Abstract
Naturally-occurring phenethyl isothiocyanate (PEITC) selectively inhibits growth of cancer cells by causing apoptosis but the mechanism of cell death induction is not fully understood. We now demonstrate, for the first time, that growth factor adapter protein p66shc is indispensable for PEITC-induced apoptosis. Mouse embryonic fibroblasts derived from p66shc knockout mice were significantly more resistant to PEITC-mediated growth inhibition, cytoplasmic histone-associated apoptotic DNA fragmentation, and caspase-3 activation compared with wild-type fibroblasts. The PEITC treatment resulted in induction as well as increased Ser36 phosphorylation of p66shc in PC-3 and LNCaP human prostate cancer cells. Knockdown of p66shc protein conferred significant protection against PEITC-mediated cytoplasmic histone-associated DNA fragmentation as well as production of reactive oxygen species in both PC-3 and LNCaP cells. The PEITC-treated PC-3 and LNCaP cells exhibited increased binding of p66shc with prolyl isomerase Pin1, a protein implicated in translocation of p66shc to mitochondria. Consistent with these results, treatment of PC-3 cells with PEITC resulted in translocation of p66shc to the mitochondria as judged by immunoblotting using cytosolic and mitochondrial fractions and immunofluorescence microscopy. Growth suppression and apoptosis induction in tumor xenografts in vivo by oral administration of PEITC to the PC-3 tumor bearing male athymic mice was accompanied by statistically significant increase in the level of Ser36 phosphorylated p66shc. Collectively, these results provide novel insight into the critical role of p66shc in regulation of PEITC-induced apoptotic cell death in human prostate cancer cells.
Keywords: Phenethyl isothiocyanate, p66shc, Apoptosis
Introduction
Despite continuous evolution of targeted therapies and significantly improved screening efforts (e.g., digital rectal examination and screening for prostate specific antigen), prostate cancer remains a leading cause of cancer-related deaths among men in the United States (1). Because many of the risk factors associated with prostate cancer development (e.g., genetic predisposition and age) are not modifiable, novel agents to prevent and treat this devastating disease are highly attractive to reduce disease-related cost, morbidity, and mortality for a large segment of population. Natural products have received increasing attention in recent years for discovery novel cancer chemopreventive/therapeutic agents (2). Epidemiological studies have suggested that dietary intake of cruciferous vegetables may lower the risk of different malignancies including cancer of the prostate (3–5). For example, a multicenter case-control study of African-American, white, Japanese, and Chinese men (n= 1619) with histologically confirmed prostate cancer and matched controls (n= 1618; matched by ethnicity, age, region of residence) showed an inverse association between intake of cruciferous vegetables and the risk of prostate cancer (4).
Anticarcinogenic effect of cruciferous vegetables is credited to naturally-occurring chemicals with an isothiocyanate moiety (-N=C=S), which are produced by myrosinase-mediated hydrolysis of corresponding glucosinolates (6,7). Phenethyl-ITC (PEITC) is one such naturally-occurring ITC compound that has garnered tremendous research interest for a variety of reasons, including anticancer effect and favorable safety profile (8–13). For example, PEITC is a potent inhibitor of pulmonary tumorigenesis in rats induced by tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (8,9). The PEITC administration was shown to offer significant protection against diethylnitrosamine-induced hepatocellular adenoma development in mice (10). The PEITC-mediated prevention of N-nitrosobenzylmethylamine-induced esophageal cancer in rats was associated with modulation of carcinogen-induced gene expression (11,12).
More recent studies, including those from our laboratory, have revealed that PEITC has the ability to suppress growth of cancer cells in culture and in vivo (14–19). Mechanism behind anticancer effect of PEITC is not fully understood but known cellular responses to this promising agent include c-Jun N-terminal kinase- and extracellular signal-regulated kinase-mediated apoptosis induction (14,15), G2/M phase cell cycle arrest (16), suppression of nuclear factor-κB-regulated gene expression (17), inhibition of angiogenesis in vitro and ex vivo (20), and Atg5-mediated autophagy (21). We have also shown previously that oral administration of PEITC significantly retards growth of PC-3 human prostate cancer cells subcutaneously implanted in male athymic mice without causing weight loss or any other side effects (19,21).
Previous studies have indicated that the PEITC-mediated proapoptotic signal transduction is intimately linked to production of reactive oxygen species (ROS) (19,22). However, the molecular circuitry of PEITC-mediated ROS production and apoptotic cell death is still not fully understood. We now demonstrate, for the first time, that growth factor adapter protein p66shc is essential for PEITC-induced ROS production as well as apoptosis in human prostate cancer cells.
Materials and Methods
Reagents
PEITC (purity >99%) was purchased from Sigma-Aldrich. Reagents for cell culture were purchased from GIBCO. Hydroethidine (HE) and 6-carboxy-2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) were purchased from Molecular Probes. The p66Shc-targeted small interfering RNA (siRNA) was procured from Santa Cruz Biotechnology and a non-specific control siRNA was from Qiagen. A kit for quantitation of cytoplasmic histone-associated DNA fragmentation was from Roche Diagnostics. Antibodies against p66Shc, phospho-(Ser36)-p66Shc, and heat shock protein 70 (HSP70) were from Santa Cruz Biotechnology; an antibody against prolyl isomerase (Pin1) was from Cell Signaling Technology; anti-cytochrome c oxidase IV (COXIV) antibody was from Molecular Probes; and an antibody against actin was from Oncogene Research Products. Caspase-3 activation was measured by flow cytometry using a kit from Cell Signaling Technology. Protein kinase Cβ (PKCβ)-selective inhibitor [3-(1-(3-imidazol-1-ylpropyl)-1H-indol-3-yl)-4-anilino-1H-pyrrole-2,5-dione; hereafter abbreviated as PKCβ-I] was purchased from EMD Biosciences.
Cell lines and cell culture
The PC-3 and LNCaP cell lines were obtained from the American Type Culture Collection, and validated by analysis of known markers (e.g., expression of p53 and androgen-responsiveness). Monolayer cultures of PC-3 (an androgen-independent cell line lacking functional p53) and LNCaP cells (an androgen-responsive cell line with wild-type p53) were maintained as described by us previously (15,23). Immortalized MEFs derived from wild-type and p66Shc−/− mice were generously provided by Dr. T. Finkel and Dr. S. Nemoto (National Institutes of Health, Bethesda, MD), and cultured in DMEM supplemented with 10% heat-inactivated fetal bovine serum, 0.1 mmol/L non-essential amino acids, 0.1 μmol/L 2-mercaptoethanol, and antibiotics.
