Abstract
Strains of Bacillus subtilis 168 lysogenic for bacteriophage phi105 transfer with deoxyribonucleic acid (DNA) isolated from bacteriophage SPO2 at a higher efficiency than non-lysogenic strains. This enhancement of transfection was not the result of recombination between bacteriophages SPO2 and phi105. Superinfection marker rescue increased transfection with DNA from bacteriophage phi105 occurred simultaneously with the addition of the transfecting DNA. Again, this enhancement of transfection was not the result of recombination but rather a protection of the transfecting DNA by the superinfecting bacteriophage. The ability of the superinfecting bacteriophage to protect the transfecting DNA from inactivation was maximal when the bacteria were just becoming competent. Bacteriophage phi1 cannot replicate after the transfection of competent bacteria lacking a functional DNA replication system, whereas bacteriophage phi1 was able to replicate after infection of competent bacteria grown under comparable conditions. These observations support the hypothesis that GAPase and an inducible repair system play an important role in the development of competence.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arwert F., Venema G. Transfection of Bacillus subtilis with bacteriophage H1 DNA: fate of transfecting DNA and transfection enhancement in B. subtilis uur+ and uur- strains. Mol Gen Genet. 1974;128(1):55–72. doi: 10.1007/BF00267294. [DOI] [PubMed] [Google Scholar]
- Boice L. B. Evidence that Bacillus subtilis bacteriophage SP02 is temperate and heteroimmune to bacteriophage phi-105. J Virol. 1969 Jul;4(1):47–49. doi: 10.1128/jvi.4.1.47-49.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylan R. J., Mendelson N. H., Brooks D., Young F. E. Regulation of the bacterial cell wall: analysis of a mutant of Bacillus subtilis defective in biosynthesis of teichoic acid. J Bacteriol. 1972 Apr;110(1):281–290. doi: 10.1128/jb.110.1.281-290.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N. C. Inhibition of bacterial DNA replication by 6-(p-hydroxyphenylazo)-uracil: differential effect on repair and semi-conservative synthesis in Bacillus subtilis. J Mol Biol. 1971 Jul 14;59(1):1–16. doi: 10.1016/0022-2836(71)90409-8. [DOI] [PubMed] [Google Scholar]
