Abstract
Increased nonenzymatic glycation of apoB-containing lipoproteins impairs uptake and metabolism by the high affinity low density lipoprotein (LDL) receptor, and is one of the post-secretory modifications contributory to accelerated atherosclerosis in diabetes. The present study evaluated in vitro and in vivo effects of 2,2-chlorophenylaminophenylacetate (CAP22) to probe the influence of glycated lipoprotein on cholesterol homeostasis. This compound prevented the increased formation of glycated products in LDL incubated with 200 mM glucose and the increased cholesteryl ester synthesis in THP-1 macrophages induced by apoB-containing lipoproteins preincubated with high glucose concentration. The elevated circulating concentrations of glycated lipoprotein and cholesterol and higher vascular levels of lipid peroxidation products observed in streptozotocin diabetic rats compared to nondiabetic controls were significantly reduced in diabetic animals treated for six months with test compound. These results are the first to demonstrate that inhibiting nonenzymatic glycation of apoB-containing lipoproteins ameliorates abnormalities contributory to hypercholesterolemia and atherogenic risk in diabetes.
Keywords: apoB, diabetes, glycation, LDL, lipid peroxidation
INTRODUCTION
Increased nonenzymatic glycation of apolipoprotein B (apoB) is one of the post-secretory modifications of low density lipoproteins (LDL) contributory to the increased susceptibility to atherosclerosis in patients with diabetes, in whom elevated levels of glycated LDL have been consistently demonstrated (1–6). Plasma concentrations of glycated LDL show positive associations with serum cholesterol and other markers of cardiovascular disease (7) and with the incidence of myocardial infarction (8). Glycation diminishes the uptake and degradation of LDL by the high affinity LDL receptor (9–14) and promotes uptake and metabolism by alternative pathways in monocyte-macrophages that give rise to cholesterol-laden foam cells (15–18). Glycated LDL has reduced ability to regulate hydroxymethyl glutaryl-CoA reductase and acyl-CoA: cholesterol transferase activities (14,16), induces functional changes in smooth muscle and endothelial cells and in monocytes (19–26), binds to arterial wall proteoglycans (6), and accelerates free radical production and lipid peroxidation that can generate glyco-oxidized and oxidized LDL and exaggerate cellular responses elicited by glycated LDL (27–31). Glycated LDL-induced stimulation of arterial smooth muscle cell proliferation is mediated by phosphorylation of extracellular signal-regulated kinase (ERK) and involves activation of protein kinase C, phospholipase C and mitogen activated protein kinase (26).
The above considerations suggest that reducing the formation of glycated LDL could decrease LDL uptake by alternative pathways, improve regulation of cholesterol synthesis and decrease oxidative stress. The present study probed this hypothesis by evaluating the in vitro and in vivo effects of 2,2-chlorophenylaminophenylacetate (CAP22), a small molecule that is structurally related to the nonsteroidal anti-inflammatory agent diclofenac but lacks the 2, 6-dichloro substitution responsible for inhibition of cyclo-oxygenase (COX) enzymes (32,33). However, CAP22 shares the anionic nature and diphenylamino structure of diclofenac, which has been shown to saturably bind to the hydrophobic core of LDL (34), suggesting that this property could affect Amadori-glucose modification of LDL and thereby provide an opportunity to explore physiologic consequences of reducing the formation of glycated LDL. We report that CAP22 prevents the accelerated formation of glycated products in vitro in LDL incubated with high glucose concentration and the increased cholesterol ester synthesis in THP-1 macrophages that is induced by lipoprotein preincubated with high glucose. Chronic administration of CAP22 to streptozotocin-diabetic rats decreased circulating glycated lipoprotein and serum cholesterol concentrations, and reduced aortic levels of lipid peroxidation products.
METHODS
In Vitro Inhibition of Nonenzymatic Glycation
ApoB-containing lipoproteins prepared from human plasma by dextran sulfate/CaCl2 precipitation (35,36) were incubated under nitrogen for 10 days at 25°C in phosphate buffered saline (PBS), pH 7.4, containing 5 mM EDTA, without or with 200 mM glucose and in the presence of absence of test compound (2:1, 4:1 and 8:1 molar ratio). For determination of the amount of glycated product, samples were delipidated and subjected to affinity chromatography on phenylboronate agarose (Helena Labs, Beaumont, TX) to separate the glycated from the nonglycated species. After addition of BSA (5:1 ratio to apoB) to ensure apoB solubility (33), the samples were applied to the columns in loading buffer (0.1M glycine, .05M MgCl2), eluting the bound (glycated) fraction with 200 mM sorbitol in 0.1M glycine, desalting on Amicon Ultra-4 centrifuge tubes (Millipore, Bellerica, MA) and measuring protein content (BioRad, Hercules, CA) in the adsorbed fraction. In the absence of apoB, application of BSA to the affinity column showed virtually no adsorption, confirming no methodologic interference. For use in cell culture, ApoB-containing lipoproteins were incubated for 10 days under nitrogen in 0.15M NaCl/5 mM EDTA under four conditions: without glucose in the absence (A) or presence (B) of test compound (8:1 molar ratio), and with 200 mM glucose in the absence (C) or presence (D) of test compound at the same molar ratio. The preincubated lipoprotein preparations were desalted by exchange into PBS, pH 7.4, containing 5 mM EDTA before use in cell cultures.
