Abstract
The high efficiency of Ag processing and presentation by B cells requires Ag-induced BCR signaling and actin cytoskeleton reorganization, although the underlying mechanism for such requirements remains elusive. In this study, we identify Bruton's tyrosine kinase (Btk) as a linker connecting BCR signaling to actin dynamics and the Ag transport pathway. Using xid mice and a Btk inhibitor, we show that BCR engagement increases actin polymerization and Wiskott-Aldrich syndrome protein activation in a Btk-dependent manner. Concurrently, we observe Btk-dependent increases in the levels of phosphatidylinositide-4,5-bisphosphate and phosphorylated Vav upon BCR engagement. The rate of BCR internalization, its movement to late endosomes, and efficiency of BCR-mediated Ag processing and presentation are significantly reduced in both xid and Btk inhibitor-treated B cells. Thus, Btk regulates actin dynamics and Ag transport by activating Wiskott-Aldrich syndrome protein via Vav and phosphatidylinositides. This represents a novel mechanism by which BCR-mediated signaling regulates BCR-mediated Ag processing and presentation.
Antigen encounter initiates two critical cellular processes in B cells: signal transduction and Ag processing and presentation. Multivalent Ags result in the translocation of the BCR into lipid rafts in the vicinity of Src kinases, inducing signaling cascades (1) and subsequent activation of transcription factors (2). The BCR internalizes and transports Ags to the endosomal compartments, where Ags are fragmented and loaded onto MHC class II, generating ligands for T cells. Together, BCR-initiated signaling and T cell help acquired through Ag presentation provide the two crucial signals required for B cell activation and subsequent T cell-dependent Ab responses.
The BCR can thus serve as both signal transducer and Ag transporter. By increasing the kinetics and specificity of Ag capture, uptake, and transport, the BCR increases the efficiency of Ag processing and presentation by B cells (3, 4), which enables B cells to present even sparsely occurring Ags. Key signaling intermediates, such as the tyrosine kinase Syk and the adaptor protein BLNK (5, 6) are involved in the timely transport of BCR-Ag complexes from the cell surface to Ag-processing compartments. BCR signaling blockade by the tyrosine kinase inhibitor genistein or PP2 (7, 8), or loss-of-function mutants for Lyn or Syk (6, 9), has been shown to impede Ag uptake. Moreover, tyrosine phosphorylation of clathrin in lipid rafts upon BCR cross-linking (XL)3 is required for BCR internalization (10), revealing the entwined nature of signaling and Ag-transport pathways of the BCR.
The binding of the BCR to Ags not only induces the reorganization of the actin cytoskeleton but also triggers its association with the BCR and signaling molecules, including Lyn, Syk, and GTP-binding proteins (11–13). Tyrosine kinase inhibitors block BCR-induced actin polymerization (14), suggesting that actin remodeling is downstream of BCR proximal signaling. Disrupting the actin cytoskeleton does not inhibit BCR-induced tyrosine phosphorylation or the translocation of the BCR into lipid rafts (15); however, it blocks BCR internalization by inhibiting the pinching off of clathrin-coated vesicles from the plasma membrane (PM) (16). An actin-dependent, but clathrin-independent, internalization pathway for the BCR has recently been observed (17), underscoring the importance of actin in BCR internalization. These studies lead to the hypothesis that a dynamic actin cytoskeleton is a determining factor for the correct intracellular routing of BCR-bound Ags and that there is a regulatory relationship between the actin cytoskeleton and BCR signaling and Ag-transport pathways.
The mechanistic links between the interrelated pathways of BCR signaling, Ag transport, and the actin cytoskeleton have not been well studied. Wiskott-Aldrich syndrome protein (WASP) is potentially one such link. WASP is a hematopoietic cell-specific actin regulator that links upstream signals to actin polymerization and branching by stabilizing Arp2/3 complexes (18). WASP contains multiple interacting domains, including WASP homology-1 (WH1), basic (B), GTPase-binding (GBD), proline-rich domains, and C-terminal verprolin homology, cofilin homology, and acidic (VCA) domains (19). The interaction of GTP-Cdc42 with the GBD, phosphatidylinositol-4,5-bisphosphate (PtdIns-4,5-P2) with the B domain, and phosphorylation at tyrosine 256 and 291 and serine 242 and 483/484 of WASP regulate its activity (20–23). Ag binding to the BCR has been shown to induce the phosphorylation of Rho family GTPase guanidine nucleotide exchange factor (GEF) Vav (24), the activation of Rho family GTPases (25), and modulation of phosphatidylinositide metabolism (26). Although all of these signaling activities potentially regulate WASP, the exact mechanism that links BCR signaling to WASP activation remains to be defined.
Bruton's tyrosine kinase (Btk) belongs to the Tec tyrosine kinase family. The significance of Btk in B cell function was revealed by the discovery that Btk mutations cause inherited immunodeficiencies in both humans (XLA for X-linked agammaglobulinemia) and mice (xid) (27). The xid mice have a point mutation at arginine 28 to cysteine (R28C) in the pleckstrin homology (PH) domain of Btk and show B cell developmental defects, with 50% reduction in mature B cells, a virtual absence of the B1-subset of cells, and a pronounced decrease in serum levels of IgM and IgG3 (28). In addition to its kinase domain, Btk has PH, Tec homology, SH3, and SH2 domains (27). Both the kinase and PH domains are indispensable for Btk activity. Upon BCR activation, Btk is recruited to the PM by its PH domain binding PtdIns-3,4,5-P3 (29). At the PM, Btk is phosphorylated by Src kinases at tyrosine 551 in the kinase activation loop and then autophosphorylates tyrosine 223 in its SH3 domain (30, 31). Phosphorylated Btk activates PLCγ2 by binding to BLNK and, thus, modulates Ca2+ influx (32). Additionally, it facilitates phosphatidylinositide metabolism by its interaction with phosphatidylinositol 4-phosphate 5-kinase (PIP5K) (33). The xid mutation prevents Btk from binding to PtdIns-3,4,5-P3 and recruiting to the PM, consequently inhibiting its activation (34, 35). Recent studies on T cells from mice deficient in Tec kinases Itk and/or Rlk show impaired actin polarization and defects in the recruitment of GTP-Cdc42 and its GEF Vav1 to the TCR at the immune synapse (36, 37). These indicate a role for Tec kinases in linking upstream signaling to actin dynamics.
In this study, we explore the role of Btk, a Tec kinase expressed in B cells, in linking BCR signaling to the actin cytoskeleton and BCR-mediated Ag-processing pathways. We report that the BCR-triggered signaling regulates the dynamics of the actin cytoskeleton through WASP in a Btk-dependent manner. Btk function is required for BCR-induced WASP and Vav activation, increased cellular PtdIns-4,5-P2 levels, actin rearrangement, and ultimately for BCR-mediated Ag processing and presentation.
Materials and Methods
Mice and cells
Splenic B cells were isolated from wild-type (wt) (CBA/CaJ) and xid (CBA/CaHN-Btkxid/J) mice (Jackson Laboratories). Mononuclear cells were obtained by Ficoll density-gradient centrifugation (Sigma-Aldrich). T cells were deleted by anti-Thy1.2 mAb (BD Biosciences) and guinea pig complement (Rockland Immunochemicals). All procedures involving mice were approved by the Animal Care and Use Committee of University of Maryland. B cell lymphoma A20 IIA1.6 cells (H-2d, IgG2a+,FcγIIBR−) were cultured in DMEM supplemented with 10% FBS.
Flow cytometric analysis
B cells were stimulated with 20 μg/ml F(ab′)2-goat-anti-mouse IgG+M [F(ab′)2-anti-Ig] (Jackson ImmunoResearch Laboratories) at 37°C for indicated times. To determine the effect of the Btk inhibitor, B cells were preincubated with LFM A-13 (100 μM; Calbiochem) at 37°C for 1 h, and the inhibitor was included in the cell medium during B cell stimulation and chase. A nonactive derivative of LFM A-13, LFM A-11 (100 μM), was used as a control (data not shown). Cells were fixed with 4% paraformaldehyde, washed, permeabilized with 0.05% saponin, and stained with Alexa Fluor (AF) 488-phalloidin (Invitrogen) or AF488-anti-phosphorylated Vav Y174 (pVav) (Santa Cruz Biotechnology). The cells were analyzed using a FACSCalibur (BD Biosciences) flow cytometer. The data are represented as mean fluorescence intensity (MFI). To compare the levels of phosphorylated WASP (pWASP) in different B cell subpopulations, splenic B cells from wt and xid mice were incubated with AF488-anti-mouse IgM at 4°C to label the surface BCR. To activate the BCR, the cells were warmed up to 37°C for 5 min in the presence of AF488-anti-mouse IgM. The cells were then washed, fixed, and incubated with PE-Cy5-anti-mouse B220 and PE-anti-mouse IgD. After fixation and permeabilization, the cells were incubated with anti-mouse pWASP (S483/S484) Ab. The cells were analyzed using CyAn (Dako) flow cytometer. Three subsets of splenic B cells were gated, including B220+IgMlowIgDhigh (mature and follicular), B220+IgMhighIgDhigh (T2), and B220+IgMhighIgDlow (T1/marginal zone (MZ)) B cells. The pWASP staining levels of different B cell subsets were determined.
