Abstract
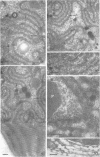
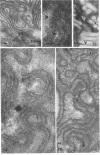
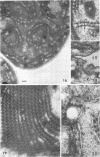
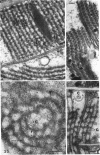
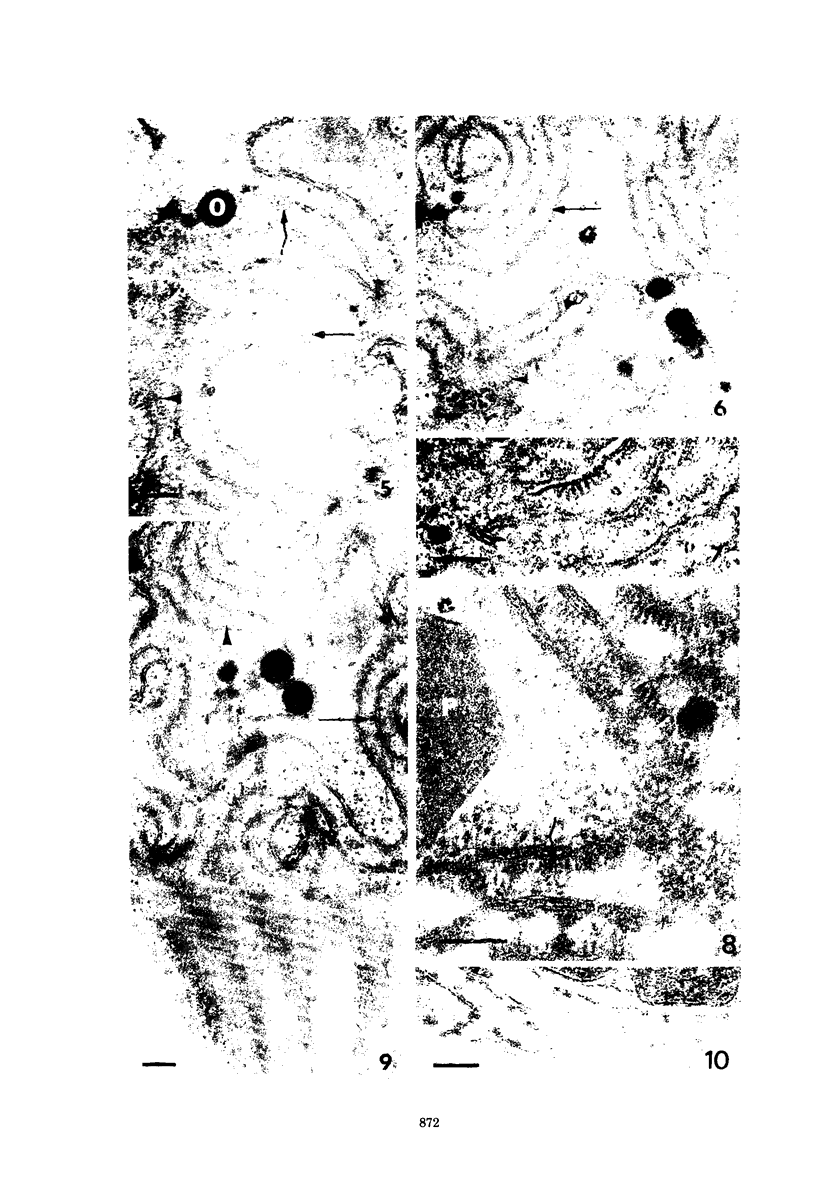
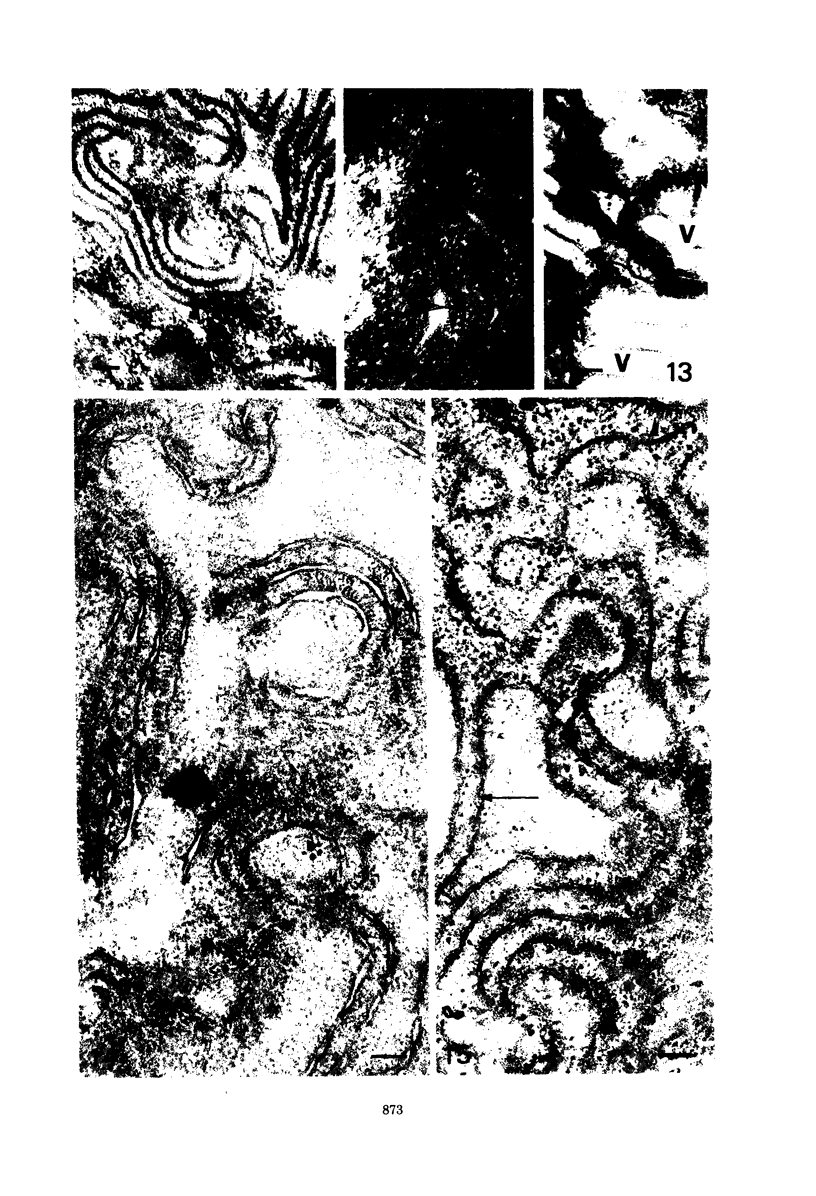
Fifteen species of freshwater blue-green algae, including unicellular, filamentous, and colonial forms, were subjected to a variety of fixatives, fixation conditions, and stains for comparison of the preservation of phycobilisomes. Absorption spectra of the corresponding in vivo and released photosynthetic pigments, in 10 of the species that were maintained in culture, demonstrated the presence of phycocyanin in all 10 species and phycoerythrin in only 2 of them. Spectroscope and electron microscope evidence was obtained for localization of phycobiliproteins in phycobilisomes of Nostoc muscorum. Phycobilisomes were observed in all species examined in situ, strenghening the hypothesis that phycobilisomes are common to all phycobiliprotein-containing photosynthetic blue-green algae.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARNOLD W., OPPENHEIMER J. R. Internal conversion in the photosynthetic mechanism of blue-green algae. J Gen Physiol. 1950 Mar;33(4):423–435. doi: 10.1085/jgp.33.4.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett A., Bogorad L. Properties of subunits and aggregates of blue-green algal biliproteins. Biochemistry. 1971 Sep 14;10(19):3625–3634. doi: 10.1021/bi00795a022. [DOI] [PubMed] [Google Scholar]
