Abstract
Set2-mediated H3 Lys36 methylation is a histone modification that has been demonstrated to function in transcriptional elongation by recruiting the Rpd3S histone deacetylase complex to repress intragenic cryptic transcription. Recently, we identified a trans-histone pathway in which the interaction between the N terminus of Set2 and histone H4 Lys44 is needed to mediate trans-histone H3 Lys36 di- and trimethylation. In the current study, we demonstrate that mutation of the lysine 44 residue in histone H4 or the Set2 mutant lacking the histone H4 interaction motif leads to intragenic cryptic transcripts, indicating that the Set2 and histone H4 interaction is important to repress intragenic cryptic transcription. We also determine that histone H2A residues (Leu116 and Leu117), which are in close proximity to histone H4 Lys44, are needed for proper trans-histone H3 Lys36 methylation. Similar to H4 Lys44 mutants, histone H2A Leu116 and Leu117 mutations exhibited decreased H3 Lys36 di- and trimethylation, increased histone H4 acetylation, increased resistance to 6-azauracil, and cryptic transcription. Interestingly, the combined histone H4 Lys44 and H2A mutations have more severe methylation defects and increased H4 acetylation levels. Furthermore, we identify that additional histone H2A and H3 core residues are also needed for H3 Lys36 di- and trimethylation. Overall, our results show and suggest that multiple H4, H2A, and H3 residues contribute to and form a Set2 docking/recognition site on the nucleosomal surface so that proper Set2-mediated H3 Lys36 di- and trimethylation, histone acetylation, and transcriptional elongation can occur.
Keywords: Chromatin, Chromatin Structure, Histone Methylation, Histone Modification, Histones, Histone Methyltransferase, Nucleosome, trans-Histone Pathway
Introduction
Histones are subject to an array of post-translational modifications, including methylation, acetylation, phosphorylation, and ubiquitination (1, 2). These covalent histone modifications function to modulate gene expression and other DNA templated processes by altering chromatin structure and/or recruiting additional effector proteins (3–5). In addition, it is believed that aberrant levels or patterns of histone modifications can alter gene expression profiles, leading to improper cell growth and/or differentiation (6).
In Saccharomyces cerevisiae, histone H3 Lys36 methylation, catalyzed by the Set2 methyltransferase, is needed for proper transcription elongation. For example, a yeast strain lacking Set2 exhibits increased resistance to 6-azauracil (6-AU),2 a drug widely used as an indicator for transcription elongation defects (7–9). Furthermore, several studies have indicated that strains carrying histone H3 Lys36 mutations all exhibited increased resistance to 6-AU, suggesting that the 6-AU resistance phenotype is caused by the inability of Set2 to methylate histone H3 Lys36 (7, 10, 11). These findings suggest that Set2-mediated histone H3 Lys36 methylation plays a pivotal role in maintaining transcription elongation.
Set2-mediated H3 Lys36 methylation is also needed so that intragenic cryptic transcription does not aberrantly occur (12, 13). The mechanism for Set2-mediated H3 Lys36 methylation in inhibiting intragenic cryptic transcription has been determined where H3 Lys36 methylation recruits the Rpd3S histone deacetylase complex by its Eaf3 and Rco1 subunits (14–16). The recruitment of Rpd3S prevents the increase in acetylation of histones H3 and H4 within the coding regions of genes so that transcription does not occur within the body of a gene (13, 16–19).
Recently, a trans-histone pathway was discovered involving Set2-mediated H3 Lys36 methylation (10). This study revealed that interaction between the N terminus of Set2 and histone H4 is needed for histone H3 Lys36 di- and trimethylation. A conserved lysine residue, Lys44, in histone H4 was identified to be important for interacting with the histone H4 interaction motif of Set2 (10). Deletion of this interaction motif resulted in the defects of H3 Lys36 di- and trimethylation, increased histone acetylation, and a 6-AU resistance phenotype (10). However, it has not been determined whether this trans-histone pathway was essential to repress cryptic transcription. In addition, we wanted to determine whether other cis- or trans-histone determinants help mediate histone H3 Lys36 methylation.
In this study, we show that the interaction between histone H4 and Set2 that is essential to mediate trans-histone H3 Lys36 di- and trimethylation is also necessary to repress intragenic cryptic transcription in vivo. More importantly, we determine that leucine 116 and leucine 117 residues in the C terminus of H2A, which are in close proximity to histone H4 Lys44, are required for trans-histone H3 Lys36 di- and trimethylation. Furthermore, both histone H4 and H2A mutants have increases in histone H4 acetylation, resistance to 6-AU, and intragenic cryptic transcription. In addition, the combined histone H4 Lys44 and H2A mutations show further decreases in histone H3 Lys36 di- and trimethylation and increases in histone H4 acetylation levels relative to single mutations. Interestingly, mutagenic analysis based on histone residues located within five angstroms of histone H4 Lys44 revealed that additional residues within the histone core of H2A and H3 are also needed for proper H3 Lys36 di- and trimethylation. Overall, our data suggest that histone residues from H4, H2A, and H3 likely contribute to and form a Set2 docking/recognition site on the nucleosome that is needed to maintain proper histone H3 Lys36 di- and trimethylation, histone acetylation levels, and transcription elongation.
EXPERIMENTAL PROCEDURES
Construction of Plasmids and Yeast Strains
Yeast strains used in this work are listed in supplemental Table S1. The set2Δ, ppr2Δ, and wild type (WT) strains of BY4741 were obtained from Open Biosystems. The SDBY1155 strain was generated using PCR amplification of the KanMX cassette from the BY4741 set2Δ strain as described previously (20). Histone H3 and H4 mutants in a CEN, TRP-based plasmid (pJH18) were described previously (21). Histone H3 mutant plasmids were generated by site-directed mutagenesis (Stratagene) using pJH18 as a template. The pHND20 construct was generated by cloning the wild type HHT2-HHF2 alleles cut from pJH18 vector by SalI and SpeI restriction sites into a CEN, LEU-based plasmid (pRS415). The pHND21 and pHND22 constructs were generated by site-directed mutagenesis (Stratagene) using the pHND20 as a template. Histone H2A mutant plasmids were generated in a CEN, HIS-based plasmid (pJH23) by site-directed mutagenesis. All of these histone constructs were confirmed by sequencing through the coding region of histone H3, H4, or H2A and are listed in supplemental Table S2. The Set2 constructs were generated as described previously (10). All of the Set2 constructs were engineered with a single HA epitope at the C terminus and are listed in supplemental Table S3.
RNA Extraction and Northern Blot Analysis
RNA extraction and Northern blot analyses were performed as described previously (13). Briefly, yeast cells were grown to mid-log phase. Total RNA were prepared by glass bead disruption and phenol extraction. RNA samples were quantified by spectrometer, and equal amounts of RNA samples were resolved on 1% agarose-formaldehyde gels running in MOPS buffer and transferred to Immobilon Nylon+ membrane (Millipore). RNA was cross-linked to the membrane by UV irradiation (Stratalinker 2400 UV cross-linker, Stratagene) and dried at 50 °C for 2 h. The membranes were prehybridized in hybridization buffer at 60 °C for 4 h. Probes (STE11 3′ nucleotides 1643–2154; SET11 5′ nucleotides 1–588; FLO8 full-length nucleotides 1–2400; and SCR1 full-length nucleotides 1–517) were PCR-amplified and labeled by random oligonucleotide priming to generate radioactive probes. The labeled probes were purified by G-25 resin column and then added into hybridization buffer to hybridize to the membrane at 60 °C overnight. After washing the membranes with 1× SSC-0.1% SDS buffer at 60 °C twice (20 min each) and at room temperature three times (5 min each), the membranes were exposed to autoradiography x-ray film or by phosphorimaging.