Western blotting
Stock solution of PEITC was prepared in dimethyl sulfoxide (DMSO) and diluted with complete medium immediately before use. An equal volume of DMSO (final concentration <0.1%) was added to the controls. Cell lysates were prepared as previously described by us (15,24). Cytosolic and mitochondrial fractions from DMSO-treated control and PEITC-treated cells were prepared using a kit from BioVision according to the manufacturer’s recommendations. Immunoblotting was performed essentially as described by us previously (24).
Cell viability assay and measurement of apoptosis
Effect of PEITC treatment on cell viability was determined by trypan blue dye exclusion assay as described by us previously (25). Apoptosis induction by PEITC was assessed by analysis of cytoplasmic histone-associated DNA fragmentation using a kit from Roche Diagnostics. Activation of caspase-3 was determined by flow cytometry using a kit from Cell Signaling Technology as recommended by the supplier.
RNA interference of p66Shc
The PC-3 or LNCaP cells (1×105) were seeded in six-well plates and allowed to attach by overnight incubation. The cells were transfected with 200 nmol/L of a control non-specific siRNA or p66Shc-targeted siRNA using Oligofectamine (Invitrogen). Twenty-four hours after transfection, the cells were treated with DMSO (control) or specified concentration of PEITC for desired time period. The cells were collected, washed with phosphate-buffered saline (PBS), and processed for immunoblotting or analysis of cytoplasmic histone-associated DNA fragmentation and ROS production as described previously (25,26).
Analysis of interaction between p66Shc and Pin1 or HSP70
Aliquots containing 300 μg of total lysate protein or mitochondria-enriched fractions from PC-3 or LNCaP cells treated with DMSO (control) or 5 μmol/L PEITC (8 h treatment) were incubated overnight at 4°C with 6 μg of anti-p66Shc antibody. Protein G-agarose beads (50 μL; Santa Cruz Biotechnology) were then added to each sample and the incubation was continued for an additional 2 h at 4°C. The immunoprecipitates were washed five times with lysis buffer and subjected to sodium-dodecyl sulfate polyacrylamide gel electrophoresis followed by immunoblotting using anti-Pin1 antibody (total lysate) or anti-HSP70 antibody (mitochondria-enriched fraction).
Immunocytochemical analysis for mitochondrial localization of p66Shc
The PC-3 cells (1×105) were plated on coverslips and treated with 5 μmol/L PEITC or DMSO (control) for 8 h. The cells were then treated with 200 nmol/L MitoTracker Red at 37°C for 30 min to stain mitochondria. After washing with PBS, the cells were fixed in 2% paraformaldehyde overnight at 4°C and permeabilized using 0.1% Triton X-100 in PBS for 10 min. The cells were washed with PBS, blocked with 0.5% bovine serum albumin in PBS for 1 h, and incubated with anti-p66Shc antibody overnight at 4°C. The cells were then washed with PBS, incubated with Alexa Fluor 488-conjugated secondary antibody (Molecular probes) for 1 h at room temperature. Subsequently, the cells were washed with PBS and treated with 10 ng/mL 4′,6-diamidino-2-phenylindole (DAPI) for 5 min at room temperature to stain nucleus. The cells were washed twice with PBS and examined under a Leica fluorescence microscope at 100× magnification.
Immunohistochemical analyses for total and phosphorylated p66Shc and Pin1 in PC-3 tumor xenografts
We used tumor tissues from a previously published xenograft study (21) to determine in vivo effect of PEITC administration on expression of total and Ser36-phosphorylated p66Shc and Pin1. Tumor tissues from the control and PEITC-treated mice were fixed in 10% neutral-buffered formalin, dehydrated, embedded in paraffin, and sectioned at 4–5 μm thickness. Immunohistochemistry was performed as described by us previously (27). At least three non-overlapping images from each tissue section were captured and analyzed using Image ProPlus software (Media Cybernetics).
Statistical analysis
Each experiment was repeated at least twice to ensure reproducibility of the results. Paired t-test or one-way ANOVA followed by Bonferroni’s multiple comparison test was used to determine significance of difference in measured variables between groups. Difference was considered significant at P<0.05.
Results
p66Shc deficiency conferred resistance toward PEITC-induced apoptosis
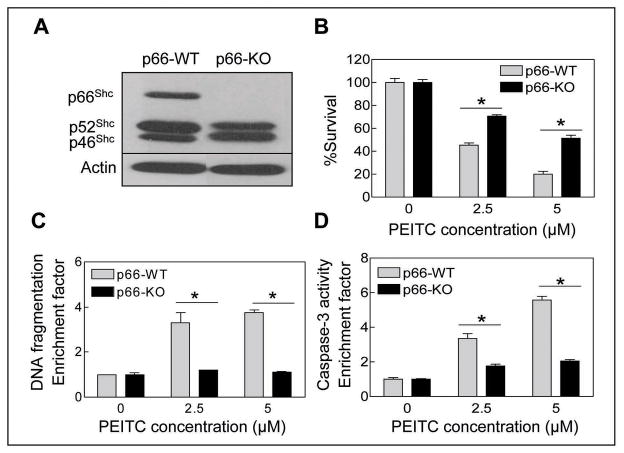
We have shown previously that the PEITC-induced apoptosis in PC-3 cell line is intimately linked to the production of ROS (19). Because electron transfer between cytochrome c and p66Shc, which is a splice variant of cytoplasmic adapter proteins p52Shc/p46Shc that are involved in signal transduction from activated tyrosine kinases to Ras (28), has been shown to cause ROS-dependent mitochondrial apoptosis (29), we raised the question of whether PEITC-induced apoptosis was mediated by the p66Shc protein. Initially, we used MEFs derived from wild-type (p66-WT) and p66Shc knockout (p66-KO) mice to test possible involvement of this protein in PEITC-induced apoptosis. Immunoblotting confirmed absence of the p66Shc isoform (but not p52Shc or p46Shc) in p66-KO MEFs (Fig. 1A). Exposure of MEFs derived from p66-WT mice to PEITC resulted in a concentration-dependent and statistically significant decrease in cell viability (Fig. 1B). The MEFs derived from the p66-KO mice were statistically significantly more resistant to PEITC-mediated growth retardation compared with p66-WT cells (Fig. 1B). The PEITC-mediated suppression of p66-WT MEF viability correlated with apoptosis induction as evidenced by cytoplasmic histone-associated DNA fragmentation (Fig. 1C) and caspase-3 activation (Fig. 1D). In agreement with cell viability data (Fig. 1B), the p66-KO MEFs were protected against PEITC-mediated cytoplasmic histone-associated apoptotic DNA fragmentation (Fig. 1C) as well as caspase-3 activation (Fig. 1D) in comparison with p66-WT MEFs.
