Abstract
Converging evidence from anatomic and physiologic studies suggests that the interaction of high-order association cortices with the thalamus is necessary to focus attention on a task in a complex environment with multiple distractions. Interposed between the thalamus and cortex, the inhibitory thalamic reticular nucleus intercepts and regulates communication between the two structures. Recent findings demonstrate that a unique circuitry links the prefrontal cortex with the reticular nucleus and may underlie the process of selective attention to enhance salient stimuli and suppress irrelevant stimuli in behavior. Unlike other cortices, some prefrontal areas issue widespread projections to the reticular nucleus, extending beyond the frontal sector to the sensory sectors of the nucleus and may influence the flow of sensory information from the thalamus to the cortex. Unlike other thalamic nuclei, the mediodorsal nucleus, which is the principal thalamic nucleus for the prefrontal cortex, has similarly widespread connections with the reticular nucleus. Unlike sensory association cortices, some terminations from prefrontal areas to the reticular nucleus are large, suggesting efficient transfer of information. We propose a model showing that the specialized features of prefrontal pathways in the reticular nucleus may allow selection of relevant information and override distractors, in processes that are deranged in schizophrenia.
Keywords: corticothalamic projections, dual mode of termination, drivers and modulators, inhibitory control, overlap of terminations, mediodorsal nucleus, association cortices
Introduction
In recent years it has become increasingly apparent that thalamic processing involves a dynamic change of peripheral or cortical input, and is thus more complex than a direct, linear relay of information /23/. Thalamocortical interactions of high-order association cortices in particular, have an important role in an array of cognitive, mnemonic, and emotional processes /11,51,52,150,151,182/.
Reciprocal circuits between the dorsal thalamus and the cortex involve first-order thalamic nuclei, which relay information from ascending pathways to the cortex, and high-order thalamic nuclei that receive input from one cortical area and transmit it to other cortical areas. Studies, based largely on the linkage of sensory systems with the thalamus, separate pathways into driver and modulatory. Driver pathways initiate activity in the next processing station, and modulatory pathways change the driving signal. Pathways from the sensory periphery that terminate in first-order thalamic nuclei, which then project to the middle layers of the cortex, are driver pathways. Pathways from the thalamus that terminate extensively in the superficial cortical layers, or the prevalent cortical projections form layer VI to the thalamus, are considered to be modulatory pathways. Some studies have provided evidence that driver pathways, at least in the thalamus, have large terminals, whereas modulatory pathways have small terminals /1,68,72,86,87,88,96,104,113,138,139,151,153/.
Interposed between the dorsal thalamus and the cortex, lies the thalamic reticular nucleus (TRN), a thin sheet of inhibitory neurons that intercept communication between the dorsal thalamus and the cortex. Thalamocortical and corticothalamic axons send collateral branches to TRN, and the TRN projects to the thalamus but not to the cortex. The strategic position of TRN and its unique functional properties that can regulate the activity of thalamocortical neurons through inhibition and disinhibition, have fueled the hypothesis that the TRN has a key role in selective attention /39,69,107,131/. Interestingly, studies in sensory systems suggest that the physiologic properties of TRN neurons change during attentional tasks, possibly modulating thalamocortical communications even at early processing stages /108,116,114,115/.
Attentional mechanisms are thought to be regulated by high-order cortical areas, like the prefrontal cortex, which has a supervisory function and a key role in selecting relevant information and discarding irrelevant information. In this review we discuss recent findings on widespread, driver- and modulatory-like prefrontal pathways in TRN /184/ and show how they differ from sensory corticoreticular projections, which appear to be exclusively modulatory. We summarize circuits suggesting novel mechanisms for cross-modal corticocortical, corticothalamic and intrathalamic interactions in TRN that may have a significant role in attentional modulation. The focus is on the interactions of prefrontal and some sensory cortices with TRN in the primate brain, with references to other mammalian species, as necessary.
Features of TRN
Anatomy and cellular architecture
The TRN develops from the ventral thalamus /84,140/ forming a thin veil that covers most of the dorsal thalamus along its entire antero-posterior extent and separates it from the cortex (Figs. 1, 2A). The nucleus is bordered laterally by the internal capsule, medially by the external medullary lamina, ventromedially by the zona incerta and posteriorly by the ventrolateral geniculate nucleus. The TRN covers the dorsal and lateral parts of the thalamus as an open umbrella with the handle positioned at the midline, between the dorsal thalamus of each hemisphere. It has a similar shape across mammals, appearing round rostrally, is progressively thinner in its central and caudal parts along the medio-lateral dimension and elongated along the dorsoventral axis, and tapers caudally into a narrow spur.
Figure 1.
Position of TRN in the rhesus monkey brain. Reconstructed hemisphere shown from the lateral surface, which was rendered transparent to show the position of the TRN (gray) surrounding the thalamus (black).
Figure 2.
The TRN nucleus and some of its neurochemical features. Brightfield photomicrographs of coronal sections from rostral (top) to caudal (bottom) levels of the rhesus monkey thalamus showing the TRN in sections treated for: Column A, acetylcholinesterase (AChE) histochemistry, showing TRN as it enveils the dorsal thalamus. Column B, High magnification of sections at the same rostrocaudal levels as in column A, immunohistochemically labeled with PV, showing that the majority of TRN neurons are PV+. Black and white arrows indicate the borders of TRN. Scale bars A, 5 mm; B, 500 μm.
The TRN consists of about 1 million neurons on each side of the adult rhesus monkey brain /184/. Unlike other thalamic nuclei, it is composed entirely of inhibitory neurons, which are GABAergic and most are parvalbumin-positive (PV+; ∼70%) /184/ (Fig. 2B). Morphologic studies of TRN in monkeys and humans have classified reticular neurons into several groups mainly according to cell body size and dendritic morphology /21,122,167/ (Fig. 3). The majority of neuronal somata have diameters of 20-50 μm and appear fusiform or ovoid with a markedly invaginated nuclear envelope and thin cytoplasm. Most neurons have long, sparsely branched, aspiny dendrites, emerging from the poles of the soma, but some have beaded structures along their dendrites /21/. The axons of TRN neurons target underlying thalamic nuclei but also send local collaterals within neighboring TRN regions, and interacting through chemical axoaxonic, axosomatic and dendrodendritic synapses inhibit each other /9,43,82,98,123,146,155,168,175,180/. Moreover, neighboring reticular neurons in rodents are electrically coupled, forming highly synchronized networks /28,95/.
Figure 3.
Features of TRN neurons. Brightfield photomicrographs showing differences in the morphology of TRN neurons from a rostral to a caudal direction (top to bottom panels), with larger multipolar neurons with round perikarya appearing rostrally (top panels), and smaller, fusiform, bipolar neurons appearing caudally (bottom panels). Column A, Coronal sections of a rhesus monkey thalamus stained for Nissl. Column B, Coronal sections of a rhesus monkey thalamus stained for PV. Column C, Coronal sections of a rhesus monkey thalamus stained for nonphosphorylated neurofilament H (SMI-32). Scale bars A, 500 μm; B, C, 200 μm.
There is no general agreement among studies about the morphology of reticular neurons across species. Some reports suggest that TRN neurons cannot be classified into separate groups based solely on dendritic morphology and orientation, which extends either dorsoventrally or rostrocaudally and is determined mainly by the shape of the nucleus /103,124/. There is general agreement that reticular neurons conform to the available space, subject to mechanical factors as in other brain regions /46,79/, having round perikarya and multipolar dendrites at the more spacious rostral pole (Fig. 3 top B, C), and elongated and flattened perikarya and dendritic trees in the more cramped central and posterior parts of the nucleus (Fig. 3 bottom B, C). Other studies in rats, rabbits and cats report diversity in the TRN neuronal population, similar to that described in primates /79/. These findings, along with the differential expression of calcium-binding proteins in subsets of TRN neurons for the monkey /184/, and other species (reviewed in /131/), suggest that TRN may be considered an architectonically heterogeneous structure.
Electrophysiologic properties
The physiologic properties of TRN neurons suggest functional heterogeneity. The TRN contains at least two types of neurons that exhibit two activity modes, burst and tonic. Importantly, the type of activity depends on the animal's behavioral state /45,74,112,119,157,159/. Dual discharge modes have also been observed in thalamic relay neurons but they differ in duration and interval between spikes /47,100/. When the animal is in a state of attentive wakefulness or REM sleep, depolarized reticular neurons fire single action potentials continuously, leading to high background activity in TRN and consequent tonic inhibition of the thalamus /108/. In contrast, during states of drowsiness or slow-wave sleep, activity in TRN is in bursts of intermittent groups of high frequency discharges separated by periods of quiescence, causing bursts of firing after a delay period in the thalamus. Interestingly, some TRN neurons have intrinsic physiologic properties that allow switch between tonic and burst firing modes based on the membrane potential /8,29,30,47,100/. In these neurons, a depolarizing current elicits tonic activity if the membrane potential is more positive than normal (>-65 mV), and a burst of action potentials, if the membrane is hyperpolarized. The ability to generate low-threshold spikes and switch from tonic to burst firing appears to be based on the presence of voltage-dependent, low-threshold, Ca2+ channels. In contrast, another population of TRN neurons, possibly lacking the low-threshold Ca2+ conductance channels can fire only tonically /30,47,81/. The functional diversity of reticular neurons has not yet been correlated with specific morphological characteristics.
Connections of TRN with cortical areas and their associated thalamic nuclei
Basic circuitry linking the cortex and dorsal thalamus with TRN
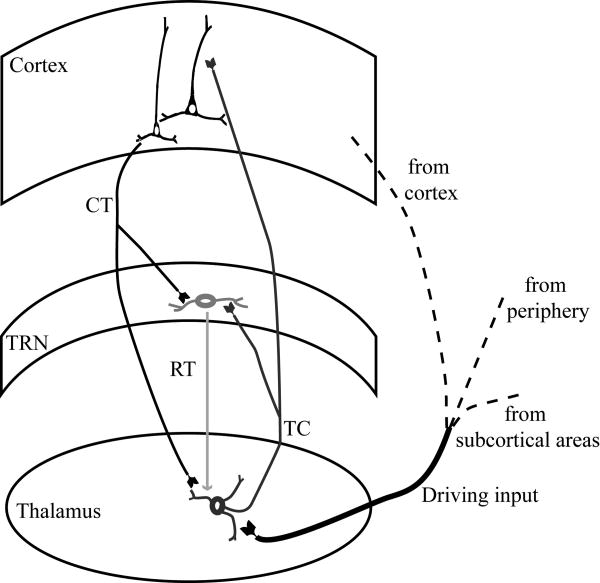
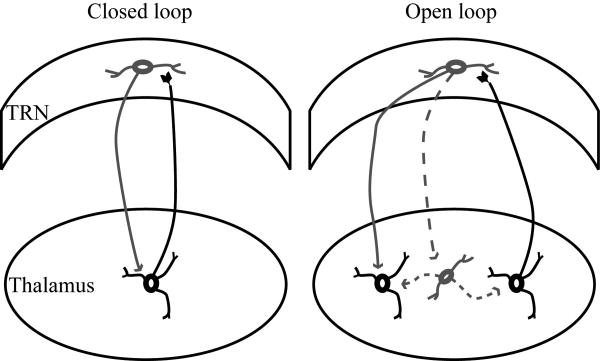
Because the TRN is interposed between the dorsal thalamus and the cortex, it is crossed by corticothalamic and thalamocortical axons, as well as axons coming to the thalamus from the striatum. The axons that pass through the mesh of inhibitory TRN neurons give the nucleus its reticulated appearance and name. The TRN receives unidirectional projections from the cortex and has bidirectional connections with the dorsal thalamus, and is thus in a unique position to modulate the flow of information between thalamus and cortex. In a simplified representation of these pathways (Fig. 4), input reaching the thalamus from the sensory periphery or the cortex, excites thalamocortical projection neurons, which then transmit that information to the cortex. On their way to the cortex, thalamocortical axons give off collateral branches that terminate in TRN. In the feedback loop of this circuit, corticothalamic axons thought to originate from layer VI /70,131/ give off collateral branches that innervate the same TRN regions. The TRN, therefore, receives excitatory input from the cortex and the thalamus and sends inhibitory projections only to the thalamus. These reticulothalamic projections can form closed or open loops (Fig. 5). The loop is considered to be closed when specific TRN and thalamic neurons are connected reciprocally, and is open when TRN neurons receive input from one part of the thalamus and send output to a neighboring region, or to local inhibitory neurons in the thalamus /131/.
