Abstract
Background
People with known risk factors for chronic obstructive pulmonary disease (COPD) are important targets for screening and early intervention. We sought to measure the prevalence of COPD among such individuals visiting a primary care practitioner for any reason. We also evaluated the accuracy of prior diagnosis or nondiagnosis of COPD and identified associated clinical characteristics.
Methods
We recruited patients from three primary care sites who were 40 years or older and had a smoking history of at least 20 pack-years. Participants were asked about respiratory symptoms and underwent postbronchodilator spirometry. COPD was defined as a ratio of forced expiratory volume in the first second of expiration to forced vital capacity (FEV1/FVC) of less than 0.7 and an FEV1 of less than 80% predicted.
Results
Of the 1459 patients who met the study criteria, 1003 (68.7%) completed spirometry testing. Of these, 208 were found to have COPD, for a prevalence of 20.7% (95% confidence interval 18.3%–23.4%). Of the 205 participants with COPD who completed the interview about respiratory symptoms before spirometry, only 67 (32.7%) were aware of their diagnosis before the study. Compared with patients in whom COPD had been correctly diagnosed before the study, those in whom COPD had been over-diagnosed or undiagnosed were similar in terms of age, sex, current smoking status and number of visits to a primary care practitioner because of a respiratory problem.
Interpretation
Among adult patients visiting a primary care practitioner, as many as one in five with known risk factors met spirometric criteria for COPD. Underdiagnosis of COPD was frequent, which suggests a need for greater screening of at-risk individuals. Knowledge of the prevalence of COPD will help plan strategies for disease management.
Chronic obstructive pulmonary disease (COPD) is a common and costly condition worldwide.1,2 A recent global population-based study estimated the prevalence of the disease to be about 10% among people 40 years of age and older.3 People with known risk factors for COPD, such as increased age and a history of smoking, are likely to have a substanially higher prevalence4–6 and to have progressive manifestations of COPD if they continue to smoke.7 They are thus appropriate targets for intervention early in the course of their disease.
People with early or undiagnosed COPD are most likely to encounter the health care system in the primary care setting. Consequently, accurate knowledge of the prevalence of COPD in this setting is critical to planning and implementing strategies for the detection and management of the disease. However, valid estimates are lacking of the prevalence of COPD among at-risk individuals in primary care practices. Access by family doctors to spirometric data confirming irreversible airway obstruction has been shown to influence patient management8 and to enable implementation of a targeted self-management plan that may include smoking cessation, pharmacotherapy and increased exercise.9–11
We sought to measure the prevalence of spirometrically confirmed COPD in an at-risk population of adults aged 40 years or more with a smoking history of at least 20 pack-years who visited a primary care practitioner (physician or nurse practitioner) for any reason and to describe their characteristics. We also evaluated the accuracy of prior diagnosis or nondiagnosis of COPD and to identify associated clinical characteristics.
Methods
The research ethics committees of the University of Toronto and the Sault Saint Marie Group Health Centre approved this study.
Recruitment and enrolment
Participants were recruited from April 2006 to February 2007 from three primary care sites in the province of Ontario representing rural, suburban and urban populations. These sites had previously participated in a provincial study of asthma. Doctors at each site were family physicians without specialization in respiratory medicine. Identification of participants varied by site. At two sites, screening questionnaires were provided immediately to all adults on attendance. At the third site, all adult attendees were identified from an electronic database and were asked to complete the screening questionnaire during a telephone interview before their visit. The sampling period was extended to include May–November 2007 to attain the necessary sample size.
The screening questionnaire ascertained age, sex, smoking history and any self-reported previous respiratory diagnosis. Patients aged 40 years or more with a smoking history of at least 20 pack-years were invited to attend an assessment session within four weeks after completing the questionnaire. They were not requested to stop using any current respiratory medications.
