Abstract
Oxysterol-binding protein (OSBP), a cytosolic receptor of cholesterol and oxysterols, is recruited to the endoplasmic reticulum by binding to the cytoplasmic major sperm protein (MSP) domain of integral endoplasmic reticulum protein VAMP-associated protein-A (VAP-A), a process essential for the stimulation of sphingomyelin synthesis by 25-hydroxycholesterol. To delineate the interaction mechanism between VAP-A and OSBP, we determined the complex structure between the VAP-A MSP domain (VAP-AMSP) and the OSBP fragment containing a VAP-A binding motif FFAT (OSBPF) by NMR. This solution structure explained that five of six conserved residues in the FFAT motif are required for the stable complex formation, and three of five, including three critical intermolecular electrostatic interactions, were not explained before. By combining NMR relaxation and titration, isothermal titration calorimetry, and mutagenesis experiments with structural information, we further elucidated the detailed roles of the FFAT motif and underlying motions of VAP-AMSP, OSBPF, and the complex. Our results show that OSBPF is disordered in the free state, and VAP-AMSP and OSBPF form a final complex by means of intermediates, where electrostatic interactions through acidic residues, including an acid patch preceding the FFAT motif, probably play a collective role. Additionally, we report that the mutation that causes the familial motor neuron disease decreases the stability of the MSP domain.
Keywords: Methods/Calorimetry, Methods/NMR, Protein/Molecular Dynamics, Protein/Binding/Lipid, Protein/Protein-Protein Interactions, Protein/Stability, Protein/Structure, Subcellular Organelles/Endoplasmic Reticulum
Introduction
Lipids are a major component of cell membranes and also act as signaling factors. A variety of cytosolic lipid-binding proteins is involved in lipid signal transduction and lipid transport (1). Because the endoplasmic reticulum (ER)2 membrane is a major site for lipid biosyntheses, many cytosolic lipid binding proteins localize at the ER membrane to perform their functions. Oxysterol-binding protein (OSBP) is a cytosolic receptor of cholesterol and oxysterols, such as 25-hydroxycholesterol, and has been implicated to play a role in vesicle transport, lipid metabolism, and signal transduction (2, 3). Wyles et al. (4) showed that OSBP localizes at the cytosolic surface of the ER membrane by interaction with integral ER protein VAMP-associated protein (VAP-A) and identified the region of OSBP required for binding to VAP-A. Shortly thereafter, Loewen et al. (5) found that the sequence EFFDAXE is conserved in OSBP and many other lipid binding proteins and is a transcriptional regulator specific to phospholipid synthesis and showed that the sequence acts as an ER-targeting determinant by interaction with VAP. Most of EFFDAXE sequences or sequences closely related to EFFDAXE have acidic flanking regions (5); therefore, these sequences are referred to as FFAT motifs because they consist of two phenylalanines (FF) in an acidic tract (AT). It has now been shown that FFAT motifs in some lipid-binding proteins play a role in targeting to the ER (6–8).