Figure 4.
Basic circuits linking TRN with the cortex and the thalamus. Driving input reaching the thalamus from the sensory periphery, subcortical regions or the cortex, activates thalamocortical projection neurons, which then transmit that information to the cortex. Thalamocortical axons, on their way to the cortex, give off collateral branches that terminate in TRN. In the feedback loop of this circuit, corticothalamic axons give off collateral branches that innervate the same TRN regions. The TRN sends inhibitory projections only to the thalamus.
Figure 5.
Reticulothalamic connections. TRN neurons send inhibitory projections (gray line) to thalamic relay neurons that excite them (black line), forming closed loops (left panel). In some cases, a TRN neuron can receive excitatory projections from one thalamic neuron (black line) and send inhibitory projections to another thalamic relay neuron (gray line) or a local inhibitory neuron (dotted gray line), forming open loops (right panel).
Topography of cortical projections and thalamic connections in TRN: sensory sectors
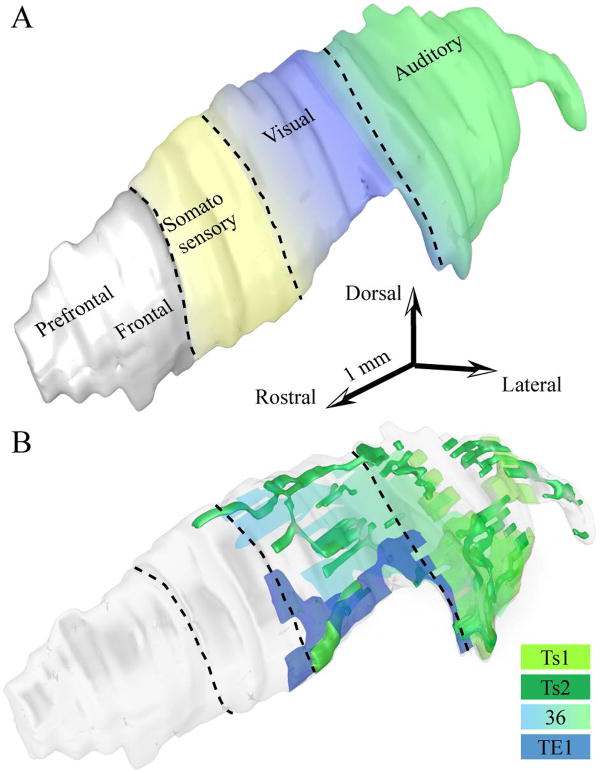
Cortical areas and their associated thalamic nuclei share the same projection sites on TRN, which are topographically organized, as shown in anatomic and physiologic studies in several species, including rats, cats, rabbits and primates (reviewed in /69/). The projections create a crude map of the cortex and the thalamus on TRN, and divide the nucleus into anatomic and functional sectors, related to motor and sensory modalities /24,26,27,31,32,33,34,35,36,38,70,83,101,102,179/. The most detailed maps are based on studies in rodents, but the relative topography of projections onto TRN seems to be similar across mammals. Along the rostrocaudal axis of TRN, the central to posterior sectors are linked consecutively with the somatosensory, visual, visceral, gustatory and auditory cortices and their associated thalamic nuclei. Available information in non-human primates places projections from somatosensory, visual and auditory cortices in comparable positions in TRN (Fig. 6A). Each of these sectors includes tiers, which are defined as subdivisions of a TRN sector having connections with different cortical or thalamic areas, or both. For example, corticoreticular terminals from visual areas V1 and V2 in the primate Galago terminate within the visual sector of TRN, but axons from V2 terminate more medially than axons from V1, forming separate tiers /26,27,166/. The topography in TRN is blurred, especially in sectors connected with high-order association cortices or thalamic nuclei, so that connection zones of nearby cortices or thalamic nuclei overlap, allowing indirect modulation of TRN inhibition (reviewed in /35,131/).
Figure 6.
Sensory sectors of the rhesus monkey TRN. A, Three-dimensional reconstruction of TRN (light gray) showing the approximate sectors receiving projections from auditory (green), visual (blue) and somatosensory (yellow) cortices in non-human primates based on available evidence. The colors between the different sectors change gradually to illustrate the blurred topography of the sectors and their overlaps. B, Three-dimensional reconstruction of TRN (light gray), which was rendered transparent to show the position of axonal terminations from temporal sensory association cortices, including auditory areas Ts1 (light green) and Ts2 (dark green), visual area TE1 (dark blue), and polymodal area 36 (light green-blue gradient).
The blurred topography is evident in the visual and auditory sectors of the monkey TRN, which are not only connected with temporal visual area TE1 and auditory area Ts1, respectively, but also with auditory association area Ts2 and polymodal area 36 /184/. The projections from Ts2 and 36 are more diffuse than projections from TE1 and Ts1 (Fig. 6B). Projections from auditory association areas Ts1 and Ts2 overlap extensively in the caudal sectors of TRN, consistent with the location of the auditory sectors of TRN in other species. In addition, terminations from polymodal area 36 and visual area TE1 are found mainly rostral to the auditory sector, in the visual sector of TRN, situated in the central-caudal parts of the nucleus. These projections are more prominent in the ventral parts of TRN, which include the perigeniculate region. The projections onto TRN in the rhesus monkey have a similar organization as projections from auditory and visual association cortices in other species /25,26,36,38/.
Topography of cortical projections and thalamic connections in TRN: the prefrontal sector
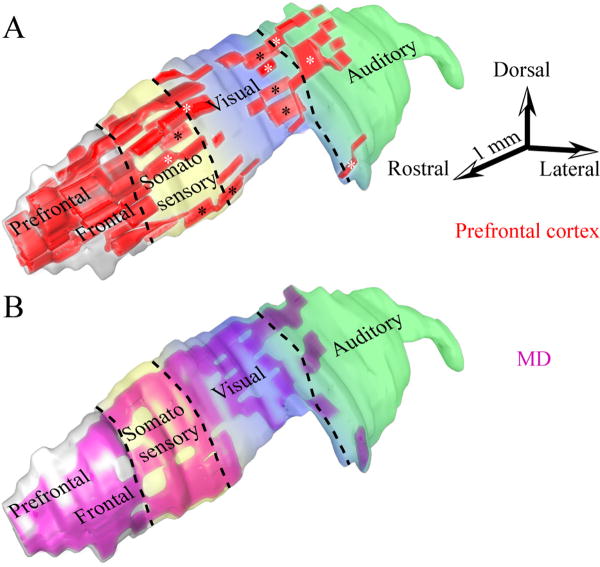
In contrast to the plethora of information on the central and caudal sectors of TRN, the anterior part has received less attention. The anterior TRN includes a motor sector, connected with motor cortex and the ventrolateral thalamic nucleus, and the rostral pole, associated with the limbic cortex and the anterior, midline and intralaminar thalamic nuclei /24,65,91,102,164,179/. Recent studies have provided evidence that the anterior quarter of the rhesus monkey TRN constitutes the prefrontal sector, overlapping extensively with the motor and limbic sectors /179,184/ (Fig. 7A). In agreement with previous studies in sensory and other systems (reviewed in /69,70/), there is considerable overlap in the terminal fields of different prefrontal areas. Projections from areas 10 and 32 terminate in the rostral sector of TRN, and are the most focal among prefrontal areas examined. Interestingly, such focal projection is unusual for area 32, which is a cingulate limbic area and has widespread cortical and subcortical connections /13,14,16,58,59,60,136/. Other prefrontal areas, including areas 46, 13, and 9, have widespread projections in TRN (Fig. 7A), which will be presented in more detail below.
Figure 7.
Widespread prefrontal terminations in TRN and projection neurons to MD overlap with projections from sensory association cortices. A, Three-dimensional reconstruction of TRN (light gray), which was rendered transparent to show the position of axonal terminations from prefrontal areas (red) and their overlaps with somatosensory (yellow), visual (blue) and auditory (green) sectors. Black asterisks indicate some projections from dorsal area 46 and white asterisks indicate some projections from orbital area 13. B, TRN neurons projecting to MD (purple) superimposed on a 3D model of TRN with color coded sensory sectors (as in A).
The same TRN regions that receive prefrontal terminations also project to the mediodorsal (MD), ventral anterior (VA) and anterior medial (AM) thalamic nuclei /184/, which together receive the majority of prefrontal cortical input /42,83,93,160,164,179/. Reticular neurons projecting to these three thalamic nuclei are most prevalent in the anterior and dorsal half of TRN, but some are also found in its ventral sector. Both VA and AM receive projections from rostral TRN, especially its medial segment, whereas projection neurons directed to MD originate from a wider region, encompassing the first three quarters of TRN (Fig. 7B). In some cases the same TRN neurons project both to MD and VA, providing an indirect anatomic link between thalamic nuclei.
Circuits that may underlie attentional regulation through prefrontal cortex and TRN
Overlap of prefrontal and cortical sensory projections in TRN
An unusually widespread pattern of termination in TRN is seen for lateral prefrontal areas 46 and 9 and orbitofrontal area 13. The projections of these prefrontal areas extend beyond the rostral sector of TRN to its second and third quarters, where they overlap extensively with terminations from temporal auditory association, visual association, and polymodal cortices /184/ (Fig. 7A). This comparison was possible by alignment and registration of brain sections containing TRN and labeled terminals on a reconstructed map of TRN. As noted above, the size of TRN in rhesus monkeys is remarkably similar across animals allowing comparisons. Simultaneous mapping of prefrontal and sensory association terminals in TRN, showed that areas 46 and 13 exhibit the most extensive overlaps with temporal sensory association cortices (Fig. 7A, asterisks), and to a lesser extent, area 9. Interestingly, among prefrontal areas examined, only area 46 has terminations that overlap with projections from anterior and medial temporal cortices within the sensory sectors of TRN. Projections from orbital area 13 overlap with temporal polymodal or auditory association areas. In contrast, terminations from area 9 show limited overlap with projections from polymodal area 36 and auditory association area Ts2, in dorsal regions of TRN /184/.
The above evidence suggests that specific prefrontal cortices have widespread projections to TRN, which overlap with other corticoreticular projections, possibly modulating them. Interestingly, none of the sensory association or polymodal areas studied have such widespread corticoreticular projections, consistent with their topography in other species, even though terminations from the polymodal area 36 extend to the auditory sector, and terminations from auditory association area Ts2 extend to the visual sector (Fig. 6B). These findings suggest that high-order sensory association cortices may have more widespread corticoreticular projections than earlier-processing sensory cortices, but are not as extensive as the prefrontal.
