Abstract
UV exposure induces skin cancer, in part by inducing immune suppression. Repairing DNA damage, neutralizing the activity of cis-urocanic acid (cis-UCA), and reversing oxidative stress abrogates UV-induced immune suppression and skin cancer induction, suggesting the DNA, UCA and lipid photo-oxidation serves as UV photoreceptors. What is not clear is whether signaling through each of these different photoreceptors activates independent pathways to induce biological effects or whether there is a common checkpoint where these pathways converge. Here we show that agents known to reverse photocarcinogenesis and photoimmune suppression, such as platelet activating factor (PAF) and serotonin (5-HT) receptor antagonists regulate DNA repair. Pyrimidine dimer repair was accelerated in UV-irradiated mice injected with PAF and 5-HT receptor antagonists. Nucleotide excision repair, as measured by unscheduled DNA synthesis, was accelerated by PAF and 5-HT receptor antagonists. Injecting PAF and 5-HT receptor antagonists into UV-irradiated Xeroderma pigmentosum complementation group A (XPA) deficient mice, which lack the enzymes responsible for nucleotide excision repair, did not accelerate photoproduct repair. Similarly, UV-induced formation of 8-oxo-deoxyguanosine (8-oxo-dG) was reduced by PAF and 5-HT receptor antagonists. We conclude that PAF and 5-HT receptor antagonists accelerate DNA repair caused by UV radiation, which prevents immune suppression and interferes with photocarcinogenesis.
Introduction
The UV radiation found in sunlight is the primary cause of non-melanoma skin cancer, the most prevalent human cancer, and UV exposure is implicated in the induction of melanoma, the most dangerous skin cancer (Boring et al., 1992). In addition to being a complete carcinogen, UV is immune suppressive, and the induction of immune suppression by UV exposure is recognized as a major risk factor for skin cancer induction (Fisher and Kripke, 1982; Yoshikawa et al., 1990). UV-irradiation induces signature mutations (C to T and CC to TT transitions) in the p53 tumor suppressor gene, a critical target for skin cancer formation (reviewed by Benjamin et al., 2008). Further, UV-induced DNA lesions, particularly cyclobutane pyrimidine dimer (CPD) formation, play an essential role in the induction of immune suppression (Applegate et al., 1989; Vink et al., 1997), identifying DNA as an important photoreceptor for UV-induced carcinogenesis and immunosuppression.
DNA is not alone in its ability to act as a photoreceptor for UV-induced immunosuppression. The isomerization of trans-UCA to the cis-isomer converts UV radiation into a biologically recognizable signal that activates immune suppression (De Fabo and Noonan, 1983). Neutralizing the activity of cis-UCA (Moodycliffe et al., 1996) or blocking the binding of cis-UCA to its receptor (5-HT2A) with selective serotonin receptor antagonists, blocks UV-induced immune suppression (Walterscheid et al., 2006). Neutralizing the activity of cis-UCA in vivo blocks photocarcinogenesis (Beissert et al., 2001).
Evidence from a number of laboratories has also indicated that membrane phospholipids can absorb UV radiation leading to lipid peroxidation and the activation of transcription factors leading to cytokine release, contributing to immune suppression (Devary et al., 1993; Simon et al., 1994). Moreover, injecting UV-irradiated phospholipids into mice activates cytokine release and immune suppression, further supporting a role for lipids as a photoreceptor (Walterscheid et al., 2006). The lipid mediator of inflammation, platelet-activating factor (PAF) is secreted by keratinocytes almost immediately after UV exposure (Pei et al., 1998), and it activates the transcription of many of the cytokines and immunomodulatory factors, such as IL-10 and prostaglandin E2, that drive UV-induced immune suppression. Blocking the binding of PAF to its receptor blocks UV-induced immune suppression (Walterscheid et al., 2002). Moreover, UV-exposure promotes oxidative stress, which induces DNA damage and the production of oxidized phospholipids, such as PAF, and treating UV-irradiated mice with anti-oxidants blocks UV-induced immune suppression and photocarcinogenesis (Black et al., 1997).
What is not clear is whether signaling through each of these different photoreceptors activates independent pathways to induce immune suppression and photocarcinogenesis or whether there is a common checkpoint where these pathways converge. Recently we demonstrated that PAF and 5-HT receptor antagonists block photocarcinogenesis (Sreevidya et al., 2008). To determine, in more detail, the mechanisms involved, we measured the numbers of CPD’s in the skin of antagonist-treated UV-irradiated mice. Initially, we noted that the numbers of CPDs in the skin of the antagonist-treated, UV-irradiated mice was similar to that seen in the UV-only controls. However, as time progressed UV-induced CPDs were repaired more rapidly in the receptor antagonist treated mice. In view of the importance that CPD formation plays in both carcinogenesis and immune suppression, we asked if the mechanism by which diverse agents, such as PAF and 5-HT receptor antagonists, reverse immune suppression and carcinogenesis is by regulating DNA repair. Here we present data demonstrating that blocking PAF and cis-UCA from binding to their receptors modulates DNA repair following exposure to UV radiation.