Immunofluorescence microscopy analysis
To analyze BCR internalization, B cells were incubated with 5 μg/ml Cy3-Fab-rabbit anti-mouse IgM (Cy3-Fab-anti-μ; Jackson ImmunoResearch Laboratories) at 4°C for 30 min in the presence of 10 μg/ml F(ab′)2-anti-Ig to label and cross-link the surface BCR. Cells were chased at 37°C for varying lengths of time and incubated with AF488-cholera toxin subunit B (CTX-B; Invitrogen) at 4°C to label the PM.
To analyze the movement of the BCR from early to late endosomes, B cells were incubated with Cy3-Fab-anti-μ in the presence or absence of F(ab′)2-anti-Ig at 18°C for 30 min to allow for BCR internalization. The cells were warmed up to 37°C for varying lengths of time. To mark early endosomes, AF488-holo-transferrin (Tf) (10 μg/ml; Invitrogen) was included in the incubation medium at both 18 and 37°C. After fixation and permeabilization, the cells were incubated with anti-CD32/CD16 mAb (BD Biosciences) to block FcγR, anti-LAMP-1 mAb (1D4B; ATCC) to mark late endosomes, and AF488-phalloidin to label F-actin. To analyze the cellular distribution of oWASP and pWASP, pVav, and PtdIns-4,5-P2, splenic B cells were pulsed with Cy3-Fab-anti-μ at 37°C for 10 min and chased in the presence or absence of F(ab′)2-anti-Ig for varying lengths of time at 37°C. The cells were fixed, permeabilized, preincubated with anti-CD32/CD16 mAb, and incubated with Ab specific for oWASP (Upstate Biotechnology), pWASP S483/S484 (Bethyl Laboratories), pVav, or PtdIns-4,5-P2 (Invitrogen), followed by their corresponding secondary Abs. Cells were analyzed under a laser-scanning confocal microscope (Zeiss LSM 510) or a Deltavision deconvolution microscope. Pearson's correlation coefficients of differently stained proteins, which measure the overlap of pixels of two staining, were determined using the LSM 510 software. Pearson's linear correlation coefficient (r) is defined as follows based on the principles described by Manders et al. (38):
with Ri and Gi the pixel values in two different channels and Ravg and Gavg the averages of all the Ri and Gi. The correlation coefficient here indicates the strength and direction of a linear relationship between the cellular locations of two proteins. The r values range between +1 and −1 with +1 being perfect colocalization, −1 being perfect exclusion, and 0 representing no significant correlation or random distribution.
Analysis of actin nucleation sites
The actin nucleation sites were labeled as previously described (39). Briefly, B cells were serum starved for 1 h and then incubated with Cy3-Fab-anti-μ and F(ab′)2-anti-Ig at 37°C for indicated times. In the last minute of incubation, cells were treated with 0.45 μM AF488-G-actin (Cytoskeleton) in the presence of 0.025% saponin and then immediately fixed. The cells were analyzed using a confocal fluorescence microscope and quantified using the LSM510 software.
Immunoblotting
B cells untreated or treated with LFM A-13 (100 μM) were activated with F(ab′)2-anti-Ig for indicated times and lysed. Cell lysates were analyzed with SDS-PAGE and Western blot, and probed for pWASP S483/484 and pVav Y174, respectively. The blots were stripped and reprobed with anti-mouse β-tubulin Ab (Sigma-Aldrich) for establishing loading controls. The blots were quantified by densitometry. The levels of pWASP and pVav were normalized against tubulin and expressed as fold increases over unstimulated levels.
Analysis of BCR internalization by flow cytometry
B cells were incubated with 10 μg/ml biotin-F(ab′)2-anti-mouse IgM at 4°C and chased for 0, 5, 10, and 30 min at 37°C. Biotin-F(ab′)2-anti-IgM left on the cell surface after the chase was stained with PE-streptavidin and quantified using a flow cytometer. The data were expressed as percentages of the cell surface-associated biotin-F(ab′)2-anti-IgM Ab at time 0. To distinguish mature and immature/transitional B cells, splenic B cells were colabeled with FITC-anti-mouse AA4.1 and PE-Cy5-anti-B220 Abs (BD Biosciences). BCR internalization in mature and immature/transitional splenic B cells was compared by gating on B220+AA4.1−(mature), B220+AA4.1+ (immature/transitional) subsets, respectively.
Ag-presentation assay
Splenic B cells from wt and xid mice and wt splenic B cells that were serum-starved and pretreated with or without LFM A-13 (100 μg/ml) were incubated with hen egg lysozyme (HEL) alone or with the following Abs in sequence at 4°C to target HEL to the BCR: anti-CD32/CD16 mAb to block FcγRs, rabbit anti-mouse IgM (5 μg/ml) to bind the BCR, goat anti-rabbit IgG (5 μg/ml) to link rabbit anti-HEL and rabbit anti-mouse IgM Abs, rabbit anti-HEL Ab (5 μg/ml), and finally HEL (0.5 or 1 μg/ml) as the Ag. Cells were warmed to 37°C with the Ag-Ab complex for 15 min, washed, and incubated at 37°C for 14 h. HEL-I-Ak complexes on the cell surface were detected by AF488-C4H3 mAb and quantified by flow cytometry. To test the ability of B cells to present Ag to T cells, splenic B cells were incubated with HEL (1 μg/ml) with or without the Ab complex for 24 h. After washing, the B cells (1 × 106) were cocultured overnight with KZH T cells (1 × 106) (a gift from Dr. Nilabh Shastri, Department of Molecular and Cell Biology, University of California, Berkeley, CA) that are specific for HEL46–61:I-Ak and express a lacZ reporter gene under the control of the IL-2 promoter (40). The lacZ activity was assayed using chlorophenol red β-galactopyranoside (41). The reaction product was quantified by its absorbance at 595 nm, with 655 nm as the reference wavelength.
Results
BCR activation induces Btk-dependent actin rearrangement
To investigate the relationship between BCR signaling pathways and the actin cytoskeleton, we determined changes in the overall levels of cellular F-actin and actin polymerization in response to BCR XL. Upon Ag binding, the levels of total F-actin, quantified by phalloidin staining and flow cytometry, increased reproducibly by 2 min followed by a decrease at later time points in both splenic and A20 B cells (Fig. 1A). This result suggests biphasic actin reorganization with polymerization and depolymerization dominated phases. To follow actin polymerization, AF488-G-actin was introduced to cells in the presence of detergent in the last minute of stimulation. The incorporation of AF488-G-actin into polymerizing ends of actin filaments marks de novo actin nucleation sites. Similar to the cellular levels of F-actin, G-actin incorporation increased significantly 1–2 min after stimulation, after which the levels of G-actin incorporation plateaued (Fig. 1, Ba–d and C). To study the subcellular location of this incorporation relative to the BCR, the correlation indices of individual BCR and G-actin pixels were calculated. XL the BCR significantly increased the correlation between staining for actin nucleation sites and the BCR (Fig. 1D). Thus, BCR activation increases actin polymerization in the vicinity of the Ag-bound BCR.
FIGURE 1.
BCR activation induces the reorganization of the actin cytoskeleton and this actin remodeling is dependent on Btk. A, Wt splenic and A20 B cells that were treated or left untreated with Btk inhibitor LFM-A13 (100 μg/ml) and untreated xid splenic B cells were stimulated with F(ab′)2-anti-mouse IgG+M (F(ab′)2-anti-Ig, 10 μg/ml) for 0, 2, 5, and 10 min. The cells were fixed, and F-actin was stained with AF488-phalloidin. The cells were analyzed using flow cytometry. Shown are the average fluorescence intensities (±SD) of phalloidin staining at the indicated times from three independent experiments. B–D, Splenic B cells from wt and xid mice were incubated with Cy3-Fab-anti-mouse μ-chain (Fab-anti-μ) and stimulated with F(ab′)2-anti-Ig for 1, 2, and 5 min at 37°C. In the last minute of the stimulation, cells were incubated with AF488-G-actin in the presence of detergent to label newly polymerizing F-actin. The cells were immediately fixed and analyzed using a confocal fluorescence microscope. Shown are representative images from three independent experiments (B). Bar, 5 μm. Images were quantitatively analyzed to determine the fluorescence intensity of cell-associated AF488-G-actin (C) and the correlation coefficients between the labeled BCR and AF488-G-actin (D). Shown are mean values (±SD) from three independent experiments where over 300 cells were individually analyzed using Zeiss LMS 510 software (*, p ≤ 0.01).
To test whether Btk plays a role in linking BCR signal transduction to the actin cytoskeleton, we used xid mice and a Btk inhibitor, LFM A-13 (42). LFM A-13, a membrane permeable inhibitor, inhibits the kinase activity of Btk. The inhibitor allows for inhibition of Btk activity independent of developmental defects seen in xid B cells (28). LFM A-13 and Btk xid mutation showed similar inhibitory effects on both BCR-triggered gross and Btk tyrosine phosphorylation (data not shown). The effect of the Btk mutation and inhibitor on BCR-induced actin rearrangement was determined. LFM A-13 inhibited BCR-induced increases in F-actin levels in both splenic and A20 B cells, while the xid mutation only slightly reduced F-actin levels (Fig. 1A). The xid mutation not only blocked BCR-induced increases in actin polymerization but also drastically reduced the basal level of actin polymerization (Fig. 1, Be–h and C) and the colocalization of the BCR with actin nucleation sites (Fig. 1D). Thus, Btk is required for both constitutive actin polymerization and BCR-induced actin reorganization in B cells.