Cell Culture
THP-1 acute monocytic leukemia cells (ATTC #T1B-202) were seeded into 24 well plastic plates (5×105 cells/well) in media containing 10% FBS, RPMI 1640 (Mediatech, Manassas, VA), 4.5 g/dL glucose, 100 mM sodium pyruvate, 0.05 mM β-mercaptoethanol, 200 mM L-glutamine, 10,000 U/ml penicillin, and 10,000 µg/ml streptomycin. Cells were induced to differentiate into macrophages by growing for 72 hours after making the above media 10 mM in phorbol 12-myristate (Sigma-Aldrich, St. Louis, MO) (38), and were fed for 18 hours with RPMI 1640 made 0.5% in lipoprotein deficient FBS and containing the above nutrients before experimental conditions were introduced. This was accomplished by adding fresh media containing 0.5% lipoprotein deficient FBS made 11 mM in glucose, and 0–100 µg/ml of lipoprotein prepared as described above.
Experimental Animals
Diabetes was induced by intravenous injection (50 mg/kg) of streptozotocin (Sigma-Aldrich, St. Louis, MO) into the tail veins of male Wistar rats (Harlan, Indianapolis, IN) aged 6 weeks and weighing between 120 and 140 g. Animals with plasma glucose concentrations ≥ 15 mM within one week after the induction of diabetes were included in the study. Age-, weight- and gender- matched Wistar rats served as nondiabetic controls. The diabetic rats were divided into two groups, one of which received test compound, as the potassium salt, by oral gavage at a total dose of 7.5 mg/kg/day from age 7 weeks through age 33 weeks, with the other group serving as diabetic controls. Commercial rodent chow and water were provided ad libitum and animals were monitored for blood glucose and weight. All diabetic rats received long-acting insulin (Lantos; Aventis, Bridgewater, NJ) every other day with adjustment of dosages to prevent ketoacidosis, improve survival, and keep animals on a positive growth curve. Animals were housed in a temperature-controlled facility and the Institutional Care and Use Committee approved the protocol and procedures. Interim and terminal blood samples were obtained, the rats were killed at the conclusion of the experimental period, and the thoracic aorta was harvested, snap frozen, and stored at −80°C until analysis.
Analytic Procedures
Glucose was measured by the glucose oxidase method. Lipid peroxide products were determined by the thiobarbituric acid assay using, as standard, the formation of malondialdehyde from 1,1,3,3-tetramethoxypropane. Aortic samples were prepared for lipid peroxide measurement by rinsing with 0.9M NaCl, grinding in a glass homogenizer and briefly sonicating, with recovery of the solubilized tissue after centrifugation. Cholesterol was measured with commercial kits according to the manufacturer’s instructions (BioVision Research Products, Mountain View, CA). For determination of glycated lipoproteins in rat serum, samples were precipitated with 10% dextran sulfate containing 0.05M CaCl2, the precipitate was collected by centrifugation, washed twice with 0.2M CaCl2 and dissolved in 5% NaCl containing 0.5% EDTA (35), following which 0.9M oxalic acid (v:v 1:2) was added and the samples were incubated at 85°C for 20 hours. After cooling, samples were made 10% in trichloroacetic acid and the supernatants were assayed for glycated products by reaction with thiobarbituric acid to yield the 5-hydroxymethylfurfuraldehyde chromogen that is generated from dehydration of glucose by boiling in oxalic acid (39), calculating concentrations from the standard curve obtained with hydroxymethylfurfuraldehyde (Sigma).
For measurement of cellular cholesterol and cholesteryl esters, cells were harvested at the end of the experimental period by scraping and transferring into tubes for centrifugation at 1000 rpm for 10 minutes. The liquid was carefully removed without disturbing the cell pellet, which was resuspended into 400 µL of chloroform:isopropanol:TritonX-100 (7:11:0.1). The solvent containing lysed cells was centrifuged at 14000 rpm for 10 minutes, and the chloroform phase was collected and air-dried at 50°C. The dried lipids were dissolved in reaction buffer provided with the assay kits for measurement of cholesterol and cholesteryl esters. Results were normalized to cell protein to correct for differences in cell number or recovery.
Statistical Analysis
Differences under different experimental conditions were analyzed by means of analysis of variance (ANOVA).
RESULTS
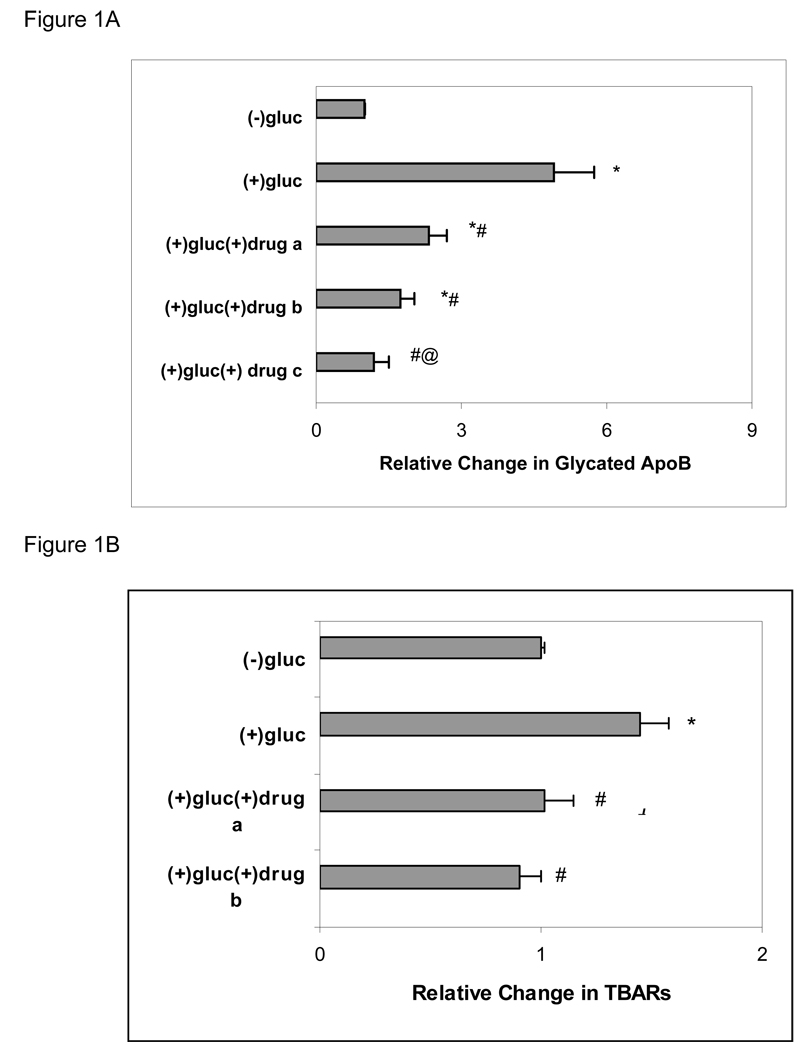
Incubation of human apoB-containing lipoprotein with 200 mM glucose for 10 days resulted in an expected increase in glycation of apoB compared to control incubations without added glucose for the same period under the same conditions. Inclusion of test compound in high glucose incubations reduced the amount of glycated product formed, with dose-responsive decreases in the relative proportion of glycated to nonglycated species (Figure 1A). Lipid peroxide products also were increased after incubation with high compared to low glucose and were similarly reduced when drug was included in the high glucose incubations (Figure 1B).
