Summary
Regulatory CD4+ T cells (Tregs) are important modulators of the immune response. Different types of Tregs have been identified based on whether they are thymically derived (natural Tregs) or induced in the periphery (adaptive Tregs). We recently reported on an adaptive Treg phenotype that can be induced by the concomitant stimulation of human CD4+ T cells through CD3 and the membrane complement regulator CD46. These complement-induced Treg cells (cTreg) potently inhibit bystander T cell proliferation through high-level secretion of IL-10. In addition, cTreg express granzyme B and exhibit cytotoxic effects towards activated effector T cells. Here we analyzed the effect of cTreg on B cell functions in a co-culture system. We found that cTreg enhance B cell antibody production. This B cell support is dependent on cell/cell contact as well as cTreg-derived IL-10. In addition, we show that T cells from a CD46-deficient patient are not capable of promoting B cell responses, whereas CD46-deficient B cells have no intrinsic defect in Ig production. This finding may relate to a subset of CD46-deficient patients who present with common variable immunodeficiency (CVID). Thus, the lack of cTreg function in optimizing B cell responses could explain why some CD46-deficient patients develop CVID.
Keywords: CD46, complement, regulatory T cells, B cell response, CVID
Introduction
CD46, or membrane cofactor protein (MCP), is a cell surface receptor, which is expressed by all human cells except erythrocytes. CD46 is a cofactor for degradation of complement fragments C3b and C4b deposited on cells and thereby protects the cell upon which CD46 is expressed from complement attack [1]. In addition to this complement regulatory function, CD46 is a receptor for a number of pathogenic bacteria and viruses [2] and can modulate adaptive immune responses [3, 4]. In particular, CD46 functions as a co-stimulator during T cell stimulation: activation of human CD4+ T cells by simultaneous antibody crosslinking of the T cell receptor and CD46 in the presence of IL-2 induces T cell proliferation [5, 6] and a phenotype resembling that of adaptive regulatory T cells [7]. Characteristics of these regulatory T cells are their high-level synthesis of IL-10, granzyme B and perforin [7, 8]. These regulatory T cells, here termed complement-induced regulatory T cells (cTreg), suppress effector CD4+ T cell (Teff) responses through release of immunosuppressive IL-10, consumption of IL-2 as well as through a contact-dependent mechanism involving granzyme B-mediated cytotoxicity [3, 9].
The cytokine pattern secreted by cTreg, although suppressing T cell responses, does not impair the maturation and T cell-stimulating function of dendritic cells [10]. This is in contrast to other regulatory T cells, which mostly exert suppressive functions towards APCs [11, 12]. This is an important finding because it provides a means to suppress/modulate T cells without affecting antigen processing. The effects of cTreg on other immunocompetent cells such as B cells has not been studied. cTreg secrete factors [10] that are known to promote B cell activation and growth, such as IL-10 and soluble CD40L [13]. In addition, cTreg produce cytokines that support Ig isotype switching, such as IFN-γ and IL-10 [14, 15]. However, cTregs also express high amounts of granzyme B and can kill a range of activated target cells [8, 16, 17]. Also, suppression of B cell responses via a cytotoxic pathway has been demonstrated for natural Tregs [18]. Thus, we could envision two possible scenarios as to how cTregs could impact antibody production during an immune response: cTregs supporting B cell activation via their strong IL-10/CD40L production but also cTreg-mediated suppression of B cell responses through granzyme B-mediated killing.
We began studying the interplay between cTreg and B cells because of the recent finding that a subset of CD46-deficient individuals presents with common variable immunodeficiency (CVID) (ref. [19–21] and unpublished data, V. F.-B.). CVID is a genetically heterogenous syndrome characterized by low serum levels of immunoglobulins (Ig) and variable T cell defects [22]. Genetic causes of CVID include mutations in B cell related genes, such as those coding for CD19 and the BAFF receptors TACI and BAFF-R, as well as mutations in T cell costimulatory molecules, such as ICOS [22]. Although a role for CD46 in B cell responses has not yet been described, the CVID phenotype indicates a possible participation of CD46 in antibody production. We therefore analyzed the effect of cross-linking CD46 on B cells on cell proliferation and Ig production as well as on the effect of CD46-induced cTregs on B cell activation and responses.
In these studies, CD46 activation of B cells did not induce changes in B cell proliferation or Ab production. In contrast, CD3/CD46-activated T cells, cTregs, induced superior B cell activation and increased Ig production compared to effector T cells. B cell support by cTregs required cTreg-derived IL-10 as well as B cell/cTreg cell contact. Interestingly, and unexpectedly, cTreg/B cell contact did not lead to B cell killing. A supportive role for cTreg in B cell responses was further suggested by the finding that CD3/CD46-activated T cells isolated from a CD46-deficient patient are unable to provide B cell help.
Results
cTreg-derived cytokines do not induce changes in B cell proliferation or Ig production
The effect of cTregs on B cell function is unknown. Because cTregs display characteristics that could either promote (IL-10/sCD40L secretion) or suppress (granzyme B/perforin production) B cell function [7, 8, 10], we analyzed the impact of cTregs on B cell activation in two systems: we first assessed the effect of cTreg-derived soluble factors/cytokines on B cell proliferation and Ig production and then studied cTreg function towards B cells in a co-culture system.
To determine if cTreg-derived cytokines provide potentially sufficient (co)stimulatory signals to drive BCR-dependent Ig production, we activated freshly isolated human B cells with pansorbin which is a BCR crosslinking mitogen in the presence of supernatants derived from either CD3-, CD3/CD28- or CD3/CD46-activated T cells and measured immunoglobulin (Ig) production at days 5 and 10. Activated B cells cultured in fresh media served as control. None of these conditions led to B cell proliferation or Ig production (data not shown). We next repeated these experiments with the addition of immobilized mAbs to CD40 to provide an essential B cell costimulatory signal [23]. As expected, B cell activation under these conditions induced vigorous B cell proliferation and antibody production in control media (Supplementary Figure 1). However, neither cTreg (CD3/CD46-activated) supernatant, nor Teff (CD3/28-activated) supernatant, nor supernatant from CD3 (alone)-activated T cells led to changes in Ig production at the end of the incubation period (Supplementary Figure 1). These results indicate that the substantial amounts of IL-10 and sCD40L [10] secreted by cTregs do not provide sufficient additional signals to drive BCR-dependent B cell activation.
Thus, although we used a number of different activation conditions known to induce B cell responses, we have not been able to show an effect of cTreg-derived cytokines/soluble factors alone on BCR-induced Ab production in vitro.
cTregs do not impair B cell survival in a co-culture system
In previous studies, we have demonstrated that cTreg upregulate adhesion molecules and express high levels of activation markers and co-stimulatory molecules, which could possibly facilitate B cell activation (ref. [7, 10, 24] and our unpublished data). However, cTreg also express high amounts of intracellular granzyme B and perforin and display potent cytotoxicity towards activated effector T cells, dendritic cells and monocytes [8, 17], making it feasible that cTregs might also kill B cells upon cell contact.
To test for the effects of direct cell-cell contact between cTreg and B cells, we developed an in vitro co-culture system in which freshly isolated autologous B and CD4+ T cells were cultured under conditions that allowed concomitant activation and interaction of both cell types. Plates were coated with antibodies to CD3 and either CD28 or CD46 to induce T cell differentiation into Teff or cTreg, respectively [7]. Anti-IgM, -IgA and -IgG Abs were coated on the same plates to elicit polyclonal B cell activation by crosslinking the BCR [23]. Autologous CD4+ T cells and B cells were mixed at a ratio of 1 T cell: 2 B cells (conditions that have previously been shown to induce effective killing of other target cells by cTreg [8]) and cultured on the antibody-coated plates for up to 5 days. Cell proliferation and cytokine production were measured. Induction of cTregs was monitored via the increase in IL-10 and granzyme B expression by T cells in the cultures activated with anti-CD46 Abs. Increases in IL-10 and granzyme B were not observed in cultures activated with antibodies to CD3 (alone) or CD3/CD28 (Figure 1A). To monitor for the cellular source of the IL-10 observed in the culture supernatants, we also performed IL-10 Secretion Assays at days 2 and 4 post activation. At day 2, about 10% of CD3/CD46-activated T cells cultured alone produced IL-10, while only a negligible number of B cells activated and cultured alone produced IL-10. In co-culture, generally a quarter to a third of T cells secreted IL-10 at day 2 of activation while 5–7 % of B cells now stained positive for IL-10. At day 4, most cells ceased to secrete IL-10 (Supplementary Figure 2). Thus, although a number of B cells produce IL-10 upon interaction with T cells, the main source of this cytokine during co-culture appear to be indeed cTreg.
