Abstract
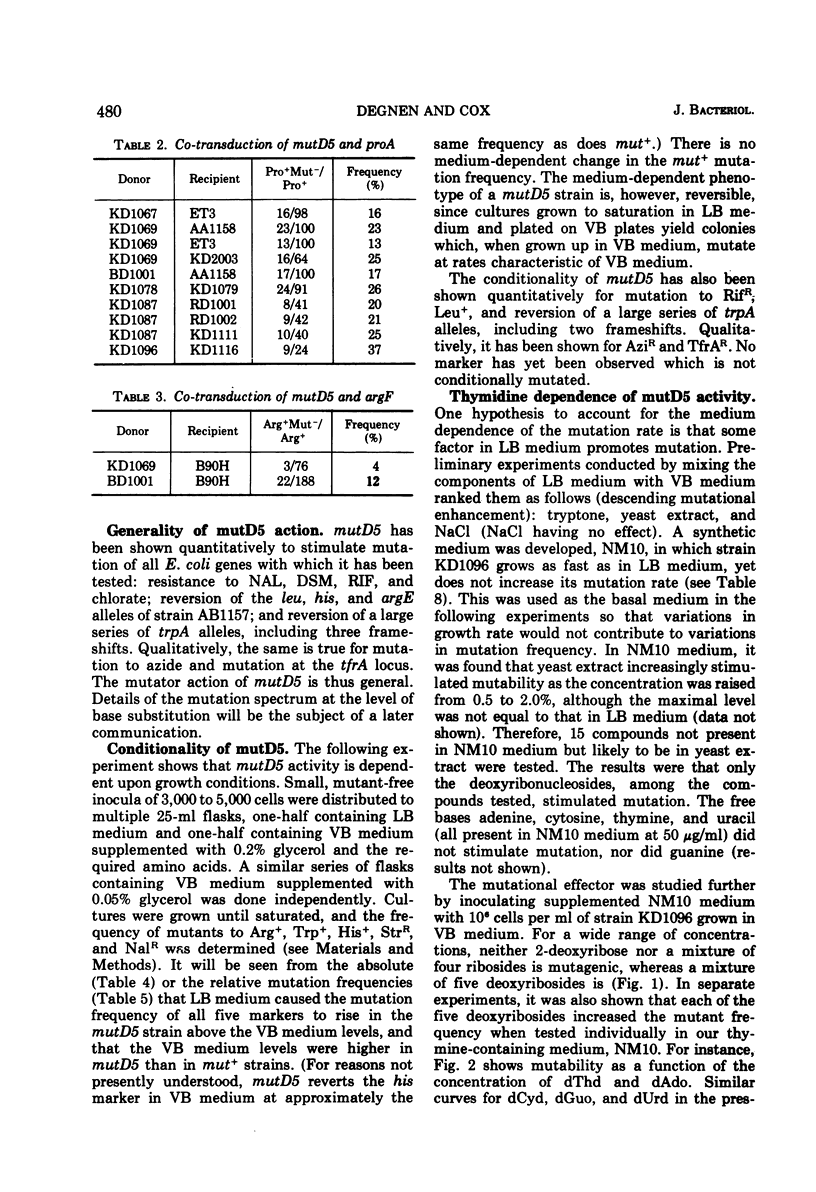
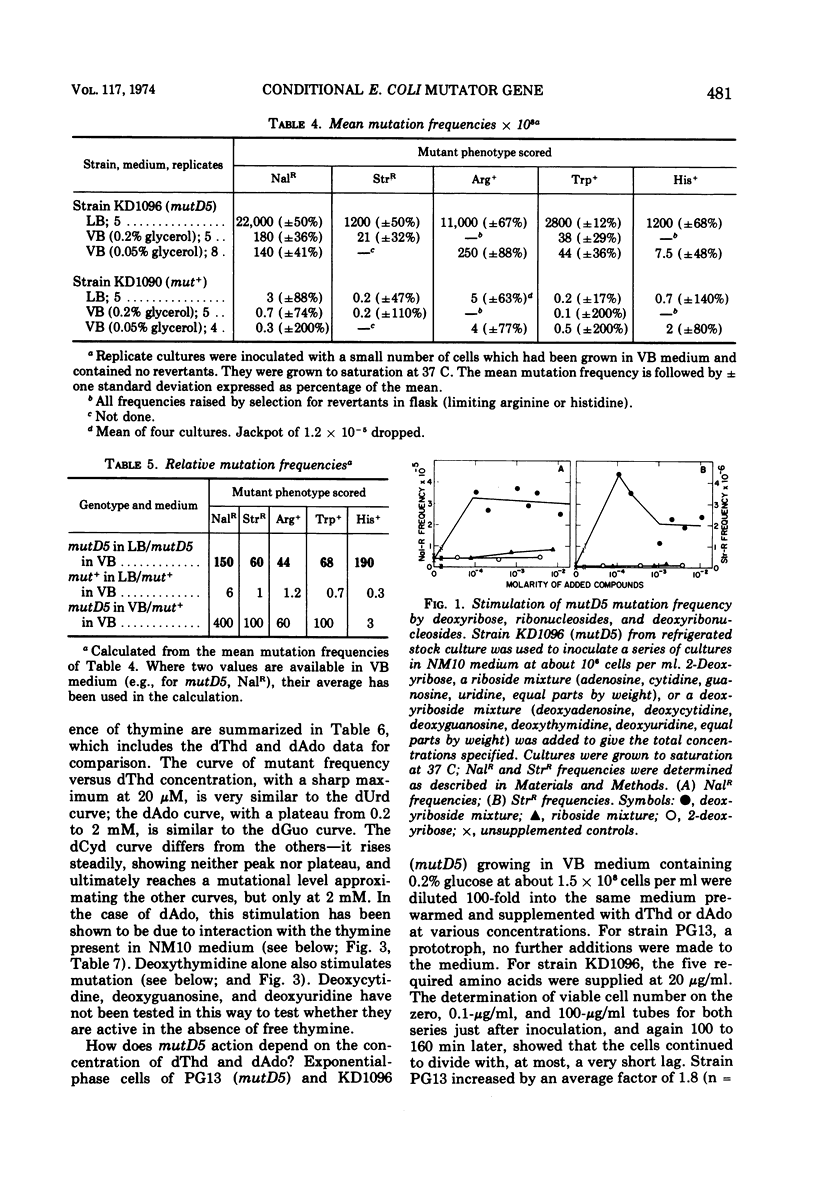
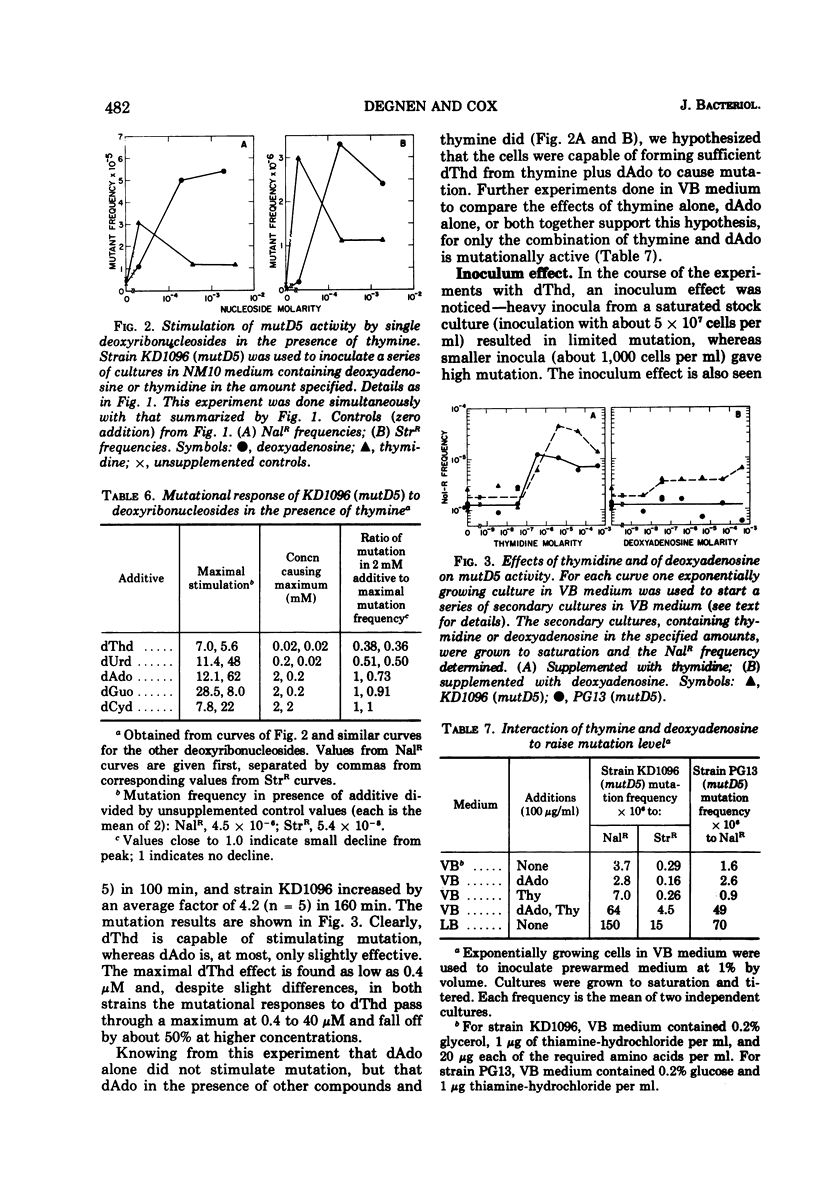
A mutator gene, mutD5, whose phenotype is conditional, has been identified in Escherichia coli. By P1 transduction it has been shown to lie at about 5.7 min on the chromosome, being co-transduced with proA and argF. In rich medium, streptomycin- and nalidixic acid-resistant mutation frequencies are 50 to 100 times higher than those in minimal medium. In minimal medium, the mutD5-induced mutation frequencies are still 50 to 100 times above co-isogenic wild-type (mut+) levels. Similar results were obtained with all markers tested. Mutant frequencies can be raised by thymidine in the medium at concentrations as low as 0.04 μM, or by the endogenous generation of thymidine from thymine plus a deoxyribosyl donor. Deoxyadenosine, various ribonucleosides, thymine, and 2-deoxyribose do not stimulate mutation. None of these effects are related to growth rate, since growth rate and mutation rate can be decoupled completely.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADELBERG E. A., BURNS S. N. Genetic variation in the sex factor of Escherichia coli. J Bacteriol. 1960 Mar;79:321–330. doi: 10.1128/jb.79.3.321-330.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonney R. J., Weinfeld H. Regulation of thymidine metabolism in Escherichia coli K-12: studies on the inducer and the coordinateness of induction of the enzymes. J Bacteriol. 1971 Jun;106(3):812–818. doi: 10.1128/jb.106.3.812-818.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budman D. R., Pardee A. B. Thymidine and thymine incorporation into deoxyribonucleic acid: inhibition and repression by uridine of thymidine phosphorylase of Escherichia coli. J Bacteriol. 1967 Nov;94(5):1546–1550. doi: 10.1128/jb.94.5.1546-1550.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPBELL A. Sensitive mutants of bacteriophage lambda. Virology. 1961 May;14:22–32. doi: 10.1016/0042-6822(61)90128-3. [DOI] [PubMed] [Google Scholar]