Assessment session
After providing informed consent, participants completed a face-to-face interview regarding limitations resulting from dyspnea (according to the Medical Research Council dyspnea scale12) and the presence of respiratory symptoms (Appendix 1, available at www.cmaj.ca/cgi/content/full/cmaj.091784/DC1). An experienced technologist performed portable spirometry. Those with spirometric evidence of COPD13,14 were requested to complete a second face-to-face interview regarding home oxygen use and comorbid health conditions (Appendix 1). Spirometry was delayed for a minimum of four weeks following resolution of any reported respiratory infection. It was not performed if a participant met specific criteria thought to compromise his or her safety.3
Spirometry
Spirometry was performed according to international guidelines15 using a calibrated digital hand-held spirometer. All participants who had a ratio of forced expiratory volume in thefirst second of expiration (FEV1) to forced vital capacity (FVC) of less than 0.7 and an FEV1 of less than 80% predicted underwent postbronchodilator spirometry 20 minutes following two puffs of salbutamol (200 μg). We defined COPD as a postbronchodilator FEV1/FVC ratio of less than 0.7 and FEV1 of less than 80% predicted (GOLD stage 2 or higher).13,14,16 Predicted values for FEV1 were calculated using the equations of Hankinson and colleagues.17
We used quality-assurance procedures based on those used in a recent large international study of COPD:18 use of the same model of spirometer at all data-collection sites; standardized training of all staff responsible for data collection; collection and review of pilot data on five clinic attendees at each site; and a minimum of two visits by the study coordinator to each site.19
For immediate use in the study, spirometry had to yield three acceptable tracings, with the two best FVC values and the two best FEV1 values within 150 mL of each other.15 Tests that yielded only one acceptable tracing, or two acceptable tracings in which the measurements were not within 150 mL of each other, were reviewed by the study coordinator, senior pulmonary function technologist and an experienced respirologist. If there was any uncertainty regarding whether the results met the criteria for a diagnosis of COPD, spirometry was repeated following advice to the site technologist regarding how to optimize the quality of the tracings.
Chart abstraction
To identify clinical characteristics that could be used by family physicians to assist in distinguishing patients with and without COPD, the charts of participants who had spirometric evidence of COPD during the initial 12-month recruitment period were reviewed and data extracted on any diagnosis of COPD and the number of visits to a primary care physician because of a respiratory problem. Each participant with COPD was matched for sex, age (within five years) and recruitment site with three participants (or fewer, if three suitable matches could not be identified) who did not meet the spirometric criteria. Participants were then classified into four groups: correctly diagnosed COPD (spirometry results positive, chart positive); undiagnosed COPD (spirometry tests positive, chart negative); overdiagnosed COPD (spirometry results negative, chart positive); and no COPD (spirometry results negative, chart negative).
Data management and analysis
Data were faxed from the participating sites to the central data-management facility at McMaster University. This facility reported inconsistencies, discrepancies or missing data to the study site every two weeks. Required corrections were made within five business days. Prospective power calculations indicated that a sample size of 1000 patients would obtain a sufficiently narrow 95% confidence interval (CI) around the proportion of participants with COPD.
Statistical analysis
The prevalence of COPD was estimated as the number of participants who met the spirometric criteria for the disease, expressed as a proportion of those for whom valid spirometric measurements were available.
We performed logistic regression analysis using age, sex, current smoking status, number of respiratory symptoms (reported during the assessment session) and number of visits to a primary care physician because of a respiratory problem (collected through chart review) as independent variables. The dependent variable was accuracy of COPD diagnosis, dichotomized in separate analyses as: undiagnosed versus no COPD; correctly diagnosed versus overdiagnosed COPD; or undiagnosed versus correctly diagnosed COPD. We considered a p value of 0.05 or less to be significant.