Yeast Extraction and Immunoblot Analysis
Yeast whole cell extracts were prepared as described previously (22). SDS-PAGE and Western blot analyses were also performed as described previously (22). Primary antibodies were used as described previously (23). The α-H3 Lys36 dimethyl-specific antibody (07-274) was obtained from Millipore and was used at a 1:1000 dilution.
GST Binding Assay
GST fusion protein GST-H2A107–131 or GST-H424–50 was expressed in Escherichia coli and purified by glutathione agarose beads. In vitro binding assays were performed as described previously (10, 23).
6-AU Growth Assay
Yeast strains expressing the histone H2A mutants were transformed with CEN, URA3-based plasmid pRS416, and 6-AU growth assays were performed as described previously (10).
Histone Methyltransferase (HMT) Assay
In vitro HMT assays were performed using yeast chromatin substrates isolated from cells expressing WT histones or histone mutants in the absence or the presence of recombinant purified CBP-Set2 (2 μg), along with 2.0 μCi of S-adenosyl-l-[methyl-3Hy]methionine at 30 °C for 30 min in a total volume of 20 μl. The reaction mixtures were analyzed by liquid scintillation counting. Yeast chromatin substrates were prepared as described previously (10, 23).
Chromatin Immunoprecipitation (ChIP) Analysis
The ChIP assays were performed as described previously using histone H3 (Abcam, ab1791, 1 μl), H3 Lys36 dimethyl (Millipore, 07-274, 2 μl), H3 Lys36 trimethyl (Abcam, ab9050, 1 μl), and H4 acetyl (provided by Dr. David Allis, 1 μl) antibodies (24). Immunoprecipitated DNA was analyzed by quantitative real time PCR (Applied Biosystems) using TaqMan probes (25). Three biological samples with three technical repeats of each were performed. The primers and probes used for ChIP assays are listed in the supplemental information.
RESULTS
The Interaction between Histone H4 and Set2 Is Important to Maintain the Repression of Intragenic Cryptic Transcription
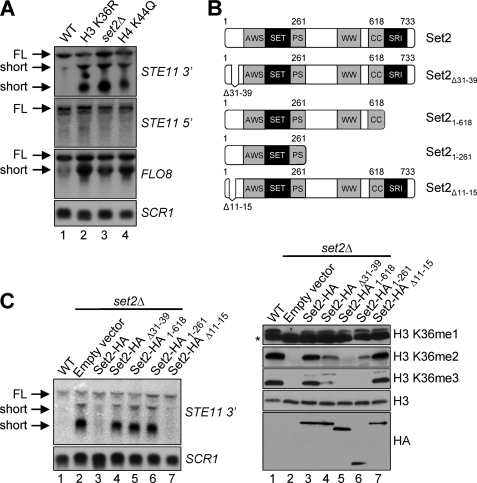
At present, lines of evidence demonstrate that Set2-mediated H3 Lys36 methylation is required for the repression of intragenic cryptic transcription in the 3′ ends of transcribed genes, such as STE11 and FLO8 (13, 26). Our recent studies show that the interaction between Set2 and histone H4 mediates the trans-histone H3 Lys36 di- and trimethylation and is needed for 6-AU resistance. However, whether this interaction between Set2 and histone H4 is needed to maintain proper transcriptional elongation is unknown. To determine this, intragenic cryptic transcription was examined at the STE11 or FLO8 gene by Northern blot analysis using SCR1 as a loading control (Fig. 1, A and C). As expected, intragenic cryptic transcripts or short transcripts are detected in a set2Δ strain or a strain expressing a histone H3 K36R mutant when using 3′ probes to STE11 or FLO8 (Fig. 1A, lanes 2 and 3). However, short transcripts are not detected when using 5′ probes to STE11 (Fig. 1A). These results are consistent with the previous reports in which a loss of H3 Lys36 methylation results in 3′ STE11 and FLO8 intragenic cryptic transcripts (13, 26). More importantly, the strain expressing a histone H4 K44Q mutant, which disrupts the binding with Set2 and histone H3 Lys36 di- and trimethylation, also generates intragenic cryptic transcripts within the 3′ region of FLO8 or STE11, but not at the 5′ region of STE11 (Fig. 1A, lane 4). The strains expressing full-length Set2-HA or Set2-HAΔ11–15, which have no defects in H3 Lys36 methylation, did not exhibit short transcripts (Fig. 1, B and C, lanes 3 and 7). In contrast, we observe that the yeast strain expressing Set2-HAΔ31–39, a mutant lacking the histone H4 interaction motif, or Set2-HA1–618, a mutant lacking the Set2-Rpb1 interacting domain, a domain needed to interact with RNA polymerase II, displays defects in histone H3 Lys36 di- and trimethylation and intragenic cryptic transcription at STE11 (Fig. 1, B and C, lanes 4 and 5). In addition, a set2Δ strain expressing a Set2 mutant containing the first 261 residues of Set2 (Set2-HA1–261), under the control of its endogenous promoter, shows decreased H3 Lys36 dimethylation, undetectable H3 Lys36 trimethylation, and intragenic cryptic transcription (Fig. 1, B and C, lanes 6). Altogether our results indicate that the interaction between Set2 and histone H4, which is needed for trans-histone H3 Lys36 di- and trimethylation, is necessary for suppressing intragenic cryptic transcription.
FIGURE 1.
Histone H4 Lys44 and the histone H4 interaction motif of Set2 are needed to maintain the repression of intragenic cryptic transcription. A, Northern blot analysis of total RNA isolated from WT or the indicated strains. For detection of cryptic transcripts, 3′ STE11 and full-length FLO8 probes are used. The 5′ STE11 probe is used as a control, and a full-length SCR1 probe is used as a loading control. The full-length transcripts (FL) and intragenic cryptic transcripts (short) are indicated. B, upper panel, schematic representation of Set2 constructs used. Lower panel, immunoblots of whole cell extracts prepared from WT cells or set2Δ cells expressing the indicated Set2-HA constructs are probed with H3 Lys36 methyl-specific antibodies. The asterisk denotes a nonspecific band. Histone H3 immunoblots are used as a loading control. The Set2 protein levels are detected by probing against anti-HA antibody. C, Northern blot analysis of total RNA isolated from WT or set2Δ cells expressing the indicated Set2-HA constructs using probes against 3′ region of STE11. SCR1 is used as a loading control.