- CUZIN F., JACOB F. [Reversible integration of the F' sexual episome in Escherichia coli K 12]. C R Hebd Seances Acad Sci. 1963 Jul 17;257:795–797. [PubMed] [Google Scholar]
- Cox E. C., Degnen G. E., Scheppe M. L. Mutator gene studies in Escherichia coli: the mutS gene. Genetics. 1972 Dec;72(4):551–567. doi: 10.1093/genetics/72.4.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox E. C. Mutator gene action and the replication of bacteriophage lambda DNA. J Mol Biol. 1970 May 28;50(1):129–135. doi: 10.1016/0022-2836(70)90109-9. [DOI] [PubMed] [Google Scholar]
- Cox E. C., Yanofsky C. Altered base ratios in the DNA of an Escherichia coli mutator strain. Proc Natl Acad Sci U S A. 1967 Nov;58(5):1895–1902. doi: 10.1073/pnas.58.5.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox E. C., Yanofsky C. Mutator gene studies in Escherichia coli. J Bacteriol. 1969 Oct;100(1):390–397. doi: 10.1128/jb.100.1.390-397.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewitt S K, Adelberg E A. The Occurrence of a Genetic Transposition in a Strain of Escherichia Coli. Genetics. 1962 May;47(5):577–585. doi: 10.1093/genetics/47.5.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doskocil J., Paces V. The effect of nucleosides on the phosphorolysis of thymidine by normal and phage-infected cells of E. coli. Biochem Biophys Res Commun. 1968 Jan 25;30(2):153–158. doi: 10.1016/0006-291x(68)90463-4. [DOI] [PubMed] [Google Scholar]
- Glansdorff N., Sand G., Verhoef C. The dual genetic control of ornithine transcarbamylase synthesis in Escherichia coli K12. Mutat Res. 1967 Nov-Dec;4(6):743–751. doi: 10.1016/0027-5107(67)90083-8. [DOI] [PubMed] [Google Scholar]
