Abstract
Hypersensitivity pneumonitis (HP) is a T-cell-driven disease that is histologically characterized by diffuse mononuclear cell infiltrates and loosely formed granulomas in the lungs. We have previously reported that interleukin-17A (IL-17A) contributes to the development of experimental HP, and that the pattern recognition receptor Toll-like receptor 6 (TLR6) might be a factor in the initiation of this response. Using a well-established murine model of Saccharopolyspora rectivirgula-induced HP, we investigated the role of TLR6 in the immunopathogenesis of this disease. In the absence of TLR6 signalling, mice that received multiple challenges with S. rectivirgula-antigen (SR-Ag) had significantly less lung inflammation compared with C57BL/6 mice (wild-type; WT) similarly challenged with SR-Ag. Flow cytometric analysis of whole lung samples from SR-Ag-challenged mice showed that TLR6−/− mice had a decreased CD4+ : CD8+ T-cell ratio compared with WT mice. Cytokine analysis at various days after the final SR-Ag challenge revealed that whole lungs from TLR6−/− mice contained significantly less IL-17A than lungs from WT mice with HP. The IL-17A-driving cytokines IL-21 and IL-23 were also expressed at lower levels in SR-Ag-challenged TLR6−/− mice, when compared with SR-Ag-challenged WT mice. Other pro-inflammatory cytokines, namely interferon-γ and RANTES, were also found to be regulated by TLR6 signalling. Anti-TLR6 neutralizing antibody treatment of dispersed lung cells significantly impaired SR-Ag-induced IL-17A and IL-6 generation. Together, these results indicate that TLR6 plays a pivotal role in the development and severity of HP via its role in IL-17A production.
Keywords: hypersensitivity pneumonitis, interleukin-17A, Toll-like receptor-6
Introduction
Hypersensitivity pneumonitis (HP) is an interstitial lung disease caused by repeated inhalation of organic antigens and low-molecular-weight inorganic particles. Hypersensitivity pneumonitis is an alveolar and interstitial disease; lung biopsy from an individual with HP is histologically characterized by poorly formed, non-necrotizing granulomas and interstitial mononuclear cell infiltration.1,2 Sources of the offending antigen are many, and include bacterial, fungal, animal, or inorganic chemicals (i.e. isocyanates).3,4 Interestingly, despite ubiquitous exposure to these factors, only 5–15% of exposed individuals ever develop HP.5 The risk factors of HP are poorly characterized and often require a high index of suspicion to make a diagnosis. The disease presents in acute, subacute, and chronic forms, depending on the amount and duration of exposure, as well as individual level of susceptibility, with the chronic form often leading to fibrotic disease.3,6
Histopathological examination of lung biopsies from patients with HP have shown B cells, but the exact role of these lymphocytes in HP pathogenesis has not been investigated.7,8 Experimental and clinical evidence point towards a T-cell-mediated mechanism driving HP. For example, athymic nude mice lacking T cells developed significantly reduced pathology upon chronic exposure to HP-inducing Thermoactinomyces vulgaris antigen.9 CD4+ and CD8+ T cells are recruited to the lungs of mice exposed to Saccharopolyspora rectivirgula antigen (SR-Ag), with CD4+ T cells predominating.1,10 These CD4+ T cells are markedly skewed toward a T helper type 1 (Th1) phenotype, and it has been shown that Th1-biased C57BL/6 mice are more susceptible to the development of HP, than are Th2-biased DBA/2 mice.11,12 Increased levels of inflammatory mediators such as interferon-γ (IFN-γ), monocyte chemoattractant protein 1, macrophage inflammatory protein 1α, interleukin-6 (IL-6) and IL-12 are also present in mice after exposure to SR-Ag, and IFN-γ (a Th1-type cytokine) has been shown to be necessary for the development of HP.13,14 Finally, adoptively transferred Th1-sensitized CD4+ T cells have the ability to induce HP in healthy mice, and the overexpression of the Th2 transcription factor GATA-3 is protective against HP.15,16 Taken together, these studies illustrate a critical Th1 component in the development of HP.
Many pathologies that were thought to be solely Th1-mediated, have more recently been shown to require IL-17A.17–19 Accordingly, we and others have shown that the gene deletion or in vivo neutralization of IL-17A in an experimental model of HP driven by repeated SR-Ag challenges results in protection from HP, indicating that IL-17A and Th17 cells are also major driving factors.20,21 The IL-17A and Th17 cells drive a pro-inflammatory immune response by inducing chemokine and chemoattractant production from resident immune and stromal cells.22 Subsequently, neutrophils and other immune cells are recruited, thereby intensifying the inflammatory response.23 Th17 differentiation requires the presence of IL-6 and transforming growth factor-β (TGF-β), while expansion and growth of this T-cell population is regulated by IL-23,24–27 and IL-21 has been shown to be necessary for induction of Th17 cells.28,29 The exact signalling mechanisms that lead to Th17 differentiation during immune responses such as HP are unclear.
It has been shown that recognition of pathogen-associated molecular patterns by cell-surface receptors called pathogen-recognition receptors (PRR) is necessary for activation of host defense mechanisms.30 In our previous studies, expression of the PRR Toll-like receptor 6 (TLR6), was found to be elevated in experimental HP.20 The TLR6 forms a heterodimer with TLR2 and recognizes bacterial diacyl lipopeptides and lipoteichoic acid, a major constituent of Gram-positive bacteria.31 In this study, we investigated the role of TLR6 in experimental HP, using a well-established farmer’s lung model, which involves repeated oropharyngeal challenges of mice with the thermophilic actinomycete S. rectivirgula. Our findings reveal that IL-17A levels and cytokines associated with the regulation of IL-17A, were significantly reduced in SR-Ag-challenged mice lacking the TLR6 gene when compared with similarly challenged wild-type (WT) mice. The absence of TLR6 resulted in decreased lung inflammation and protection against HP. Also, neutralization of TLR6 in cultures of dispersed lung cells showed decreased production of IL-17A and its regulatory cytokines. Our observations collectively suggest that TLR6 is essential for the development of experimental HP, and raise the possibility that this TLR might be a therapeutic target in clinical HP.
Methods
Mice
Male and female C57BL/6 mice (WT; 6–8 weeks old) were purchased from Taconic Farms, Inc. (Hudson, NY). The TLR6−/− mice were generated as previously described in detail by Takeuchi et al.,32 and were bred at the University of Michigan. All mice were housed under specific pathogen-free conditions in the University Laboratory Animal Medicine facility at the University of Michigan Medical School. The Animal Use Committee at the University of Michigan approved all experiments described herein.
