Abstract
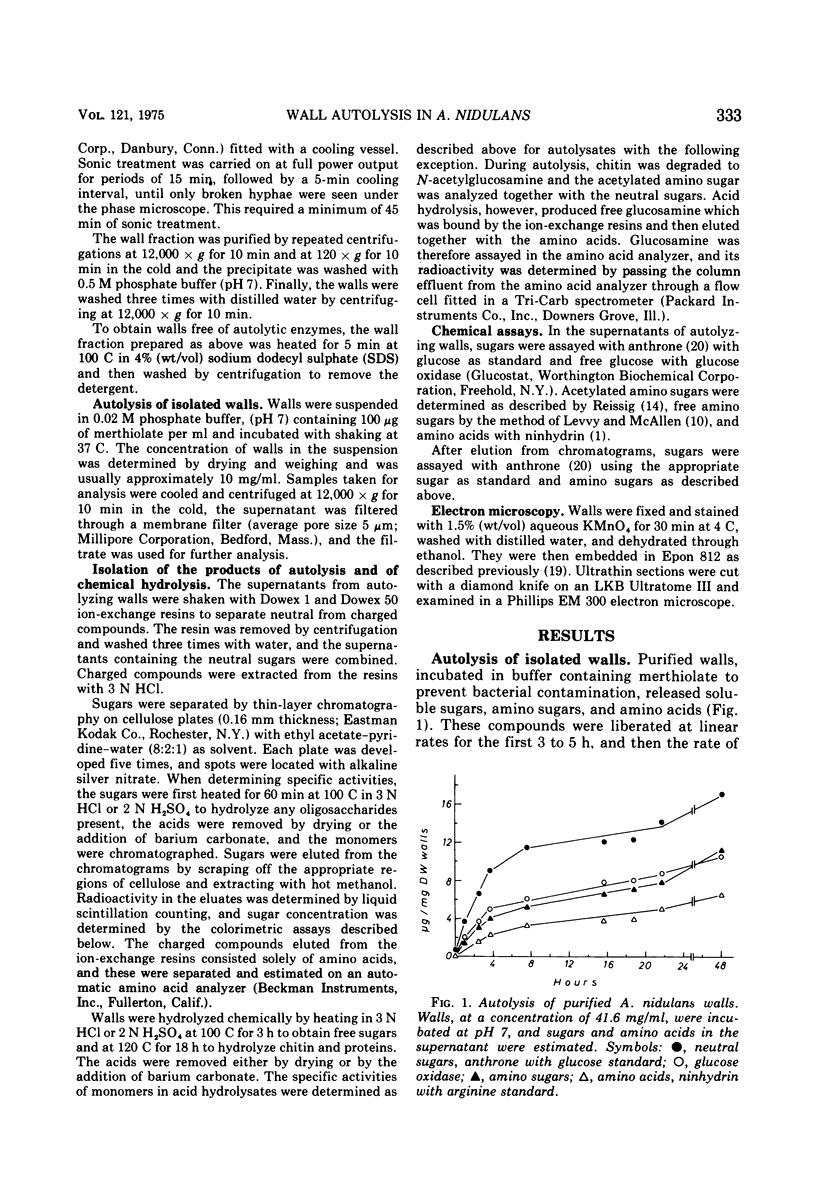
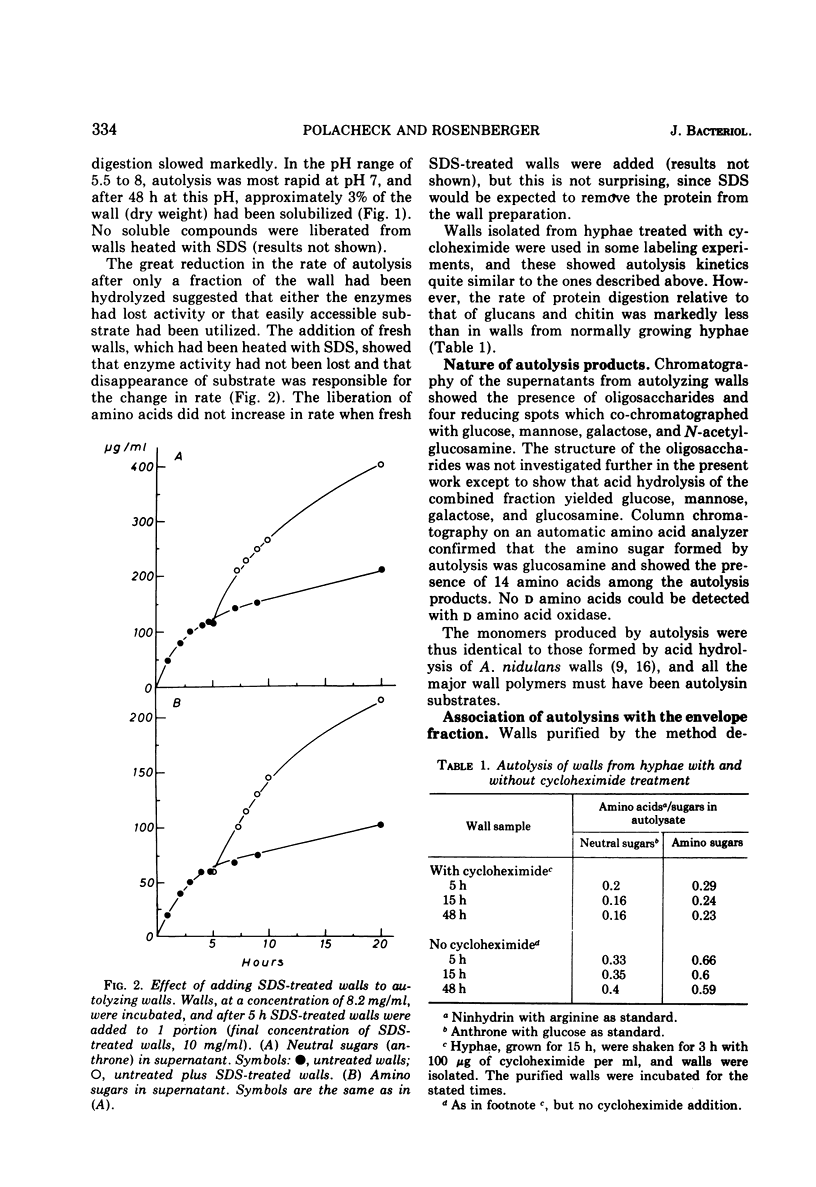
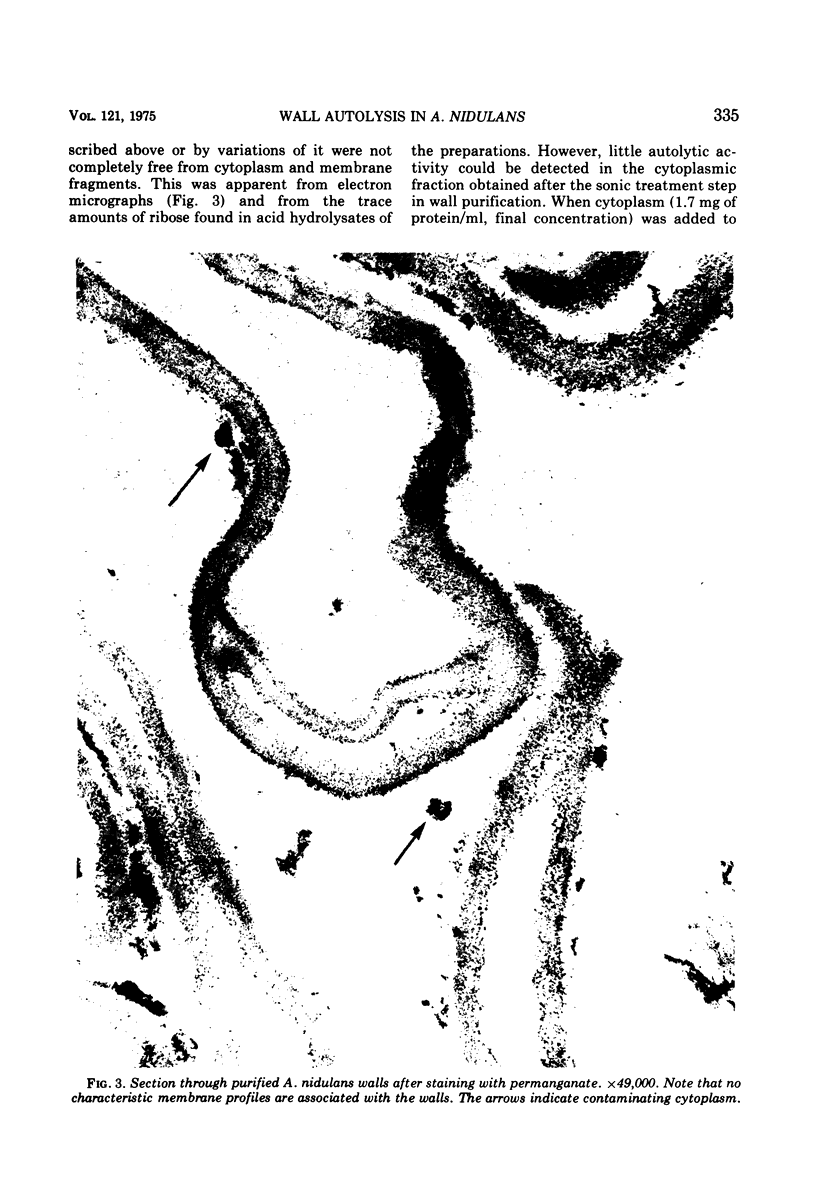
Walls, purified from hyphae of the ascomycete Aspergillus nidulans, autolyzed on incubation and liberated glucose, mannose, galactose, N-acetylglucosamine, and soluble oligosaccharides. Digestion proceeded at linear rates until approximately 3% of the wall polymers had been hydrolyzed and then slowed markedly. The change in the rate of autolysis was not due to loss of enzyme activity but was caused by the disappearance of a fraction of the wall which was highly susceptible to digestion. Radioactive labeling showed that this fraction was the newly formed wall. The new wall was highly susceptible to enzyme action both when it was deposited at the apex in growing hyphae or when deposited laterally in hyphae treated with cycloheximide. The relations between wall modification and apical growth are discussed.