Fig. 1.
A, immunoblotting for p66Shc protein using lysates from untreated MEFs derived from wild-type (p66-WT) and p66Shc knockout mice (p66-KO). The blot was stripped and re-probed with anti-actin antibody to ensure equal protein loading. Cell viability (B), cytoplasmic histone-associated DNA fragmentation (C), and caspase-3 activation (D) in p66-WT and p66-KO MEFs following 4 h treatment with DMSO (control) or the indicated concentrations of PEITC. Results are expressed as enrichment factor relative to DMSO-treated control. Each experiment was done at least twice in triplicate and representative data from a single experiment are shown. Columns, mean (n=3); bars, SE. *Significantly different (P<0.05) between the indicated groups by paired t-test.
We raised a question of whether p66Shc dependence of PEITC-induced apoptosis was unique to this compound. We addressed this question using benzyl isothiocyanate (BITC), a structural analogue of PEITC. The MEFs derived from p66-KO mice were significantly more resistant to growth inhibition (supplemental Fig. S1, panel A) as well as cytoplasmic histone-associated DNA fragmentation (supplemental Fig. S1, panel B) compared with p66-WT MEFs. These results indicated that involvement of p66Shc in apoptosis regulation is not unique to the PEITC.
PEITC-induced apoptosis and ROS production was inhibited by RNA interference of p66Shc
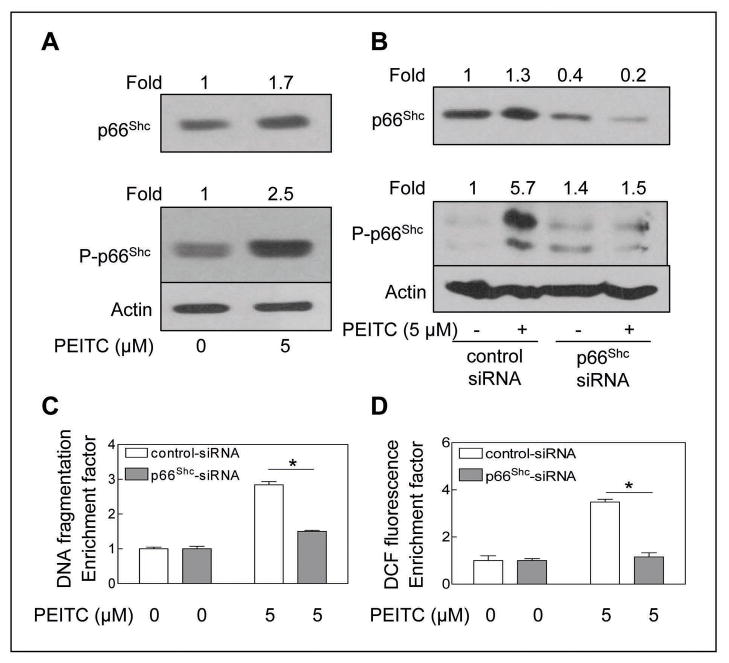
Next, we determined the effect of PEITC treatment (5 μmol/L, 24 h) on levels of total and Ser36 phosphorylated p66Shc because oxidative stress-induced Ser36 phosphorylation of p66Shc has been shown to be critical for its translocation to mitochondria after recognition by Pin1 (30). The PEITC-treated PC-3 cells exhibited a modest yet marked increase in protein level of total p66Shc, which was accompanied by ~2.5-fold increase in level of Ser36 phosphorylated p66Shc over DMSO-treated control (Fig. 2A). Next, we utilized siRNA technology to directly test contribution of p66Shc in regulation of PEITC-induced apoptosis. As can be seen in Fig. 2B, the level of p66Shc protein was decreased by about 60% by transient transfection of PC-3 cells with p66Shc-targeted siRNA compared with cells transfected with a control non-specific siRNA. Similar to un-transfected cells (Fig. 2A), the PEITC treatment (5 μmol/L, 24 h) caused an increase in levels of total and Ser36 phosphorylated p66Shc in comparison with DMSO-treated control in PC-3 cells transfected with the non-specific siRNA. The PEITC-mediated induction as well as Ser36 hyperphosphorylation of p66Shc was markedly suppressed in PC-3 cells transfected with the p66Shc-targeted siRNA (Fig. 2B). Twenty-four hour exposure of non-specific control siRNA transfected PC-3 cells to 5 μmol/L PEITC resulted in about 2.8-fold increase in cytoplasmic histone-associated DNA fragmentation compared with DMSO-treated control (Fig. 2C). The p66Shc depleted cells were nearly completely resistant to PEITC-induced apoptotic DNA fragmentation compared with cells transfected with control siRNA (Fig. 2C). Because PEITC-mediated apoptosis correlates with ROS production (19,22), we also determined ROS generation by PEITC treatment (5 μmol/L; 4 h treatment) in cells transfected with control siRNA and p66Shc-targeted siRNA. As shown in Fig. 2D, the PEITC-mediated oxidation of H2DCFDA (a measure of peroxide production) over DMSO-treated control was observed in non-specific siRNA transfected PC-3 cells but not in the p66Shc depleted cells.
Fig. 2.
A, immunoblotting for total and Ser36 phosphorylated p66Shc using lysates from PC-3 cells treated with DMSO (control) or 5 μmol/L PEITC for 24 h. B, immunoblotting for total and Ser36 phosphorylated p66Shc using lysates from PC-3 cells transiently transfected with a control non-specific siRNA or p66Shc-targeted siRNA and treated for 24 h with DMSO or 5 μmol/L PEITC. The blots were stripped and re-probed with anti-actin antibody to ensure equal protein loading. The numbers on top of the immunoreactive bands represent changes in protein levels relative to corresponding DMSO-treated control (panel A) or relative to DMSO-treated non-specific control siRNA transfected PC-3 cells (panel B). C, cytoplasmic histone-associated apoptotic DNA fragmentation, and D, DCF fluorescence in PC-3 cells transiently transfected with a control non-specific siRNA or p66Shc-targeted siRNA and treated for 24 h (panel C) or 4 h (panel D) with DMSO or 5 μmol/L PEITC. The results are expressed as enrichment factor relative to DMSO-treated control for PC-3 cells transiently transfected with the control non-specific siRNA. Each experiment was done twice and representative data from a single experiment are shown. Columns, mean (n=3); bars, SE. *Significantly different (P<0.05) between the indicated groups by paired t-test.