Saccharapolyspora rectivirgula-induced HP model
Saccharopolyspora rectivirgula (ATCC number 29034) was purchased from the American Type Culture Collection (Manassas, VA). The S. rectivirgula antigen (SR-Ag) was then prepared as previously described by Joshi et al.20 An oropharyngeal aspiration technique was used to instil 20 μg SR-Ag into the lungs of mice for 3 consecutive days per week for a total of 3 weeks, as described by Lakatos et al.33
Quantitative real-time polymerase chain reaction
At days 1, 4, 8 and 16 after the last SR-Ag challenge, right lung lobes were excised, flash frozen in liquid nitrogen and stored at − 80°. For isolation of RNA, one right lung lobe was thawed on ice. TRIzol (Invitrogen Life Technologies, Carlsbad, CA) was used for RNA extraction according to the manufacturer’s instructions. Next, RNA was converted to complementary DNA using Moloney murine leukaemia virus reverse transcriptase (Invitrogen). Real-time quantitative polymerase chain reaction analysis was carried out using an ABI PRISM 7700 detection system (Applied Biosystems, Foster City, CA). Pre-mixed primer/probe reagents were purchased from Applied Biosystems to detect TLR6, TLR2, dectin-1, IL-17A, IL-23, IL-21, IL-6, IFN-γ and regulated on activation normal T-cell expressed and secreted (RANTES) gene expression. Glyceraldehyde 3-phosphate-dehydrogenase was used as an internal control.
Detection of soluble cytokine and chemokine levels
To determine soluble cytokine and chemokine levels, a snap-frozen right lung lobe from each mouse was thawed on ice and homogenized in buffer containing Complete Mini™ (Roche Ltd, Basel, Switzerland) protease inhibitor, and 0·1% Triton-100. The samples were centrifuged, and 50 μl of cell-free supernatant was analysed using a multiplex bead-based assay. IL-13, IL-17A, IFN and RANTES were detected using Bio-Plex™ Mouse Grp I 11-plex cytokine panel from Bio-Rad (Hercules, CA). Enzyme-linked immunosorbent assay was used to measure IL-6 and TGF-β. The antibodies for the assay were purchased from R&D Systems (Minneapolis, MN).
Flow cytometry analysis
Whole left lung lobes were excised, minced and digested with collagenase (Sigma, St Louis, MO) at 37° for 30 min in a shaker. Erythrocytes were lysed using red blood cell lysis buffer and the remaining cells were washed and incubated with anti-CD16/32 to block Fc receptors (eBioscience, San Diego, CA). The following antibodies were used to stain the single cell suspensions: fluorescein isothiocyanate (FITC) -conjugated anti-CD4, phycoerythrin (PE) -conjugated anti-CD3, PECy7-conjugated anti-CD8, PECy5-conjugated anti-CD45, FITC-conjugated anti-F480 and PE-conjugated anti-CD11c. All conjugated antibodies were purchased from BD Biosciences (San Jose, CA). Beckman Coulter FC500 was used to examine the stained samples and flowjo software (Tree Star, Inc., Ashland, OR) was used to analyse the acquired data.
Histological examination
At days 1, 4, 8 and 16 after the last SR-Ag challenge, the left lobe from WT and TLR6−/− mice was excised. The lobe was then inflated with and fixed in 10% formalin. The fixed lung lobe was embedded in paraffin and 5-μm sections were then stained with haematoxylin & eosin. Images were captured using an Olympus BX40 microscope and ip lab spectrum software (Signal Analytics Corp., Vienna, VA). Lung sections were blindly scored following the method described by Hwang et al.34 At least 100 sections were examined at 200 × magnification and scored as follows: 0, no inflammation; 1, < 10% inflammation; 2, 10–30% inflammation; 3, 30–50% inflammation; 4, 50–80% inflammation; 5, > 80% inflammation.
Ex vivo lung culture for antigen restimulation
Lung lobes were excised from mice at days 1, 4, 8 and 16 after the last SR-Ag challenge. A single-cell suspension was obtained using the collagenase method (described above). We chose these time-points because they coincided with peak TLR6 (at day 1) and dectin-1 (at day 16) in WT mice after the last SR-Ag challenge. Approximately 5 × 106 to 8 × 106 cells/ml were cultured in triplicate with RPMI-1640 supplemented with 10% fetal bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin in the presence or absence of 5 μg/ml SR-Ag for 24 hr. Cell-free supernatants were stored at − 20° for further analysis by multiplex bead-based assay. RNA was extracted as previously described and analysed using real-time quantitative polymerase chain reaction analysis. One microgram per millilitre of TLR6 neutralizing antibody (InvivoGen, San Diego, CA), 1 μg/ml anti-dectin-1 neutralizing antibody (Hycult biotechnology, Uden, the Netherlands) or 1 μg/ml normal rat serum isotype control (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA), was added to other wells to examine the role of these receptors in vitro.
Statistical analysis
Three to five mice were used per group per time-point in each experiment. Student’s t-test or one-way analysis of varaince followed by Student’s Newman–Keuls post test were used to determine statistical significance. P values less than 0·05 were deemed statistically significant. Calculations were performed using prism 4.0 software for Macintosh (GraphPad Software, San Diego, CA).
Results
TLR6 and dectin-1 expression during experimental hypersensitivity pneumonitis
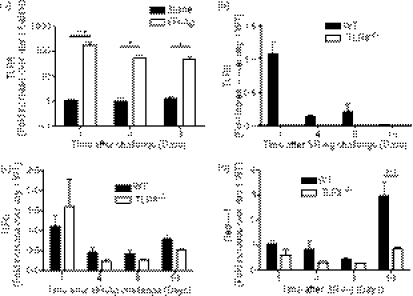
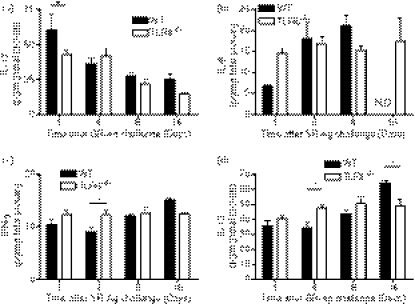
Changes in whole lung TLR6 transcript expression were first analysed during the course of experimental HP. A significant increase in TLR6 transcript expression was found in the SR-challenged mice at days 1, 4 and 8 after the last SR-Ag challenge compared with the saline-challenged control group (Fig. 1a). However, in SR-challenged mice, TLR6 transcript expression trended lower over time, with the highest expression observed at day 1 after the final SR-Ag challenge, and the lowest at day 16 (Fig. 1b). Because TLR6 forms a heterodimer with TLR2 to mediate its effects, we also analysed TLR2 transcript expression in WT and TLR6−/− mice with experimental HP.31 Again, peak TLR2 transcript expression was observed at day 1 after SR-Ag challenge and TLR2 expression was lower in the TLR6−/− mice at days 4, 8 and 16 after SR-Ag compared with the WT group (Fig. 1c). In contrast, dectin-1, a receptor noted to be involved in IL-17A generation, showed a significant increase in transcript expression at day 16 after SR-Ag in WT but not TLR6−/− mice. Dendritic cells have been shown to signal through the PRR dectin-1 to induce Th17 differentiation.35 Together, these data demonstrate that TLR6 is induced by SR-Ag, but its expression peaks early after the final SR-Ag challenge unlike dectin-1, which is induced at a much later time after the SR-Ag challenges have concluded.