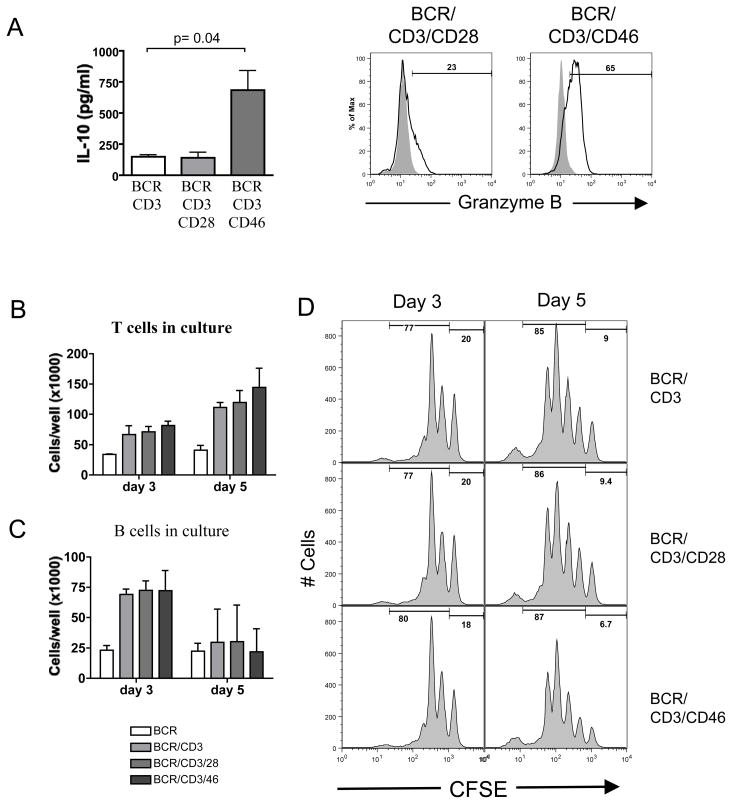
Figure 1. cTreg do not alter B cell viability or proliferation.
(A) CD46 activation induces cTreg in autologous B cell/T cell co-cultures. B cells were cultured with autologous CD4+ T cells in the presence of BCR-activating Abs and Abs to either CD3/CD28 or CD3/CD46. IL-10 content of cultures was measured at day 5 (left panel). Results shown are the combined data ± SD from three experiments. Right panel shows Granzyme B expression by T cells at day 3. Numbers above markers indicate % Granzyme B+ cells. Shown are representative data of three similar experiments. (B-D) cTreg do not affect viability of B cells. T and B cells were cultured (as under ‘A’) and numbers of viable CD4+ T cells (B) and viable (PI-negative) CD19+ B cells (C) were determined at days 3 and 5. (D) cTreg do not influence B cell proliferation. CFSE-labeled B cells were co-cultured with T cells as described under ‘A’ and analyzed for CFSE dilution at days 3 and 5 (histograms show CFSE profile of B cells, gated on live, CD4− cells). Results shown in ‘B’ and ‘C’ represent data ± SD from three independently performed experiments. ‘D’ depicts one representative result of three.
To assess for cTreg-mediated killing or suppression of B cell activation, we examined the absolute numbers of viable CD4+ T cell and B cells as well as absolute numbers of propidium iodide-positive (dead) cells at different days of culture (Figures 1B and C) and performed CFSE dilution assays to monitor B cell division (Figure 1D). As expected, T cell numbers in the co-cultures increased over time, with CD3/CD46-activated T cells proliferating slightly faster than CD3 or CD3/CD28-activated T cells (Figure 1B). This increased proliferative capacity of cTregs in the presence of IL-2 has previously been reported [5, 7, 25]. B cells underwent an initial proliferation burst peaking at day three of culture, then declined at day five with no difference in viable (Figure 1C) or dead (not shown) cell numbers in the culture conditions analyzed. Accordingly, the CFSE dilution profiles of B cells from all co-cultures with activated T cells were indistinguishable at the analyzed time points. Experiments performed with increased (equal ratios of B and T cells) numbers of Treg vs. Teff yielded similar results (data not shown).
These data suggest that cTregs do not affect the viability or proliferative behavior of activated B cells in an autologous in vitro co-culture system.
cTregs promote B cell antibody production
Having established that cTregs do not kill B cells in our co-culture system, we next analyzed if these cells impact B cell Ig production. We isolated naive CD27− B cells from PBMC and cultured them with autologous CD4+ T cells in antibody-coated plates as described above. Ig production was analyzed at day 10 of culture. For these extended culture periods, T cells were irradiated prior to co-culture with B cells to prevent an overgrowth of the faster proliferating T cells that would lead to a rapid nutrient depletion in the cultures. In addition, this method ensured equal T cell numbers throughout the culture period for each condition tested. We observed a wide range of Ig levels produced by B cells from different donors upon stimulation (Table 1). However, antibody levels were in almost all cases significantly higher in co-cultures that included CD46 stimulation, as compared to CD3 and CD3/CD28 stimulation (Figure 2A). CD46 co-stimulation induced about 9-fold higher production of IgM, 5-fold higher production of IgA and a 7-fold increase of IgG1 production in naïve B cell cultures when compared to CD3/CD28 activated cultures (Figure 2A and Table 1). Although we did not observe statistically significant differences in IgA or IgG1 production between CD3, CD3/CD28 and CD3/CD46-activated cultures in 11 experiments (Figure 2A), IgA and IgG1 levels were consistently highest in CD3/CD46-activated cultures (with the exception of one culture in which no IgG1 production was detected) (Figure 2A and Table 1). A similar trend was seen for IgM and IgA production in memory B cell cultures with autologous CD4+ T cells (not shown), although differences in Ig production were less pronounced, and the donor to donor variations considerably higher. Ig isotypes IgG2, IgG3, IgG4 and IgE were absent in most cultures tested, or produced only at low levels, displaying no significant differences between the different culture conditions (data not shown). As the high proliferative potential of cTreg represents most likely an important characteristic of their in vivo phenotype, we also performed the above-described experiments utilizing non-irradiated T cells with a number of different donors. The usage of proliferating T cells also induced strong B cell Ab production and were comparable to those obtained with irradiated T cells (data not shown).
Table 1. Ig levels are increased in B cell/T cell cultures activated in the presence of anti-CD46 antibody.
