Abstract
A concise asymmetric total synthesis of (−)-vindoline (1) is detailed based on a tandem intramolecular [4+2]/[3+2] cycloaddition cascade of a 1,3,4-oxadiazole inspired by the natural product structure, in which the tether linking the initiating dienophile and oxadiazole bears a chiral substituent that controls the facial selectivity of the initiating Diels–Alder reaction and sets absolute stereochemistry of the remaining six stereocenters in the cascade cycloadduct. This key reaction introduces three rings and four C–C bonds central to the pentacyclic ring system setting all six stereocenters and introducing essentially all the functionality found in the natural product in a single step. Implementation of the approach also required the development of a unique ring expansion reaction to provide a 6-membered ring suitably functionalized for introduction of the Δ6,7-double bond found in the core structure of vindoline and defined our use of a protected hydroxymethyl group as the substituent used to control the stereochemical course of the cycloaddition cascade.
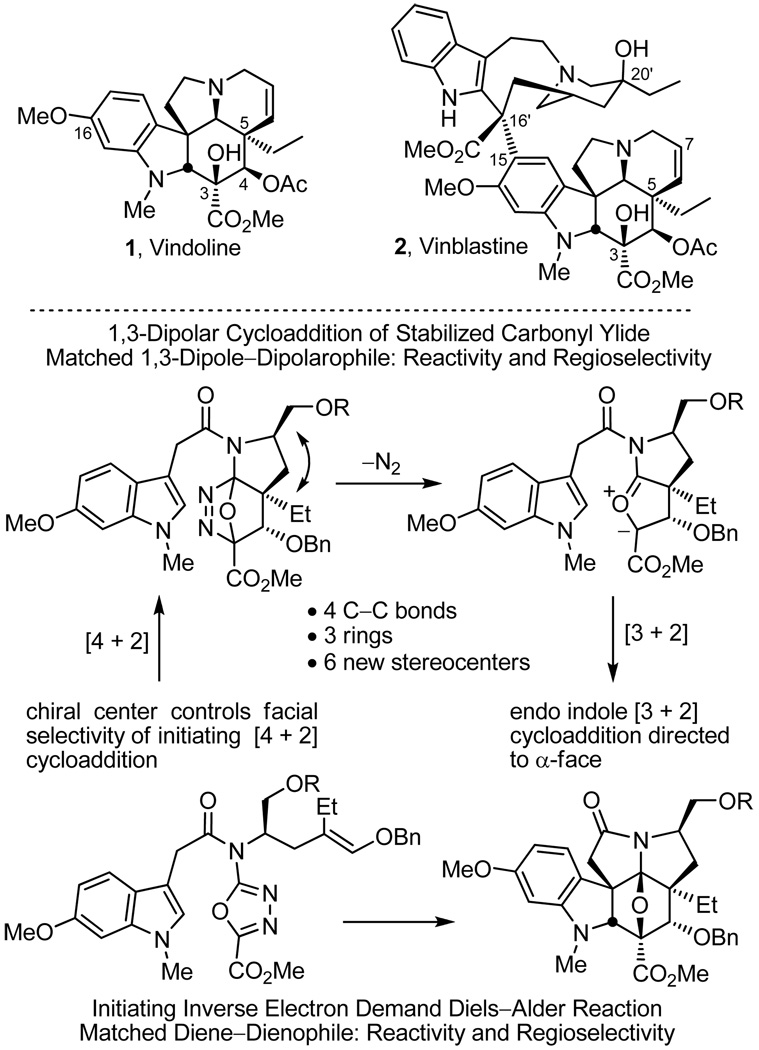
Vindoline (1),1,2 a major alkaloid of Cantharanthus roseus, constitutes the more complex lower half of vinblastine (2)2–5 and serves as both a biosynthetic3,4 and synthetic6,7 precursor to this important natural product (Figure 1). We recently reported the development of a concise total synthesis of (−)- and ent-(+)- vindoline8–10 enlisting an intramolecular tandem [4+2]/[3+2] cycloaddition cascade of 1,3,4-oxadiazoles11 with resolution of a key intermediate, its extension to the preparation of a series of related natural products including vindorosine,10,12 and the subsequent development of a biomimetic Fe(III)-promoted coupling with catharanthine for their single step incorporation into total syntheses of vinblastine and related natural products.6f