Abstract
Although several non-receptor activators of heterotrimeric G proteins have been identified, the structural features of G proteins that determine their interaction with such activators and the subsequent biological effects are poorly understood. Here we investigated the structural determinants in Gαi3 necessary for its regulation by GIV/girdin, a guanine-nucleotide exchange factor (GEF) that activates Gαi subunits. Using G protein activity and in vitro pulldown assays we demonstrate that Gαi3 is a better substrate for GIV than the highly homologous Gαo. We identified Trp-258 in the Gαi subunit as a novel structural determinant for GIV binding by comparing GIV binding to Gαi3/Gαo chimeras. Mutation of Trp-258 to the corresponding Phe in Gαo decreased GIV binding in vitro and in cultured cells but did not perturb interaction with other Gα-binding partners, i.e. Gβγ, AGS3 (a guanine nucleotide dissociation inhibitor), GAIP/RGS19 (a GTPase-activating protein), and LPAR1 (a G protein-coupled receptor). Activation of Gαi3 by GIV was also dramatically reduced when Trp-258 was replaced with Tyr, Leu, Ser, His, Asp, or Ala, highlighting that Trp is required for maximal activation. Moreover, when mutant Gαi3 W258F was expressed in HeLa cells they failed to undergo cell migration and to enhance Akt signaling after growth factor or G protein-coupled receptor stimulation. Thus activation of Gαi3 by GIV is essential for biological functions associated with Gαi3 activation. In conclusion, we have discovered a novel structural determinant on Gαi that plays a key role in defining the selectivity and efficiency of the GEF activity of GIV on Gαi and that represents an attractive target site for designing small molecules to disrupt the Gαi-GIV interface for therapeutic purposes.
Keywords: Cancer, Cell/Migration, Cytoskeleton/Actin, G Proteins/Heterotrimeric, Growth Factors, Akt
Introduction
Heterotrimeric G proteins are molecular switches that control signal transduction. G protein cycling between active and inactive states is controlled via interaction with regulatory proteins. Activation is triggered by guanine nucleotide exchange factors (GEFs),5 and deactivation is greatly enhanced by GTPase-activating proteins (GAPs) (1–3). Because the duration and extent of G protein-mediated signaling is determined by the lifetime of Gα in the GTP-bound state, it is crucial to define the molecular machinery that triggers G protein activation to understand how this signal transduction pathway functions. Ligand-occupied G protein-coupled receptors (GPCRs) are the canonical GEFs of which >800 genes have been identified in the human genome (4). They regulate a myriad of physiological functions and are the most common target for marketed drugs (∼30%) (5). Recently, a few non-receptor GEFs have been described, i.e. AGS1 (6), Ric-8 (7, 8), CSPα (9), and Arr4 (10). In contrast to GPCRs, these non-receptor GEFs are structurally unrelated, and their physiological roles are just beginning to be elucidated (8, 11–13). The lack of information on non-receptor GEFs has limited their exploitation as pharmacological targets.
We recently demonstrated that GIV is a non-receptor GEF for Gαi subunits (11). Originally GIV was identified by its ability to interact with Gαi3 in a yeast two-hybrid screen (14). Work from other groups indicated that GIV (also known as girdin) enhances Akt signaling (15) and plays a critical role in cell migration via its interaction with Akt and the actin cytoskeleton (16). GIV was shown to be required for cancer metastasis in murine models by virtue of its ability to control cell migration and actin remodeling (17). We subsequently found that active Gαi3, like GIV, promotes Akt signaling, remodeling of the actin cytoskeleton, and tumor cell migration (18).
Moreover, we recently reported that GIV activates Gαi3 subunits via an evolutionarily conserved GEF motif and that this novel regulatory motif provides the structural and biochemical basis for the pro-metastatic features of GIV (11). We identified the GEF motif of GIV based on its sequence homology with the synthetic GEF peptide KB-752 (19) and showed that mutational disruption of the ability of GIV to activate Gαi subunits via this motif abolished the enhanced Akt activation (15), actin cytoskeleton remodeling (16, 17, 20), and cell migration (16, 17) seen in metastatic tumor cells (11).
GIV is the first non-receptor GEF whose function has been shown to be governed by a defined motif. Because the GEF function of GIV appears critical for cancer metastasis, disruption of the interface formed between the GEF motif of GIV and Gαi is potentially of therapeutic significance, and defining the molecular basis and properties of this interface is crucial for the future development of pharmacological agents that target this interface. Here we investigated in depth the structural determinants in the Gαi3 subunit required for it to interact with GIV and be activated. Using the Gα selectivity of GIV to identify such determinants, we found that residues outside of the previously described Gαi-GIV interface (11) define the selectivity and efficiency of the GEF activity of GIV on Gαi in living cells and in vitro. These data provide valuable insights that can be used in the design of pharmacological agents that selectively disrupt the Gαi-GIV interface for therapeutic purposes.
EXPERIMENTAL PROCEDURES
Reagents and Antibodies
Unless otherwise indicated all reagents were of analytical grade and obtained from Sigma-Aldrich. Cell culture media were purchased from Invitrogen. All restriction endonucleases and Escherichia coli strain DH5α were purchased from New England Biolabs (Cambridge, MA). E. coli strain BL21(DE3) was purchased from Invitrogen. Pfu ultra DNA polymerase was purchased from Stratagene (La Jolla, CA). [γ-32P]GTP and [35S]GTPγS were from PerkinElmer Life Sciences. Rabbit antisera against AGS3 (21) and the coiled-coil region of GIV (14) were raised as described. Goat anti-rabbit and goat anti-mouse Alexa Fluor 680 or IRDye 800 F(ab′)2 were from Li-Cor Biosciences (Lincoln, NE). Mouse monoclonal antibodies against hexahistidine (His), FLAG (M2), and α-tubulin were obtained from Sigma-Aldrich. Rabbit anti-pan-Gβ (M-14) IgG was from Santa Cruz Biotechnology (Santa Cruz, CA) and anti-Akt and phospho-Akt (S473) IgGs were from Cell Signaling (Beverly, MA).
Plasmid Constructs and Mutagenesis
Cloning of rat Gαi3 into pGEX-4T-1 or pET28b and GIV-CT-(1623–1870) into pET28b were described previously (11, 18). Rat Gαo (isoform 1, Gαo1, hereafter referred to as Gαo) was cloned from pGBT9-Gαo (22) and inserted between the EcoRI and NotI restriction sites of the pGEX-4T-1 vector to generate GST-Gαo or between the NdeI and EcoRI restriction sites of the pET28b vector to generate His-Gαo. GIV-CT-(1623–1870) was cloned from pcDNA 3.1-GIV (16) and inserted between the EcoRI and NotI restriction sites of the pGEX-4T-1 vector to generate GST-GIV-CT-(1623–1870). GIV-CTs-(1660–1870, “s” stands for “short”) was cloned from pcDNA 3.1-GIV and inserted between the NdeI and EcoRI restriction sites of the pET28b vector to generate His-GIV-CTs-(1660–1870) used in the GTPase and GTPγS binding assays. To generate Gαi3 with three FLAG sequences fused to the C terminus of the protein (Gαi3-FLAG), rat Gαi3 was amplified by PCR from pET28b-Gαi3 with primers designed to add HindIII and BamHI restrictions sites at the 5′- and 3′-ends, respectively, and to replace the stop codon of the original cDNA with a glycine codon. The PCR product was digested and inserted between the HindIII and BamHI restriction sites of p3XFLAG-CMV-14. Untagged rat Gαi3 cloned into pcDNA3.1 was described previously (18). Mouse LPA receptor 1 (LPAR1) cloned into pFLAG-CMV-1 was a gift from Dr. Jerold Chun (Scripps Research Institute) and was described previously (23). Gαi3 and Gαo mutants were generated using specific primers (sequences available upon request) following the manufacturer's instructions (QuikChange II, Stratagene). All constructs were checked by DNA sequencing (University of California at San Diego Moores Cancer Center Sequencing Facility).
GST-Gαi3/o chimeras were generated by using an overlapping PCR strategy (24). pGEX-4T-1-Gαi3 and pGEX-4T-1-Gαo were used as templates to amplify the following sequences in the first PCR: Gαi3-(1–59), Gαi3-(1–177), Gαi3-(1–270), Gαi3-(178–354), Gαi3-(271–354), Gαo-(60–178), Gαo-(60–178), Gαo-(179–354), Gαo-(179–271), and Gαo-(272–354). These cDNA fragments, which contained overlapping sequences in the internal boundaries of the chimeras, were used as templates in successive PCR reactions to generate the full-length chimeras. The full-length chimeras were digested and inserted between the EcoRI and NotI restriction sites of pGEX-4T-1.
Protein Purification
GST, GST-Gαi3, GST-Gαo, GST-Gαi3/o chimeras, His-Gαi3, His-Gαo, His-GIV-CT, or His-GIV-CTs fusion constructs were expressed in E. coli strain BL21(DE3) (Invitrogen) as described previously (11) and induced overnight at 25 °C with 1 mm 1-isopropyl-β-d-thiogalactopyranoside. Pelleted bacteria from 1 liter of culture were resuspended in 10 ml of GST-lysis buffer (25 mm Tris-HCl, pH 7.5, 20 mm NaCl, 1 mm EDTA, 20% (v/v) glycerol, 1% (v/v) Triton X-100, 2× protease inhibitor mixture (Complete EDTA-free, Roche Diagnostics)) or His-lysis buffer (50 mm NaH2PO4, pH 7.4, 300 mm NaCl, 10 mm imidazole, 1% (v/v) Triton X-100, 2× protease inhibitor mixture (Complete EDTA-free, Roche Diagnostics)) for GST- or His-fused proteins, respectively. After sonication (4 × 20 s, 1 min between cycles), lysates were centrifuged at 12,000 × g at 4 °C for 20 min. Solubilized proteins were affinity-purified on glutathione-Sepharose 4B beads (Amersham Biosciences) or HisPur Cobalt Resin (Pierce). Proteins were eluted, dialyzed overnight against phosphate-buffered saline, and stored at −80 °C. His-Gαi3 and His-Gαo were buffer-exchanged into G protein storage buffer (20 mm Tris-HCl, pH 7.4, 200 mm NaCl, 1 mm MgCl2, 1 mm DTT, 10 μm GDP, 5% (v/v) glycerol) prior to storage at −80 °C.