Ag engagement of the BCR induces Btk-dependent activation of WASP
To understand the mechanism for BCR-induced actin reorganization, we examined the cellular behavior of WASP, an actin nucleation promoting factor. The activation of WASP was followed by changes in its conformation and phosphorylation using Abs specific for WASP in its open, active conformation (oWASP) or WASP phosphorylated at S483/S484 (pWASP), respectively. Immunofluorescence microscopic studies found that upon antigenic stimulation, oWASP was relocated from the cytoplasm to cell surface under BCR caps (Fig. 2, Aa–f). At 10 min after the stimulation, oWASP was recruited to BCR+-vesicles at the perinuclear location (Fig. 2, Ag–i). By 30 min, while some of oWASP remained with BCR+-vesicles, the rest appeared to move into the nuclei (Fig. 2, Aj–l). Consistent with these results, BCR activation significantly increased the correlation coefficient between the staining of oWASP and the BCR by 5 min and it remained high until at least 30 min (Fig. 2Ca). Although the BCR colocalized with F-actin in both stimulated and unstimulated cells, BCR XL further increased this colocalization (Fig. 2, Ba–h and Cb). Furthermore, BCR XL increased the correlation between the staining of oWASP and F-actin from negative to positive values (Fig. 2Cc).
FIGURE 2.
BCR stimulation induces Btk-dependent WASP activation. A, The surface BCR on wt splenic B cells was labeled with Cy3-Fab-anti-μ and either left unstimulated (−XL) or stimulated with F(ab′)2-anti-Ig at 37°C for indicated times. The cells were fixed, permeabilized, and stained with an Ab specific for oWASP. Cells were analyzed using the Deltavision deconvolution microscope. Shown are representative images from three independent experiments. Bar, 3 μm. B, The surface BCR of splenic B cells from wt (a–h) and xid (i–p) mice were labeled and stimulated as described in A. After fixation and permeabilization, cells were labeled with AF488-phalloidin and an Ab specific for oWASP, and analyzed using a confocal fluorescence microscope. Bar, 3 μm. C, The colocalization coefficients between oWASP and BCR (a), BCR and F-actin (b), and oWASP and F-actin (c) for wt and xid B cells were quantified using the LSM 510 software. Shown are the average values (±SD) from three independent experiments of ≥300 cells (*, p ≤ 0.01).
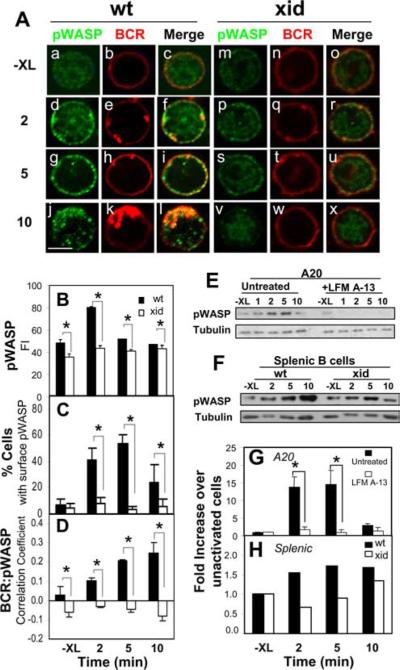
The phosphorylation of WASP was analyzed by both immunofluorescence microscopy and Western blot. Immunofluorescence microscopic studies showed a significant increase in pWASP staining over time in the splenic B cells upon BCR XL (Fig. 3, Aa, Ad, Ag, Aj, and B). The average level of pWASP staining peaked at 2 min (Fig. 3B). Meanwhile, pWASP was relocated from the cytoplasm to the PM where it colocalized with the surface BCR (Fig. 3, Aa–i, C, and D). At 5 min, ~50% of cells showed predominant cell surface staining of pWASP in contrast to ~5% of unstimulated cells (Fig. 3C). By 10 min, the number of cells showing significant surface staining of pWASP were reduced to ~25% (Fig. 3C). Correlation analyses showed continued increase in the colocalization between pWASP and the BCR with time (Fig. 3D). pWASP colocalized with BCRs at the cell surface at early time points (Fig. 3, Ad–i, C, and D) and with internalized BCRs at later time points (Fig. 3, Aj–l and D). Quantitative analysis of pWASP by Western blot showed that XL the BCR significantly increased the level of pWASP in both A20 (Fig. 3, E and G) and splenic B cells (Fig. 3, F and H), which peaked at 2 min after stimulation. Thus, BCR activation induces phosphorylation and conformational changes in WASP and the recruitment of WASP to the cell surface and BCR+-vesicles.
FIGURE 3.
BCR activation increases the phosphorylation of WASP and colocalization of pWASP with the BCR in a Btk-dependent manner. A, Splenic B cells from wt and xid mice were stained with Cy3-Fab-anti-μ for the BCR and stimulated with F(ab′)2-anti-Ig at 37°C for indicated times. The cells were fixed, permeabilized, and stained with an Ab specific for WASP phosphorylated at S483/S484 (pWASP). The cells were analyzed using a confocal fluorescence microscope. Shown are representative images of three independent experiments. Bar, 3 μm. B, Shown are the means (±SD) of pWASP fluorescence intensity of >300 cells from three independent experiments (*, p ≤ 0.005). C and D, Cells showing membrane redistribution of pWASP were visually determined and quantified. The data were plotted as percentages of total cells in images (C). The correlation coefficients between the BCR and pWASP in wt and xid B cells were determined using the LSM 510 software (D). Shown are the average results of three independent experiments where >300 cells were analyzed (*, p ≤ 0.005). E–H, A20 B cells that were treated with or without LFM A-13 (E and G) and splenic B cells from wt and xid mice (F and H) were stimulated with F(ab′)2-anti-Ig for indicated times. The cells were lysed, and the cell lysates were analyzed using SDS-PAGE and Western blot, and probed for pWASP S483/S484. The blots were stripped and reprobed for tubulin as loading controls. The blots were analyzed by densitometry. pWASP levels were normalized against tubulin levels, and the data were plotted as fold increases over unstimulated levels (G and H). Shown are representative blots and plots of three independent experiments (*, p ≤ 0.05).
The induction of WASP activation by BCR XL suggests that BCR-derived signals regulate the actin cytoskeleton through WASP. The lack of BCR-triggered actin reorganization in the Btk-deficient models implicates Btk in regulating WASP functions. We thus measured the effect of the xid mutation and Btk inhibitor on the cellular distribution and levels of oWASP and pWASP. In comparison with wt splenic B cells, the staining level of oWASP in xid splenic B cells was much lower (Fig. 2B), suggesting a defect in WASP activation in xid B cells. Although BCR activation did cause an initial increase in the colocalization between the BCR and oWASP in xid B cells, the correlation was significantly lower than in wt B cells (Fig. 2Ca). In contrast to wt splenic B cells, where the colocalization of the BCR and oWASP was sustained, this colocalization declined in xid B cells over time (Fig. 2Ca). Although the xid mutation had less of an inhibitory effect on the correlation of the BCR with F-actin (Fig. 2Cb), it significantly decreased the correlation of oWASP with F-actin (Fig. 2Cc). BCR activation failed to significantly increase the staining level of pWASP (Fig. 3, Am–x and B) or induce comparable redistribution of the pWASP to the cell surface in xid B cells (Fig. 3, Am–x and C), similar to the results with oWASP. In xid B cells, the BCR and pWASP showed a negative correlation (Fig. 3D), indicating that the BCR and pWASP do not colocalize, rather they exclude from each other. Western blot analysis further confirmed that LFM A-13 (Fig. 3, E and G) and the xid mutation (Fig. 3, F and H) both block BCR-induced phosphorylation of WASP. Thus inhibition of Btk blocks BCR-induced activation and recruitment of WASP to the BCR, indicating a role for Btk in regulating WASP activity.
To test whether the inhibition of WASP activation in xid mice is an indirect effect of B cell developmental defects caused by the Btk mutation, we compared the levels of pWASP between different subpopulations of splenic B cells by flow cytometry. We found that BCR XL increased pWASP levels in all the splenic B cell subpopulations from wt mice, including IgDhighIgMlow mature follicular B cells, IgDhighIgMhigh T2 B cells, and IgDlowIgMhigh T1 and MZ B cells, but failed to increase the pWASP levels in all the splenic B cell subpopulations from xid mice (Fig. 4). This indicates that it is the xid mutation, but not delayed B cell development, which inhibits BCR-induced WASP activation.
FIGURE 4.