- Chestukhin A. V., Shemyakin M. F., Kalinina N. A., Prozorov A. A. Some properties of ATP dependent deoxyribonucleases from normal and rec-mutant strains of Bacillus subtilis. FEBS Lett. 1972 Jul 15;24(1):121–125. doi: 10.1016/0014-5793(72)80841-x. [DOI] [PubMed] [Google Scholar]
- Clark A. J. Recombination deficient mutants of E. coli and other bacteria. Annu Rev Genet. 1973;7:67–86. doi: 10.1146/annurev.ge.07.120173.000435. [DOI] [PubMed] [Google Scholar]
- Cosloy S. D., Oishi M. Genetic transformation in Escherichia coli K12. Proc Natl Acad Sci U S A. 1973 Jan;70(1):84–87. doi: 10.1073/pnas.70.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosloy S. D., Oishi M. The nature of the transformation process in Escherichia coli K12. Mol Gen Genet. 1973 Jul 31;124(1):1–10. doi: 10.1007/BF00267159. [DOI] [PubMed] [Google Scholar]
- Defais M., Fauquet P., Radman M., Errera M. Ultraviolet reactivation and ultraviolet mutagenesis of lambda in different genetic systems. Virology. 1971 Feb;43(2):495–503. doi: 10.1016/0042-6822(71)90321-7. [DOI] [PubMed] [Google Scholar]
- Doly J., Sasarman E., Anagnostopoulos C. ATP-dependent deoxyribonuclease in Bacillus subtilis and a mutant deficient in this activity. Mutat Res. 1974 Jan;22(1):15–23. doi: 10.1016/0027-5107(74)90003-7. [DOI] [PubMed] [Google Scholar]
- Dubnau D., Cirigliano C. Fate of transforming DNA following uptake by competent Bacillus subtilis. Formation and properties of products isolated from transformed cells which are derived entirely from donor DNA. J Mol Biol. 1972 Feb 28;64(1):9–29. doi: 10.1016/0022-2836(72)90318-x. [DOI] [PubMed] [Google Scholar]
- Dubnau D., Davidoff-Abelson R. Fate of transforming DNA following uptake by competent Bacillus subtilis. I. Formation and properties of the donor-recipient complex. J Mol Biol. 1971 Mar 14;56(2):209–221. doi: 10.1016/0022-2836(71)90460-8. [DOI] [PubMed] [Google Scholar]