Figure 1. Effect of CAP22 on Lipoprotein Glycation and Peroxidation.
A. Human ApoB containing lipoproteins prepared by dextran sulfate precipitation were incubated in 0 or 200 mM glucose without or with test compound as described in Methods. Results were calculated as µg/mg apoB and represent mean ± SEM of 4 experiments under each condition. The amount of glycated apoB with no added glucose was assigned an arbitrary value of 1. a, b, c represent molar ratios of drug to ApoB of 2:1, 4:1 and 8:1, respectively. *p ≤ 0.05 compared to (−) glucose; #p ≤ 0.05 compared to (+) glucose; @p = .05 compared to (+) glucose (+) drug a.
B. Lipid peroxidation in human apoB-containing lipoprotein incubated in 0 or 200 mM glucose without or with test compound as described in Methods. Results calculated as nm TBARs/mg apoB, with 0 glucose assigned an arbitrary value of 1, and represent mean ± SEM of 4 experiments under each condition. *p = 0.05 compared to (−) glucose; #p = 0.05 compared to (+) glucose.
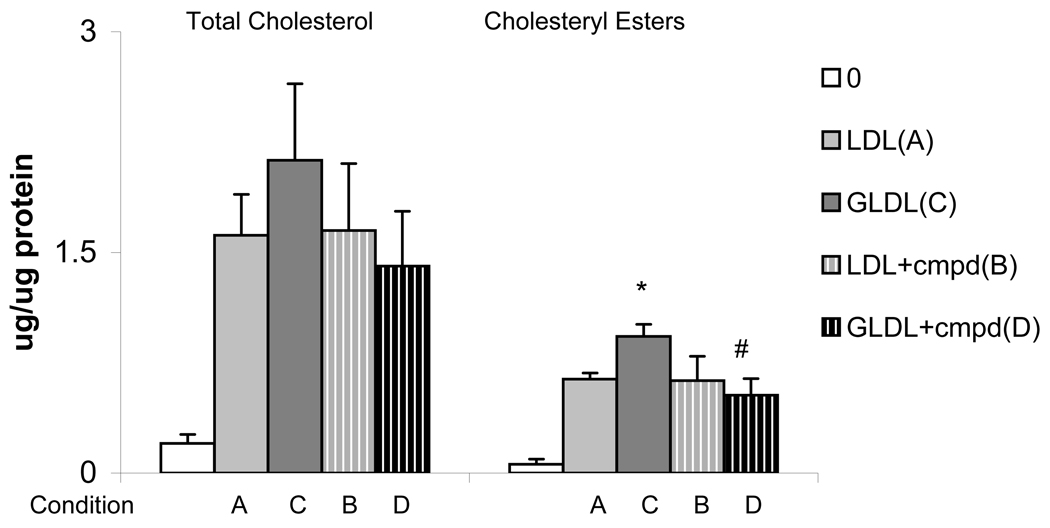
Macrophages exposed to lipoprotein preincubated with high glucose (condition C) showed a significant increase in cholesteryl esters compared to cells exposed to control lipoprotein (condition A or B), without change in total cholesterol content (Figure 2). In contrast, cholesteryl esters in cells exposed to lipoprotein preincubated with high glucose in the presence of test compound (condition D) did not differ from control (Figure 2). Macrophages incubated without added lipoprotein showed negligible amounts of cholesteryl esters, indicating that the measured cholesteryl esters represented new synthesis.
Figure 2. Macrophage Cholesteryl Esters.
THP-1 macrophages were incubated for 3 hours with human apoB-containing lipoproteins that had been preincubated without (condition A, B) or with (condition C, D) 200 mM glucose in the absence (condition A, C) or presence (condition B, D) of test compound (8:1 molar ratio). Results are mean ± SEM of 3 experiments. *p ≤ 0.05 compared to condition A and C; #p ≤ 0.05 compared to condition B.
