Abstract
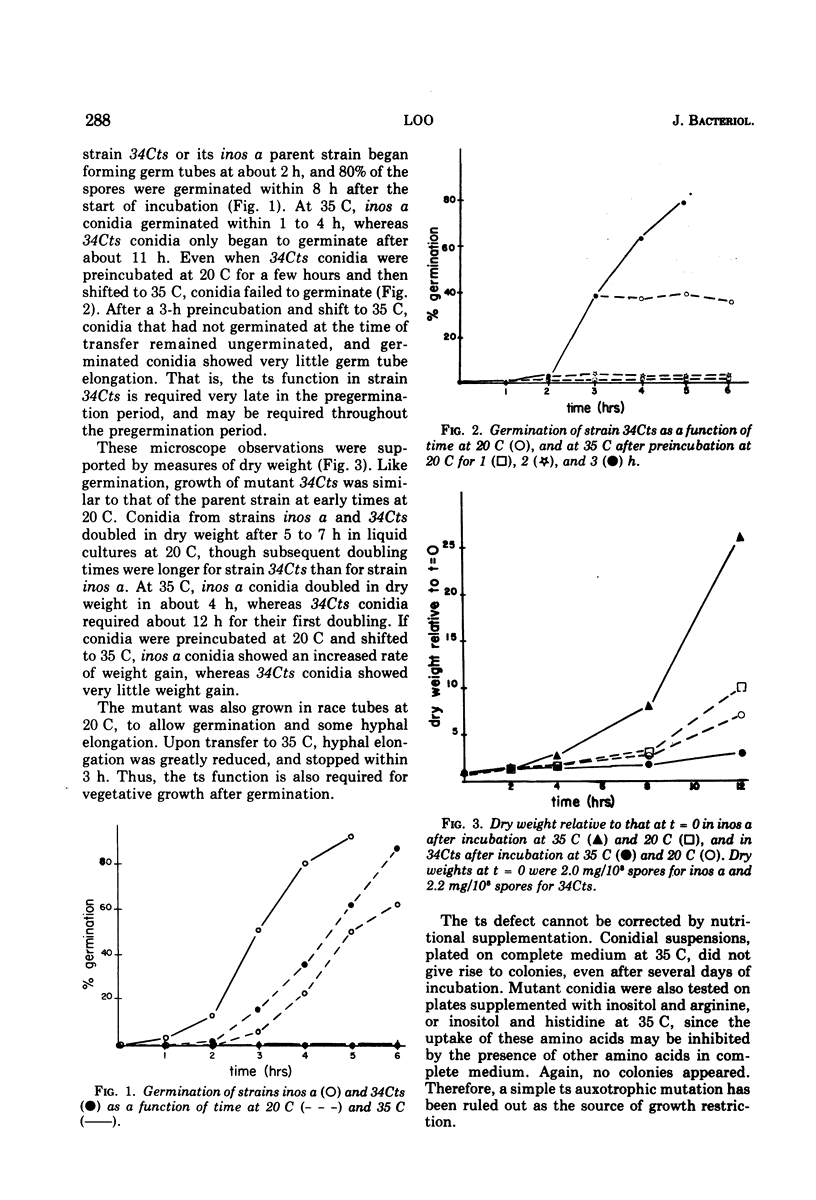
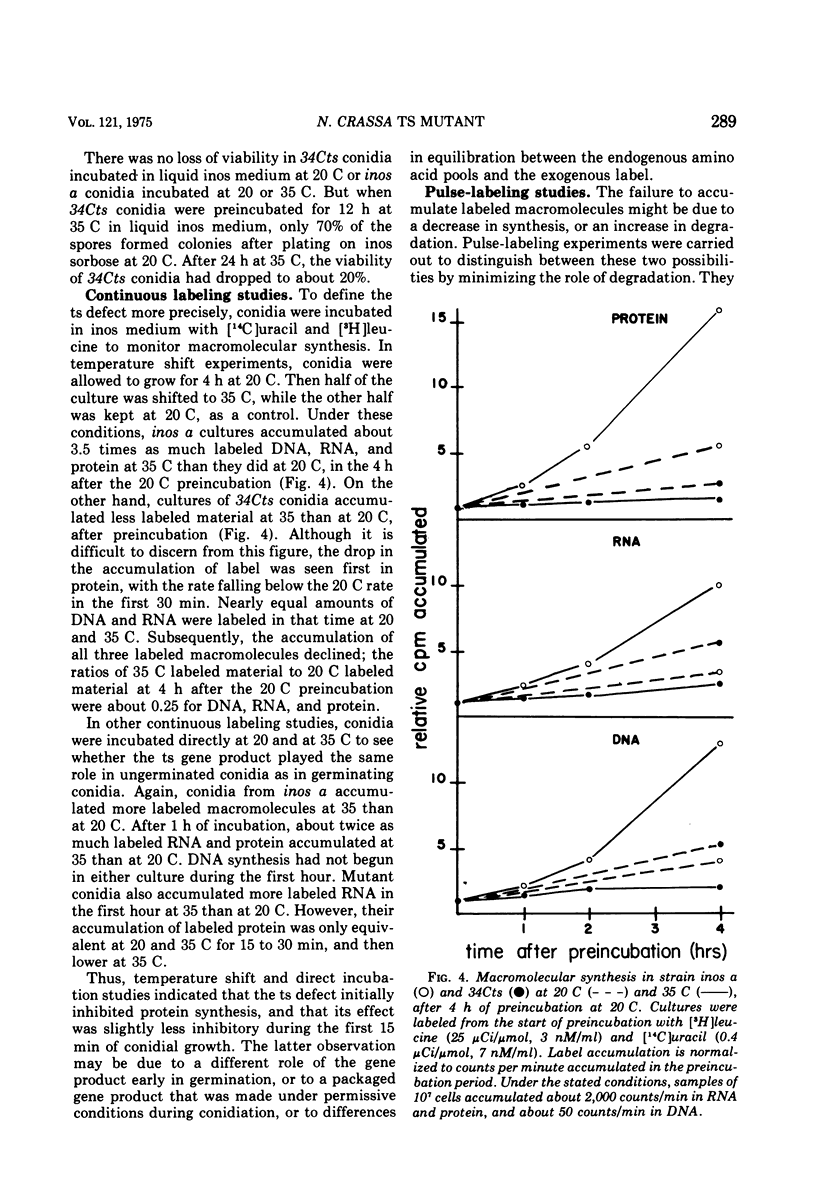
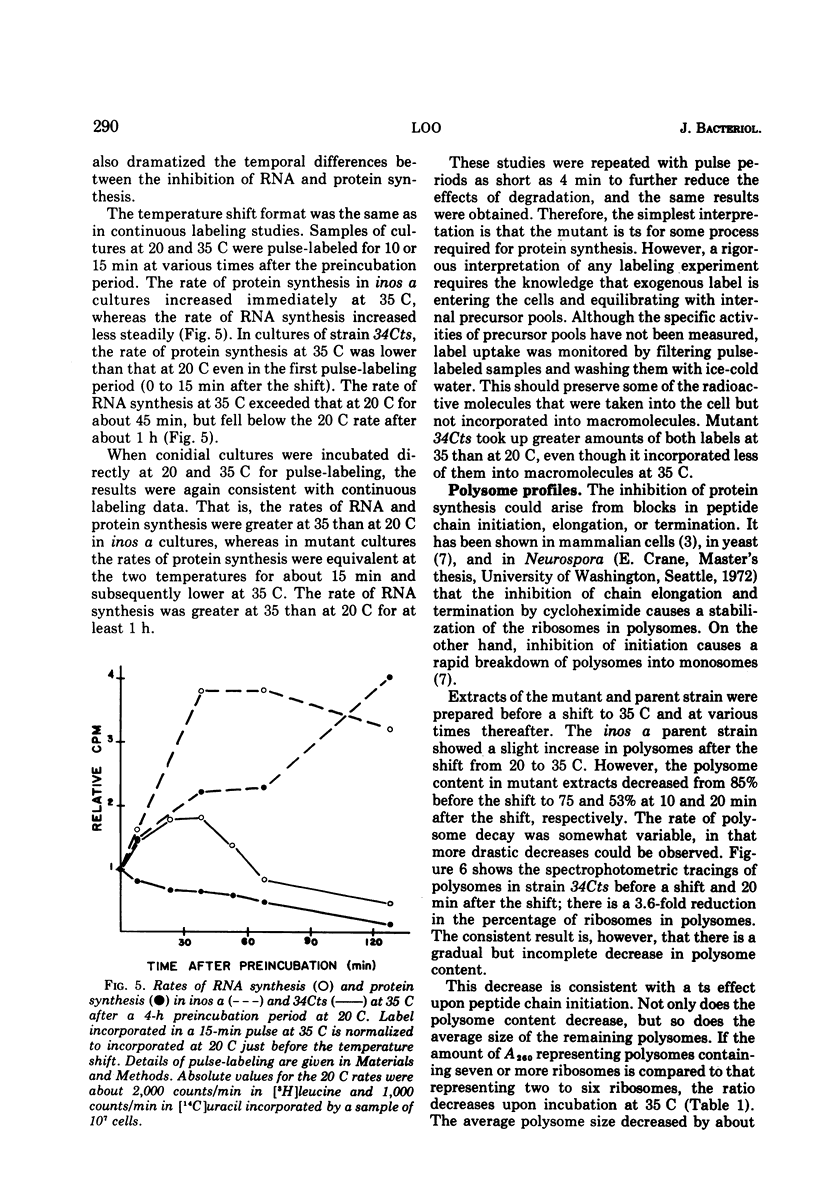
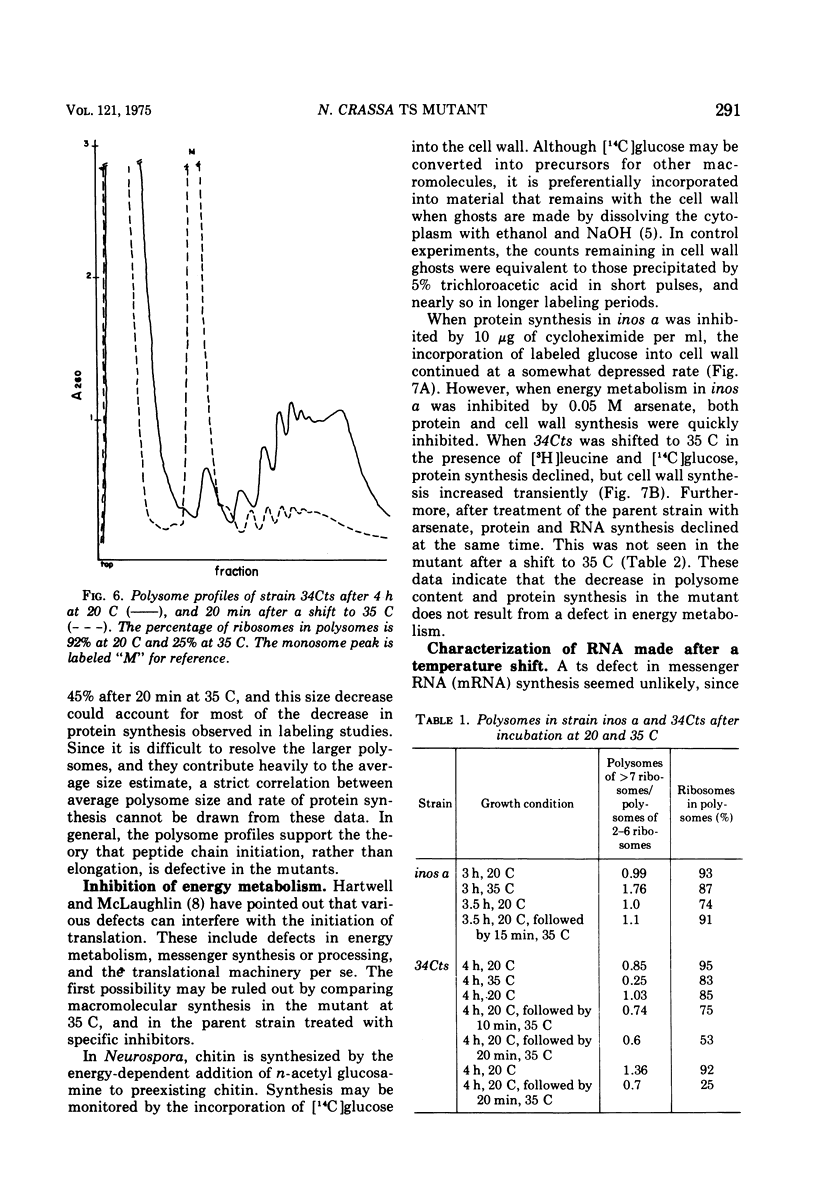
A temperature-sensitive mutant of Neurospora was isolated which appeared to be defective in the initiation of protein synthesis. The defect in mutant 34Cts was apparently due to a single gene mutation, and was recessive in heterokaryons. Conidial germination was normal and hyphal growth was nearly so in the mutant at 20 C, but both were greatly inhibited at 35 C. After 15 min at 35 C there was a reduced rate of protein synthesis, followed by decreases in ribonucleic acid and deoxyribonucleic acid synthesis. The percentage of ribosomes in polysomes declined at 35 C and the average size of polysomes decreased. Because the decrease in protein synthesis, it was believed that some part of the translational system may be affected by the mutation. Mutant 34Cts was given the designation psi-1.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adesnik M., Salditt M., Thomas W., Darnell J. E. Evidence that all messenger RNA molecules (except histone messenger RNA) contain Poly (A) sequences and that the Poly(A) has a nuclear function. J Mol Biol. 1972 Oct 28;71(1):21–30. doi: 10.1016/0022-2836(72)90397-x. [DOI] [PubMed] [Google Scholar]