Widespread TRN connections with the mediodorsal thalamic nucleus
The vast majority of projection neurons in TRN directed to the principal thalamic nucleus of the prefrontal cortex, the mediodorsal nucleus, are found in anterior loci, but also originate in posterior TRN sectors, albeit to a lesser extent /164,184/. In contrast to other thalamic nuclei connected with prefrontal areas, such as AM and VA, projection neurons in TRN directed to MD extend beyond the rostral sector, encompassing the first three quarters of TRN /184/ (Fig. 7B). Simultaneous mapping in 3D of terminals from prefrontal cortices in TRN, and neurons in TRN that project to MD, reveal considerable overlap in the two projection systems. Superimposed terminations from auditory, visual and polymodal association cortices on the same map show partial overlap with projections from dorsal area 46 and area 13 in the third quarter of TRN, at sites that also project to MD (Fig. 7A, B).
In concert with prefrontal corticoreticular projections, TRN connections with MD may influence relay cells in other high-order or first-order thalamic nuclei situated beneath the central and posterior sectors of TRN. This extensive prefrontal and MD network may thus exert indirect control over other cortices or subcortical structures through their thalamic nuclei that are connected bidirectionally with TRN.
The fine structure of cortical terminations in TRN
Examination of the fine structure of the projections from the cortex to TRN reveals further specialization. Previous studies in rats, cats and monkeys have identified three types of terminals synapsing on TRN neurons /98,123,176/. The first type of terminal is GABAergic, where axonal boutons form symmetric synapses and have flattened vesicles. These GABAergic synapses are from TRN recurrent collaterals, or from projections originating outside the thalamus, including the substancia nigra, globus pallidus or pretectum /5,40,127/. The second type of terminal consists of large glutamatergic boutons with round vesicles that form asymmetric, presumed excitatory synapses, and originate in the dorsal thalamus. The third type of terminal consists of small glutamatergic terminals with round vesicles that form asymmetric (and presumed excitatory synapses) that originate in the cortex. Here we focus on the corticoreticular axonal terminals, which are the most abundant and have special characteristics.
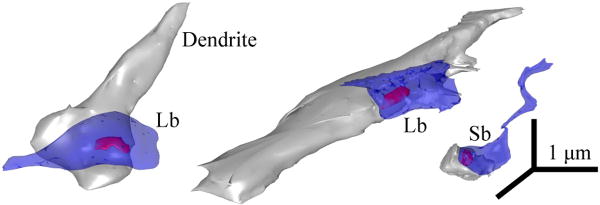
Several studies have indicated that projections from sensory or motor cortices to TRN terminate exclusively as small boutons and are thought to originate from layer VI /67,68,69,70,71,89,142,143,144,151/, which is also the predominant pattern of cortical innervation in other thalamic nuclei. In contrast to projections from sensory association cortices, prefrontal axons in TRN have a dual mode of termination, consisting mostly of small, but also a significant proportion of large boutons (∼10% of the total population; /184/) (Fig. 8). Somatosensory corticoreticular terminations are reported to have dual morphology as well /176/, but the large boutons are significantly smaller than the prefrontal terminals, well within the range of the small terminals from temporal visual and auditory association cortices in TRN /184/.
Figure 8.
Large and small bouton populations in TRN. 3D-reconstructions of large (Lb) and small (Sb) boutons (blue), synapsing (red) on PV+ dendrites of TRN neurons (gray).
Prefrontal cortices, therefore, unlike other cortices, issue some driver-like projections to TRN, which terminate as large boutons and could originate from cortical layer V. Prefrontal cortices, in particular, issue substantial projections to the thalamus from layer V in comparison with other cortices. Projection neurons in layer V of prefrontal cortices account for about 20% of all neurons directed to the anterior thalamic nucleus /178/, and about half of those projecting to the ventral anterior thalamic nucleus /179/.
Large boutons have more synaptic vesicles /56,130,148/ and are more likely to undergo multivesicular release upon stimulation /121,141/, and could thus be more efficient in activating TRN neurons. Large boutons may be present in highly active networks, consistent with their increased mitochondrial content /57/, which is activity dependent /165/. Moreover, because neighboring reticular neurons communicate synaptically and can inhibit adjacent TRN regions /183/, subsets of thalamic neurons could be inhibited and others disinhibited, facilitating discrimination of incoming information. In rodents, and possibly other mammals, reticular neurons are additionally coupled by electrical synapses /94,95/, suggesting that even a small number of large boutons could initiate widespread activation in TRN.
Cholinergic and monoaminergic input to TRN
In addition to the glutamatergic projections from the cortex and thalamus, or the GABAergic projections from the substancia nigra, globus pallidus, and pretectum, the TRN receives strong cholinergic and monoaminergic inputs from the brainstem /6,24,76,92/. These ascending brainstem projections arise primarily from mesopontine cholinergic nuclei /31,128,129,134,162/ and the monoaminergic nuclei locus coeruleus and dorsal raphe /118/. The monoaminergic projection slightly depolarizes TRN neurons, favoring a switch to tonic firing mode, whereas cholinergic input hyperpolarizes them, enabling a switch to burst firing /18,90,111,112,132/. The effect of cholinergic hyperpolarization of TRN neurons is the removal of their tonic inhibition on thalamocortical projection neurons, leading to increased spontaneous firing in the thalamus. On the contrary, noradrenergic depolarization of TRN neurons, enables a switch to tonic firing mode, and leads to decreased spontaneous firing of thalamic neurons, reducing noise /80/.
The same neurotransmitter systems and dopamine also innervate robustly thalamic nuclei connected with prefrontal cortices. In contrast to the differential effects on TRN neurons, thalamocortical cells are depolarized by either acetylcholine or norepinephrine /109/. The neurotransmitter projections in the dorsal thalamus have been implicated in the sleep-wake cycle, arousal, attentional mechanisms and the control of vigilance /7,55,75,110,145,161/. A delicate balance of norepinephrine, acetylcholine and dopamine is essential for prefrontal cortical function /4,111,145/ and its disruption is implicated in several diseases, including attention deficit hyperactivity disorder and schizophrenia. The interaction of these neurotransmitter systems with cortical or thalamic afferents in TRN, in conjunction with the widespread prefrontal projections in TRN, may allow extensive modulation of corticothalamic communication.
Cortical activation and functional flexibility of TRN neurons
Thalamic, cortical, and other projections to TRN play a critical role in controlling the firing patterns of thalamocortical relay neurons, affecting corticothalamic rhythmic activity and corticocortical communication (for reviews see /47,131/). Recent studies indicate that the predominant synaptic input in TRN is from the cortex, suggesting that the cortex has the greatest modulatory influence on TRN activity /98/. Physiologic studies and computational models of reconstructed reticular neurons have shown that the number of glutamate receptor subunits is significantly higher in corticoreticular synapses compared to other corticothalamic synapses. Moreover, the dendrites of TRN neurons have high densities of the low-threshold Ca2+ current channel, rendering them highly sensitive to cortical excitatory postsynaptic potentials (EPSPs), but only when the dendrites are hyperpolarized /44,47,64/. As a result, corticothalamic EPSPs easily evoke bursts in reticular neurons and have a net inhibitory effect on thalamic relay cells. Hyperpolarized thalamic projection neurons then respond after a short delay to incoming input with a long-lasting burst followed by prolonged inhibition. This neuronal activity is observed during the early stages of sleep /161/. On the other hand, when the dendrites of TRN neurons are depolarized, thalamic reticular neurons switch to the tonic mode. In this state, the cortical influence on thalamic relay cells is mostly excitatory, manifested through single spike tonic activity, resulting in desynchronization of thalamocortical oscillations and transition to the alert state.
Comparison among some species
Studies in monkeys have shown that about half of synapses between TRN axons in the anterior and mediodorsal thalamic nuclei are on thalamic GABAergic neurons, enabling disinhibition of thalamic relay cells /93,164/. By contrast, in the somatosensory and visual systems of cats, reticular neurons target preferentially projection neurons in several thalamic nuclei, including the ventroposterior, dorsal lateral geniculate, medial interlaminar, lateral posterior, and the pulvinar, and tend to avoid intrinsic inhibitory neurons /99,171/. This evidence suggests that there is a significant difference in the corticothalamic systems of primates and cats. However, one cannot rule out the possibility that these differences are due to examination of different thalamic nuclei in each species.
Thalamic chemoarchitecture may affect the outcome of TRN activation by the cortex
Local circuit inhibitory neurons are found in dorsal thalamic nuclei of primates and cats as well as the dorsal lateral geniculate nucleus of rats, but are absent in rodents /10,20,73,84,105,117,120,125,126,156,158,172/. Consequently, cortical activation of hyperpolarized TRN neurons in rodents would inhibit or reduce the excitability of targeted thalamic neurons.
Another significant difference in the thalamus of primates and other mammals is in the dual neurochemical character of the thalamic relay cells, which, in primates, includes neurons positive for the calcium binding protein calbindin or parvalbumin, which often have complementary distributions. These proteins likely play an important role in the regulation of neuronal responses, since they are implicated in the buffering and transport of calcium and in the regulation of related enzyme systems /3,77,78/. In the primate thalamus, these two calcium-binding proteins are not expressed by intrinsic GABAergic neurons and only parvalbumin is expressed by the reticular neurons /85,87,88,184/. Similar neurochemical specificity is present at least in the medial geniculate complex of rabbits /41/. In contrast, in the cat thalamus, most projection neurons are calbindin-positive, while parvalbumin is expressed in intrinsic GABAergic neurons as well as in reticular neurons, but not the relay neurons of the intralaminar nuclei (reviewed in /87/). In rodents, most thalamic projection neurons are calbindin-positive but many do not express either calbindin or parvalbumin, but some express a third calcium-binding protein, calretinin (reviewed in /87/). These species-specific differences in the expression of calcium-binding proteins may underlie significant differences in the properties of thalamic neurons and the way they respond to cortical and reticular innervation.
Attentional modulation in TRN
Through its extensive linkage with the cortex, thalamus and brainstem, the TRN may have a unique role among brain structures in attentional processes. The TRN can potentially regulate the activity of thalamocortical neurons through inhibitory/disinhibitory mechanisms, and determine whether information is relayed through the thalamus unchanged or processed. This gate-keeping role is a result of the circuitry of TRN and depends on the physiologic state. The functional activation of TRN is determined largely by the cortex, and by cholinergic and monoaminergic inputs from the brainstem that regulate the membrane potential of TRN neurons. These control mechanisms provide the substrate for the fine-tuning of the excitability of neurons in TRN, endowing this system with the functional flexibility necessary to modulate attention in a constantly changing environment. The involvement of TRN in attentional modulation is exemplified after lesion of the visual sector of TRN, resulting in impairment in selection of relevant targets in attentional orienting tasks /174/. Other studies in the visual system of rats show that when a focus of attention is transmitted from the cortex to the thalamus generating a core of excitation, there is a concomitant increase in Fos-positive neurons in the associated TRN sector /114,115/. Similarly, there is evidence for an increase in TRN activity during attentional tasks in monkeys /108/ and humans /163,170,173/.