Results
Agents that reverse immune suppression accelerate photoproduct removal
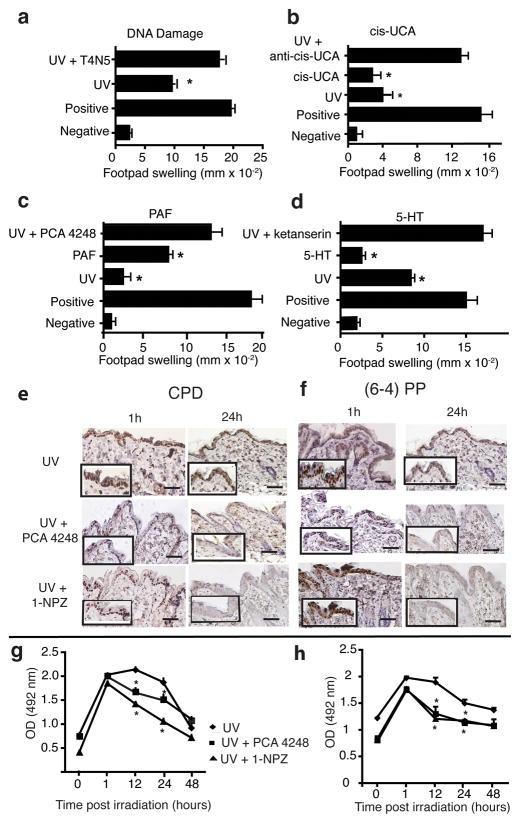
Over the years a variety of structurally and mechanistically diverse agents have been used to overcome UV-induced immune suppression. This is illustrated in Figure 1. Repairing CPD formation (Fig. 1A) by applying T4N5-containing liposomes (Kripke et al., 1992), neutralizing the activity of cis-UCA (Fig. 1B) (El-Ghorr and Norval, 1995), blocking PAF receptor binding (Fig. 1C) (Walterscheid et al., 2002), and blocking 5-HT receptor binding (Walterscheid et al., 2006) (Fig. 1D), all interfere with UV-induced immune suppression. Alternatively, treating mice with PAF, 5-HT and cis-UCA all activate immune suppression. We measured the effect these agents had on the numbers of CPD and pyrimidine (6-4) pyrimidinone photoproduct (6-4 photoproduct) in the skin. Mice were exposed to 2 kJ/m2 of UVB radiation and immediately injected with a PAF receptor antagonist (PCA-4248), or a 5-HT receptor antagonist 1-(1-Napthyl) piperazine (1-NPZ), as described previously (Sreevidya et al., 2008; Walterscheid et al., 2006). At various times post irradiation, skin samples were acquired; DNA was isolated and CPD and 6-4 photoproducts numbers were determined by immunohistochemistry (Fig 1E & F) and ELISA (Fig 1G & H). Note that 1h post irradiation, the number of CPDs and 6-4 photoproducts found in the skin of the mice injected with the receptor antagonists were similar to the numbers found in mice only exposed to UV radiation. At 12 and 24h post irradiation we saw a significant (P < 0.05) decrease in the numbers of CPD and (6-4) photoproducts found in the skin of UV-irradiated and PAF- and/or 5-HT-antagonist injected mice, compared to the UV-irradiated control. By 48h, no significant difference in the numbers of CPD and 6-4 photoproducts found in the skin of the UV-irradiated and PAF and/or 5-HT injected mice, versus the number of CPD and 6-4 photoproducts found in the skin of the UV-only controls was noted. These data indicate that treating UV-irradiated mice with PAF and 5-HT receptor antagonists accelerate the repair of UV-induced photoproducts.
Figure 1.
Reversal of immune suppression and acceleration of DNA repair by PAF and 5-HT receptor antagonists. Effect of agents that repair DNA damage, neutralize cis-UCA activity or block PAF and 5-HT from binding to their receptors on immune suppression. Mice were exposed to 15 kJ/m2 of UVB radiation and then treated with: (a) T4N5-containing liposomes, (b) monoclonal anti-cis-UCA (c) PAF receptor antagonist (d) 5-HT receptor antagonist. The effect the various treatments had on UV-induced immune suppression was determined using a DTH assay. The data are expressed as mean footpad swelling ± SEM. The asterisk denotes a significant difference (p < 0.05) from the positive control. Effect of PAF and 5-HT receptor antagonists on the numbers of CPD and 6-4 photoproducts. Mice were exposed to 2 kJ/m2 of UVB radiation and then immediately injected with 1 μM of PCA-4248 or 1 μM of 1-NPZ. One and 24h post irradiation, skin samples were removed and (e) CPD or (f) 6-4 PP were visualized by immunohistochemistry. Bar = 50 μm. High magnification views (400X) are shown in the inserts. At various times post irradiation skin samples were taken, DNA was isolated and (g) CDP and (h) 6-4 photoproducts were measured by ELISA. * indicates a significant difference (p < 0.05) from the UV only control (Student’s t-test, n = 5).
Effect of PAF and 5-HT receptor antagonists on unscheduled DNA repair
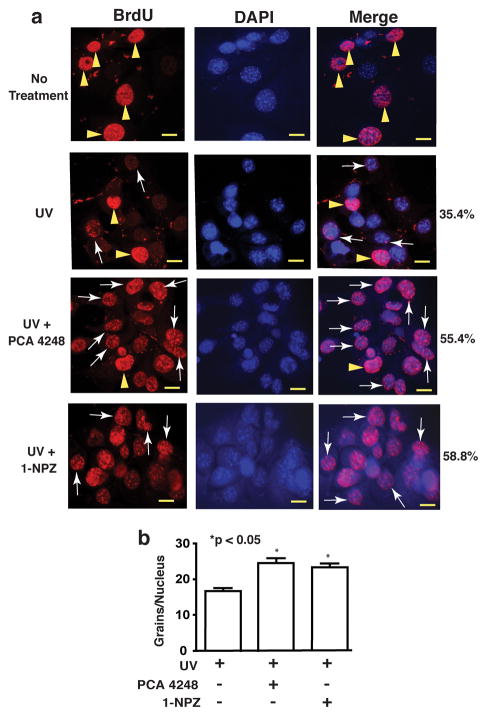
UV-induced photoproducts are removed by nucleotide excision repair (NER), which is routinely detected by unscheduled DNA synthesis (UDS) (Hanawalt et al., 2003). Therefore, we asked whether accelerated photoproduct repair was due to NER. Keratinocytes were pretreated with a PAF (PCA-4248, 50 μM) or a 5-HT (1-NPZ; 25 μM) receptor antagonist, washed, and then UV-irradiated (200 J/m2). The cells were then incubated with BrdU and then stained with anti-BrdU antibody (Fig 2A). The left panel shows anti-BrdU staining, the middle panel shows DAPI staining to illuminate the nuclei, and the right panel shows a merge of the DAPI and BrdU panels. Compared to un-irradiated control cells, punctate nuclear staining (arrows), indicative of UDS was found in cells exposed to UV radiation (Fig 2A). Treating the cells with the PAF or the 5-HT receptor antagonist further increased the number of cells under going UDS (35.4% UV only vs. 55.4% UV + PCA 4248, vs. 58.8% UV + 1-NPZ). In addition, the number of grains per nuclei was counted and we found that treating the cells with the PAF or 5-HT receptor antagonists increased the grains per nuclei (Fig. 2B). These data indicate that PAF and 5-HT receptor antagonists accelerate NER.
Figure 2.
Unscheduled DNA synthesis is accelerated by treating UV-irradiated mice with PAF and 5-HT receptor antagonists. (a) Pam 212 cells were treated with a PAF (PCA-4248; 50 μM) or a 5-HT (1-NPZ; 25 μM) receptor antagonist, washed and then exposed 200 J/m2 of UVB radiation. The cells were incubated with BrdU (3h, 37°) and then incubated with anti-BrdU antibody. The left panels show anti-BrdU staining (red). The center panels show DAPI staining of the nuclei. The right panel shows a merge of the BrdU and DAPI staining. Arrowheads illustrate nuclei in S phase; arrows indicate punctate nuclear staining characteristic of cells undergoing UDS. The percentage of cells undergoing UDS in each group is indicated. Bar = 50 μm. (b) Quantification of UDS. The number of individual grains/nuclei was counted. * indicates a significant difference (p < 0.05) from the UV only control (Student’s t-test, 50 cells per sample).