Co-cultures of naïve B cells and irradiated CD4+ T cells were tested for the presence of IgM (top), IgA (middle) and IgG1 (bottom) at day 10 of culture. Ig concentrations are given in ng/ml. Fold increases of Ig levels in BCR/CD3/CD46-activated co-cultures over that of BCR/CD3/CD28-activated co-cultures are displayed in the right column. For donor 56, the fold increase in IgM was measured as BCR/CD3/CD46 over BCR/CD3 because of the unusual low levels of Ig produced in the BCR/CD3/CD28 condition (marked by an asterix, *).
| IgM | |||||
|---|---|---|---|---|---|
| Donor | BCR/CD3 | BCR/CD3/CD28 | BCR/CD3/CD46 | Fold increase over BCR/CD3/CD28 | |
| 49 | 1563 | 1406 | 4603 | 3.3 | |
| 50 | 8309 | 7020 | 20845 | 3.0 | |
| 52 | 881 | 1274 | 3633 | 2.9 | |
| 53 | 1105 | 1758 | 6535 | 3.7 | |
| 55 | 926 | 1636 | 22144 | 13.5 | |
| 56 | 541 | 85 | 8826 | 16.3* | |
| 60 | 390 | 398 | 11570 | 29.1 | |
| 61 | 3185 | 12093 | 17888 | 1.5 | |
| 67 | 9304 | 7842 | 22584 | 2.9 | |
| 68 | 6099 | 4969 | 12678 | 2.6 | |
| 69 | 164 | 171 | 3546 | 20.7 | |
| Mean: | 9.0 | ||||
| IgA | |||||
| Donor | BCR/CD3 | BCR/CD3/CD28 | BCR/CD3/CD46 | Fold increase over BCR/CD3/CD28 | |
| 49 | 422 | 677 | 1411 | 2.1 | |
| 50 | 1588 | 1691 | 10747 | 6.4 | |
| 52 | 266 | 61 | 1130 | 11.8 | |
| 53 | 0 | 109 | 1103 | 10.1 | |
| 55 | 453 | 985 | 4774 | 4.9 | |
| 56 | 150 | 282 | 565 | 2.0 | |
| 60 | 28 | 1057 | 3028 | 2.9 | |
| 61 | 358 | 1228 | 1108 | 0.9 | |
| 67 | 3137 | 2284 | 14970 | 6.6 | |
| 68 | 6197 | 5063 | 4025 | 0.8 | |
| 69 | 0 | 0 | 0 | n.a. | |
| Mean: | 4.9 | ||||
| IgG1 | |||||
| Donor | BCR/CD3 | BCR/CD3/CD28 | BCR/CD3/CD46 | Fold increase over BCR/CD3/CD28 | |
| 49 | 129 | 87 | 437 | 5.0 | |
| 50 | 2866 | 2370 | 19675 | 8.3 | |
| 52 | 146 | 178 | 1342 | 7.5 | |
| 53 | 104 | 101 | 1074 | 10.7 | |
| 55 | 96 | 34 | 417 | 12.4 | |
| 56 | 17 | 72 | 81 | 1.1 | |
| 60 | 46 | 36 | 1017 | 28.2 | |
| 61 | 528 | 614 | 1806 | 2.9 | |
| 67 | 296 | 2225 | 3764 | 1.7 | |
| 68 | 371 | 353 | 439 | 1.2 | |
| 69 | 0 | 8 | 17 | 2.0 | |
| Mean: | 7.4 | ||||
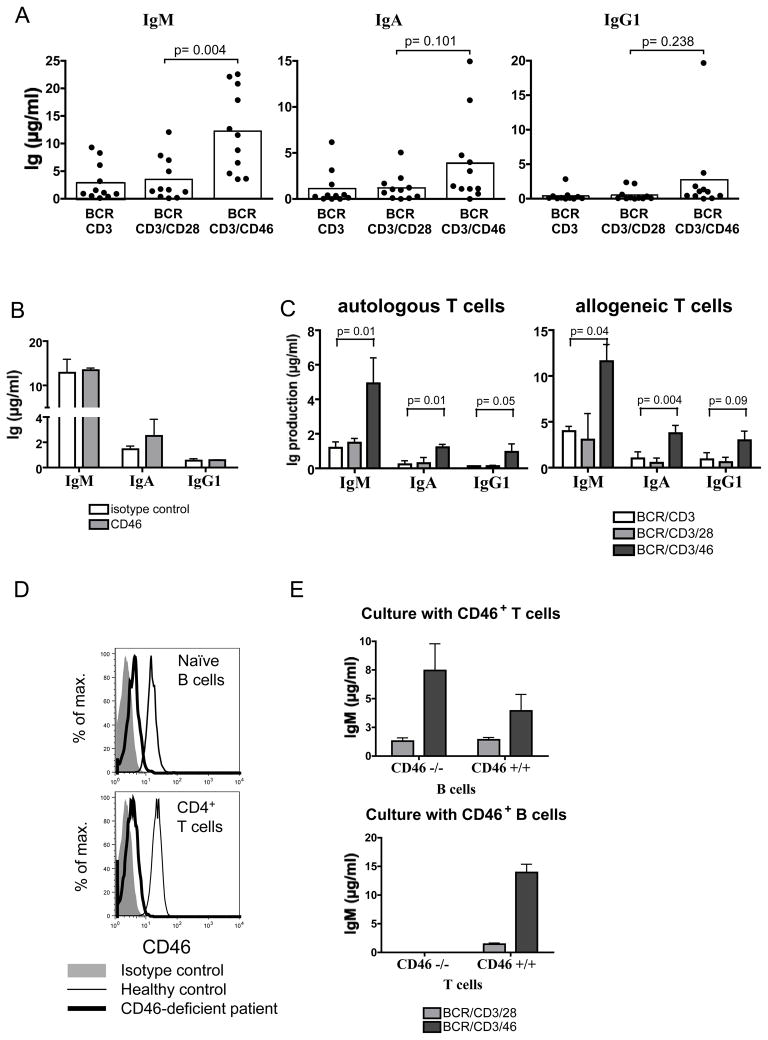
Figure 2. cTregs support B cell responses.
(A) cTregs enhance antibody production in B/T cell co-cultures. Naïve B cells were co-cultured with CD4+ T cells in the presence of BCR-stimulating Abs and Abs to CD3 alone, CD3/CD28, or CD3/CD46. IgM and IgA content of cultures were determined at day 10. Shown are data obtained from 11 donors. (B) Engagement of CD46 on B cells has no effect on Ig production. Purified B cells were activated with pansorbin and anti-CD40 in the presence of anti-CD46 mAb or an isotype-matched control antibody and Ig levels determined (day 10). Shown is one representative out of four similarly performed experiments. (C) Autologous and allogeneic B/T cell cultures induce comparable Ig production. B cells were co-cultured with irradiated autologous or allogeneic T cells activated as indicated and Ig amounts measured. Shown are combined results from three independently performed experiments, +/− SD. (D) CD46 expression on B and T cells from a CD46-deficient patient and a healthy donor. (E) T cells from a CD46-deficient patient fail to provide efficient B cell help. Naïve B cells from a healthy individual or from a CD46-deficient patient were co-cultured with irradiated CD46-sufficient T cells (upper graph) as described under ‘B’ and IgM production measured. Similarly, irradiated CD46+ or CD46-deficient T cells were co-cultured with CD46+ B cells and IgM production assessed (lower graph).
In conclusion, in contrast to their suppressive effects on effector T cell populations, cTreg did not inhibit B cell functions in our autologous T cell/B cell co-cultures. Furthermore, cTreg appeared more potent in enhancing Ig production than conventional effector T cells.
CD46 engagement on B cells does not enhance Ig production
The above results demonstrate that Ab-mediated activation of CD46 during B/T cell co-cultures increases Ig responses. However, since B cells also express CD46, CD46 is not only engaged on T cells, but also on B cells in our experimental setup. To discriminate whether the heightened B cell response is mediated via CD46-induced signals directly on the B cells or indirectly via CD46-activation of T cells, we cultured purified B cells (without T cells) in the presence of crosslinking Abs to the BCR, with or without simultaneous CD46 activation. No B cell proliferation or Ig production was observed in either condition, indicating that concurrent BCR and CD46 crosslinking is not sufficient to induce B cell blasting or Ig production (data not shown). We next modified this system and introduced additional stimulatory signals by activating the B cells via anti-CD40 antibody and pansorbin; conditions known to induce polyclonal B cell activation. Additional cross-linking of CD46 in these cultures did not alter IgM or IgG production, while IgA production was marginally (and non-significantly) increased (Figure 2B).
cTreg from a CD46-deficient patient do not provide efficient B cell help
The previous experiments suggest that direct crosslinking of CD46 on B cells is likely not responsible for the increased Ig production observed in our cultures but rather is due to signals provided by CD46-activated T cells. We tried to substantiate this notion by using CD46-deficient B cells in this assay. Although we have not yet succeeded in silencing CD46 expression in primary human B cells, we were able to obtain a blood sample from a CD46-deficient patient [20].