Model for enhancement of salient stimuli and suppression of distractors
By what mechanism are relevant stimuli selected and distractors suppressed? There is general agreement that in order to modulate attention, TRN should either enhance relevant stimuli or dampen the noise, increasing the signal-to-noise ratio /39,69,131/. Figure 9 shows a model of circuits through which salient or distracting stimuli may gain access to the cortex or may be dampened in the awake brain in primates (Fig. 9A-D). The proposed wiring underlying these circuits is based on the circuitry known from anatomic studies and on physiologic studies examining how salient or distracting input entering the thalamus can reach the cortex and initiate a cascade of cortico-reticulo-thalamic activation through open loops (Fig. 9A, C, D) or closed loops (Fig. 9B, C, D). Open loops allow prolonged activation between the thalamus and cortex, and closed loops allow brief thalamo-cortical activation. Figure 9 shows the possible combinations of salient and distracting input interacting with open or closed loops in the bidirectional linkage between the dorsal thalamus and TRN, and also shows the unidirectional link from sensory and prefrontal cortex onto TRN. Behaviorally salient inputs can have prolonged access to the cortex through open reticulothalamic loops, as shown in Figure 9A, C. In this case, an increase in TRN activity, elicited by the incoming salient signal, would lead to surround inhibition of neighboring thalamic neurons, enhancing the incoming signal, and suppressing distractors (Fig. 9A, C) /93,131,133,164/. In the case of closed loop reticulothalamic projections, a behaviorally salient input would gain brief access to the cortex, before an increase in TRN activity inhibits the thalamic relay neurons that transmit the salient stimulus and prevent further relay to the cortex (Fig. 9B, D). This brief access to the cortex may be enough to guide attention. Alternatively, it has been suggested that during wakefulness, the rhythmic burst firing in thalamic relay neurons initiated after feedback inhibition from TRN may serve as a wake up call to alert the cortex of the presence of behaviorally relevant sensory input /149,152/.
Figure 9.
Schematic diagram summarizing the involvement of prefrontal and sensory systems in attentional mechanisms through TRN. A-D depict all possible combinations of salient (black) and distracting (brown) inputs interacting with open or closed reticulothalamic loops. Reticulo-MD loops can be either closed or open. A, Salient and distracting inputs interact with open reticulothalamic loops. Salient and distracting input is relayed from the thalamus to the cortex (green dots and lines) and back (gray triangles and lines); Activated TRN neurons inhibit other thalamic neurons (dotted red lines) or thalamic GABAergic neurons (red squares), allowing prolonged access of the stimulus to the cortex. The sensory system can suppress distracting stimuli if reticular neurons activated by the salient signal inhibit neighboring thalamic neurons that transmit distracting signals (1a). Dimorphic prefrontal input (blue), through small and large terminals, and input from MD (cyan) can also activate neighboring reticular neurons and inhibit the thalamic neurons that relay distractors (2) and disinhibit thalamic neurons relaying relevant information (1b). B, Salient and distracting inputs interact with closed reticulothalamic loops, reaching the cortex briefly before they are inhibited by TRN neurons. Input from prefrontal cortex and MD can reverse this outcome by activating neighboring TRN neurons that inhibit the TRN neurons, that prevent transmission of the salient input (3a), or by inhibiting thalamic GABAergic neurons and disinhibiting the relay of salient input (3b). Prefrontal-MD input could also lead to increased inhibition of distractors (3c). C, Salient input has prolonged access to the cortex, interacting with open reticulothalamic loops, and distracting input reaches the cortex briefly, interacting with closed reticulothalamic loops. This is the best case scenario for selection of relevant information and suppression of distractors, and can occur even at early processing stages. Input from the prefrontal cortex and MD to TRN results in enhancement of selection of relevant stimuli by activating TRN neurons that suppress distractors (4a, 5) or disinhibit neurons relaying salient input (4b). D, Salient input reaches the cortex briefly, before TRN feedback inhibition through closed loops, and distracting input has prolonged access to the cortex, interacting with open reticulothalamic loops. Projections from prefrontal cortex and MD to TRN provide a mechanism to allow prolonged passage of relevant input, through lateral inhibition in TRN (6a) or disinhibition of the thalamus (6b). The same projections have a concomitant consequence of suppressing distractors (6c).
The model also explains how incoming distracting stimuli can be suppressed, or reach the cortex. In the open reticulothalamic loop depicted in Figure 9A, D a distracting stimulus gains access to the cortex for a prolonged period through activation of thalamocortical pathways. Distractors can also reach the cortex, in the closed loop mode, but only briefly, before a feedback projection from a TRN neuron inhibits the thalamic relay neuron (Fig. 9B, C). The best case scenario for the selection of relevant information and suppression of distractors is presented in Figure 9C, where salient input gains prolonged access to the cortex, through activation of open reticulothalamic loops, and distracting input accesses the cortex only briefly, through activation of closed reticulothalamic loops. Figure 9D presents the worst case scenario, in which the transmission of salient and distracting inputs is reversed.
Model of prefrontal influence in attentional modulation through TRN
The above discussion shows how salient stimuli may reach the cortex and distractors suppressed or pass on to the cortex by virtue of activity in sensory cortices through the thalamus, TRN and cortex, consistent with previous findings /108,116,114,115/. Prefrontal pathways can perform both functions more effectively, as summarized on the basis of recent findings /184/, in Figure 9 (blue pathways). Prefrontal pathways serve three key functions. First, through their widespread small and large terminals onto TRN prefrontal pathways can increase the drive for the salient stimuli and ensure passage to the cortex (Fig. 9A-D). Second, through the same mechanism prefrontal pathways to TRN can increase the drive of reticular neurons, effectively inhibiting distractors in both open loop (Fig. 9A, D) and closed loop (Fig. 9B, C) circuits. Third, prefrontal activation of MD, which maps broadly on the same TRN sites as the prefrontal pathways, also enhances transmission of relevant signals and eliminates distractors. Given that sensory and other systems can modulate attention at early processing stages, interactions of the prefrontal system with TRN may be especially necessary in complex situations involving cognitive and emotional processing.
Classic studies have shown that detection of novel stimuli evokes large negative slow potentials in the prefrontal cortex and correlated positive slow potentials in rostral TRN /154,181/. According to these authors (reviewed in /22/), selective intermodal attention might be realized by prefrontal control over TRN through the inferior thalamic peduncle. A potential problem for this hypothesis was thought to be in the topography of prefrontal cortical input to the rostral part of TRN, while the processing of salient or distracting sensory stimuli takes place in more posterior parts of the thalamus, in sensory related nuclei. Since there are no direct connections between dorsal thalamic nuclei, another link between the anterior and the posterior part of TRN is needed to provide an anatomic substrate for selective attention. The recent findings of strong and widespread prefrontal projections in TRN that overlap extensively with projections from other cortical and thalamic pathways provide a link between different sectors of TRN /184/ (Figs. 7, 9). Moreover, some TRN neurons project simultaneously to more than one thalamic nucleus, like the MD and VA /184/. Consistent with the circuitry, there is evidence that stimulation of neurons in sensory- or motor-related dorsal thalamic nuclei robustly inhibits other dorsal thalamic nuclei of the same or different modalities through disynaptic inhibition mediated by TRN /35,37/.
The prefrontal corticoreticular pathway has two unique characteristics that other corticoreticular pathways lack. The first is its dimorphic termination into many small but also a significant proportion of large terminals, which may be more efficient in affecting information processing. Large terminals could override input from small terminals arising in sensory, motor or even other prefrontal cortical areas promoting the relay of relevant stimuli over distracting stimuli. The nature of prefrontal corticoreticular interactions, combined with the properties of TRN neurons, favor the searchlight hypothesis proposed by Crick in 1984 /39/. According to Crick's hypothesis, the TRN acts as an attentional searchlight in the brain that can discover action ‘hotspots’ in the cortex and the thalamus, intensify the ‘hotspot’ activity and then rapidly turn off and repeat the same process for the next field of attention. This integration and intensification of relevant cues was thought to occur through rapidly modifiable synapses that are ‘strengthened’ to temporarily coordinate two already connected neurons, when those neurons fire in a highly correlated manner. The large terminals of prefrontal pathways may facilitate selection of specific loops through TRN and provide the flexibility needed to direct attention to relevant stimuli, creating ‘hotspots’ through the thalamus and cortex.
The second unique feature of some prefrontal corticoreticular pathways is their widespread extent, poised to modulate relevant signals from several modalities, as shown in Figure 7. The evidence presented suggests involvement of these pathways in attentional mechanisms through direct interaction of prefrontal terminals, MD, and temporal sensory association areas in TRN. It seems plausible that, through these overlaps in TRN, prefrontal areas 46, 13, and 9 may help modulate signals conveyed by projections from temporal sensory association cortices in the same TRN sites, guiding the selection of relevant and motivationally significant signals.
The uniquely widespread circuitry of some prefrontal cortices and MD with TRN may contribute to the functional specialization of the prefrontal cortex in executive control. Pathways linking area 46 with MD, in particular, are active when animals must hold information temporarily in mind to solve the task at hand /2,48,49,50,53,62/. The interdependence of prefrontal cortex and MD is exemplified after cooling and incapacitating lateral prefrontal cortex, leading to disruption of activity in MD related to working memory /2/. In addition, area 46 processes detailed sensory information, necessary for discrimination, and has connections with premotor cortices for action /15,106/. Interestingly, axonal terminations from area 46 overlap in TRN with projections from inferior temporal cortices associated with visual perception and visual memory /54,66/. On the other hand, area 13 has robust connections with limbic structures and is associated with emotional processing and evaluation of reward contingencies /12,17/. The widespread prefrontal projections to TRN from these cortices may help focus attention on relevant and motivationally salient stimuli. In schizophrenia, both lateral prefrontal cortex and MD are disrupted /19,61,63,97,135,137,147,169,177/, potentially impairing the ability to effectively select relevant stimuli and suppress distractors.
Acknowledgments
The authors' work was supported by NIH grants from NIMH and NINDS.