Failure of PAF and 5HT receptor antagonists to accelerate DNA repair in XPA-deficient mice
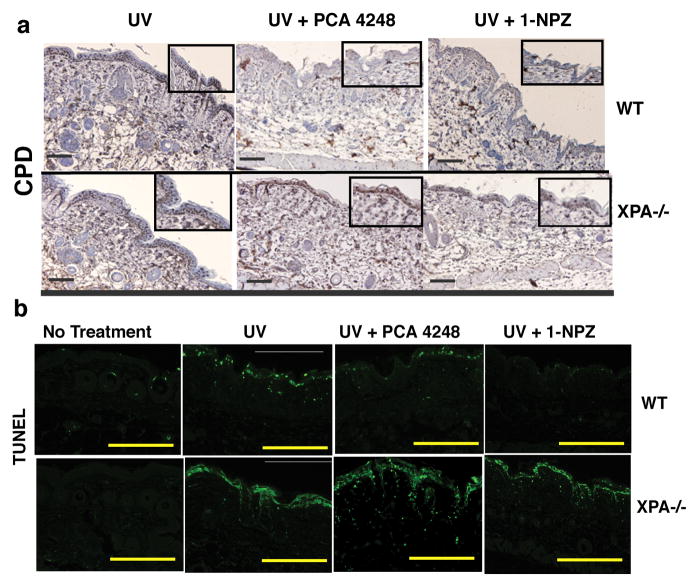
NER is absent in XPA−/− mice (Hanawalt, 2001). To confirm that NER is involved in the accelerated DNA repair induced by PAF and 5-HT receptor antagonists, we measured CPD removal in XPA−/− mice. Wild type and XPA−/− mice were UV-irradiated and then injected with PAF and 5-HT receptor antagonists. CPD was observed in wild type mice 24h after UV irradiation. Treating the wild type mice with a PAF and/or a 5-HT receptor antagonist accelerated the rate of DNA repair (Fig 3A). No removal of CPD was noted when the PAF and/or the 5-HT receptor antagonists were injected into UV-irradiated XPA−/− mice. In addition, we saw no inhibition of apoptosis in XPA−/− mice injected with PAF or 5-HT receptor antagonists (Fig 3B). These findings support our hypothesis that PAF and 5-HT receptors antagonists accelerate the rate of DNA repair via a mechanism involving NER.
Figure 3.
Failure to reverse CPD formation in PAF and 5-HT receptor antagonist-treated XPA−/−mice. Wild type (WT) or XPA−/− mice were exposed to 2 kJ/m2 of UVB radiation and immediately injected with 1 μM of PCA-4248 or 1 μM of 1-NPZ. (a) CPDs were detected by immunohistochemistry. Bar = 50 μm. High magnification views (400X) are shown in the inserts. (b) Apoptosis was detected by TUNEL. Bar = 200 μm.
Induction of Reactive Oxygen Species (ROS) and DNA damage following treatment with PAF, 5-HT and cis-UCA
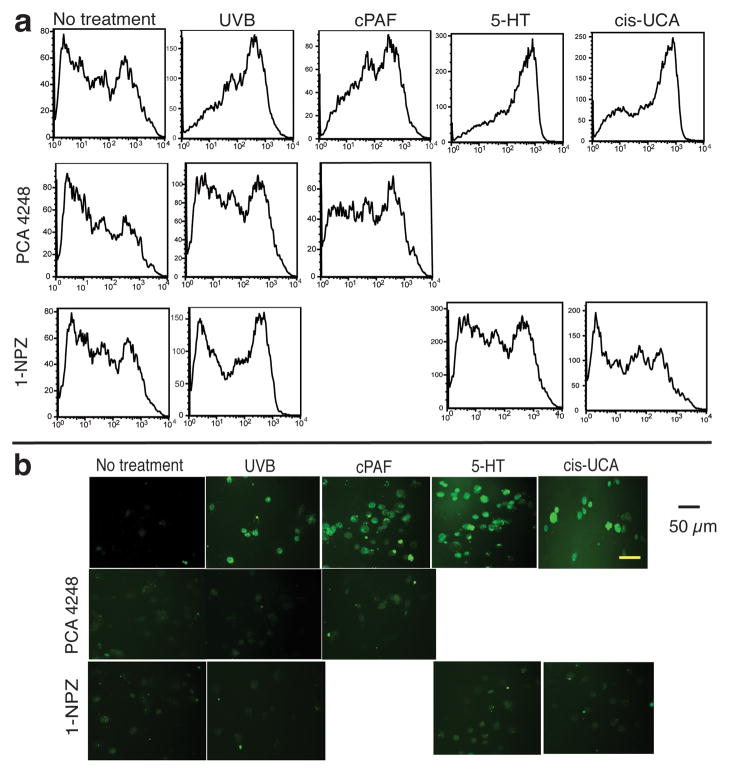
UV induces oxidative stress, which can lead to free radical formation and ultimately DNA damage (Bertram and Hass, 2008). Although PAF and 5-HT have been shown to activate ROS and apoptosis (Barber et al., 1998; Breard et al., 2007), it is not clear if cis-UCA can induce ROS and promote DNA damage. To address this question, keratinocytes were treated with UV, cPAF (the metabolically stable analog of PAF), 5-HT, and cis-UCA. The cells were then incubated with 2′, 7′-dichlorodihydro-fluorescein diacetate, a dye that is oxidized into the highly fluorescent product dichlorofluorescein by ROS (Brubacher and Bols, 2001). Fluorescence was measured by flow cytometry. The positive controls (UV radiation, cPAF and 5-HT) activated ROS production as measured by the increased numbers of highly fluorescent cells (Fig. 4A). Treatment with cis-UCA also induced ROS formation. Treating the cells with PCA-4248, or 1-NPZ, reduced the number of intensely staining fluorescent cells, indicating that these agents block ROS production. This observation was confirmed by fluorescent microscopy (Fig. 4B). No fluorescence was found in normal cells, but many fluorescent cells were found following UV and/or cPAF exposure. Keratinocytes treated with 5-HT or cis-UCA also emitted a strong fluorescent signal. Treating the cells with PAF and 5-HT receptor antagonists reduced the fluorescent signal, supporting the conclusion that PAF, 5-HT and cis-UCA activate ROS production.
Figure 4.