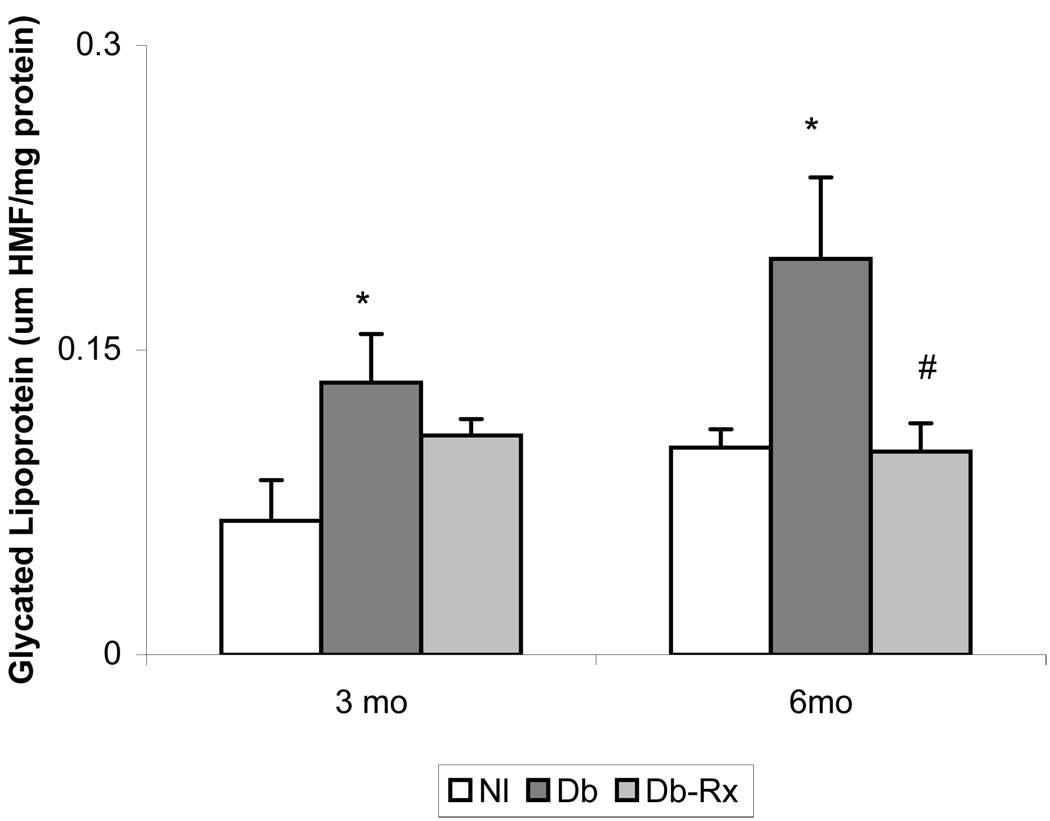
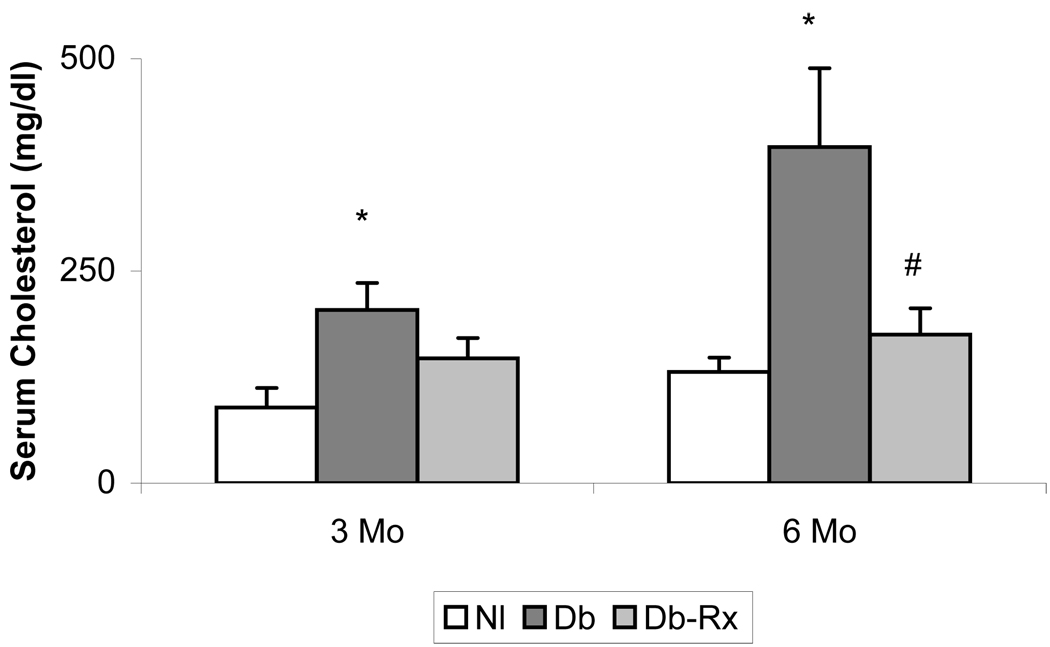
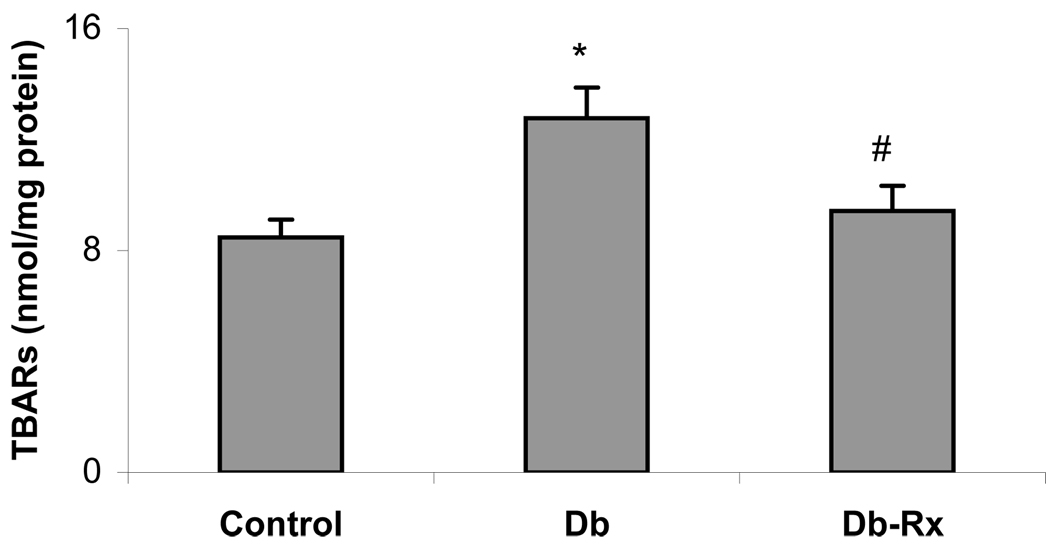
General characteristics of the experimental animals at the conclusion of the study period are shown in Table 1. Diabetic rats weighed significantly less than nondiabetic controls, but body weights and blood glucose levels did not differ in diabetic controls compared with diabetic rats receiving test compound. The growth curves and glucose levels in the animals in this study were similar to those reported by others using streptozotocin diabetic rats receiving insulin without or with co-administration of an agent that does not affect hyperglycemia (40). Serum concentrations of glycated lipoprotein were significantly higher in diabetic compared to nondiabetic controls, and were significantly reduced in diabetic rats receiving test compound, consistent with an effect on nonenzymatic glycation despite prevailing hyperglycemia (Figure 3). Serum cholesterol concentrations were about 3-fold higher in diabetic than in nondiabetic rats, but were not significantly different from nondiabetic controls in diabetic rats treated with test compound (Figure 4). Levels of lipid peroxidation products in aortas of diabetic rats were significantly higher than in nondiabetic controls, and were significantly less in diabetic animals receiving test compound than in the diabetic controls (Figure 5).
Table 1.
Experimental Animal Data
| Nondiabetic (n = 8) |
Diabetic Control (n = 10) |
Diabetic-CAP22 (n = 11) |
|
|---|---|---|---|
| Body Wt. (g) | |||
| Interim | 510 ± 24 | 330 ± 7* | 346 ± 11* |
| Final | 584 ± 17 | 366 ± 6* | 394 ± 12* |
| Blood glucose (mM) | |||
| Interim | 6.9 ± 0.9 | 27.6 ± 1.0* | 24.7 ± 0.8* |
| Final | 7.0 ± 0.9 | 24.7 ± 0.6* | 26.9 ± 1.2* |
p < 0.05 compared to nondiabetic
Interim values ≈ week 17 of protocol
Figure 3. Glycated Lipoprotein in Rat Serum.
Serum lipoproteins in interim and terminal samples were precipitated with dextran sulfate followed by determination of hydroxymethylfurfuraldehyde chromogen content as described in text. *p ≤ 0.05 compound to nondiabetic; #p ≤ 0.05 compared to diabetic control.
Figure 4. Rat Serum Cholesterol Concentrations.
Samples collected at midpoint and conclusion of experimental period. Results are mean ± SEM from animals described in Table 1. *p ≤ .05 compared to normal; #p ≤ .05 compared to diabetic control.
Figure 5. Aortic Lipid Peroxidation Products.
