Abstract
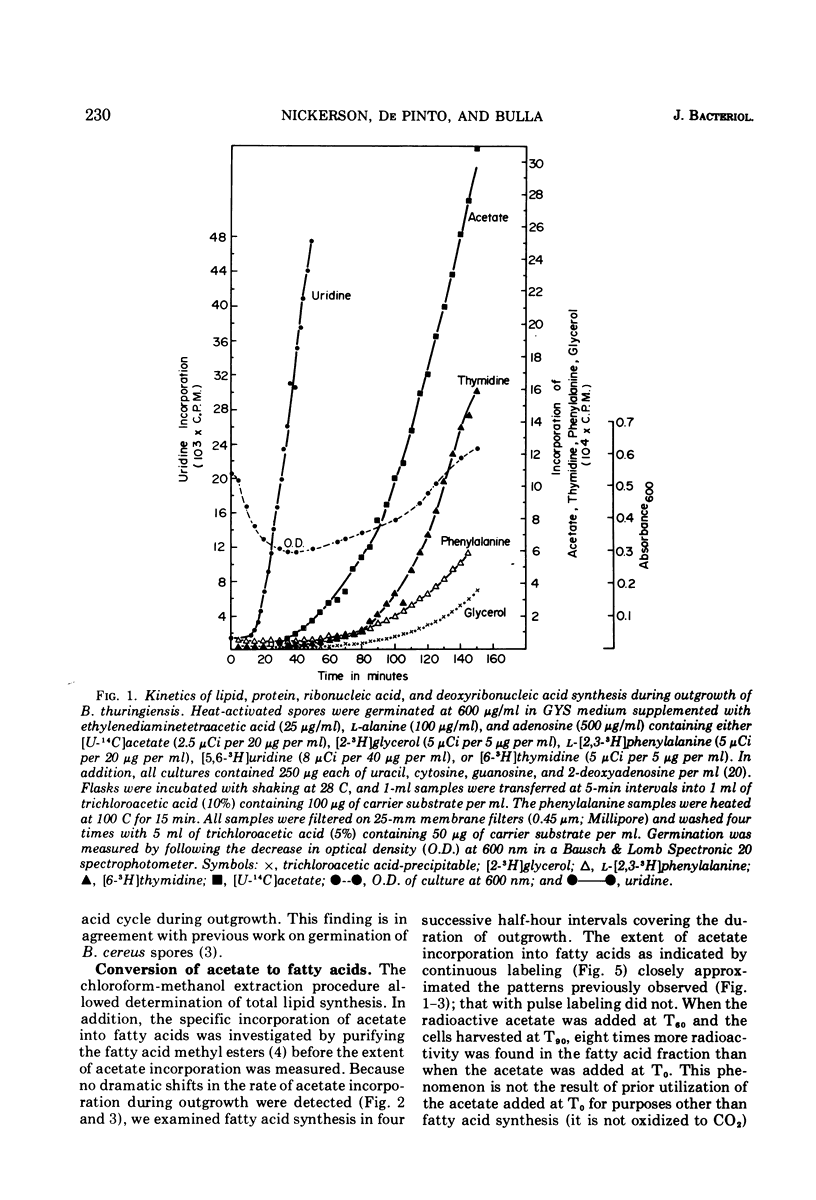
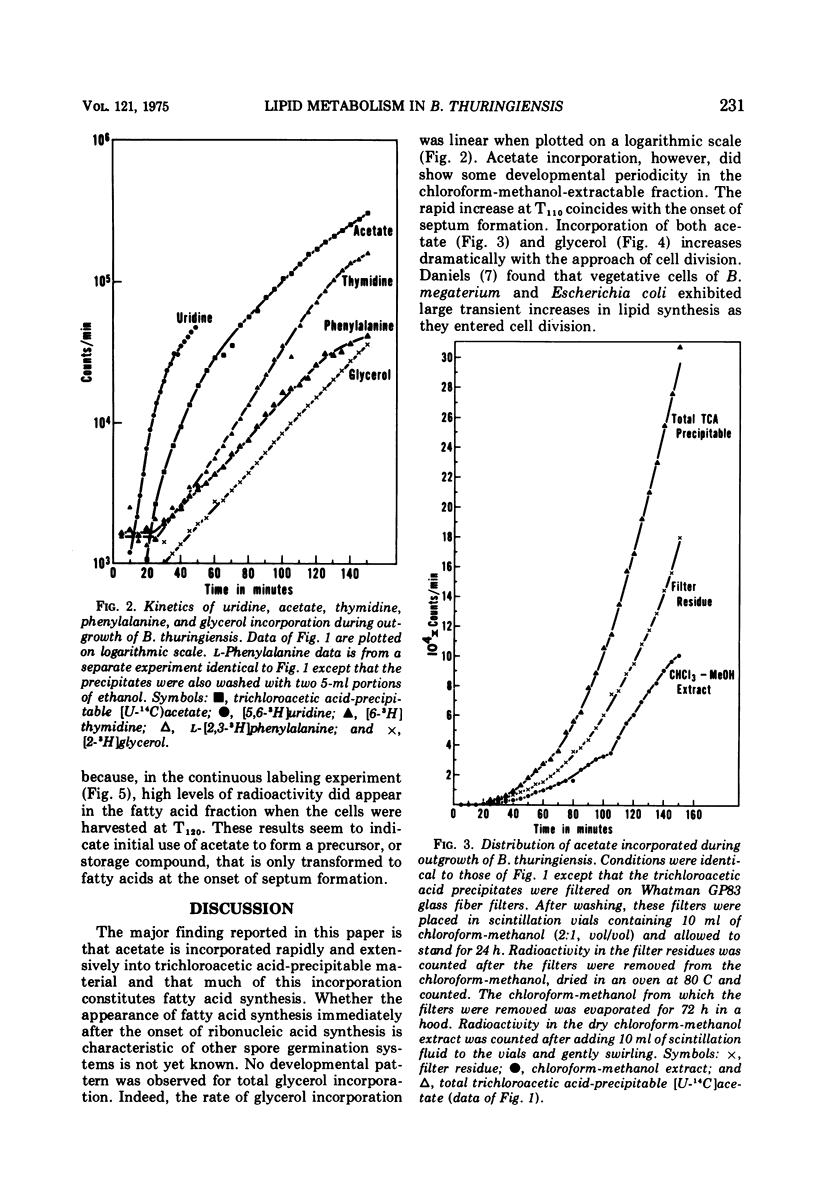
The timing and kinetics of fatty acid synthesis are delineated for Bacillus thuringiensis spore germination and outgrowth by analyzing [U-14C]acetate and [2-3H]glycerol incorporation into chloroform-methanol-extractable and trichloroacetic acid-precipitable lipids. In addition to measurement of pulsed and continuous labeling of fatty acids, monitoring the incorporation of radioactive phenylalanine, thymidine, and uridine from the onset of germination through first cell division provides a profile of biochemical activities related to membrane differentiation and cellular development. Upon germination, ribonucleic acid synthesis is initiated, immediately followed by rapid and extensive fatty acid synthesis that in turn precedes protein, deoxyribonucleic acid and triglyceride synthesis. Significantly, formation of fatty acids from acetate exhibits further developmental periodicity in which a large transient increase in fatty acid synthetic activity coincides with the approach of cell division. Radiorespirometric analyses indicates only slight oxidative decarboxylation of acetate and corroborates the extreme involvement of acetate in specific fatty acid biosynthetic reactions throughout cellular modification. These findings graphically demonstrate an intimate association of fatty acid metabolism with commitment to spore outgrowth and subsequent cell division.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong R. L., Sueoka N. Phase transitions in ribonucleic acid synthesis during germination of Bacillus subtilis spores. Proc Natl Acad Sci U S A. 1968 Jan;59(1):153–160. doi: 10.1073/pnas.59.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertsch L. L., Bonsen P. P., Kornberg A. Biochemical studies of bacterial sporulation and germination. XIV. Phospholipids in Bacillus megaterium. J Bacteriol. 1969 Apr;98(1):75–81. doi: 10.1128/jb.98.1.75-81.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulla L. A., Bennett G. A., Shotwell O. L. Physiology of Sporeforming Bacteria Associated with Insects II. Lipids of Vegetative Cells. J Bacteriol. 