5-HT and cis-UCA induced ROS formation. Keratinocytes were exposed to UVB radiation (200 J/m2), or cultured with 10 μM of cPAF, 50 μM of 5-HT or 50 μM of cis-UCA. Some of the cells were pre-treated with PCA-4248 (50 μM) or 1-NPZ (25 μM). (a) ROS induction was measured by flow cytometry, or (b) fluorescence microscopy. Bar = 50 μm.
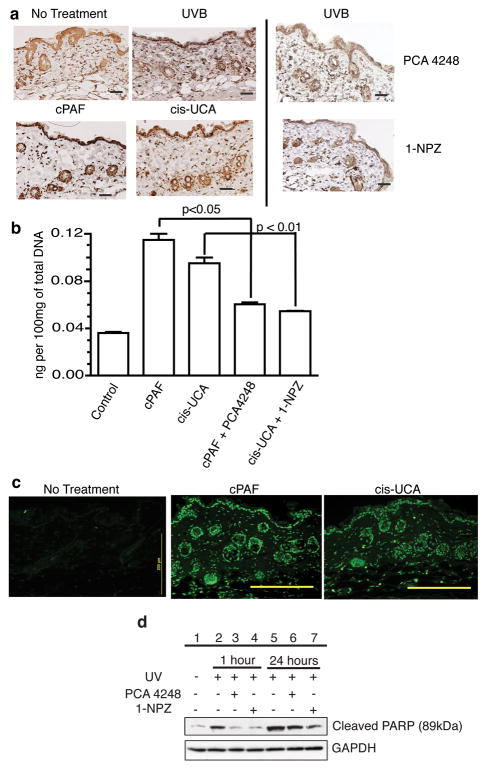
ROS induces 8-oxo-deoxyguanosine (8-oxo-dG), which is pre-mutagenic and has been implicated in photocarcinogenesis (Le Page et al., 1995). We measured 8-oxo-dG in the skin of mice injected with PAF and cis-UCA (Fig. 5A). Background levels of 8-oxo-dG, were observed in samples from untreated mice, which is expected because cells express 8-oxo-dG under normal physiological conditions (Wiseman and Halliwell, 1996). UV irradiation increased 8-oxo-dG formation. Treating the mice with, cPAF and cis-UCA also increased 8-oxo-dG formation. Treating the mice with PCA-4248 or 1-NZP reduced UV-induced 8-oxo-dG production in the skin. 8-oxo-dG production was measured by ELISA (Fig. 5B). We observed a statistically significant induction of 8-oxo-dG by cPAF and cis-UCA treatment (P < 0.001 vs. normal skin), which was inhibited when the mice were treated with the respective receptor antagonist. Since severe DNA damage leads to apoptosis, we also asked if cPAF and cis-UCA induced apoptosis (Fig. 5C). A high number of TUNEL-positive apoptotic cells were observed in the skin of the treated mice. Because Beneke et al., (2000) demonstrated that the cleavage of endogenous Poly (ADP-ribose) polymerase 1 is a marker for apoptotic cell death, we measured the effect of PAF and 5-HT receptor antagonists on PARP cleavage. Western blotting with an antibody specific for the cleaved form (89kDa) of PARP indicated that UV exposure activated PARP cleavage (Fig 5D: lanes 2 & 5). Treating the cells with a PAF (lanes 3 & 6), or a 5-HT (lanes 4 & 7) receptor antagonist inhibited PARP cleavage. These results confirm that both PAF and cis-UCA can induce DNA damage, and blocking the binding of these inflammatory mediators to their receptors, will block DNA damage.
Figure 5.
Reduced 8-oxo-dG lesions following treatment with PAF and 5-HT receptor antagonists. Mice were exposed to 2 kJ/m2 of UVB radiation, or injected with 500 nM of cPAF or 5 μM of cis-UCA. Some mice were injected with 1μM of PCA-4248 or 1μM of 1-NPZ immediately following irradiation. Skin samples were also collected from after dermal injection of 500 nM of cPAF or 5μM of cis-UCA. All samples were collected 24h post treatment. (a) 8-oxo-dG was detected by immunohistochemistry. (b) 8-oxo-dG was measured by ELISA. Data is expressed as ng of 8-oxo-dG/100mg total DNA. P values determined by Student’s t-test (n = 5). (c) Apoptosis (TUNEL) in response to dermal cPAF and cis-UCA injection. Bar = 200 μm. (d) PARP cleavage is blocked by PAF and 5-HT receptor antagonists. Keratinocytes were pre-treated with PCA 4248 (50 μM) or 1-NPZ (25 μM), washed and then exposed to 200 J/m2 of UVB radiation. Total cellular protein was isolated 1 and 24h post irradiation. Cleaved PARP was measured by Western blotting. Equal gel loading was confirmed by staining with a GAPDH-specific antibody.
Discussion
UV-induced DNA mutations are a critical step in the induction of skin cancer, and compelling evidence from Kripke and colleagues demonstrated that DNA serves as a photoreceptor for UV-induced immune suppression (Applegate et al., 1989; Kripke et al., 1992; Vink et al., 1997). Compelling data also exists demonstrating that cis-UCA is a UV photoreceptor (De Fabo and Noonan, 1983) and neutralizing its function will also impair photocarcinogenesis (Beissert et al., 2001) and block the induction of immune suppression (Moodycliffe et al., 1996; Walterscheid et al., 2006). Moreover, injecting PAF-receptor antagonists into UV-irradiated mice blocks both immune suppression and photocarcinogenesis (Sreevidya et al., 2008; Walterscheid et al., 2002). Conventional wisdom suggests that the reason anti-cis-UCA antibody, PAF receptor, and 5-HT receptor antagonists block photocarcinogenesis is by blocking immune suppression (as illustrated in Fig 1). Undoubtedly blocking immune suppression is contributing to some of the anti-cancer effects, but our findings illustrate a novel and as yet unappreciated mechanism to describe the anti-cancer effects described previously. We demonstrate that blocking the binding of cis-UCA to its receptor (5-HT2A), or blocking the binding of PAF to its receptor, agents that reverse UV-induced immune suppression and photocarcinogenesis also modulate DNA repair. Both UV-induced photoproducts (CPD and 6-4 photoproducts) were removed following treatment with PAF and 5-HT receptor antagonists. Since this is an NER dependent mechanism, we measured UDS. Treatment with the PAF and 5-HT receptor antagonists accelerated UDS, indicating that these agents accelerated NER. Failure of the PAF and 5-HT receptor antagonists to accelerate DNA repair in XPA−/− mice, further supports our hypothesis that these agents affect NER. The implication of these findings is that UV-induced immunosuppressive agents, such as PAF and cis-UCA inhibit DNA repair in vivo. It is interesting to note that Yarosh and colleagues demonstrated that two classic immunosuppressive drugs, cyclosporine and tacrolimus, also inhibit the removal of CPD in UV-irradiated keratinocytes (Yarosh et al., 2005).