References
- 1.Abramson BP, Chalupa LM. The laminar distribution of cortical connections with the tecto- and cortico-recipient zones in the cat's lateral posterior nucleus. Neuroscience. 1985;15:81–95. doi: 10.1016/0306-4522(85)90125-3. [DOI] [PubMed] [Google Scholar]
- 2.Alexander GE, Fuster JM. Effects of cooling prefrontal cortex on cell firing in the nucleus medialis dorsalis. Brain Res. 1973;61:93–105. doi: 10.1016/0006-8993(73)90518-0. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez P, Squire LR. Memory consolidation and the medial temporal lobe: a simple network model. Proc Natl Acad Sci U S A. 1994;91:7041–7045. doi: 10.1073/pnas.91.15.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnsten AF, Li BM. Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biol Psychiatry. 2005;57:1377–1384. doi: 10.1016/j.biopsych.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 5.Asanuma C. GABAergic and pallidal terminals in the thalamic reticular nucleus of squirrel monkeys. Exp Brain Res. 1994;101:439–451. doi: 10.1007/BF00227337. [DOI] [PubMed] [Google Scholar]
- 6.Asanuma C. Noradrenergic innervation of the thalamic reticular nucleus: a light and electron microscopic immunohistochemical study in rats. J Comp Neurol. 1992;319:299–311. doi: 10.1002/cne.903190209. [DOI] [PubMed] [Google Scholar]
- 7.Aston-Jones G, Rajkowski J, Cohen J. Role of locus coeruleus in attention and behavioral flexibility. Biol Psychiatry. 1999;46:1309–1320. doi: 10.1016/s0006-3223(99)00140-7. [DOI] [PubMed] [Google Scholar]
- 8.Bal T, McCormick DA. Mechanisms of oscillatory activity in guinea-pig nucleus reticularis thalami in vitro: a mammalian pacemaker. J Physiol. 1993;468:669–691. doi: 10.1113/jphysiol.1993.sp019794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bal T, von Krosigk M, McCormick DA. Synaptic and membrane mechanisms underlying synchronized oscillations in the ferret lateral geniculate nucleus in vitro. J Physiol. 1995;483(Pt 3):641–663. doi: 10.1113/jphysiol.1995.sp020612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barbaresi P, Spreafico R, Frassoni C, Rustioni A. GABAergic neurons are present in the dorsal column nuclei but not in the ventroposterior complex of rats. Brain Res. 1986;382:305–326. doi: 10.1016/0006-8993(86)91340-5. [DOI] [PubMed] [Google Scholar]
- 11.Barbas H. Complementary role of prefrontal cortical regions in cognition, memory and emotion in primates. Adv Neurol. 2000;84:87–110. [PubMed] [Google Scholar]
- 12.Barbas H. Connections underlying the synthesis of cognition, memory, and emotion in primate prefrontal cortices. Brain Res Bull. 2000;52:319–330. doi: 10.1016/s0361-9230(99)00245-2. [DOI] [PubMed] [Google Scholar]
- 13.Barbas H, Henion TH, Dermon CR. Diverse thalamic projections to the prefrontal cortex in the rhesus monkey. J Comp Neurol. 1991;313:65–94. doi: 10.1002/cne.903130106. [DOI] [PubMed] [Google Scholar]
- 14.Barbas H, Hilgetag CC, Saha S, Dermon CR, Suski JL. Parallel organization of contralateral and ipsilateral prefrontal cortical projections in the rhesus monkey. BMC Neurosci. 2005;6:32. doi: 10.1186/1471-2202-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barbas H, Pandya DN. Architecture and frontal cortical connections of the premotor cortex (area 6) in the rhesus monkey. J Comp Neurol. 1987;256:211–218. doi: 10.1002/cne.902560203. [DOI] [PubMed] [Google Scholar]
- 16.Barbas H, Saha S, Rempel-Clower N, Ghashghaei T. Serial pathways from primate prefrontal cortex to autonomic areas may influence emotional expression. BMC Neurosci. 2003;4:25. doi: 10.1186/1471-2202-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barbas H, Zikopoulos B. Sequential and parallel circuits for emotional processing in primate orbitofrontal cortex. In: Zald David, Rauch Scott., editors. The Orbitofrontal Cortex. 2006. pp. 57–91. [Google Scholar]
- 18.Ben Ari Y, Dingledine R, Kanazawa I, Kelly JS. Inhibitory effects of acetylcholine on neurones in the feline nucleus reticularis thalami. J Physiol. 1976;261:647–671. doi: 10.1113/jphysiol.1976.sp011579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benes FM, Berretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology. 2001;25:1–27. doi: 10.1016/S0893-133X(01)00225-1. [DOI] [PubMed] [Google Scholar]
- 20.Benson DL, Isackson PJ, Gall CM, Jones EG. Contrasting patterns in the localization of glutamic acid decarboxylase and Ca2+/calmodulin protein kinase gene expression in the rat central nervous system. Neuroscience. 1992;46(4):825–849. doi: 10.1016/0306-4522(92)90188-8. [DOI] [PubMed] [Google Scholar]
- 21.Berezhnaya LA. Neuronal organization of the reticular nucleus of the thalamus in adult humans. Neurosci Behav Physiol. 2006;36:519–525. doi: 10.1007/s11055-006-0049-1. [DOI] [PubMed] [Google Scholar]
- 22.Brunia CH, van Boxtel GJ. Wait and see. Int J Psychophysiol. 2001;43:59–75. doi: 10.1016/s0167-8760(01)00179-9. [DOI] [PubMed] [Google Scholar]
- 23.Casagrande VA, Guillery RW, Sherman SM. Cortical function: A view from the thalamus. Cortical Function: A View from the Thalamus. 2005;149:IX–XIV. [Google Scholar]
- 24.Cicirata F, Angaut P, Serapide MF, Panto MR. Functional organization of the direct and indirect projection via the reticularis thalami nuclear complex from the motor cortex to the thalamic nucleus ventralis lateralis. Exp Brain Res. 1990;79:325–337. doi: 10.1007/BF00608242. [DOI] [PubMed] [Google Scholar]
- 25.Coleman KA, Mitrofanis J. Organization of the visual reticular thalamic nucleus of the rat. Eur J Neurosci. 1996;8:388–404. doi: 10.1111/j.1460-9568.1996.tb01222.x. [DOI] [PubMed] [Google Scholar]
- 26.Conley M, Diamond IT. Organization of the Visual Sector of the Thalamic Reticular Nucleus in Galago. Eur J Neurosci. 1990;2:211–226. doi: 10.1111/j.1460-9568.1990.tb00414.x. [DOI] [PubMed] [Google Scholar]
- 27.Conley M, Kupersmith AC, Diamond IT. The Organization of Projections from Subdivisions of the Auditory Cortex and Thalamus to the Auditory Sector of the Thalamic Reticular Nucleus in Galago. Eur J Neurosci. 1991;3:1089–1103. doi: 10.1111/j.1460-9568.1991.tb00044.x. [DOI] [PubMed] [Google Scholar]
- 28.Connors BW, Long MA. Electrical synapses in the mammalian brain. Annu Rev Neurosci. 2004;27:393–418. doi: 10.1146/annurev.neuro.26.041002.131128. [DOI] [PubMed] [Google Scholar]
- 29.Contreras D, Curro DR, Steriade M. Electrophysiological properties of cat reticular thalamic neurones in vivo. J Physiol. 1993;470:273–294. doi: 10.1113/jphysiol.1993.sp019858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Contreras D, Curro DR, Steriade M. Bursting and tonic discharges in two classes of reticular thalamic neurons. J Neurophysiol. 1992;68:973–977. doi: 10.1152/jn.1992.68.3.973. [DOI] [PubMed] [Google Scholar]
- 31.Cornwall J, Cooper JD, Phillipson OT. Projections to the rostral reticular thalamic nucleus in the rat. Exp Brain Res. 1990;80:157–171. doi: 10.1007/BF00228857. [DOI] [PubMed] [Google Scholar]
- 32.Crabtree JW. The Somatotopic Organization Within the Cat's Thalamic Reticular Nucleus. Eur J Neurosci. 1992;4:1352–1361. doi: 10.1111/j.1460-9568.1992.tb00160.x. [DOI] [PubMed] [Google Scholar]
- 33.Crabtree JW. The Somatotopic Organization Within the Rabbit's Thalamic Reticular Nucleus. Eur J Neurosci. 1992;4:1343–1351. doi: 10.1111/j.1460-9568.1992.tb00159.x. [DOI] [PubMed] [Google Scholar]
- 34.Crabtree JW. Organization in the somatosensory sector of the cat's thalamic reticular nucleus. J Comp Neurol. 1996;366:207–222. doi: 10.1002/(SICI)1096-9861(19960304)366:2<207::AID-CNE2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 35.Crabtree JW. Intrathalamic sensory connections mediated by the thalamic reticular nucleus. Cell Mol Life Sci. 1999;56:683–700. doi: 10.1007/s000180050462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crabtree JW. Organization in the auditory sector of the cat's thalamic reticular nucleus. J Comp Neurol. 1998;390:167–182. [PubMed] [Google Scholar]
- 37.Crabtree JW, Collingridge GL, Isaac JTR. A new intrathalamic pathway linking modalityrelated nuclei in the dorsal thalamus. Nat Neurosci. 1998;1:389–394. doi: 10.1038/1603. [DOI] [PubMed] [Google Scholar]
- 38.Crabtree JW, Killackey HP. The Topographic Organization and Axis of Projection within the Visual Sector of the Rabbit's Thalamic Reticular Nucleus. Eur J Neurosci. 1989;1:94–109. doi: 10.1111/j.1460-9568.1989.tb00777.x. [DOI] [PubMed] [Google Scholar]
- 39.Crick F. Function of the thalamic reticular complex: the searchlight hypothesis. Proc Natl Acad Sci U S A. 1984;81:4586–4590. doi: 10.1073/pnas.81.14.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cucchiaro JB, Uhlrich DJ, Sherman SM. Ultrastructure of synapses from the pretectum in the A-laminae of the cat's lateral geniculate nucleus. J Comp Neurol. 1993;334:618–630. doi: 10.1002/cne.903340409. [DOI] [PubMed] [Google Scholar]
- 41.de Venecia RK, Smelser CB, Lossman SD, McMullen NT. Complementary expression of parvalbumin and calbindin D-28k delineates subdivisions of the rabbit medial geniculate body. J Comp Neurol. 1995;359:595–612. doi: 10.1002/cne.903590407. [DOI] [PubMed] [Google Scholar]
- 42.Dermon CR, Barbas H. Contralateral thalamic projections predominantly reach transitional cortices in the rhesus monkey. J Comp Neurol. 1994;344:508–531. doi: 10.1002/cne.903440403. [DOI] [PubMed] [Google Scholar]
- 43.Deschenes M, Madariaga-Domich A, Steriade M. Dendrodendritic synapses in the cat reticularis thalami nucleus: a structural basis for thalamic spindle synchronization. Brain Res. 1985;334:165–168. doi: 10.1016/0006-8993(85)90580-3. [DOI] [PubMed] [Google Scholar]
- 44.Destexhe A. Modelling corticothalamic feedback and the gating of the thalamus by the cerebral cortex. J Physiol Paris. 2000;94:391–410. doi: 10.1016/s0928-4257(00)01093-7. [DOI] [PubMed] [Google Scholar]
- 45.Domich L, Oakson G, Steriade M. Thalamic burst patterns in the naturally sleeping cat: A comparison between cortically projecting and reticularis neurones. J Physiol. 1986;379:429–449. doi: 10.1113/jphysiol.1986.sp016262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feldman ML, Peters A. The forms of non-pyramidal neurons in the visual cortex of the rat. J Comp Neurol. 1978;179:761–793. doi: 10.1002/cne.901790406. [DOI] [PubMed] [Google Scholar]