Herein, we report the development of an asymmetric total synthesis of (−)-vindoline based on an additional implementation of the tandem [4+2]/[3+2] cycloaddition reaction in which the tether linking the initiating dienophile and oxadiazole bears a chiral substituent that sets absolute stereochemistry of the remaining six stereocenters in the cascade cycloadduct. Relative to our earlier work,10 the dienophile linking tether was reduced in length by one carbon permitting the effective control of the facial selectivity of the initiating Diels–Alder reaction and subsequent transmission of the attached substituent stereochemistry throughout the newly constructed pentacyclic ring system that was not observed in our studies with a four atom tether to the initiating dienophile.10 Moreover, this insured that the initiating Diels–Alder reaction could be conducted under milder conditions than previously observed.11 The approach required that the activating acyl chain carbonyl now reside in the dipolarophile tether and that the [4+2] cycloaddition afford a fused 5-membered versus 6-membered ring. A subsequent, unique ring expansion reaction was developed to provide a 6-membered ring suitably functionalized for introduction of the Δ 6,7-double bond found in the core structure of vindoline and defined our use of a protected hydroxymethyl group as the substituent used to control the stereochemical course of the cycloaddition cascade.
Figure 1.
Natural product and key cycloaddition cascade.
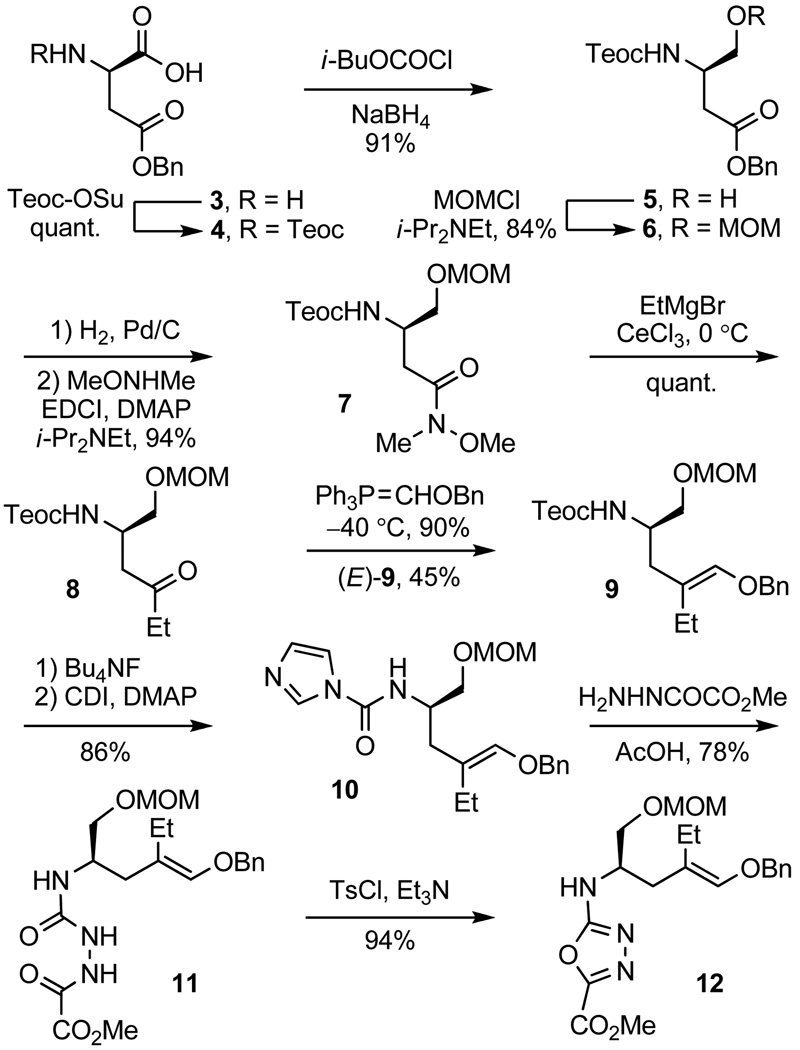
The most important question addressed in initial studies was the stereochemical fate of the key cycloaddition cascade. Accordingly, substrate 13 was prepared and examined in detail. Although 13 lacks the aryl methoxy substituent required for the synthesis of vindoline, it was judged to be an ideal surrogate for examination of the key