Figure 1.
Pathogen recognition receptor expression in experimental hypersensitivity pneumonitis (HP). Whole lung samples were analysed by Taqman for gene expression. (a) Transcript expression of Toll-like receptor 6 (TLR6) in whole lungs of wild-type (WT) mice at 1, 4 and 8 days after their last exposure to Saccharopolyspora rectivirgula antigen (SR-Ag) or saline. Transcript expression for TLR6 (b), TLR2 (c), and Dectin-1 (d), in whole lungs from WT and TLR6−/− mice at 1, 4, 8 and 16 days after final SR-Ag challenge. Results are expressed as fold change over transcript expression in WT samples collected at day 1 after the last saline (a), or SR-Ag (b–d) challenge. Data represent the mean ± SEM, with n = 4 or n = 5 for each group. One-way analysis of variance and Newman–Keuls multiple comparison test were used to analyse significance between groups. *P ≤ 0·05, ***P ≤ 0·001.
Histopathological examination of TLR6+/+ and TLR6−/− lungs
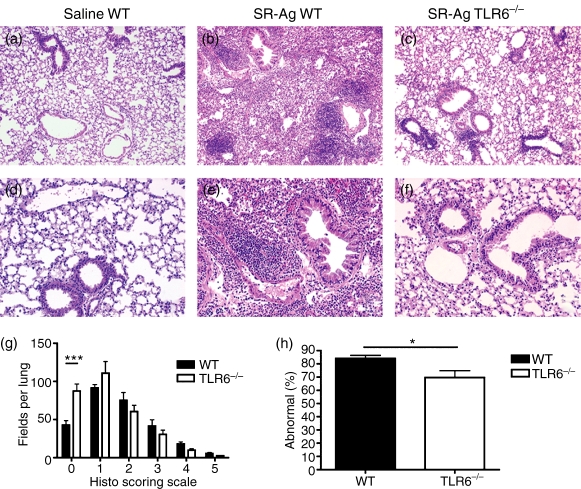
The TLR6 appeared to peak at day 1 after SR-Ag so histological changes in the lungs of SR-challenged mice were examined at this time. At day 1 after SR-Ag challenge, lungs from both WT (Fig. 2b,e) and TLR6−/− (Fig. 2c,f) mice exhibited mononuclear cell infiltration around the airways and vasculature when compared with lungs from saline-challenged WT mice (Fig. 2a,d). Overall, lung sections from SR-Ag-challenged TLR6−/− mice displayed a reduced inflammatory response. To quantify the difference in mononuclear cell infiltration, the lung sections were blindly scored. Significantly greater areas in the lungs of SR-Ag-challenged TLR6−/− mice were devoid of inflammatory infiltrate when compared with SR-Ag-challenged WT mice (Fig. 2g). When analysed as percentage of inflamed fields, TLR6−/− lung sections had a significantly lower percentage of inflamed areas compared with WT mice at day 1 after SR-Ag challenge (Fig. 2h). Taken together, these data suggest that the absence of TLR6 is protective in a murine model of HP, particularly at the very acute stages after SR-Ag challenge.
Figure 2.
Histological examination of whole lung samples from wild-type (WT) and Toll-like receptor-6-deficient (TLR6−/−) mice 1 day after the last Saccharopolyspora rectivirgula antigen (SR-Ag) challenge: magnification, 100 × (a–c), and 200 × (d–f). SR-Ag-challenged lungs from TLR6−/− mice (c,f) and saline-challenged lungs from WT mice (a,d), show reduced inflammation when compared with SR-Ag-challenged WT lungs (b,e). Histological scoring of whole lung sections revealed a higher number of fields devoid of inflammatory infiltrate (g) and a lower percentage of abnormal fields (h) in SR-Ag-challenged TLR6−/− mice when compared with SR-Ag-challenged WT mice. Data shown are mean ± SEM, with n = 4 or n = 5 for each group. One-way analysis of variance and Newman–Keuls multiple comparison test were used to analyse histological score significance between groups, and Student’s t-test was used to determine significance between WT and TLR6−/− lungs. *P ≤ 0·05, ***P ≤ 0·001.
Deletion of TLR6 affects lung cellularity
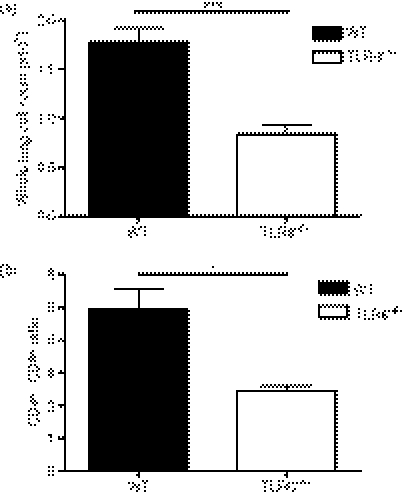
Cell suspensions from whole lung lobes were analysed by flow cytometry to determine the role of TLR6 in cell recruitment into the lung during HP. The cells were subsequently counted and the number of cells per mouse was averaged. At day 1 after SR-Ag challenge, TLR6−/− mice had significantly fewer lung cells per mouse when compared with SR-Ag-challenged WT mice (Fig. 3a). Previous studies have shown an increase in the CD4+ : CD8+ ratio in SR-Ag-induced murine HP.15,36 Using flow cytometry, we observed that the CD4+ : CD8+ ratio in SR-challenged TLR6−/− mice was significantly lower when compared with SR-challenged WT mice at day 1 after SR-Ag challenge (Fig. 3b). Hence the absence of TLR6 markedly impacted the recruitment of cells, particularly CD4+ T cells in the lungs of mice with experimental HP.
Figure 3.
Role of Toll-like receptor 6 (TLR6) in whole lung cellularity during experimental hypersensitivity pneumonitis (HP). (a) Total cell count of dispersed whole lung cells from mice at 1 day after the last Saccharopolyspora rectivirgula antigen (SR-Ag) challenge showed decreased number of cells from TLR6−/− mice compared with SR-Ag-challenged wild-type (WT) mice. (b) Flow cytometric analysis of dispersed lung cells showed a decreased CD4+ : CD8+ ratio in the lungs of TLR6−/− mice at 4 days after the last SR-Ag antigen challenge, when compared with SR-Ag-challenged WT mice. Data shown are mean ± SEM, with n = 4 or n = 5 for each group. Student’s t-test was used to determine significance between groups *P ≤ 0·05, ***P ≤ 0·001.