Tissue collected at conclusion of experimental period. Results are mean ± SEM from animals described in Table 1. *p ≤ .05 compared to normal; #p = .05 compared to diabetic control.
DISCUSSION
The results of this study indicate that glycated lipoproteins stimulate cholesteryl ester synthesis in macrophages and that increased levels of glycated apoB-containing lipoproteins profoundly influence serum cholesterol levels and enhance the propensity for lipid peroxidation in the aorta of streptozotocin diabetic rats. The test compound was shown to inhibit the nonenzymatic glycation of LDL in vitro by reducing the formation of Amadori-glucose adducts in human apoB-containing lipoproteins that were exposed to high glucose concentrations and in vivo by decreasing plasma levels of glycated lipoprotein in the face of persistent hyperglycemia in diabetic rats. The finding that lowering the elevated levels of glycated lipoprotein in streptozotocin diabetic rats was accompanied by a reduction in serum cholesterol and in aortic lipid peroxidation, a proathogenic accelerator (28–31,41,42), is interpreted to reflect improved cholesterol homeostasis consequent to decreased stimulation of cholesteryl ester synthesis and decreased oxidative stress resulting from lessening the burden of glycated LDL . The ability of CAP22 to inhibit LDL glycation is presumed to relate to lipoprotein binding properties analogous to those described for diclofenac (34), which interacts with several classes of binding sites in the lipoprotein core protein, although other mechanisms cannot be excluded. Since glycation of apoB occurs at reactive lysine-lysine or lys-x-lys sequences in proximity to a catalytic proton donor/acceptor group (4,43), one explanation for the observed inhibition of nonenzymatic glycation is that structural consequences of drug binding impede the ability of lysine amino groups to undergo the Amadori rearrangement necessary for the formation of the stable glucose adduct. Appropriately located positively charged amino acid residues promote local acid base catalysis that fosters stabilization following Schiff base condensation of glucose with protein (44), suggesting that drug binding compromises local closeness of charged amino groups that act catalytically in the Amadori rearrangement at lysine residues susceptible to nonenzymatic reaction with glucose. Direct interaction of the anionic side chain in CAP22 with potentially glycatable lysine amino groups is also possible, analogous to the direct inhibition of glycation consequent to binding of acetylsalicylic acid to albumin (45,46).