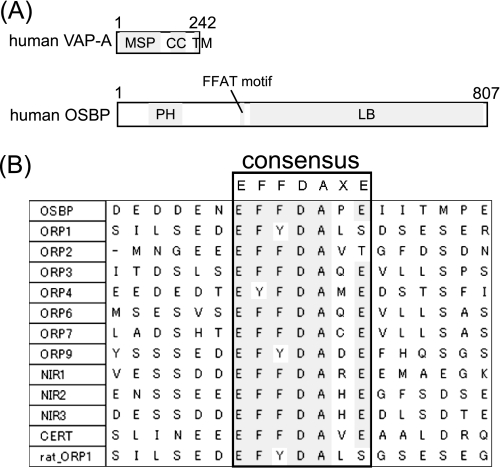
VAPs are type II membrane proteins that are conserved from yeast to human. Three VAP isoforms have been identified in humans: VAP-A, VAP-B, and VAP-C (a splicing variant of VAP-B) (9). VAPs generally localize at the ER, although they can localize at other subcellular organelles in some species and cell types (10–15). Most VAPs are composed of three domains; a major sperm protein (MSP) domain, a coiled-coil domain, and a transmembrane (TM) domain (Fig. 1A). The N-terminal region contains the highly conserved MSP domain. VAPs bind to FFAT motifs by the MSP domain. VAPs are involved in a diverse range of cellular events. Before discovery of the FFAT motif, VAPs were mainly considered to play a role in vesicle trafficking given that VAPs were shown to interact with proteins involved in vesicular fusion, although precise details of their functions were unclear (12, 16–19). After discovery of the FFAT motif, VAPs were shown to have important roles in non-vesicular lipid transport (8), lipid metabolism (20), the regulation of ER structure (7, 21), and the unfolded protein response (14) through interaction with FFAT motifs. Additionally, the P56S mutation in human VAP-B causes autosomal dominant motoneuronal diseases and familial amyotrophic lateral sclerosis 8 (ALS8), and P56S mutants of human VAP-B form aggregations in cells (22, 23). VAPs are important proteins whose functions are mediated by interaction of MSP domains with FFAT motifs.
FIGURE 1.
A, domain structures of human VAP-A and human OSBP are shown. MSP, major sperm protein; CC, coiled-coil; TM, transmembrane; PH, pleckstrin homology; LB, lipid binding. B, alignment of FFAT motifs of human lipid binding proteins and rat ORP1 is shown.
OSBP, which was the first lipid binding protein shown to interact with VAP-A, requires interaction of the FFAT motif with VAP-A to perform its function. In addition to the FFAT motif, OSBP has an N-terminal pleckstrin homology domain and a C-terminal lipid binding domain (Fig. 1A). OSBP usually localizes in a vesicular compartment and the cytosol and translocates to the Golgi apparatus surface in response to increases in cellular 25-hydroxycholesterol or depletion of cellular cholesterol (24). 25-Hydroxycholesterol stimulates sphingomyelin synthesis (25), and the interaction between the FFAT motif of OSBP and VAP-A is essential for stimulation of sphingomyelin synthesis by 25-hydroxycholesterol (26). Proteins displaying homology to the C-terminal lipid binding domain of OSBP are referred to as OSBP-related proteins (ORPs) (2, 27). Humans possess 12 ORP genes including OSBP (28). Four of the 12 human ORPs contain the EFFDAXE sequence, and 4 contain sequences closely related to EFFDAXE. In addition to these 8 human ORPs, human lipid binding proteins containing FFAT motifs (Fig. 1B) have been investigated with respect to their interaction with VAPs and subcellular localization (Table 1). These results suggest that the EFFDAXE sequence alone is not sufficient for strong binding to VAP, and crucial residues may be present in regions other than the EFFDAXE sequence. It should be noted that details of the interaction between OSBP and VAP-A remain unknown.
TABLE 1.
Summary of binding assays of VAP and human proteins containing FFAT motifs
PDA, glutatione S-transferase pulldown assay; Y2H, yeast two-hybrid; IP, coimmunoprecipitation; S, strong binding; W, weak binding; ND, no data; ○, the protein binds to VAP or localizes at ER; ×, the protein does not bind to VAP or does not localize at ER.
In this study we have determined the solution structure of the complex between the human VAP-A MSP domain (VAP-AMSP) and human OSBP fragment containing the FFAT motif (OBSPF) in an effort to delineate the interaction between VAP-A and OSBP. The detailed binding mechanism was further investigated by NMR and isothermal titration calorimetry (ITC) combined with mutagenesis. We have successfully examined 1) the stoichiometry between VAP-AMSP and OSBPF and 2) the disorder property of OSBPF and 3) identified residues within and at the N-terminal side of the FFAT motif that contribute to the binding to VAP-AMSP. Additionally, we have investigated the effect of the mutation that causes the disease ALS8 using NMR and differential scanning calorimetry (DSC).