Histone H2A Residues Leu116 and Leu117 Are Needed for trans-Histone H3 Lys36 Di- and Trimethylation and 6-AU Sensitivity
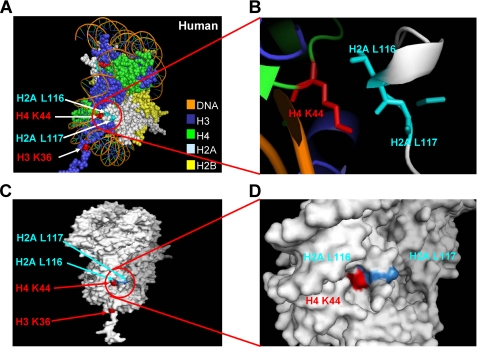
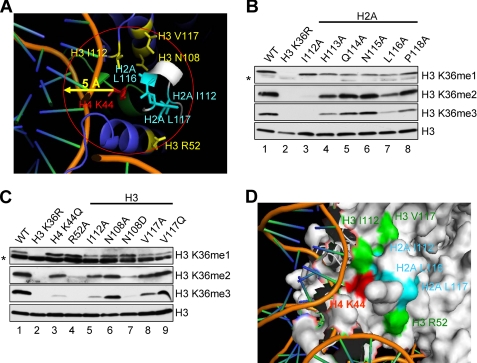
Our previous study found that H4 Lys44 is essential for H3 Lys36 methylation in vivo, in which the strains expressing H4 K44Q or K44E severely reduce H3 Lys36 di- and trimethylation (10). As shown in both yeast and human nucleosome structures, H4 Lys44 is located at the entry and exit point of the nucleosomal DNA, and the side chain of H4 Lys44 appears to be surrounded by the C terminus of H2A (Fig. 2A). Upon surveying the nucleosomal region surrounding histone H4 Lys44, we determined that two H2A residues, leucines 116 and 117 of histone H2A, are in close proximity to the histone H4 Lys44 residue with a distance of approximately five angstroms (Fig. 2B). Interestingly, as viewed by a surface picture of the nucleosome core particle, H4 Lys44 and H2A Leu116 and Leu117 are located on the surface of nucleosomes within or near a pocket (Fig. 2, C and D). This observation indicates that these basic and hydrophobic residues may form a binding patch or pocket that allows the histone H4 interaction motif within Set2 to bind so that subsequent trans-histone H3 Lys36 di- and trimethylation can occur.
FIGURE 2.
X-ray crystal structure of the nucleosome core particle indicating the positions of histone H4 Lys44 and H2A Leu116 and Leu117. A, the structure of the human nucleosome core particle is shown (H3, cyan; H4, green; H2A, gray; H2B, yellow). Histone H3 Lys36 and histone H4 Lys44 are marked in red, and histone H2A Leu116 and Leu117 are marked in blue. B, a zoomed in view of the nucleosome is shown indicating the close proximity of histone H4 Lys44 and histone H2A Leu116 and Leu117 residues. H4 Lys44 is marked in red, and H2A Leu116 and Leu117 are marked in blue. C and D, histone H4 Lys44 and H2A Leu116 and Leu117 form a patch on the surface of the nucleosome within or near a pocket. Histone H3 Lys36 and histone H4 Lys44 are marked in red, and histone H2A Leu116 and Leu117 are marked in blue. All of the figures were generated using Pymol.
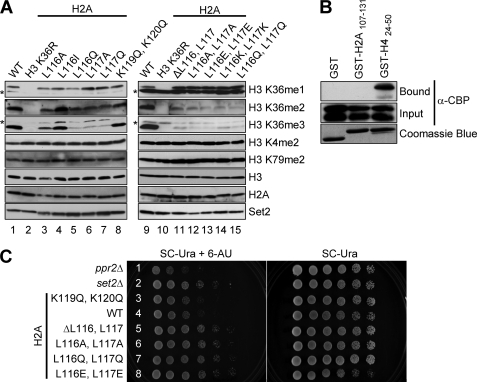
To test these ideas, we first examined whether Leu116 and Leu117 residues within the C-terminal tail of histone H2A are required for H3 Lys36 methylation by generating yeast strains that express various H2A mutants. Western blot analyses show that cells expressing H2A single mutation L116A, L116Q, L117A, or L117Q have significantly reduced H3 Lys36 trimethylation and only moderately affected H3 Lys36 dimethylation (Fig. 3A, lanes 3 and 5–7). Interestingly, mutating H2A Leu116 to isoleucine (H2A L116I) does not appear to change H3 Lys36 di- and trimethylation levels (Fig. 3A, lane 4). In addition, similar to what we have previously published with H4 Lys44 mutations, H3 Lys36 monomethylation is intact in these H2A mutants, indicating that H2A Leu116 and Leu117 and H4 Lys44 residues are working in the same trans-histone pathway (Fig. 3A). Importantly, the impact that the H2A Leu116 and Leu117 substitutions have on H3 Lys36 methylation is specific, because those strains do not display any detectable changes in global histone H3 Lys4 or Lys79 methylation or H3 or H2A levels (Fig. 3A). Moreover, H3 Lys36 di- and trimethylation defects in these H2A mutant strains are not due to lower levels of Set2 protein because immunoblots with an α-Set2 antibody show nearly equivalent levels in yeast expressing WT or mutant histones (Fig. 3A).
FIGURE 3.
Histone H2A Leu116 and Leu117 are needed for trans-histone H3 Lys36 di- and trimethylation in vivo and for appropriate 6-AU sensitivity. A, whole cell extracts prepared from cells expressing WT histones or the indicated histone mutants are immunoblotted with H3 Lys4, H3 Lys36, and H3 Lys79 methyl-specific antibodies. The asterisks denote nonspecific bands. Immunoblots for histone H3 and H2A serve as loading controls. The Set2 proteins levels are detected by probing with an α-Set2 antibody. B, C-terminal tail of histone H2A does not bind to recombinant Set2. In vitro binding assays are performed using GST, GST-H424–50, or GST-H2A107–131, incubated with CBP-Set2 extracts. Bound and input amounts of CBP-Set2 protein are detected by immunoblotting with an α-CBP antibody. The amount of GST, GST-H4, or GST-H2A fusion protein used in the assays is analyzed by Coomassie Blue staining. C, strains with H3 Lys36 methylation defects shown in A exhibit 6-AU resistance. Yeast cells expressing WT or the indicated histone H2A mutants are assayed for growth in the presence or absence of 6-AU (150 μg/ml). The ppr2Δ and set2Δ strains serve as controls for 6-AU sensitivity and resistance, respectively.
Given that H2A Leu116 or Leu117 single mutations only moderately affect H3 Lys36 dimethylation, we wanted to determine whether both H2A Leu116 and Leu117 residues are necessary for H3 Lys36 dimethylation. Interestingly, yeast strains that express H2A Leu116 and Leu117 double deletion (H2A ΔL116,L117) or various H2A double mutants (L116A,L117A; L116E,L117E; L116K,L117K; and L116Q,L117Q) had a further reduction in H3 Lys36 dimethylation and almost abolished H3 Lys36 trimethylation (Fig. 3A, lanes 11–15). Similar to H2A Leu116 or Leu117 single mutations, H2A double mutations do not affect H3 Lys36 monomethylation, as well as H3 Lys4 or H3 Lys79 methylation in vivo. Importantly, H3 Lys36 di- and trimethylation defects in the H2A double mutants are not due to histone H3 loading or changes in Set2 protein levels (Fig. 3A). Taken together, these data indicate that both H2A Leu116 and Leu117 are important for mediating in vivo trans-histone H3 Lys36 di- and trimethylation.
To determine whether other residues in the C terminus of histone H2A could disrupt H3 Lys36 methylation, a histone H2A K119Q,K120Q double mutant was generated and expressed in yeast. Interestingly, Lys119 has been identified to be an H2A ubiquitination site in higher eukaryotes (27). Up to now, no evidence has been provided to show that H2A is ubiquitinated in S. cerevisiae. Nonetheless, we still wanted to test whether these conserved lysine residues could play a role in H3 Lys36 methylation (28). Immunoblots show that this strain has no detectable changes in global histone H3 Lys4, Lys36 or Lys79 methylation, indicating that these conserved lysine residues are not essential for H3 Lys36 histone methylation (Fig. 3A, lane 8). These results further support that H2A Leu116 and Leu117 play an important and specific role in H3 Lys36 di- and trimethylation.