- Fan H., Penman S. Regulation of protein synthesis in mammalian cells. II. Inhibition of protein synthesis at the level of initiation during mitosis. J Mol Biol. 1970 Jun 28;50(3):655–670. doi: 10.1016/0022-2836(70)90091-4. [DOI] [PubMed] [Google Scholar]
- Goldstein E. S., Penman S. Regulation of protein synthesis in mammalian cells. V. Further studies on the effect of actinomycin D on translation control in HeLa cells. J Mol Biol. 1973 Oct 25;80(2):243–254. doi: 10.1016/0022-2836(73)90170-8. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H., Hutchison H. T., Holland T. M., McLaughlin C. S. The effect of cycloheximide upon polyribosome stability in two yeast mutants defective respectively in the initiation of polypeptide chains and in messenger RNA synthesis. Mol Gen Genet. 1970;106(4):347–361. doi: 10.1007/BF00324052. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H. Macromolecule synthesis in temperature-sensitive mutants of yeast. J Bacteriol. 1967 May;93(5):1662–1670. doi: 10.1128/jb.93.5.1662-1670.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H., McLaughlin C. S. Temperature-sensitive mutants of yeast exhibiting a rapid inhibition of protein synthesis. J Bacteriol. 1968 Nov;96(5):1664–1671. doi: 10.1128/jb.96.5.1664-1671.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobbágy A. J., Wagner R. P. Changes in enzyme activity of germinating conidia of Neurospora crassa. Dev Biol. 1973 Apr;31(2):264–274. doi: 10.1016/0012-1606(73)90263-7. [DOI] [PubMed] [Google Scholar]