cycloaddition reaction. The side chain chirality was set using aspartic acid as the starting material (Scheme 1, both enantiomers prepared, natural enantiomer series shown). Teoc protection of d-H2N-Asp(OBn)-OH (TeocOSu, quant.) followed by mixed anhydride formation (i-BuOCOCl, NMM, DME, −15 °C) and reduction (NaBH4, H2O) provided the alcohol 5 (91%), which was protected as its MOM ether 6 (MOMCl, i-Pr2NEt, CH2Cl2, 84%). Benzyl ester hydrogenolysis (H2, 10% Pd/C, THF), coupling of the crude carboxylic acid with N,O-dimethylhydroxylamine (EDCI, DMAP, i-Pr2NEt, CH2Cl2, 94% from 6), and reaction of the Weinreb amide 7 with EtMgBr (3 equiv, 3 equiv of CeCl3, THF, 0 °C, 1 h, quant.)13 cleanly provided the ethyl ketone 8.
Scheme 1.
Wittig olefination of 8 with Ph3P=CHOBn provided a 1:1 mixture of the separable (E) and (Z) enol ethers 9. Teoc deprotection (Bu4NF) and treatment of the liberated amine with carbonyldiimidazole (CDI) afforded 10 (86%, two steps) that was converted to the oxadiazole precursor 12 by treatment with methyl oxalylhydrazide14 in the presence of HOAc (78%) and cyclization of 11 to form the corresponding oxadiazole (TsCl, Et3N, CH2Cl2, 94%). Coupling of 12 with (1-methylindol-3-yl)-2-acetic acid provided the substrate 13 with which the initial examination of the cycloaddition cascade was conducted.
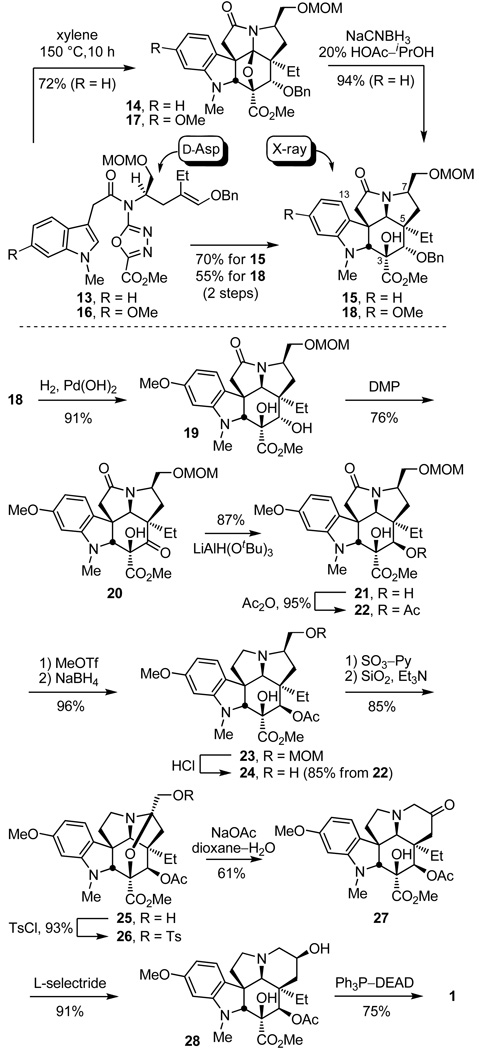
Cyclization of 13 proceeded effectively providing essentially or predominantly a single cascade cyloaddition diastereomer 14 in superb conversions (72%, xylene, 145–150 °C, 10 h) and only small amounts (0–13%) of a second diastereomer were detected, Scheme 2. The temperature required to initiate the cycloaddition cascade is lower (145 vs 180 °C) and the reaction time required for complete reaction is shorter (10 vs 24 h) than those observed with substrates bearing a longer dienophile tether.10
Scheme 2.