Our findings also indicate that treating mice with cis-UCA, PAF, and 5-HT, induces ROS, leading to oxygen radical formation and DNA damage, as evidenced by 8-oxo-dG formation. The induction of ROS by PAF and 5-HT, which are well known inflammatory mediators, is documented (Breard et al., 2007; Bussolati et al., 1998). Although others have suggested that it is theoretically possible for cis-UCA to induce ROS (Shen and Ji, 2008), here we provide direct evidence for cis-UCA-induced ROS and DNA damage. In addition, we provide data demonstrating that blocking the binding of cis-UCA and PAF to their receptors, blocks ROS and 8-oxo-dG formation. These findings suggest that agents used here that reverse UV-induced immune suppression and photocarcinogenesis may do so by regulating DNA repair.
Kripke and colleagues clearly demonstrated the role of UV-induced CPD formation in activating immune suppression. However, it is equally important to remember that other types of DNA damage, particularly double strand breaks will also induce immunosuppression and the production of immune suppressive cytokines in vivo (Nishigori et al., 1998; O’Connor et al., 1996). We suggest that this is one pathway by which cis-UCA and PAF are activating immune suppression, in absence of the typical UV-induced DNA damage (Fig. 1). 8-oxo-dG formation is indicative of oxidative DNA damage. Treating keratinocytes, or skin with PAF, cis-UCA or 5-HT activates DNA damage, as evidenced by the up-regulation of 8-oxo-dG. Moreover, PAF and 5-HT receptor antagonists decrease 8-oxo-dG formation in vivo. Our data indicate that the PAF and 5-HT receptor antagonists can work at two levels to repair DNA lesions. We see accelerated repair of both UV-induced photoproducts (CPD and 6-4 photoproducts) and reduced numbers of ROS-induced lesions (8-oxo-dG formation) in mice treated with PAF and 5-HT receptor antagonists. Indeed, these two observations may be related. Others have demonstrated that ROS can adversely affect NER. Langie and colleagues found that NER capacity as well as the expression of some of the genes involved in NER is modulated by oxidative stress. Specifically, they noted an inhibition of NER after exposure to H2O2 (Langie et al., 2007). It may be possible that UV-induced cis-UCA and PAF are contributing to immune suppression and photocarcinogenesis by not only inducing DNA damage via the production of reactive oxygen, but also by inhibiting NER, and allowing for the persistence of CPD and 6-4 photoproducts through a ROS-dependent mechanism. This may help explain why these seemingly disparate agents block both UV-induced and cis-UCA-induced immune suppression.
Interleukin-12 is a classic immune modulatory cytokine, one that has been shown to reverse UV-induced immune suppression (Schmitt et al., 1995). Schwarz and colleagues demonstrated that IL-12 treatment reverses UV-induced apoptosis and immune suppression, in part, by activating DNA repair (Schwarz et al., 2005; Schwarz et al., 2002). Our findings are reminiscent of the dual effects of IL-12. For example, blocking PAF from binding to its receptor restores immune competence, by suppressing prostaglandin E2 and IL-10 production (Walterscheid et al., 2002) and as shown here, PAF receptor antagonists accelerate DNA repair. The implication of this finding is that inflammatory mediators, such as PAF, depress DNA repair, thus providing another mechanistic link between inflammation and carcinogenesis. Supporting this idea are the observations that PAF receptor binding has been implicated in a variety of cancers, including melanoma (Melnikova et al., 2006), ovarian (Aponte et al., 2008), breast (Montrucchio et al., 1998), brain (Denizot et al., 2006) and leukemia (Denizot et al., 2004).
In summary our data demonstrate that agents that block UV-induced immune suppression do so by modulating DNA repair. Damage caused by a direct result of UV radiation (CPD and 6-4 photoproducts), as well as damage due to UV-induced ROS (8-oxo-dG) was reduced when the binding of PAF to its receptor, and the binding of cis-UCA to its receptor was blocked. Moreover, treating keratinocytes with inflammatory mediators induced DNA damage. Impaired DNA repair has serious consequences in humans, as evidenced by the clinical features associated with XP, Cockayne syndrome and trichothiodystrophy (van Steeg and Kraemer, 1999). The findings reported here confirm the important role that DNA damage and DNA repair play in UV-induced immune suppression. Further, they provide a mechanism describing how different UV-induced mediators, such as PAF and cis-UCA, can regulate DNA repair, which affects immune suppression and contributes to UV-induced carcinogenesis.
Materials and Methods
Mice
C57BL/6 mice were purchased from the NCI-Frederick Cancer Research Facility, (Frederick, MD). XPA−/− mice (Nakane et al., 1995) were bred at the Kobe University Graduate School of Medicine. All animals were maintained with alternating 12-hour light and dark cycles and controlled temperature and humidity in facilities approved by the Association for Assessment and Accreditation of Laboratory Animal Care International. The University of Texas M.D. Anderson Cancer Center Institutional Animal Care and Use Committee and the Animal Ethics Committee of the approved all the animal procedures described here.
Reagents
Carbamyl-PAF (cPAF), PCA-4248, 1-(1-Napthyl) piperazine (1-NPZ) and ketanserin were purchased from Biomol Research Labs. Dichlorofluorescein diacetate, OPD Peroxidase substrate, and cis-UCA were purchased from Sigma-Aldrich (St Louis, MO). The murine keratinocyte cell line Pam 212, was a gift from Dr. Stuart Yuspa, NCI. Tissue culture medium was obtained from Gibco BRL (Grand Island, NY). Biotin-SP conjugated goat anti-mouse immunoglobulin and peroxidase-conjugated streptavidin were purchased from Jackson ImmunoResearch Labs (West Grove, PA). An antibody specific for cleaved PARP was purchased from Cell Signaling Technology (Danvers, MA). TUNEL positive cells were detected using a commercial kit provided by Promega (Madison, WI).
Radiation source
Mice were exposed to UV radiation supplied by a 1000 W xenon UV solar simulator (Oriel Inc, Stratford, CT) (Sreevidya et al., 2008). To irradiate keratinocytes in culture a single FS-40 sunlight was used (Rivas and Ullrich, 1992). The intensity and spectral output of the radiation sources was measured with an Optronics model OL-754 scanning spectrophotometer (Optronics Labs, Orlando, FL).