EXPERIMENTAL PROCEDURES
Protein Expression and Purification
The expression and purification of unlabeled 13C,15N-labeled and 15N-labeled VAP-AMSP (human VAP-A E5-E128) and C-terminal hexahistidine-tagged OSBPF (human OSBP G346-S379) with an N-terminal tryptophan were as previously reported (32). Briefly, both VAP-AMSP and OSBPF were overexpressed in Escherichia coli Rosetta (DE3). Cells were lysed by sonication and centrifuged, and the supernatant was loaded onto a glutathione-Sepharose 4B resin (GE Healthcare). The column eluate was then applied to a Superdex 75 (26/60) gel filtration column (GE Healthcare). The fractions obtained were concentrated, and the glutathione S-transferase tag was cleaved using PreScission Protease (GE Healthcare). Finally, the sample was applied to a Superdex 75 (26/60) gel filtration column. For OSBPF, nickel-Sepharose (GE Healthcare) was used in lieu of the aforementioned final gel-filtration step, and protein was eluted with a buffer containing 300 mm imidazole.
Expression vectors for the site-directed mutants of OSBPF (E356K, E358A, F360A, E360Q, D361A, E364K, and E356K/E364K) were prepared using QuikChange (Stratagene). The following primers were designed to introduce the mutations: E356K forward (5′-gcgatgaagatgataagaatgaattttttg-3′) and reverse (5′-caaaaaattcattcttatcatcttcatcgc-3′); E358A forward (5′-agatgatgagaatgcattttttgatgcac-3′) and reverse (5′-gtgcatcaaaaaatgcattctcatcatct-3′); F360A forward (5′-gatgagaatgaatttgctgatgcacctgagat-3′) and reverse (5′-atctcaggtgcatcagcaaattcattctcatc-3′); F360Q forward (5′-tgatgagaatgaatttcaagatgcacctgagatc-3′) and reverse (5′-gatctcaggtgcatcttgaaattcattctcatca-3′); D361A forward (5′-gaatgaattttttgctgcacctgagatca-3′) and reverse (5′-tgatctcaggtgcaccaaaaaattcattc-3′); E364K forward (5′-tttttgatgcacctaagatcatcaccatg-3′) and reverse (5′-catggtgatgatcttaggtgcatcaaaaa-3′). To generate the E356K/E364K double mutant, E356K was initially prepared, and then the E364K mutation was introduced into E356K. All mutant OSBPFs were overexpressed in E. coli Rosetta (DE3) grown in LB medium and then purified using the same method as described above.
The expression vector for the P56S mutant of VAP-AMSP was prepared using QuikChange (Stratagene) using forward primer 5′-cggtactgtgtgaggtccaacagtggaatta-3′ and reverse primer 5′-taattccactgttggacctcacacagtaccg-3′. 15N-Labeled P56S VAP-AMSP was overexpressed in E. coli Rosetta (DE3) grown in M9 medium containing 15NH4Cl and unlabeled glucose and then purified using the same method as described above.
NMR Samples of the Complex
NMR samples of the complex were prepared in 93% H2O, 7% D2O (v/v) containing 50 mm potassium phosphate (pH 6.9), 100 mm KCl, 1 mm DTT, and 0.1 mm EDTA. Six complexes between VAP-AMSP and OSBPF were prepared: 13C,15N or 15N-labeled VAP-AMSP with unlabeled OSBPF, unlabeled VAP-AMSP with 13C,15N or 15N-labeled OSBPF, 13C,15N -labeled VAP-AMSP with 13C,15N-labeled OSBPF, and 15N-labeled VAP-AMSP with 15N-labeled OSBPF. Complex formation was monitored using the 15N HSQC spectrum of the mixture of VAP-AMSP and OSBPF. Peak positions of the complex were identified by the 1H,15N HSQC spectrum of 15N-labeled VAP-AMSP or 15N-labeled OSBPF in the presence of an excess of unlabeled OSBPF or unlabeled VAP-AMSP, respectively.