Diastereoselective reductive cleavage of the oxido bridge was effected by treatment with NaCNBH3 (2 equiv, 20% HOAc–i-PrOH, 0–25 °C, 40 min, 94%) in a reaction that proceeds by acid-catalyzed generation of an acyliminium ion flanked by two quaternary centers that is reduced by hydride addition to the less hindered convex face, and provided 15 whose structure and stereochemistry were confirmed in a single crystal X-ray structure determination.15 Following initial studies characterizing the cascade cycloaddition reaction of 13, it proved most convenient to run the cycloaddition and subsequent reductive oxido bridge cleavage without the intermediate purification of 14, which proved sensitive to silica gel exposure, providing 15 directly in good overall conversions (57–70% for two steps).
The reaction cascade is initiated by [4+2] cycloaddition of the 1,3,4-oxadiazole with the tethered electron-rich enol ether whose reactivity and regioselectivity are matched to react with the electron-deficient oxadiazole in an inverse electron demand Diels–Alder reaction (Figure 1). Loss of N2 from the initial cycloadduct provides a carbonyl ylide, which undergoes a subsequent 1,3-dipolar cycloaddition with the tethered indole.16 The diene and dienophile substituents reinforce the [4+2] cycloaddition regioselectivity dictated by the linking tether, the intermediate 1,3-dipole is stabilized by the complementary substitution at the dipole termini, and the intrinsic regioselectivity of the attached dipolarophile (indole) complements the [3+2] cycloaddition regioselectivity that is set by its linking chain. The dienophile tether substituent effectively controls the facial selectivity of the initiating [4+2] cycloaddition reaction dictating that the protected hydroxylmethyl group at C7 and the C5 ethyl group reside trans to one another on the newly formed 5-membered ring avoiding a cis pseudodiaxial-1,3-interaction on the sterically more congested concave face of the transition state leading to the initial [4+2] cycloadduct. This establishes the absolute stereochemistry at C5, which in turn is transmitted throughout the cascade cycloadduct where the remaining relative stereochemistry is controlled by a combination of the dienophile geometry (C4 and C5 stereochemistry) and an endo indole [3+2] cycloaddition sterically directed to the face of the 1,3-dipole opposite the newly formed 5-membered ring.10–12 The minor diastereomer occasionally observed in the cycloaddition of 13 appears to be derived from endo indole [3+2] cycloaddition on the same face of the 1,3-dipole as the newly formed 5-membered ring (C2/C11 diastereomer) suggesting the facial selectivity for the initiating Diels–Alder reaction is >20:1 (detection limits).17
The substrate 16, required for the synthesis of vindoline and bearing the indole methoxy group participated in the cycloaddition cascade (130 °C, 8 h and 175 °C, 8 h, xylene) in an analogous fashion and, although the initial cascade cycloadduct 17 was isolated and characterized, it was most conveniently subjected to reductive oxido bridge cleavage (NaCNBH3, 10% HOAc/i-PrOH) prior to purification providing 18 directly (55% for two steps, Scheme 2). The product 18 was converted to the key intermediate 22 by benzyl ether hydrogenolysis (91%), oxidation of the free alcohol 19 (DMP, pyridine–CH2Cl2, 0 °C, 3 h, 76%) and diastereoselective ketone reduction (LiAlH(OtBu)3, THF, 0 °C, 10 h, 87%, 30:1 dr) from the less hindered convex face of 20, followed by C4 alcohol 21 acetylation (Ac2O, DMAP, pyridine, 95%) to provide 22 (Scheme 2). O-Methylation and reductive removal of the lactam carbonyl (MeOTf, 2,6-di-tbutylpyridine, CH2Cl2, 25 °C, 2 h; NaBH4, MeOH, 25 °C, 5 min) followed by MOM ether deprotection (HCl, MeOH, 25 °C, 16 h) liberated the primary alcohol 24 (85% for two steps from 22). Oxidation (3 equiv of SO3–Py, 3 equiv of Et3N, CH2Cl2/DMSO, 25 °C, 1–2 h) of 24 provided an unstable α-aminoaldehyde that not only rapidly epimerized, but was found to be prone to hydrate and enol formation. Moreover, we found that simply exposing the crude aldehyde to silica gel in the presence of Et3N (1% Et3N/EtOAc) in the course of conventional purification led to clean conversion to the stable N,O-ketal 25 (85%), equation 1.