- 47.Fuentealba P, Steriade M. The reticular nucleus revisited: Intrinsic and network properties of a thalamic pacemaker. Prog Neurobiol. 2005;75:125–141. doi: 10.1016/j.pneurobio.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 48.Funahashi S, Kubota K. Working memory and prefrontal cortex. Neurosci Res. 1994;21:1–11. doi: 10.1016/0168-0102(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 49.Funahashi S, Takeda K. Information processes in the primate prefrontal cortex in relation to working memory processes. Rev Neurosci. 2002;13:313–345. doi: 10.1515/revneuro.2002.13.4.313. [DOI] [PubMed] [Google Scholar]
- 50.Fuster JM. Network memory. Trends Neurosci. 1997;20:451–459. doi: 10.1016/s0166-2236(97)01128-4. [DOI] [PubMed] [Google Scholar]
- 51.Fuster JM. Frontal lobes. Curr Opin Neurobiol. 1993;3:160–165. doi: 10.1016/0959-4388(93)90204-c. [DOI] [PubMed] [Google Scholar]
- 52.Fuster JM. Executive frontal functions. Exp Brain Res. 2000;133:66–70. doi: 10.1007/s002210000401. [DOI] [PubMed] [Google Scholar]
- 53.Fuster JM, Alexander GE. Firing changes in cells of the nucleus medialis dorsalis associated with delayed response behavior. Brain Res. 1973;61:79–91. doi: 10.1016/0006-8993(73)90517-9. [DOI] [PubMed] [Google Scholar]
- 54.Fuster JM, Bauer RH, Jervey JP. Effects of cooling inferotemporal cortex on performance of visual memory tasks. Exp Neurol. 1981;71:398–409. doi: 10.1016/0014-4886(81)90098-4. [DOI] [PubMed] [Google Scholar]
- 55.Garcia-Cabezas MA, Rico B, Sanchez-Gonzalez MA, Cavada C. Distribution of the dopamine innervation in the macaque and human thalamus. Neuroimage. 2006 doi: 10.1016/j.neuroimage.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 56.Germuska M, Saha S, Fiala J, Barbas H. Synaptic distinction of laminar specific prefrontal-temporal pathways in primates. Cereb Cortex. 2006;16:865–875. doi: 10.1093/cercor/bhj030. [DOI] [PubMed] [Google Scholar]
- 57.Germuska M, Saha S, Fiala JC, Barbas H. Synaptic distinction of laminar specific prefrontal-temporal pathways in primates. Cereb Cortex. 2005 doi: 10.1093/cercor/bhj030. [DOI] [PubMed] [Google Scholar]
- 58.Ghashghaei HT, Barbas H. Neural interaction between the basal forebrain and functionally distinct prefrontal cortices in the rhesus monkey. Neuroscience. 2001;103:593–614. doi: 10.1016/s0306-4522(00)00585-6. [DOI] [PubMed] [Google Scholar]
- 59.Ghashghaei HT, Barbas H. Pathways for emotions: Interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002;115:1261–1279. doi: 10.1016/s0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- 60.Ghashghaei HT, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2006 doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gold JM, Weinberger DR. Cognitive deficits and the neurobiology of schizophrenia. Curr Opin Neurobiol. 1995;5:225–230. doi: 10.1016/0959-4388(95)80030-1. [DOI] [PubMed] [Google Scholar]
- 62.Goldman-Rakic PS. Regional and cellular fractionation of working memory. Proc Natl Acad Sci U S A. 1996;93:13473–13480. doi: 10.1073/pnas.93.24.13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goldman-Rakic PS, Selemon LD. Functional and anatomical aspects of prefrontal pathology in Schizophrenia. Schizophr Bull. 1997;23:437–458. doi: 10.1093/schbul/23.3.437. [DOI] [PubMed] [Google Scholar]
- 64.Golshani P, Liu XB, Jones EG. Differences in quantal amplitude reflect GluR4- subunit number at corticothalamic synapses on two populations of thalamic neurons. Proc Natl Acad Sci U S A. 2001;98:4172–4177. doi: 10.1073/pnas.061013698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gonzalo-Ruiz A, Lieberman AR. Topographic organization of projections from the thalamic reticular nucleus to the anterior thalamic nuclei in the rat. Brain Res Bull. 1995;37:17–35. doi: 10.1016/0361-9230(94)00252-5. [DOI] [PubMed] [Google Scholar]
- 66.Gross CG. How inferior temporal cortex became a visual area. Cereb Cortex. 1994;5:455–469. doi: 10.1093/cercor/4.5.455. [DOI] [PubMed] [Google Scholar]
- 67.Guillery RW. Anatomical pathways that link perception and action. Cortical Function: A View from the Thalamus. 2005;149:235–256. doi: 10.1016/S0079-6123(05)49017-2. [DOI] [PubMed] [Google Scholar]
- 68.Guillery RW. Anatomical evidence concerning the role of the thalamus in corticocortical communication: a brief review. J Anat. 1995;187(Pt 3):583–592. [PMC free article] [PubMed] [Google Scholar]
- 69.Guillery RW, Feig SL, Lozsadi DA. Paying attention to the thalamic reticular nucleus. Trends Neurosci. 1998;21:28–32. doi: 10.1016/s0166-2236(97)01157-0. [DOI] [PubMed] [Google Scholar]
- 70.Guillery RW, Harting JK. Structure and connections of the thalamic reticular nucleus: Advancing views over half a century. J Comp Neurol. 2003;463:360–371. doi: 10.1002/cne.10738. [DOI] [PubMed] [Google Scholar]
- 71.Guillery RW, Sherman SM. The thalamus as a monitor of motor outputs. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences. 2002;357:1809–1821. doi: 10.1098/rstb.2002.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Guillery RW, Sherman SM. Thalamic relay functions and their role in corticocortical communication: generalizations from the visual system. Neuron. 2002;33:163–175. doi: 10.1016/s0896-6273(01)00582-7. [DOI] [PubMed] [Google Scholar]
- 73.Harris RM, Hendrickson AE. Local circuit neurons in the rat ventrobasal thalamus--a GABA immunocytochemical study. Neuroscience. 1987;21:229–236. doi: 10.1016/0306-4522(87)90335-6. [DOI] [PubMed] [Google Scholar]
- 74.Hartings JA, Temereanca S, Simons DJ. State-dependent processing of sensory stimuli by thalamic reticular neurons. J Neurosci. 2003;23:5264–5271. doi: 10.1523/JNEUROSCI.23-12-05264.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hasselmo ME. Neuromodulation: acetylcholine and memory consolidation. Trends Cogn Sci. 1999;3:351–359. doi: 10.1016/s1364-6613(99)01365-0. [DOI] [PubMed] [Google Scholar]
- 76.Heckers S, Geula C, Mesulam MM. Cholinergic innervation of the human thalamus: dual origin and differential nuclear distribution. J Comp Neurol. 1992;325:68–82. doi: 10.1002/cne.903250107. [DOI] [PubMed] [Google Scholar]
- 77.Heizmann CW. Calcium-binding proteins: Basic concepts and clinical implications. Gen Physiol Biophys. 1992;11:411–425. [PubMed] [Google Scholar]
- 78.Heizmann CW, Braun K. Changes in Ca(2+)-binding proteins in human neurodegenerative disorders. Trends Neurosci. 1992;15:259–264. doi: 10.1016/0166-2236(92)90067-i. [DOI] [PubMed] [Google Scholar]
- 79.Hilgetag CC, Barbas H. Role of mechanical factors in the morphology of the primate cerebral cortex. PLoS Comput Biol. 2006;2:e22. doi: 10.1371/journal.pcbi.0020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hirata A, Aguilar J, Castro-Alamancos MA. Noradrenergic activation amplifies bottom-up and top-down signal-to-noise ratios in sensory thalamus. J Neurosci. 2006;26:4426–4436. doi: 10.1523/JNEUROSCI.5298-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huguenard JR. Low-threshold calcium currents in central nervous system neurons. Annu Rev Physiol. 1996;58:329–348. doi: 10.1146/annurev.ph.58.030196.001553. [DOI] [PubMed] [Google Scholar]
- 82.Huntsman MM, Porcello DM, Homanics GE, DeLorey TM, Huguenard JR. Reciprocal inhibitory connections and network synchrony in the mammalian thalamus. Science. 1999;283:541–543. doi: 10.1126/science.283.5401.541. [DOI] [PubMed] [Google Scholar]
- 83.Ilinsky IA, Ambardekar AV, Kultas-Ilinsky K. Organization of projections from the anterior pole of the nucleus reticularis thalami (NRT) to subdivisions of the motor thalamus: light and electron microscopic studies in the rhesus monkey. J Comp Neurol. 1999;409:369–384. [PubMed] [Google Scholar]
- 84.Jones EG, editor. The Thalamus. New York (NY): Plenum Press; 1985. [Google Scholar]
- 85.Jones EG. The thalamic matrix and thalamocortical synchrony. Trends Neurosci. 2001;24:595–601. doi: 10.1016/s0166-2236(00)01922-6. [DOI] [PubMed] [Google Scholar]
- 86.Jones EG. A new view of specific and nonspecific thalamocortical connections. Adv Neurol. 1998;77:49–71. [PubMed] [Google Scholar]
- 87.Jones EG. Viewpoint: the core and matrix of thalamic organization. Neuroscience. 1998;85:331–345. doi: 10.1016/s0306-4522(97)00581-2. [DOI] [PubMed] [Google Scholar]
- 88.Jones EG, Hendry SHC. Differential calcium binding protein immunoreactivity distinguishes classes of relay neurons in monkey thalamic nuclei. Eur J Neurosci. 1989;1:222–246. doi: 10.1111/j.1460-9568.1989.tb00791.x. [DOI] [PubMed] [Google Scholar]
- 89.Kakei S, Na J, Shinoda Y. Thalamic terminal morphology and distribution of single corticothalamic axons originating from layers 5 and 6 of the cat motor cortex. J Comp Neurol. 2001;437:170–185. doi: 10.1002/cne.1277. [DOI] [PubMed] [Google Scholar]
- 90.Kayama Y, Negi T, Sugitani M, Iwama K. Effects of locus coeruleus stimulation on neuronal activities of dorsal lateral geniculate nucleus and perigeniculate reticular nucleus of the rat. Neuroscience. 1982;7:655–666. doi: 10.1016/0306-4522(82)90071-9. [DOI] [PubMed] [Google Scholar]
- 91.Kolmac CI, Mitrofanis J. Organisation of the reticular thalamic projection to the intralaminar and midline nuclei in rats. J Comp Neurol. 1997;377:165–178. doi: 10.1002/(sici)1096-9861(19970113)377:2<165::aid-cne2>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 92.Kolmac CI, Mitrofanis J. Patterns of brainstem projection to the thalamic reticular nucleus. J Comp Neurol. 1998;396:531–543. [PubMed] [Google Scholar]
- 93.Kultas-Ilinsky K, Yi H, Ilinsky IA. Nucleus reticularis thalami input to the anterior thalamic nuclei in the monkey: a light and electron microscopic study. Neurosci Lett. 1995;186:25–28. doi: 10.1016/0304-3940(95)11273-y. [DOI] [PubMed] [Google Scholar]
- 94.Landisman CE, Connors BW. Long-term modulation of electrical synapses in the mammalian thalamus. Science. 2005;310:1809–1813. doi: 10.1126/science.1114655. [DOI] [PubMed] [Google Scholar]
- 95.Landisman CE, Long MA, Beierlein M, Deans MR, Paul DL, Connors BW. Electrical synapses in the thalamic reticular nucleus. J Neurosci. 2002;22:1002–1009. doi: 10.1523/JNEUROSCI.22-03-01002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Larkum ME, Senn W, Luscher HR. Top-down dendritic input increases the gain of layer 5 pyramidal neurons. Cereb Cortex. 2004;14:1059–1070. doi: 10.1093/cercor/bhh065. [DOI] [PubMed] [Google Scholar]