Results
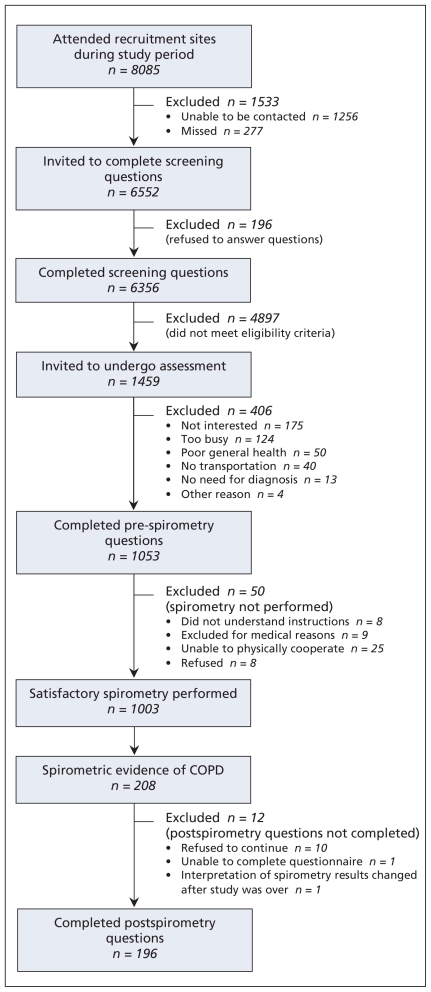
During the study period, 8085 patients attended the three recruitment sites. A total of 6552 were approached to complete the screening questionnaire, of whom 6356 (97.0%) agreed to answer the questions. Of the 1459 patients who met the eligibility criteria, 406 (27.8%) declined participation and 50 (3.4%) were unable to undergo spirometry (Figure 1). Compared with the 1053 participants, nonparticipants were more likely to be female (54.2% v. 48.2%; p = 0.047) and current smokers (53.2% v. 43.3%; p < 0.001). Of the 1003 participants for whom valid spirometric data were available, 613 (61.1%), 337 (33.6%) and 53 (5.3%) were recruited from the suburban, rural and urban sites, respectively.
Figure 1.
Recruitment and flow of participants.
Of the 1003 participants who completed spirometry, 208 met the spirometric criteria for COPD, for a prevalence of 20.7% (95% CI 18.3% to 23.4%). The characteristics of the participants who completed spirometry are summarized in Table 1. Of the 196 participants with COPD who completed the interview after spirometry, 58 (29.6%) reported a previous diagnosis of heart disease, 100 (51.0%) hypertension, 43 (21.9%) diabetes, 12 (6.1%) stroke, 3 (1.5%) lung cancer and 3 (1.5%) tuberculosis. Four (2.0%) of the 196 participants were using home oxygen. Of the 205 participants with positive spirometry results who completed the interview about respiratory symptoms before spirometry, 67 (32.7%) reported having received a prior diagnosis of COPD. Cough was the most common respiratory symptom, reported by 110 individuals (53.7%).
Table 1.
Characteristics of 1003 participants aged 40 years and older for whom results of spirometry were available
| Characteristic | Result of spirometry | ||
|---|---|---|---|
| No COPD n = 795 | GOLD stage 2 COPD*n = 164 | GOLD stage 3 or 4 COPD†n = 44 | |
| Age, yr, mean (SD) | 59.1 (10.5) | 64.0 (9.9) | 68.0 (8.7) |
| Sex, male:female ratio | 418:377 | 80:84 | 23:21 |
| Current smoker, no. (%) | 337 (42.4) | 72 (43.9) | 24 (54.5) |
| FEV1/FVC ratio, mean (SD) | 0.74 (0.07) | 0.59 (0.07) | 0.42 (0.10) |
| FEV1, % predicted, mean (SD) | 89.0 (13.2) | 67.8 (7.7) | 38.1 (7.7) |
| Smoking history, pack-years, mean (SD) | 33.0 (14.0) | 38.3 (16.4) | 46.8 (23.4) |
| Body mass index, mean (SD) | 29.7 (6.4) | 27.9 (5.7) | 25.0 (5.5) |
| White, no. (%) | 791 (99.5) | 163 (99.4) | 43 (97.7) |
| Medical Research Council dyspnea score, median (IQR) | 1 (1–2) | 2 (1–2) | 2 (2–3) |
| Self-reported diagnosis of COPD, no. (%) | 43 (5.6)‡ | 43 (26.7)§ | 24 (54.5) |
| ≥ 1 respiratory symptom, no. (%) | 519 (65.6)** | 121 (74.7)†† | 39 (90.7)‡‡ |
Note: COPD = chronic obstructive pulmonary disease, FEV1 = forced expiratory volume in first second of expiration, FVC = forced vital capacity, IQR = interquartile range, SD = standard deviation.