ApoB is the protein determinant for cellular recognition of LDL by the LDL receptor, which promotes internalization, catabolism and clearance of LDL from the plasma, and regulates cellular cholesterol biosynthesis. Impaired recognition of glycated apoB by the classical LDL receptor, enhanced uptake by cells expressing alternative receptors, and faulty regulation of cellular cholesterol homeostasis favor cholesterol accumulation within the cells and compromise plasma clearance. Small dense LDL, which associate with coronary disease, have been reported to undergo more glycation than other LDL subfractions, even in nondiabetic subjects, and to be more susceptible to glycation in vivo when exposed to high glucose concentration (47,48). This aspect was not examined in the present study, nor was the effect of test compound on HDL glycation, but it is worth noting that nonenzymatic glycation of HDL, which is also increased in the diabetic milieu, increases the transfer of cholesteryl esters to apoB-containing lipoproteins, exaggerating cholesterol transport abnormalities contributory to hypercholesterolemia (36).
In summary, we report that inhibiting the nonenzymatic glycation of apoB-containing lipoproteins in vitro prevents increased cholesteryl ester synthesis observed in macrophages exposed to glycated LDL, and reduces circulating levels of glycated lipoprotein, serum cholesterol and vascular lipid peroxidation in streptozotocin diabetic rats. These findings encourage clinical studies of CAP22 to evaluate its potential for reducing atherogenic risk in human diabetes.
Acknowledgements
Supported in part by Grant No. 72587 from the National Institutes of Health and by Glycadia, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: The authors are employed by Glycadia, a funding source for this study, and are inventors on a relevant patent application. Dr. Cohen is a stockholder in Glycadia.
Institutional approval: The Institutional Care and Use Committee approved the protocol and procedures for this research on experimental animals.
REFERENCES
- 1.Lyons TJ, Baynes JW, Patrick JS, Colwell JA, Lopez-Virella MF. Glycosylation of low-density lipoproteins in patients with type 1 (insulin-dependent) diabetes: Correlation with other parameters of glycemic control. Diabetologia. 1986;29:685–689. doi: 10.1007/BF00870276. [DOI] [PubMed] [Google Scholar]
- 2.Jack CM, Sheridan B, Kennedy L, Stout RW. Nonenzymatic glycosylation of low-density lipoproteins. Results of an affinity chromatography method. Diabetologia. 1988;31:126–128. doi: 10.1007/BF00395561. [DOI] [PubMed] [Google Scholar]
- 3.Tames FJ, Mackness MI, Anol S, Liang I, Durrington PN. Nonenzymatic glycation of apolipoprotein B in the sera of diabetic and nondiabetic subjects. Atherosclerosis. 1972;93:237–244. doi: 10.1016/0021-9150(92)90260-n. [DOI] [PubMed] [Google Scholar]
- 4.Curtiss LK, Witztum JL. Plasma apolipoprotein A1, A11, B, Cl and E are glycosylated in hyperglycemic diabetic subjects. Diabetes. 1985;34:452–461. doi: 10.2337/diab.34.5.452. [DOI] [PubMed] [Google Scholar]
- 5.Cohen MP, Lautenslager GT, Shea E. Glycated LDL concentrations in non-diabetic and diabetic subjects measured with monoclonal antibodies reactive with glycated apolipoprotein B epitopes. Eur. J. Clin. Chem. Clin. Biochem. 1993;31:707–713. doi: 10.1515/cclm.1993.31.11.707. [DOI] [PubMed] [Google Scholar]
- 6.Edwards IJ, Wagner JD, Litwak KN, Rudel LL, Cefalu WT. Glycation of plasma low density lipoproteins increases interaction with arterial proteoglycans. Diabetes Res. Clin. Pract. 1999;46:9–18. doi: 10.1016/s0168-8227(99)00074-1. [DOI] [PubMed] [Google Scholar]
- 7.Cohen MP, Jin Y, Lautenslager GT. Increased plasma glycated low-density lipoprotein concentrations in diabetes: A marker of atherogenic risk. Diabetes Technol. Therapeut. 2004;6:348–356. doi: 10.1089/152091504774198043. [DOI] [PubMed] [Google Scholar]
- 8.Misciagna G, Logroscino G, DeMichele G, et al. Glycated apoliproptein B and myocardial infarction. Nutr. Metab. & Cardiovasc. Dis. 2007;17:6–12. doi: 10.1016/j.numecd.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Lopez-Virella MF, Sherer GK, Lees AM, et al. Surface binding, internalization and degradation by cultured human fibroblasts of low-density lipoproteins from type 1 (insulin dependent) diabetic patients: Changes with metabolic control. Diabetologia. 1982;22:430–436. doi: 10.1007/BF00282585. [DOI] [PubMed] [Google Scholar]
- 10.Sasaki J, Cottam GL. Glycosylation of LDL decreases its ability to interact with high affinity receptors of human fibroblasts in vitro and decreases its clearance from rabbit plasma in vivo. Biochim. Biophys. Acta. 1982;713:119–207. doi: 10.1016/0005-2760(82)90237-5. [DOI] [PubMed] [Google Scholar]