- 97.Lewis DA, Cruz DA, Melchitzky DS, Pierri JN. Lamina-specific deficits in parvalbumin-immunoreactive varicosities in the prefrontal cortex of subjects with schizophrenia: evidence for fewer projections from the thalamus. Am J Psychiatry. 2001;158:1411–1422. doi: 10.1176/appi.ajp.158.9.1411. [DOI] [PubMed] [Google Scholar]
- 98.Liu XB, Jones EG. Predominance of corticothalamic synaptic inputs to thalamic reticular nucleus neurons in the rat. J Comp Neurol. 1999;414:67–79. [PubMed] [Google Scholar]
- 99.Liu XB, Warren RA, Jones EG. Synaptic distribution of afferents from reticular nucleus in ventroposterior nucleus of cat thalamus. J Comp Neurol. 1995;352:187–202X. doi: 10.1002/cne.903520203. [DOI] [PubMed] [Google Scholar]
- 100.Llinas RR. The intrinsic electrophysiological properties of mammalian neurons: insights into central nervous system function. Science. 1988;242:1654–1664. doi: 10.1126/science.3059497. [DOI] [PubMed] [Google Scholar]
- 101.Lozsadi DA. Organization of cortical afferents to the rostral, limbic sector of the rat thalamic reticular nucleus. J Comp Neurol. 1994;341:520–533. doi: 10.1002/cne.903410408. [DOI] [PubMed] [Google Scholar]
- 102.Lozsadi DA. Organization of connections between the thalamic reticular and the anterior thalamic nuclei in the rat. J Comp Neurol. 1995;358:233–246. doi: 10.1002/cne.903580206. [DOI] [PubMed] [Google Scholar]
- 103.Lubke J. Morphology of neurons in the thalamic reticular nucleus (TRN) of mammals as revealed by intracellular injections into fixed brain slices. J Comp Neurol. 1993;329:458–471. doi: 10.1002/cne.903290404. [DOI] [PubMed] [Google Scholar]
- 104.Lund JS, Lund RD, Hendrickson AE, Hunt AB, Fuchs AF. The origin of efferent pathways from the primary visual cortex, area 17, of the macaque monkey as shown by retrograde transport of horseradish peroxidase. J Comp Neurol. 1976;164:287–304. doi: 10.1002/cne.901640303. [DOI] [PubMed] [Google Scholar]
- 105.Marini G, Ceccarelli P, Mancia M. Thalamocortical dysrhythmia and the thalamic reticular nucleus in behaving rats. Clin Neurophysiol. 2002;113:1152–1164. doi: 10.1016/s1388-2457(02)00111-6. [DOI] [PubMed] [Google Scholar]
- 106.Matelli M, Camarda R, Glickstein M, Rizzolatti G. Afferent and efferent projections of the inferior area 6 in the Macaque monkey. J Comp Neurol. 1986;251:281–298. doi: 10.1002/cne.902510302. [DOI] [PubMed] [Google Scholar]
- 107.McAlonan K, Brown VJ. The thalamic reticular nucleus: more than a sensory nucleus? Neuroscientist. 2002;8:302–305. doi: 10.1177/107385840200800405. [DOI] [PubMed] [Google Scholar]
- 108.McAlonan K, Cavanaugh J, Wurtz RH. Attentional modulation of thalamic reticular neurons. J Neurosci. 2006;26:4444–4450. doi: 10.1523/JNEUROSCI.5602-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.McCormick DA. Neurotransmitter actions in the thalamus and cerebral cortex and their role in neuromodulation of thalamocortical activity. Prog Neurobiol. 1992;39:337–388. doi: 10.1016/0301-0082(92)90012-4. [DOI] [PubMed] [Google Scholar]
- 110.McCormick DA. Cholinergic and noradrenergic modulation of thalamocortical processing. Trends Neurosci. 1989;12:215–221. doi: 10.1016/0166-2236(89)90125-2. [DOI] [PubMed] [Google Scholar]
- 111.McCormick DA, Pape HC, Williamson A. Actions of norepinephrine in the cerebral cortex and thalamus: implications for function of the central noradrenergic system. Prog Brain Res. 1991;88:293–305. doi: 10.1016/s0079-6123(08)63817-0. [DOI] [PubMed] [Google Scholar]
- 112.McCormick DA, Prince DA. Acetylcholine induces burst firing in thalamic reticular neurones by activating a potassium conductance. Nature. 1986;319:402–405. doi: 10.1038/319402a0. [DOI] [PubMed] [Google Scholar]
- 113.McFarland NR, Haber SN. Thalamic relay nuclei of the basal ganglia form both reciprocal and nonreciprocal cortical connections, linking multiple frontal cortical areas. J Neurosci. 2002;22:8117–8132. doi: 10.1523/JNEUROSCI.22-18-08117.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Montero VM. Amblyopia decreases activation of the corticogeniculate pathway and visual thalamic reticularis in attentive rats: a ‘focal attention’ hypothesis. Neuroscience. 1999;91:805–817. doi: 10.1016/s0306-4522(98)00632-0. [DOI] [PubMed] [Google Scholar]
- 115.Montero VM. Attentional activation of the visual thalamic reticular nucleus depends on ‘top-down’ inputs from the primary visual cortex via corticogeniculate pathways. Brain Res. 2000;864:95–104. doi: 10.1016/s0006-8993(00)02182-x. [DOI] [PubMed] [Google Scholar]
- 116.Montero VM. c-fos induction in sensory pathways of rats exploring a novel complex environment: shifts of active thalamic reticular sectors by predominant sensory cues. Neuroscience. 1997;76:1069–1081. doi: 10.1016/s0306-4522(96)00417-4. [DOI] [PubMed] [Google Scholar]
- 117.Montero VM, Zempel J. The proportion and size of GABA-immunoreactive neurons in the magnocellular and parvocellular layers of the lateral geniculate nucleus of the rhesus monkey. Exp Brain Res. 1986;62:215–223. doi: 10.1007/BF00237420. [DOI] [PubMed] [Google Scholar]
- 118.Morrison JH, Foote SL. Noradrenergic and serotoninergic innervation of cortical, thalamic, and tectal visual structures in Old and New World monkeys. J Comp Neurol. 1986;243:117–138. doi: 10.1002/cne.902430110. [DOI] [PubMed] [Google Scholar]
- 119.Mukhametov LM, Rizzolatti G, Tradardi V. Spontaneous activity of neurones of nucleus reticularis thalami in freely moving cats. J Physiol. 1970;210:651–667. doi: 10.1113/jphysiol.1970.sp009233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Munoz A, Huntsman MM, Jones EG. GABA(B) receptor gene expression in monkey thalamus. J Comp Neurol. 1998;394:118–126. doi: 10.1002/(sici)1096-9861(19980427)394:1<118::aid-cne9>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 121.Murthy VN, Sejnowski TJ, Stevens CF. Heterogeneous release properties of visualized individual hippocampal synapses. Neuron. 1997;18:599–612. doi: 10.1016/s0896-6273(00)80301-3. [DOI] [PubMed] [Google Scholar]
- 122.Nakatani T. The degree of GABA immunoreactivity is related to neuronal size in the thalamic reticular nucleus of the Japanese monkey, Macaca fuscata. Kaibogaku Zasshi. 1993;68:180–189. [PubMed] [Google Scholar]
- 123.Ohara PT. Synaptic organization of the thalamic reticular nucleus. J Electron Microsc Tech. 1988;10:283–292. doi: 10.1002/jemt.1060100306. [DOI] [PubMed] [Google Scholar]
- 124.Ohara PT, Havton LA. Dendritic arbors of neurons from different regions of the rat thalamic reticular nucleus share a similar orientation. Brain Res. 1996;731:236–240. doi: 10.1016/0006-8993(96)00706-8. [DOI] [PubMed] [Google Scholar]
- 125.Ohara PT, Lieberman AR. Some aspects of the synaptic circuitry underlying inhibition in the ventrobasal thalamus. J Neurocytol. 1993;22:815–25X. doi: 10.1007/BF01181326. [DOI] [PubMed] [Google Scholar]
- 126.Ohara PT, Lieberman AR, Hunt SP, Wu JY. Neural elements containing glutamic acid decarboxylase (GAD) in the dorsal lateral geniculate nucleus of the rat; immunohistochemical studies by light and electron microscopy. Neuroscience. 1983;8:189–211. doi: 10.1016/0306-4522(83)90060-x. [DOI] [PubMed] [Google Scholar]
- 127.Pare D, Hazrati LN, Parent A, Steriade M. Substantia nigra pars reticulata projects to the reticular thalamic nucleus of the cat: a morphological and electrophysiological study. Brain Res. 1990;535:139–146. doi: 10.1016/0006-8993(90)91832-2. [DOI] [PubMed] [Google Scholar]
- 128.Pare D, Smith Y, Parent A, Steriade M. Projections of brainstem core cholinergic and non-cholinergic neurons of cat to intralaminar and reticular thalamic nuclei. Neuroscience. 1988;25:69–86. doi: 10.1016/0306-4522(88)90007-3. [DOI] [PubMed] [Google Scholar]
- 129.Parent A, Pare D, Smith Y, Steriade M. Basal forebrain cholinergic and noncholinergic projections to the thalamus and brainstem in cats and monkeys. J Comp Neurol. 1988;277:281–301. doi: 10.1002/cne.902770209. [DOI] [PubMed] [Google Scholar]
- 130.Pierce JP, Lewin GR. An ultrastructural size principle. Neuroscience. 1994;58:441–446. doi: 10.1016/0306-4522(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 131.Pinault D. The thalamic reticular nucleus: structure, function and concept. Brain Res Brain Res Rev. 2004;46:1–31. doi: 10.1016/j.brainresrev.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 132.Pinault D, Deschenes M. Muscarinic inhibition of reticular thalamic cells by basal forebrain neurones. NeuroReport. 1992;3:1101–1104. doi: 10.1097/00001756-199212000-00017. [DOI] [PubMed] [Google Scholar]
- 133.Pinault D, Deschenes M. Anatomical evidence for a mechanism of lateral inhibition in the rat thalamus. Eur J Neurosci. 1998;10:3462–3469. doi: 10.1046/j.1460-9568.1998.00362.x. [DOI] [PubMed] [Google Scholar]
- 134.Raczkowski D, Fitzpatrick D. Organization of cholinergic synapses in the cat's dorsal lateral geniculate and perigeniculate nuclei. J Comp Neurol. 1989;288:676–690. doi: 10.1002/cne.902880412. [DOI] [PubMed] [Google Scholar]
- 135.Rajkowska G, Selemon LD, Goldman-Rakic PS. Neuronal and glial somal size in the prefrontal cortex: a postmortem morphometric study of schizophrenia and Huntington disease. Arch Gen Psychiatry. 1998;55:215–224. doi: 10.1001/archpsyc.55.3.215. [DOI] [PubMed] [Google Scholar]
- 136.Rempel-Clower NL, Barbas H. Topographic organization of connections between the hypothalamus and prefrontal cortex in the rhesus monkey. J Comp Neurol. 1998;398:393–419. doi: 10.1002/(sici)1096-9861(19980831)398:3<393::aid-cne7>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 137.Reynolds GP, Zhang ZJ, Beasley CL. Neurochemical correlates of cortical GABAergic deficits in schizophrenia: selective losses of calcium binding protein immunoreactivity. Brain Res Bull. 2001;55:579–584. doi: 10.1016/s0361-9230(01)00526-3. [DOI] [PubMed] [Google Scholar]
- 138.Rockland KS. Two types of corticopulvinar terminations: round (type 2) and elongate (type 1) J Comp Neurol. 1996;368:57–87. doi: 10.1002/(SICI)1096-9861(19960422)368:1<57::AID-CNE5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 139.Rockland KS, Andresen J, Cowie RJ, Robinson DL. Single axon analysis of pulvinocortical connections to several visual areas in the macaque. J Comp Neurol. 1999;406:221–250. doi: 10.1002/(sici)1096-9861(19990405)406:2<221::aid-cne7>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 140.Rose JE. The ontogenic development of the rabbit's diencephalon. J Comp Neurol. 1942;77:61–129. [Google Scholar]