Whole lung cytokine transcript and protein levels from mice with experimental hypersensitivity pneumonitis
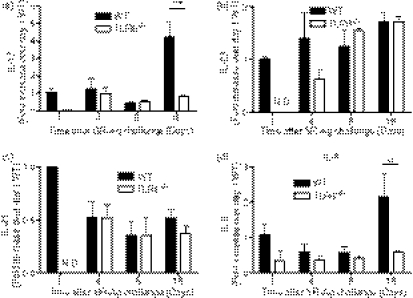
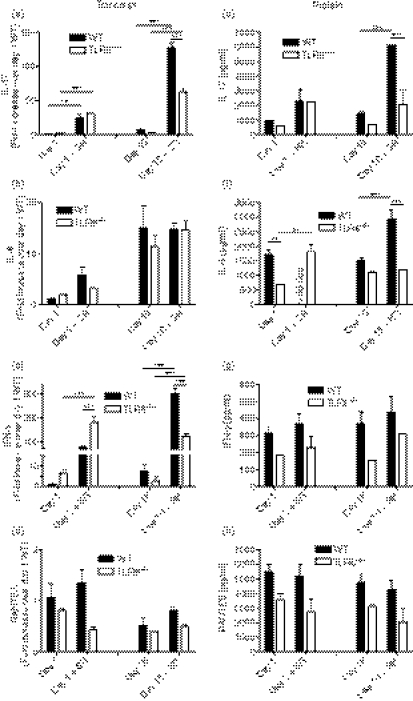
Transcript levels of various cytokines were next analysed to determine whether TLR6 altered the cytokine profile of mice exposed to SR-Ag. We and others have recently discovered that IL-17A and its related pathway play a major role in the immunological response of experimental SR-Ag-induced HP,20,21 and we first focused on this factor and its associated cytokines in the present study. As shown in Fig. 4(a), SR-Ag-challenged TLR6−/− mice showed decreased IL-17A transcripts at all days and a significant decrease at day 16 after the final SR-Ag challenge, when compared with SR-Ag-challenged WT mice (Fig. 4a). In SR-Ag-challenged TLR6−/− mice, IL-23 transcripts were lower on days 1 and day 4 after SR-Ag (Fig. 4b), and IL-21 (Fig. 4c) transcripts were decreased on day 1, compared with SR-Ag-challenged WT mice. Both cytokines play major roles in Th17 differentiation; IL-23 has a key role in the expansion and maintenance of Th17 cells whereas IL-21 is involved in Th17 differentiation.26,28,29,37,38 Transforming growth factor-β in the presence of IL-6 has been shown to be necessary for the generation of Th17 cells.25,27,39 Interleukin-6 transcript levels from SR-Ag-challenged TLR6−/− mice were decreased at all time-points, and significantly so on day 16, when compared with SR-Ag-challenged WT mice. Interestingly, TGF-β transcript expression was not different between the groups at any time-point after the last SR-Ag challenge (not shown). Together, these findings suggest that IL-17A and Th17-associated cytokines were decreased in SR-Ag-challenged TLR6−/− mice when compared with SR-Ag-challenged WT mice, suggesting that this PRR is required for full elaboration of IL-17A, and related cytokines.
Figure 4.
Interleukin-17A (IL-17A) and IL-17A-associated cytokine transcripts were decreased in Toll-like receptor-6-deficient (TLR6−/−) mice with experimental hypersensitivity pneumonitis (HP). Cyokine transcript analysis of whole lung samples was performed by Taqman. Transcript expression of IL-17A (a), IL-23 (b), IL-21 (c) and IL-6 (d), in whole lungs of wild-type (WT) and TLR6−/− mice at 1, 4, 8 and 16 days after the last Saccharopolyspora rectivirgula antigen (SR-Ag) challenge was determined. Results are expressed as fold change over transcript expression in WT samples collected at day 1 after the last SR-Ag challenge. Data represent the mean ± SEM, with n = 4 or n = 5 for each group. One-way analysis of variance and Newman–Keuls multiple comparison test were used to analyse significance between groups. **P ≤ 0·01, ***P ≤ 0·001.
We next examined the soluble cytokine levels from lung homogenate using a multiplex immunobead assay. The IL-17A protein levels in SR-Ag-challenged mice were decreased significantly at day 1 and were lower at day 16, after the final challenge, when compared with SR-Ag-challenged WT mice (Fig. 5a). Protein levels of IL-6 peaked at day 8 after the final SR-Ag challenge in whole lungs from WT mice and levels of this cytokine were not detected at day 16 in this group (Fig. 5b). In contrast, levels of IL-6 were constant throughout the time–course of this SR-Ag HP model in the TLR6−/− groups. These data suggest that changes in IL-6 levels in the whole lung depend upon the expression of TLR6.
Figure 5.
Protein levels in lungs of mice with experimental hypersensitivity pneumonitis (HP). Lungs were excised from Toll-like receptor-6-deficient (TLR6−/−)and wild-type (WT) mice at 1, 4, 8 and 16 days after the last Saccharopolyspora rectivirgula antigen challenge and analysed using a multiplex bead-based assay or enzyme-linked immunosorbent assay. Interleukin-17A (IL-17A) (a), IL-6 (b), interferon-γ (IFN-γ) (c) and IL-13 (d) protein levels were measured. Data represent the mean ± SEM, with n = 4 or n = 5 for each group. One-way analysis of variance and Newman–Keuls multiple comparison test were used to analyse significance between groups. *P ≤ 0·05.
Previous studies have demonstrated that IFN-γ expression is necessary for the development of the granulomatous inflammatory response in the lungs of mice with SR-induced HP.13 Gocke et al.40 have shown that Th1 and Th17 regulation is linked through the transcription factor T-bet. As shown in Fig. 5(c), IFN-γ was significantly increased at day 4 after SR-Ag challenge in TLR6−/− mice when compared with WT mice at the same time after SR-Ag (Fig. 5c). Finally, SR-Ag-challenged TLR6−/− mice showed increased levels of the Th2 cytokine, IL-13, at day 4, but significantly less IL-13 at day 16 when compared with SR-Ag-challenged WT mice, indicating that different signalling pathways could be used over time (Fig. 5d). Together, these data suggest that the absence of TLR6 has a major effect on the generation of IL-17A, IL-6, IFN-γ, and IL-13 during experimental HP.