Cell Culture, Transfection, and Lysis
COS7 and HeLa cells were grown at 37 °C in DMEM supplemented with 10% fetal bovine serum, 100 units/ml penicillin, 100 μg/ml streptomycin, 1% l-glutamine, and 5% CO2. siRNA transfection of HeLa cells was carried out using Oligofectamine (Invitrogen) following the manufacturer's protocol. Oligonucleotides against human Gαi3 were from Santa Cruz Biotechnology. When reversal of phenotype was attempted, pcDNA3.1-Gαi3 (untagged) transfection was carried out 8–10 h post-siRNA transfection using GeneJuice (Novagen) following the manufacturer's protocol, and cells were analyzed after ∼38–40 h. Transfection of COS7 cells with Gαi3-FLAG was also carried out using GeneJuice. Lysates used as a source for GIV for in vitro protein binding assays or for immunoprecipitation were prepared by resuspending the cells in lysis buffer (20 mm HEPES, pH 7.2, 5 mm Mg(CH3COO)2, 125 mm K(CH3COO), 0.4% Triton X-100, 1 mm DTT) supplemented with phosphatase (Sigma) and protease (Roche Applied Science) inhibitor mixtures, passed through a 28-gauge needle at 4 °C, and cleared (10,000 × g for 10 min) before use in subsequent experiments.
In Vitro Protein Binding Assays
Purified GST fusion proteins or GST alone (5–10 μg) were immobilized on glutathione-Sepharose beads and incubated in binding buffer (50 mm Tris-HCl, pH 7.4, 100 mm NaCl, 0.4% (v/v) Nonidet P-40, 10 mm MgCl2, 5 mm EDTA, 2 mm DTT, protease inhibitor mixture) containing either 30 μm GDP or 30 μm GDP, 30 μm AlCl3, 10 mm NaF for 90 min at room temperature. ∼250 μg of COS7 cell lysate or 3 μg of purified His-GIV-CT or His-Gαi3 was added to each tube, and binding reactions were carried out overnight at 4 °C with constant tumbling. Beads were washed (×4) with 1 ml of wash buffer (4.3 mm Na2HPO4, 1.4 mm KH2PO4, pH 7.4, 137 mm NaCl, 2.7 mm KCl, 0.1% (v/v) Tween 20, 10 mm MgCl2, 5 mm EDTA, 2 mm DTT, supplemented with GDP or GDP, AlCl3, and NaF as during binding) and boiled in sample buffer for SDS-PAGE.
Immunoprecipitation
COS7 cell lysates (∼1–2 mg of protein) were incubated 4 h at 4 °C with 2 μg of anti-FLAG monoclonal antibody (Sigma) followed by incubation with protein G-agarose beads (Invitrogen) at 4 °C for an additional 60 min. Beads were washed (×4) with 1 ml of wash buffer (4.3 mm Na2HPO4, 1.4 mm KH2PO4, pH 7.4, 137 mm NaCl, 2.7 mm KCl, 0.1% (v/v) Tween 20, 10 mm MgCl2, 5 mm EDTA, 2 mm DTT), and the bound immune complexes were eluted by boiling in SDS sample buffer. For immunoprecipitation of FLAG-LPAR1 the boiling step was omitted.
Steady-state GTPase Assay
This assay was performed as described previously (11). Briefly, His-Gαi3 or His-Gαo (100 nm) was preincubated with different concentrations of His-GIV-CTs-(1660–1870), for 15 min at 30 °C in assay buffer (20 mm sodium HEPES, pH 8, 100 mm NaCl, 1 mm EDTA, 2 mm MgCl2, 1 mm DTT, 0.05% (w/v) C12E10). GTPase reactions were initiated at 30 °C by adding an equal volume of assay buffer containing 1 μm [γ-32P]GTP (∼50 cpm/fmol). Duplicate aliquots (50 μl) were removed at 10 min, and reactions were stopped with 950 μl of ice-cold 5% (w/v) activated charcoal in 20 mm H3PO4, pH 3. Samples were then centrifuged for 10 min at 10,000 × g, and 500 μl of the resultant supernatant was scintillation-counted to quantify released [32P]Pi. To determine the specific Pi produced, the background [32P]Pi detected at 10 min in the absence of G protein was subtracted from each reaction. The % reduction in G protein activation of Gαo and Gαi3 mutants compared with wt Gαi3 was determined using the formula, 100 − [100*(x − 1)/(y − 1)], where x is the -fold increase in G protein activity for Gαo and Gαi3 mutants and y is the -fold increase for wt Gαi3.
Single-turnover GTPase Assay
This assay was performed as previously described (25). Briefly, His-Gαi3 (500 nm) was loaded for 30 min at 30 °C with GTP in the absence of magnesium to prevent the hydrolysis of the nucleotide (20 mm sodium HEPES, pH 8, 5 mm EDTA, 1 mm DTT, 4 μm [γ-32P]GTP (∼100 cpm/fmol)). After transferring the tubes to ice, the reaction was initiated by diluting ten times the GTP-loaded G protein with assay buffer (20 mm sodium HEPES, pH 8, 80 mm NaCl, 5 mm EDTA, 7.5 mm MgCl2, 1 mm DTT, 200 μm GTP, 0.05% (w/v) C12E10) containing the desired amount of protein to be tested (His-GIV-CTs, 2 μm, GST-GAIP, 1 μm, or equal volume of buffer in the control reaction). The reactions were carried out on ice. Aliquots (50 μl) were removed at different time points (0, 20, 40, 60, 90, 120, 180, 240, and 300 s), and reactions were stopped with 950 μl of ice-cold 5% (w/v) activated charcoal in 20 mm H3PO4, pH 3. Samples were then centrifuged for 10 min at 10,000 × g, and 500 μl of the resultant supernatant was scintillation-counted to quantify released [32P]Pi. To determine the specific Pi produced, the background [32P]Pi detected at 0 s was subtracted from each reaction.
GTPγS Binding Assay
GTPγS binding was measured using a filter binding method (8, 26). His-Gαi3 (100 nm) was preincubated with different concentrations of wild-type His-GIV-CTs-(1660–1870) or His-GIV-CTs-(1660–1870) F1685A mutant, for 15 min at 30 °C in assay buffer (20 mm sodium HEPES, pH 8, 100 mm NaCl, 1 mm EDTA, 25 mm MgCl2, 1 mm DTT, 0.05% (w/v) C12E10). Reactions were initiated at 30 °C by adding an equal volume of assay buffer containing 1 μm [35S]GTPγS (∼50 cpm/fmol). Duplicate aliquots (50 μl) were removed at 15 min, and binding of radioactive nucleotide was stopped by addition of 3 ml of ice-cold wash buffer (20 mm Tris-HCl, pH 8.0, 100 mm NaCl, 25 mm MgCl2). The quenched reactions were rapidly passed through BA-85 nitrocellulose filters (Amersham Biosciences) and washed with 4 ml wash buffer. Filters were dried and subjected to liquid scintillation counting. To determine the specific nucleotide binding, the background [35S]GTPγS detected in the absence of G protein was subtracted from each reaction.
Trypsinization of Gα subunits
His-Gαi3, His-Gαo, or the indicated His-Gαi3 mutants (0.5 mg/ml) were incubated for 120 min at 30 °C in the presence of GDP (30 μm) or GDP·AlF4− (30 μm GDP, 30 μm AlCl3, 10 mm NaF). After incubation, trypsin was added to the tubes (final concentration, 12.5 μg/ml), and samples were incubated for an additional 10 min at 30 °C. Reactions were stopped by adding SDS-PAGE sample buffer and boiling. Proteins were resolved by SDS-PAGE and stained with Coomassie Blue.
Immunoblotting
Proteins samples were separated on 10% SDS-PAGE and transferred to polyvinylidene difluoride membranes (Millipore, Billerica, MA). Membranes were blocked with phosphate-buffered saline supplemented with 5% nonfat milk (or 5% bovine serum albumin when probing for Akt) before sequential incubation with primary and secondary antibodies. Infrared imaging with two-color detection was performed using an Odyssey imaging system (Li-Cor Biosciences, Lincoln, NE). Primary antibodies were diluted as follows: anti-GIV (coiled-coil), 1:500; anti-AGS3, 1:1000; anti-His, 1:2000; anti-panGβ, 1:200; anti-pAkt (S473), 1:200; anti-Akt, 1:400; and anti-α-tubulin, 1:2500.
Cell Migration Assays
Scratch wound assays were done as described previously (18). Briefly, 1-mm wounds were created in monolayer cell cultures (∼100% confluent) with a 1-ml sterile pipette tip, and the cells were subsequently monitored by phase-contrast microscopy over the succeeding 24 h. To quantify cell migration (expressed as % wound area covered), images were analyzed using ImageJ (National Institutes of Health) software to calculate the difference between the wound area at 0 h and at the end of the migration assay divided by the area at 0 h × 100.
Statistical Analysis
Experiments were repeated at least three times, and results were presented either as one representative experiment or as mean ± S.E. when data from multiple independent experiments were pooled. Statistical significance between various conditions was assessed with the Student's t test. p < 0.05 was considered significant.