To date, there are eight known cases of homozygous CD46-deficiency [19–21, 26, 27]. These patients lack cell surface expression of CD46, which renders them more susceptible to complement attack under inflammatory conditions. All patients with complete CD46 deficiency develop hemolytic uremic syndrome (HUS), a triad of hemolytic anemia, thrombocytopenia and renal failure caused by thrombotic microangiopathy of the kidney [21]. Surprisingly, in three out of the eight known cases of complete CD46 deficiency, the patients also developed CVID ([20] and unpublished data, V. F.-B.). Although the five remaining patients do not meet all criteria for a CVID diagnosis, two of them present with subnormal IgG1 levels ([19, 20] and unpublished data, V. F.-B.). Based on our observation that cTreg provide B cell help in our co-culture system, we hypothesized that T cells from CD46-deficient individuals fail to differentiate into cTregs and that these patients are missing a potential B cell activation pathway.
We obtained cells from a CD46-deficient patient with HUS but without CVID [20] to assess the role of CD46 activation on B and/or T cells in Ig induction. This patient has a mutation in an invariant dinucleotide of the splice site (IVS2 +2T>G) between exon 2 and 3 resulting in the deletion of 144 bp and 48 amino acids in phase with the wild-type sequence. Expression of the mutated CD46 protein on granulocytes from this patient was less than 10% compared to healthy donors. The patient displayed normal CD4+/CD8+ T and B cell numbers, naïve vs. memory B cell populations as well as normal proportions of IgM+, IgA+ and IgG+ B cells in peripheral blood (data not shown). To co-culture CD46-deficient patient B/T cells with CD46-sufficient normal donor T/B cells, we first established that an allogeneic B/T cell co-culture system leads to similar results as those obtained with the autologous system. Figure 2C shows that in both autologous and allogeneic B/T cell co-cultures Ig levels are significantly increased in the presence of cTregs. Similar to our previous experiments, cTreg induction under allogeneic culture conditions was monitored by high levels of IL-10 and granzyme B production (data not shown). Figure 2D shows CD46 expression on B and T cells from a healthy donor and the minimal residual expression on those from the CD46-deficient patient.
CD46-deficient B cells produced similar amounts of IgM compared to CD46-sufficient B cells upon co-culture with CD46-sufficient T cells (Figure 2E, upper panel). This observation supports our initial observation that CD46 cross-linking directly on B cells does not enhance Ig production (Figure 2B) and substantiates our interpretation that the B cell support in our co-culture systems is independent of CD46 signaling on B cells but delivered via signals from the CD46-activated T cells. Accordingly, CD46-deficient T cells from this patient did not develop into IL-10-producing cTreg upon CD3/CD46 engagement (data not shown) and were impaired in their ability to provide help to normal CD46-sufficient B cells under the tested culture conditions (Figure 2E, lower panel). However, we noted that CD3/CD28-activated T cells from this patient also failed to provide B cell support (Figure 2E, lower panel). At present, it is unknown whether T cells from this CD46-deficient patient have other defects that contribute to the lack of B cell help from both cTreg and Teff.
cTreg-mediated B cell help requires IL-10 and cell-cell contact
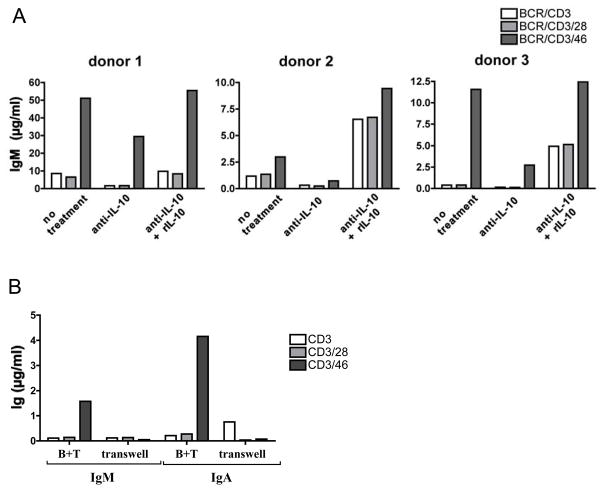
Our results indicate that cTreg promote antibody responses upon co-culture with naive B cells. cTreg B cell help could be mediated by either soluble factor(s) released by cTreg, by a cell-cell contact dependent mechanism, or by a combination of both. Because IL-10 is a major factor required for optimal Ig production [13], we assessed if cTreg-derived IL-10 mediates B cell activation in our co-culture system. B cells were cultured with cTregs or Teff with and without the addition of function-neutralizing Abs to IL-10 and reconstitution with rIL-10 (Figure 3A). IgM levels were measured at day 10 of culture. Neutralization of IL-10 decreased cTreg-induced IgM production from B cells on average by about 60% (range: 42–84%). Reconstitution of cultures with rIL-10 not only restored IgM responses in CD3/CD46-activation settings but also increased IgM production in cultures with CD3 or CD3/CD28-activated T cells in most of the experiments performed (Figure 3A). These results demonstrate the importance of cTreg-derived IL-10 in this system but also suggest that additional factors are involved because Ig production was never completely abolished by IL-10 neutralization.
Figure 3. B cell help by cTregs is dependent on IL-10 secretion and cell/cell contact.
(A) cTreg-derived IL-10 enhances B cell Ig production. Co-cultures of naïve B and T cells were prepared +/− function-blocking anti-IL-10 Ab or anti-IL-10 Ab in combination with excess amounts of rhuman IL-10. IgM content was measured from pooled triplicate activation conditions at day 10. Shown are results obtained from three independent experiments. An isotype control mAb showed no effect (not shown). (B) Usage of a transwell system abrogates B cell help by cTreg. T cells and naïve B cells were co-cultured, either by allowing direct cell-cell contact (B+T), or by separating B cells from T cells in a transwell system and activated via BCR-stimulating mAbs and mAbs to CD3, CD3/CD28 or CD3/CD46. Culture supernatants were analyzed for Ig production (day 10). Shown is one representative of three similarly performed experiments.
To address whether direct T cell/B cell contact or cross-talk is necessary for the cTreg-mediated B cell help, we set up co-cultures in a transwell system, which prevents direct cell contact but allows B/T cell cross-talk via soluble factors. Separation of B and T cells abolished the enhancing effects of cTreg on Ig production, indicating that cell-contact dependent mechanisms are required for optimal B cell activation (Figure 3B).
These experiments suggest that B cell activation supported by cTregs is dependent on direct cell-cell contact but is further enhanced through IL-10 secretion.