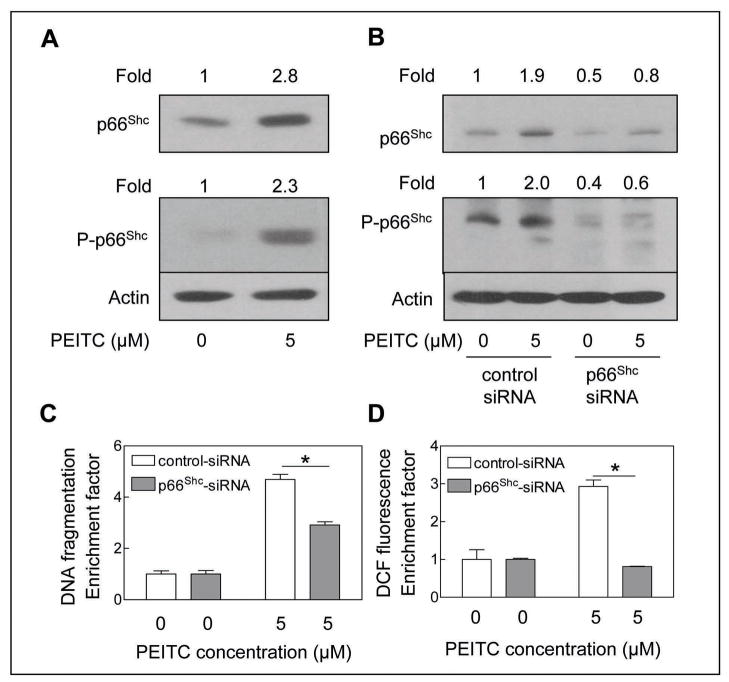
We raised the question of whether involvement of p66Shc protein in regulation of PEITC-induced apoptosis was unique to the PC-3 cell line or influenced by androgen responsiveness and p53 status. We addressed these questions using LNCaP cell line. The p53 connection was particularly intriguing because p66Shc protein has been shown to be a downstream target of the tumour suppressor p53 (31). Similar to PC-3 cells, the PEITC treatment (5 μmol/L; 24 h) resulted in induction as well as increased Ser36 phosphorylation of p66Shc in LNCaP cells (Fig. 3A). Moreover, a 50% depletion of p66Shc protein level by transient transfection of LNCaP cells with the p66Shc-targeted siRNA (Fig. 3B) caused statistically significant protection against PEITC-induced DNA fragmentation (Fig. 3C) as well as peroxide production (Fig. 3D) compared with cells transfected with non-specific siRNA. Collectively, these results indicated that the p66Shc protein was essential for apoptosis resulting from PEITC treatment in prostate cancer cells irrespective of their androgen-responsiveness or the p53 status. Based on these results, we also conclude that p66Shc protein functions upstream of ROS production in the molecular circuitry of PEITC-induced apoptosis.
Fig. 3.
A, immunoblotting for total and Ser36 phosphorylated p66Shc using lysates from LNCaP cells treated with DMSO (control) or 5 μmol/L PEITC for 24 h. B, immunoblotting for total and Ser36 phosphorylated p66Shc using lysates from LNCaP cells transiently transfected with a control non-specific siRNA or p66Shc-targeted siRNA and treated for 24 h with DMSO or 5 μmol/L PEITC. The blots were stripped and re-probed with anti-actin antibody to ensure equal protein loading. The numbers on top of the immunoreactive bands represent changes in protein levels relative to DMSO-treated control (panel A) or relative to DMSO-treated non-specific control siRNA transfected cells (panel B). C, cytoplasmic histone-associated apoptotic DNA fragmentation, and D, DCF fluorescence in LNCaP cells transiently transfected with a control non-specific siRNA or p66Shc-targeted siRNA and treated for 24 h (panel C) and 4 h (panel D) with DMSO or 5 μmol/L PEITC. The results are expressed as enrichment factor relative to DMSO-treated control for LNCaP cells transiently transfected with the non-specific siRNA. Each experiment was done twice and representative data from a single experiment are shown. Columns, mean (n=3); bars, SE. *Significantly different (P<0.05) between the indicated groups by paired t-test.
PEITC-induced apoptosis was inhibited by pharmacologic inhibition of PKCβ
We used a cell permeable pharmacologic inhibitor of PKCβ to determine its role in PEITC-mediated increase in Ser36 phosphorylation of p66Shc. As shown in supplemental Fig. S2 (panel A), increased Ser36 phosphorylation of p66Shc resulting from PEITC exposure (5 μmol/L; 24 h) was significantly inhibited in the presence of PKCβ-I in PC-3 cells. Moreover, co-treatment of PC-3 cells with PKCβ-I conferred statistically significant protection against PEITC-induced cytoplasmic histone-associated apoptotic DNA fragmentation (supplemental Fig. S2, panel B). These results indicated that the PEITC-mediated Ser36 hyperphosphorylation of p66Shc was mediated by PKCβ.
PEITC treatment promoted mitochondrial translocation of p66Shc
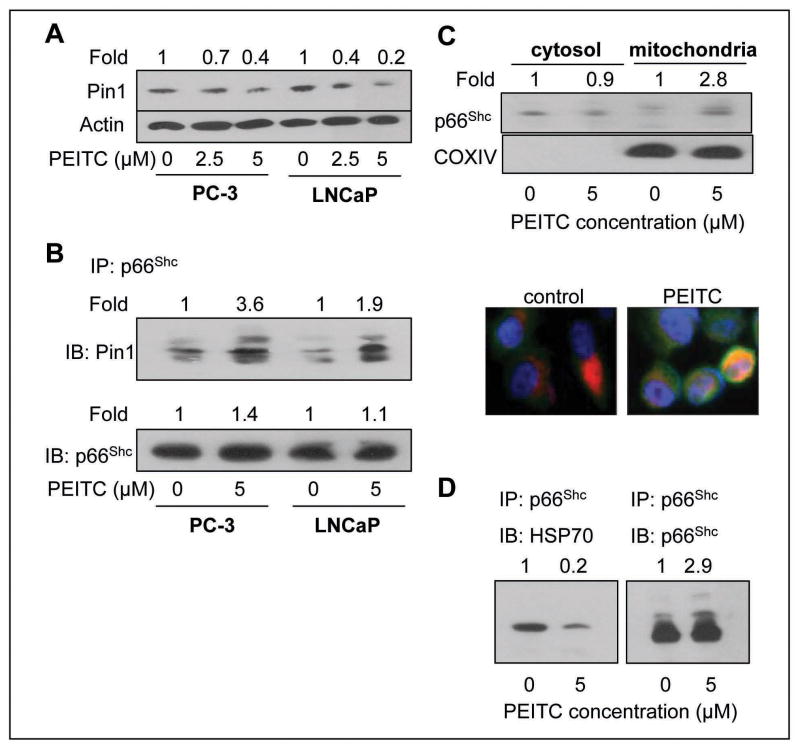
Prolyl isomerase Pin1 has been shown to play an important role in mitochondrial translocation of p66Shc (30). Initially, we determined the effect of PEITC treatment on protein level of Pin1 by immunoblotting (Fig. 4A). The protein level of Pin1 was decreased by about 30–80% by a 24 h treatment of PC-3 and LNCaP cells with 2.5 and 5 μmol/L PEITC (Fig. 4A). Despite reduction in Pin1 protein level, the PEITC treatment (5 μmol/L, 8 h) resulted in increased binding of p66Shc protein with Pin1 in both cell lines as revealed by immunoprecipitation using anti-p66Shc antibody followed by immunoblotting with anti-Pin1 antibody (Fig. 4B). The blot was also probed with anti-p66Shc antibody to ensure equal immunoprecipitation (Fig. 4B).