FEV1/FVC < 0.7 and FEV1 ≥ 50% predicted but < 80% predicted.
FEV1/FVC < 0.7 and FEV1 < 50% predicted.
Data available for 774.
Data available for 161.
Data available for 791.
Data available for 162.
Data available for 43.
Table 2 presents the characteristics of the 382 (38.1%) participants for whom chart abstraction was undertaken, including 107 (93.9%) of 114 who had positive spirometry results and 275 matched controls. Table 3 presents the results of the logistic regression analysis of the association between patient characteristics and the accuracy of the COPD diagnosis. The median time between spirometry and the most recent visit to a primary care practitioner was 1.1 (interquartile range 0.4–2.9) years.
Table 2.
Characteristics of 382 participants for whom chart abstraction was undertaken
| Characteristic | COPD correctly diagnosed*n = 58 | COPD undiagnosed†n = 49 | COPD overdiagnosed‡n = 45 | No COPD§n = 230 |
|---|---|---|---|---|
| Age, yr, mean (SD) | 64.2 (8.2) | 65.0 (10.3) | 65.5 (9.3) | 62.6 (8.6) |
| Sex, male:female ratio | 26:32 | 26:23 | 25:20 | 118:112 |
| Current smoker | 31 (53.4) | 14 (28.6) | 23 (51.1) | 62 (27.0) |
| ≥ 2 respiratory symptoms | 43 (74.1) | 19 (38.8) | 22 (50.0)†† | 84 (36.5) |
| No. of visits to a primary care physician because of respiratory problem, rate per patient-year** | 0.92 | 0.45 | 0.74 | 0.32 |
| Underwent previous spirometry** | 49 (84.5) | 13 (26.5) | 29 (64.4) | 60 (26.1) |
| Had been prescribed any respiratory medication** | 51 (87.9) | 19 (38.8) | 30 (66.7) | 63 (27.4) |
Note: COPD = chronic obstructive pulmonary disease, SD = standard deviation.
Positive spirometry result, diagnosis of COPD in chart.
Positive spirometry result, no diagnosis of COPD in chart.
Negative spirometry result, diagnosis of COPD in chart.
Negative spirometry result, no diagnosis of COPD in chart.
Data extracted from charts.
Data available for n = 44.
Table 3.
Association between patient characteristics and accuracy of COPD diagnosis
| Characteristic | COPD diagnosis; OR (95% CI)* | ||
|---|---|---|---|
| Undiagnosed COPD v. no COPD | Correctly diagnosed COPD v. overdiagnosed COPD | Undiagnosed COPD v. correctly diagnosed COPD | |
| Age > 63 yr | 1.30 (0.67–2.54) | 1.00 (0.40–2.46) | 0.58 (0.24–1.41) |
| Female sex | 0.91 (0.48–1.74) | 1.64 (0.70–3.83) | 0.85 (0.36–1.99) |
| Current smoker | 1.12 (0.52–2.44) | 0.76 (0.30–1.91) | 0.44 (0.17–1.11) |
| No. of respiratory symptoms* | 1.05 (0.82–1.35) | 1.34 (1.01–1.79) | 0.62 (0.46–0.84) |
| No. of visits to primary care physician because of respiratory problem | 1.51 (0.93–2.45) | 1.25 (0.77–2.04) | 1.01 (0.97–1.06) |
Note: CI = confidence interval, COPD = chronic obstructive pulmonary disease, OR = odds ratio.