The Btk xid mutation inhibits BCR-induced WASP phosphorylation in all subsets of splenic B cells. Splenic B cells from wt and xid mice were incubated with AF488-anti-mouse IgM at 4°C. To activate the BCR, cells were warmed up to 37°C for 5 min in the presence of AF488-anti-mouse IgM. The cells were then washed, fixed, and stained with PE-Cy5-anti-mouse B220 and PE-anti-mouse IgD. After fixation and permeabilization, cells were incubated with anti-mouse pWASP (S483/S484) Ab. The cells were analyzed using flow cytometry. Three subsets of splenic B cells were gated, including B220+IgMlowIgDhigh mature follicular (FO), B220+IgMhighIgDhigh transitional T2, and B220+IgMhighIgDlow T1 and MZ B cells (A). Shown are representative histograms of pWASP levels in each B cell subset from wt and xid spleens, with (+XL) and without (−XL) BCR XL, from three independent experiments (B).
BCR-induced biogenesis of PtdIns-4,5-P2 depends on Btk
To examine the mechanism for Btk-mediated activation of WASP, we determined the effect of Btk deficiency on the cellular level and distribution of PtdIns-4,5-P2, a coactivator of WASP, using immunofluorescence microscopy and flow cytometry. Significant increases in PtdIns-4,5-P2 staining levels were observed in both activated splenic (Fig. 5, Aa–i) and A20 B cells (Fig. 5C) compared to unstimulated cells (−XL). Additionally, PtdIns-4,5-P2 appeared to be recruited to the cell periphery and BCR+-vesicles after activation for 10 min (Fig. 5, Ag–i). The correlation analysis showed an increased colocalization between BCR and PtdIns-4,5-P2 staining (Fig. 5B). This BCR-induced increase in the levels of PtdIns-4,5-P2 and its redistribution were severely dampened in xid splenic B cells (Fig. 5, Aj–r and B). LFM A-13 treatment not only blocked BCR-triggered increases in PtdIns-4,5-P2 but also dramatically reduced constitutive levels of PtdIns-4,5-P2 in unstimulated A20 B cells (Fig. 5C). Thus, cellular biogenesis of PtdIns-4,5-P2 in response to BCR stimulation is dependent on the unimpaired activity of Btk.
FIGURE 5.

BCR activation induces Btk-dependent increase in PdtIns-4,5-P2 levels. Splenic B cells from wt (Aa-i) and xid (Aj-r) mice were incubated with Cy3-Fab-anti-Ig to label the BCR and activated with F(ab′)2-anti-Ig for indicated times at 37°C. The cells were fixed, permeabilized, and stained with an anti-PtdIns-4,5-P2 mAb followed by a Cy2-conjugated secondary Ab. The cells were analyzed by a confocal fluorescence microscope. Shown are representative images from three independent experiments (A). Bar, 3 μm. The colocalization between the BCR and PtdIns-4,5-P2 (PIP2) staining in wt and xid B cells was quantified as correlation coefficients using the LSM 510 software (B). Shown are the average results (±SE) of two independent experiments where >200 cells were analyzed. PtdIns-4,5-P2 (PIP2) levels in untreated and LFM A-13-treated A20 cells were analyzed by flow cytometry (C). Shown are the MFI (±SD) of PtdIns-4,5-P2 that were plotted against time (*, p ≤ 0.01).
BCR-triggered Vav activation requires Btk
In addition to PtdIns-4,5-P2 binding to the B domain, the coordinated binding of GTP-Cdc42 to the GBD domain of WASP is required for WASP activation (23). Vav serves as a GEF for Cdc42 (43). To test whether Btk-dependent WASP activation is mediated through Vav, we determined the effect of the xid mutation and LFM A-13 on Vav activation. Vav activation was followed by its recruitment to the cell surface and its phosphorylation in response to BCR stimulation (44) using an Ab specific for Vav phosphorylated at Y174 (pVav) by immunofluorescence microscopy, flow cytometry, and Western blot. In wt splenic B cells, BCR XL noticeably increased the staining levels of pVav, compared with unstimulated B cells (−XL) (Fig. 6, Aa–i). This BCR-triggered increase in pVav staining was drastically reduced in the xid B cells (Fig. 6, Aj–r). pVav accumulated primarily at the cell surface where it heavily colocalized with the BCR at early times after activation (~2 min) (Fig. 6, Ad–f). At later times (>10 min), pVav colocalized not only with the surface BCR but also the internalized BCR at the perinuclear location (Fig. 6, Ag–i). Correlation analysis showed an increase in the colocalization of pVav and the BCR upon BCR XL (data not shown). Both flow cytometry (Fig. 6B) and Western blot (Fig. 6, C–F) analyses confirmed that BCR XL increased pVav levels in wt splenic and A20 B cells, which were reduced by the xid mutation and LFM A-13 treatment. Thus, BCR-triggered Vav phosphorylation and colocalization of pVav with the BCR are dependent on the activity of Btk.
FIGURE 6.
BCR activation induces Btk-dependent phosphorylation of Vav and recruitment of phosphorylated Vav to the BCR. A, The surface BCR of splenic B cells from wt (a–i) and xid mice (j–r) were labeled with Cy3-Fab-anti-μ and activated with F(ab′)2-anti-Ig for varying lengths of time. The cells were fixed, permeabilized, and stained with an Ab specific for phosphorylated Vav at Y174 (pVav). Images were acquired using a confocal fluorescence microscope. Shown are representative images from three independent experiments. Bar, 3 μm. B, The wt splenic B cells that were treated or untreated with LFM A-13 and xid splenic B cells were activated with F(ab′)2-anti-Ig for varying lengths of time. The cells were fixed, permeabilized, and stained with an Ab specific for pVav Y174. The MFI of pVav was quantified using flow cytometry. Shown is a representative plot of pVav MFI vs the time from three independent experiments. C–F, A20 B cells that were treated with LFM A-13 or left untreated (C and D)as well as splenic wt and xid B cells (E and F) were stimulated with F(ab′)2-anti-Ig for indicated times and lysed. The lysates were analyzed by SDS-PAGE and Western blot, and probed for pVav Y174. The blots were stripped and reprobed for tubulin as loading controls. The blots were analyzed using densitometry. pVav levels were normalized against tubulin levels, presented as fold increases over unstimulated B cells, and plotted as a function of time. Shown are representative blots and average pVav levels from three independent experiments.
Btk inhibitor and deficiency inhibit BCR-mediated Ag internalization and transport to Ag-processing compartments
BCR-mediated Ag uptake and transport is dependent upon the integrity of signaling pathways and the actin cytoskeleton. Btk's roles in modulating both signaling and the actin cytoskeleton implicate its role in BCR-mediated Ag processing. To test this hypothesis, we determined the effects of Btk deficiency on the internalization and movement of the BCR from the cell surface to Ag-processing compartments. The surface-labeled BCR was chased for 30 min at 37°C. The cell surface was identified with CTX-B which binds GM1, early endosomes with holo-Tf, and late endosomes/lysosomes by LAMP-1. After 30 min of Ab XL, the BCR moved from the PM to a perinuclear location (Fig. 7, Aa and Ac), where most of BCRs colocalized with LAMP-1 (Fig. 7, Bg–i) while a small portion was found with Tf (Fig. 7, Ba–c). In contrast, after the same treatment, the surface labeled BCR in splenic xid B cells remained colocalized with CTX-B at the PM (Fig. 7Ad) and showed little to no internalization and colocalization with Tf (Fig. 7, Bd–f) or LAMP-1 (Fig. 7, Bj–l). The correlation between the BCR and LAMP-1 staining increased with time in wt splenic B cells, but this increase was abrogated in xid splenic B cells (Fig. 7C). We next determined the effect of Btk deficiency on the kinetics of BCR internalization, which was followed by decreases in the levels of surface-labeled BCR after the chase using flow cytometry. We found that the rate of BCR internalization was dramatically reduced in the xid B cells, in comparison with the wt B cells (Fig. 7D). Similarly, LFM A-13 significantly reduced BCR internalization rates in both splenic and A20 B cells (Fig. 7D). Furthermore, by gating on B220+AA4.1+ immature/transitional B cells and B220+AA4.1− mature B cells, we found that both mature and immature B cells from the spleen of xid mice showed reduced rates of BCR internalization compared with respective wt B cell subsets (Fig. 8), indicating that the decrease in the rate of BCR internalization is not the result of B cell developmental defects in xid mice. These results demonstrate a requirement for Btk-mediated signals in efficient BCR internalization and transport of Ag to Ag-processing compartments.
FIGURE 7.
Btk inhibitor and xid mutation inhibit BCR internalization and intracellular movement to late endosomes. A–C, Splenic B cells from wt and xid mice were incubated with Cy3-Fab-anti-μ at 4°C to label the surface BCR and treated with or without F(ab′)2-anti-Ig for 30 min at 37°C. Then cells were incubated with AF488-CTX-B at 4°C to demarcate the cell surface (A). The cells were fixed, permeabilized, and stained for LAMP-1 using a mAb (ID4B) for marking late endosomes (Bg-l). To mark early endosomes, splenic B cells from wt and xid mice were labeled with Cy3-Fab-anti-μ in the presence of F(ab′)2-anti-Ig at 18°C for 30 min and chased at 37°C for 30 min in the presence of AF488-holo-Tf (Ba-f). The cells were analyzed using a confocal fluorescence microscope. Representative images from three independent experiments are shown. Bar, 3 μm. C, The correlation coefficients between BCR and LAMP-1 staining were determined from images of ≥300 cells from three independent experiments using the Zeiss LSM 510 software (*, p ≤ 0.05).D, Splenic B cells from wt and xid mice and LFM A-13-treated wt and A20 B cells were incubated with biotinylated F(ab′)2-anti-Ig at 4°C to label the surface BCR. After washing, cells were incubated at 37°C for indicated times. Biotin-F(ab′)2-anti-Ig remaining on the cell surface after the chase was detected with PE-streptavidin and quantified using flow cytometry. Shown are the average percentages (±SD) of biotin-F(ab′)2-anti-Ig remaining on the cell surface from three independent experiments (*, p ≤ 0.05).