- Guerola N., Ingraham J. L., Cerdá-Olmedo E. Induction of closely linked multiple mutations by nitrosoguanidine. Nat New Biol. 1971 Mar 24;230(12):122–125. doi: 10.1038/newbio230122a0. [DOI] [PubMed] [Google Scholar]
- Hammer-Jespersen K., Munch-Petersen A., Schwartz M., Nygaard P. Induction of enzymes involed in the catabolism of deoxyribonucleosides and ribonucleosides in Escherichia coli K 12. Eur J Biochem. 1971 Apr 30;19(4):533–538. doi: 10.1111/j.1432-1033.1971.tb01345.x. [DOI] [PubMed] [Google Scholar]
- Hill R. F. Location of genes controlling excision repair of UV damage and mutator activity in Escherichia coli WP2. Mutat Res. 1970 Mar;9(3):341–344. doi: 10.1016/0027-5107(70)90135-1. [DOI] [PubMed] [Google Scholar]
- Ikeda H., Tomizawa J. I. Transducing fragments in generalized transduction by phage P1. I. Molecular origin of the fragments. J Mol Biol. 1965 Nov;14(1):85–109. doi: 10.1016/s0022-2836(65)80232-7. [DOI] [PubMed] [Google Scholar]
- Jacoby G. A. Mapping the gene determining ornithine transcarbamylase and its operator in Escherichia coli B. J Bacteriol. 1971 Nov;108(2):645–651. doi: 10.1128/jb.108.2.645-651.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammen H. O., Strand M. Thymine metabolism in Escherichia coli. II. Altered uptake of thymine after bacteriophage infection. J Biol Chem. 1967 Apr 25;242(8):1854–1863. [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Liberfarb R. M., Bryson V. Isolation, characterization, and genetic analysis of mutator genes in Escherichia coli B and K-12. J Bacteriol. 1970 Oct;104(1):363–375. doi: 10.1128/jb.104.1.363-375.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low B. Low recombination frequency for markers very near the origin in conjugation in E. coli. Genet Res. 1965 Nov;6(3):469–473. doi: 10.1017/s0016672300004341. [DOI] [PubMed] [Google Scholar]
