biochem Med Metab Biol. 1988;39:319–329. doi: 10.1016/0885-4505(88)90092-8. [DOI] [PubMed] [Google Scholar]
- 60.Weinberg JM, Buchanan DN, Davis JA, Abarzua M. Metabolic aspects of protection by glycine against hypoxic injury to isolated proximal tubules. J Am Soc Nephrol. 1991;1:949–958. doi: 10.1681/ASN.V17949. [DOI] [PubMed] [Google Scholar]
- 61.Weinberg JM, Nissim I, Roeser NF, Davis JA, Schultz S, Nissim I. Relationships between intracellular amino acid levels and protection against injury to isolated proximal tubules. Am J Physiol Renal Fluid Electrolyte Physiol. 1991;260:F410–F419. doi: 10.1152/ajprenal.1991.260.3.F410. [DOI] [PubMed] [Google Scholar]
- 62.Weinberg JM, Roeser NF, Davis JA, Venkatachalam MA. Glycine-protected, hypoxic, proximal tubules develop severely compromised energetic function. Kidney Int. 1997;52:140–151. doi: 10.1038/ki.1997.313. [DOI] [PubMed] [Google Scholar]
- 63.Weinberg JM, Venkatachalam MA, Roeser NF, Nissim I. Mechanisms for proximal tubule mitochondrial deenergization during hypoxia/reoxygenation and their reversal by succinate (Abstract) J Am Soc Nephrol. 2000;11:610A. [Google Scholar]
- 64.Weinberg JM, Venkatachalam MA, Roeser NF, Nissim I. Mitochondrial dysfunction during hypoxia/reoxygenation and its correction by anaerobic metabolism of citric acid cycle intermediates. Proc Natl Acad Sci USA. 2000;97:2826–2831. doi: 10.1073/pnas.97.6.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wiesner RH, Rosen P, Grieshaber MK. Pathways of succinate formation and their contribution to improvement of cardiac function in hypoxic heart. Biochem Med Metab Biol. 1988;40:19–34. doi: 10.1016/0885-4505(88)90100-4. [DOI] [PubMed] [Google Scholar]
- 66.Williamson DH, Brosnan JT. Concentrations of metabolites in animal tissues. In: Bergmeyer HU, editor. Methods of enzymatic analysis. 2. Vol. 4. Weinheim: Verlag Chemie; 1974. pp. 2266–2302. [Google Scholar]
- 67.Wirthensohn G, Guder G. Renal substrate metabolism. Physiol Rev. 1986;66:469–497. doi: 10.1152/physrev.1986.66.2.469. [DOI] [PubMed] [Google Scholar]
- 68.Woodfield K, Rück A, Brdiczka D, Halestrap AP. Direct demonstration of a specific interaction between cyclophilin-D and the adenine nucleotide translocase confirms their role in the mitochondrial permeability transition. Biochem J. 1998;336:287–290. doi: 10.1042/bj3360287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang J, Liu XS, Bhalla K, Kim CN, Ibrado AM, Cai JY, Peng TI, Jones DP, Wang XD. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]