For age, ORs are for participants older than the median age of 63 years; for the number of respiratory symptoms, ORs are for each additional symptom; for the number of visits to a primary care physician because of respiratory problem, ORs are for each additional visit per patient-year.
Compared with patients whose COPD was undiagnosed (n = 49), those correctly found not to have COPD (n = 230) were similar in terms of age (p = 0.44), sex (p = 0.78), current smoking status (p = 0.77), number of respiratory symptoms (p = 0.70) and number of visits to a primary care physician because of a respiratory problem (p = 0.10). Compared with patients whose COPD was correctly diagnosed (n = 58), those in whom COPD was overdiagnosed (n = 45) described fewer respiratory symptoms (p = 0.045) but otherwise were similar in terms of age (p = 0.99), sex (p = 0.25), current smoking status (p = 0.56) and number of visits to a primary care physician because of a respiratory problem (p = 0.37). The same was true when we compared patients whose COPD was correctly diagnosed and those with undiagnosed COPD, with the latter describing fewer respiratory symptoms (p = 0.002) but otherwise being similar in terms of age (p = 0.23), sex (p = 0.70), current smoking status (p = 0.08) and number of visits because of a respiratory problem (p = 0.44).
Interpretation
We identified COPD in approximately one of every five adults who visited a primary care practitioner for any reason, were 40 years or older and had a smoking history of at least 20 pack-years. Although more than three-quarters of the patients with COPD reported at least one respiratory symptom, two-thirds were unaware of their diagnosis. These findings suggest that adults who attend a primary care practice with known risk factors for COPD are important targets for screening and early intervention.
The prevalence of COPD in our cohort was considerably higher than the 10.1% reported in the Burden of Obstructive Lung Disease (BOLD) study that recruited participants of similar age and used identical diagnostic criteria.3 However, unlike previous studies that used population-based sampling,3,18 we recruited only patients with a smoking history of at least 20 pack-years who had visited a primary care practitioner. In the BOLD study, the prevalence of COPD in the subgroup of heavy smokers was 15.5%–33.9% among men and 2.7%–29.7% among women across various countries.3 Our estimate of COPD prevalence in a sample of more than 1000 individuals is more precise (20.7%, 95% CI 18.3%–23.4%) and agrees with earlier reports of a high prevalence of COPD among people with known risk factors.4–6
Although underdiagnosis of COPD has been previously reported,20 the extent of it in our cohort is especially striking given that all of the patients had two important risk factors for COPD.3 Moreover, almost 50% of the patients with COPD were still smoking and therefore at increased risk for progression to more severe disease. Early diagnosis in this group is particularly important because spirometric evidence of airflow obstruction may help health care professionals to optimize smoking cessation.21,22
Differences in respiratory symptoms between the patients with correctly diagnosed COPD and those with overdiagnosed disease were modest and did not reflect differences in age, sex, smoking status or visits to a primary care physician. Nor did the clinical characteristics differ between the patients with undiagnosed COPD and those without the disease, although the former group reported fewer respiratory symptoms than those with correctly diagnosed COPD. The absence of distinguishing clinical characteristics within the population tested should encourage the use of spirometry in patients with known risk factors for COPD who visit a primary care practitioner for any reason.
Our selective recruitment of patients with known risk factors for COPD who had visited a primary care practitioner for any reason allowed us to find novel evidence that this population has a high prevalence of COPD. In addition, prior diagnosis of COPD was based on their family doctor’s diagnosis, validated by chart extraction, rather than based on self-report as in most studies.