Unscheduled DNA synthesis
Pam 212 cells were treated with 50 μM of PCA-4248 and 25 μM of 1-NPZ for 30 min at 37°. These doses were chosen based on previous studies (Sreevidya et al., 2008; Walterscheid et al., 2006; Walterscheid et al., 2002). The cells were washed extensively, overlayed with PBS, and then exposed to UV radiation (200 J/m2). The cells were incubated for 3 hr at 37° with 10 μM of BrdU. The cells were then fixed in methanol, and UDS was analyzed as described (Nakagawa et al., 1998). UDS was visualized by counting punctuated spots in nuclei, in contrast to the uniformly and intense staining found in ordinary DNA synthesis. The grains per nuclei counted in at least 50 cells undergoing UDS were recorded. The percentage of cells undergoing UDS was calculated by counting at least 10 fields/sample.
Measurement of CPD, 6-4 PP and 8-oxo-dG
C57BL/6 mice were exposed to 2 kJ/m2 of UVB radiation and injected i.p. with 1μM of PCA-4248 or 1μM of 1-NPZ. These doses were chosen based on previous studies (Sreevidya et al., 2008; Walterscheid et al., 2006; Walterscheid et al., 2002). Skin sections were collected at various times post exposure and fixed as described previously (Sreevidya et al., 2008). CPD was detected using a monoclonal anti-thymine dimer (clone H3, Sigma-Aldrich). 6-4 photoproducts were detected using monoclonal antibody, D195-1 clone 64M2 (MBL International, Woburn, MA). 8-oxo-dG was detected using monoclonal antibody N45.1 (MBL International). Genomic DNA was purified using DNeasy kit (Qiagen, Valencia, CA). CPD and 6-4 photoproducts were also measured by ELISA (MBL International). An ELISA to detect 8-oxo-dG was acquired from Genox (Baltimore, MD).
Measurement of ROS
Pam 212 cells were treated with 50 μM of PCA-4248 and 25 μM of 1-NPZ. After 30 min, the cells were washed, overlayed with PBS and exposed to 200 J/m2 of UVB radiation. Alternatively, some cells were treated with 10 μM of cPAF, 50 μM of 5-HT or 50 μM of cis-UCA. The cells were washed, trypsinized and re-suspended in 5% FBS/HBSS. Dichlorofluorescein diacetate (2 μM) was added, and after 1hr at room temperature, fluorescence was measured by flow cytometry (FACS Caliber, Becton Dickenson, San Jose, CA). Alternatively, 1×105 Pam 212 cells were added to Lab-Tek chamber slides, and cultured overnight. The cells treated with PAF and 5-HT receptor antagonists, and then exposed to UVB radiation, as described above. 5μM of aminophenyl fluorescein and 5μM of hydroxyphenyl fluorescein (Sigma-Aldrich) was added. After 30 minutes the monolayers were washed and fluorescence was detected by microscopy.
Immune suppression
Suppression of the induction of a delayed-type hypersensitivity (DTH) reaction, as described in detail elsewhere (Nghiem et al., 2002), was used to measure the effect of UV, cis-UCA, 5-HT and PAF on the immune response. C57Bl/6 mice were first exposed to 15 kJ/m2 of UVB radiation, and then 5 days later immunized with formalin-fixed Candida albicans. DTH, as determined by an increase in footpad swelling in response to challenge with C. albicans specific antigen (Alerchek, Portland, ME) was measured 10 days post immunization. T4N5-containing liposomes were a gift from Dan Yarosh (AGI-Dermatics, Freeport, NY) and were used as described previously (Nishigori et al., 1996). Mice were injected i.p. with 5 μM cis-UCA (Walterscheid et al., 2006), 250 pM of 5-HT (Walterscheid et al., 2006) and 500 pM of cPAF (Walterscheid et al., 2002) to induce immune suppression, and 10 μg of anti-cis-UCA antibody (Moodycliffe et al., 1996), 1μM of PCA-4248 (Walterscheid et al., 2002) and 1μM of ketanserin (Walterscheid et al., 2006) to block immune suppression. Anti-cis-UCA, PCA-4248 and ketanserin were injected i.p. immediately following UV exposure. There were at least 5 mice per group, the mean footpad thickness for the group ± the SEM was calculated. A statistical difference between the positive control and the experimental groups was determined using a one-way ANOVA followed by the Dunnett’s Multiple Comparison test (GraphPad Prism Software, GraphPad, Inc. San Diego, CA).
Acknowledgments
S.S.C. and A.F. contributed equally to this work. This work was funding by grants from the National Cancer Institute (CA112660 & CA131207). C.S.S. was supported by an NCI training grant (T32-CA-09598-15). The animal, histology, and flow cytometry facilities at UTMDACC were supported in part by a NCI Cancer Center Support Grant (CA16672). We thank Dr. Mary Norval for the gifts of cis-UCA and anti-cis-UCA monoclonal antibody.
Abbreviations
- cPAF
carbamyl-PAF cis-UCA, cis-urocanic acid
- CPD
cyclobutane pyrimidine dimers
- DTH
delayed type hypersensitivity
- NER
nucleotide excision repair
- 1-NPZ
1-(1-Napthyl)piperazine
- 8-oxo-dG
8-oxo-deoxyguanosine;
- 6-4 photoproducts;
pyrimidine (6-4) pyrimidinone photoproducts
- PAF
platelet activating factor
- PARP
poly(ADP-ribose) polymerase
- 6-4 photoproducts
pyrimidine (6-4) pyrimidinone photoproducts
- ROS
reactive oxygen species
- 5-HT
serotonin
- UDS
unscheduled DNA synthesis
- XPA
Xeroderma pigmentosum complementation group A
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
References
- Aponte M, Jiang W, Lakkis M, Li MJ, Edwards D, Albitar L, et al. Activation of platelet-activating factor receptor and pleiotropic effects on tyrosine phospho-EGFR/Src/FAK/paxillin in ovarian cancer. Cancer Res. 2008;68:5839–5848. doi: 10.1158/0008-5472.CAN-07-5771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Applegate LA, Ley RD, Alcalay J, Kripke ML. Identification of the molecular target for the suppression of contact hypersensitivity by ultraviolet radiation. J Exp Med. 1989;170:1117–1131. doi: 10.1084/jem.170.4.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber LA, Spandau DF, Rathman SC, Murphy RC, Johnson CA, Kelley SW, et al. Expression of the platelet-activating factor receptor results in enhanced ultraviolet B radiation-induced apoptosis in a human epidermal cell line. J Biol Chem. 1998;273:18891–18897. doi: 10.1074/jbc.273.30.18891. [DOI] [PubMed] [Google Scholar]