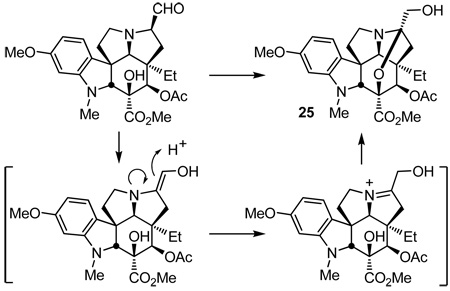 |
(1) |
Formation of the primary tosylate 26 (TsCl, DMAP, Et3N, CH2Cl2, 25 °C, 16 h, 93%) and its subjection to conditions developed for ring expansion18 (NaOAc, dioxane–H2O, 70 °C) provided the key 6-membered ring ketone 17 (61%). Although several mechanistic possibilities can be envisioned for this transformation, some of which proceed through an aziridiniumion, it is most simply and formally represented as hydrolysis of the N,O-ketal to release a reactive α-tosyloxymethyl ketone followed by its intramolecular N-alkylation to provide the 6-membered ketone 17 (equation 2).
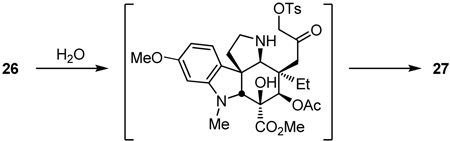 |
(2) |
Diastereoselective reduction of 27 (L-selectride, THF, −78 °C, 0.5 h) provided the penultimate secondary alcohol 28 (91%, >30:1 dr),12c which turn underwent regioselective elimination as previously described10 to provide vindoline (1) upon Mitsunobu activation in the absence of added nucleophiles, Scheme 2.19
Exploration of additional means to effect the key ring expansion reaction, extensions to the preparation of additional Aspidosperma alkaloids and key vindoline analogues, and their incorporation (e.g., 24 and 28) into vinblastine analogues are in progress and will be reported in due course.
Supplementary Material
Acknowledgments
We gratefully acknowledge the financial support of the National Institutes of Health (CA115526 and CA042056) and the Skaggs Institute for Chemical Biology. We wish to thank Dr. Raj Chadha for the X-ray crystal structures and the Uehara Memorial Foundation for fellowship support (Y.S.). D.K. is a Skaggs Fellow.
Footnotes
Supporting Information Available: Full experimental details are provided. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Gorman M, Neuss N, Biemann K. J. Am. Chem. Soc. 1962;84:1058. [Google Scholar]
- 2.Moncrief JW, Lipscomb WN. J. Am. Chem. Soc. 1965;84:4963. doi: 10.1021/ja00949a056. [DOI] [PubMed] [Google Scholar]
- 3.(a) Noble RL, Beer CT, Cutts JH. Ann. N. Y. Acad. Sci. 1958;76:882. doi: 10.1111/j.1749-6632.1958.tb54906.x. [DOI] [PubMed] [Google Scholar]; (b) Noble RL. Lloydia. 1964;27:280. [Google Scholar]; (c) Svoboda GH, Nuess N, Gorman M. J. Am. Pharm. Assoc. Sci. Ed. 1959;48:659. doi: 10.1002/jps.3030481115. [DOI] [PubMed] [Google Scholar]
- 4.Review: Neuss N, Neuss MN. In: The Alkaloids. Brossi A, Suffness M, editors. Vol. 37. San Diego: Academic; 1990. p. 229.