NMR Experiments
All NMR experiments were performed using a Bruker AV500 or DRX800 spectrometer at 303 K. All spectra were processed using NMRpipe (33) and analyzed using SPARKY (T. D. Goddard and D. G. Kneller, University of California, San Francisco). NMR spectra used for resonance assignments of the complex were as previously reported (32). For backbone resonance assignments of free VAP-AMSP and free OSBPF, HNCACB, HN(CO)CACB, HN(CA)CO, and HNCO spectra (34, 35) of 13C,15N-labeled free VAP-AMSP and 13C,15N-labeled free OSBPF were recorded, respectively. To obtain the distance restraints of the complex, the three-dimensional 13C-edited NOESY spectrum of 13C,15N-labeled complex, three-dimensional 15N-edited NOESY, and two-dimensional 15N- filtered 1H,1H NOESY spectra of 15N-labeled complex and 13C-edited (F2)/13C-filtered (F1) NOESY spectrum of the complex between 13C,15N-labeled VAP-AMSP and unlabeled OSBPF (34, 35) were recorded. To obtain χ1 angles, HNHB and HN(CO)HB spectra (36, 37) and three bond JC′Cγ and JNCγ couplings (38) were measured.
The 15N longitudinal spin-relaxation rates (R1) were measured with relaxation delays of 16.5*, 38.5, 60.5*, 115.5, 203.5, 335.5, 555.5, and 885.5 ms (35). The 15N transverse relaxation rates (R2) were measured with relaxation delays of 16*, 32, 48*, 64, 96, 112, and 128 ms (35). In these measurements, time points marked with an asterisk were duplicated for error estimations. 1H,15N hetero-NOEs were recorded with and without proton saturation in an interleaved fashion (35).
Titration experiments of 15N-labeled VAP-AMSP with unlabeled OSBPF (WT) were performed in 90% H2O, 10% D2O (v/v) containing 50 mm potassium phosphate (pH 6.9), 100 mm KCl, 1 mm DTT, and 0.1 mm EDTA and in 90% H2O, 10% D2O (v/v) containing 50 mm potassium phosphate (pH 6.9), 500 mm KCl, 1 mm DTT, and 0.1 mm EDTA. Titration experiments of 15N-labeled VAP-AMSP with unlabeled OSBPF (E356K) was performed in 90% H2O, 10% D2O (v/v) containing 50 mm potassium phosphate (pH 6.9), 100 mm KCl, 1 mm DTT, and 0.1 mm EDTA. For all titration experiments, the initial concentration of VAP-AMSP was set to 0.17 mm. For the titration with OSBPF (WT), 3.17 mm OSBPF (WT) was added to VAP-AMSP at molar ratios to VAP-AMSP of 0.15, 0.30, 0.44, 0.59, 0.73, 0.88, 1.02, 1.17, 1.46, and 1.76. For the titration with OSBPF (E356K), 2.30 mm OSBPF (E356K) was added to VAP-AMSP with molar ratios to VAP-AMSP of 0.15, 0.30, 0.59, 0.88, 1.17, 1.46, 1.76, 2.34, 3.51, and 5.85. Finally, at 500 mm KCl, 1.69 mm OSBPF (WT) was added to VAP-AMSP with molar ratios to VAP-AMSP of 0.15, 0.30, 0.44, 0.59, 0.73, 0.88, 1.02, 1.17, 1.46, 2.34, 3.51, 5.85, and 10.5. For the line-shape analysis, the LineShapeKin Simulation 4.1.1 software (39) was used with the following parameters; Ka = 2.8 × 105 m−1, koff = 20, 40, 80, 100, 120, 140, 160, 180, 200, 250, 300, 350, 400, 450, 500, 700, 1000, and 2000 s−1, frequency for 15N of Glu-91 = 40,748 and 41,237 s−1 for free and complex, respectively, R2 = 11 and 17 s−1 for free and complex, respectively, receptor concentration = 0.16–0.17 mm, and the ligand/receptor ratio = 0.15, 0.30, 0.44, 0.59, 0.73, 0.88, 1.02, 1.17, 1.46, and 1.76. The dilution effects were corrected using the LineShapeKin software for the peaks with non-linear behavior.