- Chao L., Bowen C. C. Purification and properties of glycogen isolated from a blue-green alga, Nostoc muscorum. J Bacteriol. 1971 Jan;105(1):331–338. doi: 10.1128/jb.105.1.331-338.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Bazire G., Lefort-Tran M. Fixation of phycobiliproteins to photosynthetic membranes by glutaraldehyde. Arch Mikrobiol. 1970;71(3):245–257. doi: 10.1007/BF00410158. [DOI] [PubMed] [Google Scholar]
- DUYSENS L. N. M. Transfer of light energy within the pigment systems present in photosynthesizing cells. Nature. 1951 Sep 29;168(4274):548–550. doi: 10.1038/168548a0. [DOI] [PubMed] [Google Scholar]
- Edwards M. R., Gantt E. Phycobilisomes of the thermophilic blue-green alga Synechococcus lividus. J Cell Biol. 1971 Sep;50(3):896–900. doi: 10.1083/jcb.50.3.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. L., Allen M. M. Phycobilisomes in Anacystis nidulans. J Bacteriol. 1973 Jan;113(1):403–408. doi: 10.1128/jb.113.1.403-408.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Krien S., Brown R. M., Jr Simultaneous glutaraldehyde-osmium tetroxide fixation with postosmication. An improved fixation procedure for electron microscopy of plant and animal cells. Histochemie. 1969;19(2):162–164. doi: 10.1007/BF00281096. [DOI] [PubMed] [Google Scholar]