- 5.Reviews: Pearce HL. In: The Alkaloids. Brossi A, Suffness M, editors. Vol. 37. San Diego: Academic; 1990. p. 145. Borman LS, Kuehne ME. In: The Alkaloids. Brossi A, Suffness M, editors. Vol. 37. San Diego: Academic; 1990. p. 133.
- 6.(a) Mangeney P, Andriamialisoa RZ, Langlois N, Langlois Y, Potier P. J. Am. Chem. Soc. 1979;101:2243. doi: 10.1021/jo01336a006. [DOI] [PubMed] [Google Scholar]; (b) Kutney JP, Choi LSL, Nakano J, Tsukamoto H, McHugh M, Boulet CA. Heterocycles. 1988;27:1845. [Google Scholar]; (c) Kuehne ME, Matson PA, Bornmann WG. J. Org. Chem. 1991;56:513. [Google Scholar]; Bornmann WG, Kuehne ME. J. Org. Chem. 1992;57:1752. [Google Scholar]; Kuehne ME, Zebovitz TC, Bornmann WG, Marko I. J. Org. Chem. 1987;52:4340. [Google Scholar]; (d) Magnus P, Mendoza JS, Stamford A, Ladlow M, Willis P. J. Am. Chem. Soc. 1992;114:10232. [Google Scholar]; (e) Yokoshima S, Ueda T, Kobayashi S, Sato A, Kuboyama T, Tokuyama H, Fukuyama T. J. Am. Chem. Soc. 2002;124:2137. doi: 10.1021/ja0177049. [DOI] [PubMed] [Google Scholar]; Kuboyama T, Yokoshima S, Tokuyama H, Fukuyama T. Proc. Natl. Acad. Sci. USA. 2004;101:11966. doi: 10.1073/pnas.0401323101. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Ishikawa H, Colby DA, Boger DL. J. Am. Chem. Soc. 2008;130:420. doi: 10.1021/ja078192m. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ishikawa H, Colby DA, Seto S, Va P, Tam A, Kakei H, Rayl TJ, Hwang I, Boger DL. J. Am. Chem. Soc. 2009;131:4904. doi: 10.1021/ja809842b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Review: Kuehne ME, Marko I. In: The Alkaloids. Brossi A, Suffness M, editors. Vol. 37. San Diego: Academic; 1990. p. 77.
- 8.Racemic total syntheses: Ando M, Büchi G, Ohnuma T. J. Am. Chem. Soc. 1975;97:6880. Kutney JP, Bunzli-Trepp U, Chan KK, De Souza JP, Fujise Y, Honda T, Katsube J, Klein FK, Leutwiler A, Morehead S, Rohr M, Worth BR. J. Am. Chem. Soc. 1978;100:4220. Andriamialisoa RZ, Langlois N, Langlois Y. J. Org. Chem. 1985;50:961. doi: 10.1021/jo01336a006. Formal total syntheses: (d) Ban Y, Sekine Y, Oishi T. Tetrahedron Lett. 1978;2:151. Takano S, Shishido K, Sato M, Yuta K, Ogasawara K. J. Chem. Soc., Chem. Commun. 1978:943. Takano S, Shishido K, Matsuzaka J, Sato M, Ogasawara K. Heterocycles. 1979;13:307. Danieli B, Lesma G, Palmisano G, Riva R. J. Chem. Soc., Chem. Commun. 1984:909. Danieli B, Lesma G, Palmisano G, Riva R. J. Chem. Soc., Perkin Trans. 1. 1987:155. Zhou S, Bommeziijn S, Murphy JA. Org. Lett. 2002;4:443. doi: 10.1021/ol0171618.