Expression of co-stimulatory molecules by CD3/CD46-stimulated T cells
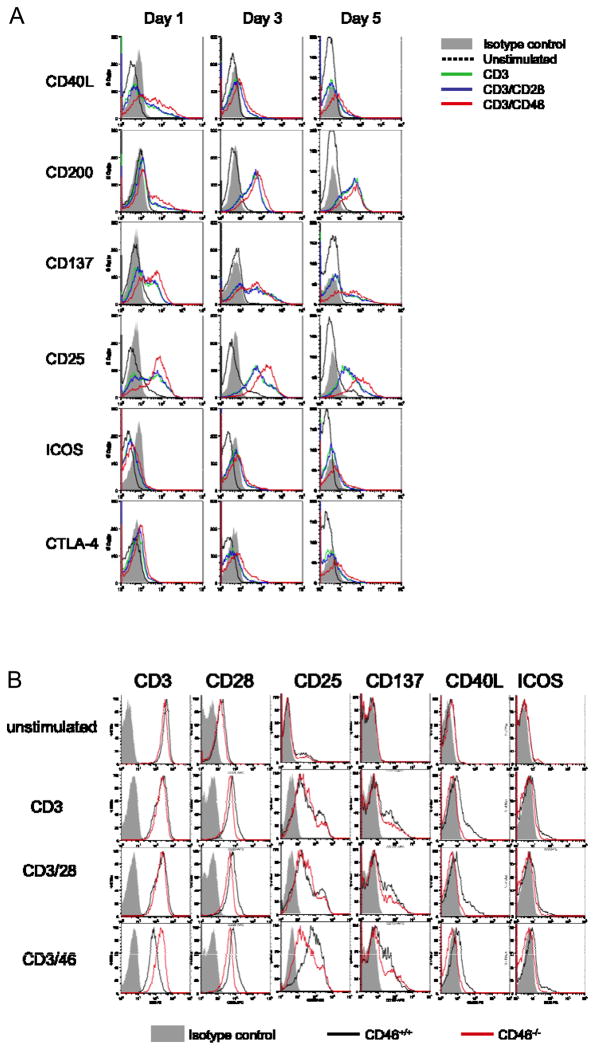
Potential cell-cell contact dependent mechanisms during T cell/B cell interaction include the engagement of costimulatory molecules on B and T cells. To determine if cTregs provide superior B cell help via the expression of a distinct co-stimulator profile, we compared the cell surface expression of a number of known T cell co-stimulation and activation markers on cTregs and Teff after one, three and five days of culture (Figure 4A). CD46 co-stimulation did not induce significantly different expression levels of the costimulatory and activation markers CD40L, CD28, CTLA-4, CD200, OX40, and ICOS [28, 29] (Figure 4A and not shown). We did, however, note a consistently higher and prolonged expression of CD137, a member of the tumor necrosis receptor family (TNFR) known to be involved in T cell activation/costimulation [30], and higher expression of CD25 on cTregs. We are currently investigating whether the changes in the expression of these two molecules account for the better B cell help provided by cTregs compared to Teff.
Figure 4. Expression pattern of co-stimulatory molecules on cTregs, Teff and activated CD46-deficient T cells.
(A) cTreg express higher levels of CD25 and CD137 as compared to Teff. CD4+ T cells were cultured on plates +/− anti-CD3, CD3/CD28 or CD3/CD46 mAbs, and analyzed for the expression of the depicted surface markers on days 1, 3 and 5. One representative experiment of three is shown. (B) Costimulatory markers expressed by activated CD46-deficient T cells. CD4+ T cells from the CD46-deficient patient or a healthy control were cultured on coated plates as under ‘A’. On day 3, cultures were analyzed for surface expression of depicted markers.
The analysis of the co-stimulatory surface marker expression pattern of the T cells isolated from the CD46-deficient patient showed the expected down-regulation of CD3, upregulation of CD28 and no significant differences in the up-regulation or expression of CTLA-4, CD200, OX40, LFA-1, ICOS or CD137 following stimulation with anti-CD3 antibody (Figure 4B and not shown). However, we observed two differences: T cells from this patient did not express CD40L on the surface, a major hallmark for T cell activation. And, as expected, CD3/CD46 activation did not induce the high levels of CD25 and CD137 observed on CD4+ T cells from healthy donors – although CD46-deficient T cells responded normally to CD3 and CD3/CD28 activation with intermediate levels of CD25 and CD137 expression (Figure 4B). The functional consequences of these observations are unclear and their further investigation is currently hampered by the limited access to blood samples from CD46-deficient patients.
Discussion
In this study we show that despite their suppressive effect on effector T cells activation and function, CD3/CD46-activated T cells supported B cell responses during in vitro B cell/T cell co-culture experiments. B cell help by cTreg required their IL-10 production but also cell/cell contact. T cells isolated from a CD46-deficient patient did not provide B cell help upon CD3/CD46 stimulation.
IL-10-secreting adaptive regulatory CD4+ T cells represent an anti-inflammatory T cell subpopulation with the capacity to down-modulate both Th1 and Th2 effector T cell responses [11, 31, 32]. Recent work suggests that they play a central role in the maintenance of tolerance at specific locations involving host/environment interfaces, such as the gut, skin and the lung [33]. Adaptive Tregs are thus of considerable interest because of their therapeutic potential for the treatment of immune-mediated pathologies [34].
In humans, IL-10-secreting regulatory T cells can be induced ex vivo via polyclonal stimuli or antigen presented by APC [11, 35], in the presence of the glucocorticoid, dexamethasone (Dex) and the active form of vitamin D, 1a 25-dihydroxyvitamin D3 (1a25VitD3) (Tr1 cells) [35, 36], through stimulation via tolerogenic APCs (Tr1 cells) [37] or through the concurrent activation of the TCR and the complement regulator CD46 in the presence of IL-2 (cTreg) [7]. Although we and others have demonstrated the induction of cTreg by natural and pathogen-derived ligands for CD46 [38–40], their in vivo existence and function has not yet been verified conclusively. This is mostly due to the lack of a suitable small animal model for the analysis of CD46 in T cell function. Rodents such as mice, rats and guinea pigs do not express CD46 on their somatic cells [4, 41], suggesting that a CD46-induced cTreg pathway does not exist in these species. CD4+ T cells from transgenic mice expressing human CD46 in a human-like isoform pattern do not demonstrate increased IL-10 or granzyme B production upon concurrent TCR and hCD46 stimulation [42] (and data not shown). We therefore are currently primarily expanding our understanding of CD46-induced Tregs using human in vitro culture systems. A recent observation, however, strengthens the case for an in vivo role of cTreg in immune regulation: A study by Astier et al. established a connection between defects in the CD46-mediated induction of IL-10 in CD4+ T cells with multiple sclerosis (MS) in a proportion of patients indicating that CD46 may indeed play a role in the prevention of autoimmunity in man [38, 40]. Excitingly, a connection between dysfunctional CD46-mediated IL-10 production in T cells has now also been linked with acute experimental autoimmune encephalomyelitis (EAE), a mimic model fur human MS, in cynomolgus monkeys (which express CD46 naturally) [43].
An in vivo involvement of CD46 also in B cell responses is suggested by the observation that one third of the patients with complete CD46 deficiency develop CVID ([20] and unpublished results, V. F.-B.). The phenotype of these patients raises an immediate question: Do these individuals show defects in Ig production because of the lack of CD46 signaling directly on B cells or because of ‘missing’ CD46-activated T cells? To answer this, we performed experiments using a variety of B cell stimulatory conditions. We were unable to show that signaling through B cell-expressed CD46 directly affected B cell Ab responses under the conditions tested (Figure 2B, E). Accordingly, B cells isolated from a CD46-deficient patient responded with normal Ig production upon proper T cell help in our co-culture system. We cannot exclude that CD46 crosslinking and signaling directly on B cells modulates Ab responses under alternative activation conditions or in vivo. However, the data here suggest that the increased Ig production observed in our system is due to B cell help provided by CD3/CD46-induced cTreg. This interpretation is supported by a recent study performed by Drouet and colleagues. In their study, Douet et al. show that a C3-deficient patient (C3b is a principal ligand for CD46) presents with severe defects in DC function, reduced numbers of switched memory B cells and a defect in cTreg induction. The authors suggest that the poor cTreg function might contribute to the impaired B cell responses seen in this patient [44]. The B lymphocyte sample from the CD46-deficient patient was helpful in determining that CD46 signaling within B cells is not vital to Ig production. The interpretation of the data from these assays regarding cTreg in vivo support of B cell responses are much less straight forward; this patient did not develop CVID despite that fact that CD28- and CD46-activated T cells failed to provide B cell help in our assays. The ability to mount quick and effective antibody responses upon infections is crucial. Thus, such important immunological functions are ‘covered’ by several redundant pathways (one of which possibly being CD46-dependent) and it seems that one or several of these alternative T cell help pathways (in addition to CD46) are affected in CD46-deficient patients with CVID. This however complicates a delineation of cTreg function using T cell samples from these patients.
Also, the experiments performed here utilized B cells isolated from freshly drawn blood samples as this is so far the most reliable and accessible source for human naïve B cells. However, the phenotype of these B cells will likely differ from lymphoid tissue-resident B cells, representing the B cell population directly interacting with activated T cells. Although we observed in experiments using B cells isolated from tonsils (which are part of the mucosal-associated lymphoid tissue, MALT), that blood-derived cTreg also support activation and Ab production by tonsillar naïve B cells in a comparable fashion (data not shown), cTreg may have distinct in vivo effects on other lymphoid B cells or on B cells that are activated in locations with a specialized environmental milieu.