Fig. 4.
A, immunoblotting for Pin1 using lysates from PC-3 and LNCaP cells following 24 h treatment with DMSO or the indicated concentrations of PEITC. The blot was stripped and reprobed with anti-actin antibody to ensure equal protein loading. The numbers on top of the immunoreactive bands represent change in protein levels relative to corresponding DMSO-treated control. B, immunoblotting for Pin1 using p66Shc immunoprecipitates from PC-3 and LNCaP cells treated for 8 h with DMSO (control) or 5 μmol/L PEITC. The numbers on top of the immunoreactive bands represent change in levels relative to DMSO-treated control for each cell line. C, immunoblotting for p66Shc using isolated cytosolic and mitochondrial fractions from PC-3 cells following 8 h treatment with DMSO or 5 μmol/L PEITC. The blot was reprobed with anti-COXIV antibody to ensure purity of the mitochondrial fraction. The numbers on top of the immunoreactive bands represent change in levels relative to DMSO-treated control. Immunofluorescence microscopic analysis for p66Shc localization in PC-3 cells following 8 h treatment with DMSO or 2.5 μmol/L PEITC (magnification 100×). The staining for mitochondria (MitoTracker red), p66Shc, and nuclei are indicated by red, green and blue fluorescence, respectively. D, immunoblotting for HSP70 using p66Shc immunoprecipitates (using anti-p66Shc antibody) from control (DMSO, 8 h) or PEITC-treated (5 μmol/L, 8 h) PC-3 cells. The numbers on top of the immunoreactive bands represent change in levels relative to DMSO-treated control.
Next, we proceeded to test whether PEITC treatment caused mitochondrial translocation of p66Shc by immunoblotting using isolated cytosolic and mitochondrial fractions from PC-3 cells. As can be seen in Fig. 4C, the p66Shc protein was detectable in both cytosolic and mitochondrial fractions of DMSO-treated control PC-3 cells. However, the mitochondrial level of p66Shc protein was increased by about 2.8-fold upon 8 h treatment of PC-3 cells with 5 μmol/L PEITC compared with DMSO-treated control (Fig. 4C, upper panel). Probing of the blot with anti-COXIV antibody confirmed purity of the mitochondrial fraction. The PEITC-mediated translocation of p66Shc to the mitochondria was confirmed by immunofluorescence microscopy (Fig. 4C, lower panel). Intensity of the p66Shc-associated green fluorescence was very weak in DMSO-treated control PC-3 cells. On the other hand, 8 h treatment of PC-3 cells with 2.5 μmol/L PEITC resulted in marked increase in green fluorescence due to induction of p66Shc protein (Fig. 4C), which was consistent with the immunoblotting data shown in Fig. 2A. Moreover, the PEITC-treated PC-3 cells exhibited increased mitochondrial localization of p66Shc as evidenced by appearance of yellow-orange color due to the merging of MitoTracker-associated red fluorescence and p66Shc-associated green fluorescence (Fig. 4C, lower panel). Collectively, these results indicated that PEITC treatment caused increased interaction of p66Shc with Pin1 and resulted in mitochondrial translocation of p66Shc.
PEITC treatment inhibited interaction between p66Shc and mitochondrial HSP70
Previous studies have shown that mitochondrial p66Shc complexes with HSP70, and disruption of this interaction is necessary for regulation of trans-membrane potential (32). We therefore tested the possibility whether PEITC treatment affected interaction between p66Shc and HSP70. As can be seen in Fig. 4D, the p66Shc-HSP70 complex was detectable in DMSO-treated control PC-3 cells as evidenced by immunoprecipitation using anti-p66Shc antibody followed by immunoblotting with the anti-HSP70 antibody (Fig. 4D). The level of p66Shc-HSP70 complex was decreased by about 80% upon 8 h treatment of PC-3 cells with 5 μmol/L PEITC (Fig. 4D). These results showed that PEITC treatment disrupted interaction between p66Shc and HSP70.
Oral administration of PEITC increased Ser36 phosphorylation of p66Shc in PC-3 xenografts
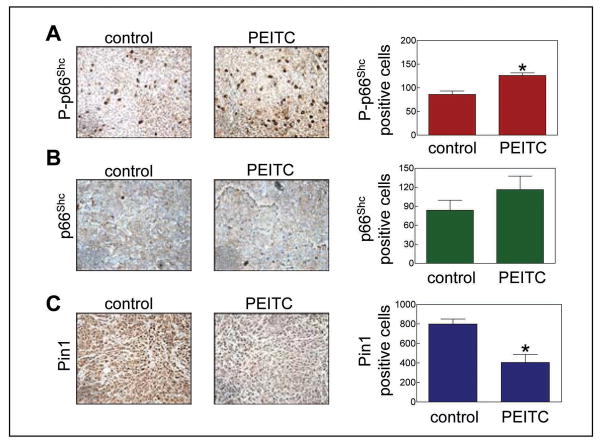
We have shown previously that oral administration of PEITC inhibits growth of PC-3 cells implanted in male athymic mice in association with apoptosis induction (21). The average tumor volume on the day of sacrifice (38 days after tumor cell implantation) in mice gavaged with 9 μmol PEITC was lower by about 48% compared with the vehicle-treated control mice (21). Fraction of apoptotic bodies was about 4-fold higher in tumor sections from PEITC-treated mice compared with control tumors (21). In the present study, we used tumor sections from these mice to determine in vivo significance of our cellular findings. As shown in Fig. 5A, the tumor sections from PEITC-treated mice exhibited statistically significant increase in the level of Ser36 phosphorylated p66Shc compared with control tumor sections, which was consistent with data in cultured PC-3 cells (Fig. 2A). Even though PEITC treatment resulted in a modest increase in the level of total p66Shc (Fig. 5B), the difference did not reach statistical significance. In agreement with cellular data (Fig. 4A), the level of Pin1 protein was ~50% lower (P<0.05 compared with control by paired t-test) in the tumor sections from PEITC-treated mice compared with control tumor sections (Fig. 5C). These results showed that oral administration of PEITC resulted in increased Ser36 phosphorylation of p66Shc as well as down-regulation of Pin1 protein in PC-3 tumor xenograft in vivo.