Limitations
Our diagnostic criteria for COPD, based on the FEV1/FVC ratio and FEV1 percent predicted, can be questioned, particularly among elderly patients, in whom the FEV1/FVC ratio may be normally reduced as a function of aging.23–25 However, our classification, which represents those with stage 2 COPD or higher according to the GOLD classification,16 is consistent with the definitions of COPD provided by international guidelines10,13,26 and is the definition used in the BOLD study.3 This classification is supported by epidemiologic data27 and allows grouping of people according to the severity of disease, which is linked to outcomes such as death, hospital admission and comorbid conditions.28–30 By not including GOLD stage 1 COPD, or mild COPD according to Canadian guidelines,10 we minimized the likelihood of including people with asthma and provided a more conservative estimate than in some studies.18,31,32 Furthermore, by excluding people with GOLD stage 1 disease, we optimized the likelihood of identifying individuals with disease-specific impairment in health status.33
Although we selected rural, suburban and urban populations, our sample was almost exclusively white, which limits the generalizability of our findings to other ethnic groups. There was some disparity in prevalence estimates among the recruitment sites; however, we did not include a detailed assessment of variables that might account for these differences, such as literacy, socio-economic status and occupational exposures. The small proportion of participants from the urban site was most likely due to the very high proportion of international immigrants in this region and their reluctance to participate in a study regarding lung health.
Our rates of participation are concordant with those reported in previous studies.3,18 Participants and nonparticipants differed in some characteristics in a manner which, if nonresponse bias was introduced, might have led to under-estimation of COPD prevalence. Although the measurement properties of the questions about the presence of respiratory symptoms have not been investigated, they are basic questions commonly used in clinical practice to screen for cases of COPD.
Conclusions
Our findings show that early detection of COPD in high-risk patients in a primary care setting is feasible. For health policy-makers, this should provide a stimulus for the development of preventive care programs designed for COPD patients in family practice. Success of early intervention in this population could result in important reductions in mortality, morbidity and health care expenditures related to COPD, although this remains to be demonstrated in future evaluations. For practitioners, our results call for a higher index of suspicion for the presence of COPD in patients aged 40 years or older with a substantial smoking history, as well as a wider use of spirometry for diagnosis. Further research is indicated to define more clearly the reasons for underdiagnosis and overdiagnosis of COPD in primary care settings.
Supplementary Material
Acknowledgements
The authors thank the following people for their assistance with this project: Peggy Austin, Teresa To, Karen Jones, Christina Dolgowicz, Anjie Valgardson, Janice Belanger, Jennifer Zufelt, Josh McColman, Nancy Juby, Deana Juby and Nataliya Vasylevych.
Footnotes
Competing interests: Richard Hodder has attended advisory board meetings and provided continuing medical education for which he has received honoraria from various pharmaceutical companies, including AstraZeneca, Boehringer-Ingelheim, GlaxoSmithKline, Novartis, Nycomed, Merck Frosst and Pfizer. Matthew Stanbrook is a member of the executive of the Ontario Thoracic Society. No competing interests declared by Kylie Hill, Roger Goldstein, Gordon Guyatt, Maria Blouin, Wan Tan, Lori Davis, Diane Heels-Ansdell, Marko Erak, Pauline Bragaglia or Itamar Tamari.
Disclaimer: Matthew Stanbrook is a coauthor of this article. As a deputy editor of CMAJ, he was not involved in the selection of peer reviewers or the vetting of the manuscript before its acceptance.
Contributors: Kylie Hill contributed to the interpretation of the study results and drafted the article. Roger Goldstein, Gordon Guyatt and Wan Tan contributed to the study design and interpretation of the results. Maria Blouin, Lori Davis, Marko Erak, Pauline Bragaglia and Itamar Tamari contributed to the study design and acquisition of data. Diane Heels-Ansdell performed the data analyses and contributed to the interpretation of the results. Richard Hodder contributed to the study design. Matthew Stanbrook contributed to the study design and interpretation of the results. All of the authors revised the manuscript critically for important intellectual content and approved the final version submitted for publication.