In our previous study, we determine by in vitro binding assays that histone H4 Lys44 is the major determinant for Set2 binding. In addition, we also identified a histone H4 interaction motif located within the N terminus of Set2. Given that H2A Leu116 and Leu117 are in close proximity to H4 Lys44 and are required for proper H3 Lys36 di- and trimethylation, we wondered whether H2A Leu116 and Leu117 are also required for interaction with Set2. Therefore, in vitro binding assays were performed using purified GST-H2A fusion protein, coding for GST-H2A107–131, incubated with bacterial extracts of recombinant CBP-Set2. Surprisingly, Set2 does not bind to H2A107–131 but binds to H424–50 efficiently (Fig. 3B). Moreover, incubating GST-H2A107–131 with GST-H424–50 does not enhance the binding of Set2 (data not shown). Although at this point it is unclear how H2A Leu116 and Leu117 contribute to histone H3 Lys36 di- and trimethylation, it is likely that our in vitro binding assay is not sensitive enough to detect interactions, or these residues do not directly contribute to binding but are needed to help correctly position H4 Lys44.
Set2-mediated H3 Lys36 methylation has been demonstrated to play an important role in transcription elongation (13, 17, 18). One of the approaches used to examine this function is the 6-AU sensitivity assay (7, 8). Our previous studies have shown that strains with H3 Lys36 methylation defects exhibit increased resistance to 6-AU when compared with WT cells (10). To examine whether mutations of H2A Leu116 and Leu117 would result in a 6-AU resistance phenotype, cells expressing WT histones or various H2A double deletion or mutations (K119Q,K120Q; ΔL116,L117; L116A,L117A; L116Q,L117Q; and L116E,L117E) were grown on plates with or without 6-AU. As a control for 6-AU sensitivity, a ppr2Δ strain was used as a negative control. The gene product of PPR2, also called DST1, is known as TFIIS, a known general transcription elongation factor that helps RNA polymerase II to read through blocks that occur during transcriptional elongation (29, 30). As expected, the ppr2Δ strain shows a sensitivity to 6-AU, whereas a set2Δ strain shows resistance to 6-AU when compared with WT cells (Fig. 3C, rows 1 and 2). Consistent with our previous data, we observe that expression of a H2A K119Q,K120Q double mutant, which has no changes in H3 Lys36 methylation, has WT levels of 6-AU sensitivity (Fig. 3, A, lane 8, and C, row 3). In contrast, cells expressing H2A ΔL116,L117 deletion or various H2A Leu116 and Leu117 mutants (L116A,L117A; L116E,L117E; L116K,L117K; and L116Q,L117Q), which have defects in H3 Lys36 di- and trimethylation, exhibit resistance to 6-AU, similar to the phenotype of a set2Δ strain (Fig. 3, A, lanes 11–15, and C, rows 5–8). Taken together, we conclude that histone H2A Leu116 and Leu117 along with H4 Lys44 are needed to mediate trans-histone H3 Lys36 di- and trimethylation and maintain proper transcription elongation.
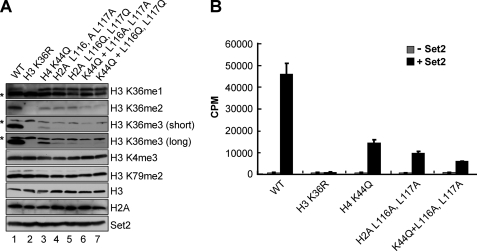
Both H4 Lys44 and H2A Leu116 and Leu117 Are Needed to Maintain trans-Histone H3 Lys36 Methylation in Vivo and in Vitro
Because H4 Lys44 or H2A Leu116 and Leu117 mutants only affect H3 Lys36 di- and trimethylation, we asked whether combination of these residues would disrupt H3 Lys36 monomethylation. Therefore, the methylation states of H3 Lys36 were examined in cells expressing triple mutants in which a H4 K44Q mutation was combined with a H2A L116A,L117A mutation (K44Q + L116A,L117A) or a H2A L116Q,L117Q mutation (K44Q + L116Q,L117Q). Strikingly, the triple histone mutants all show WT levels of H3 Lys36 monomethylation (Fig. 4A, lanes 6 and 7). However, these triple histone mutants completely abolish H3 Lys36 trimethylation and reduce H3 Lys36 dimethylation compared with either H4 K44Q single mutant or H2A Leu116 and Leu117 double mutants (Fig. 4A, lanes 6 and 7 versus lanes 3–5), suggesting that both H4 Lys44 and H2A Leu116 and Leu117 are needed to mediate H3 Lys36 di- and trimethylation. Again, the contribution of H4 Lys44 and H2A Leu116 and Leu117 to H3 Lys36 methylation is specific, because cells expressing the triple histone mutants do not exhibit defects in H3 Lys4 and Lys79 methylation. In addition, histone H3, H2A, and Set2 proteins levels are similar in WT and all of the histone mutants examined (Fig. 4A).
FIGURE 4.
Both histone H4 Lys44 and H2A Leu116 and Leu117 are needed for trans-histone H3 Lys36 di- and trimethylation in vivo and in vitro. A, yeast whole cell extracts generated from cells expressing WT or the indicated histone mutants, including histone H4 single mutations, H2A double mutations, or combination of H4 and H2A triple mutations, are immunoblotted against H3 Lys4, H3 Lys36, and H3 Lys79 methyl-specific antibodies. The asterisks denote nonspecific bands. Immunoblots for histone H3 and H2A are used as loading controls. For detecting H3 Lys36 trimethylation, both short and long exposures to film are shown. The Set2 protein levels are detected by probing with an α-Set2 antibody. B, in vitro HMT assays are performed using the indicated yeast chromatin substrates incubated with or without recombinant Set2. The amounts of chromatin substrates used in the assays are normalized by the Branford method. HMT reactions are analyzed by scintillation counting.
Next, we examined whether H2A Leu116 and Leu117 are necessary for in vitro Set2 HMT activity on yeast chromatin substrates. Soluble chromatin substrates were isolated from the nuclei of yeast strains that express WT or mutant histones (H3 K36R, H4 K44Q, H2A L116A,L117A, and H4 K44Q with H2A L116A,L117A). In vitro HMT assays were performed using these chromatin substrates incubated with or without purified recombinant Set2. After the addition of S-adenosyl-l-[methyl-3H]methionine, total [3H]-methyl incorporation is measured. As shown in Fig. 4B, Set2 is not active on H3 K36R substrate, whereas on a H4 K44Q substrate Set2 shows an approximately 3-fold decrease in activity when compared with WT chromatin, which is consistent with our previous report (10). In support of our in vivo histone methylation results, when Set2 is incubated with H2A L116A,L117A chromatin substrate, a 4.5-fold decrease in HMT activity is observed (Fig. 4B). In addition, when compared with either a H4 K44Q or a H2A L116A,L117A chromatin substrate, a triple histone mutant chromatin substrate, a H4 K44Q mutation with a H2A L116A,L117A mutation, exhibits a further decrease in Set2 activity (Fig. 4B). Because H4 K44Q, H2A L116A,L117A, and the triple histone mutant show similar levels of H3 Lys36 monomethylation in vivo when compared with the WT strain, some of the remaining Set2 in vitro activity is likely due to H3 Lys36 monomethylation on unmodified histone H3 (Fig. 4B). Taken together, our in vivo and in vitro methylation data are consistent with each other and indicate that both H4 Lys44 and H2A Leu116 and Leu117 contribute to maintaining the proper H3 Lys36 di- and trimethylated states.
