Abstract
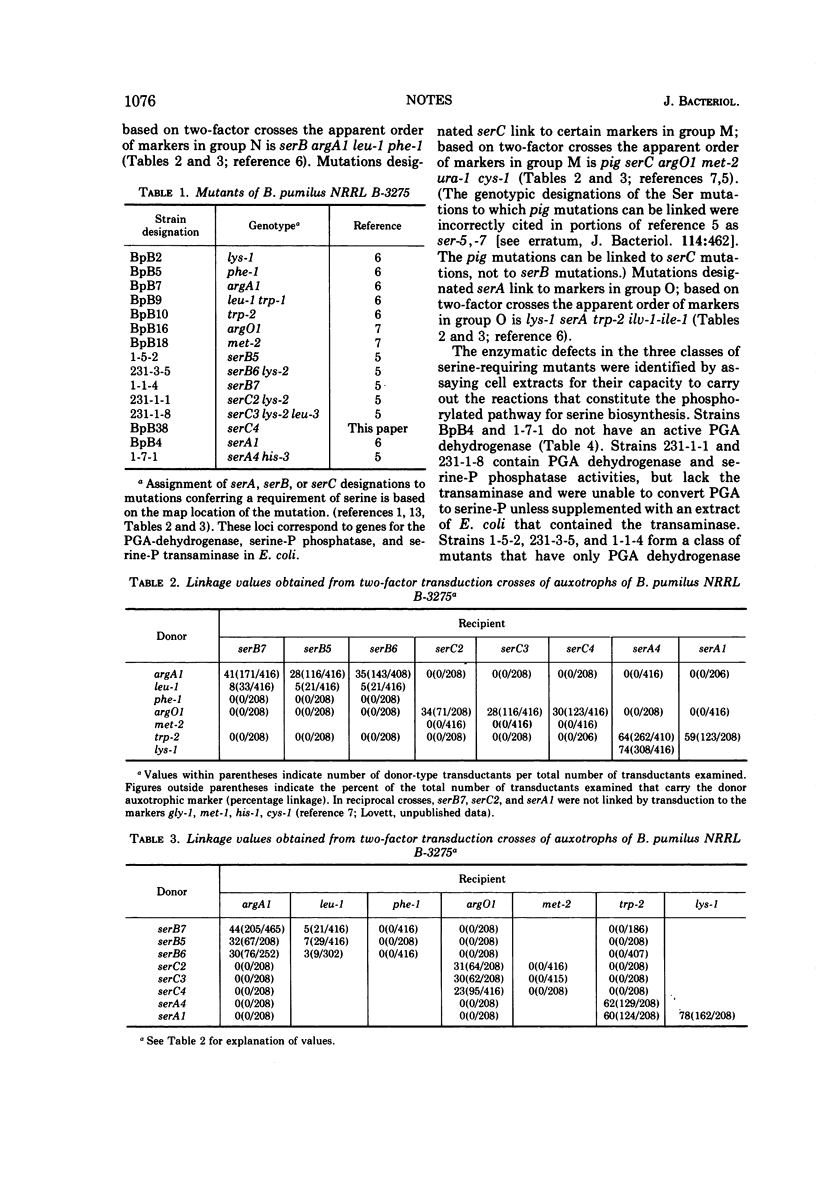
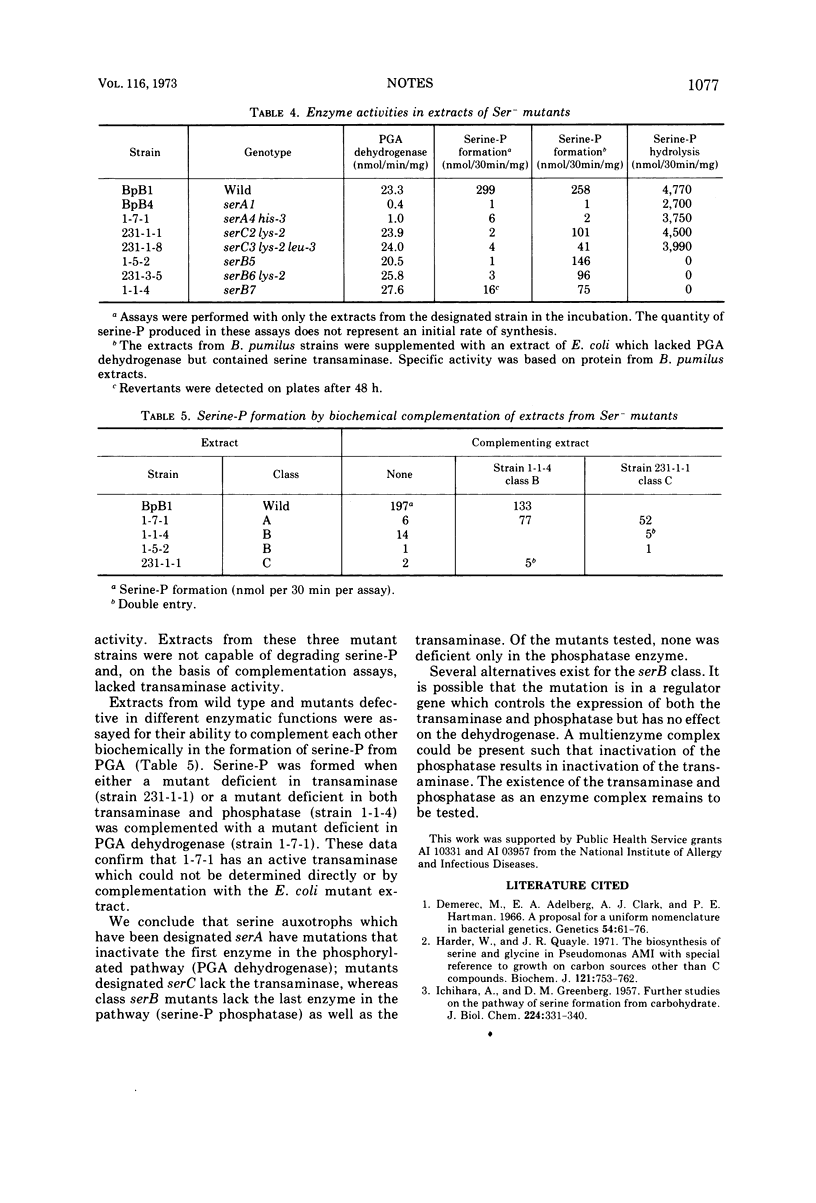
Serine-requiring mutants of Bacillus pumilus NRRL B-3275 have been divided into three groups based on the position of the mutant loci on the linkage map of this organism. Representatives of each group were found deficient in enzymatic activities that constitute the phosphorylated pathway for serine biosynthesis. The evidence suggests that the genes coding for the enzymes of the phosphorylated pathway of serine biosynthesis are not clustered in B. pumilus.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENBERG D. M., ICHIHARA A. Further studies on the pathway of serine formation from carbohydrate. J Biol Chem. 1957 Jan;224(1):331–340. [PubMed] [Google Scholar]
- Harder W., Quayle J. R. The biosynthesis of serine and glycine in Pseudomonas AM1 with special reference to growth on carbon sources other than C1 compounds. Biochem J. 1971 Mar;121(5):753–762. doi: 10.1042/bj1210753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lovett P. S. Spontaneous auxotrophic and pigmented mutants occurring at high frequency in Bacillus pumilus NRRL B-3275. J Bacteriol. 1972 Nov;112(2):977–985. doi: 10.1128/jb.112.2.977-985.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett P. S., Young F. E. Genetic analysis in Bacillus pumilus by PBSI-mediated transduction. J Bacteriol. 1970 Feb;101(2):603–608. doi: 10.1128/jb.101.2.603-608.