- LESTER H. E., GROSS S. R. Efficient method for selection of auxotrophic mutants of Neurospora. Science. 1959 Feb 27;129(3348):572–572. doi: 10.1126/science.129.3348.572. [DOI] [PubMed] [Google Scholar]
- Menninger J. R., Walker C., Tan P. F. Studies on the metabolic role of peptidyl-tRNA hydrolase. I. Properties of a mutant E. coli with temperature-sensitive peptidyl-tRNA hydrolase. Mol Gen Genet. 1973 Mar 19;121(4):307–324. doi: 10.1007/BF00433230. [DOI] [PubMed] [Google Scholar]
- Mirkes P. E. Polysomes, ribonucleic acid, and protein synthesis during germination of Neurospora crassa conidia. J Bacteriol. 1974 Jan;117(1):196–202. doi: 10.1128/jb.117.1.196-202.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson D., Gillespie D. Effect of elevated temperatures on protein synthesis in Escherichia coli. J Bacteriol. 1972 Dec;112(3):1177–1183. doi: 10.1128/jb.112.3.1177-1183.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rho H. M., DeBusk A. G. NH2-terminal methionine in nascent peptides from Neurospora crassa. Biochem Biophys Res Commun. 1971 Jan 22;42(2):319–325. doi: 10.1016/0006-291x(71)90105-7. [DOI] [PubMed] [Google Scholar]