FIGURE 8.
The Btk xid mutation decreases the rate of BCR internalization in both mature and transitional splenic B cells. Splenic B cells from wt and xid mice were labeled with PE-Cy5 anti-mouse B220, FITC-anti-mouse AA4.1 (CD93), and biotin-F(ab′)2-anti-mouse IgM at 4°C and chased at 37°C for varying lengths of time. BCR internalization was analyzed as described in Fig. 7. BCR internalization in mature and immature B cell subsets (B) was measured by gating for B220+AA4.1− (mature) and B220+AA4.1+ (immature/transitional B cells) (A). Shown are data from two independent experiments.
Btk-deficiency and Btk inhibition reduce BCR-mediated Ag presentation
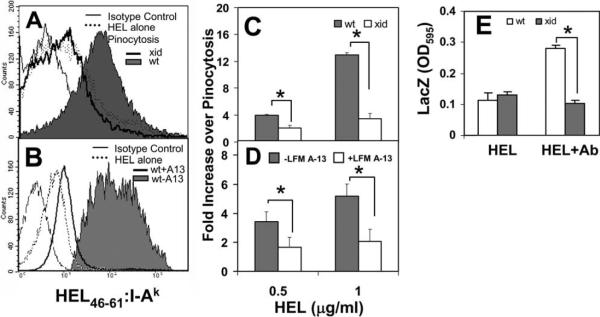
The inhibitory effect on BCR internalization and transport to late endosomes suggests that Btk deficiency interferes with BCR-mediated Ag processing. To test this, we compared the efficiencies of BCR-mediated Ag presentation by wt and xid B cells and by wt B cells treated with or without Btk inhibitor LFM A-13. A model Ag, HEL, was targeted to the BCR for BCR-mediated Ag processing and presentation using an Ab complex. The surface levels of HEL46–61-loaded MHC class II I-Ak (HEL46–61:I-Ak) was determined by mAb C4H3 (45) and flow cytometry. To test the sensitivity and efficiency of Ag processing and presentation, splenic B cells were pulsed with HEL (0.5 or 1 μg/ml) either alone (for pinocytosis-mediated Ag processing) or in presence of the Ab complex (for BCR-mediated Ag processing) for 15 min, washed, and incubated at 37°C for 14 h. The wt B cells pulsed with HEL plus the Ab complex displayed much higher levels of surface HEL46–61:I-Ak than those pulsed with HEL alone (Fig. 9A). This indicates that BCR-mediated Ag processing and presentation has a higher efficiency than nonspecific pinocytosis. The wt and xid B cells pulsed with HEL alone showed similar surface levels of HEL46–61:I-Ak (Fig. 9A, dotted line), suggesting that Btk deficiency does not significantly affect pinocytic rates of Ag processing and presentation. Although both wt and xid B cells show similar levels of MHC class II I-Ak on their surfaces (data not shown), the surface levels of HEL46–61:I-Ak on xid B cells pulsed with HEL-Ab complex were much lower than those on wt B cells, even though they were slightly higher than those of xid B cells pulsed with HEL alone (Fig. 9, A and C). Although both wt and xid B cells increased surface HEL46–61: I-Ak levels as the Ag concentration increased, the increase shown by xid B cells was much smaller than that by wt B cells (Fig. 9C). Similarly, the Btk inhibitor, LFM A-13, significantly decreased the surface levels of HEL46–61:I-Ak in treated B cells compared to untreated B cells (Fig. 9, B and D). To further compare the abilities of wt and xid B cells to present Ag and activate T cells, splenic B cells were first incubated with HEL or HEL-Ab complex for 24 h, washed, and then incubated with KZH T cells. The KZH T cells, specific for the same peptide-MHC class II complex recognized by the C4H3 mAb, express a lacZ reporter gene under the control of the IL-2 promoter (40). T cell activation was monitored by the expression of the reporter lacZ. Similar to the surface HEL46–47: I-Ak levels, wt and xid B cells pulsed with HEL alone activated the KZH T cells to similar extents (Fig. 9E). However, when HEL was targeted to the BCR by the Ab complex, wt B cells stimulated the KZH T cells to a much higher level than xid B cells (Fig. 9E). These data demonstrate a role for Btk in regulating BCR-mediated Ag processing and presentation.
FIGURE 9.
The Ag-presentation efficiency is reduced in both Btk-deficient B cells and Btk inhibitor-treated B cells. A and B, Splenic B cells from xid and wt mice (A) or splenic B cells from wt mice that were serum starved and treated with or without LFM A-13 (B) were pulsed with HEL (1 μg/ml) alone or with the Ab complex that targets HEL to the BCR at 37°C for 15 min. After washing, cells were incubated at 37°C for 14 h. MHC class II I-Ak loaded with HEL peptides (HEL46–61: I-Ak) on the cell surface was detected using a mAb (C4H3) and quantified using flow cytometry. Shown are representative histograms of three independent experiments. C and D, Shown are the ratios of MFI of HEL46–61:I-Ak on the surface of B cells that were pulsed with the HEL-Ab complex, which targets HEL to the BCR, vs those B cells that were pulsed with HEL alone, where HEL was internalized through pinocytosis. Shown are average values (±SD) of three independent experiments (*, p ≤ 0.01). E, Splenic B cells (1 × 106) from wt and xid mice were either incubated with HEL (1 μg/ml) alone or with the Ab complex for 24 h. After washing, the B cells (1×106) were cocultured overnight with KZH T cells (1 × 106). The activity of LacZ that is under the control of IL-2 promoter in the T cells was measured using a colorimetric LacZ substrate. Shown is the OD of the LacZ enzymatic product over time and representative data of three independent experiments with triplicates (*, p ≤ 0.005).
Discussion
The binding of Ags to the BCR induces a series of cellular events that are essential for B cell activation, including signaling cascades, actin reorganization, and Ag internalization for processing and presentation. Although there is a regulatory relationship between these cellular events, no distinct link between these cellular pathways has been defined. In this study, we identify Btk as a linker that transduces signals from the BCR into actin reorganization by controlling the activity of WASP, Vav, and PtdIns-4,5-P2 biogenesis. Significantly, Btk-dependent actin cytoskeleton remodeling is required for the high efficiency of BCR-mediated Ag uptake, processing, and presentation.
BCR activation is known to trigger changes in the actin cytoskeleton (12, 46). We further characterize it as a biphasic process with a rapid but transient increase in cellular F-actin in the first few minutes post BCR XL, followed by a decline during the next few minutes. Moreover, XL of the BCR triggers site-directed actin polymerization near the BCR. Localized actin polymerization shown here provides an explanation for the dependency of BCR internalization on the actin cytoskeleton we reported previously (16). BCR activation induces actin assembly at BCR internalization sites and BCR+-vesicles, where this polymerization may provide the driving force for fission of clathrin-coated vesicles and inward movement of BCR+-vesicles. BCR-triggered depolymerization of F-actin, in contrast, may loosen the “fence” formed by the cortical actin cytoskeleton, allowing for inward movement of BCR-containing vesicles. Thus, these biphasic actin dynamics may be essential for restructuring the actin cytoskeleton in response to BCR activation.
The abrogation of BCR-triggered actin cytoskeleton rearrangement in the presence of tyrosine kinase inhibitors and Syk deficiency (6, 7, 9) indicates a regulatory role for BCR-mediated signaling in actin dynamics. Using two model systems, xid mice and Btk inhibitor LFM A-13, we demonstrate that BCR-dependent actin polymerization and even the constitutive level of actin polymerization is dependent on the functionality of Btk. This study reveals for the first time that Btk is the major signaling component that links BCR signals to the actin cytoskeleton in B cells.
Although the Btk xid mutation and LFM A-13 exhibit similar inhibitory effects on Btk activity and actin dynamics in B cells, each of them could influence actin dynamics through a mechanism different from Btk, as the xid mutation causes B cell developmental delays (47) and LFM A-13 can inhibit Jak2 (48). Our finding that both mature and immature/transitional subsets of splenic B cells increase the level of pWASP to similar degrees and exhibit similar BCR internalization rates demonstrates that the observed inhibitory effect of the xid mutation is not caused by B cell developmental defects. Because BCR activation does not induce Jak2 phosphorylation and BCR-induced STAT activation is independent of Jaks (49), the effect of LFM A-13 on actin polymerization is unlikely due to its inhibition of Jak2.