- Beissert S, Ruhlemann D, Mohammad T, Grabbe S, El-Ghorr A, Norval M, et al. IL-12 prevents the Inhibitory effects of cis-Urocanic acid on tumor antigen presentation by Langerhans cells: Implications for photocarcinogenesis. J Immunol. 2001;167:6232–6238. doi: 10.4049/jimmunol.167.11.6232. [DOI] [PubMed] [Google Scholar]
- Beneke R, Geisen C, Zevnik B, Bauch T, Muller WU, Kupper JH, et al. DNA excision repair and DNA damage-induced apoptosis are linked to Poly(ADP-ribosyl)ation but have different requirements for p53. Mol Cell Biol. 2000;20:6695–6703. doi: 10.1128/mcb.20.18.6695-6703.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin CL, Ullrich SE, Kripke ML, Ananthaswamy HN. p53 tumor suppressor gene: a critical molecular target for UV induction and prevention of skin cancer. Photochem Photobiol. 2008;84:55–62. doi: 10.1111/j.1751-1097.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- Bertram C, Hass R. Cellular responses to reactive oxygen species-induced DNA damage and aging. Biol Chem. 2008;389:211–220. doi: 10.1515/BC.2008.031. [DOI] [PubMed] [Google Scholar]
- Black HS, deGruijl FR, Forbes PD, Cleaver JE, Ananthaswamy HN, deFabo EC, et al. Photocarcinogenesis: an overview. J Photochem Photobiol B. 1997;40:29–47. doi: 10.1016/s1011-1344(97)00021-3. [DOI] [PubMed] [Google Scholar]
- Boring CC, Squires TS, Tong T. Cancer Statistics. CA Cancer J Clin. 1992;42:19–38. doi: 10.3322/canjclin.42.1.19. [DOI] [PubMed] [Google Scholar]
- Breard M, Sari MA, Frapart Y, Boucher JL, Ducrocq C, Grillon C. The endogenous neurotransmitter, serotonin, modifies neuronal nitric oxide synthase activities. Free Radic Res. 2007;41:413–423. doi: 10.1080/10715760601105681. [DOI] [PubMed] [Google Scholar]
- Brubacher JL, Bols NC. Chemically de-acetylated 2′,7′-dichlorodihydrofluorescein diacetate as a probe of respiratory burst activity in mononuclear phagocytes. J Immunol Methods. 2001;251:81–91. doi: 10.1016/s0022-1759(01)00308-8. [DOI] [PubMed] [Google Scholar]
- Bussolati B, Mariano F, Cignetti A, Guarini A, Cambi V, Foa R, et al. Platelet-activating factor synthesized by IL-12-stimulated polymorphonuclear neutrophils and NK cells mediates chemotaxis. J Immunol. 1998;161:1493–1500. [PubMed] [Google Scholar]
- De Fabo EC, Noonan FP. Mechanism of immune suppression by ultraviolet irradiation in vivo. I. Evidence for the existence of a unique photoreceptor in skin and its role in photoimmunology. J Exp Med. 1983;157:84–98. doi: 10.1084/jem.158.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denizot Y, De Armas R, Caire F, Pommepuy I, Truffinet V, Labrousse F. Platelet-activating factor and human meningiomas. Neuropathol Appl Neurobiol. 2006;32:674–678. doi: 10.1111/j.1365-2990.2006.00775.x. [DOI] [PubMed] [Google Scholar]
- Denizot Y, Donnard M, Guglielmi L, Faucher JL, Jaccard A, Bordessoule D, et al. Detection of functional platelet-activating factor receptors on leukemic B cells of chronic lymphocytic leukemic patients. Leuk Lymphoma. 2004;45:515–518. doi: 10.1080/1042819032000141293. [DOI] [PubMed] [Google Scholar]
- Devary Y, Rosette C, DiDonato JA, Karin M. NF-κB activation by ultraviolet light is not dependent on a nuclear signal. Science. 1993;261:1442–1445. doi: 10.1126/science.8367725. [DOI] [PubMed] [Google Scholar]
- El-Ghorr AA, Norval M. A monoclonal antibody to cis-urocanic acid prevents the UV-induced changes in Langerhans cells and DTH responses in mice, although not preventing dendritic cell accumulation in lymph nodes draining the site of irradiation and contact hypersensitivity responses. J Invest Dermatol. 1995;105:264–268. doi: 10.1111/1523-1747.ep12318410. [DOI] [PubMed] [Google Scholar]
- Fisher MS, Kripke ML. Suppressor T lymphocytes control the development of primary skin cancers in ultraviolet-irradiated mice. Science. 1982;216:1133–1134. doi: 10.1126/science.6210958. [DOI] [PubMed] [Google Scholar]
- Hanawalt PC. Controlling the efficiency of excision repair. Mutat Res. 2001;485:3–13. doi: 10.1016/s0921-8777(00)00071-9. [DOI] [PubMed] [Google Scholar]
- Hanawalt PC, Ford JM, Lloyd DR. Functional characterization of global genomic DNA repair and its implications for cancer. Mutat Res. 2003;544:107–114. doi: 10.1016/j.mrrev.2003.06.002. [DOI] [PubMed] [Google Scholar]
- Kripke ML, Cox PA, Alas LG, Yarosh DB. Pyrimidine dimers in DNA initiate systemic immunosuppression in UV-irradiated mice. Proc Natl Acad Sci U S A. 1992;89:7516–7520. doi: 10.1073/pnas.89.16.7516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langie SAS, Knaapen AM, Houben JMJ, van Kempen FC, de Hoon JPJ, Gottschalk RWH, Godschalk RWL, van Schooten FJ. The role of glutathione in the regulation of nucleotide excision repair during oxidative stress. Toxicol Lett. 2007;168:302–309. doi: 10.1016/j.toxlet.2006.10.027. [DOI] [PubMed] [Google Scholar]
- Le Page F, Margot A, Grollman AP, Sarasin A, Gentil A. Mutagenicity of a unique 8-oxoguanine in a human Ha-ras sequence in mammalian cells. Carcinogenesis. 1995;16:2779–2784. doi: 10.1093/carcin/16.11.2779. [DOI] [PubMed] [Google Scholar]
- Melnikova VO, Mourad-Zeidan AA, Lev DC, Bar-Eli M. Platelet-activating factor mediates MMP-2 expression and activation via phosphorylation of cAMP-response element-binding protein and contributes to melanoma metastasis. J Biol Chem. 2006;281:2911–2922. doi: 10.1074/jbc.M508683200. [DOI] [PubMed] [Google Scholar]