15N Relaxation Analysis
All R1, R2, and 1H,15N hetero-NOE values were measured using the AV500 spectrometer at 303 K. Peak height intensities in each spectrum were measured using SPARKY. 1H,15N hetero-NOE values were obtained by calculating the ratios of the peak height intensities in the spectra with and without 1H saturation. For determination of the overall rotational correlation time (τC) of the complex, R1 and R2 values were obtained using the Sparky2rate software (Loria Lab), which employs a relaxation peak intensity file generated by Sparky and fits the rate constants using the nonlinear least-squares fitting program CURVEFIT (40). Then, τC for the complex was calculated using the program TENSOR 2.0 (41) assuming isotropic, axial symmetric, and fully anisotropic motion, and the isotropic model was selected. For determination of the residue-specific order parameters (S2) of VAP-AMSP in the bound and unbound states, the exponential decay curves of R1 and R2 were fitted to a two-parameter exponential equation using in-house developed software. The uncertainties of the relaxation rates were estimated by 500 Monte-Carlo simulations using the intensity deviations of duplicated time points. Base-line noise levels were used for the uncertainties in hetero-NOE experiments. Rotational diffusion tensors and Lipari-Szabo model-free analyses for VAP-AMSP in the unbound and bound states were achieved using TENSOR 2.0 (41). Only residues having values higher than 0.7 in the hetero-NOE data were considered for determination of the rotational diffusion tensor. Anisotropic and isotropic rotational diffusion tensors were assumed for the unbound and bound states of VAP-AMSP, respectively. Using these tensors, S2 were calculated.
Structure Calculation
The NOE cross-peaks in the three-dimensional 13C-edited NOESY, three-dimensional 15N-edited NOESY, and two-dimensional 1H,1H NOESY spectra were assigned using the CANDID algorithm of CYANA (42). A total of 2796 upper distance restraints were obtained by CANDID. The NOE cross-peaks in the three-dimensional 13C-edited (F2)/13C-filtered (F1) NOESY spectrum were assigned manually, and 29 intermolecular upper distance limits were obtained. All other intermolecular NOEs were assigned through CANDID/CYANA cycles and confirmed by visual inspections. 141 backbone torsion angle restraints were derived using the TALOS program (43), 10 χ1 angle restraints were obtained from HNHB and HN(CO)HB spectra, and 3 χ1 angle restraints were obtained by three bond JC′Cγ and JNCγ couplings. With these restraints, a total of 100 structures that did not show significant violations were generated by 20,000 time-step dynamics using CYANA (44, 45), and these were further refined by AMBER 9 (46) using an all-atom force field (ff99SB). The AMBER refinement consists of three stages: 1500 steps of energy minimization, 20-ps molecular dynamics, and 1500 steps of energy minimization. To take into account the electrostatic and solvent-solute interactions precisely, a cut-off value for the non-bonded interaction was set to infinity (cut = 999.0), and a generalized Born model (igb = 5 and gbsa = 1) (47) was used. The best 20 structures were selected and analyzed using AQUA and PROCHECK-NMR software (48). A structure closest to the lowest energy was employed as being representative.
ITC
Before the ITC experiments, purified protein and peptides were dialyzed against 50 mm potassium phosphate buffer (pH 6.9) containing 100 mm KCl, 1 mm DTT, and 0.1 mm EDTA. This dialysis buffer was used to measure heats of dilution. ITC experiments were performed at 293 K on a VP-ITC or an Omega Micro Calorimeter (Microcal Inc.). The experimental data were analyzed by employing the Origin-ITC software package. Heats of dilution were subtracted from the raw data before analysis.
The concentrations of OSBPF (WT and mutants) were determined by measuring the absorbance at 280 nm, although that of VAP-AMSP was not determined accurately due to trace contamination with other proteins. From the ITC data analysis, the apparent stoichiometry was 1:1.17 for OSBPF:VAP-AMSP. Here, we have assumed that this difference (17%) is derived from an overestimation of the VAP-AMSP concentration due to contamination, and therefore, the concentration of VAP-AMSP was re-calibrated for all experiments.