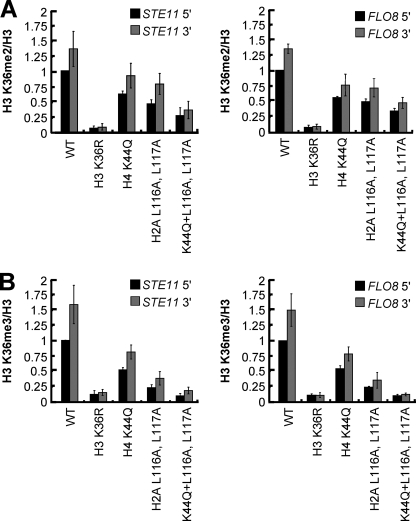
Both H4 Lys44 and H2A Leu116 and Leu117 Are Needed for trans-Histone H3 Lys36 Di- and Trimethylation at Gene-specific Loci
To determine whether H3 Lys36 di- and trimethylation are affected at different coding regions at gene-specific loci, ChIP assays were performed using H3 Lys36 di- and trimethyl-specific antibodies. Relative levels of H3 Lys36 di- and trimethylation were determined by quantitative real time PCR using TaqMan primer and probe pairs specific to the 5′ and 3′ regions of STE11 or FLO8 loci. In our ChIP analysis, we determine that STE11 and FLO8 open reading frames in WT cells have H3 Lys36 di- and trimethylation present at the 5′ and 3′ regions with an enrichment of trimethylation at the 3′ ends. The detected H3 Lys36 methylation is specific because the H3 K36R mutant is used as a negative control (Fig. 5). In addition, the observed changes in histone H3 Lys36 methylation are specific and not due to changes in histone levels because all of the ChIP assays are normalized to histone H3 levels. This pattern of H3 Lys36 di- and trimethylation is also consistent with results determined by genome-wide high resolution ChIP-ChIP studies (14, 15). Interestingly, when compared with WT cells, there is an approximately 2-fold decrease of H3 Lys36 dimethylation in the H4 K44Q mutant or the H2A L116A,L117A mutant, and a 3-fold decrease in the H4 K44Q and H2A L116A,L117A triple mutant (Fig. 5A and supplemental Tables S4 and S5). In a similar manner, H3 Lys36 trimethylation is decreased 2-fold in the H4 K44Q mutant, decreased over 4-fold in the H2A L116A,L117A mutant, and decreased ∼10-fold in the H4 K44Q and H2A L116A,L117A triple mutant (Fig. 5B and supplemental Tables S6 and S7). Overall, these gene-specific methylation patterns determined by ChIP analysis are in strong agreement with the global methylation patterns that we observed by immunoblot analysis. Therefore, this indicates that both H4 Lys44 and H2A Leu116 and Leu117 residues contribute to maintaining the trans-histone H3 Lys36 di- and trimethylation pathway at gene-specific loci.
FIGURE 5.
Both H4 Lys44 and H2A Leu116 and Leu117 are needed to mediate trans-histone H3 Lys36 di- and trimethylation at gene-specific loci. A and B, ChIP assays from the indicated strains are performed using antibodies specific to H3 Lys36 dimethyl (A) and trimethyl (B) and histone H3. Eluted DNA is analyzed by quantitative real time PCR using TaqMan primer and probe sets specifically recognizing the 5′ or 3′ regions of STE11 or FLO8. The ChIP data are normalized to histone H3 levels. The standard errors are provided for values representing three independent biological samples with three technical repeats each.
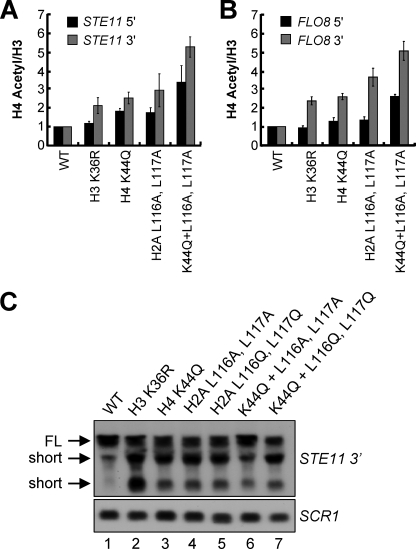
The Histone H4 and H2A Mutants with H3 Lys36 Di- and Trimethylation Defects Exhibit Increases in H4 Acetylation and Intragenic Cryptic Transcription
Previous studies have correlated Set2-mediated H3 Lys36 methylation with Rpd3S-dependent deacetylation of chromatin (13, 17, 18). Disruption of all H3 Lys36 mono-, di-, and trimethylation levels, such as set2Δ or H3 K36A mutant, increases histone acetylation levels of chromatin in the transcribed regions of specific loci (13, 17, 18). In addition, our previous study has also shown that decreases in H3 Lys36 di- and trimethylation in Set2 mutants lacking the histone H4 interaction motif or the Set2-Rpb1 interacting domain of Set2 increase histone H4 acetylation (10). Therefore, we wanted to determine whether a H4 K44Q mutant or H2A Leu116 and Leu117 mutants, which have H3 Lys36 di- and trimethyl defects, would have increases in histone H4 acetylation. To test this, ChIP assays were performed using an α-H4 acetyl antibody. An α-histone H3 antibody was used to normalize for changes in nucleosome density. Relative levels of H4 acetylation were measured by quantitative real time PCR analysis using the TaqMan primer probe sets previously used for the H3 Lys36 di- and trimethylation ChIP analysis. When compared with a WT strain, the strains expressing H4 K44Q mutant, the H2A L116A,L117A mutant, and the triple mutants bearing H4 K44Q and H2A L116A,L117A show an increase in histone H4 acetylation predominantly within the 3′ ends of STE11 and FLO8 (Fig. 6, A and B). Particularly, there is a 2–3-fold increase in histone H4 acetylation in the H4 K44Q and H2A L116A,L117A mutants and an over 5-fold increase in histone H4 acetylation the triple histone H4 and H2A mutant (H4 K44Q + L116A,L117A) when compared with WT (Fig. 6, A and B, and supplemental Tables S8 and S9). Consistent with previous observations, the levels of histone H4 acetylation do not increase significantly at the 5′ ends of STE11 or FLO8 loci in all of the strains examined here, except in the H4 and H2A triple mutant (Fig. 6, A and B). The H4 and H2A triple mutant shows an approximately 2–3-fold increase in histone H4 acetylation as compared with the levels of WT cells, indicating that this mutant might play another role in regulating histone H4 acetylation independent of Rpd3S complex at the 5′ ends of transcribed genes. Intriguingly, abolishing H3 Lys36 methylation in H3 K36R mutant did not substantially increase the levels of histone H4 acetylation at the 3′ region of STE11 or FLO8 loci (Fig. 6, A and B), although this mutant did exhibit short transcripts on STE11 or FLO8 genes (Figs. 1A and 6C, lane 2). Therefore, it appears that small increases in histone H4 acetylation can contribute to cryptic transcription.
FIGURE 6.