TLR6 regulates SR-Ag recall responses in vitro
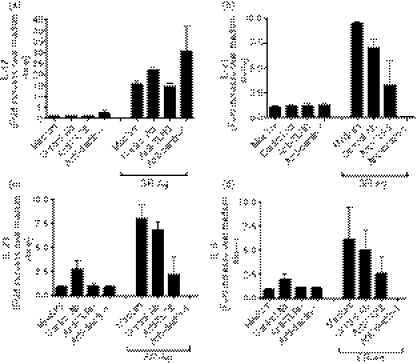
We next examined the synthetic ability of dispersed lung cells to generate cytokines after a 24-hr culture with or without SR-Ag. Transcript expression within the cells and protein levels in the cell-free culture supernatants were measured. Dispersed lung cells from SR-Ag-challenged mice that were restimulated in vitro with SR-Ag showed significantly elevated levels of IL-17A transcripts on days 1 and 16 when compared with lung cells cultured in medium alone (Fig. 6a). There was no difference in IL-17A transcript expression between the restimulated TLR6−/− cells and WT cells harvested from mice 1 day after the final SR-Ag challenge. However, at day 16 the lung cells from restimulated TLR6−/− mice showed significantly less IL-17A transcript levels when compared with lung cells from restimulated WT cells (Fig. 6a). Interleukin-6 transcripts were increased on day 1 in the dispersed lung cells that were restimulated with SR-Ag, whereas the lung cells restimulated with SR-Ag from SR-Ag-challenged TLR6−/− mice demonstrated a decrease in transcript expression on day 1 when compared with lung cells from SR-Ag-challenged WT mice (Fig. 6b). At day 16, SR-Ag restimulation did not result in elevated IL-6 transcript levels over that from cells cultured in medium alone. The IFN-γ transcript levels were significantly increased at days 1 and 16 in dispersed lung cells restimulated with SR-Ag, when compared with lung cells cultured in medium alone. However, TLR6−/− lung cells showed significantly increased transcript levels when compared with WT lung cells at day 1, and decreased transcripts compared with WT lung cells at day 16 (Fig. 6c). It has previously been shown that the IFN-γ-inducible chemotactic factor for T cells, RANTES, is secreted at elevated levels in patients and mice with HP.36,41 In our model, RANTES transcript levels were decreased in lung cells from all SR-Ag-challenged TLR6−/− mouse groups when compared with SR-Ag-challenged WT groups (Fig. 6d).
Figure 6.
Toll-like receptor 6 (TLR6) regulation of cytokine generation in Saccharopolyspora rectivirgula antigen (SR-Ag) -driven recall responses by dispersed lung cells. At days 1, 4, 8 and 16 days after the final SR-Ag challenge, dispersed lung cells were cultured for 24 hr with and without SR-Ag. Cell-free supernatants were collected for protein analysis and cells were processed for Taqman analysis. interelukin-17A (IL-17A) (a), IL-6 (b), interferon-γ (IFN-γ) (c), and regulated on activation normal T-cell expressed and secreted (RANTES) (d) transcript analyses of cell cultures were carried out by Taqman. Results are expressed as fold change over transcript expression in wild-type (WT) samples collected at day 1 after the last SR-Ag challenge. Fifty microlitres of cell-free supernatant was analysed using a multiplex bead-based assay or enzyme-linked immunosorbent assay, to determine protein levels of IL-17A (e), IL-6 (f), IFN-γ (g) and RANTES (h). Data represent the mean ± SEM, with n = 3 for each group. One-way analysis of variance and Newman–Keuls multiple comparison test were used to analyse significance between the WT and TLR6−/− groups. *P ≤ 0·05, **P ≤ 0·01, ***P ≤ 0·001.
Interleukin-17A protein levels in the cell-free supernatants followed similar trends as observed with transcript levels. The addition of SR-Ag to lung cultures at day 1 after the last in vivo SR-Ag elicited major changes in IL-17A and IL-6, but not IFN-γ or RANTES, in cultures from WT mice (Fig. 6e,h). The SR-Ag-challenged TLR6−/− lung cells showed significantly decreased levels of IL-17A in the SR-Ag-restimulated group at day 16 when compared with SR-Ag-challenged WT lung cells. Similarly, IL-6 protein levels were also decreased in SR-Ag-challenged cultures of TLR6−/− cells compared with day 16 SR-Ag-challenged WT lung cells (Fig. 6f). However, decreased levels of IFN-γ were observed in all SR-Ag-challenged TLR6−/− versus SR-Ag-challenged WT treatment groups (Fig. 6g). Protein levels of RANTES were also consistent with transcript expression, with decreased levels measured in all SR-Ag-challenged TLR6−/− lung cells when compared with SR-Ag-challenged WT lung cells (Fig. 6h). The TGF-β was analysed, and similar to the in vivo observations, no significant changes were observed (not shown). Together, these data highlight that SR-Ag drives the synthesis of both IL-17A and IL-6, but not IFN-γ or RANTES. Also, the generation of all four cytokines is TLR6-dependent in vitro.
Neutralization of TLR6 results in decreased expression of IL-17A and IL-17A-associated cytokines
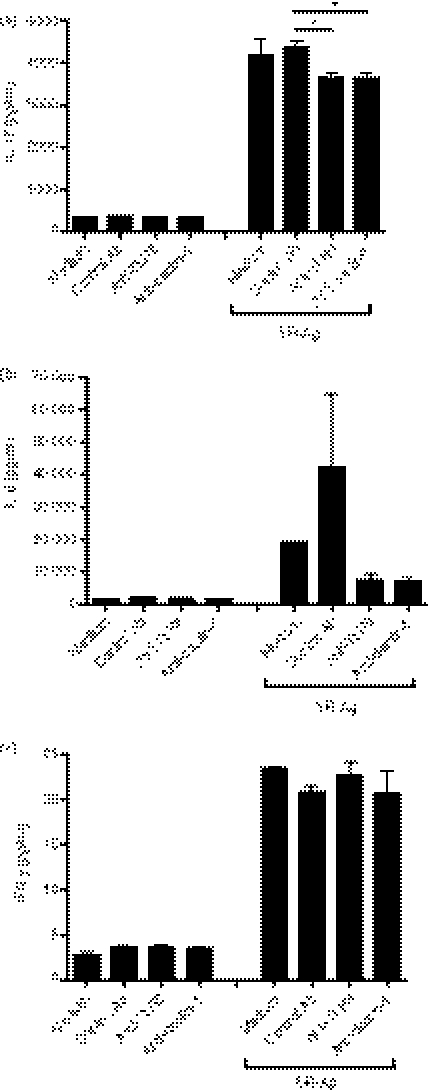
We next determined the role of TLR6 and dectin-1 in IL-17A and IL-17A-associated cytokines generated by cultures of lung cells from SR-Ag-challenged WT mice at day 1 after the last SR-Ag challenge with and without SR-Ag restimulation, using anti-TLR6 and anti-dectin-1 neutralizing antibodies. Transcript expression for IL-17A, IL-21, IL-23 and IL-6 was up-regulated in SR-Ag-restimulated lung cells for all groups when compared with lung cells cultured in medium alone (Fig. 7a–d). The transcript levels of all four cytokines in the lung cells cultured with SR-Ag and anti-TLR6 neutralizing antibody were down-regulated in comparison with lung cells cultured with SR-Ag and the isotype antibody control. Similarly, lung cells treated with the anti-dectin-1 neutralizing antibody showed reduced expression of IL-21, IL-23 and IL-6 transcripts (Fig. 7b–d). Interestingly, IL-17 transcript levels were unaffected by anti-dectin-1 treatment (Fig. 7a). Together, these data highlight the importance of TLR6 in the generation of IL-17A and its associated cytokines following SR-Ag challenge.
Figure 7.