- 141.Rosenmund C, Stevens CF. Definition of the readily releasable pool of vesicles at hippocampal synapses. Neuron. 1996;16:1197–1207. doi: 10.1016/s0896-6273(00)80146-4. [DOI] [PubMed] [Google Scholar]
- 142.Rouiller EM, Durif C. The dual pattern of corticothalamic projection of the primary auditory cortex in macaque monkey. Neurosci Lett. 2004;358:49–52. doi: 10.1016/j.neulet.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 143.Rouiller EM, Welker E. Morphology of corticothalamic terminals arising from the auditory cortex of the rat: a Phaseolus vulgaris-leucoagglutinin (PHA-L) tracing study. Hear Res. 1991;56:179–190. doi: 10.1016/0378-5955(91)90168-9. [DOI] [PubMed] [Google Scholar]
- 144.Rouiller EM, Welker E. A comparative analysis of the morphology of corticothalamic projections in mammals. Brain Res Bull. 2000;53:727–741. doi: 10.1016/s0361-9230(00)00364-6. [DOI] [PubMed] [Google Scholar]
- 145.Sanchez-Gonzalez MA, Garcia-Cabezas MA, Rico B, Cavada C. The primate thalamus is a key target for brain dopamine. J Neurosci. 2005;25:6076–6083. doi: 10.1523/JNEUROSCI.0968-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sanchez-Vives MV, Bal T, McCormick DA. Inhibitory interactions between perigeniculate GABAergic neurons. J Neurosci. 1997;17:8894–8908. doi: 10.1523/JNEUROSCI.17-22-08894.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Selemon LD, Rajkowska G, Goldman-Rakic PS. Elevated neuronal density in prefrontal area 46 in brains from schizophrenic patients: application of a three-dimensional, stereologic counting method. J Comp Neurol. 1998;392:402–412. [PubMed] [Google Scholar]
- 148.Shepherd GM, Harris KM. Three-dimensional structure and composition of CA3-->CA1 axons in rat hippocampal slices: implications for presynaptic connectivity and compartmentalization. J Neurosci. 1998;18:8300–8310. doi: 10.1523/JNEUROSCI.18-20-08300.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sherman SM. A wake-up call from the thalamus. Nat Neurosci. 2001;4:344–346. doi: 10.1038/85973. [DOI] [PubMed] [Google Scholar]
- 150.Sherman SM. Thalamic relays and cortical functioning. Prog Brain Res. 2005;149:107–126. doi: 10.1016/S0079-6123(05)49009-3. [DOI] [PubMed] [Google Scholar]
- 151.Sherman SM, Guillery RW. The role of the thalamus in the flow of information to the cortex. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences. 2002;357:1695–1708. doi: 10.1098/rstb.2002.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sherman SM, Guillery RW. Functional organization of thalamocortical relays. J Neurophysiol. 1996;76:1367–1395. doi: 10.1152/jn.1996.76.3.1367. [DOI] [PubMed] [Google Scholar]
- 153.Sherman SM, Guillery RW. On the actions that one nerve cell can have on another: distinguishing “drivers” from “modulators”. Proc Natl Acad Sci U S A. 1998;95:7121–7126. doi: 10.1073/pnas.95.12.7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Skinner JE, Yingling CD. Regulation of slow potential shifts in nucleus reticularis thalami by the mesencephalic reticular formation and the frontal granular cortex. Electroencephalogr Clin Neurophysiol. 1976;40:288–296. doi: 10.1016/0013-4694(76)90152-8. [DOI] [PubMed] [Google Scholar]
- 155.Sohal VS, Huntsman MM, Huguenard JR. Reciprocal inhibitory connections regulate the spatiotemporal properties of intrathalamic oscillations. J Neurosci. 2000;20:1735–1745. doi: 10.1523/JNEUROSCI.20-05-01735.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Spacek J, Lieberman AR. Ultrastructure and three-dimensional organization of synaptic glomeruli in rat somatosensory thalamus. J Anat. 1974;117:487–516. [PMC free article] [PubMed] [Google Scholar]
- 157.Spreafico R, de Curtis M, Frassoni C, Avanzini G. Electrophysiological characteristics of morphologically identified reticular thalamic neurons from rat slices. Neuroscience. 1988;27:629–638. doi: 10.1016/0306-4522(88)90294-1. [DOI] [PubMed] [Google Scholar]
- 158.Steriade M. Local gating of information processing through the thalamus. Neuron. 2004;41:493–494. doi: 10.1016/s0896-6273(04)00076-5. [DOI] [PubMed] [Google Scholar]
- 159.Steriade M, Domich L, Oakson G. Reticularis thalami neurons revisited: activity changes during shifts in states of vigilance. J Neurosci. 1986;6:68–81. doi: 10.1523/JNEUROSCI.06-01-00068.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Steriade M, Jones EG, McCormick DA, editors. Thalamus - Organisation and function. Vol. 1. Oxford: Elsevier Science; 1997. [Google Scholar]
- 161.Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- 162.Steriade M, Parent A, Pare D, Smith Y. Cholinergic and non-cholinergic neurons of cat basal forebrain project to reticular and mediodorsal thalamic nuclei. Brain Res. 1987;408:372–376. doi: 10.1016/0006-8993(87)90408-2. [DOI] [PubMed] [Google Scholar]
- 163.Suffczynski P, Kalitzin S, Pfurtscheller G, Lopes da Silva FH. Computational model of thalamo-cortical networks: dynamical control of alpha rhythms in relation to focal attention. Int J Psychophysiol. 2001;43:25–40. doi: 10.1016/s0167-8760(01)00177-5. [DOI] [PubMed] [Google Scholar]
- 164.Tai Y, Yi H, Ilinsky IA, Kultas-Ilinsky K. Nucleus reticularis thalami connections with the mediodorsal thalamic nucleus: a light and electron microscopic study in the monkey. Brain Res Bull. 1995;38:475–488. doi: 10.1016/0361-9230(95)02018-m. [DOI] [PubMed] [Google Scholar]
- 165.Thomson AM. Molecular frequency filters at central synapses. Prog Neurobiol. 2000;62:159–196. doi: 10.1016/s0301-0082(00)00008-3. [DOI] [PubMed] [Google Scholar]
- 166.Uhlrich DJ, Manning KA, Feig SL. Laminar and cellular targets of individual thalamic reticular nucleus axons in the lateral geniculate nucleus in the prosimian primate Galago. J Comp Neurol. 2003;458:128–143. doi: 10.1002/cne.10568. [DOI] [PubMed] [Google Scholar]
- 167.Ulfig N, Nickel J, Bohl J. Transient features of the thalamic reticular nucleus in the human foetal brain. Eur J Neurosci. 1998;10:3773–3784. doi: 10.1046/j.1460-9568.1998.00390.x. [DOI] [PubMed] [Google Scholar]
- 168.Ulrich D, Huguenard JR. GABAB receptor-mediated responses in GABAergic projection neurones of rat nucleus reticularis thalami in vitro. J Physiol. 1996;493(Pt 3):845–854. doi: 10.1113/jphysiol.1996.sp021427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Volk DW, Lewis DA. Impaired prefrontal inhibition in schizophrenia: relevance for cognitive dysfunction. Physiol Behav. 2002;77:501–505. doi: 10.1016/s0031-9384(02)00936-8. [DOI] [PubMed] [Google Scholar]
- 170.Vuilleumier P, Hester D, Assal G, Regli F. Unilateral spatial neglect recovery after sequential strokes. Neurology. 1996;46:184–189. doi: 10.1212/wnl.46.1.184. [DOI] [PubMed] [Google Scholar]
- 171.Wang S, Bickford ME, Van Horn SC, Erisir A, Godwin DW, Sherman SM. Synaptic targets of thalamic reticular nucleus terminals in the visual thalamus of the cat. J Comp Neurol. 2001;440:321–341. doi: 10.1002/cne.1389. [DOI] [PubMed] [Google Scholar]
- 172.Webster MJ, Rowe MH. Morphology of identified relay cells and interneurons in the dorsal lateral geniculate nucleus of the rat. Exp Brain Res. 1984;56:468–474. doi: 10.1007/BF00237987. [DOI] [PubMed] [Google Scholar]
- 173.Weddell RA. Subcortical modulation of spatial attention including evidence that the Sprague effect extends to man. Brain Cogn. 2004;55:497–506. doi: 10.1016/j.bandc.2004.02.075. [DOI] [PubMed] [Google Scholar]
- 174.Weese GD, Phillips JM, Brown VJ. Attentional orienting is impaired by unilateral lesions of the thalamic reticular nucleus in the rat. J Neurosci. 1999;19:10135–10139. doi: 10.1523/JNEUROSCI.19-22-10135.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Williamson AM, Ohara PT, Ralston DD, Milroy AM, Ralston HJ. Analysis of gamma-aminobutyric acidergic synaptic contacts in the thalamic reticular nucleus of the monkey. J Comp Neurol. 1994;349:182–192. doi: 10.1002/cne.903490203. [DOI] [PubMed] [Google Scholar]
- 176.Williamson AM, Ohara PT, Ralston HJ. Electron microscopic evidence that cortical terminals make direct contact onto cells of the thalamic reticular nucleus in the monkey. Brain Res. 1993;631:175–179. doi: 10.1016/0006-8993(93)91207-9. [DOI] [PubMed] [Google Scholar]
- 177.Winterer G, Weinberger DR. Genes, dopamine and cortical signal-to-noise ratio in schizophrenia. Trends Neurosci. 2004;27:683–690. doi: 10.1016/j.tins.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 178.Xiao D, Barbas H. Pathways for emotions and memory II. afferent input to the anterior thalamic nuclei from prefrontal, temporal, hypothalamic areas and the basal ganglia in the rhesus monkey. Thalamus and Related Systems. 2002;2:33–48. [Google Scholar]
- 179.Xiao D, Barbas H. Circuits through prefrontal cortex, basal ganglia, and ventral anterior nucleus map pathways beyond motor control. Thalamus & Related Systems. 2004;2:325–343. [Google Scholar]
- 180.Yen CT, Conley M, Hendry SH, Jones EG. The morphology of physiologically identified GABAergic neurons in the somatic sensory part of the thalamic reticular nucleus in the cat. J Neurosci. 1985;5:2254–2268. doi: 10.1523/JNEUROSCI.05-08-02254.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yingling CD, Skinner JE. Regulation of unit activity in nucleus reticularis thalami by the mesencephalic reticular formation and the frontal granular cortex. Electroencephalogr Clin Neurophysiol. 1975;39:635–642. doi: 10.1016/0013-4694(75)90076-0. [DOI] [PubMed] [Google Scholar]
- 182.Yukie M, editor. The association cortex, structure, and function. Amsterdam: Hardwood Academic; 1997. [Google Scholar]
- 183.Zhang L, Jones EG. Corticothalamic inhibition in the thalamic reticular nucleus. J Neurophysiol. 2004;91:759–766. doi: 10.1152/jn.00624.2003. [DOI] [PubMed] [Google Scholar]
- 184.Zikopoulos B, Barbas H. Prefrontal projections to the thalamic reticular nucleus form a unique circuit for attentional mechanisms. J Neurosci. 2006;26:7348–7361. doi: 10.1523/JNEUROSCI.5511-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]