- 11.Klein RL, Laimins M, Lopez-Virella MF. Isolation, characterization and metabolism of the glycated and nonglycated subfractions of low-density lipoproteins isolated from type 1 diabetic patients and nondiabetic subjects. Diabetes. 1995;44:1093–1098. doi: 10.2337/diab.44.9.1093. [DOI] [PubMed] [Google Scholar]
- 12.Witztum JL, Mahoney EM, Branks MJ, Fisher M, Elam R, Steinberg D. Nonenzymatic glycosylation of low-density lipoproteins alters its biologic activity. Diabetes. 1982;31:283–291. doi: 10.2337/diab.31.4.283. [DOI] [PubMed] [Google Scholar]
- 13.Lorenzi M, Cagliero E, Markey B. Interaction of human endothelial cells with elevated glucose concentrations and native and glycosylated low-density lipoproteins. Diabetologia. 1984;26:218–222. doi: 10.1007/BF00252411. [DOI] [PubMed] [Google Scholar]
- 14.Steinbrecher UP, Witztum JL. Glucosylation of low-density lipoproteins to an extent comparable to that seen in diabetes slows their catabolism. Diabetes. 33:130–134. doi: 10.2337/diab.33.2.130. [DOI] [PubMed] [Google Scholar]
- 15.Klein RL, Lyons TS, Lopez-Virella MF. Interaction of very low-density lipoproteins isolated from type 1 (insulin dependent) diabetic subjects with human monocyte derived macrophages. Metabolism. 1989;38:1108–1113. doi: 10.1016/0026-0495(89)90048-6. [DOI] [PubMed] [Google Scholar]
- 16.Lopez-Virella MF, Klein RL, Lyons TJ, Stevenson HC, Witztum JL. Glycation of low-density lipoproteins enhances cholesteryl ester synthesis in human monocyte-derived macrophages. Diabetes. 1988;37:550–557. doi: 10.2337/diab.37.5.550. [DOI] [PubMed] [Google Scholar]
- 17.Brown MS, Goldstein JL. Scavenging for receptors. Nature (Lond.) 1990;343:508–509. doi: 10.1038/343508a0. [DOI] [PubMed] [Google Scholar]
- 18.Makita T, Tanaka A, Nakano T, Nakajima K, Numano F. Importance of glycation in the acceleration of low-density lipoprotein (LDL) uptake into macrophages in patients with diabetes mellitus. Int. Angiol. 1999;18:149–153. [PubMed] [Google Scholar]
- 19.Millican SA, Schultz D, Bagga M, Coussons PJ, Muller K, Hunt JV. Glucose-modified low-density lipoprotein enhances human monocyte chemotaxis. Free Rad. Res. 1998;28:533–542. doi: 10.3109/10715769809066890. [DOI] [PubMed] [Google Scholar]
- 20.Zoltowska M, Delvin E, Ziv E, Peretti N, Chartre M, Levy E. Impact of in vivo LDL glycation on platelet aggregation and monocyte chemotaxis in psammomys obesus. Lipids. 2004;39:81–85. doi: 10.1007/s11745-004-1205-7. [DOI] [PubMed] [Google Scholar]
- 21.Makita T, Tanaka A, Numano F. Effect of glycated low-density lipoprotein on smooth muscle cell proliferation. Int. Angiol. 1999;18:3314. [PubMed] [Google Scholar]
- 22.Taguchi S, Oinuma T, Yamada T. A Comparative study of cultured smooth muscle cell proliferation and injury, utilizing glycated low-density lipoproteins with slight oxidation, auto-oxidation, or extensive oxidation. J. Atheroscler. Thromb. 2000;3:132–137. doi: 10.5551/jat1994.7.132. [DOI] [PubMed] [Google Scholar]
- 23.Velarde V, Jenkins AJ, Christopher J, Lyons TJ, Jaffa AA. Activation of MAPK by modified low-density lipoproteins in vascular smooth muscle cells. J. Appl. Physiol. 2000;91:1412–1420. doi: 10.1152/jappl.2001.91.3.1412. [DOI] [PubMed] [Google Scholar]
- 24.Brizzi MF, Dentelli P, Gambino R, et al. STAT5 activation induced by diabetic LDL depends on LDL glycation and occurs via src kinase activity. Diabetes. 2002;51:3311–3317. doi: 10.2337/diabetes.51.11.3311. [DOI] [PubMed] [Google Scholar]
- 25.Sonoki K, Yoshinari M, Iwase M, et al. Glycoxidized low-density lipoprotein enhances monocyte chemoattractant protein-1 mRNA expression in human endothelial cells: Relation to lysophosphatidlycholine content and inhibition by nitric oxide donor. Metabolism. 2002;51:1135–1142. doi: 10.1053/meta.2002.34703. [DOI] [PubMed] [Google Scholar]
- 26.Cho HM, Choi SH, Hwang KC, et al. The Src/PLC/PKC/MEK/ERK signaling pathway is involved in aortic smooth muscle cell proliferation induced by glycated LDL. Mol. Cells. 2005;19:60–66. [PubMed] [Google Scholar]
- 27.Gillery P, Monboisse JC, Marquart FX, Borel JP. Glycation of proteins as a source of superoxide. Diabete Metabolisme. 1988;14:25–40. [PubMed] [Google Scholar]
- 28.Mullarkey CJ, Edelstein D, Brownlee M. Free radical generation by the early glycation products: A mechanism for accelerated atherogenesis in diabetes. Biochem. Biophys. Res. Comm. 1990;173:932–939. doi: 10.1016/s0006-291x(05)80875-7. [DOI] [PubMed] [Google Scholar]