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett P. S., Young F. E. Linkage groups in Bacillus pumilus determined by bacteriophage PBS1-mediated transduction. J Bacteriol. 1971 May;106(2):697–699. doi: 10.1128/jb.106.2.697-699.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson J. D., Jr, Naylor H. B. The synthesis of L-serine by Micrococcus lysodeikticus. Can J Microbiol. 1971 Jan;17(1):73–77. doi: 10.1139/m71-012. [DOI] [PubMed] [Google Scholar]
- PIZER L. I. THE PATHWAY AND CONTROL OF SERINE BIOSYNTHESIS IN ESCHERICHIA COLI. J Biol Chem. 1963 Dec;238:3934–3944. [PubMed] [Google Scholar]
- Pizer L. I., Ponce-de-Leon M., Michalka J. Serine biosynthesis and regulation in Haemophilus influenzae. J Bacteriol. 1969 Mar;97(3):1357–1361. doi: 10.1128/jb.97.3.1357-1361.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponce-de-Leon M. M., Pizer L. I. Serine biosynthesis and its regulation in Bacillus subtilis. J Bacteriol. 1972 Jun;110(3):895–904. doi: 10.1128/jb.110.3.895-904.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson K. E. Linkage map of Salmonella typhimurium, edition IV. Bacteriol Rev. 1972 Dec;36(4):558–586. doi: 10.1128/br.36.4.558-586.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UMBARGER H. E., UMBARGER M. A., SIU P. M. BIOSYNTHESIS OF SERINE IN ESCHERICHIA COLI AND SALMONELLA TYPHIMURIUM. J Bacteriol. 1963 Jun;85:1431–1439. doi: 10.1128/jb.85.6.1431-1439.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]