RESULTS
Validation of GIV as a Bona Fide GEF for Gαi subunits
In our previous work (11) we demonstrated that GIV was capable of increasing the steady-state GTPase activity of Gαi3. The steady-state GTP hydrolysis by Gα subunits is a reaction with two major steps, nucleotide exchange (i.e. release of GDP and loading of GTP) and GTP hydrolysis. The GTP hydrolysis step is 10- to 100-fold faster than the nucleotide exchange step. For this reason nucleotide exchange is rate-limiting for the steady-state GTPase reaction (27). Because GIV increases the steady-state GTPase activity of Gαi3 and this activity directly depends on the rate of nucleotide exchange, we proposed that GIV is a GEF for Gαi subunits.
To rule out an effect of GIV on GTP hydrolysis we performed single-turnover GTPase assays. Under these experimental conditions the nucleotide exchange step is bypassed and the GTPase activity depends solely on the rate of GTP hydrolysis (28). We measured the single-turnover GTPase activity of purified His-Gαi3 in the absence or presence of GIV-CTs-(1660–1870) (“s” stands for “short”), which contains the GEF motif of GIV. His-GIV-CTs was used, because it behaves the same as His-GIV-CT-(1623–1870) (13) in terms of its binding to Gαi3 (data not shown) and modulation of steady-state GTPase activity (Fig. 1A) but gives greater protein yields in E. coli. As a positive control we used GST-GAIP, a well characterized GAP (1) that accelerates the rate of GTP hydrolysis by Gαi subunits. GST-GAIP dramatically increased the single-turnover GTPase activity of Gαi3, whereas GIV-CTs had no effect (Fig. 1B) even at concentrations (2 μm) that provoke a maximal increase of the steady-state GTPase activity (Fig. 1A). This result demonstrates that GIV does not affect GTP hydrolysis in the steady-state GTPase reaction.
FIGURE 1.
GIV is a bona fide GEF for Gαi3. A, His-GIV-CT (aa 1623–1870) and His-GIV-CTs (aa 1660–1870) are equally efficient in increasing the steady-state GTPase activity of Gαi3. The steady-state GTPase activity of purified His-Gαi3 (50 nm) was determined in the presence of the indicated amounts (0, 0.1, 0.25, 0.5, 1, and 2 μm) of purified His-GIV-CT (aa 1623–1870, closed circles) or His-GIV-CTs (aa 1660–1870, open circles) by quantification of the amount of [γ-32P]GTP (0.5 μm, ∼50 cpm/fmol) hydrolyzed in 10 min. Data are expressed as % of GTP hydrolyzed by the G protein alone (0 μm His-GIV-CT or His-GIV-CTs). Results are shown as mean ± S.E. of n = 3 independent experiments. B, His-GIV-CTs does not affect the rate of GTP hydrolysis by Gαi3. Single-turnover GTPase assays for Gαi3 (50 nm) were performed as described under “Experimental Procedures” in the presence of His-GIV-CTs (2 μm, open circles), GST-GAIP (1 μm, “x”), or buffer (closed circles). GST-GAIP increases the rate of GTP hydrolysis by Gαi3, whereas His-GIV-CTs has no effect. One representative experiment of four is shown (C) His-GIV-CTs increases GTPγS binding to Gαi3. Nucleotide binding activity of purified His-Gαi3 (50 nm) was determined in the presence of the indicated amounts (0, 0.1, 0.5, 1, and 2 μm) of purified wild-type His-GIV-CTs (closed circles) or His-GIV-CTs F1685A mutant (open circles) by quantification of the amount of [35S]GTPγS (0.5 μm, ∼50 cpm/fmol) bound in 15 min. Data are expressed as % of GTPγS bound by the G protein alone in the absence of His-GIV-CTs. His-GIV-CTs increases GTPγS binding up to 2.2-fold over basal binding, whereas His-GIV-CTs F1685A has no significant effect. Results are shown as mean ± S.E. of n = 3 independent experiments.
To further validate the role of GIV as a GEF, we performed GTPγS binding experiments, which directly measure nucleotide exchange activity (8, 26). Purified His-Gαi3 was incubated in the presence of increasing amounts of wild-type His-GIV-CTs or the Gαi3 binding-deficient His-GIV-CTs F1685A mutant (11). GTPγS binding to Gαi3 was increased by His-GIV-CTs in a dose-dependent manner but was not significantly affected by His-GIV-CTs F1685A (Fig. 1C). At the maximal concentration of His-GIV-CTs tested (2 μm) GTPγS binding was increased up to ∼2.2-fold over basal binding. This result indicates that GIV increases nucleotide exchange by Gαi3 via its previously described GEF motif (11). Taken together these results demonstrate that GIV is a bona fide GEF for Gαi3 and validates the steady-state GTPase activity as a direct measure of its GEF activity.
Comparative Binding of GIV to Gαi3 and Gαo
We previously reported that GIV interacts with members of the Gi (includes Gαi1, Gαi2, Gαi3, and Gαo) and Gs subfamilies of G proteins in two-hybrid assays (14); however, in in vitro pulldown assays GIV preferentially binds to Gαi3 versus Gαs and binds as efficiently to Gαi1 and Gαi2 (11, 18) as to Gαi3. To gain insights into the structural features responsible for the preferential binding of GIV to Gαi subunits, we compared GIV binding to Gαi3 and Gαo1 (hereafter referred as Gαo), which also belongs to the Gi family and is the closest to Gαi subunits in sequence and structure (29). We found that both GST-Gαi3 and GST-Gαo interact with endogenous GIV in pulldown assays on COS7 lysates when the G protein is preloaded with GDP (inactive state (Fig. 2A)); however, GST-Gαi3 bound ∼15- to 20-fold more GIV than GST-Gαo. Neither Gαi3 nor Gαo bound GIV when preloaded with GDP and AlF4− (mimicking the active state) (Fig. 2A).
FIGURE 2.
GIV binds to and activates Gαi3 more efficiently than Gαo. A, GST-Gαi3·GDP (lane 1) binds ∼15- to 20-fold more endogenous GIV than GST-Gαo·GDP (lane 3). No binding of either Gαi3 or Gαo is detected in the presence of AlF4− (lanes 2 and 4). COS7 cell lysates (∼250 μg of protein) were incubated with ∼10 μg of purified GST-Gαi3 (lanes 1 and 2) or GST-Gαo (lanes 3 and 4) pre-loaded with GDP (lanes 1 and 3) or GDP·AlF4− (lanes 2 and 4) immobilized on glutathione beads. After extensive washing, bound proteins were separated by SDS-PAGE and analyzed by immunoblotting (IB) for GIV. Equal loading of GST proteins was confirmed by Ponceau S staining (lower panel). B, GST-Gαi3·GDP (lane 1) binds ∼10-fold more His-GIV-CT than GST-Gαo·GDP (lane 3). His-GIV-CT binding to both GST-Gαi3 and GST-Gαo is dramatically reduced in the presence of AlF4− (lanes 2 and 4). ∼3 μg of purified His-GIV-CT (aa 1623–1870) was incubated with ∼10 μg of purified GST-Gαi3 (lanes 1 and 2) or GST-Gαo (lanes 3 and 4) pre-loaded with GDP (lanes 1 and 3) or GDP·AlF4− (lanes 2 and 4) immobilized on glutathione beads. After extensive washing, bound proteins were separated by SDS-PAGE and analyzed by immunoblotting (IB) for His. Equal loading of GST proteins was confirmed by Ponceau S-staining (lower panel). C, His-GIV-CTs increases steady-state GTP hydrolysis of His-Gαi3 at least 3-fold more than His-Gαo at all concentrations tested. Results are shown as mean ± S.E. of nine independent experiments. The steady-state GTPase activity of purified His-Gαi3 (closed circles, 50 nm) or His-Gαo (open circles, 50 nm) was determined in the presence of the indicated amounts (0, 0.1, 0.5, and 2 μm) of purified His-GIV-CTs (aa 1660–1870) by quantification of the amount of [γ-32P]GTP (0.5 μm, ∼50 cpm/fmol) hydrolyzed in 10 min. Data is expressed as % of GTP hydrolyzed by the G protein alone in the absence of His-GIV-CTs.
Because the state-dependent interaction of GIV with inactive Gαi is mediated by the GEF motif located in the C terminus, we next investigated the ability of both GST-Gαi3 and GST-Gαo immobilized on beads to bind purified His-GIV-CT-(1623–1870) in vitro. The findings were similar to those obtained for endogenous GIV from cell lysates: His-GIV-CT bound preferentially to inactive (GDP-bound) Gαi3 and Gαo, and binding to Gαi3 was ∼10-fold greater than to Gαo (Fig. 2B). These results indicate that, although GIV-CT can bind to both Gαi3·GDP and Gαo·GDP, binding to Gαo·GDP is much less efficient.
Comparative Activation of Gαi3 and Gαo by GIV
Based on our recent finding (11) that binding of the GEF motif of GIV to the Gα subunit is required for GIV to exert its GEF function, we hypothesized that its decreased binding to Gαo·GDP might affect its GEF activity. To test if this is the case, we measured the steady-state GTPase activity of purified His-Gαi3 and His-Gαo in the presence of increasing amounts of purified GIV-CTs. The relative increase in the steady-state GTP hydrolysis exerted by GIV-CTs on Gαo was significantly reduced compared to its effect on Gαi3 at all concentrations tested (Fig. 2C). At the maximal concentration of GIV-CTs tested (2 μm), activation of Gαo was ∼75% less than Gαi3 (Table 1). Taken together, these results demonstrate that GIV is a more efficient GEF for Gαi3 than for Gαo, and thus is capable of discriminating among Gα subunits of the Gi subfamily.
TABLE 1.