- 29.Moro E, Alessandrini P, Zambon C, et al. Is glycation of low-density lipoproteins in patients with type 2 diabetes mellitus a LDL pre-oxidative condition? Diabetic Med. 1999;16:663–669. doi: 10.1046/j.1464-5491.1999.00136.x. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi K, Watanabe J, Umeda F, Nawata H. Glycation accelerates the oxidation of low-density lipoprotein by copper ions. Endocrinol. J. 1995;42:461–465. doi: 10.1507/endocrj.42.461. [DOI] [PubMed] [Google Scholar]
- 31.Bowie A, Owens D, Collins P, Johnson A, Tomkin GH. Glycosylated low-density lipoprotein is more sensitive to oxidation: Implications for diabetic patients. Atherosclerosis. 1993;102:63–67. doi: 10.1016/0021-9150(93)90084-8. [DOI] [PubMed] [Google Scholar]
- 32.Moser P, Sallman P, Weisenberg I. Synthesis and quantitative structure-activity relationships of diclofenac. J. Med. Chem. 1990;33:2358–2368. doi: 10.1021/jm00171a008. [DOI] [PubMed] [Google Scholar]
- 33.Cohen MP, Hud E, Shea E, Shearman CW. Normalizing glycated albumin reduces increased urinary collagen IV and prevents renal insufficiency in diabetic db/db mice. Metabolism. 2002;51:901–905. doi: 10.1053/meta.2002.33359. [DOI] [PubMed] [Google Scholar]
- 34.Chamouard JM, Barre J, Urien S, Houin G, Tillement J-P. Diclofenac binding to albumin and lipoproteins in human serum. Biochem. Pharmacol. 1985;34:1695–1700. doi: 10.1016/0006-2952(85)90636-7. [DOI] [PubMed] [Google Scholar]
- 35.Cornwell DG, Kruger FA. Molecular complexes in the isolation and characterization of plasma lipoproteins. J. Lipid Res. 1961;2:110–134. [PubMed] [Google Scholar]
- 36.Passarelli M, Catanozi S, Nakandakare ER, et al. Plasma lipoproteins from patients with poorly controlled diabetes mellitus and “in vitro” glycation of lipoproteins enhance the transfer rate of cholesteryl ester from HDL to apo-β-containing lipoproteins. Diabetologia. 1997;40:1085–1093. doi: 10.1007/s001250050791. [DOI] [PubMed] [Google Scholar]
- 37.Shireman RB, Fisher WR. Apoliprotein B: its role in the control of fibroblast cholesterol biosynthesis and in the regulation of its own binding to cellular receptors. J. Lipid Res. 1979;20:594–598. [PubMed] [Google Scholar]
- 38.Wilson ME, Sonzoku T, Alfred KF. Estrogen prevents cholesteryl ester accumulation in macrophages induced by the protease inhibitor retonavir. J. Cell. Biochem. 2008;103:1598–1606. doi: 10.1002/jcb.21546. [DOI] [PubMed] [Google Scholar]
- 39.Fluckiger R, Gallop P. Measurement of nonenzymatic protein glycation. Meth. Enzymol. 1984;106:77–87. doi: 10.1016/0076-6879(84)06009-2. [DOI] [PubMed] [Google Scholar]
- 40.Thallas-Bonke V, Thorpe SR, Coughlan MT, et al. Inhibition of NADPH oxidase prevents advanced glycation end product-mediated damage in diabetic nephropathy through a protein kinase Co-α-dependent pathway. Diabetes. 2008;57:460–469. doi: 10.2337/db07-1119. [DOI] [PubMed] [Google Scholar]
- 41.Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL. Beyond cholesterol. Modifications of low-density lipoproteins that increase its atherogenicity. New Engl. J. Med. 1989;320:915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- 42.Esterbauer H, Dieber-Rothender M, Waeg G, Striegl G, Jurgens C. Biochemical structural and functional properties of oxidized low-density lipoproteins. Chem. Res. Toxicol. 1990;3:77–92. doi: 10.1021/tx00014a001. [DOI] [PubMed] [Google Scholar]
- 43.Fluckiger R. Glycosylation of lipoproteins: Chemistry and biological implications. Monogr. Atheroscl. 1985;13:53–62. [PubMed] [Google Scholar]
- 44.Iberg N, Fluckiger R. Nonenzymatic glycosylation of albumin in vivo. Identification of multiple glycosylated sites. J. Biol. Chem. 1986;12:13542–13545. [PubMed] [Google Scholar]
- 45.Walker JE. Lysine residue 199 of human serum albumin is modified by acetylsalicylic acid. F.E.B.S. Lett. 1976;66:173–175. doi: 10.1016/0014-5793(76)80496-6. [DOI] [PubMed] [Google Scholar]
- 46.Nakajou K, Watanabe H, Kragh-Hansen U, Maruyama T, Otagiri M. The effect of glycation on the structure, function and biological fate of human serum albumin as revealed by recombinant mutants. Biochim. Biophys. Acta. 2003;1623:88–97. doi: 10.1016/j.bbagen.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 47.Youni N, Charlton-Menys V, Sharma R, Soran H, Durrington PN. Glycation of LDL in nondiabetic people: Small dense LDL is preferentially glycated both in vivo and in vitro. Atherosclerosis. 2009;202:162–168. doi: 10.1016/j.atherosclerosis.2008.04.036. [DOI] [PubMed] [Google Scholar]
- 48.Younis N, Sharma R, Soran H, Charlton-Menys V, Elseweidy M, Durrington PN. Glycation as an atherogenic modification of LDL. Curr. Opin. Lipidol. 2008;19:378–384. doi: 10.1097/MOL.0b013e328306a057. [DOI] [PubMed] [Google Scholar]