Previously published at www.cmaj.ca
Funding: Funding for this study was provided by the Government of Ontario and the Ontario Lung Association.
This article has been peer reviewed.
REFERENCES
- 1.Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet. 1997;349:1498–503. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 2.Chapman KR, Mannino DM, Soriano JB, et al. Epidemiology and costs of chronic obstructive pulmonary disease. Eur Respir J. 2006;27:188–207. doi: 10.1183/09031936.06.00024505. [DOI] [PubMed] [Google Scholar]
- 3.Buist AS, McBurnie MA, Vollmer WM, et al. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet. 2007;370:741–50. doi: 10.1016/S0140-6736(07)61377-4. [DOI] [PubMed] [Google Scholar]
- 4.Zielinski J, Bednarek M. Early detection of COPD in a high-risk population using spirometric screening. Chest. 2001;119:731–6. doi: 10.1378/chest.119.3.731. [DOI] [PubMed] [Google Scholar]
- 5.Bednarek M, Gorecka D, Wielgomas J, et al. Smokers with airway obstruction are more likely to quit smoking. Thorax. 2006;61:869–73. doi: 10.1136/thx.2006.059071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maleki-Yazdi MR, Lewczuk CK, Haddon JM, et al. Early detection and impaired quality of life in COPD GOLD stage 0: a pilot study. COPD. 2007;4:313–20. doi: 10.1080/15412550701595740. [DOI] [PubMed] [Google Scholar]
- 7.Fletcher C, Peto R. The natural history of chronic airflow obstruction. BMJ. 1977;1:1645–8. doi: 10.1136/bmj.1.6077.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dales RE, Vandemheen KL, Clinch J, et al. Spirometry in the primary care setting: influence on clinical diagnosis and management of airflow obstruction. Chest. 2005;128:2443–7. doi: 10.1378/chest.128.4.2443. [DOI] [PubMed] [Google Scholar]
- 9.Walker PP, Mitchell P, Diamantea F, et al. Effect of primary-care spirometry on the diagnosis and management of COPD. Eur Respir J. 2006;28:945–52. doi: 10.1183/09031936.06.00019306. [DOI] [PubMed] [Google Scholar]
- 10.O’Donnell DE, Aaron S, Bourbeau J, et al. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease — 2007 update. Can Respir J. 2007;14 (Suppl B):5B–32B. doi: 10.1155/2007/830570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bourbeau J, van der Palen J. Promoting effective self-management programmes to improve COPD. Eur Respir J. 2009;33:461–3. doi: 10.1183/09031936.00001309. [DOI] [PubMed] [Google Scholar]
- 12.Bestall JC, Paul EA, Garrod R, et al. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54:581–6. doi: 10.1136/thx.54.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The COPD Guidelines Group of the Standards of Care Committee of the BTS. BTS guidelines for the management of chronic obstructive pulmonary disease. Thorax. 1995;(Suppl 5):S1–28. [PMC free article] [PubMed] [Google Scholar]
- 14.National Collaborating Centre for Chronic Conditions. Chronic obstructive pulmonary disease. National clinical guideline on management of chronic obstructive pulmonary disease in adults in primary and secondary care. Thorax. 2004;59(Suppl 1):1–232. [PMC free article] [PubMed] [Google Scholar]
- 15.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 16.Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–55. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 17.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general US population. Am J Respir Crit Care Med. 1999;159:179–87. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 18.Menezes AM, Perez-Padilla R, Jardim JR, et al. Chronic obstructive pulmonary disease in five Latin American cities (the PLATINO study): a prevalence study. Lancet. 2005;366:1875–81. doi: 10.1016/S0140-6736(05)67632-5. [DOI] [PubMed] [Google Scholar]