1970 Dec;104(3):1246–1253. doi: 10.1128/jb.104.3.1246-1253.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulla L. A., Jr, St Julian G., Rhodes R. A. Physiology of sporeforming bacteria associated with insects. 3. Radiorespirometry of pyruvate, acetate, succinate, and glutamate oxidation. Can J Microbiol. 1971 Aug;17(8):1073–1079. doi: 10.1139/m71-170. [DOI] [PubMed] [Google Scholar]
- Daniels M. J. Lipid synthesis in relation to the cell cycle of Bacillus megaterium KM and Escherichia coli. Biochem J. 1969 Dec;115(4):697–701. doi: 10.1042/bj1150697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delafield F. P., Somerville H. J., Rittenberg S. C. Immunological homology between crystal and spore protein of Bacillus thuringiensis. J Bacteriol. 1968 Sep;96(3):713–720. doi: 10.1128/jb.96.3.713-720.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FITZ-JAMES P. C. Participation of the cytoplasmic membrane in the growth and spore fromation of bacilli. J Biophys Biochem Cytol. 1960 Oct;8:507–528. doi: 10.1083/jcb.8.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrick-Silversmith L., Torriani A. Macromolecular syntheses during germination and outgrowth of Bacillus subtilis spores. J Bacteriol. 1973 May;114(2):507–516. doi: 10.1128/jb.114.2.507-516.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halvorson H. O., Vary J. C., Steinberg W. Developmental changes during the formation and breaking of the dormant state in bacteria. Annu Rev Microbiol. 1966;20:169–188. doi: 10.1146/annurev.mi.20.100166.001125. [DOI] [PubMed] [Google Scholar]
- Hansen J. N., Spiegelman G., Halvorson H. O. Bacterial spore outgrowth: its regulation. Science. 1970 Jun 12;168(3937):1291–1298. doi: 10.1126/science.168.3937.1291. [DOI] [PubMed] [Google Scholar]