Anti-Toll-like receptor 6 (TLR6) neutralizing antibody reduced interleukin-17A (IL-17A) and IL-17A-associated transcript levels. At day 1 after the final Saccharopolyspora rectivirgula antigen (SR-Ag) challenge, dispersed lung cells were cultured with and without SR-Ag restimulation. The role of TLR6 and dectin-1 were analysed using anti-TLR6 or anti-dectin-1 neutralizing antibody. Immunoglobulin G was used as a control for these experiments. Results are expressed as fold change over transcript expression in samples cultured in medium alone. Cytokine transcript analysis of IL-17A (a), IL-21 (b), IL-23 (c), and IL-6 (d) were determined by Taqman. Data represent the mean ± SEM, with n = 3 for each group.
Analysis of protein levels in the cell-free supernatant of dispersed whole lung cultures revealed elevated levels of IL-17A, IL-6 and IFN-γ in the groups restimulated with SR-Ag when compared with the medium-alone groups. Neutralizing TLR6 or dectin-1 in SR-Ag restimulation cultures resulted in significantly lower levels of IL-17A and decreased levels of IL-6, compared with isotype control (Fig. 8a,b). No differences were found in IFN-γ protein levels, suggesting that neutralization of TLR6 and anti-dectin-1 was insufficient to block IFN-γ production (Fig. 8c). Together, these data showed that activation via TLR6 was required for the generation of IL-17A and IL-6 during experimental HP.
Figure 8.
Anti-Toll-like receptor 6 (TLR6) neutralizing antibody reduced interleukin-17A (IL-17A) and IL-17A-associated cytokine protein levels. At day 1 after the final Saccharopolyspora rectivirgula antigen (SR-Ag) challenge, dispersed lung cells were cultured with and without SR-Ag restimulation. The roles of TLR6 and dectin-1 were analysed by using anti-TLR6 or anti-dectin-1 neutralizing antibody. Immunoglobulin G was used as a control for these experiments. IL-17A (a), IL-6 (b), and interferion-γ (IFN-γ) (c) protein levels were measured using a multiplex bead-based assay or enzyme-linked immunosorbent assay. Data represent the mean ± SEM, with n = 3 for each group. One-way analysis of variance and Newman–Keuls multiple comparison test were used to analyse significance between groups. *P ≤ 0·05.
Discussion
We have previously reported that SR-Ag-induced experimental HP was mediated by IL-17A. While it was clear that SR-Ag was a potent inducer of IL-17A, it was not clear which receptor(s) were responsible for this induction. The present study specifically addressed the role of TLR6 in the immunopathogenesis associated with SR-Ag-induced murine HP. This TLR was selected because our analysis of TLR expression showed that this TLR was among the most strongly induced TLRs in HP lungs. Further impetus for the present study came from an earlier study by Nance et al.,42 which demonstrated that MyD88, the adaptor protein for most TLRs, was necessary for SR-Ag-induced lung inflammation in mice. In the present study, lung inflammation was significantly reduced in SR-Ag-challenged TLR6−/− mice when compared with SR-Ag-challenged WT mice. Analysis of whole lung cytokine levels revealed that IL-17A was expressed at significantly lower levels in TLR6−/− mice, again compared with the WT counterparts. Together, the present findings highlight the major role of TLR6 in the generation of IL-17A during SR-Ag-induced HP.
Activation of Th-17-mediated pathways and production of IL-17A is regulated by cell surface receptors that detect pathogen-associated molecular patterns and trigger host responses. The C-type lectin, dectin-1, is a PRR that has been shown to initiate a pathway promoting an IL-17A response, particularly in dendritic cells.43 Production of IL-17A during pulmonary responses to Aspergillus fumigatus in mice is dependent upon dectin-1, and in this context IL-17A has a marked protective effect.19 Our results suggest that TLR6 might be the major receptor responsible for the initiation of IL-17A production in SR-induced murine HP. Analysis of transcript levels from lungs of TLR−/− mice challenged with SR-Ag consistently demonstrated decreased transcript levels for IL-17A and IL-17A-associated cytokines such as IL-6, IL-21 and IL-23. Our studies on restimulation of ex vivo lung cultures with SR-Ag show that IL-17, IL-6, IFN-γ and RANTES protein expression was TLR6 dependent in lung cells harvested from mice at both days 1 and 16 after the last SR-Ag challenge. Transcript analysis of these factors in these same cultures showed variable effects of TLR6 deficiency on transcript expression, particularly for IL-6 and IFN-γ. One explanation for enhanced IFN-γ gene expression by TLR6−/− cells might be the importance of TLR6 signalling in the translation of IFN-γ into protein. Overall, however, the lack of TLR6 through gene deficiency had a major effect on the ability of dispersed cells to express IL-17A.
In ex vivo cultures of dispersed lung cells, the addition of an anti-TLR6 neutralizing antibody resulted in lower IL-17A and IL-6 levels compared with lung cells treated with isotype control. Lung cells treated with anti-dectin-1 neutralizing antibody showed little change in IL-17A transcript levels when compared with isotype control groups, but protein levels of this cytokine were significantly decreased using a similar comparison. These data suggest that both TLR6 and dectin-1 contribute to IL-17A production but the difference in the timing of expression of these receptors could be important for disease initiation and maintenance. It should also be noted that the expression of dectin-1 was dependent upon TLR6 expression. Finally, the expression of IL-17A at the intermediate time-points of days 4 and 8 after SR-Ag appeared to be independent of TLR6, suggesting that other receptors/mechanisms contribute to the generation of IL-17A during the course of experimental HP. Which additional innate immune receptors contribute to the immunopathology of HP is currently under investigation in our laboratory. Taken together, these results suggest that TLR6 is a critical PRR in recognizing SR-Ag, and that TLR6–SR-Ag interactions lead to de novo generation of IL-17A in the lung.
This study also provides some insight into the cross-regulation between Th17 cells and Th1 cells. It has been demonstrated that Th1 responses are increased in mice lacking the IL-17A gene.44 Also, others have shown that the Th1-mediating transcription factor, T-bet, negatively regulates IL-17A, providing further evidence of cross-regulation between Th17 and Th1 responses.45 Consistent with these reports, in our experimental model, IFN-γ in whole lung was increased in SR-Ag-challenged TLR6−/− mice, when compared with SR-Ag-challenged WT mice. Nance et al.14 have shown that IFN-γ production from neutrophils is critical during the immunopathogenesis of HP. Interestingly, our study shows that an increase in IFN-γ levels in TLR6−/− mice did not result in increased pathology underscoring the pivotal role of IL-17 in HP pathogenesis.
In an effort to better understand the role of TLRs in a clinical setting, various studies have investigated the role of genetic alterations in disease pathogenesis. Single nucleotide polymorphisms (SNPs) in TLR genes from cohort studies have been linked to certain diseases. Relevant to this study, certain loss of function in TLR6 SNPs have been shown to be associated with a susceptibility to asthma, an increased risk of non-Hodgkin lymphoma, and in some cases, increased risk of prostate cancer.46–49 Future studies are required to investigate TLR6 SNPs because they may play a role in the pathogenesis of HP, perhaps influencing the susceptibility of a patient to environmental antigens and the severity of disease progression.