- Epstein H. T., Mahler I. Mechanisms of enhancement of SP82 transfection. J Virol. 1968 Jul;2(7):710–715. doi: 10.1128/jvi.2.7.710-715.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein H. T. Source of the nonlinear dependence of bacteriophage SP82 transfection on deoxyribonucleic acid concentration. J Virol. 1971 Jun;7(6):749–752. doi: 10.1128/jvi.7.6.749-752.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein H. T. Transfection enhancement by ultraviolet irradiation. Biochem Biophys Res Commun. 1967 Apr 20;27(2):258–262. doi: 10.1016/s0006-291x(67)80071-8. [DOI] [PubMed] [Google Scholar]
- FOELDES J., TRAUTNER T. A. INFECTIOUS DNA FROM A NEWLY ISOLATED B. SUBTILIS PHAGE. Z Vererbungsl. 1964 Apr 10;95:57–65. doi: 10.1007/BF00898184. [DOI] [PubMed] [Google Scholar]
- GREEN D. M. INFECTIVITY OF DNA ISOLATED FROM BACILLUS SUBTILIS BACTERIOPHAGE, SP82. J Mol Biol. 1964 Dec;10:438–451. doi: 10.1016/s0022-2836(64)80065-6. [DOI] [PubMed] [Google Scholar]
- Gass K. B., Low R. L., Cozzarelli N. R. Inhibition of a DNA polymerase from Bacillus subtilis by hydroxyphenylazopyrimidines. Proc Natl Acad Sci U S A. 1973 Jan;70(1):103–107. doi: 10.1073/pnas.70.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George J., Devoret R., Radman M. Indirect ultraviolet-reactivation of phage lambda. Proc Natl Acad Sci U S A. 1974 Jan;71(1):144–147. doi: 10.1073/pnas.71.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D. M., Urban M. I. Recombination and transfection mapping of cistron 5 of bacteriophage sp82g. Genetics. 1972 Feb;70(2):187–203. doi: 10.1093/genetics/70.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris W. J., Barr G. C. Mechanism of transformation in B. subtilis. Mol Gen Genet. 1971;113(4):331–344. doi: 10.1007/BF00272333. [DOI] [PubMed] [Google Scholar]
- Harris W. J., Barr G. C. Some properties of DNA in competent Bacillus subtilis. J Mol Biol. 1969 Jan;39(2):245–255. doi: 10.1016/0022-2836(69)90314-3. [DOI] [PubMed] [Google Scholar]
- Harris W. J., Barr G. C. Structural features of DNA in competent Bacillus subtilis. Mol Gen Genet. 1971;113(4):316–330. doi: 10.1007/BF00272332. [DOI] [PubMed] [Google Scholar]
- Israel V., Woodworth-Gutai M., Levine M. Inhibitory effect of bacteriophage P22 infection on host cell deoxyribonuclease activity. J Virol. 1972 May;9(5):752–757. doi: 10.1128/jvi.9.5.752-757.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karu A. E., MacKay V., Goldmark P. J., Linn S. The recBC deoxyribonuclease of Escherichia coli K-12. Substrate specificity and reaction intermediates. J Biol Chem. 1973 Jul 25;248(14):4874–4884. [PubMed] [Google Scholar]