We have initiated a screen of cTregs for specific activation markers or co-stimulatory molecules that could explain the increased B cell stimulatory properties of these cells in comparison to Teff (Figure 4A). One difference we noted between cTreg and Teff was a slightly higher and more prolonged expression of CD25 and CD137 upon CD46-stimulation. However, whether these molecules (in combination with the high IL-10 production by cTregs) are involved in the increased Ig production upon cTreg/B cell contact needs to be addressed in future experiments.
Similar to other adaptive Tregs, CD46-induced Tregs suppress Teff via high IL-10 production [7]. However, cTregs display features that set them apart from the ‘classic’ adaptive Tregs [3]. CD3/CD46-activation induces strong granzyme B and perforin expression in CD4+ T cells [8]. Granzyme A expression has been observed for natural CD25/Foxp3-positive Tregs but not for adaptive Tregs [8]. Although granzyme B expressed by natural Tregs is important in tolerance induction in transplantation [45], the functional role of CD46-mediated granzyme B expression is not clear. In addition, in contrast to other adaptive Tregs, cTregs support DC responses [10] and, as shown in this study, also B cell responses. Tregs generally inhibit DC maturation [11] and, although the current knowledge of the effect of Tregs on B cells is sparse, the few existing studies indicate a suppressive effect of Tregs towards B lymphocytes [18, 46–48]. Thus, one could argue that cTregs are distinct from other adaptive Tregs in such that they specifically down-modulate Teff responses but support the other arms of the immune response.
The complement system is generally seen as a first line defense against invading pathogens. Its major function is to sense danger, destroy pathogens directly and immediately induce protective responses involving innate and adaptive immunity [49]. Intuitively, complement proteins such as CD46 should transmit signals that support APC function, Ig production and effector T cell responses. Thus, the observation that cTregs not only support but also amplify APC and B cell functions, which are essential in pathogen clearance, is therefore indeed in line with the idea that complement activation is a vital part in the initiation of an immune response. However, it is now clear that the proper and timely resolution (or suppression) of an immune response is also vital in the prevention of inflammation-mediated tissue damage and autoimmunity and cTregs may participate in this equally important down-modulatory phase in immunity/immune homeostasis. Thus, the interesting characteristics of CD46-induced cTregs may be an intriguing example as to how the complement system evolved to direct the initiation, effector and resolution phase of immune responses.
Material and methods
Patient and donor samples
Patients and healthy donors participating in this study provided written consent in accordance with the Declaration of Helsinki. Blood was collected and processed with the approval and in accordance with the Washington University Medical Center Human Studies Committee guidelines and the Hôpital Européen Georges Pompidou Hospital Paris Review Board. The CD46-deficient patient has been described in a previous study [20].
Cell lines and antibodies
T cells and B cells were maintained in RPMI 1640 medium (Invitrogen, Carlsbad, CA) with 10% FCS (Hyclone, Logan, UT) and 200 mM L-glutamine in the presence of 25 U/ml recombinant human IL-2 (BioSource International, Camarilla, CA) or additional components as indicated. CD40L-transfected J558L cells were a kind gift from Marco Colonna (Washington University). The anti-CD46 mAb TRA-2-10 [50] was labeled with FITC (Sigma Aldrich, St. Louis, MO) using standard protocols. Unconjugated antibodies to CD3 and CD28, and conjugated CD4, CD19, CD27, CD154, CD25, CD152, granzyme B, isotype control Abs and function-neutralizing mAb to human IL-10 were bought from BD Biosciences (San Diego, CA). ICOS, CD137, CD134 and anti-CD40 clone 5C3 were obtained from eBioscience (San Diego, CA). CD200 was from Coulter Immunotech and F(ab′)2 anti-human IgM/G/A from Jackson ImmunoResearch (West Grove, PA). Recombinant human IL-10 was obtained from BD Biosciences.
Isolation of human T cells and B cells
CD4+ T cells and B cells were isolated from PBMC using CD4 and CD19 MicroBeads (Miltenyi Biotec, Auburn, CA), respectively, according to the manufacturer’s instructions. Where indicated, naïve B cells were isolated using the Naïve B cell Isolation Kit (Miltenyi Biotec). Purity of isolated lymphocyte fractions were typically >95%.
T cell activation
In vitro stimulation of isolated CD4+ T-cells was performed in 96-well culture plates coated with mAbs to CD3, CD28 and/or CD46, each at 2.5 μg/ml. Purified CD4+ lymphocytes (1.5 – 2.0 × 105 cells/well) were added in 100 μl culture medium supplemented with 25 U/ml recombinant human IL-2. The plates were centrifuged at 50 x g for 2 min, cultured and cell supernatants harvested at day 3 for cytokine analysis and for use in B cell cultures.
B cell activation
To assess B cell activation in the presence of T cell supernatant, B cells were plated at 1 × 105 cells/well in culture medium containing 50 ng/ml rIL-10, 50 U/ml IL-2, 0.5 μg/ml anti-CD40 and pansorbin (heat-inactivated Staphylococcus aureus, used 1:2500, EMD Chemicals, Gibbstown, NJ) in 50 μl volume. T cell supernatant (100 μl) was then added and cell mixtures cultured at 37 °C. Culture supernatants were collected at day 5 (wells then replenished with fresh media) and day 10 of cultures to measure Ig production. The effects of direct CD46 engagement on B cells were assessed by culturing B cells as described above but with or without the addition of pansorbin (Sigma Aldrich) and in 96-well plates that had been coated overnight with 5 μg/ml anti-CD46 antibody.
T cell/B cell co-cultures
B cells (50,000) were cultured with 100,000 irradiated (2000 rad) CD4+ T cells on 96-well plates coated with 5 μg/ml F(ab′)2 anti-human IgM/G/A and combinations of anti-CD3, CD3/CD28 or CD3/CD46. Culture media (containing 50 U/ml IL-2) was replaced on day 5 of co-culture and supernatants were analyzed on day 5 for cytokine content and on day 10 for Ig content. For neutralization of IL-10, 2.5 μg/ml soluble anti-IL-10 antibody was added at the start of the culture. Specificity of IL-10 neutralization was monitored via simultaneous treatment with anti-IL-10 antibody and excess amounts of rIL-10 (125 ng/ml). For assessing B and T cell proliferation and B cell survival, B cells were labeled with CFSE (Sigma Aldrich). Labeled B cells (100,000) were cultured with 50,000 autologous CD4+ T cells. CFSE dilution and numbers of live and dead B and T cells were assessed by FACS using anti-CD4 and PI staining.
Cytokine and Ig measurements
IL-10 production by T or B cells was measured using either the Th1/Th2 Cytometric Bead Array (BD Biosciences) or the Human IL-10 Secretion Assay Kit (Miltenyi Biotec) according to the manufacturer’s suggestions. IgM, IgA, IgG2, IgG3 and IgG4 were measured using the Human Ig FlexSet System (BD Biosciences). Samples were analyzed on a FACS Calibur and cytokine/Ig concentrations determined using the CBA software and the FCAP software (all BD Biosciences), respectively. IgG1, and in some experiments, also IgA and IgM, were analysed by ELISA using antibody pairs from BD Biosciences and IgE levels were measured by ELISA (Bethyl Laboratories, Montgomery, TX).
Statistical analysis
Statistical analyses were performed using the Student’s two-tailed t-test (Excel software (Microsoft, Redmond, WA).
Supplementary Material
Acknowledgments
This work was supported in part by grants from the Sandler Program for Asthma Research (SPAR) and the NIH grants U19 AI070489 and RO1 AI037618.