Comparative activation of Gαi3 and Gαo by GIV-CTs
Experiments were performed as described for Fig 2C. Reduction in G protein activation by GIV-CTs was calculated as percent decrease in the -fold activation of Gαo compared to -fold activation of Gαi3 as described under “Experimental Procedures.” All parameters are expressed as mean ± S.E. of n = 9 independent experiments.
| Basal GTP hydrolysis in 10 min | G protein activation by GIV-CTsa | Reduction in G protein activation by GIV-CTs compared to wt Gαi3a | |
|---|---|---|---|
| mol GTP/mol Gα | -fold increase | % | |
| Gαi3 | 0.11 ± 0.01 | 3.17 ± 0.18 | |
| Gαo | 0.54 ± 0.03 | 1.47 ± 0.14 | 78.4 ± 6.5 |
a GIV-CTs concentration = 2 μm.
Mapping the Region of the Gα Subunit That Determines Preferential Binding of GIV to Gαi
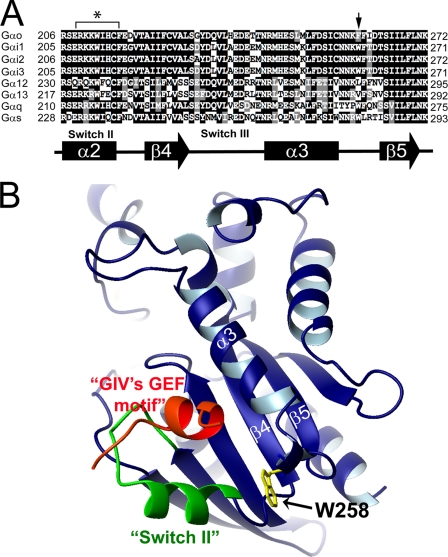
Gαi1, Gαi2, Gαi3, and Gαo, share ∼75% sequence identity and have a similar tertiary structure (29). In our previous work we found that the GEF motif of GIV binding to the switch II on Gαi subunits (11), but this region is 100% identical in Gαo. We reasoned that another region of Gαi3 must be responsible for GIV binding. To localize the region that specifies the strength of the interaction of Gαi3 with GIV, we generated a number of GST-Gαi3/Gαo chimeras (Fig. 3A). wt Gαi3 and Gαi3/o chimera 1 (contains the all-helical domain of Gαo), bound similar amounts of endogenous GIV from COS7 lysates, whereas Gαi3/o chimera 2 (contains the Ras-like domain of Gαo), showed dramatically reduced GIV binding (Fig. 3B). In addition, chimera 3 (contains the C-terminal half, aa 272–354) but not chimera 4 (contains the N-terminal half, aa 178–271 of the Ras-like domain of Gαo) bound as much GIV as wt Gαi3 (Fig. 3B). None of the chimeras bound GIV when they were preloaded with GDP·AlF4− (Fig. 3B). These results indicate that aa 178–270 of Gαi3 (corresponding to 179–271 of Gαo) where switches I, II, and III are located (Fig. 3A) contains the determinants that specify the preferential binding of GIV to Gαi3 versus Gαo.
FIGURE 3.
Amino acids 178–270 of Gαi3 are responsible for the preferential binding of GIV to Gαi3versus Gαo. A, schematic showing the Gαi3/o chimeras (1–4) used in B. In B: Upper panel, GDP-loaded GST-Gαi3/o chimera 1 (lane 6) and chimera 3 (lane 10) bind as much full-length GIV as GST-Gαi3 (lane 4), whereas GST-Gαi3/o chimera 2 (lane 8) and chimera 4 (lane 12) show dramatically reduced GIV binding. Binding of GIV to GDP·AlF4−-loaded G proteins is absent in all cases (lanes 3, 5, 7, 9, 11, and 13). COS7 cell lysates were incubated with purified GST (lanes 2 and 3), GST-Gαi3 (lanes 4 and 5) or the indicated GST-Gαi3/o chimeras (lanes 6–13) pre-loaded with GDP (lanes 2, 4, 6, 8, 10, and 12) or GDP·AlF4− (lanes 3, 5, 7, 9, 11, and 13) immobilized on glutathione beads and analyzed as in Fig. 2A. A higher exposure of the same immunoblot (middle panel) shows the weak binding observed for GST-Gαi3/o chimera 2 (lane 8) and 4 (lane 12). Equal loading of GST proteins was confirmed by Ponceau S staining (lower panel).
Identification of a Single Residue That Determines GIV Preferential Binding and Activation of Gαi3
We reasoned that one or several of the residues within aa 178–270 of Gαi3 that are conserved among Gαi1, Gαi2, and Gαi3 but different in Gαo must be responsible for the preferential binding of GIV to Gαi subunits. To identify such residues we aligned the sequences of Gαo, Gαi1, Gαi2, and Gαi3 (Fig. 4A). We mutated residues conserved among Gαi subunits but differing from Gαo to the corresponding amino acids of Gαo (specified in Fig. 4A) and tested their ability to bind endogenous GIV in GST pulldown assays on COS7 cell lysates. The Gαi3 W258F/T260I double mutant and the Gαi3 W258F single mutant showed a dramatic reduction in GIV binding, whereas the remainder of the mutants were the same as wt Gαi3 (Fig. 4B). Importantly, the GST-Gαi3 W258F mutant bound as much AGS3 and Gβγ as wt Gαi3 (data not shown), suggesting that the decreased GIV binding is specific and not due to an overall effect on the structure of Gα subunits. In addition to the mutants specified in Fig. 4A, two other mutants (Gαi3 E193N/Y195H/K197R/M198L and Gαi3 D229G/L232Q) were used in similar pulldown assays, but no differences in GIV binding were observed from wt Gαi3 (data not shown).
FIGURE 4.
Trp-258 of Gαi3 is responsible for GIV binding and activation of Gαi3. A, sequence alignment of Gαo, Gαi1, Gαi2, and Gαi3 indicating the Gαi3 mutants studied. Rat Gαo, Gαi1, Gαi2, and Gαi3 sequences corresponding to Gαi3 aa 178–270 were obtained form the NCBI data base and aligned using ClustalW. Conserved identical residues are in black; similar residues are shaded in gray. The secondary structure elements (α = α-helix, β = β-sheet) indicated below the alignment are named according to their crystal structures (29, 33). Residues conserved among Gαi1, Gαi2, and Gαi3 but different in Gαo within the 178–270 region were mutated in GST-Gαi3 to the corresponding residue in Gαo (indicated above with arrows). B, mutations W258F/T260I and W258F, in the α3/β5 loop, impair endogenous GIV binding to GDP-loaded GST-Gαi3, whereas mutations G217D, L232Q, A235H/E239T/M240T, and K248M do not affect binding (upper panels). GIV binding to GDP·AlF4−-loaded G proteins is virtually absent in all cases (lower panels). COS7 cell lysates were incubated with ∼5 μg of purified GST-Gαi3 or the indicated GST-Gαi3 mutants pre-loaded with GDP (upper panels) or GDP·AlF4− (lower panels) immobilized on glutathione beads and analyzed as in Fig. 2A. Equal loading of GST proteins was confirmed by Ponceau S staining. C, binding of GST-GIV-CT to His-Gαi3 W258F (lane 6) is reduced (∼80%) compared with wt His-Gαi3 (lane 3). ∼3 μg of purified wt His-Gαi3 (lanes 1–3) or His-Gαi3 W258F (lanes 4–6) pre-loaded with GDP were incubated with ∼9 μg of GST (lanes 2 and 5) or GST-GIV-CT (aa 1623–1870, lanes 3 and 6) immobilized on glutathione beads. Bound proteins were analyzed by immunoblotting (IB) for His. Equal loading of GST proteins was confirmed by Ponceau S staining (lower panel). D, mutation of Trp-258 to Phe reduces (∼60–70%) activation of His-Gαi3 by His-GIV-CTs at all concentrations tested. The steady-state GTPase activity of purified His-Gαi3 (closed circles, 50 nm) or His-Gαi3 W258F (open circles, 50 nm) was determined as in Fig. 2C. Results are shown as mean ± S.E. of n = 9 (Gαi3) or n = 5 (Gαi3 W258F) independent experiments. E, mutation of Phe-259 to Trp increases (∼2-fold) activation of His-Gαo by His-GIV-CTs at all concentrations tested. The steady-state GTPase activity of purified His-Gαo (closed circles, 50 nm) or His-Gαo F259W (open circles, 50 nm) was determined as in Fig. 2C. Results are shown as mean ± S.E. of three independent experiments.
To further confirm that the W258F mutation directly affects interaction between GIV and the G protein we carried out protein interaction assays with purified recombinant proteins. GST-GIV-CT was immobilized on glutathione-agarose beads and incubated with His-tagged wt Gαi3 or Gαi3 W258F. Binding of His-Gαi3 W258F was dramatically reduced compared to wt Gαi3 (Fig. 4C). Thus, Trp-258, which is located in the α3/β5 loop of Gαi, is a critical determinant of its interaction with GIV.
We next investigated the GEF activity of GIV on Gαi3 W258F and found that the relative increase in steady-state GTP hydrolysis exerted by GIV-CTs on Gαi3 W258F was significantly reduced compared with wt Gαi3 (Fig. 4D). At the maximal concentration of the GEF tested (2 μm), activation of Gαi3 W258F was reduced >65% (Table 2), which is comparable to that observed for Gαo (Fig. 2C and Table 1). In addition, we tested the GEF activity of GIV on a Gαo mutant in which Phe-259 was replaced by the corresponding aa (Trp-258) in Gαi3. We found that mutation of Phe-259 to Trp enhanced the relative increase in steady-state GTP hydrolysis exerted by GIV-CTs on Gαo ∼2-fold (Fig. 4E), indicating that the F259W mutation is sufficient to make Gαo a better substrate for the GEF activity of GIV. From these results we conclude that mutation of Trp-258 in the α3/β5 loop of Gαi3 to the corresponding amino acid (Phe) in Gαo dramatically reduces GIV binding and accounts for the reduced GEF activity of GIV on Gαo.