- 19.Perez-Padilla R, Vazquez-Garcia JC, Marquez MN, et al. Spirometry quality-control strategies in a multinational study of the prevalence of chronic obstructive pulmonary disease. Respir Care. 2008;53:1019–26. [PubMed] [Google Scholar]
- 20.Mannino DM, Gagnon RC, Petty TL, et al. Obstructive lung disease and low lung function in adults in the Unites States: data from the National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med. 2000;160:1683–9. doi: 10.1001/archinte.160.11.1683. [DOI] [PubMed] [Google Scholar]
- 21.Stratelis G, Molstad S, Jakobsson P, et al. The impact of repeated spirometry and smoking cessation advice on smokers with mild COPD. Scand J Prim Health Care. 2006;24:133–9. doi: 10.1080/02813430600819751. [DOI] [PubMed] [Google Scholar]
- 22.Parkes G, Greenhalgh T, Griffin M, et al. Effect on smoking quit rates of telling patients their lung age: the Step2quit randomised controlled trial. BMJ. 2008;336:598–600. doi: 10.1136/bmj.39503.582396.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberts SD, Farber MO, Knox KS, et al. FEV1/FVC ratio of 70% misclassifies patients with obstruction at the extremes of age. Chest. 2006;130:200–6. doi: 10.1378/chest.130.1.200. [DOI] [PubMed] [Google Scholar]
- 24.Schermer TR, Smeele IJ, Thoonen BP, et al. Current clinical guideline definitions of airflow obstructive and COPD overdiagnosis in primary care. Eur Respir J. 2008;32:945–52. doi: 10.1183/09031936.00170307. [DOI] [PubMed] [Google Scholar]
- 25.Swanney MP, Ruppel G, Enright PL, et al. Using the lower limit of normal for the FEV1/FVC ratio reduces misclassification of airway obstruction. Thorax. 2008;63:1046–51. doi: 10.1136/thx.2008.098483. [DOI] [PubMed] [Google Scholar]
- 26.McKenzie DK, Abramson M, Crockett AJ, et al. The COPD-X plan: Australia and New Zealand guidelines for the management of chronic obstructive pulmonary disease 2009. Bowen Hills (Australia): The Australian Lung Foundation; 2009. [(accessed June 2009)]. Available: www.copdx.org.au/guidelines/index.asp. [Google Scholar]
- 27.Johannessen A, Lehmann S, Omenass ER, et al. Post-bronchodilator spirometry reference values in adults and implications for disease management. Am J Respir Crit Care Med. 2006;173:1316–25. doi: 10.1164/rccm.200601-023OC. [DOI] [PubMed] [Google Scholar]
- 28.Mannino DM, Doherty DE, Buist AS. Global Initiative on Obstructive Lung Disease (GOLD) classification of lung disease and mortality: findings from the Atherosclerosis Risk in Communities (ARIC) study. Respir Med. 2006;100:115–22. doi: 10.1016/j.rmed.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 29.Mannino DM, Thorn D, Swensen A, et al. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur Respir J. 2008;32:962–9. doi: 10.1183/09031936.00012408. [DOI] [PubMed] [Google Scholar]
- 30.Mannino DM, Davis KJ, Kiri VA. Chronic obstructive pulmonary disease and hospitalizations for pneumonia in a US cohort. Respir Med. 2009;103:224–9. doi: 10.1016/j.rmed.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 31.Shahab L, Jarvis MJ, Britton J, et al. Prevalence, diagnosis and relation to tobacco dependence of chronic obstructive pulmonary disease in a nationally representative population sample. Thorax. 2006;61:1043–7. doi: 10.1136/thx.2006.064410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhong N, Wang C, Yao W, et al. Prevalence of chronic obstructive pulmonary disease in China: a large, population-based survey. Am J Respir Crit Care Med. 2007;176:753–60. doi: 10.1164/rccm.200612-1749OC. [DOI] [PubMed] [Google Scholar]
- 33.Antonelli-Incalzi R, Imperiale C, Bellia V, et al. Do GOLD stages of COPD severity really correspond to difference in health status? Eur Respir J. 2003;22:444–9. doi: 10.1183/09031936.03.00101203. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.