In summary, the present study demonstrates that the deletion of TLR6 in experimental HP significantly impairs IL-17A generation and markedly reduces disease severity. Moreover, neutralization of TLR6 in ex vivo lung cultures results in reduced transcript and protein levels of IL-17A and IL-17A-associated genes. Recently, it has been reported that IL-17A plays a protective role in a model of HP that involves repeated exposure to live Bacillus subtilis.50 Further studies are required to address the role of TLR6 in this model. In conclusion, we report a critical role for TLR6 in the development of HP, and also suggest that this might be a therapeutic target in clinical HP.
Acknowledgments
This work was supported by a grant from The University of Michigan Medical School.
Glossary
Abbreviations:
- FITC
fluorescein isothiocyanate
- HP
hypersensitivity pneumonitis
- IFN
interferon
- IL-6
interleukin-6
- PE
phycoerythrin
- PRR
pathogen-recognition receptors
- RANTES
regulated on activation normal T-cell expressed and secreted
- SR-Ag
Saccharopolyspora rectivirgula-antigen
- TGF
transforming growth factor
- Th1
T helper type 1
- TLR
Toll-like receptor
- WT
wild-type
Disclosures
None of the authors of this paper have conflicts of interest to disclose.
References
- 1.Barrios RJ. Hypersensitivity pneumonitis: histopathology. Arch Pathol Lab Med. 2008;132:199–203. doi: 10.5858/2008-132-199-HPH. [DOI] [PubMed] [Google Scholar]
- 2.Takemura T, Akashi T, Ohtani Y, Inase N, Yoshizawa Y. Pathology of hypersensitivity pneumonitis. Curr Opin Pulm Med. 2008;14:440–54. doi: 10.1097/MCP.0b013e3283043dfa. [DOI] [PubMed] [Google Scholar]
- 3.Churg A, Muller NL, Flint J, Wright JL. Chronic hypersensitivity pneumonitis. Am J Surg Pathol. 2006;30:201–8. doi: 10.1097/01.pas.0000184806.38037.3c. [DOI] [PubMed] [Google Scholar]
- 4.Madison JM. Hypersensitivity pneumonitis: clinical perspectives. Arch Pathol Lab Med. 2008;132:195–8. doi: 10.5858/2008-132-195-HPCP. [DOI] [PubMed] [Google Scholar]
- 5.Bourke SJ, Dalphin JC, Boyd G, McSharry C, Baldwin CI, Calvert JE. Hypersensitivity pneumonitis: current concepts. Eur Respir J Suppl. 2001;32:81s–92s. [PubMed] [Google Scholar]
- 6.Story RE, Grammer LC. Hypersensitivity pneumonitis. Allergy Asthma Proc. 2004;25:S40–1. [PubMed] [Google Scholar]
- 7.Suda T, Chida K, Hayakawa H, Imokawa S, Iwata M, Nakamura H, Sato A. Development of bronchus-associated lymphoid tissue in chronic hypersensitivity pneumonitis. Chest. 1999;115:357–63. doi: 10.1378/chest.115.2.357. [DOI] [PubMed] [Google Scholar]
- 8.McClellan JS, Albers GM, Noyes BE, Sotelo C, Petterchak JA, Knutsen AP. B-lymphocyte aggregates in alveoli from a child with hypersensitivity pneumonitis (bird breeders lung) Ann Allergy Asthma Immunol. 1999;83:357–60. doi: 10.1016/S1081-1206(10)62831-1. [DOI] [PubMed] [Google Scholar]
- 9.Takizawa H, Ohta K, Horiuchi T, et al. Hypersensitivity pneumonitis in athymic nude mice. Additional evidence of T cell dependency. Am Rev Respir Dis. 1992;146:479–84. doi: 10.1164/ajrccm/146.2.479. [DOI] [PubMed] [Google Scholar]
- 10.Woda BA. Hypersensitivity pneumonitis: an immunopathology review. Arch Pathol Lab Med. 2008;132:204–5. doi: 10.5858/2008-132-204-HPAIR. [DOI] [PubMed] [Google Scholar]
- 11.Butler NS, Monick MM, Yarovinsky TO, Powers LS, Hunninghake GW. Altered IL-4 mRNA stability correlates with Th1 and Th2 bias and susceptibility to hypersensitivity pneumonitis in two inbred strains of mice. J Immunol. 2002;169:3700–9. doi: 10.4049/jimmunol.169.7.3700. [DOI] [PubMed] [Google Scholar]
- 12.Schuyler M, Gott K, Cherne A. Mediators of hypersensitivity pneumonitis. J Lab Clin Med. 2000;136:29–38. doi: 10.1067/mlc.2000.107694. [DOI] [PubMed] [Google Scholar]
- 13.Gudmundsson G, Hunninghake GW. Interferon-γ is necessary for the expression of hypersensitivity pneumonitis. J Clin Invest. 1997;99:2386–90. doi: 10.1172/JCI119420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nance S, Cross R, Yi AK, Fitzpatrick EA. IFN-γ production by innate immune cells is sufficient for development of hypersensitivity pneumonitis. Eur J Immunol. 2005;35:1928–38. doi: 10.1002/eji.200425762. [DOI] [PubMed] [Google Scholar]
- 15.Matsuno Y, Ishii Y, Yoh K, et al. Overexpression of GATA-3 protects against the development of hypersensitivity pneumonitis. Am J Respir Crit Care Med. 2007;176:1015–25. doi: 10.1164/rccm.200612-1887OC. [DOI] [PubMed] [Google Scholar]
- 16.Schuyler M, Gott K, Cherne A, Edwards B. Th1 CD4+ cells adoptively transfer experimental hypersensitivity pneumonitis. Cell Immunol. 1997;177:169–75. doi: 10.1006/cimm.1997.1107. [DOI] [PubMed] [Google Scholar]
- 17.Chiang EY, Kolumam GA, Yu X, et al. Targeted depletion of lymphotoxin-alpha-expressing T(H)1 and T(H)17 cells inhibits autoimmune disease. Nat Med. 2009;15:766–73. doi: 10.1038/nm.1984. [DOI] [PubMed] [Google Scholar]
- 18.Monari C, Bevilacqua S, Piccioni M, et al. A microbial polysaccharide reduces the severity of rheumatoid arthritis by influencing th17 differentiation and proinflammatory cytokines production. J Immunol. 2009;183:191–200. doi: 10.4049/jimmunol.0804144. [DOI] [PubMed] [Google Scholar]
- 19.Werner JL, Metz AE, Horn D, et al. Requisite role for the dectin-1 β-glucan receptor in pulmonary defense against Aspergillus fumigatus. J Immunol. 2009;182:4938–46. doi: 10.4049/jimmunol.0804250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joshi AD, Fong DJ, Oak SR, Trujillo G, Flaherty KR, Martinez FJ, Hogaboam CM. Interleukin-17-mediated immunopathogenesis in experimental hypersensitivity pneumonitis. Am J Respir Crit Care Med. 2009;179:705–16. doi: 10.1164/rccm.200811-1700OC. [DOI] [PubMed] [Google Scholar]
- 21.Simonian PL, Roark CL, Wehrmann F, Lanham AK, Diaz del Valle F, Born WK, O’Brien RL, Fontenot AP. Th17-polarized immune response in a murine model of hypersensitivity pneumonitis and lung fibrosis. J Immunol. 2009;182:657–65. [PMC free article] [PubMed] [Google Scholar]