TABLE 2.
Comparative activation of wt Gαi3 and Gαi3 W258 mutants by GIV-CTs
Experiments were performed as described for Fig 5A. Reduction in G protein activation by GIV-CTs was calculated as percent decrease in the -fold activation of each Gαi3 mutant compared to -fold activation of wt Gαi3 as described under “Experimental Procedures.” All parameters are expressed as mean ± S.E. of n = 3–9 independent experiments.
| Basal GTP hydrolysis in 10 min | G protein activation by GIV-CTsa | Reduction in G protein activation by GIV-CTs compared to wt Gαi3a | |
|---|---|---|---|
| mol GTP/mol Gα | -fold increase | % | |
| Gαi3 wt | 0.11 ± 0.01 | 3.17 ± 0.18 | |
| Gαi3 W258F | 0.11 ± 0.01 | 1.72 ± 0.03 | 66.9 ± 1.3 |
| Gαi3 W258A | 0.14 ± 0.01 | 1.13 ± 0.03 | 93.7 ± 1.4 |
| Gαi3 W258L | 0.12 ± 0.01 | 1.58 ± 0.18 | 73.3 ± 9.4 |
| Gαi3 W258S | 0.11 ± 0.01 | 1.47 ± 0.04 | 78.1 ± 1.9 |
| Gαi3 W258Y | 0.11 ± 0.01 | 1.84 ± 0.04 | 61.1 ± 1.8 |
| Gαi3 W258D | 0.11 ± 0.01 | 1.11 ± 0.03 | 94.8 ± 1.6 |
| Gαi3 W258H | 0.14 ± 0.02 | 1.42 ± 0.06 | 80.6 ± 2.8 |
a GIV-CTs concentration = 2 μm.
Trp-258 Is Critical for Activation of Gαi3 by GIV
Mutation of Trp-258 to Phe (aromatic to an aromatic side chain) is a conservative mutation; yet it significantly reduces Gαi3 activation by GIV. Based on this finding we reasoned that functional Gαi3-GIV coupling should be very sensitive to alterations in aa 258 of Gαi3. To test if this is the case, we performed further mutational analysis by replacing Trp-258 with amino acids of different nature, i.e. tyrosine (aromatic), leucine (aliphatic), serine (polar), histidine (basic), aspartate (acidic), and alanine (small). The mutants were then tested for their response to GIV in steady-state GTPase assays. All the Gαi3 Trp-258 mutants showed reduced activation by GIV-CTs compared to wt Gαi3, but the relative decrease in GTPase hydrolysis varied (Fig. 5A). At the maximal concentration of GIV-CTs tested (2 μm), G protein activation was as follows: W258A ∼ W258D < W258S ∼ W258H ∼ W258L < W258Y (Table 2). The W258A and W258D mutants showed a ∼90–95% reduction and W258Y a ∼60% reduction in activation, a value very similar to that observed for W258F (Table 2). These results highlight the specific requirement for Trp in position 258 to achieve maximal activation by GIV.
FIGURE 5.
Gαi3 activation by GIV is reduced when Trp-258 is replaced by Tyr, Leu, Ser, His, Asp, or Ala. A, all the Gαi3 Trp-258 mutants investigated show reduced activation by His-GIV-CT ranging from a ∼60% (W258Y) to ∼95% (W258D) reduction. Trp-258 of His-Gαi3 was mutated to amino acids of different nature, i.e. tyrosine (aromatic), leucine (aliphatic), serine (polar), histidine (basic), aspartate (acidic), and alanine (small). The steady-state GTPase activity of purified His-Gαi3 (closed circles, 50 nm) or the indicated His-Gαi3 mutants (open circles, 50 nm) was determined as described in Fig. 1C. Results are shown as mean ± S.E. of 4–9 independent experiments. B, binding of GST-GIV CT to His-Gαi3 mutants W258F (lane 3), W258A (lane 9), and W258D (lane 12) is reduced compared to wt His-Gαi3 (lane 6). ∼3 μg of purified His-Gαi3 (lanes 4–6) or the indicated His-Gαi3 mutants (W258F: lanes 1–3, W258A: lanes 7–9, W258D: lanes 10–12) pre-loaded with GDP were incubated with ∼9 μg of GST (lanes 2, 5, 8, and 11) or GST-GIV-CT (aa 1623–1870, lanes 3, 6, 9, and 12) immobilized on glutathione beads. Bound proteins were analyzed by immunoblotting (IB) for His. Equal loading of GST proteins was confirmed by Ponceau S staining (lower panel).
Results from pulldown assays with purified recombinant proteins were consistent with the results of the GTPase assay: binding of GST-GIV-CT to His-Gαi3 W258A or W258D was virtually abolished (Fig. 5B) and less than to His-Gαi3 W258F (Fig. 5B). Thus the extent of GIV binding parallels the extent of Gαi3 activation (Figs. 4D and 5A and Table 2).
Some mutations that cause reduced activation of Gα subunits by GPCRs also decrease their activation by AlF4− (30–32), which activates G proteins by mimicking the γ-phosphate of GTP in the transition state (33). To test the ability of the Gα mutants to be activated by AlF4− we took advantage of a well established assay based on differential resistance to proteolysis (31, 34). When Gα subunits are in the inactive GDP-bound conformation, they are readily digested by trypsin, whereas upon AlF4− binding and adoption of the active conformation only a short sequence can be cleaved, and the remainder of the protein remains trypsin-resistant (34). All the His-Gαi3 Trp-258 mutants (see Table 2) as well as His-Gαo behaved like wt His-Gαi3 in that they were hydrolyzed by trypsin when pre-loaded with GDP but generated a trypsin-resistant form when preloaded with GDP·AlF4− (Fig. 6). Thus all the Gα subunits tested can efficiently adopt the active conformation upon AlF4− binding. Because activation by AlF4− requires the nucleotide binding site to contain GDP and the G protein to be in an appropriate conformation, these results also indicate that the Gα proteins are properly folded. In addition, all the Gαi3 Trp-258 mutants had similar basal rates of steady-state GTPase hydrolysis (see Table 2), suggesting that the spontaneous exchange of nucleotide is unaffected and that they fold properly and maintain their native properties. Collectively, these results support the conclusion that mutations in position 258 of Gαi3 specifically alter GIV-catalyzed activation without causing global structural changes in the Gα subunit.
FIGURE 6.
Mutations at Trp-258 do not affect the trypsin sensitivity of Gαi3 after activation by AlF4−. Gαi3 wt and all the Gα mutants tested are digested when pre-loaded with GDP, and all adopt the trypsin-resistant conformation after incubation with GDP·AlF4−. His-Gαi3 and the indicated His-Gαi3 mutants or His-Gαo (0.5 mg/ml) were incubated in the presence of GDP or GDP·AlF4− and treated or not with trypsin as described under “Experimental Procedures.” A Coomassie Blue-stained gel of a representative experiment is shown. The arrowhead denotes the position of the non-trypsinized full-length proteins loaded, and the asterisk arrowhead denotes the trypsin-resistant form of the active, GDP·AlF4−-loaded His-Gα subunit.
Mutation of Trp-258 Impairs Gαi3 Binding to GIV in Cultured Cells
Next, we investigated the effect of mutating Trp-258 on the interaction between GIV and the G protein in cultured cells. COS7 cells were transfected with FLAG-tagged wt Gαi3 and Gαi3 mutants, and immunoprecipitation was carried out using anti-FLAG IgG followed by immunoblotting for GIV. We found that the amount of endogenous GIV that co-immunoprecipitated with the Gαi3 mutants W258F, W258A, and W258D was dramatically reduced compared with wt Gαi3 (Fig. 7). These results demonstrate that mutation of Trp-258 impairs the interaction of Gαi3 with endogenous GIV in cultured cells, corroborating our observations in vitro.
FIGURE 7.
Mutation of Trp-258 to Asp, Ala, or Phe impairs the interaction between Gαi3 and endogenous GIV in cultured cells. Upper panel: co-immunoprecipitation of GIV, Gβγ, and AGS3 with FLAG-tagged Gαi3 mutants W258D (lane 2) or W258A (lane 3) is dramatically reduced compared with wt Gαi3 (lane 4). By contrast, with Gαi3 W258F (lane 5) only co-immunoprecipitation of GIV, but not Gβγ and AGS3 is reduced compared to controls. COS7 cells were transfected with empty vector (lane 1) or plasmids encoding FLAG-tagged Gαi3 W258D (lane 2), Gαi3 W258A (lane 3), wt Gαi3 (lane 4), or Gαi3 W258F (lane 5). 48 h after transfection cells were harvested, and lysates were used for immunoprecipitation (IP) with anti-FLAG IgG (∼2 μg) as described under “Experimental Procedures.” IP was followed by immunoblotting (IB) for FLAG (Gαi3), GIV, AGS3, and Gβ (pan-Gβ). Equal IgG loading was confirmed by Ponceau S staining. Lower panel: aliquots of the lysates (10%) were analyzed by immunoblotting (IB) to confirm the equal loading of Gαi3, GIV, AGS3, and Gβ and the expression of Gαi3-FLAG constructs in the different transfected samples.