The authors would like to thank Prof. Jean-Pierre Grunfeld (Hopital Necker, Paris) and the CD46-deficient patient for generously providing us with a blood sample for our studies. We thank Alex Braun and Chris Evagora (Queen Mary University of London, ICMS Core Pathology), Graham Lord, Nicholas Powell and James Canavan (Biomedical Research Centre, King’s College London) for expert help in immunohistology, and Daniel Hoft and Steven Truscott (Saint Louis University in St. Louis, MO), and Tim Wilson (Washington University in St. Louis) for helpful discussion of the results and critical reading of the manuscript.
This study was funded by the American Asthma Foundation (formerly SPAR) (A.F., J.P.A. and C.K.), the National Institute of Health grants 5 RO1 AI037618 (J.P.A.) and U19 AI070489 (J.P.A. and C.K.), the Kidney Patient Association UK (C.K.), and the Assistance Publique-Hopitaux de Paris - Programme Hôspitalier de Recherche Clinique [AOM 08198] 2008 (V. F.-B.).
Abbreviations
- cTreg
complement(CD46)-induced regulatory T cell
- CVID
common variable immunodeficiency
- Teff
effector T cells
Footnotes
Conflict of interest
The authors declare that they have no competing financial interest.
References
- 1.Seya T, Turner JR, Atkinson JP. Purification and characterization of a membrane protein (gp45-70) that is a cofactor for cleavage of C3b and C4b. J Exp Med. 1986;163:837–855. doi: 10.1084/jem.163.4.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cattaneo R. Four viruses, two bacteria, and one receptor: membrane cofactor protein (CD46) as pathogens’ magnet. J Virol. 2004;78:4385–4388. doi: 10.1128/JVI.78.9.4385-4388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kemper C, Atkinson JP. T-cell regulation: with complements from innate immunity. Nat Rev Immunol. 2007;7:9–18. doi: 10.1038/nri1994. [DOI] [PubMed] [Google Scholar]
- 4.Riley-Vargas RC, Gill DB, Kemper C, Liszewski MK, Atkinson JP. CD46: expanding beyond complement regulation. Trends Immunol. 2004;25:496–503. doi: 10.1016/j.it.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Astier A, Trescol-Biemont MC, Azocar O, Lamouille B, Rabourdin-Combe C. Cutting edge: CD46, a new costimulatory molecule for T cells, that induces p120CBL and LAT phosphorylation. J Immunol. 2000;164:6091–6095. doi: 10.4049/jimmunol.164.12.6091. [DOI] [PubMed] [Google Scholar]
- 6.Sanchez A, Feito MJ, Rojo JM. CD46-mediated costimulation induces a Th1-biased response and enhances early TCR/CD3 signaling in human CD4+ T lymphocytes. Eur J Immunol. 2004;34:2439–2448. doi: 10.1002/eji.200324259. [DOI] [PubMed] [Google Scholar]
- 7.Kemper C, Chan AC, Green JM, Brett KA, Murphy KM, Atkinson JP. Activation of human CD4+ cells with CD3 and CD46 induces a T-regulatory cell 1 phenotype. Nature. 2003;421:388–392. doi: 10.1038/nature01315. [DOI] [PubMed] [Google Scholar]
- 8.Grossman WJ, Verbsky JW, Barchet W, Colonna M, Atkinson JP, Ley TJ. Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity. 2004;21:589–601. doi: 10.1016/j.immuni.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Kemper C, Verbsky JW, Price JD, Atkinson JP. T-cell stimulation and regulation: with complements from CD46. Immunol Res. 2005;32:31–43. doi: 10.1385/IR:32:1-3:031. [DOI] [PubMed] [Google Scholar]
- 10.Barchet W, Price JD, Cella M, Colonna M, MacMillan SK, Cobb JP, Thompson PA, Murphy KM, Atkinson JP, Kemper C. Complement-induced regulatory T cells suppress T-cell responses but allow for dendritic-cell maturation. Blood. 2006;107:1497–1504. doi: 10.1182/blood-2005-07-2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3:253–257. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 12.Waldmann H, Cobbold S. How do monoclonal antibodies induce tolerance? A role for infectious tolerance? Annu Rev Immunol. 1998;16:619–644. doi: 10.1146/annurev.immunol.16.1.619. [DOI] [PubMed] [Google Scholar]
- 13.Liu YJ, Banchereau J. Regulation of B-cell commitment to plasma cells or to memory B cells. Semin Immunol. 1997;9:235–240. doi: 10.1006/smim.1997.0080. [DOI] [PubMed] [Google Scholar]
- 14.Malisan F, Briere F, Bridon JM, Harindranath N, Mills FC, Max EE, Banchereau J, Martinez-Valdez H. Interleukin-10 induces immunoglobulin G isotype switch recombination in human CD40-activated naive B lymphocytes. J Exp Med. 1996;183:937–947. doi: 10.1084/jem.183.3.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Snapper CM, Paul WE. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987;236:944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- 16.Gondek DC, Lu LF, Quezada SA, Sakaguchi S, Noelle RJ. Cutting edge: contact-mediated suppression by CD4+CD25+ regulatory cells involves a granzyme B-dependent, perforin-independent mechanism. J Immunol. 2005;174:1783–1786. doi: 10.4049/jimmunol.174.4.1783. [DOI] [PubMed] [Google Scholar]
- 17.Grossman WJ, Verbsky JW, Tollefsen BL, Kemper C, Atkinson JP, Ley TJ. Differential expression of granzymes A and B in human cytotoxic lymphocyte subsets and T regulatory cells. Blood. 2004;104:2840–2848. doi: 10.1182/blood-2004-03-0859. [DOI] [PubMed] [Google Scholar]
- 18.Zhao DM, Thornton AM, DiPaolo RJ, Shevach EM. Activated CD4+CD25+ T cells selectively kill B lymphocytes. Blood. 2006;107:3925–3932. doi: 10.1182/blood-2005-11-4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caprioli J, Noris M, Brioschi S, Pianetti G, Castelletti F, Bettinaglio P, Mele C, Bresin E, Cassis L, Gamba S, Porrati F, Bucchioni S, Monteferrante G, Fang CJ, Liszewski MK, Kavanagh D, Atkinson JP, Remuzzi G. Genetics of HUS: the impact of MCP, CFH, and IF mutations on clinical presentation, response to treatment, and outcome. Blood. 2006;108:1267–1279. doi: 10.1182/blood-2005-10-007252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fremeaux-Bacchi V, Moulton EA, Kavanagh D, Dragon-Durey MA, Blouin J, Caudy A, Arzouk N, Cleper R, Francois M, Guest G, Pourrat J, Seligman R, Fridman WH, Loirat C, Atkinson JP. Genetic and functional analyses of membrane cofactor protein (CD46) mutations in atypical hemolytic uremic syndrome. J Am Soc Nephrol. 2006;17:2017–2025. doi: 10.1681/ASN.2005101051. [DOI] [PubMed] [Google Scholar]
- 21.Kavanagh D, Richards A, Atkinson J. Complement regulatory genes and hemolytic uremic syndromes. Annu Rev Med. 2008;59:293–309. doi: 10.1146/annurev.med.59.060106.185110. [DOI] [PubMed] [Google Scholar]
- 22.Park MA, Li JT, Hagan JB, Maddox DE, Abraham RS. Common variable immunodeficiency: a new look at an old disease. Lancet. 2008;372:489–502. doi: 10.1016/S0140-6736(08)61199-X. [DOI] [PubMed] [Google Scholar]