- Montrucchio G, Sapino A, Bussolati B, Ghisolfi G, Rizea-Savu S, Silvestro L, et al. Potential angiogenic role of platelet-activating factor in human breast cancer. Am J Pathol. 1998;153:1589–1596. doi: 10.1016/S0002-9440(10)65747-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moodycliffe AM, Bucana CD, Kripke ML, Norval M, Ullrich SE. Differential effects of a monoclonal antibody to cis-urocanic acid on the suppression of delayed and contact hypersensitivity following ultraviolet irradiation. J Immunol. 1996;157:2891–2899. [PubMed] [Google Scholar]
- Nakagawa A, Kobayashi N, Muramatsu T, Yamashina Y, Shirai T, Hashimoto MW, et al. Three-dimensional visualization of ultraviolet-induced DNA damage and its repair in human cell nuclei. J Invest Dermatol. 1998;110:143–148. doi: 10.1046/j.1523-1747.1998.00100.x. [DOI] [PubMed] [Google Scholar]
- Nakane H, Takeuchi S, Yuba S, Saijo M, Nakatsu Y, Murai H, et al. High incidence of ultraviolet-B- or chemical-carcinogen induced skin tumors in mice lacking the xeroderma pigmentosum group gene. Nature. 1995;377:165–168. doi: 10.1038/377165a0. [DOI] [PubMed] [Google Scholar]
- Nghiem DX, Walterscheid JP, Kazimi N, Ullrich SE. Ultraviolet radiation-induced immunosuppression of delayed-type hypersensitivity in mice. Methods. 2002;28:25–33. doi: 10.1016/s1046-2023(02)00207-4. [DOI] [PubMed] [Google Scholar]
- Nishigori C, Yarosh D, O’Connor A, Shreedhar VK, Ullrich SE, Cox P, et al. HindIII liposomes suppress delayed-type hypersensitivity responses in vivo and induce epidermal IL-10 in vitro. J Immunol. 1998;161:2684–2691. [PubMed] [Google Scholar]
- Nishigori C, Yarosh DB, Ullrich SE, Vink AA, Bucana CD, Roza L, et al. Evidence that DNA damage triggers interleukin 10 cytokine production in UV-irradiated murine keratinocytes. Proc Natl Acad Sci U S A. 1996;93:10354–10359. doi: 10.1073/pnas.93.19.10354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor A, Nishigori C, Yarosh D, Alas L, Kibitel J, Burley L, et al. DNA double strand breaks in epidermal cells cause immune suppression in vivo and cytokine production in vitro. J Immunol. 1996;157:271–278. [PubMed] [Google Scholar]
- Pei Y, Barber LA, Murphy RC, Johnson CA, Kelley SW, Dy LC, et al. Activation of the epidermal platelet-activating factor receptor results in cytokine and cyclooxygenase-2 biosynthesis. J Immunol. 1998;161:1954–1961. [PubMed] [Google Scholar]
- Rivas JM, Ullrich SE. Systemic suppression of delayed-type hypersensitivity by supernatants from UV-irradiated keratinocytes. An essential role for keratinocyte-derived IL-10. J Immunol. 1992;149:3865–3871. [PubMed] [Google Scholar]
- Schmitt DA, Owen-Schaub L, Ullrich SE. Effect of IL-12 on immune suppression and suppressor cell induction by ultraviolet radiation. J Immunol. 1995;154:5114–5120. [PubMed] [Google Scholar]
- Schwarz A, Maeda A, Kernebeck K, van Steeg H, Beissert S, Schwarz T. Prevention of UV radiation-induced immunosuppression by IL-12 is dependent on DNA repair. J Exp Med. 2005;201:173–179. doi: 10.1084/jem.20041212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz A, Stander S, Berneburg M, Bohm M, Kulms D, van Steeg H, et al. Interleukin-12 suppresses ultraviolet radiation-induced apoptosis by inducing DNA repair. Nat Cell Biol. 2002;4:26–31. doi: 10.1038/ncb717. [DOI] [PubMed] [Google Scholar]
- Shen L, Ji HF. Theoretical investigation of the photosensitization mechanisms of urocanic acid. J Photochem Photobiol B. 2008;91:96–98. doi: 10.1016/j.jphotobiol.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Simon MM, Aragane Y, Schwarz A, Luger TA, Schwarz T. UVB light induces a nuclear factor κB (NFκB) activity independently from chromosomal DNA damage in cell-free cytosolic extracts. J Invest Dermatol. 1994;102:422–427. doi: 10.1111/1523-1747.ep12372194. [DOI] [PubMed] [Google Scholar]
- Sreevidya CS, Khaskhely NM, Fukunaga A, Khaskina P, Ullrich SE. Inhibition of photocarcinogenesis by platelet-activating factor or serotonin receptor antagonists. Cancer Res. 2008;68:3978–3984. doi: 10.1158/0008-5472.CAN-07-6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Steeg H, Kraemer KH. Xeroderma pigmentosum and the role of UV-induced DNA damage in skin cancer. Mol Med Today. 1999;5:86–94. doi: 10.1016/s1357-4310(98)01394-x. [DOI] [PubMed] [Google Scholar]
- Vink AA, Moodycliffe AM, Shreedhar V, Ullrich SE, Roza L, Yarosh DB, et al. The inhibition of antigen-presenting activity of dendritic cells resulting from UV irradiation of murine skin is restored by in vitro photorepair of cyclobutane pyrimidine dimers. Proc Natl Acad Sci U S A. 1997;94:5255–5260. doi: 10.1073/pnas.94.10.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walterscheid JP, Nghiem DX, Kazimi N, Nutt LK, McConkey DJ, Norval M, et al. Cis-urocanic acid, a sunlight-induced immunosuppressive factor, activates immune suppression via the 5-HT2A receptor. Proc Natl Acad Sci U S A. 2006;103:17420–17425. doi: 10.1073/pnas.0603119103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walterscheid JP, Ullrich SE, Nghiem DX. Platelet-activating factor, a molecular sensor for cellular damage, activates systemic immune suppression. J Exp Med. 2002;195:171–179. doi: 10.1084/jem.20011450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman H, Halliwell B. Damage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer. Biochem J. 1996;313:17–29. doi: 10.1042/bj3130017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarosh DB, Pena AV, Nay SL, Canning MT, Brown DA. Calcineurin inhibitors decrease DNA repair and apoptosis in human keratinocytes following ultraviolet B irradiation. J Invest Dermatol. 2005;125:1020–1025. doi: 10.1111/j.0022-202X.2005.23858.x. [DOI] [PubMed] [Google Scholar]
- Yoshikawa T, Rae V, Bruins-Slot W, Van den Berg JW, Taylor JR, Streilein JW. Susceptibility to effects of UVB radiation on induction of contact hypersensitivity as a risk factor for skin cancer in humans. J Invest Dermatol. 1990;95:530–536. doi: 10.1111/1523-1747.ep12504877. [DOI] [PubMed] [Google Scholar]