Mutation of Trp-258 to Phe Does Not Affect Binding of Gβγ, AGS3, GAIP/RGS19, or LPAR1 to Gαi3
We next investigated if mutation of Trp-258 of Gαi3 to Phe interferes with its interaction with other binding partners such as Gβγ and AGS3 (a Gαi-guanine nucleotide dissociation inhibitor (21, 35)). We also investigated the behavior of Gαi3 W258A and W258D, because these two mutants have reduced GIV binding both in vitro and in cultured cells (Figs. 5 and 7) and show the most dramatic reduction in activation by GIV (Fig. 5 and Table 2). We found that Gαi3 W258F binds Gβγ and AGS3 as efficiently as wt Gαi3 in co-immunoprecipitation assays (Fig. 7), whereas binding of Gαi3 W258A or W258D to Gβγ and AGS3 was dramatically reduced (Fig. 7). From these results we conclude that mutation of Trp-258 to Phe specifically impairs Gαi3 interaction with GIV without affecting its interaction with Gβγ and AGS3, whereas this is not the case for mutation of Trp-258 to Asp or Ala.
Thus mutation of Trp-258 specifically to Phe can be tolerated for the interaction of the G protein with binding partners other than GIV (e.g. Gβγ and AGS3), whereas mutation of Trp-258 to Ala or Asp most likely affects the structural properties of the G protein such that they impair its interaction with Gβγ and AGS3. We further investigated the ability of Gαi3 W258F to interact with GAIP (RGS19) (22), a GAP for Gαi subunits (36). Identical results were obtained with wt Gαi3 and Gαi3 W258F: GST-GAIP bound robustly to Gαi3 preloaded with GDP-AlF4− but showed virtually no binding to inactive, GDP-loaded Gαi3 (22) (Fig. 8A), demonstrating that this mutation does not compromise the interaction with GAIP.
FIGURE 8.
Gαi3 W258F binds GAIP and LPAR1 as efficiently as wt Gαi3. A, mutation of Trp-258 to Phe does not affect Gαi3 interaction with GAIP. GDP·AlF4−-preloaded Gαi3 W258F (lane 6, upper panel) binds GAIP to the same extent as wt Gαi3 (lane 3, upper panel). Neither of the GDP-loaded G proteins binds GAIP (lanes 3 and 6, lower panel). ∼2 μg of purified wt His-Gαi3 (lanes 1–3) or His-Gαi3 W258F (lanes 4–6) pre-loaded with GDP·AlF4− (upper panel) or GDP (lower panel) were incubated with ∼5 μg of GST (lanes 2 and 5) or GST-GAIP (lanes 3 and 6) immobilized on glutathione beads. Bound proteins were analyzed by immunoblotting (IB) for His. 10% of the input His-Gα proteins were loaded in lanes 1 and 4. Equal loading of GST proteins was confirmed by Ponceau S staining. In B: Upper panel, FLAG-tagged LPAR1 (lanes 4–6) co-immunoprecipitates with both exogenously expressed wt Gαi3 (lane 5) and Gαi3 W258F mutant (lane 6). Endogenous Gαi3 (lane 4) is not detected in FLAG-LPAR1 immunoprecipitates, and no Gαi3 is present in FLAG-immunoprecipitates from controls (lanes 1–3). 48 h after transfection cells were harvested and lysates used for immunoprecipitation (IP) with anti-FLAG IgG (∼2 μg) as described under “Experimental Procedures.” IP was followed by immunoblotting (IB) for FLAG (LPAR1) and Gαi3. Equal IgG loading was confirmed by Ponceau S staining. Lower panel, aliquots of cell lysates (∼5%) were analyzed for expression of FLAG (LPAR1), Gαi3, and α-tubulin by immunoblotting (IB) to confirm the expression of the analyzed proteins.
We also investigated the ability of Gαi3 W258F to interact with LPAR1, a GPCR that couples to Gαi/o subunits (23, 37, 38). COS7 cells were co-transfected with FLAG-tagged LPAR1 and untagged wt Gαi3 or Gαi3 W258F, and immunoprecipitation was carried out using anti-FLAG IgG followed by immunoblotting for Gαi3. We found that the amount of Gαi3 that co-immunoprecipitated with the receptor from lysates of COS7 cells transfected with either wt Gαi3 or Gαi3 W258F was virtually the same (Fig. 8B), indicating that mutation of Trp-258 to Phe does not affect the interaction of Gαi3 with LPAR1, a GPCR. Taken together, these results indicate that the W258F, but not the W258A or W258D mutation, specifically impairs activation of Gαi3 by GIV without perturbing other known interactions of the G protein.
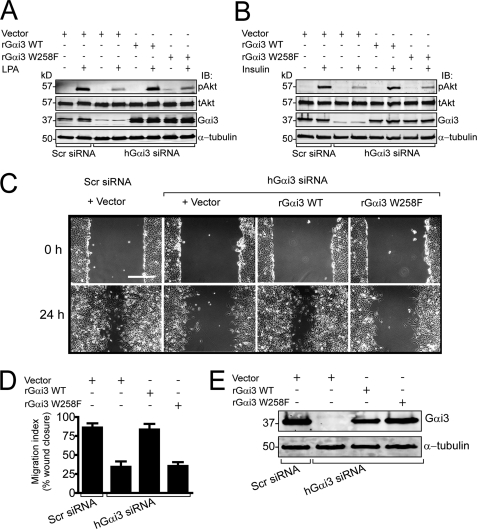
Gαi3 W258F Fails to Enhance LPA- and Insulin-stimulated Akt Activation and to Promote Cell Migration
We have previously shown that activation of Gαi3 enhances Akt signaling after stimulation of both GPCRs and receptor tyrosine kinases (18) and that the GEF motif of GIV is required for these functions (11). These effects might be triggered directly by activation of the G protein by GIV, or alternatively, they could be enhanced by GIV-independent activation of Gαi3. To distinguish between these two possibilities we took advantage of the GIV-insensitive Gαi3 W258F mutant. Gαi3 was depleted (>95%) in HeLa cells using siRNA oligonucleotides that specifically target the human sequence of Gαi3 (18), and Akt activation was measured in response to stimulation of either the LPA receptor, a GPCR that enhances Akt signaling by activating Gi proteins (37, 38), or the insulin receptor, a receptor tyrosine kinase. When serum-starved HeLa cells were stimulated with either LPA (Fig. 9A) or insulin (Fig. 9B), activation of Akt was dramatically reduced (∼70%) in Gαi3-depleted cells compared with controls, and this effect could be reversed by expression of wt rat Gαi3 (which is insensitive to human Gαi3-specific siRNA oligonucleotides (18)). Gαi3 depletion also impaired the ability of HeLa cells to migrate efficiently in scratch-wound assays (Fig. 9, C–E), and this effect was restored by expression of rat Gαi3 wt. By contrast, expression of rat Gαi3 W258F failed to restore Akt activation in response to either LPA or insulin (Fig. 9, A and B), and to reverse the defect on cell migration (Fig. 9, C–E). From these results we conclude that direct activation of Gαi3 by GIV is required to enhance Akt signaling and to promote cell migration after growth factor or GPCR stimulation.
FIGURE 9.
Gαi3 W258F fails to rescue Akt activation and cell migration defects observed upon depletion of endogenous Gαi3. A and B, HeLa cells treated with scrambled (Scr) or hGαi3 siRNA oligonucleotides and the indicated DNA plasmids (empty vector, rGαi3 WT, or rGαi3 W258F) were serum-starved for 6 h and stimulated with 10 μm LPA (A) or 100 nm insulin (B) for 5 min. Cell lysates were analyzed by immunoblotting (IB) for total (tAkt) and S473 phospho-Akt (pAkt), Gαi3, and α-tubulin. Depletion of endogenous Gαi3 reduces LPA-stimulated (A) and insulin-stimulated (B) Akt activation by ∼70%, which is restored upon transfection of wt rGαi3 but not rGαi3 W258F. C, in controls (Scr siRNA), HeLa cells cover the majority of the experimental wound area after 24 h, whereas in Gαi3-depleted cells wound closure is greatly impaired. The ability to migrate and close the wound area at 24 h is restored by transfection of rGαi3 wt but not rGαi3 W258F. HeLa cell monolayers treated with the indicated siRNA oligonucleotides, and DNA plasmids were scratch-wounded and examined by light microscopy immediately (0 h) or 24 h after wounding. Scale bar = 500 μm. D, bar graph showing quantification of the wound area covered by cells in C. The area covered by cells was determined by calculating the difference between the wound area at 0 and 24 h expressed as percent of the wound area at 0 h. Results are shown as mean ± S.D. of 8–12 randomly chosen fields from three independent experiments. E, cell lysates from cells treated as in C were immunoblotted to assess the efficiency of siRNA depletion of Gαi3 (∼95%) and the expression of rGαi3 WT or rGαi3 W258F.
DISCUSSION
GIV is a recently characterized non-receptor GEF that can activate Gαi3 (11). The major finding in this work is the identification of a novel structural determinant on Gαi3 that renders the G protein sensitive to activation by GIV. This structural determinant is required to promote efficient cell migration and Akt signaling, two cell functions that we have previously shown to be triggered by active Gαi3 (18). Using site-directed mutagenesis, we demonstrate here that Trp-258 located in the α3/β5 loop of the Ras-like domain of Gαi subunits is required to establish an efficient interaction with GIV and to activate the G protein. When Trp-258 is mutated to Phe, Gαi3 is less efficiently activated by GIV, but it retains its ability to interact with Gβγ subunits, AGS3, GAIP (RGS19), and LPAR1, to change conformation upon activation and to efficiently hydrolyze GTP (Figs. 6–8 and Table 2). In addition, the GIV-insensitive Gαi3 W258F mutant fails to enhance LPA- and insulin-stimulated activation of Akt and to promote cell migration (Fig. 9). These results are consistent with our previous work in that the GEF-deficient F1685A mutant of GIV also fails to promote LPA- and insulin-stimulated activation of Akt and cell migration (11).