The histone H4 and H2A mutants showing trans-histone H3 Lys36 di- and trimethylation defects exhibit increased histone H4 acetylation and intragenic cryptic transcription. A and B, ChIP assays from the indicated strains are performed using antibodies specific to acetylated histone H4 and histone H3. Eluted DNA is analyzed by quantitative real time PCR using TaqMan primer and probe sets specifically recognizing the 5′ or 3′ regions of STE11 or FLO8. The data are normalized to histone H3 levels. The standard errors are provided for values representing three independent biological samples with three technical repeats each. C, total RNA isolated from strains used in A is subjected to Northern blot analysis using probes against 3′ region of STE11. SCR1 serves as a loading control. The full-length transcripts (FL) and short internal cryptic transcripts (short) are indicated.
We have demonstrated that a H4 K44Q mutant with H3 Lys36 di- and trimethylation defects also generates intragenic cryptic transcripts (Figs. 1A and 4A). Next, we asked whether the strains expressing H2A mutants that have H3 Lys36 di- and trimethylation defects show a cryptic transcription phenotype. To address this issue, Northern blot analyses were performed to examine the occurrence of intragenic initiation within the STE11 gene using the H4 K44Q mutant, H2A double mutants L116A,L117A and L116Q,L117Q, and the histone H4 and H2A triple mutants H4 K44Q with H2A L116A,L117A and H4 K44Q with H2A L116Q,L117Q. We observed that all strains that have defects in H3 Lys36 di- and trimethylation and increases in histone H4 acetylation exhibit short transcripts on the 3′ region of STE11 (Fig. 6C, lanes 3–7), whereas intragenic cryptic transcription is repressed in the WT strain (Fig. 6C, lane 1). Altogether, these observations show that this trans-histone H3 Lys36 methylation pathway mediated by histone H4 Lys44 and H2A Leu116 and Leu117 is essential to maintain proper H3 Lys36 di- and trimethylation, prevent increases in histone H4 acetylation, and repress intragenic cryptic transcription.
Multiple Histone H2A and H3 Residues Located near Histone H4 Lys44 Are Needed for Proper H3 Lys36 Di- and Trimethylation
Because histone H2A residues Leu116 and Leu117 are needed for proper H3 Lys36 di- and trimethylation and are in close proximity to H3 Lys44, we analyzed the nucleosome structure for additional histone residues located within 5 angstrom of histone H4 Lys44. This analysis determined that histone H2A Ile112, His113, Gln114, Asn115, and Pro118 and H3 Leu48, Ile51, Arg52, Ile112, Asn108, and Val117 and H4 Arg39 and Arg45 are all within 5 angstroms of histone H4 Lys44 (Fig. 7A) (31, 32). In addition, many of these residues are exposed at the surface of the nucleosome with the exception of H3 Ile51 and Asn108 and H4 Arg39 (31, 32). To test whether these cis- and trans-histone residues were required for proper Set2-mediated H3 Lys36 methylation, histone H2A and H3 mutants were generated and expressed in yeast. Consistent with what has been reported, cells expressing H3 L48A, H3 I51A, H4 R39A, or H4 R45A are not viable (data not shown) (10, 33, 34). However, strains expressing histone H2A I112A, H2A H113A, H2A Q114A, H2A N115A, H2A P118A, H3 R52A, H3 I112A, H3 N108A, H3 N108D, H3 V117A, or H3 V117Q mutations are viable. Whole cell extracts from viable H2A and H3 mutant strains were analyzed for their histone H3 Lys36 methylation status by immunoblot analysis (Fig. 7, B and C). Interestingly, several of the histone H2A and H3 mutants were defective in H3 Lys36 di- and trimethylation (Fig. 7, B and C). Consistent with the data shown in Fig. 3A, a strain expressing H2A I116A has significantly reduced H3 Lys36 trimethylation and only moderately affects H3 Lys36 dimethylation (Fig. 7B, lane 7). A strain expressing H2A I112A completely abolishes H3 Lys36 di- and trimethylation (Fig. 7B, lane 3), suggesting that H2A Ile112 may play a key role in mediating H3 Lys36 di- and trimethylation. In addition, strains expressing H2A H113A or H2A P118A moderately reduce H3 Lys36 di- and trimethylation levels (Fig. 7B, lanes 4 and 8), whereas strains expressing H2A Q114A or N115A did not have noticeable changes in H3 Lys36 methylation (Fig. 7B, lanes 5 and 6). Cells expressing histone H3 R52A or H3 N108D mutant almost abolish H3 Lys36 di- and trimethylation (Fig. 7C, lanes 4 and 7). However, cells expressing a H3 I112A mutant significantly reduce H3 Lys36 trimethylation and moderately affect H3 Lys36 dimethylation (Fig. 7C, lane 5). A H3 V117A mutant strain only moderately affects H3 Lys36 trimethylation (Fig. 7C, lane 8), whereas cells expressing H3 N108A or H3 V117Q mutants do not globally change H3 Lys36 methylation (Fig. 7C, lanes 6 and 9). Again, similar to what we have previously published with other histone mutations, H3 Lys36 monomethylation is intact in all of the histone H2A and H3 mutant strains examined (Fig. 7, B and C). In addition, these histone H2A and H3 core residues are also likely to be important for maintaining proper histone acetylation and repression of cryptic transcription similar to histone H4 Lys44 and histone H2A Leu116 and Leu117. Based on our results and the nucleosome structure, histone H4, H2A, and H3 residues appear to form a region on the nucleosomal surface that is optimal for Set2 binding and/or recognition (Fig. 7D). Future studies exploring the co-crystal structure of Set2 and the nucleosome would be interesting and will help determine the precise mechanism of interaction between Set2 and this region of the nucleosome.
FIGURE 7.
Multiple histone H2A and H3 residues located near histone H4 Lys44 are needed for proper H3 Lys36 di- and trimethylation. A, a zoomed in view of the nucleosome is shown indicating multiple residues in the close proximity within 5 angstroms of histone H4 Lys44 residue. Representative residues that are needed for H3 Lys36 di- and trimethylation are labeled. H4 Lys44 is marked in red; H2A Ile112, Leu116, and Leu117 are marked in blue; and H3 Arg52, Ile112, Asn108, and Val117 are marked in yellow. B and C, whole cell extracts prepared from cells expressing WT histones or the indicated histone mutants are immunoblotted with H3 Lys36 methyl-specific antibodies. The asterisks denote nonspecific bands. Immunoblot for histone H3 serve as loading controls. D, the histone residues with H3 Lys36 di- and trimethyl defects have been shown to form a patch on the surface of the nucleosome. H4 Lys44 is marked in red; and H2A Ile112, Leu116, and Leu117 are marked in blue; and H3 Arg52, Ile112, and Val117 are marked in green. H3 Asn108 is not exposed at the surface of the nucleosome. These two structural figures were generated by Pymol.
DISCUSSION
In this report, we identify new cis- and trans-histone requirements for Set2-mediated H3 Lys36 methylation and show that residues in histone H4, H2A, and H3 are needed for proper H3 Lys36 di- and trimethylation. We also show that a H4 K44Q mutant and H2A C-terminal tail Leu116 and Leu117 mutants result in decreased H3 Lys36 di- and trimethylation, increased histone H4 acetylation, and resistance to 6-AU. In addition, this pattern of decreased H3 Lys36 di- and trimethylation and increased H4 acetylation observed in yeast strains expressing H4 Lys44 or H2A Leu116 and Leu117 mutants consistently show aberrant intragenic cryptic transcription. Furthermore, yeast strains expressing triple mutations consisting of histone H4 Lys44 and H2A Leu116 and Leu117 substitution mutations have lower levels of H3 Lys36 di- and trimethylation and increased levels of histone H4 acetylation than either the single H4 Lys44 mutant or the double H2A Leu116 and Leu117 mutants. Finally, we identify several additional histone H2A and H3 core residues that are also required for proper H3 Lys36 di- and trimethylation. Altogether, our results suggest that Set2 likely recognizes and docks on a H4, H2A, and H3 nucleosomal surface so that subsequent histone H3 Lys36 di- and trimethylation allows for proper maintenance of histone acetylation and transcriptional elongation.