- Roth R. M., Dampier C. Dependence of ribonucleic acid synthesis on continuous protein synthesis in yeast. J Bacteriol. 1972 Feb;109(2):773–779. doi: 10.1128/jb.109.2.773-779.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreier M. H., Staehelin T. Initiation of eukaryotic protein synthesis: (Met-tRNA f -40S ribosome) initiation complex catalysed by purified initiation factors in the absence of mRNA. Nat New Biol. 1973 Mar 14;242(115):35–38. doi: 10.1038/newbio242035a0. [DOI] [PubMed] [Google Scholar]
- Sheldon R., Jurale C., Kates J. Detection of polyadenylic acid sequences in viral and eukaryotic RNA(polu(U)-cellulose columns-poly(U) filters-fiberglass-HeLa cells-bacteriophage T4). Proc Natl Acad Sci U S A. 1972 Feb;69(2):417–421. doi: 10.1073/pnas.69.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki D. T. Temperature-sensitive mutations in Drosophila melanogaster. Science. 1970 Nov 13;170(3959):695–706. doi: 10.1126/science.170.3959.695. [DOI] [PubMed] [Google Scholar]
- Tisdale J. H., DeBusk A. G. Developmental regulation of amino acid transport in Neurospora crassa. J Bacteriol. 1970 Nov;104(2):689–697. doi: 10.1128/jb.104.2.689-697.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuveson R. W., West D. J., Barratt R. W. Glutamic acid dehydrogenases in quiescent and germinating conidia of Neurospora crassa. J Gen Microbiol. 1967 Aug;48(2):235–248. doi: 10.1099/00221287-48-2-235. [DOI] [PubMed] [Google Scholar]