In our previous work (11, 14, 18) we found that GIV interacts with Gα subunits of the Gi subfamily (Gαi1, Gαi2, Gαi3, and Gαo) and, to a lesser extent, the Gs subfamily (Gαs) but not those of the Gq and G12 (Gα12 and Gα13) subfamilies. Here we demonstrate that GIV can also discriminate within the Gi subfamily, because Gαi3 is a better substrate for the binding and GEF activity of GIV than the highly homologous Gαo (Fig. 2).
Based on structural studies (39–43) the α3/β5 loop has been proposed to be one of the critical elements that determines the binding specificity of Gα subunits of different families to their respective effectors (42). Our data suggest that the α3/β5 loop may be important not only for determining the effector binding specificity of different Gα subunits, but also for its interaction with GIV, a non-receptor GEF. For example, Gα subunits of the G12 subfamily, which do not bind GIV, have aliphatic amino acids (Val or Leu in Gα12 and Gα13) in the position corresponding to Trp-258 of Gαi3 (Fig. 10A). Our finding that mutation of Trp-258 to aliphatic amino acids impairs activation by GIV suggests that the inability of Gα12 and Gα13 to bind GIV (14) is, at least in part, a consequence of this difference within the α3/β5 loop. The sequence of Gα12 and Gα13 also differs from Gi in the Switch II region, which is a previously described binding site for GIV (11) and thus could contribute to their inability to bind GIV.
FIGURE 10.
Sequence comparison and three-dimensional view of the newly identified structural determinant in Gαi3 required for its activation by GIV. A, representative members of different Gα subfamilies were aligned to compare the sequence corresponding to the newly identified structural determinant in Gαi3. Alignment of rat Gαo, Gαi1, Gαi2, Gαi3, Gα12, Gα13, Gαq, and Gαs sequences obtained form the NCBI data base was performed using ClustalW. Conserved identical residues are in black; similar residues are shaded in gray. The secondary structure elements (α = α-helix, β = β-sheet) corresponding to these sequences are named according to previously reported crystal structures (29, 33) and are indicated below the alignment. The arrow denotes the position corresponding to the Trp-258 of Gαi3, and the asterisk denotes the previously identified binding site for GIV (11). B, three-dimensional view of the GEF motif of GIV bound to Gαi3 is shown to depict the relative location of the newly identified structural determinant (Trp-258 in the α3/β5 loop), which is positioned C-terminal to the GEF motif. The homology model of GDP·Gαi3 in complex with the GEF motif of GIV generated as described previously (11) using the structure of the synthetic peptide KB-752 bound to Gαi1 (PDB: 1Y3A) as a template (18). The “Ras-like” domain of Gαi3 is shown in blue, the “switch II” region in green, and Trp-258 in yellow. The GEF motif of GIV is shown in red.
Analysis of the sequence of Gαq and Gαs reveals that they have a Trp conserved in the positions corresponding to the Trp-258 of Gαi3 (Fig. 10A), yet they interact poorly with GIV (14, 18). Therefore, in these cases the Gα specificity of GIV cannot be simply attributed to this single residue. However, Gαq and Gαs show significant sequence divergence from Gαi in the residues of the α3/β5 loop that surround the position corresponding to Trp-258 of Gαi3 (Fig. 10A). We propose that this difference in the sequence of the α3/β5 loop could modify its structural properties and account for the decreased GIV binding observed for Gq and Gs (14, 18). In the case of Gαs, this idea is supported by the fact that the α3/β5 loop adopts a conformation completely different from Gαi subunits (40, 41). In addition, subtle differences between Gαq/Gαs and Gαi in some of the residues close to the GIV binding site in the Switch II region (Fig. 10A) may also contribute to the binding specificity of GIV.
Mutants that selectively abolish the ability of Gαi subunits to be regulated by GAPs (44) or guanine nucleotide dissociation inhibitors (45) have been described and used to evaluate the role of these regulators in Gi functions (44–50). Gαi3 W258F represents a new addition to the growing battery of mutants that can be used to finely dissect how different regulators of G protein activity control cell fate. Although Gi-coupled GPCRs also couple efficiently to Gαo subunits (51–54), here we show that replacement of a single residue (Trp-258) in Gαi3 for the corresponding residue in Gαo (Phe) dramatically and specifically reduces G protein coupling to GIV. Using this mutant we provide evidence that cell migration and Akt signaling, cell functions previously described to be promoted by constitutively active Gαi3 mutants (18), require Gαi3 to be specifically activated by GIV. The Gαi3 W258F mutant will also be useful in the future to distinguish other functions of Gαi subunits controlled by the GEF activity of GIV.
The data presented here suggest that the footprint of the GIV GEF domain on Gαi3 probably extends from the previously described binding site within switch II (11) to make contact with an additional binding site located in the α3/β5 loop. Based on our homology modeling (11) depicted in Fig. 10B, the GEF motif of GIV docks within the groove formed between the switch II and the α3 helix of the G protein. The location of the novel structural determinant in Gαi3 required for binding of GIV raises the interesting possibility that GIV residues C-terminal to the previously described GEF motif may be involved in making direct contact with the α3/β5 loop region surrounding Trp-258. However, at this point allosteric effects cannot be ruled out to explain the decreased interaction between GIV and Gαi3 upon mutation of Trp-258. Nevertheless, our unpublished work6 favors the possibility of a direct contact site, because mutation of GIV residues C-terminal to the previously described GEF motif impairs the Gαi3-GIV interaction.
Our identification of a novel structural determinant in Gαi3 required for GIV binding provides insights that may help in the design of selective pharmacological agents that disrupt the Gαi-GIV interface for therapeutic purposes. We previously found that expression of full-length GIV is induced severalfold in cancer cell lines that are highly metastatic (18) and that mutational disruption of the GIV binding to switch II of Gαi abolishes Akt signaling and tumor cell migration (11), which are hallmarks of cancer metastasis (55–58). Here we describe that GIV binding and these functions are similarly abolished by mutational disruption of the α3/β5 loop of Gαi3. Because alterations in the switch II may also impair the binding of other molecules to the G protein (59–61), the α3/β5 loop represents a more attractive target than the previously described switch II binding site. Moreover, we show here that alterations in aa 258 of Gαi3 can impair GIV binding and abolish cell functions controlled by GIV without affecting the interaction of Gαi3 with its other binding partners Gβγ, AGS3, GAIP/RGS19, and LPAR1. Thus we envision that small molecules that target this site might work as anti-metastatic agents by specifically disrupting GIV-Gαi interaction.
Acknowledgments
We thank Patrick Kietrsunthorn, Michelle Adia, and Jasmine Wong for valuable technical support; Ruben Abagyan and Irina Kufareva (University of California, San Diego) for helpful discussions on the design of mutants; and Timo Meerloo for assistance with the preparation of the figures.
This work was supported, in whole or in part, by National Institutes of Health Grants DKI7780 and CA100768 (to M. G. F.).
M. Garcia-Marcos, P. Ghosh, J. Ear, and M. G. Farquhar, unpublished observations.
- GEF
- guanine nucleotide exchange factor
- GIV
- Gα-interacting, vesicle-associated protein
- GPCR
- G protein-coupled receptor
- GAP
- GTPase-activating protein
- RGS
- regulator of G protein signaling
- GAIP
- Gα-interacting protein
- AGS3
- activator of G protein signaling 3
- GST
- glutathione S-transferase
- IP
- immunoprecipitation
- IB
- immunoblot
- siRNA
- small interfering RNA
- LPA
- lysophosphatidic acid
- LPA1
- LPA receptor 1
- DTT
- dithiothreitol
- wt
- wild type
- aa
- amino acid(s)
- GTPγS
- guanosine 5′-3-O-(thio)triphosphate
- CMV
- cytomegalovirus.