The results presented here demonstrate that BCR-triggered, Btk-dependent actin cytoskeleton rearrangement in B cells is mediated through WASP. Binding of Ag to the BCR increases the levels of oWASP, triggers its phosphorylation at S483/484, and recruits activated WASP to the PM and the BCR. The concordance of Ag-bound BCR, actin nucleation sites, and active WASP strongly suggests that WASP mediates actin polymerization and branching at BCR internalization sites. A fraction of the active WASP was found to maintain its colocalization with the BCR, even after it had been internalized, suggesting a role for WASP beyond internalization, possibly in driving the inward movement and/or membrane fusion of BCR+-vesicles to late endosomes. The relationship of defects in BCR-triggered WASP activation and actin reorganization in xid and Btk inhibitor-treated B cells reinforces that Btk can funnel BCR signaling cues to the cytoskeleton through WASP.
The activation mechanism for WASP has been extensively studied and a general model for its activation has emerged. In the absence of stimuli, WASP is present in an autoinhibited state mediated by the interaction of its GBD with VCA region. This autoinhibition is released when its GBD, B, and proline-rich domains coordinately bind to GTP-Cdc42, PtdIns-4,5-P2, and a SH3 domain-containing protein, respectively, freeing the VCA region for binding Arp2/3 (19). The phosphorylation of WASP at Y291 and S483/484 (22) increases the actin polymerization activity of WASP, by stabilizing its open, active conformation. In B cells, BCR activation has been shown to induce transient tyrosine phosphorylation of WASP (50), and an interaction of WASP with Btk in vitro has been reported (50–52). Our results show that BCR activation induces the cell surface recruitment and phosphorylation of Cdc42 GEF Vav and increases the cellular biogenesis of PtdIns-4,5 P2 and the surface recruitment and serine phosphorylation of WASP, all of which occur in a Btk-dependent manner. These results suggest that Btk activates WASP by several related mechanisms, by regulating the activity of Vav, the generation of PtdIns-4,5-P2, and the phosphorylation of WASP. The direct interaction of WASP with Btk reported previously (50–52) provides another mechanism for Btk to regulate the phosphorylation and subcellular location of WASP, whereby Btk recruits WASP to the PM where WASP interacts with PtdIns-4,5-P2 and is phosphorylated by kinases.
Reports on the involvement of Tec kinases in regulating Vav activity in T cells (37, 53) and our study support a general function of Tec kinases in regulating WASP activity by controlling the activity of Vav. How Btk activates Vav in B cells remains to be elucidated. Possible mechanisms include direct phosphorylation of Vav by Btk or Btk-mediated recruitment of Vav to BCR signaling microdomains, where it is phosphorylated by Src or Syk. The adaptor function of Btk brings to the cell surface PIP5K, the primary PtdIns-4,5-P2-generating lipid kinase (33). In this study, we show that Btk is able to regulate the local metabolism of PtdIns-4,5-P2 that in turn is a substrate or coactivator for downstream effectors of Btk such as PLCγ2 and WASP (23, 32). Btk-dependent PtdIns-4,5-P2 generation activates WASP in cooperation with GTP-Cdc42, the product of Btk-dependent Vav activation.
Btk's capacity in BCR signaling has been well characterized (54–57). The results of this study show for the first time that Btk is part of a regulatory mechanism for efficient Ag uptake and transport that leads to Ag processing and presentation. This appears to contradict previous findings that xid and Btk−/− mice have profound defects in response to T-independent Ags but not in their T-dependent responses to protein Ags. The high efficiency of B cells to process and present Ag relies on the abilities of the BCR to capture Ags with high specificity and affinity, to rapidly internalize them, and specifically target them to the Ag-processing compartment. This allows B cells to present Ags even when exposed to low levels of Ags for short periods of time. The disruption of Btk function, either by the R28C mutation or the inhibitor, reduces the rates of BCR internalization and its movement to the Ag-processing compartment. This consequently decreases the amount of Ag available to the processing and presentation machinery. It is important to note that the processing and presentation of Ags endocytosed by pinocytosis is not significantly affected by the Btk xid mutation. This implies that Btk's role in this process is instigated by the BCR binding to cognate Ags. Since the processing and presentation of Ag internalized through nonspecific pinocytosis was not affected by the Btk xid mutation, T-dependent Ab response will not be completely blocked or significantly affected, especially when Ag is abundant as in the case of animal immunization models. The specific effect of the Btk xid mutation and Btk inhibitor on BCR-induced Ag presentation would reduce the sensitivity and efficiency of B cells to process and present Ag, especially when Ag is not in abundance like at the beginning of an infection. This notion is supported by an early report that the activation of B cells from xid mice is sensitive to the concentration of T-dependent Ag (58). The defects of BCR-mediated Ag processing and BCR-triggered actin cytoskeleton rearrangement in the xid B cells suggest that Btk connects BCR signaling activity to its Ag transport and processing functions by mobilizing the actin cytoskeleton.
A working model for the interactions among BCR signaling, the actin cytoskeleton, and BCR Ag-processing pathway, thus, emerges. The binding of the BCR by multivalent Ags triggers the formation of surface signaling microdomains. The production of PtdIns-3,4,5-P3 by PI3K recruits Btk to the signaling microdomains where Btk is activated by phosphorylation. Subsequently, Btk recruits WASP to the signaling microdomains and activates it by inducing its phosphorylation, activating Vav and consequently Cdc42, and increasing local PtdIn-4,5-P2 levels. Activated WASP promotes actin assembly and branching in the vicinity of the BCR. This provides the driving force for the formation of BCR+ clathrin-coated vesicles and the inward movement and fusion of these Ag-containing vesicles with the Ag-processing compartment. This cross-talk mechanism between signaling, actin cytoskeleton, and membrane transport machineries might be important for all lymphocytes to transduce antigenic signals into cellular responses. The functional and physical interaction between Btk and WASP may not be the only link between BCR signaling and the actin cytoskeleton. B cells from WASP knockout mice only show mild defects in general (59), suggesting the presence of additional links. Other members of the WASP family proteins, N-WASP and WAVE (60), potentially compensate for WASP downstream of BCR signaling. BCR signaling could also regulate the actin cytoskeleton through other actin regulators that do not belong to the WASP family, such as the regulation of HS1, an actin-binding protein and a homologue of cortactin expressed in lymphocytes, by Syk kinases (61) and actin-binding protein 1 by BCR signaling (62). Moreover, redundant functions provided by other Tec kinases cannot be eliminated, as the recently created Btk/Tec double knockout mice (63) display defects that are more severe than Btk knockout alone. Future studies will examine additional molecular links and interaction mechanisms between the actin cytoskeleton and BCR-mediated signaling and Ag-1processing pathways.
Acknowledgments
We thank Drs. Kenneth Frauwirth, Lian-Yong Gao, and Silvia Bolland for critical reading of the manuscript and Amy Beaven for technical assistance on confocal fluorescence microscope.
This work is supported by a National Institutes of Health Grant AI059617 (to W.S.).
Footnotes
Abbreviations used in this paper: XL, cross-linking; AF, Alexa Fluor; B, basic; Btk, Bruton's tyrosine kinase; CTX-B, cholera toxin subunit B; GBD, GTPase-binding domain; GEF, guanidine nucleotide exchange factor; HEL, hen egg lysozyme; PH, pleckstrin homology; PM, plasma membrane; PRD, proline-rich domain; PtdIns-4,5-P2, phosphatidylinositol-4,5-bisphosphate; Tf, transferrin; VCA domains, verprolin homology, cofilin homology and acidic; WASP, Wiskott-Aldrich syndrome protein; oWASP, WASP with open and active conformation; pWASP, phosphorylated WASP; MFI, mean fluorescence intensity; wt, wild type; MZ, marginal zone.
Disclosures The authors have no financial conflict of interest.