Our recent studies have determined that Dot1 and Set2 methylate histone H3 Lys79 and Lys36, respectively, by two different trans-histone methylation pathways (10, 23, 35). In the Dot1 trans-histone methylation pathway, an electrostatic interaction between the C-terminal tail of Dot1 and histone H4 basic patch is need for proper H3 Lys79 di- and trimethylation (23). In a similar but distinct manner, we have identified that a histone H4 interaction motif found in the N terminus of Set2 interacts with histone H4 Lys44 to mediate H3 Lys36 di- and trimethylation (10). To further understand the Set2-mediated trans-histone pathway, we analyzed the crystal structures of human and yeast nucleosome core particles. Intriguingly, we noticed that the histone H4 Lys44 residue appeared to be surrounded by the C terminus of histone H2A, and we predicted that residues in the H2A C terminus might be needed for H3 Lys36 methylation. We tested this hypothesis and determined that histone H2A residues Leu116 and Leu117 that are in close proximity to the H4 Lys44 residue are critical for Set2-mediated methylation. We also analyzed the nucleosome structure for additional residues located within 5 angstroms of histone H4 Lys44 and found that several H2A and H3 core residues fit this criterion. More importantly, several of these histone core residues were also needed for H3 Lys36 di- and trimethylation. Overall, our studies have identified the first region on the nucleosome surface involving histone H4, H2A, and H3 residues that are required for Set2-mediated H3 Lys36 di- and trimethylation. Given that H4 Lys44 and H2A C-terminal tail mutations increase histone acetylation levels and result in intragenic cryptic transcription, we would expect that H2A and H3 core residues that disrupt H3 Lys36 methylation would also be important in maintaining proper histone acetylation and repression of cryptic transcription. In addition, the histone H3 N-terminal tail was recently determined to help mediate Set2 activity but not binding, suggesting other cis-tail determinants may exist for mediating Set2-mediated H3 Lys36 methylation (36).
Because H4 Lys44 and H2A Leu116 and Leu117 are closely located on the surface of the nucleosome, these residues likely form a docking site for the histone H4 interaction motif of Set2. Although Set2 can bind histone H4 peptides containing H4 Lys44, we were unable to detect Set2 binding to the C terminus of H2A. Therefore, our assay may not be sensitive enough to detect weak Set2 and H2A interactions, or it is quite possible that H2A residues Leu116 and Leu117 are not directly involved in binding to Set2 but are needed to help correctly position H4 Lys44 to form a binding pocket. Further structural studies will be needed to determine precisely how Set2 engages this part of the nucleosome surface and what residues are needed for direct interaction. Because all of the histone H4, H2A, and H3 mutations that disrupt H3 Lys36 di- and trimethylation do not abolish H3 Lys36 monomethylation, additional structural, genetic, and biochemical studies will be needed to understand the determinants for histone H3 Lys36 monomethylation and its function in the cell.
Previous studies have indicated that Set2-mediated H3 Lys36 methylation is needed to recruit the Rpd3S complex and deacetylate histones so that intragenic cryptic transcription within the 3′ region of genes is prevented (13, 17, 18). Consistent with these observations, we show that our previously identified trans-histone methylation pathway involving an interaction between Set2 and histone H4 Lys44 is also needed for repression of intragenic cryptic transcription. In addition, we show that histone H2A C-terminal residues are also required for repression of intragenic cryptic transcription. Recently published reports have indicated that histone H3 Lys36 dimethylation is sufficient for repressing cryptic transcription (37, 38). However, we consistently show that Set2 deletion mutants and histone H4 and H2A mutations still undergo intragenic cryptic transcription, even though these mutants still maintain some level of global and gene-specific H3 Lys36 di- and trimethylation. Therefore, we favor the idea that a combination of H3 Lys36 di- and trimethylation is needed for the proper recruitment or activity of the Rpd3S complex. This idea would also be consistent with the ability of the Rpd3S complex to equally bind H3 Lys36 di- and trimethylated nucleosomes and peptides (17, 38, 39). In addition, it has been reported that other unknown mechanisms besides histone H3 Lys36 methylation may contribute to intragenic cryptic transcription (40). Therefore, additional histone modifications and chromatin factors may help to regulate and control cryptic transcription (40). Although more work will be needed to establish what is required for cryptic transcription, further investigation is also needed to determine why there are so many cryptic promoters and whether they play a functional role in the cell.
Interestingly, during the preparation of this manuscript, a new report showed that a human homologue of yeast Set2, NSD2, exhibits severe reduced activity on H3 Lys36 methylation in vitro on recombinant nucleosome substrates where histone H4 Lys44 was mutated to Gln or Glu (41). These observations reinforce and support our previously published observation regarding Set2-mediated trans-histone pathway in budding yeast (10). Based on our new data and given that histones are highly conserved from yeast to human, it is likely that NSD2 and other Set2-like methyltransferases require this nucleosome surface for proper methyltransferase activity. Therefore, studying how histone H4, H2A, and H3 residues contribute toward maintaining proper H3 Lys36 methylation in yeast may potentially help our understanding of how proper H3 Lys36 methylation and transcription elongation occur in humans. In addition, several Set2 human homologues, NSD1, NSD2, and NSD3, have been found overexpressed or mutated in various human cancers such as acute myeloid leukemia, multiple myeloma, and breast cancer (42–44). Therefore, our studies may also provide insight into how these histone methyltransferases contribute to oncogenesis.
Supplementary Material
Acknowledgments
We thank Dr. Bing Li (University of Texas Southwestern Medical School), Dr. Ian Fingerman (NLM, NCBI, National Institutes of Health), Dr. James Forney (Purdue University), and Dr. Steven Broyles (Purdue University) for technical help and suggestions. We also thank Dr. David Allis (Rockefeller University), Dr. Brian Strahl (University of North Carolina), and Dr. Fred Winston (Harvard Medical School) for kindly providing the H4 Acetyl antibody, Set2 antibody, and FY406 yeast strain, respectively. We are also grateful to the members of the Briggs lab, Dr. Douglas Mersman, Paul South, and Kayla Harmeyer for critical review of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grant GM74183 (to S. D. B.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental text and Tables S1–S9.
- 6-AU
- 6-azauracil
- WT
- wild type
- HMT
- histone methyltransferase
- ChIP
- chromatin immunoprecipitation
- MOPS
- 4-morpholinepropanesulfonic acid
- GST
- glutathione S-transferase.