- Lang D. R., Lundgren D. G. Lipid composition of Bacillus cereus during growth and sporulation. J Bacteriol. 1970 Feb;101(2):483–489. doi: 10.1128/jb.101.2.483-489.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKATA H. M. EFFECT OF PH ON INTERMEDIATES PRODUCED DURING GROWTH AND SPORULATION OF BACILLUS CEREUS. J Bacteriol. 1963 Sep;86:577–581. doi: 10.1128/jb.86.3.577-581.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata H. M. Role of acetate in sporogenesis of Bacillus cereus. J Bacteriol. 1966 Feb;91(2):784–788. doi: 10.1128/jb.91.2.784-788.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitel D. W., Gilvarg C. Timing of mucopeptide and phospholipid synthesis in sporulating Bacillus megaterium. J Biol Chem. 1971 Jun 10;246(11):3720–3724. [PubMed] [Google Scholar]
- Rana R. S., Halvorson H. O. Method for restricting incorporation of radioactivity from 3 H-thymidine into deoxyribonucleic acid only during outgrowth of spores of Bacillus cereus T. J Bacteriol. 1972 Feb;109(2):599–605. doi: 10.1128/jb.109.2.599-605.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana R. S., Halvorson H. O. Nature of deoxyribonucleic acid synthesis and its relationship to protein synthesis during outgrowth of Bacillus cereus T. J Bacteriol. 1972 Feb;109(2):606–615. doi: 10.1128/jb.109.2.606-615.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodenberg S., Steinberg W., Piper J., Nickerson K., Vary J., Epstein R., Halvorson H. O. Relationship between protein and ribonucleic acid synthesis during outgrowth of spores of Bacillus cereus. J Bacteriol. 1968 Aug;96(2):492–500. doi: 10.1128/jb.96.2.492-500.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scandella C. J., Kornberg A. Biochemical studies of bacterial sporulation and germination. XV. Fatty acids in growth, sporulation, and germination of Bacillus megaterium. J Bacteriol. 1969 Apr;98(1):82–86. doi: 10.1128/jb.98.1.82-86.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P. Deoxyribonucleic acid synthesis and deoxynucleotide metabolism during bacterial spore germination. J Bacteriol. 1973 Jun;114(3):1099–1107. doi: 10.1128/jb.114.3.1099-1107.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H., Wilson T. H. The role of energy coupling in the transport of beta-galactosides by Escherichia coli. J Biol Chem. 1966 May 25;241(10):2200–2211. [PubMed] [Google Scholar]