References
- 1.Cheng PC, Cherukuri A, Dykstra M, Malapati S, Sproul T, Chen MR, Pierce SK. Floating the raft hypothesis: the roles of lipid rafts in B cell antigen receptor function. Semin. Immunol. 2001;13:107–114. doi: 10.1006/smim.2000.0302. [DOI] [PubMed] [Google Scholar]
- 2.Reth M, Wienands J. Initiation and processing of signals from the B cell antigen receptor. Annu. Rev. Immunol. 1997;15:453–479. doi: 10.1146/annurev.immunol.15.1.453. [DOI] [PubMed] [Google Scholar]
- 3.Song W, Cho H, Cheng P, Pierce SK. Entry of B cell antigen receptor and antigen into class II peptide-loading compartment is independent of receptor cross-linking. J. Immunol. 1995;155:4255–4263. [PubMed] [Google Scholar]
- 4.Cheng PC, Steele CR, Gu L, Song W, Pierce SK. MHC class II antigen processing in B cells: accelerated intracellular targeting of antigens. J. Immunol. 1999;162:7171–7180. [PubMed] [Google Scholar]
- 5.Siemasko K, Skaggs, J. B, Kabak S, Williamson E, Brown BK, Song W, Clark, R. M. Receptor facilitated antigen presentation requires the recruitment of B cell linker protein to Igα. J. Immunol. 2002;168:2127–2138. doi: 10.4049/jimmunol.168.5.2127. [DOI] [PubMed] [Google Scholar]
- 6.Le Roux D, Lankar D, Yuseff MI, Vascotto F, Yokozeki T, Faure-Andre G, Mougneau E, Glaichenhaus N, Manoury B, Bonnerot C, Lennon-Dumenil AM. Syk-dependent actin dynamics regulate endocytic trafficking and processing of antigens internalized through the B-cell receptor. Mol. Biol. Cell. 2007;18:3451–3462. doi: 10.1091/mbc.E06-12-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dykstra ML, Longnecker R, Pierce SK. Epstein-Barr virus coopts lipid rafts to block the signaling and antigen transport functions of the BCR. Immunity. 2001;14:57–67. doi: 10.1016/s1074-7613(01)00089-9. [DOI] [PubMed] [Google Scholar]
- 8.Wagle NM, Kim JH, Pierce SK. Signaling through the B cell antigen receptor regulates discrete steps in the antigen processing pathway. Cell. Immunol. 1998;184:1–11. doi: 10.1006/cimm.1998.1264. [DOI] [PubMed] [Google Scholar]
- 9.Ma H, Yankee TM, Hu J, Asai DJ, Harrison ML, Geahlen RL. Visualization of Syk-antigen receptor interactions using green fluorescent protein: differential roles for Syk and Lyn in the regulation of receptor capping and internalization. J. Immunol. 2001;166:1507–1516. doi: 10.4049/jimmunol.166.3.1507. [DOI] [PubMed] [Google Scholar]
- 10.Stoddart A, Dykstra ML, Brown BK, Song W, Pierce SK, Brodsky FM. Lipid rafts unite signaling cascades with clathrin to regulate BCR internalization. Immunity. 2002;17:451–462. doi: 10.1016/s1074-7613(02)00416-8. [DOI] [PubMed] [Google Scholar]
- 11.Jugloff LS, Jongstra-Bilen J. Cross-linking of the IgM receptor induces rapid translocation of IgM-associated Igα, Lyn, and Syk tyrosine kinases to the membrane skeleton. J. Immunol. 1997;159:1096–1106. [PubMed] [Google Scholar]
- 12.Albrecht DL, Noelle RJ. Membrane Ig-cytoskeletal interactions, I: flow cytofluorometric and biochemical analysis of membrane IgM-cytoskeletal interactions. J. Immunol. 1988;141:3915–3922. [PubMed] [Google Scholar]
- 13.Melamed I, Downey GP, Aktories K, Roifman CM. Microfilament assembly is required for antigen-receptor-mediated activation of human B lymphocytes. J. Immunol. 1991;147:1139–1146. [PubMed] [Google Scholar]
- 14.Melamed I, Downey GP, Roifman CM. Tyrosine phosphorylation is essential for microfilament assembly in B lymphocytes. Biochem. Biophys. Res. Commun. 1991;176:1424–1429. doi: 10.1016/0006-291x(91)90445-d. [DOI] [PubMed] [Google Scholar]
- 15.Cheng PC, Brown BK, Song W, Pierce SK. Translocation of the B cell antigen receptor into lipid rafts reveals a novel step in signaling. J. Immunol. 2001;166:3693–3701. doi: 10.4049/jimmunol.166.6.3693. [DOI] [PubMed] [Google Scholar]
- 16.Brown BK, Song W. The actin cytoskeleton is required for the trafficking of the B cell antigen receptor to the late endosomes. Traffic. 2001;2:414–427. doi: 10.1034/j.1600-0854.2001.002006414.x. [DOI] [PubMed] [Google Scholar]
- 17.Stoddart A, Jackson AP, Brodsky FM. Plasticity of B cell receptor internalization upon conditional depletion of clathrin. Mol. Biol. Cell. 2005;16:2339–2348. doi: 10.1091/mbc.E05-01-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Millard TH, Machesky LM. The Wiskott-Aldrich syndrome protein (WASP) family. Trends Biochem. Sci. 2001;26:198–199. doi: 10.1016/s0968-0004(01)01788-1. [DOI] [PubMed] [Google Scholar]
- 19.Thrasher AJ. WASp in immune-system organization and function. Nat. Rev. Immunol. 2002;2:635–646. doi: 10.1038/nri884. [DOI] [PubMed] [Google Scholar]
- 20.Cory GO, Garg R, Cramer R, Ridley AJ. Phosphorylation of tyrosine 291 enhances the ability of WASp to stimulate actin polymerization and filopodium formation. Wiskott-Aldrich Syndrome protein. J. Biol. Chem. 2002;277:45115–45121. doi: 10.1074/jbc.M203346200. [DOI] [PubMed] [Google Scholar]
- 21.Yokoyama N, Lougheed J, Miller WT. Phosphorylation of WASP by the Cdc42-associated kinase ACK1: dual hydroxyamino acid specificity in a tyrosine kinase. J. Biol. Chem. 2005;280:42219–42226. doi: 10.1074/jbc.M506996200. [DOI] [PubMed] [Google Scholar]
- 22.Cory GO, Cramer R, Blanchoin L, Ridley AJ. Phosphorylation of the WASP-VCA domain increases its affinity for the Arp2/3 complex and enhances actin polymerization by WASP. Mol. Cell. 2003;11:1229–1239. doi: 10.1016/s1097-2765(03)00172-2. [DOI] [PubMed] [Google Scholar]
- 23.Higgs HN, Pollard TD. Activation by Cdc42 and PIP2 of Wiskott-Aldrich syndrome protein (WASp) stimulates actin nucleation by Arp2/3 complex. J. Cell Biol. 2000;150:1311–1320. doi: 10.1083/jcb.150.6.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bustelo XR, Barbacid M. Tyrosine phosphorylation of the vav proto-oncogene product in activated B cells. Science. 1992;256:1196–1199. doi: 10.1126/science.256.5060.1196. [DOI] [PubMed] [Google Scholar]
- 25.Arana E, Vehlow A, Harwood NE, Vigorito E, Henderson R, Turner M, Tybulewicz VL, Batista FD. Activation of the small GTPase Rac2 via the B cell receptor regulates B cell adhesion and immunological-synapse formation. Immunity. 2008;28:88–99. doi: 10.1016/j.immuni.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 26.O'Rourke LM, Tooze R, Turner M, Sandoval DM, Carter RH, Tybulewicz VL, Fearon DT. CD19 as a membrane-anchored adaptor protein of B lymphocytes: costimulation of lipid and protein kinases by recruitment of Vav. Immunity. 1998;8:635–645. doi: 10.1016/s1074-7613(00)80568-3. [DOI] [PubMed] [Google Scholar]
- 27.Lindvall JM, Blomberg KE, Valiaho J, Vargas L, Heinonen JE, Berglof A, Mohamed AJ, Nore BF, Vihinen M, Smith CI. Bruton's tyrosine kinase: cell biology, sequence conservation, mutation spectrum, siRNA modifications, and expression profiling. Immunol. Rev. 2005;203:200–215. doi: 10.1111/j.0105-2896.2005.00225.x. [DOI] [PubMed] [Google Scholar]
- 28.Satterthwaite AB, Witte ON. The role of Bruton's tyrosine kinase in B-cell development and function: a genetic perspective. Immunol. Rev. 2000;175:120–127. [PubMed] [Google Scholar]
- 29.Salim K, Bottomley MJ, Querfurth E, Zvelebil MJ, Gout I, Scaife R, Margolis RL, Gigg R, Smith CI, Driscoll PC, et al. Distinct specificity in the recognition of phosphoinositides by the pleckstrin homology domains of dynamin and Bruton's tyrosine kinase. EMBO J. 1996;15:6241–6250. [PMC free article] [PubMed] [Google Scholar]
- 30.Park H, Wahl MI, Afar DE, Turck CW, Rawlings DJ, Tam C, Scharenberg AM, Kinet JP, Witte ON. Regulation of Btk function by a major autophosphorylation site within the SH3 domain. Immunity. 1996;4:515–525. doi: 10.1016/s1074-7613(00)80417-3. [DOI] [PubMed] [Google Scholar]
- 31.Rawlings DJ, Scharenberg AM, Park H, Wahl MI, Lin S, Kato RM, Fluckiger AC, Witte ON, Kinet JP. Activation of BTK by a phosphorylation mechanism initiated by SRC family kinases. Science. 1996;271:822–825. doi: 10.1126/science.271.5250.822. [DOI] [PubMed] [Google Scholar]
- 32.Kurosaki T, Tsukada S. BLNK: connecting Syk and Btk to calcium signals. Immunity. 2000;12:1–5. doi: 10.1016/s1074-7613(00)80153-3. [DOI] [PubMed] [Google Scholar]
- 33.Saito K, Tolias KF, Saci A, Koon HB, Humphries LA, Scharenberg A, Rawlings DJ, Kinet JP, Carpenter CL. BTK regulates PtdIns-4,5-P2 synthesis: importance for calcium signaling and PI3K activity. Immunity. 2003;19:669–678. doi: 10.1016/s1074-7613(03)00297-8. [DOI] [PubMed] [Google Scholar]