Full text
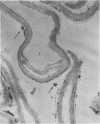
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Forsberg C., Rogers H. J. Autolytic enzymes in growth of bacteria. Nature. 1971 Jan 22;229(5282):272–273. doi: 10.1038/229272a0. [DOI] [PubMed] [Google Scholar]
- Ghuysen J. M. Use of bacteriolytic enzymes in determination of wall structure and their role in cell metabolism. Bacteriol Rev. 1968 Dec;32(4 Pt 2):425–464. [PMC free article] [PubMed] [Google Scholar]
- Gull K., Trinci A. P. Detection of areas of wall differentiation in fungi using fluorescent staining. Arch Mikrobiol. 1974 Mar 1;96(1):53–57. doi: 10.1007/BF00590162. [DOI] [PubMed] [Google Scholar]
- Higgins M. L., Shockman G. D. Model for cell wall growth of Streptococcus faecalis. J Bacteriol. 1970 Feb;101(2):643–648. doi: 10.1128/jb.101.2.643-648.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L., Shockman G. D. Procaryotic cell division with respect to wall and membranes. CRC Crit Rev Microbiol. 1971 May;1(1):29–72. doi: 10.3109/10408417109104477. [DOI] [PubMed] [Google Scholar]
- Katz D., Goldstein D., Rosenberger R. F. Model for branch initiation in Aspergillus nidulans based on measurements of growth parameters. J Bacteriol. 1972 Mar;109(3):1097–1100. doi: 10.1128/jb.109.3.1097-1100.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz D., Rosenberger R. F. A mutation in Aspergillus nidulans producing hyphal walls which lack chitin. Biochim Biophys Acta. 1970 Jun;208(3):452–460. doi: 10.1016/0304-4165(70)90218-7. [DOI] [PubMed] [Google Scholar]
- LEVVY G. A., MCALLAN A. The N-acetylation and estimation of hexosamines. Biochem J. 1959 Sep;73:127–132. doi: 10.1042/bj0730127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadevan P. R., Mahadkar U. R. Role of enzymes in growth and morphology of Neurospora crassa: cell-wall-bound enzymes and their possible role in branching. J Bacteriol. 1970 Mar;101(3):941–947. doi: 10.1128/jb.101.3.941-947.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederpruem D. J., Wessels J. G. Cytodifferentiation and morphogenesis in Schizophyllum commune. Bacteriol Rev. 1969 Dec;33(4):505–535. doi: 10.1128/br.33.4.505-535.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooley H. M., Shockman G. D. Relationship between the location of autolysin, cell wall synthesis, and the development of resistance to cellular autolysis in Streptococcus faecalis after inhibition of protein synthesis. J Bacteriol. 1970 Aug;103(2):457–466. doi: 10.1128/jb.103.2.457-466.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REISSIG J. L., STORMINGER J. L., LELOIR L. F. A modified colorimetric method for the estimation of N-acetylamino sugars. J Biol Chem. 1955 Dec;217(2):959–966. [PubMed] [Google Scholar]
- Ruiz-Herrera J. Chemical components of the cell wall of Aspergillus species. Arch Biochem Biophys. 1967 Oct;122(1):118–125. doi: 10.1016/0003-9861(67)90130-0. [DOI] [PubMed] [Google Scholar]
- Sayare M., Daneo-Moore L., Shockman G. D. Influence of macromolecular biosynthesis on cellular autolysis in Streptococcus faecalis. J Bacteriol. 1972 Oct;112(1):337–344. doi: 10.1128/jb.112.1.337-344.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D., Pooley H. M., Thompson J. S. Autolytic enzyme system of Streptococcus faecalis. 3. Localization of the autolysin at the sites of cell wall synthesis. J Bacteriol. 1967 Nov;94(5):1525–1530. doi: 10.1128/jb.94.5.1525-1530.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternlicht E., Katz D., Rosenberger R. F. Subapical wall synthesis and wall thickening induced by cycloheximide in hyphae of Aspergillus nidulans. J Bacteriol. 1973 May;114(2):819–823. doi: 10.1128/jb.114.2.819-823.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREVELYAN W. E., HARRISON J. S. Studies on yeast metabolism. I. Fractionation and microdetermination of cell carbohydrates. Biochem J. 1952 Jan;50(3):298–303. doi: 10.1042/bj0500298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonneveld B. J. A new type of enzyme, and exo-splitting -1,3 glucanase from non-induced cultures of Aspergillus nidulans. Biochim Biophys Acta. 1972 Feb 28;258(2):541–547. doi: 10.1016/0005-2744(72)90245-8. [DOI] [PubMed] [Google Scholar]