DSC
Before the DSC experiments, WT and P56S VAP-AMSP were dialyzed against 50 mm potassium phosphate buffer (pH 6.9) containing 100 mm KCl, 1 mm DTT, and 0.1 mm EDTA and against 50 mm potassium phosphate buffer (pH 6.9) containing 100 mm KCl and 1 mm DTT, respectively. The dialysate buffer was used as a reference solution for the DSC scan. Both calorimetric scans were performed using nanoDSC (Calorimetry Sciences Corp.). WT (1.5 mg/ml) and P56S (1.8 mg/ml) were scanned from 273 to 353 K and from 273 to 343 K, respectively, with a heating rate of 1 K/min.
RESULTS
Chemical Shift Assignments
All resonances of backbone nuclei (1HN, 15N, 13Cα, and 13C′) of the complex between VAP-AMSP and OSBPF were assigned, and more than 90% of side-chain 1H and 13C resonances of the complex were also assigned. Details of the resonance assignments of the complex (Biological Magnetic Resonance Bank ID 7025) have been previously described (32). Resonances of all backbone nuclei of free VAP-AMSP and 97.6% of backbone nuclei of free OSBPF were also assigned using conventional triple-resonance techniques (35).
Stoichiometry between VAP-AMSP and OSBPF
Initially, the ITC experiment for the interaction of OSBPF and VAP-AMSP was performed (supplemental Fig. S1). The result indicated that the molar ratio of the complex is 1:1. The apparent molecular mass of the complex was then determined by NMR relaxation spectroscopy. From the R1, R2, and 1H,15N hetero-NOE values, the τc value of the complex at 303 K was determined to be 12.6 ± 0.1 ns using TENSOR 2.0. Because the τc value of a globular protein is roughly proportional to the apparent molecular mass, a linear correlation plot between τc and apparent molecular mass values was obtained as shown in supplemental Fig. S2. According to the obtained plot (supplemental Fig. S2), the τc value of 12.6 ns indicates that the apparent molecular mass value is between 19.6 and 32.2 kDa (95% accuracy). The molecular mass of the complex comprising of one VAP-AMSP and one OSBPF is 20,249.7 Da. Therefore, the apparent molecular mass value of the complex together with the ITC study indicates that the complex consists of one VAP-AMSP and one OSBPF.
Structure Determination of the Complex of VAP-A MSP Domain with OSBP Fragment
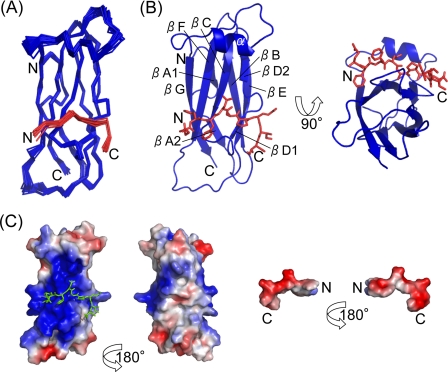
The solution structure of the complex of VAP-AMSP with OSBPF was determined. For structure calculations of the complex, the NOE cross-peaks observed in most NOESY spectra were assigned using the CANDID algorithm of CYANA, although the intermolecular NOE cross-peaks observed in the three-dimensional 13C-edited (F2)/13C-filtered (F1) NOESY spectrum were assigned manually (supplemental Fig. S3). A total of 2825 distance restraints were obtained, and 29 of 83 intermolecular NOEs were derived from the three-dimensional 13C-edited (F2)/13C-filtered (F1) NOESY spectrum (Table 2). The other restraints for structure calculations are shown in Table 2. Using these restraints, structures of the complex were calculated. A superimposed representation of the final 20 lowest energy structures obtained is shown in Fig. 2A. The root mean square deviations of backbone and heavy atoms over residues 6–125 of VAP-AMSP and residues 358–366 of OSBPF were 0.66 and 1.19 Å, respectively (Table 2). Other structural statistics are provided in Table 2.
TABLE 2.