- 23.Galibert L, Burdin N, de Saint-Vis B, Garrone P, Van Kooten C, Banchereau J, Rousset F. CD40 and B cell antigen receptor dual triggering of resting B lymphocytes turns on a partial germinal center phenotype. J Exp Med. 1996;183:77–85. doi: 10.1084/jem.183.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alford SK, Longmore GD, Stenson WF, Kemper C. CD46-induced immunomodulatory CD4+ T cells express the adhesion molecule and chemokine receptor pattern of intestinal T cells. J Immunol. 2008;181:2544–2555. doi: 10.4049/jimmunol.181.4.2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zaffran Y, Destaing O, Roux A, Ory S, Nheu T, Jurdic P, Rabourdin-Combe C, Astier AL. CD46/CD3 costimulation induces morphological changes of human T cells and activation of Vav, Rac, and extracellular signal-regulated kinase mitogen-activated protein kinase. J Immunol. 2001;167:6780–6785. doi: 10.4049/jimmunol.167.12.6780. [DOI] [PubMed] [Google Scholar]
- 26.Couzi L, Contin-Bordes C, Marliot F, Sarrat A, Grimal P, Moreau JF, Merville P, Fremeaux-Bacchi V. Inherited deficiency of membrane cofactor protein expression and varying manifestations of recurrent atypical hemolytic uremic syndrome in a sibling pair. Am J Kidney Dis. 2008;52:e5–9. doi: 10.1053/j.ajkd.2008.02.359. [DOI] [PubMed] [Google Scholar]
- 27.Sellier-Leclerc AL, Fremeaux-Bacchi V, Dragon-Durey MA, Macher MA, Niaudet P, Guest G, Boudailliez B, Bouissou F, Deschenes G, Gie S, Tsimaratos M, Fischbach M, Morin D, Nivet H, Alberti C, Loirat C. Differential impact of complement mutations on clinical characteristics in atypical hemolytic uremic syndrome. J Am Soc Nephrol. 2007;18:2392–2400. doi: 10.1681/ASN.2006080811. [DOI] [PubMed] [Google Scholar]
- 28.Barclay AN, Wright GJ, Brooke G, Brown MH. CD200 and membrane protein interactions in the control of myeloid cells. Trends Immunol. 2002;23:285–290. doi: 10.1016/s1471-4906(02)02223-8. [DOI] [PubMed] [Google Scholar]
- 29.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 30.Croft M. Costimulation of T cells by OX40, 4-1BB, and CD27. Cytokine Growth Factor Rev. 2003;14:265–273. doi: 10.1016/s1359-6101(03)00025-x. [DOI] [PubMed] [Google Scholar]
- 31.Fehervari Z, Sakaguchi S. CD4+ Tregs and immune control. J Clin Invest. 2004;114:1209–1217. doi: 10.1172/JCI23395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Garra A, Vieira PL, Vieira P, Goldfeld AE. IL-10-producing and naturally occurring CD4+ Tregs: limiting collateral damage. J Clin Invest. 2004;114:1372–1378. doi: 10.1172/JCI23215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, Treuting P, Siewe L, Roers A, Henderson WR, Jr, Muller W, Rudensky AY. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 34.Walsh PT, Taylor DK, Turka LA. Tregs and transplantation tolerance. J Clin Invest. 2004;114:1398–1403. doi: 10.1172/JCI23238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Groux H, O’Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 36.Barrat FJ, Cua DJ, Boonstra A, Richards DF, Crain C, Savelkoul HF, de Waal-Malefyt R, Coffman RL, Hawrylowicz CM, O’Garra A. In vitro generation of interleukin 10-producing regulatory CD4(+) T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)-and Th2-inducing cytokines. J Exp Med. 2002;195:603–616. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levings MK, Gregori S, Tresoldi E, Cazzaniga S, Bonini C, Roncarolo MG. Differentiation of Tr1 cells by immature dendritic cells requires IL-10 but not CD25+CD4+ Tr cells. Blood. 2005;105:1162–1169. doi: 10.1182/blood-2004-03-1211. [DOI] [PubMed] [Google Scholar]
- 38.Martinez-Forero I, Garcia-Munoz R, Martinez-Pasamar S, Inoges S, Lopez-Diaz de Cerio A, Palacios R, Sepulcre J, Moreno B, Gonzalez Z, Fernandez-Diez B, Melero I, Bendandi M, Villoslada P. IL-10 suppressor activity and ex vivo Tr1 cell function are impaired in multiple sclerosis. Eur J Immunol. 2008;38:576–586. doi: 10.1002/eji.200737271. [DOI] [PubMed] [Google Scholar]
- 39.Price JD, Schaumburg J, Sandin C, Atkinson JP, Lindahl G, Kemper C. Induction of a regulatory phenotype in human CD4+ T cells by streptococcal M protein. J Immunol. 2005;175:677–684. doi: 10.4049/jimmunol.175.2.677. [DOI] [PubMed] [Google Scholar]
- 40.Astier AL, Meiffren G, Freeman S, Hafler DA. Alterations in CD46-mediated Tr1 regulatory T cells in patients with multiple sclerosis. J Clin Invest. 2006;116:3252–3257. doi: 10.1172/JCI29251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsujimura A, Shida K, Kitamura M, Nomura M, Takeda J, Tanaka H, Matsumoto M, Matsumiya K, Okuyama A, Nishimune Y, Okabe M, Seya T. Molecular cloning of a murine homologue of membrane cofactor protein (CD46): preferential expression in testicular germ cells. Biochem J. 1998;330(Pt 1):163–168. doi: 10.1042/bj3300163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marie JC, Astier AL, Rivailler P, Rabourdin-Combe C, Wild TF, Horvat B. Linking innate and acquired immunity: divergent role of CD46 cytoplasmic domains in T cell induced inflammation. Nat Immunol. 2002;3:659–666. doi: 10.1038/ni810. [DOI] [PubMed] [Google Scholar]
- 43.Ma A, Xiong Z, Hu Y, Qi S, Song L, Dun H, Zhang L, Lou D, Yang P, Zhao Z, Wang X, Zhang D, Daloze P, Chen H. Dysfunction of IL-10-producing type 1 regulatory T cells and CD4(+)CD25(+) regulatory T cells in a mimic model of human multiple sclerosis in Cynomolgus monkeys. Int Immunopharmacol. 2009;9:599–608. doi: 10.1016/j.intimp.2009.01.034. [DOI] [PubMed] [Google Scholar]
- 44.Ghannam A, Pernollet M, Fauquert JL, Monnier N, Ponard D, Villiers MB, Peguet-Navarro J, Tridon A, Lunardi J, Gerlier D, Drouet C. Human C3 deficiency associated with impairments in dendritic cell differentiation, memory B cells, and regulatory T cells. J Immunol. 2008;181:5158–5166. doi: 10.4049/jimmunol.181.7.5158. [DOI] [PubMed] [Google Scholar]
- 45.Gondek DC, Devries V, Nowak EC, Lu LF, Bennett KA, Scott ZA, Noelle RJ. Transplantation survival is maintained by granzyme B+ regulatory cells and adaptive regulatory T cells. J Immunol. 2008;181:4752–4760. doi: 10.4049/jimmunol.181.7.4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Janssens W, Carlier V, Wu B, VanderElst L, Jacquemin MG, Saint-Remy JM. CD4+CD25+ T cells lyse antigen-presenting B cells by Fas-Fas ligand interaction in an epitope-specific manner. J Immunol. 2003;171:4604–4612. doi: 10.4049/jimmunol.171.9.4604. [DOI] [PubMed] [Google Scholar]
- 47.Lim HW, Hillsamer P, Banham AH, Kim CH. Cutting edge: direct suppression of B cells by CD4+ CD25+ regulatory T cells. J Immunol. 2005;175:4180–4183. doi: 10.4049/jimmunol.175.7.4180. [DOI] [PubMed] [Google Scholar]
- 48.Ludwig-Portugall I, Hamilton-Williams EE, Gottschalk C, Kurts C. Cutting edge: CD25+ regulatory T cells prevent expansion and induce apoptosis of B cells specific for tissue autoantigens. J Immunol. 2008;181:4447–4451. doi: 10.4049/jimmunol.181.7.4447. [DOI] [PubMed] [Google Scholar]
- 49.Volanakis JE, Frank MM. The Human Complement System in Health and Disease. Marcel Dekker, Inc; New York: 1998. [Google Scholar]
- 50.Liszewski MK, Leung M, Cui W, Subramanian VB, Parkinson J, Barlow PN, Manchester M, Atkinson JP. Dissecting sites important for complement regulatory activity in membrane cofactor protein (MCP; CD46) J Biol Chem. 2000;275:37692–37701. doi: 10.1074/jbc.M004650200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.