- MAAS R., MAAS W. K. Introduction of a gene from Escherichia coli B into HFR and F-strains of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1962 Nov 15;48:1887–1893. doi: 10.1073/pnas.48.11.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONOD J., CHANGEUX J. P., JACOB F. Allosteric proteins and cellular control systems. J Mol Biol. 1963 Apr;6:306–329. doi: 10.1016/s0022-2836(63)80091-1. [DOI] [PubMed] [Google Scholar]
- Matsuhashi M., Strominger J. L. Thymidine diphosphate 4-acetamido-2,6-dideoxyhexoses. 3. Purification and properties of thymidine diphosphate 4-keto-6-deoxy-D-glucose transaminase from Escherichia coli strain B. J Biol Chem. 1966 Oct 25;241(20):4738–4744. [PubMed] [Google Scholar]
- Munch-Petersen A. On the catabolism of deoxyribonucleosides in cells and cell extracts of Escherichia coli. Eur J Biochem. 1968 Nov;6(3):432–442. doi: 10.1111/j.1432-1033.1968.tb00465.x. [DOI] [PubMed] [Google Scholar]
- NIKAIDO H., MIKAIDO K., SUBBAIAH T. V., STOCKER B. A. ROUGH MUTANTS OF SALMONELLA TYPHIMURIUM. III. ENZYMATIC SYNTHESIS OF NUCLEOTIDE-SUGAR COMPOUNDS. Nature. 1964 Mar 28;201:1301–1302. doi: 10.1038/2011301a0. [DOI] [PubMed] [Google Scholar]
- Newton W. A., Beckwith J. R., Zipser D., Brenner S. Nonsense mutants and polarity in the lac operon of Escherichia coli. J Mol Biol. 1965 Nov;14(1):290–296. doi: 10.1016/s0022-2836(65)80250-9. [DOI] [PubMed] [Google Scholar]
- RACHMELER M., GERHART J., ROSNER J. Limited thymidine uptake in Escherichia coli due to an inducible thymidine phosphorylase. Biochim Biophys Acta. 1961 Apr 29;49:222–225. doi: 10.1016/0006-3002(61)90888-5. [DOI] [PubMed] [Google Scholar]