REFERENCES
- 1.Kouzarides T. (2007) Cell 128, 693–705 [DOI] [PubMed] [Google Scholar]
- 2.Vaquero A., Loyola A., Reinberg D. (2003) Sci. Aging Knowledge Environ. 14, RE4, 10.1126/sageke.2003.14.re4 [DOI] [PubMed] [Google Scholar]
- 3.Li B., Carey M., Workman J. L. (2007) Cell 128, 707–719 [DOI] [PubMed] [Google Scholar]
- 4.Taverna S. D., Li H., Ruthenburg A. J., Allis C. D., Patel D. J. (2007) Nat. Struct. Mol. Biol. 14, 1025–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin C., Zhang Y. (2005) Nat. Rev. Mol. Cell Biol. 6, 838–849 [DOI] [PubMed] [Google Scholar]
- 6.Lan F., Nottke A. C., Shi Y. (2008) Curr. Opin. Cell Biol. 20, 316–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kizer K. O., Phatnani H. P., Shibata Y., Hall H., Greenleaf A. L., Strahl B. D. (2005) Mol. Cell. Biol. 25, 3305–3316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li B., Howe L., Anderson S., Yates J. R., 3rd, Workman J. L. (2003) J. Biol. Chem. 278, 8897–8903 [DOI] [PubMed] [Google Scholar]
- 9.Biswas D., Dutta-Biswas R., Mitra D., Shibata Y., Strahl B. D., Formosa T., Stillman D. J. (2006) EMBO J. 25, 4479–4489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du H. N., Fingerman I. M., Briggs S. D. (2008) Genes Dev. 22, 2786–2798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krogan N. J., Kim M., Ahn S. H., Zhong G., Kobor M. S., Cagney G., Emili A., Shilatifard A., Buratowski S., Greenblatt J. F. (2002) Mol. Cell. Biol. 22, 6979–6992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strahl B. D., Grant P. A., Briggs S. D., Sun Z. W., Bone J. R., Caldwell J. A., Mollah S., Cook R. G., Shabanowitz J., Hunt D. F., Allis C. D. (2002) Mol. Cell. Biol. 22, 1298–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carrozza M. J., Li B., Florens L., Suganuma T., Swanson S. K., Lee K. K., Shia W. J., Anderson S., Yates J., Washburn M. P., Workman J. L. (2005) Cell 123, 581–592 [DOI] [PubMed] [Google Scholar]
- 14.Rao B., Shibata Y., Strahl B. D., Lieb J. D. (2005) Mol. Cell. Biol. 25, 9447–9459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pokholok D. K., Harbison C. T., Levine S., Cole M., Hannett N. M., Lee T. I., Bell G. W., Walker K., Rolfe P. A., Herbolsheimer E., Zeitlinger J., Lewitter F., Gifford D. K., Young R. A. (2005) Cell 122, 517–527 [DOI] [PubMed] [Google Scholar]
- 16.Li B., Gogol M., Carey M., Lee D., Seidel C., Workman J. L. (2007) Science 316, 1050–1054 [DOI] [PubMed] [Google Scholar]
- 17.Joshi A. A., Struhl K. (2005) Mol. Cell 20, 971–978 [DOI] [PubMed] [Google Scholar]
- 18.Keogh M. C., Kurdistani S. K., Morris S. A., Ahn S. H., Podolny V., Collins S. R., Schuldiner M., Chin K., Punna T., Thompson N. J., Boone C., Emili A., Weissman J. S., Hughes T. R., Strahl B. D., Grunstein M., Greenblatt J. F., Buratowski S., Krogan N. J. (2005) Cell 123, 593–605 [DOI] [PubMed] [Google Scholar]
- 19.Li B., Gogol M., Carey M., Pattenden S. G., Seidel C., Workman J. L. (2007) Genes Dev. 21, 1422–1430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldstein A. L., McCusker J. H. (1999) Yeast 15, 1541–1553 [DOI] [PubMed] [Google Scholar]
- 21.Briggs S. D., Bryk M., Strahl B. D., Cheung W. L., Davie J. K., Dent S. Y., Winston F., Allis C. D. (2001) Genes Dev. 15, 3286–3295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fingerman I. M., Wu C. L., Wilson B. D., Briggs S. D. (2005) J. Biol. Chem. 280, 28761–28765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fingerman I. M., Li H. C., Briggs S. D. (2007) Genes Dev. 21, 2018–2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuo M. H., Allis C. D. (1999) Methods 19, 425–433 [DOI] [PubMed] [Google Scholar]
- 25.Mersman D. P., Du H. N., Fingerman I. M., South P. F., Briggs S. D. (2009) Genes Dev. 23, 951–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chu Y., Simic R., Warner M. H., Arndt K. M., Prelich G. (2007) EMBO J. 26, 4646–4656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hensold J. O., Swerdlow P. S., Housman D. E. (1988) Blood 71, 1153–1156 [PubMed] [Google Scholar]
- 28.Swerdlow P. S., Schuster T., Finley D. (1990) Mol. Cell. Biol. 10, 4905–4911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kerppola T. K., Kane C. M. (1991) FASEB J. 5, 2833–2842 [DOI] [PubMed] [Google Scholar]
- 30.Exinger F., Lacroute F. (1992) Curr. Genet. 22, 9–11 [DOI] [PubMed] [Google Scholar]
- 31.Luger K., Mäder A. W., Richmond R. K., Sargent D. F., Richmond T. J. (1997) Nature 389, 251–260 [DOI] [PubMed] [Google Scholar]
- 32.White C. L., Suto R. K., Luger K. (2001) EMBO J. 20, 5207–5218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dai J., Hyland E. M., Yuan D. S., Huang H., Bader J. S., Boeke J. D. (2008) Cell 134, 1066–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakanishi S., Sanderson B. W., Delventhal K. M., Bradford W. D., Staehling-Hampton K., Shilatifard A. (2008) Nat. Struct. Mol. Biol. 15, 881–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Altaf M., Utley R. T., Lacoste N., Tan S., Briggs S. D., Côté J. (2007) Mol. Cell 28, 1002–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Psathas J. N., Zheng S., Tan S., Reese J. C. (2009) Mol. Cell. Biol. 29, 6413–6426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Youdell M. L., Kizer K. O., Kisseleva-Romanova E., Fuchs S. M., Duro E., Strahl B. D., Mellor J. (2008) Mol. Cell. Biol. 28, 4915–4926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li B., Jackson J., Simon M. D., Fleharty B., Gogol M., Seidel C., Workman J. L., Shilatifard A. (2009) J. Biol. Chem. 284, 7970–7976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi X., Kachirskaia I., Walter K. L., Kuo J. H., Lake A., Davrazou F., Chan S. M., Martin D. G., Fingerman I. M., Briggs S. D., Howe L., Utz P. J., Kutateladze T. G., Lugovskoy A. A., Bedford M. T., Gozani O. (2007) J. Biol. Chem. 282, 2450–2455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheung V., Chua G., Batada N. N., Landry C. R., Michnick S. W., Hughes T. R., Winston F. (2008) PLoS Biol. 6, e277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Y., Trojer P., Xu C. F., Cheung P., Kuo A., Drury W. J., 3rd, Qiao Q., Neubert T. A., Xu R. M., Gozani O., Reinberg D. (2009) J. Biol. Chem. 284, 34283–34295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stec I., Wright T. J., van Ommen G. J., de Boer P. A., van Haeringen A., Moorman A. F., Altherr M. R., den Dunnen J. T. (1998) Hum. Mol. Genet. 7, 1071–1082 [DOI] [PubMed] [Google Scholar]
- 43.Chesi M., Nardini E., Lim R. S., Smith K. D., Kuehl W. M., Bergsagel P. L. (1998) Blood 92, 3025–3034 [PubMed] [Google Scholar]
- 44.Angrand P. O., Apiou F., Stewart A. F., Dutrillaux B., Losson R., Chambon P. (2001) Genomics 74, 79–88 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.