- 22.Fossiez F, Djossou O, Chomarat P, et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183:2593–603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laan M, Cui ZH, Hoshino H, Lotvall J, Sjostrand M, Gruenert DC, Skoogh BE, Linden A. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J Immunol. 1999;162:2347–52. [PubMed] [Google Scholar]
- 24.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 25.Kimura A, Naka T, Kishimoto T. IL-6-dependent and -independent pathways in the development of interleukin 17-producing T helper cells. Proc Natl Acad Sci U S A. 2007;104:12099–104. doi: 10.1073/pnas.0705268104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langrish CL, Chen Y, Blumenschein WM, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–40. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mangan PR, Harrington LE, O’Quinn DB, et al. Transforming growth factor-β induces development of the T(H)17 lineage. Nature. 2006;441:231–4. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 28.Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–7. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nurieva R, Yang XO, Martinez G, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–3. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 30.Werling D, Jungi TW. TOLL-like receptors linking innate and adaptive immune response. Vet Immunol Immunopathol. 2003;91:1–12. doi: 10.1016/s0165-2427(02)00228-3. [DOI] [PubMed] [Google Scholar]
- 31.Takeuchi O, Kawai T, Muhlradt PF, Morr M, Radolf JD, Zychlinsky A, Takeda K, Akira S. Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int Immunol. 2001;13:933–40. doi: 10.1093/intimm/13.7.933. [DOI] [PubMed] [Google Scholar]
- 32.Takeuchi O, Kawai T, Sanjo H, Copeland NG, Gilbert DJ, Jenkins NA, Takeda K, Akira S. TLR6: a novel member of an expanding toll-like receptor family. Gene. 1999;231:59–65. doi: 10.1016/s0378-1119(99)00098-0. [DOI] [PubMed] [Google Scholar]
- 33.Lakatos HF, Burgess HA, Thatcher TH, Redonnet MR, Hernady E, Williams JP, Sime PJ. Oropharyngeal aspiration of a silica suspension produces a superior model of silicosis in the mouse when compared to intratracheal instillation. Exp Lung Res. 2006;32:181–99. doi: 10.1080/01902140600817465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hwang SJ, Kim S, Park WS, Chung DH. IL-4-secreting NKT cells prevent hypersensitivity pneumonitis by suppressing IFN-γ-producing neutrophils. J Immunol. 2006;177:5258–68. doi: 10.4049/jimmunol.177.8.5258. [DOI] [PubMed] [Google Scholar]
- 35.Tassi I, Cella M, Castro I, Gilfillan S, Khan WN, Colonna M. Requirement of phospholipase C-γ2 (PLCγ2) for Dectin-1-induced antigen presentation and induction of TH1/TH17 polarization. Eur J Immunol. 2009;39:1369–78. doi: 10.1002/eji.200839313. [DOI] [PubMed] [Google Scholar]
- 36.Thorne PS, Adamcakova-Dodd A, Kelly KM, O’Neill ME, Duchaine C. Metalworking fluid with mycobacteria and endotoxin induces hypersensitivity pneumonitis in mice. Am J Respir Crit Care Med. 2006;173:759–68. doi: 10.1164/rccm.200405-627OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem. 2003;278:1910–4. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 38.Zhou L, Ivanov II, Spolski R, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–74. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 39.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGF-β in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–89. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 40.Gocke AR, Cravens PD, Ben LH, Hussain RZ, Northrop SC, Racke MK, Lovett-Racke AE. T-bet regulates the fate of Th1 and Th17 lymphocytes in autoimmunity. J Immunol. 2007;178:1341–8. doi: 10.4049/jimmunol.178.3.1341. [DOI] [PubMed] [Google Scholar]
- 41.Oshima M, Maeda A, Ishioka S, Hiyama K, Yamakido M. Expression of C-C chemokines in bronchoalveolar lavage cells from patients with granulomatous lung diseases. Lung. 1999;177:229–40. doi: 10.1007/pl00007643. [DOI] [PubMed] [Google Scholar]
- 42.Nance SC, Yi AK, Re FC, Fitzpatrick EA. MyD88 is necessary for neutrophil recruitment in hypersensitivity pneumonitis. J Leukoc Biol. 2008;83:1207–17. doi: 10.1189/jlb.0607391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.LeibundGut-Landmann S, Gross O, Robinson MJ, et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007;8:630–8. doi: 10.1038/ni1460. [DOI] [PubMed] [Google Scholar]
- 44.O’Connor W, Jr, Kamanaka M, Booth CJ, Town T, Nakae S, Iwakura Y, Kolls JK, Flavell RA. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat Immunol. 2009;10:603–9. doi: 10.1038/ni.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rangachari M, Mauermann N, Marty RR, Dirnhofer S, Kurrer MO, Komnenovic V, Penninger JM, Eriksson U. T-bet negatively regulates autoimmune myocarditis by suppressing local production of interleukin 17. J Exp Med. 2006;203:2009–19. doi: 10.1084/jem.20052222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cerhan JR, Ansell SM, Fredericksen ZS, et al. Genetic variation in 1253 immune and inflammation genes and risk of non-Hodgkin lymphoma. Blood. 2007;110:4455–63. doi: 10.1182/blood-2007-05-088682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kormann MS, Depner M, Hartl D, et al. Toll-like receptor heterodimer variants protect from childhood asthma. J Allergy Clin Immunol. 2008;122:86–92. doi: 10.1016/j.jaci.2008.04.039. 92 e81-88. [DOI] [PubMed] [Google Scholar]
- 48.Purdue MP, Lan Q, Wang SS, et al. A pooled investigation of Toll-like receptor gene variants and risk of non-Hodgkin lymphoma. Carcinogenesis. 2009;30:275–81. doi: 10.1093/carcin/bgn262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun J, Wiklund F, Zheng SL, et al. Sequence variants in Toll-like receptor gene cluster (TLR6-TLR1-TLR10) and prostate cancer risk. J Natl Cancer Inst. 2005;97:525–32. doi: 10.1093/jnci/dji070. [DOI] [PubMed] [Google Scholar]
- 50.Simonian PL, Roark CL, Wehrmann F, Lanham AM, Born WK, O’Brien RL, Fontenot AP. IL-17A-expressing T cells are essential for bacterial clearance in a murine model of hypersensitivity pneumonitis. J Immunol. 2009;182:6540–9. doi: 10.4049/jimmunol.0900013. [DOI] [PMC free article] [PubMed] [Google Scholar]