Fig. 5.
Immunohistochemical analysis for expression of (A) Ser36 phosphorylated p66Shc, (B) total p66Shc, and (C) Pin1 (200× magnification) in representative tumor section of a vehicle-treated control mouse and a PEITC-treated mouse. Right panel, quantitation of immunohistochemical data. Columns, mean (n= 3); bars, SE. *Significantly different (P<0.05) compared with control by paired t-test.
Discussion
Previous studies have shown that cells lacking p66Shc protein resist oxidation of ROS-sensitive chemical probes and display reduced accumulation of endogenous oxidative stress marker 8-oxo-guanosine (31,33). Furthermore, the p66Shc knockout mice exhibit diminished levels of systemic (isoprostane) as well as intracellular (8-oxo-guanosine) markers of oxidative stress (31,33,34). Genetic deletion of p66Shc in cells also confers protection against apoptosis induced by ultraviolet radiation, staurosporin, and growth factor deprivation (32,35,36). Moreover, the p66Shc knockout mice are resistant to apoptosis induction by paraquat and ischemia (34,35,37). Because proapoptotic effect of PEITC in cancer cells is linked to ROS production (19,22), we raised the question of whether molecular circuitry of PEITC-induced apoptosis was dependent on p66Shc. Results presented herein indicate that the p66Shc protein is indeed essential for PEITC-induced apoptosis. This conclusion is substantiated by the observed resistance of immortalized MEFs derived from p66Shc−/− mice to growth suppression and apoptosis induction by PEITC compared with WT fibroblasts. Moreover, knockdown of p66Shc protein by RNA interference significantly inhibits PEITC-induced apoptosis in PC-3 and LNCaP cells. Another important conclusion from the present study is that p66Shc protein functions upstream of ROS production in execution of PEITC-induced apoptosis. Previous studies have shown that electron transfer between cytochrome c and p66Shc leads to production of hydrogen peroxide (29). It is probable that PEITC treatment promotes p66Shc-mediated oxidation of reduced cytochrome c to trigger ROS production in our model. Further work is needed to directly test this possibility.
The PC-3 and LNCaP cells were used in the present study not only to authenticate generality of the p66Shc-dependence of PEITC-induced apoptosis but also to determine possible influence of p53 in this response. The p53 status is known to influence stability of the p66Shc protein in response to treatment with UV and hydrogen peroxide (31). The PEITC-mediated induction of p66Shc protein expression was relatively more pronounced in the LNCaP cell line with intact p53 compared with PC-3 cells lacking functional p53. Because PEITC treatment results in modest induction of p66Shc protein in the PC-3 cell line, we can conclude that the induction of p66Shc protein in our model is not solely dependent on the p53 status. The LNCaP cell line is relatively more sensitive to PEITC-mediated apoptotic DNA fragmentation compared with PC-3 cells. On the other hand, the PC-3 cells express nearly 4-fold higher level of p66Shc protein compared with LNCaP C-33 cells (38). A role for p66Shc and ROS in androgen-induced prostate cancer cell proliferation has also been documented by the same group of investigators (39). Because the p66Shc protein promotes PEITC-induced apoptosis in our model, we conclude that differential sensitivity of PC-3 and LNCaP cells to proapoptotic effect of PEITC per se is not related to difference in p66Shc protein level.
Protein kinase Cβ-mediated Ser36 phosphorylation of p66Shc has been documented to promote its mitochondrial accumulation in a prooxidant environment even though this protein lacks conventional mitochondria-targeting sequence (30). Instead, the oxidative stress-induced (e.g., H2O2) mitochondrial enrichment of p66Shc is facilitated by interaction with peptidyl-prolyl isomerase Pin1 (30). The precise mechanism to explain Pin1-dependent mitochondrial import of p66Shc is still not clear. The present study reveals that PEITC treatment not only intensifies Ser36 phosphorylation of p66Shc in prostate cancer cells but also increases its binding with Pin1 as evidenced by immunoprecipitation-immunoblotting experiments. Consistent with these results mitochondrial enrichment of p66Shc is evident upon treatment with PEITC. We also found that PEITC treatment reduces protein level of Pin1 in both PC-3 and LNCaP cell lines. Interestingly, Pin1 has been shown to facilitate cytokine-induced survival of eosinophils by suppressing Bax activation (40). It is plausible that down-regulation of Pin1 contributes to Bax activation in PEITC-induced apoptosis. This possibility is likely because cell death resulting from PEITC exposure is inhibited in cells lacking Bax (18).
In vivo validation of the cellular mechanistic observations is critical for identification of biomarkers(s) of response potentially useful in future clinical trials. Biomarker discovery is especially critical because cancer incidence is too rigorous of an end point for chemoprevention trials. We found that oral administration of PEITC to the PC-3 xenograft-bearing male athymic mice not only retards tumor growth in association with apoptosis induction (21) but also increases Ser36 phosphorylation of p66Shc and down-regulates Pin1 protein level (present study). These observations suggest that Ser36 phosphorylated p66Shc and Pin1 protein level may be viable biomarkers of PEITC response.
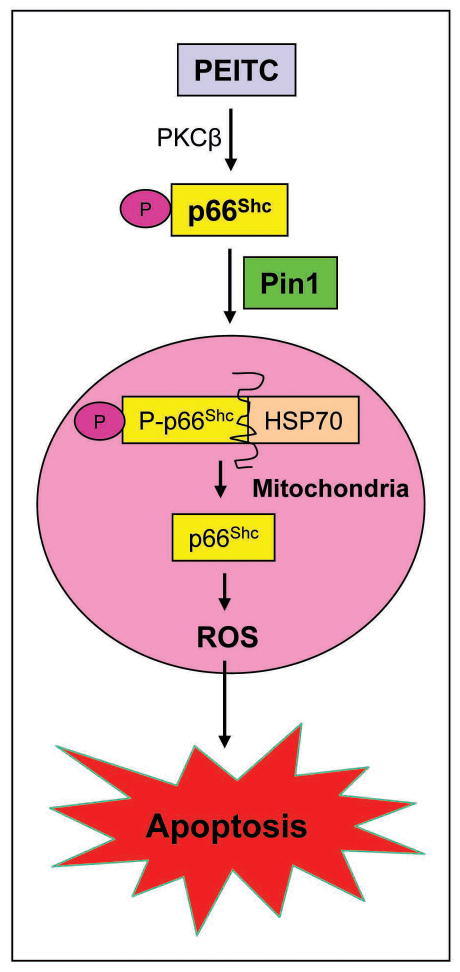
In conclusion, as summarized in Fig. 6 the results presented herein indicate that PEITC treatment enhances PKCβ-mediated Ser36 phosphorylation of p66Shc, which increases its binding with Pin1. The binding of p66Shc with Pin1 promotes translocation of p66Shc to the mitochondria. The p66Shc protein localized to the mitochondria is released from HSP70 and causes ROS production leading to caspase-3 activation and apoptotic cell death.