REFERENCES
- 1.De Vries L., Zheng B., Fischer T., Elenko E., Farquhar M. G. (2000) Annu. Rev. Pharmacol. Toxicol. 40, 235–271 [DOI] [PubMed] [Google Scholar]
- 2.Gilman A. G. (1987) Annu. Rev. Biochem. 56, 615–649 [DOI] [PubMed] [Google Scholar]
- 3.Ross E. M., Wilkie T. M. (2000) Annu. Rev. Biochem. 69, 795–827 [DOI] [PubMed] [Google Scholar]
- 4.Oldham W. M., Hamm H. E. (2008) Nat. Rev. Mol. Cell Biol. 9, 60–71 [DOI] [PubMed] [Google Scholar]
- 5.Overington J. P., Al-Lazikani B., Hopkins A. L. (2006) Nat. Rev. Drug Discov. 5, 993–996 [DOI] [PubMed] [Google Scholar]
- 6.Cismowski M. J., Takesono A., Ma C., Lizano J. S., Xie X., Fuernkranz H., Lanier S. M., Duzic E. (1999) Nat. Biotechnol. 17, 878–883 [DOI] [PubMed] [Google Scholar]
- 7.Tall G. G., Krumins A. M., Gilman A. G. (2003) J. Biol. Chem. 278, 8356–8362 [DOI] [PubMed] [Google Scholar]
- 8.Afshar K., Willard F. S., Colombo K., Johnston C. A., McCudden C. R., Siderovski D. P., Gönczy P. (2004) Cell 119, 219–230 [DOI] [PubMed] [Google Scholar]
- 9.Natochin M., Campbell T. N., Barren B., Miller L. C., Hameed S., Artemyev N. O., Braun J. E. (2005) J. Biol. Chem. 280, 30236–30241 [DOI] [PubMed] [Google Scholar]
- 10.Lee M. J., Dohlman H. G. (2008) Curr. Biol. 18, 211–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia-Marcos M., Ghosh P., Farquhar M. G. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 3178–3183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malik S., Ghosh M., Bonacci T. M., Tall G. G., Smrcka A. V. (2005) Mol. Pharmacol. 68, 129–136 [DOI] [PubMed] [Google Scholar]
- 13.Nishimura A., Okamoto M., Sugawara Y., Mizuno N., Yamauchi J., Itoh H. (2006) Genes Cells 11, 487–498 [DOI] [PubMed] [Google Scholar]
- 14.Le-Niculescu H., Niesman I., Fischer T., DeVries L., Farquhar M. G. (2005) J. Biol. Chem. 280, 22012–22020 [DOI] [PubMed] [Google Scholar]
- 15.Anai M., Shojima N., Katagiri H., Ogihara T., Sakoda H., Onishi Y., Ono H., Fujishiro M., Fukushima Y., Horike N., Viana A., Kikuchi M., Noguchi N., Takahashi S., Takata K., Oka Y., Uchijima Y., Kurihara H., Asano T. (2005) J. Biol. Chem. 280, 18525–18535 [DOI] [PubMed] [Google Scholar]
- 16.Enomoto A., Murakami H., Asai N., Morone N., Watanabe T., Kawai K., Murakumo Y., Usukura J., Kaibuchi K., Takahashi M. (2005) Dev. Cell 9, 389–402 [DOI] [PubMed] [Google Scholar]
- 17.Jiang P., Enomoto A., Jijiwa M., Kato T., Hasegawa T., Ishida M., Sato T., Asai N., Murakumo Y., Takahashi M. (2008) Cancer Res. 68, 1310–1318 [DOI] [PubMed] [Google Scholar]
- 18.Ghosh P., Garcia-Marcos M., Bornheimer S. J., Farquhar M. G. (2008) J. Cell Biol. 182, 381–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnston C. A., Willard F. S., Jezyk M. R., Fredericks Z., Bodor E. T., Jones M. B., Blaesius R., Watts V. J., Harden T. K., Sondek J., Ramer J. K., Siderovski D. P. (2005) Structure 13, 1069–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kitamura T., Asai N., Enomoto A., Maeda K., Kato T., Ishida M., Jiang P., Watanabe T., Usukura J., Kondo T., Costantini F., Murohara T., Takahashi M. (2008) Nat. Cell Biol. 10, 329–337 [DOI] [PubMed] [Google Scholar]
- 21.De Vries L., Fischer T., Tronchère H., Brothers G. M., Strockbine B., Siderovski D. P., Farquhar M. G. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 14364–14369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Vries L., Elenko E., Hubler L., Jones T. L., Farquhar M. G. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 15203–15208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishii I., Contos J. J., Fukushima N., Chun J. (2000) Mol. Pharmacol. 58, 895–902 [DOI] [PubMed] [Google Scholar]
- 24.Vázquez-Prado J., Miyazaki H., Castellone M. D., Teramoto H., Gutkind J. S. (2004) J. Biol. Chem. 279, 54283–54290 [DOI] [PubMed] [Google Scholar]
- 25.Krumins A. M., Gilman A. G. (2002) Methods Enzymol. 344, 673–685 [DOI] [PubMed] [Google Scholar]
- 26.Sternweis P. C., Robishaw J. D. (1984) J. Biol. Chem. 259, 13806–13813 [PubMed] [Google Scholar]
- 27.Mukhopadhyay S., Ross E. M. (2002) Methods Enzymol. 344, 350–369 [DOI] [PubMed] [Google Scholar]
- 28.Berman D. M., Wilkie T. M., Gilman A. G. (1996) Cell 86, 445–452 [DOI] [PubMed] [Google Scholar]
- 29.Slep K. C., Kercher M. A., Wieland T., Chen C. K., Simon M. I., Sigler P. B. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 6243–6248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Codina J., Birnbaumer L. (1994) J. Biol. Chem. 269, 29339–29342 [PubMed] [Google Scholar]
- 31.Grishina G., Berlot C. H. (1998) J. Biol. Chem. 273, 15053–15060 [DOI] [PubMed] [Google Scholar]
- 32.Warner D. R., Weinstein L. S. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 4268–4272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coleman D. E., Berghuis A. M., Lee E., Linder M. E., Gilman A. G., Sprang S. R. (1994) Science 265, 1405–1412 [DOI] [PubMed] [Google Scholar]
- 34.Kleuss C., Raw A. S., Lee E., Sprang S. R., Gilman A. G. (1994) Proc. Natl. Acad. Sci. U.S.A. 91, 9828–9831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bernard M. L., Peterson Y. K., Chung P., Jourdan J., Lanier S. M. (2001) J. Biol. Chem. 276, 1585–1593 [DOI] [PubMed] [Google Scholar]
- 36.Huang C., Hepler J. R., Gilman A. G., Mumby S. M. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 6159–6163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moolenaar W. H., van Meeteren L. A., Giepmans B. N. (2004) BioEssays 26, 870–881 [DOI] [PubMed] [Google Scholar]
- 38.Ye X., Ishii I., Kingsbury M. A., Chun J. (2002) Biochim. Biophys. Acta 1585, 108–113 [DOI] [PubMed] [Google Scholar]
- 39.Chen Z., Singer W. D., Sternweis P. C., Sprang S. R. (2005) Nat. Struct. Mol. Biol. 12, 191–197 [DOI] [PubMed] [Google Scholar]
- 40.Sunahara R. K., Tesmer J. J., Gilman A. G., Sprang S. R. (1997) Science 278, 1943–1947 [DOI] [PubMed] [Google Scholar]
- 41.Tesmer J. J., Sunahara R. K., Gilman A. G., Sprang S. R. (1997) Science 278, 1907–1916 [DOI] [PubMed] [Google Scholar]
- 42.Tesmer V. M., Kawano T., Shankaranarayanan A., Kozasa T., Tesmer J. J. (2005) Science 310, 1686–1690 [DOI] [PubMed] [Google Scholar]
- 43.Slep K. C., Kercher M. A., He W., Cowan C. W., Wensel T. G., Sigler P. B. (2001) Nature 409, 1071–1077 [DOI] [PubMed] [Google Scholar]
- 44.Lan K. L., Sarvazyan N. A., Taussig R., Mackenzie R. G., DiBello P. R., Dohlman H. G., Neubig R. R. (1998) J. Biol. Chem. 273, 12794–12797 [DOI] [PubMed] [Google Scholar]
- 45.Willard F. S., Zheng Z., Guo J., Digby G. J., Kimple A. J., Conley J. M., Johnston C. A., Bosch D., Willard M. D., Watts V. J., Lambert N. A., Ikeda S. R., Du Q., Siderovski D. P. (2008) J. Biol. Chem. 283, 36698–36710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clark M. J., Harrison C., Zhong H., Neubig R. R., Traynor J. R. (2003) J. Biol. Chem. 278, 9418–9425 [DOI] [PubMed] [Google Scholar]
- 47.Fu Y., Huang X., Zhong H., Mortensen R. M., D'Alecy L. G., Neubig R. R. (2006) Circ. Res. 98, 659–666 [DOI] [PubMed] [Google Scholar]
- 48.Fu Y., Zhong H., Nanamori M., Mortensen R. M., Huang X., Lan K., Neubig R. R. (2004) Methods Enzymol. 389, 229–243 [DOI] [PubMed] [Google Scholar]
- 49.Huang X., Charbeneau R. A., Fu Y., Kaur K., Gerin I., MacDougald O. A., Neubig R. R. (2008) Diabetes 57, 77–85 [DOI] [PubMed] [Google Scholar]
- 50.Clark M. J., Neubig R. R., Traynor J. R. (2004) J. Pharmacol. Exp. Ther. 310, 215–222 [DOI] [PubMed] [Google Scholar]
- 51.Cavalli A., Druey K. M., Milligan G. (2000) J. Biol. Chem. 275, 23693–23699 [DOI] [PubMed] [Google Scholar]
- 52.Frank M., Thümer L., Lohse M. J., Bünemann M. (2005) J. Biol. Chem. 280, 24584–24590 [DOI] [PubMed] [Google Scholar]
- 53.Lane J. R., Powney B., Wise A., Rees S., Milligan G. (2008) J. Pharmacol. Exp. Ther. 325, 319–330 [DOI] [PubMed] [Google Scholar]
- 54.Wise A., Sheehan M., Rees S., Lee M., Milligan G. (1999) Biochemistry 38, 2272–2278 [DOI] [PubMed] [Google Scholar]
- 55.Altomare D. A., Testa J. R. (2005) Oncogene 24, 7455–7464 [DOI] [PubMed] [Google Scholar]
- 56.Ju X., Katiyar S., Wang C., Liu M., Jiao X., Li S., Zhou J., Turner J., Lisanti M. P., Russell R. G., Mueller S. C., Ojeifo J., Chen W. S., Hay N., Pestell R. G. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 7438–7443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qiao M., Iglehart J. D., Pardee A. B. (2007) Cancer Res. 67, 5293–5299 [DOI] [PubMed] [Google Scholar]
- 58.Qiao M., Sheng S., Pardee A. B. (2008) Cell Cycle 7, 2991–2996 [DOI] [PubMed] [Google Scholar]
- 59.Kimple R. J., Kimple M. E., Betts L., Sondek J., Siderovski D. P. (2002) Nature 416, 878–881 [DOI] [PubMed] [Google Scholar]
- 60.Tesmer J. J., Berman D. M., Gilman A. G., Sprang S. R. (1997) Cell 89, 251–261 [DOI] [PubMed] [Google Scholar]
- 61.Wall M. A., Coleman D. E., Lee E., Iñiguez-Lluhi J. A., Posner B. A., Gilman A. G., Sprang S. R. (1995) Cell 83, 1047–1058 [DOI] [PubMed] [Google Scholar]