- 9.Enantioselective total syntheses: Feldman PL, Rapoport H. J. Am. Chem. Soc. 1987;109:1603. Kuehne ME, Podhorez DE, Mulamba T, Bornmann WG. J. Org. Chem. 1987;52:347. Cardwell K, Hewitt B, Ladlow M, Magnus P. J. Am. Chem. Soc. 1988;110:2242. Kobayashi S, Ueda T, Fukuyama T. Synlett. 2000:883.
- 10.(a) Ishikawa H, Elliott GI, Velcicky J, Choi Y, Boger DL. J. Am. Chem. Soc. 2006;128:10596. doi: 10.1021/ja061256t. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Choi Y, Ishikawa H, Velcicky J, Elliott GI, Miller MM, Boger DL. Org. Lett. 2005;7:4539. doi: 10.1021/ol051975x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wilkie GD, Elliott GI, Blagg BSJ, Wolkenberg SE, Soenen DB, Miller MM, Pollack S, Boger DL. J. Am. Chem. Soc. 2002;124:11292. doi: 10.1021/ja027533n. Elliott GI, Fuchs JR, Blagg BSJ, Ishikawa H, Tao H, Yuan Z, Boger DL. J. Am. Chem. Soc. 2006;128:10589. doi: 10.1021/ja0612549. For reviews of heterocyclic azadiene cycloaddition reactions, see: Boger DL. Tetrahedron. 1983;39:2869. Boger DL. Chem. Rev. 1986;86:781. Boger DL, Weinreb SM. Hetero Diels–Alder Methodology in Organic Synthesis. San Diego: Academic; 1987.
- 12.(a) Elliott GI, Velcicky J, Ishikawa H, Li Y, Boger DL. Angew. Chem. Int. Ed. 2006;45:620. doi: 10.1002/anie.200503024. [DOI] [PubMed] [Google Scholar]; (b) Yuan Z, Ishikawa H, Boger DL. Org. Lett. 2005;7:741. doi: 10.1021/ol050017s. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Ishikawa H, Boger DL. Heterocycles. 2007;72:95. [Google Scholar]
- 13.Imamoto T, Takiyama N, Nakamura K, Hatajima T, Kamiya Y. J. Am. Chem. Soc. 1989;111:4392. [Google Scholar]
- 14.Christl M, Lanzendoerfer U, Groetsch MM, Ditterich E, Hegmann J. Chem. Ber. 1990;123:2031. [Google Scholar]
- 15.The structure and stereochemistry of 15 (CCDC 758511) and the MOM-deprotected primary alcohol of 18 (CCDC 758510) were established by X-ray and conducted on the unnatural and natural enantiomer series, respectively.
- 16.Padwa A, Price AT. J. Org. Chem. 1998;63:556. doi: 10.1021/jo971424n. and 1995, 60, 6258. [DOI] [PubMed] [Google Scholar]
- 17.The substrate 13 bearing the (Z)-enol ether also participates in the cycloaddition cascade, but not as effectively as the (E)-enol ether (51–57% vs 72%) and requires more dilute reaction conditions (0.1 vs 1–5 mM).
- 18.(a) Frehel D, Badorc A, Pereillo J-M, Maffrand J-M. J. Heterocyclic Chem. 1985;22:1011. [Google Scholar]; (b) Kavadias G, Velkof S, Belleau B. Can. J. Chem. 1979;57:1861. [Google Scholar]
- 19.Abbreviations: EDCI = 1-[3-(dimethylamino)propyl]-3-ethylcarbodiimide hydrochloride; DMP = Dess-Martin periodinane; MOM = methoxymethyl; DMAP = 4-dimethylaminopyridine; DMSO = dimethylsulfoxide; NMM = N-methylmorpholine; Teoc = 2-(trimethylsilyl)ethoxycarbonyl.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.