Abstract
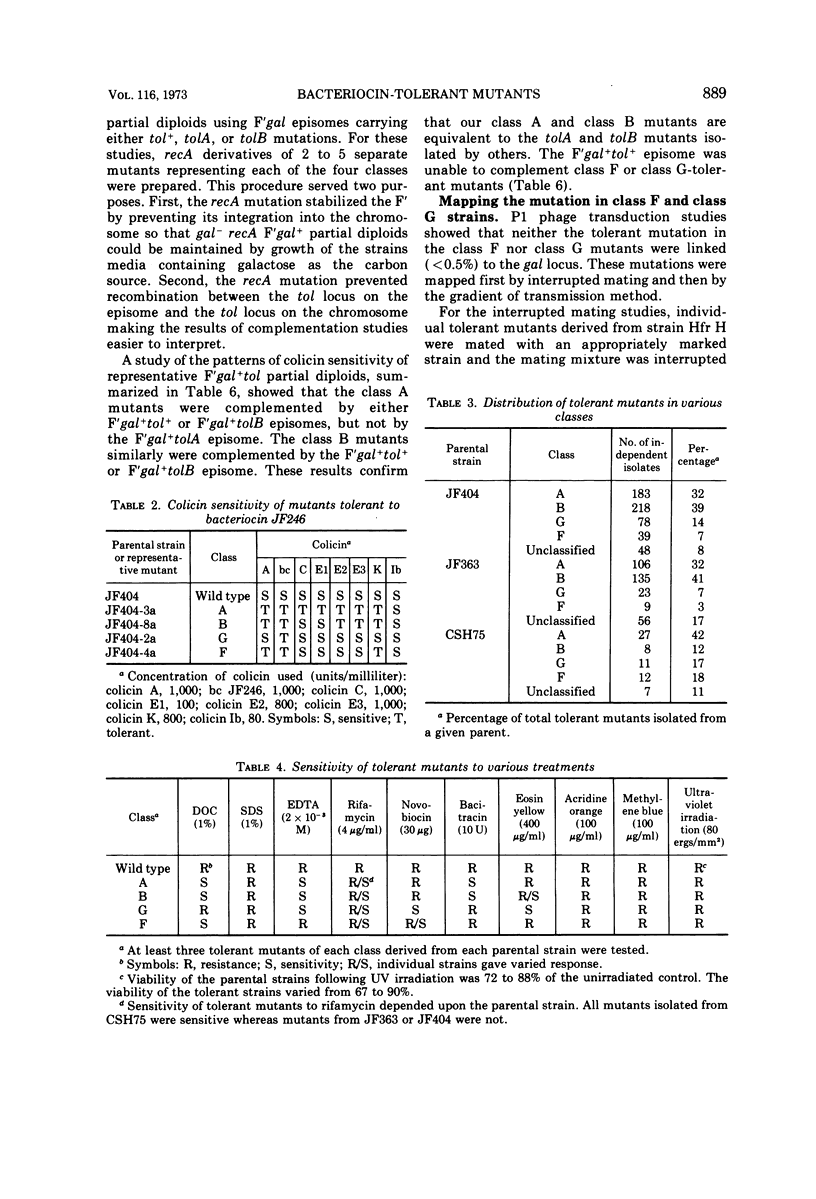
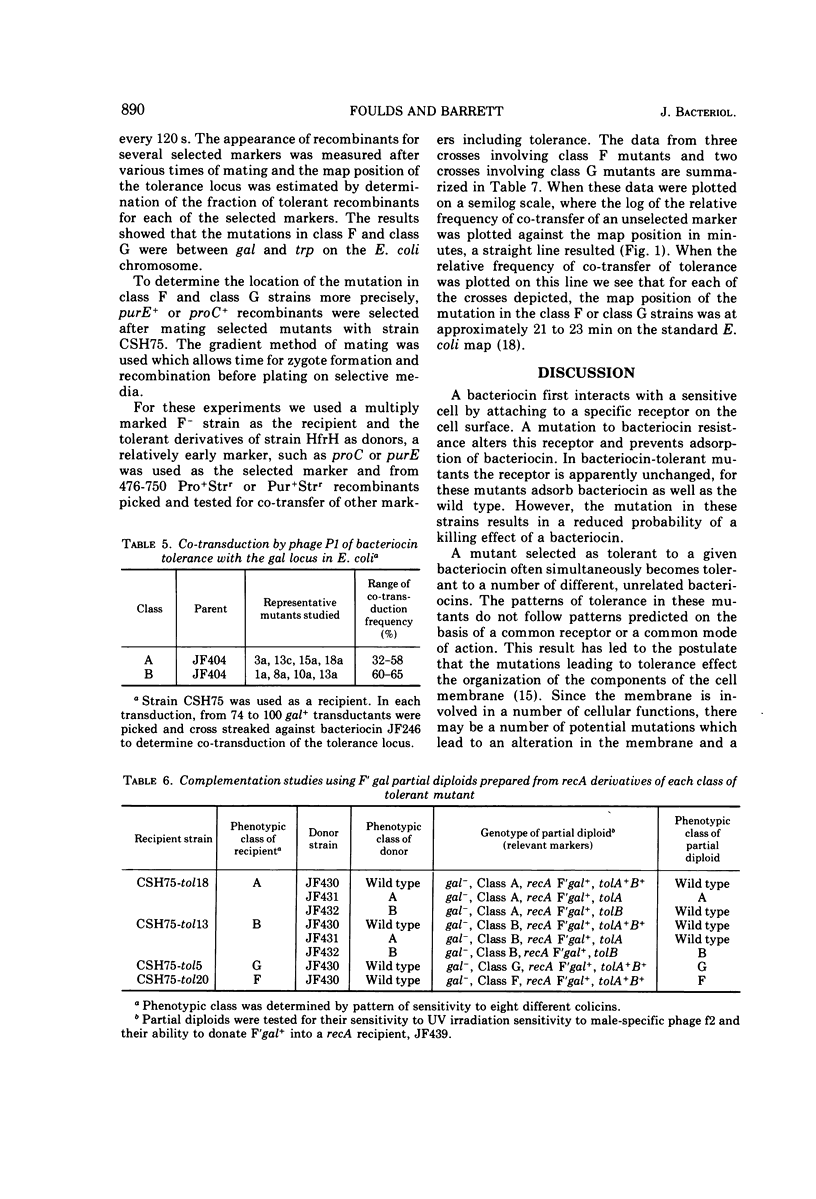
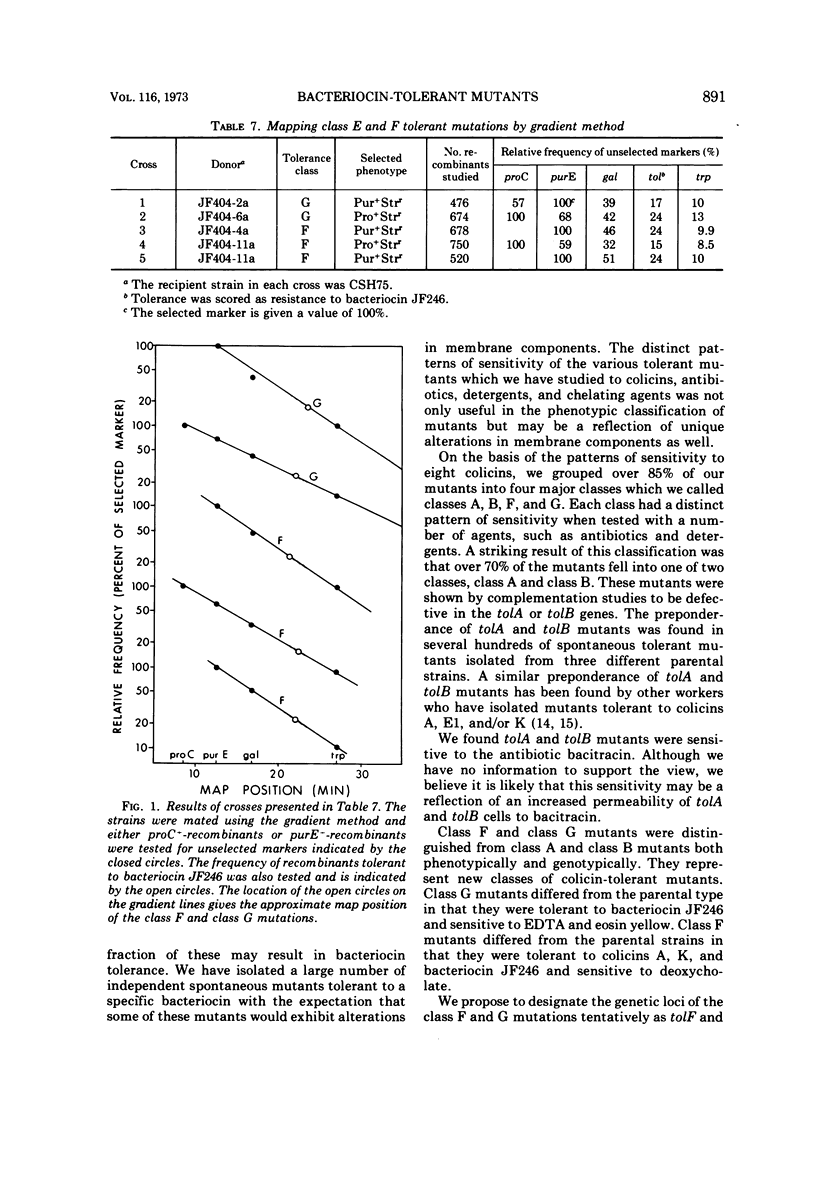
Several hundred independent bacteriocin-tolerant mutants have been isolated without mutagenesis from three strains of Escherichia coli. On the basis of patterns of sensitivity to eight different colicins, over 85% of these mutants could be grouped into four classes. Two classes of mutants, class A and class B, are equivalent to tolA and tolB type mutants. We found tolA and tolB mutants were sensitive to the antibiotic bacitracin. The other two classes of bacteriocin-tolerant mutants, class F and class G, are distinguished from other types of colicin-tolerant mutants on the basis of sensitivity to colicins, dyes, detergents, antibiotics, and chelating agents. The mutation in class F and class G mutants is located between 21 to 23 min on the E. coli chromosome. We propose to designate the loci of these mutations as tolF and tolG, respectively.
Full text
PDF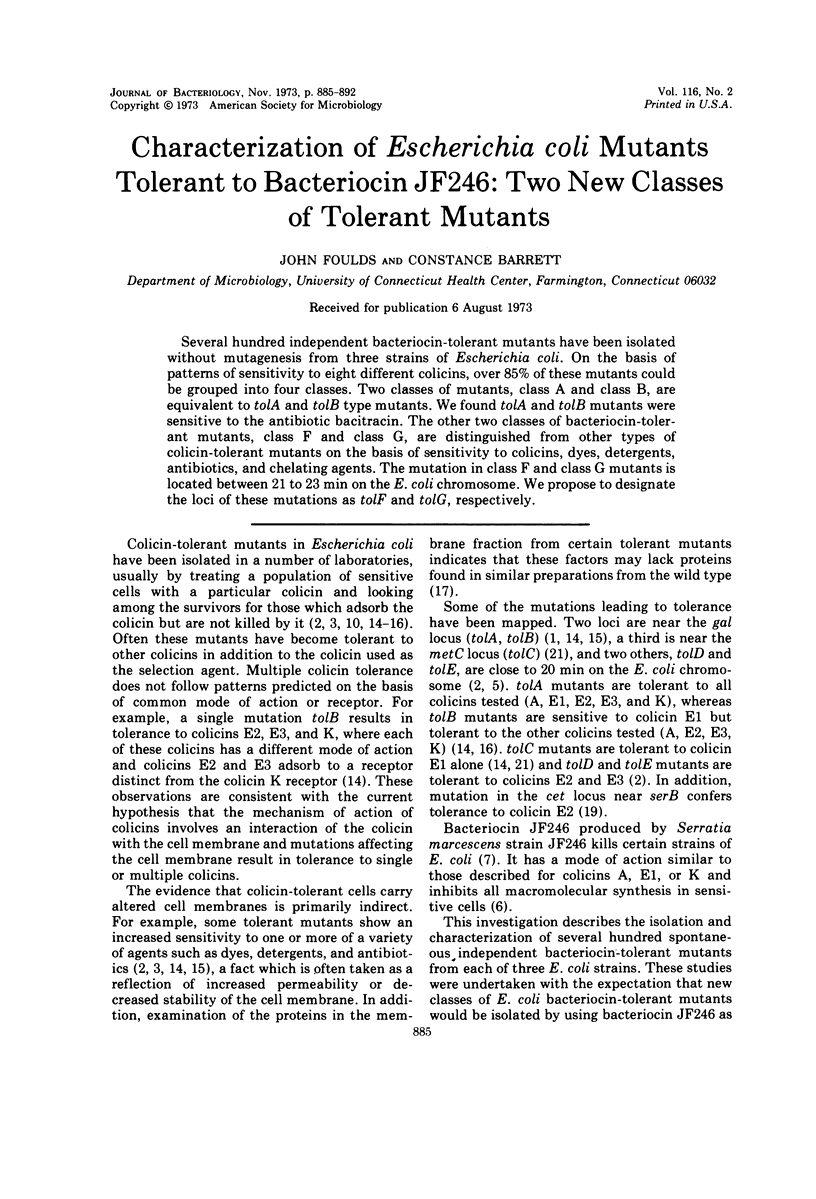