Fig. 6.
A mechanistic model explaining role of p66Shc in PEITC-induced apoptosis. Based on the results of the present study, we conclude that PEITC treatment increases Ser36 phosphorylation of p66Shc, which increases its binding with Pin1. The binding of p66Shc with Pin1 promotes translocation of p66Shc to the mitochondria. The p66Shc protein localized to the mitochondria is released from HSP70 and causes ROS production leading to caspase-3 activation and eventual apoptotic cell death.
Supplementary Material
Acknowledgments
Grant support: This investigation was supported by the USPHS grant CA101753-07 awarded by the National Cancer Institute.
Immortalized mouse embryonic fibroblast derived from wild-type and p66Shc−/− were generously provided by Dr. T. Finkel and S. Nemoto to J. Antosiewicz (a post-doctoral research associate in our laboratory) and S.V. Singh. Authors thank Julie A. Arlotti and Yan Zeng for technical assistance.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer Statistics. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nature Rev Cancer. 2003;3:768–80. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 3.Verhoeven DT, Goldbohm RA, van Poppel G, Verhagen H, van den Brandt PA. Epidemiological studies on brassica vegetables and cancer risk. Cancer Epidemiol Biomarkers Prev. 1996;5:733–48. [PubMed] [Google Scholar]
- 4.Kolonel LN, Hankin JH, Whittemore AS, et al. Vegetables, fruits, legumes and prostate cancer: a multiethnic case-control study. Cancer Epidemiol Biomarkers Prev. 2000;9:795–804. [PubMed] [Google Scholar]
- 5.Ambrosone CB, McCann SE, Freudenheim JL, Marshall JR, Zhang Y, Shields PG. Breast cancer risk in premenopausal women is inversely associated with consumption of broccoli, a source of isothiocyanates, but is not modified by GST genotype. J Nutr. 2004;134:1134–8. doi: 10.1093/jn/134.5.1134. [DOI] [PubMed] [Google Scholar]
- 6.Hecht SS. Inhibition of carcinogenesis by isothiocyanates. Drug Metab Rev. 2000;32:395–411. doi: 10.1081/dmr-100102342. [DOI] [PubMed] [Google Scholar]
- 7.Fahey JW, Zalcmann AT, Talalay P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochem. 2001;56:5–51. doi: 10.1016/s0031-9422(00)00316-2. [DOI] [PubMed] [Google Scholar]
- 8.Morse MA, Wang CX, Stoner GD, et al. Inhibition of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone induced DNA adduct formation and Tumorigenicity in lung of F344 rats by dietary phenethyl isothiocyanate. Cancer Res. 1989;49:549–53. [PubMed] [Google Scholar]
- 9.Hecht SS, Trushin N, Rigotty J, et al. Complete inhibition of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced rat lung tumorigenesis and favorable modification of biomarkers by phenethyl isothiocyanate. Cancer Epidemiol Biomarkers Prev. 1996;5:645–52. [PubMed] [Google Scholar]
- 10.Pereira MA. Chemoprevention of diethylnitrosamine-induced liver foci and hepatocellular adenomas in C3H mice. Anticancer Res. 1995;15:1953–6. [PubMed] [Google Scholar]
- 11.Stoner GD, Morrissey DT, Heur YH, Galati AJ, Daniel EM, Wagner SA. Inhibitory effects of Phenethyl isothiocyanate on N-nitrosobenzylmethylamine carcinogenesis in the rat esophagus. Cancer Res. 1991;51:2063–8. [PubMed] [Google Scholar]
- 12.Stoner GD, Dombkowski AA, Reen RK, et al. Carcinogen-altered genes in rat esophagus positively modulated to normal levels of expression by both black raspberries and phenylethyl isothiocyanate. Cancer Res. 2008;68:6460–7. doi: 10.1158/0008-5472.CAN-08-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khor TO, Cheung WK, Prawan A, Reddy BS, Kong AN. Chemoprevention of familial adenomatous polyposis in Apc(Min/+) mice by phenethyl isothiocyanate (PEITC) Mol Carcin. 2008;47:321–5. doi: 10.1002/mc.20390. [DOI] [PubMed] [Google Scholar]
- 14.Chen YR, Han J, Kori R, Kong AN, Tan TH. Phenylethyl isothiocyanate induces apoptotic signaling via suppressing phosphatase activity against c-Jun N-terminal kinase. J Biol Chem. 2002;277:39334–42. doi: 10.1074/jbc.M202070200. [DOI] [PubMed] [Google Scholar]
- 15.Xiao D, Singh SV. Phenethyl isothiocyanate-induced apoptosis in p53-deficient PC-3 human prostate cancer cell line is mediated by extracellular signal-regulated kinases. Cancer Res. 2002;62:3615–9. [PubMed] [Google Scholar]
- 16.Xiao D, Johnson CS, Trump DL, Singh SV. Proteasome-mediated degradation of cell division cycle 25C and cyclin-dependent kinase 1 in Phenethyl isothiocyanate-induced G2-M-phase cell cycle arrest in PC-3 human prostate cancer cells. Mol Cancer Ther. 2004;3:567–75. [PubMed] [Google Scholar]
- 17.Xu C, Shen G, Chen C, Gelinas C, Kong AN. Suppression of NF-kappaB and NF-kappaB-regulated gene expression by sulforaphane and PEITC through lkappaBalpha, IKK pathway in human prostate cancer PC-3 cells. Oncogene. 2005;24:4486–95. doi: 10.1038/sj.onc.1208656. [DOI] [PubMed] [Google Scholar]
- 18.Xiao D, Zeng Y, Choi S, Lew KL, Nelson JB, Singh SV. Caspase-dependent apoptosis induction by phenethyl isothiocyanate, a cruciferous vegetable-derived cancer chemopreventive agent, is mediated by Bak and Bax. Clin Cancer Res. 2005;11:2670–9. doi: 10.1158/1078-0432.CCR-04-1545. [DOI] [PubMed] [Google Scholar]
- 19.Xiao D, Lew KL, Zeng Y, et al. Phenethyl isothiocyanate-induced apoptosis in PC-3 human prostate cancer cells is mediated by reactive oxygen species-dependent disruption of the mitochondrial membrane potential. Carcinogenesis. 2006;27:2223–34. doi: 10.1093/carcin/bgl087. [DOI] [PubMed] [Google Scholar]