- Marsden H. S., Pollard E. C., Ginoza W., Randall E. P. Involvement of recA and exr genes in the in vivo inhibition of the recBC nuclease. J Bacteriol. 1974 May;118(2):465–470. doi: 10.1128/jb.118.2.465-470.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister W. T., Green D. M. Bacteriophage SP82G inhibition of an intracellular deoxyribonucleic acid inactivation process in Bacillus subtilis. J Virol. 1972 Jul;10(1):51–59. doi: 10.1128/jvi.10.1.51-59.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKUBO S., STRAUSS B., STODOLSKY M. THE POSSIBLE ROLE OF RECOMBINATION IN THE INFECTION OF COMPETENT BACILLUS SUBTILIS BY BACTERIOPHAGE DEOXYRIBONUCLEIC ACID. Virology. 1964 Dec;24:552–562. doi: 10.1016/0042-6822(64)90207-7. [DOI] [PubMed] [Google Scholar]
- Oishi M., Cosloy S. D. The genetic and biochemical basis of the transformability of Escherichia coli K12. Biochem Biophys Res Commun. 1972 Dec 18;49(6):1568–1572. doi: 10.1016/0006-291x(72)90520-7. [DOI] [PubMed] [Google Scholar]
- REILLY B. E., SPIZIZEN J. BACTERIOPHAGE DEOXYRIBONUCLEATE INFECTION OF COMPETENT BACILLUS SUBTILIS. J Bacteriol. 1965 Mar;89:782–790. doi: 10.1128/jb.89.3.782-790.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudin L., Sjöström J. E., Lindberg M., Philipson L. Factors affecting competence for transformation in Staphylococcus aureus. J Bacteriol. 1974 Apr;118(1):155–164. doi: 10.1128/jb.118.1.155-164.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutberg B., Rutberg L. Growth of bacteriophage phi 105 and its deoxyribonucleic acid in radiation-sensitive mutants of Bacillus subtilis. J Virol. 1971 Dec;8(6):919–921. doi: 10.1128/jvi.8.6.919-921.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutberg L., Armentrout R. W., Jonasson J. Unrelatedness of temperate Bacillus subtilis bacteriophages SP02 and phi105. J Virol. 1972 May;9(5):732–737. doi: 10.1128/jvi.9.5.732-737.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutberg L., Hoch J. A., Spizizen J. Mechanism of transfection with deoxyribonucleic acid from the temperate Bacillus bacteriophage phi-105. J Virol. 