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernstein A., Rolfe B., Onodera K. Pleiotropic properties and genetic organization of the tolA,B locus of Escherichia coli K-12. J Bacteriol. 1972 Oct;112(1):74–83. doi: 10.1128/jb.112.1.74-83.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Haan P. G., Hoekstra W. P., Verhoef C., Felix H. S. Recombination in Escherichia coli. 3. Mapping by the gradient of transmission. Mutat Res. 1969 Nov-Dec;8(3):505–512. doi: 10.1016/0027-5107(69)90067-0. [DOI] [PubMed] [Google Scholar]
- Eriksson-Grennberg K. G., Nordström K. Genetics and physiology of a tolE mutant of Escherichia coli K-12 and phenotypic suppression of its phenotype by galactose. J Bacteriol. 1973 Sep;115(3):1219–1222. doi: 10.1128/jb.115.3.1219-1222.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds J. Mode of action of a bacteriocin from Serratia marcescens. J Bacteriol. 1971 Sep;107(3):833–839. doi: 10.1128/jb.107.3.833-839.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds J. Purification and partial characterization of a bacteriocin from Serratia marcescens. J Bacteriol. 1972 Jun;110(3):1001–1009. doi: 10.1128/jb.110.3.1001-1009.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guterman S. K., Luria S. E. Escherichia coli: strains that excrete an inhibitor of colicin B. Science. 1969 Jun 20;164(3886):1414–1414. doi: 10.1126/science.164.3886.1414. [DOI] [PubMed] [Google Scholar]