- Siegel E. C., Bryson V. Mutator gene of Escherichia coli B. J Bacteriol. 1967 Jul;94(1):38–47. doi: 10.1128/jb.94.1.38-47.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnov G. B., Filkova E. V., Skavronskaya A. G. The mutator property of uvr502 mutation affecting UV sensitivity of Escherichia coli. Mol Gen Genet. 1972;118(1):51–56. doi: 10.1007/BF02428332. [DOI] [PubMed] [Google Scholar]
- Taylor A. L. Current linkage map of Escherichia coli. Bacteriol Rev. 1970 Jun;34(2):155–175. doi: 10.1128/br.34.2.155-175.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Revised linkage map of Escherichia coli. Bacteriol Rev. 1967 Dec;31(4):332–353. doi: 10.1128/br.31.4.332-353.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treffers H. P., Spinelli V., Belser N. O. A Factor (or Mutator Gene) Influencing Mutation Rates in Escherichia Coli. Proc Natl Acad Sci U S A. 1954 Nov;40(11):1064–1071. doi: 10.1073/pnas.40.11.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Ward C. B., Hane M. W., Glaser D. A. Synchronous reinitiation of chromosome replication in E. coli B-r after nalidixic acid treatment. Proc Natl Acad Sci U S A. 1970 Jun;66(2):365–369. doi: 10.1073/pnas.66.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky C., Cox E. C., Horn V. The unusual mutagenic specificity of an E. Coli mutator gene. Proc Natl Acad Sci U S A. 1966 Feb;55(2):274–281. doi: 10.1073/pnas.55.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky C., Ito J., Horn V. Amino acid replacements and the genetic code. Cold Spring Harb Symp Quant Biol. 1966;31:151–162. doi: 10.1101/sqb.1966.031.01.023. [DOI] [PubMed] [Google Scholar]
- Zamenhof P. J. A genetic locus responsible for generalized high mutability in Escherichia coli. Proc Natl Acad Sci U S A. 1966 Sep;56(3):845–852. doi: 10.1073/pnas.56.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]