- Gantt E., Conti S. F. Granules associated with the chloroplast lamellae of Porphyridium cruentum. J Cell Biol. 1966 Jun;29(3):423–434. doi: 10.1083/jcb.29.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantt E., Conti S. F. Phycobiliprotein localization in algae. Brookhaven Symp Biol. 1966;19:393–405. [PubMed] [Google Scholar]
- Gantt E., Conti S. F. Ultrastructure of blue-green algae. J Bacteriol. 1969 Mar;97(3):1486–1493. doi: 10.1128/jb.97.3.1486-1493.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantt E., Lipschultz C. A. Energy transfer in phycobilisomes from phycoerythrin to allophycocyanin. Biochim Biophys Acta. 1973 Apr 5;292(3):858–861. doi: 10.1016/0005-2728(73)90036-4. [DOI] [PubMed] [Google Scholar]
- Gantt E., Lipschultz C. A. Phycobilisomes of Porphyridium cruentum. I. Isolation. J Cell Biol. 1972 Aug;54(2):313–324. doi: 10.1083/jcb.54.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray B. H., Lipschultz C. A., Gantt E. Phycobilisomes from a blue-green alga Nostoc species. J Bacteriol. 1973 Oct;116(1):471–478. doi: 10.1128/jb.116.1.471-478.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori A., Crespi H. L., Katz J. J. Effect of side-chain deuteration on protein stability. Biochemistry. 1965 Jul;4(7):1213–1225. doi: 10.1021/bi00883a002. [DOI] [PubMed] [Google Scholar]