- 34.Saito K, Scharenberg AM, Kinet JP. Interaction between the Btk PH domain and phosphatidylinositol-3,4,5-trisphosphate directly regulates Btk. J. Biol. Chem. 2001;276:16201–16206. doi: 10.1074/jbc.M100873200. [DOI] [PubMed] [Google Scholar]
- 35.Varnai P, Rother KI, Balla T. Phosphatidylinositol 3-kinase-dependent membrane association of the Bruton's tyrosine kinase pleckstrin homology domain visualized in single living cells. J. Biol. Chem. 1999;274:10983–10989. doi: 10.1074/jbc.274.16.10983. [DOI] [PubMed] [Google Scholar]
- 36.Labno CM, Lewis CM, You D, Leung DW, Takesono A, Kamberos N, Seth A, Finkelstein LD, Rosen MK, Schwartzberg PL, Burkhardt JK. Itk functions to control actin polymerization at the immune synapse through localized activation of Cdc42 and WASP. Curr. Biol. 2003;13:1619–1624. doi: 10.1016/j.cub.2003.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schaeffer EM, Debnath J, Yap G, McVicar D, Liao XC, Littman DR, Sher A, Varmus HE, Lenardo MJ, Schwartzberg PL. Requirement for Tec kinases Rlk and Itk in T cell receptor signaling and immunity. Science. 1999;284:638–641. doi: 10.1126/science.284.5414.638. [DOI] [PubMed] [Google Scholar]
- 38.Manders EMM, Verbeek FJ, Aten JA. Measurement of co-localization of object in dual-colour confocal images. J. Microsc. 1993;169:375–382. doi: 10.1111/j.1365-2818.1993.tb03313.x. [DOI] [PubMed] [Google Scholar]
- 39.Chan AY, Raft S, Bailly M, Wyckoff JB, Segall JE, Condeelis JS. EGF stimulates an increase in actin nucleation and filament number at the leading edge of the lamellipod in mammary adenocarcinoma cells. J. Cell. Sci. 1998;111:199–211. doi: 10.1242/jcs.111.2.199. [DOI] [PubMed] [Google Scholar]
- 40.Karttunen J, Shastri N. Measurement of ligand-induced activation in single viable T cells using the lacZ reporter gene. Proc. Natl. Acad. Sci. USA. 1991;88:3972–3976. doi: 10.1073/pnas.88.9.3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanderson S, Shastri N. LacZ inducible, antigen/MHC-specific T cell hybrids. Int. Immunol. 1994;6:369–376. doi: 10.1093/intimm/6.3.369. [DOI] [PubMed] [Google Scholar]
- 42.Mahajan S, Ghosh S, Sudbeck EA, Zheng Y, Downs S, Hupke M, Uckun FM. Rational design and synthesis of a novel anti-leukemic agent targeting Bruton's tyrosine kinase (BTK), LFM-A13 [α-cyano-β-hydroxy-β-methyl-N-(2, 5-dibromophenyl)propenamide] J. Biol. Chem. 1999;274:9587–9599. doi: 10.1074/jbc.274.14.9587. [DOI] [PubMed] [Google Scholar]
- 43.Heo J, Thapar R, Campbell SL. Recognition and activation of Rho GTPases by Vav1 and Vav2 guanine nucleotide exchange factors. Biochemistry. 2005;44:6573–6585. doi: 10.1021/bi047443q. [DOI] [PubMed] [Google Scholar]
- 44.Lopez-Lago M, Lee H, Cruz C, Movilla N, Bustelo XR. Tyrosine phosphorylation mediates both activation and downmodulation of the biological activity of Vav. Mol. Cell. Biol. 2000;20:1678–1691. doi: 10.1128/mcb.20.5.1678-1691.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhong G, Reis e Sousa C, Germain RN. Production, specificity, and functionality of monoclonal antibodies to specific peptide-major histocompatibility complex class II complexes formed by processing of exogenous protein. Proc. Natl. Acad. Sci. USA. 1997;94:13856–13861. doi: 10.1073/pnas.94.25.13856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hao S, August A. Actin depolymerization transduces the strength of B-cell receptor stimulation. Mol. Biol. Cell. 2005;16:2275–2284. doi: 10.1091/mbc.E04-10-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Satterthwaite AB, Li Z, Witte ON. Btk function in B cell development and response. Semin. Immunol. 1998;10:309–316. doi: 10.1006/smim.1998.0123. [DOI] [PubMed] [Google Scholar]
- 48.van den Akker E, van Dijk TB, Schmidt U, Felida L, Beug H, Lowenberg B, von Lindern M. The Btk inhibitor LFM-A13 is a potent inhibitor of Jak2 kinase activity. Biol. Chem. 2004;385:409–413. doi: 10.1515/BC.2004.045. [DOI] [PubMed] [Google Scholar]
- 49.Wang L, Kurosaki T, Corey SJ. Engagement of the B-cell antigen receptor activates STAT through Lyn in a Jak-independent pathway. Oncogene. 2007;26:2851–2859. doi: 10.1038/sj.onc.1210092. [DOI] [PubMed] [Google Scholar]
- 50.Baba Y, Nonoyama S, Matsushita M, Yamadori T, Hashimoto S, Imai K, Arai S, Kunikata T, Kurimoto M, Kurosaki T, et al. Involvement of wiskott-aldrich syndrome protein in B-cell cytoplasmic tyrosine kinase pathway. Blood. 1999;93:2003–2012. [PubMed] [Google Scholar]
- 51.Cory GO, MacCarthy-Morrogh L, Banin S, Gout I, Brickell PM, Levinsky RJ, Kinnon C, Lovering RC. Evidence that the Wiskott-Aldrich syndrome protein may be involved in lymphoid cell signaling pathways. J. Immunol. 1996;157:3791–3795. [PubMed] [Google Scholar]
- 52.Morrogh LM, Hinshelwood S, Costello P, Cory GO, Kinnon C. The SH3 domain of Bruton's tyrosine kinase displays altered ligand binding properties when auto-phosphorylated in vitro. Eur. J. Immunol. 1999;29:2269–2279. doi: 10.1002/(SICI)1521-4141(199907)29:07<2269::AID-IMMU2269>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 53.Finkelstein LD, Shimizu Y, Schwartzberg PL. Tec kinases regulate TCR-mediated recruitment of signaling molecules and integrin-dependent cell adhesion. J. Immunol. 2005;175:5923–5930. doi: 10.4049/jimmunol.175.9.5923. [DOI] [PubMed] [Google Scholar]
- 54.Craxton A, Jiang A, Kurosaki T, Clark EA. Syk and Bruton's tyrosine kinase are required for B cell antigen receptor-mediated activation of the kinase Akt. J. Biol. Chem. 1999;274:30644–30650. doi: 10.1074/jbc.274.43.30644. [DOI] [PubMed] [Google Scholar]
- 55.Fluckiger AC, Li Z, Kato RM, Wahl MI, Ochs HD, Longnecker R, Kinet JP, Witte ON, Scharenberg AM, Rawlings DJ. Btk/Tec kinases regulate sustained increases in intracellular Ca2+ following B-cell receptor activation. EMBO J. 1998;17:1973–1985. doi: 10.1093/emboj/17.7.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Inabe K, Miyawaki T, Longnecker R, Matsukura H, Tsukada S, Kurosaki T. Bruton's tyrosine kinase regulates B cell antigen receptor-mediated JNK1 response through Rac1 and phospholipase C-γ2 activation. FEBS Lett. 2002;514:260–262. doi: 10.1016/s0014-5793(02)02375-x. [DOI] [PubMed] [Google Scholar]
- 57.Mohamed AJ, Nore BF, Christensson B, Smith CI. Signalling of Bruton's tyrosine kinase, Btk. Scand. J. Immunol. 1999;49:113–118. doi: 10.1046/j.1365-3083.1999.00504.x. [DOI] [PubMed] [Google Scholar]
- 58.Boswell HS, Nerenberg MI, Scher I, Singer A. Role of accessory cells in B cell activation. III. Cellular analysis of primary immune response deficits in CBA/N mice: presence of an accessory cell-B cell interaction defect. J. Exp. Med. 1980;152:1194–1309. doi: 10.1084/jem.152.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Snapper SB, Rosen FS, Mizoguchi E, Cohen P, Khan W, Liu CH, Hagemann TL, Kwan SP, Ferrini R, Davidson L, et al. Wiskott-Aldrich syndrome protein-deficient mice reveal a role for WASP in T but not B cell activation. Immunity. 1998;9:81–91. doi: 10.1016/s1074-7613(00)80590-7. [DOI] [PubMed] [Google Scholar]
- 60.Miki H, Takenawa T. Regulation of actin dynamics by WASP family proteins. J. Biochem. 2003;134:309–313. doi: 10.1093/jb/mvg146. [DOI] [PubMed] [Google Scholar]
- 61.Hao JJ, Carey GB, Zhan X. Syk-mediated tyrosine phosphorylation is required for the association of hematopoietic lineage cell-specific protein 1 with lipid rafts and B cell antigen receptor signalosome complex. J. Biol. Chem. 2004;279:33413–33420. doi: 10.1074/jbc.M313564200. [DOI] [PubMed] [Google Scholar]
- 62.Onabajo OO, Seeley MK, Kale A, Qualmann B, Kessels M, Han J, Tan TH, Song W. Actin-binding protein 1 regulates B cell receptor-mediated antigen processing and presentation in response to B cell receptor activation. J. Immunol. 2008;180:6685–6695. doi: 10.4049/jimmunol.180.10.6685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ellmeier W, Jung S, Sunshine MJ, Hatam F, Xu Y, Baltimore D, Mano H, Littman DR. Severe B cell deficiency in mice lacking the tec kinase family members Tec and Btk. J. Exp. Med. 2000;192:1611–1624. doi: 10.1084/jem.192.11.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]