1969 Jul;4(1):50–57. doi: 10.1128/jvi.4.1.50-57.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaki Y., Karu A. E., Linn S., Echols H. Purification and properties of the gamma-protein specified by bacteriophage lambda: an inhibitor of the host RecBC recombination enzyme. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2215–2219. doi: 10.1073/pnas.70.8.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spatz H. C., Trautner T. A. The role of recombination in transfection of B. subtilis. Mol Gen Genet. 1971;113(2):174–190. doi: 10.1007/BF00333191. [DOI] [PubMed] [Google Scholar]
- Tanner D., Oishi M. The effect of bacteriophage T4 infection on an ATP-dependent deoxyribonuclease in Escherichia coli. Biochim Biophys Acta. 1971 Feb 11;228(3):767–769. doi: 10.1016/0005-2787(71)90747-7. [DOI] [PubMed] [Google Scholar]
- Trautner T. A., Spatz H. C. Transfection in B. subtilis. Curr Top Microbiol Immunol. 1973;62:61–88. doi: 10.1007/978-3-642-65772-6_3. [DOI] [PubMed] [Google Scholar]
- Witkin E. M., George D. L. Ultraviolet mutagenesis in polA and UvrA polA derivatives of Escherichia coli B-R: evidence for an inducible error-prone repair system. Genetics. 1973 Apr;73(Suppl):91–10. [PubMed] [Google Scholar]
- Yasbin R. E., Ganesan A. T., Young F. E. Bacteriophage interference in Bacillus subtilis 168. J Virol. 1974 Apr;13(4):916–921. doi: 10.1128/jvi.13.4.916-921.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
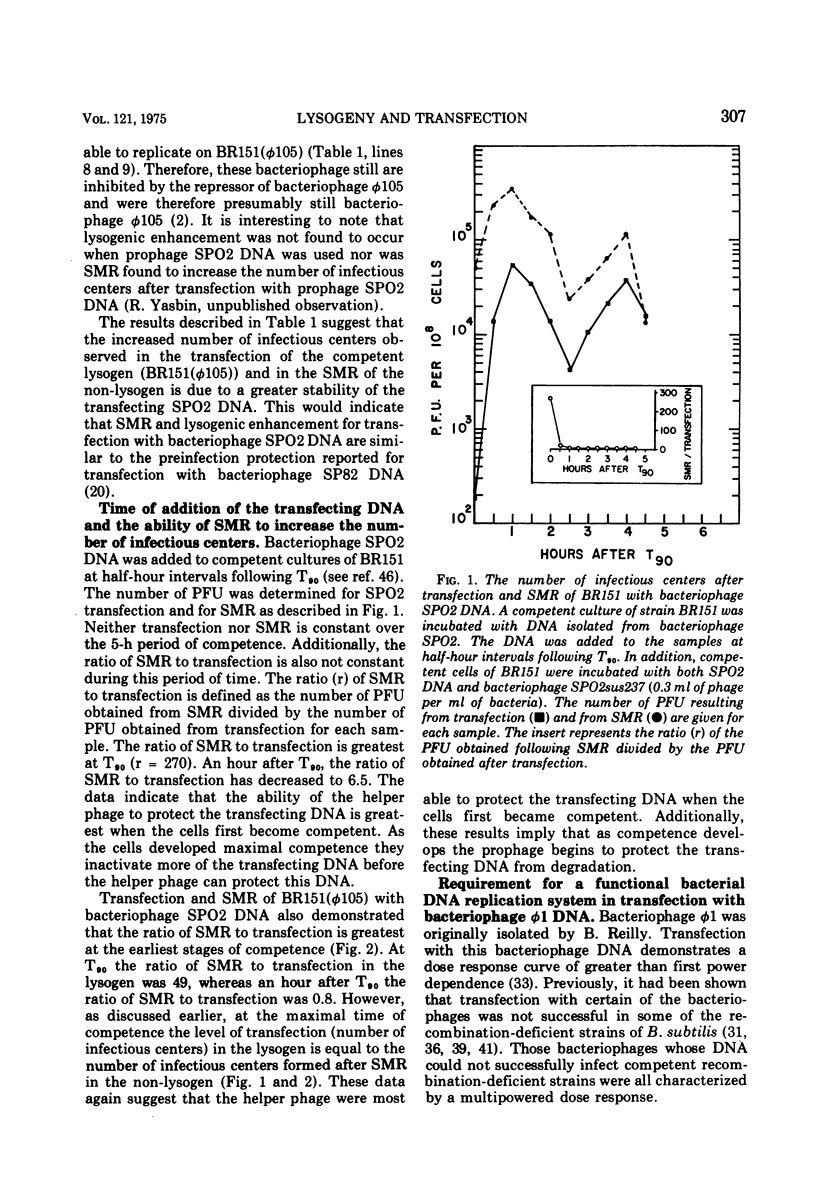
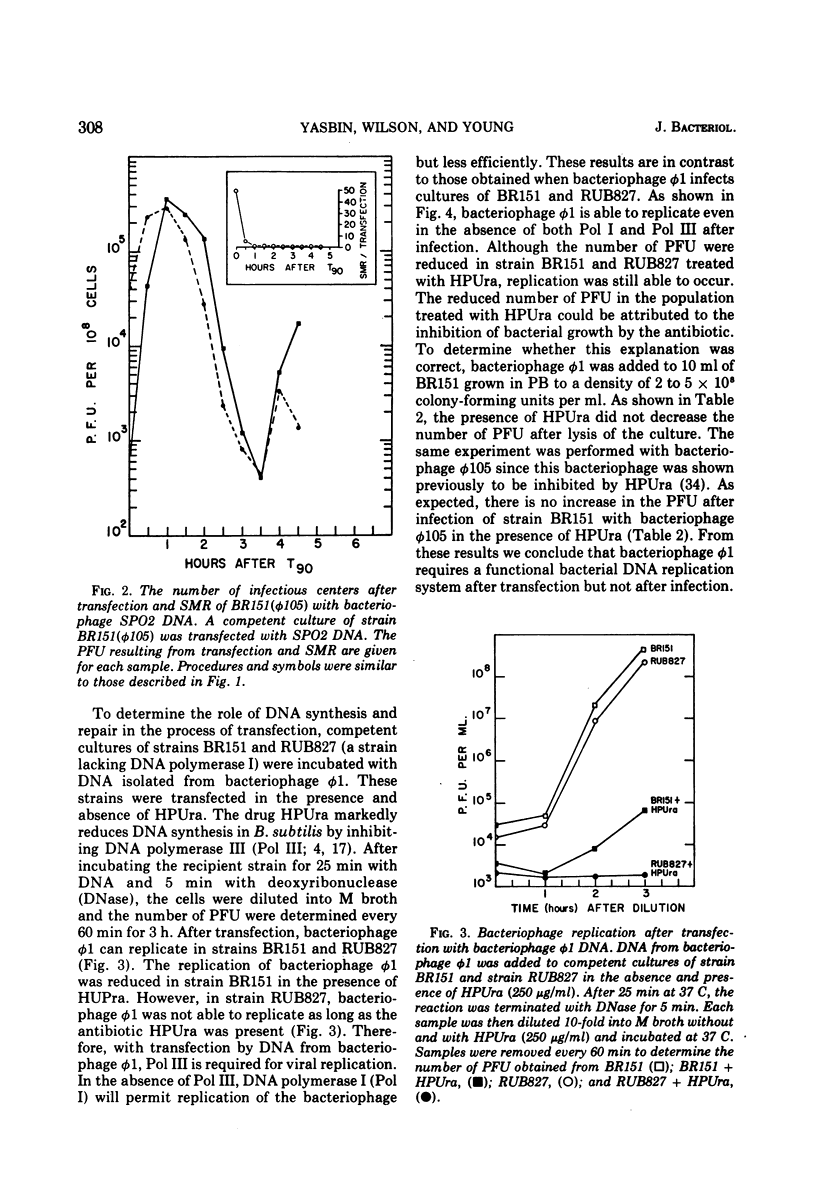
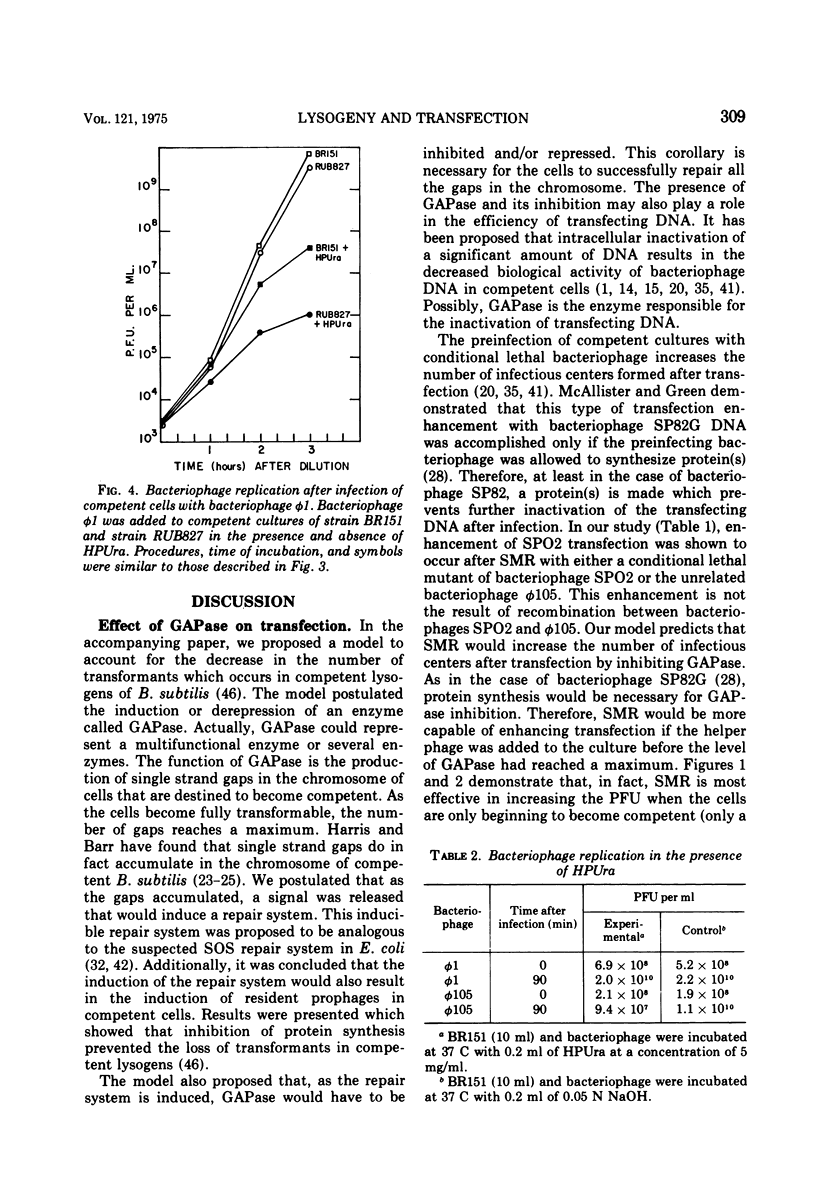
- Yasbin R. E., Wilson G. A., Young F. E. Transformation and transfection in lysogenic strains of Bacillus subtilis 168. J Bacteriol. 1973 Feb;113(2):540–548. doi: 10.1128/jb.113.2.540-548.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasbin R. E., Wilson G. A., Young F. E. Transformation and transfection in lysogenic strains of Bacillus subtilis: evidence for selective induction of prophage in competent cells. J Bacteriol. 1975 Jan;121(1):296–304. doi: 10.1128/jb.121.1.296-304.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