- Holt S. C., Edwards M. R. Fine structure of the thermophilic blue-green alga Synechococcus lividus Copeland. A study of frozen-fractured-etched cells. Can J Microbiol. 1972 Feb;18(2):175–181. doi: 10.1139/m72-028. [DOI] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOLLENHAUER H. H. PLASTIC EMBEDDING MIXTURES FOR USE IN ELECTRON MICROSCOPY. Stain Technol. 1964 Mar;39:111–114. [PubMed] [Google Scholar]
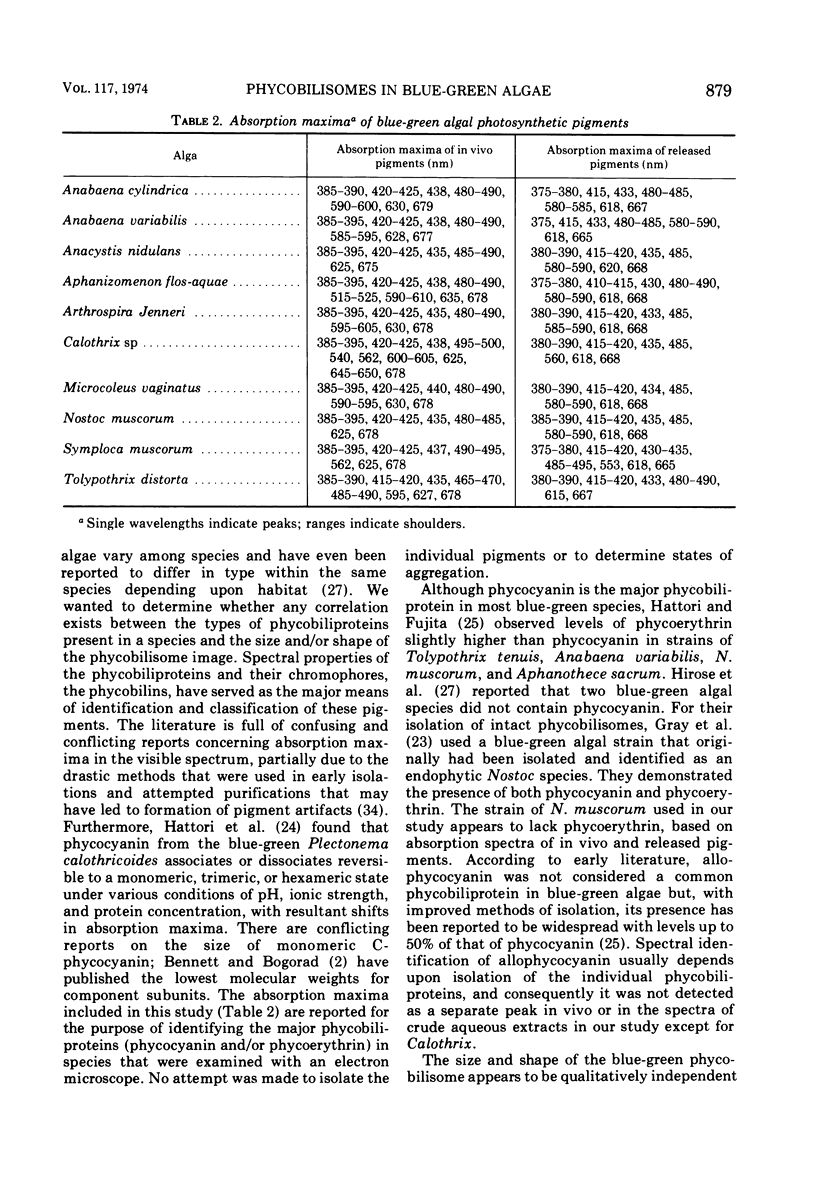
- OHEOCHA C. Spectral properties of the phycobilins. I. Phycocyanobilin. Biochemistry. 1963 Mar-Apr;2:375–382. doi: 10.1021/bi00902a034. [DOI] [PubMed] [Google Scholar]
- SABATINI D. D., BENSCH K., BARRNETT R. J. Cytochemistry and electron microscopy. The preservation of cellular ultrastructure and enzymatic activity by aldehyde fixation. J Cell Biol. 1963 Apr;17:19–58. doi: 10.1083/jcb.17.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEMPAK J. G., WARD R. T. AN IMPROVED STAINING METHOD FOR ELECTRON MICROSCOPY. J Cell Biol. 1964 Sep;22:697–701. doi: 10.1083/jcb.22.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trüper H. G., Yentsch C. S. Use of glass fiber filters for the rapid preparation of in vivo absorption spectra of photosynthetic bacteria. J Bacteriol. 1967 Oct;94(4):1255–1256. doi: 10.1128/jb.94.4.1255-1256.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VENABLE J. H., COGGESHALL R. A SIMPLIFIED LEAD CITRATE STAIN FOR USE IN ELECTRON MICROSCOPY. J Cell Biol. 1965 May;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]