- 20.Xiao D, Singh SV. Phenethyl isothiocyanate inhibits angiogenesis in vitro and ex vivo. Cancer Res. 2007;67:2239–46. doi: 10.1158/0008-5472.CAN-06-3645. [DOI] [PubMed] [Google Scholar]
- 21.Bommareddy A, Hahm ER, Xiao D, et al. Atg5 regulates phenethyl isothiocyanate-induced autophagic and apoptotic cell death in human prostate cancer cells. Cancer Res. 2009;69:3704–12. doi: 10.1158/0008-5472.CAN-08-4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trachootham D, Zhou Y, Zhang H, et al. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell. 2006;10:241–52. doi: 10.1016/j.ccr.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 23.Kim YA, Xiao D, Xiao H, et al. Mitochondria-mediated apoptosis by diallyl trisulfide in human prostate cancer cells is associated with generation of reactive oxygen species and regulated by Bax/Bak. Mol Cancer Ther. 2007;6:1599–609. doi: 10.1158/1535-7163.MCT-06-0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiao D, Srivastava SK, Lew KL, et al. Allyl isothiocyanate, a constituent of cruciferous vegetables, inhibits proliferation of human prostate cancer cells by causing G2/M arrest and inducing apoptosis. Carcinogenesis. 2003;24:891–7. doi: 10.1093/carcin/bgg023. [DOI] [PubMed] [Google Scholar]
- 25.Xiao D, Choi S, Johnson DE, et al. Diallyl trisulfide-induced apoptosis in human prostate cancer cells involves c-Jun N-terminal kinase and extracellular-signal regulated kinase-mediated phosphorylation of Bcl-2. Oncogene. 2004;23:5594–606. doi: 10.1038/sj.onc.1207747. [DOI] [PubMed] [Google Scholar]
- 26.Singh SV, Srivastava SK, Choi S, et al. Sulforaphane-induced cell death in human prostate cancer cells is initiated by reactive oxygen species. J Biol Chem. 2005;280:19911–24. doi: 10.1074/jbc.M412443200. [DOI] [PubMed] [Google Scholar]
- 27.Singh SV, Powolny AA, Stan SD, et al. Garlic constituent diallyl trisulfide prevents development of poorly-differentiated prostate cancer and pulmonary metastasis multiplicity in TRAMP mice. Cancer Res. 2008;68:9503–11. doi: 10.1158/0008-5472.CAN-08-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pelicci G, Lanfrancone L, Grignani F, et al. A novel transforming protein (SHC) with an SH2 domain is implicated in mitogenic signal transduction. Cell. 1992;70:93–104. doi: 10.1016/0092-8674(92)90536-l. [DOI] [PubMed] [Google Scholar]
- 29.Giorgio M, Migliaccio E, Orsini F, et al. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell. 2005;122:221–33. doi: 10.1016/j.cell.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 30.Pinton P, Rimessi A, Marchi S, et al. Protein kinase C Beta and prolyl isomerase 1 regulate mitochondrial effects of the life-span determinant p66Shc. Science. 2007;315:659–63. doi: 10.1126/science.1135380. [DOI] [PubMed] [Google Scholar]
- 31.Trinei M, Giorgio M, Cicalese A, et al. A p53-p66Shc signalling pathway controls intracellular redox status, levels of oxidation-damaged DNA and oxidative stress-induced apoptosis. Oncogene. 2002;21:3872–8. doi: 10.1038/sj.onc.1205513. [DOI] [PubMed] [Google Scholar]
- 32.Orsini F, Migliaccio E, Moroni M, et al. The life span determinant p66Shc localizes to mitochondria where it associates with mitochondrial heat shock protein 70 and regulates trans-membrane potential. J Biol Chem. 2004;279:25689–95. doi: 10.1074/jbc.M401844200. [DOI] [PubMed] [Google Scholar]
- 33.Nemoto S, Finkel T. Redox regulation of forkhead proteins through a p66shc-dependent signaling pathway. Science. 2002;295:2450–2. doi: 10.1126/science.1069004. [DOI] [PubMed] [Google Scholar]
- 34.Napoli C, Martin-Padura I, de Nigris F, et al. Deletion of the p66Shc longevity gene reduces systemic and tissue oxidative stress, vascular cell apoptosis, and early atherogenesis in mice fed a high-fat diet. Proc Natl Acad Sci USA. 2003;100:2112–6. doi: 10.1073/pnas.0336359100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Migliaccio E, Giorgio M, Mele S, et al. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature. 1999;402:309–13. doi: 10.1038/46311. [DOI] [PubMed] [Google Scholar]
- 36.Pacini S, Pellegrini M, Migliaccio E, et al. P66Shc promotes apoptosis and antagonizes mitogenic signaling in T cells. Mol Cell Biol. 2004;24:1747–57. doi: 10.1128/MCB.24.4.1747-1757.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zaccagnini G, Martelli F, Fasanaro P, et al. P66ShcA modulates tissue response to hindlimb ischemia. Circulation. 2004;109:2917–23. doi: 10.1161/01.CIR.0000129309.58874.0F. [DOI] [PubMed] [Google Scholar]
- 38.Veeramani S, Igawa T, Yuan TC, et al. Expression of p66Shc protein correlates with proliferation of human prostate cancer cells. Oncogene. 2005;24:7203–12. doi: 10.1038/sj.onc.1208852. [DOI] [PubMed] [Google Scholar]
- 39.Veeramani S, Yuan TC, Lin FF, Lin MF. Mitochondrial redox signaling by p66Shc is involved in regulating androgenic growth stimulation of human prostate cancer cells. Oncogene. 2008;27:5057–68. doi: 10.1038/onc.2008.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen Z, Esnault S, Schinzel A, Borner C, Malter JS. The peptidyl-prolyl isomerase Pin1 facilitates cytokine-induced survival of eosinophils by suppressing Bax activation. Nature Immunol. 2009;10:257–65. doi: 10.1038/ni.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.