- Hill C., Holland I. B. Genetic basis of colicin E susceptibility in Escherichia coli. I. Isolation and properties of refractory mutants and the preliminary mapping of their mutations. J Bacteriol. 1967 Sep;94(3):677–686. doi: 10.1128/jb.94.3.677-686.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland I. B. Properties of Escherichia coli K12 mutants which show conditional refractivity to colicin E2. J Mol Biol. 1968 Jan 28;31(2):267–275. doi: 10.1016/0022-2836(68)90443-9. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- LOW B., WOOD T. H. A QUICK AND EFFICIENT METHOD FOR INTERRUPTION OF BACTERIAL CONJUGATION. Genet Res. 1965 Jul;6:300–303. doi: 10.1017/s001667230000416x. [DOI] [PubMed] [Google Scholar]
- Low B. Formation of merodiploids in matings with a class of Rec- recipient strains of Escherichia coli K12. Proc Natl Acad Sci U S A. 1968 May;60(1):160–167. doi: 10.1073/pnas.60.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel de Zwaig R., Luria S. E. Genetics and physiology of colicin-tolerant mutants of Escherichia coli. J Bacteriol. 1967 Oct;94(4):1112–1123. doi: 10.1128/jb.94.4.1112-1123.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel de Zwaig R. New class of conditional colicin-tolerant mutants. J Bacteriol. 1969 Jul;99(1):78–84. doi: 10.1128/jb.99.1.78-84.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M., Witten C. Interaction of colicins with bacterial cells. 3. Colicin-tolerant mutations in Escherichia coli. J Bacteriol. 1967 Oct;94(4):1093–1111. doi: 10.1128/jb.94.4.1093-1111.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfe B., Onodera K. Demonstration of missing membrane proteins in a colicin-tolerant mutant of E. coli K12. Biochem Biophys Res Commun. 1971 Aug 20;44(4):767–773. doi: 10.1016/0006-291x(71)90776-5. [DOI] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlfall E. J., Holland I. B. Co-transduction with serB of a pleiotropic mutation affecting colicin E2 refractivity, ultraviolet sensitivity, recombination proficiency and surface properties of Escherichia coli K12. J Gen Microbiol. 1970 Aug;62(3):383–398. doi: 10.1099/00221287-62-3-383. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Whitney E. N. The tolC locus in Escherichia coli K12. Genetics. 1971 Jan;67(1):39–53. doi: 10.1093/genetics/67.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]