Structural statistics for the complex between VAP-AMSP and OSBPF
| NOE upper distance restraints | |
| Intramolecular | |
| Intraresidual (|i − j| = 0) | 691 |
| Sequential (|i − j| = 1) | 824 |
| Medium range (1 < |i − j| < 5) | 365 |
| Long range (≤|i − j|) | 858 |
| Total | 2742 |
| Intermolecular | 83(29a) |
| Dihedral angle restraints | |
| Φ | 69 |
| ψ | 68 |
| χ1 | 13 |
| AMBER GB energies (kcal/mol) | |
| Total | −5825 ± 6 |
| Constraints | 15 ± 1 |
| Maximum violations | |
| Distance (Å) | 0.216 |
| Angle (°) | 4.868 |
| Mean deviations from ideal geometry | |
| Bond lengths (Å) | 0.0098 ± 0.0001 |
| Bond angles (°) | 2.29 ± 0.02 |
| RMSD (VAP-A 6–125, OSBP 358–366) (Å) | |
| Backbone atoms | 0.66 ± 0.14 |
| Heavy atoms | 1.19 ± 0.16 |
| Ramachandran analysis (VAP-A 6–125, OSBP 358–366) (%) | |
| Most favored regions | 90.6 |
| Additional allowed region | 8.1 |
| Generously allowed regions | 1.4 |
| Disallowed regions | 0.0 |
a Distance restraints obtained from three-dimensional 13C-edited (F2)/13C-filtered (F1) NOESY.
FIGURE 2.
Solution structure of the complex between VAP-A (6–125) and OSBP (358–366). A, shown is a superimposed representation of 20 lowest energy structures. B, shown is a ribbon representation of VAP-AMSP. OSBPF is shown as a stick model. C, shown are the electrostatic surfaces of VAP-AMSP (left) and OSBPF (right) contoured from −3 kT (red) to +3 kT (blue). The electrostatic potential was calculated using an amber force field.
Global Structure
VAP-AMSP has an immunoglobulin-like β-sandwich fold consisting of seven β-strands and one α-helix (Fig. 2B). The structure of VAP-AMSP is similar to MSP (PDB ID 1MSP), and the positional root mean square deviation of superimposed Cα atoms is 2.0 Å. The FFAT motif of OSBPF adopts an extended β-strand-like conformation and is bent at the C terminus of the FFAT motif (Fig. 2, A and B). OSBPF has an overall negatively charged surface and binds to VAP-AMSP at a positively charged surface (Fig. 2C).
Interaction between VAP-A and OSBP Fragment
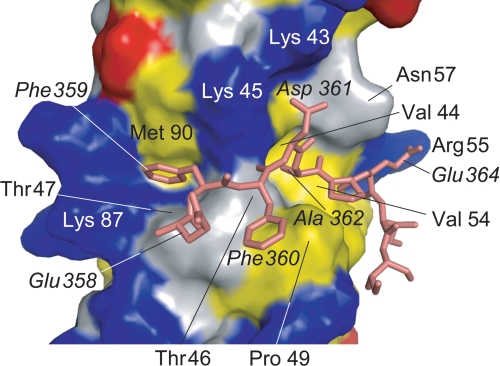
Details of the intermolecular interactions were analyzed using MONSTER (49), and interactions observed in more than 12 structures of the final 20 structures were adopted. The FFAT motif and C-terminal side of OSBPF interact with VAP-AMSP β strands C, D, E, and F and a loop connecting β-strands C and D. The complex of VAP-AMSP and OSBPF is stabilized by electrostatic, hydrophobic, and hydrogen-bond interactions. Fig. 3 shows the interaction site of VAP-AMSP and OSBPF. The carboxyl groups of Asp-361 and Glu-364 of OSBPF interact electrostatically with the side chains of VAP-AMSP Lys-43/Lys-45 and Arg-55, respectively (supplemental Fig. S4). These electrostatic interactions were well defined by 13 intermolecular NOEs observed between the electrostatic interaction pair and its neighboring residues; that is, 5 NOEs between Thr-46 and Ala-362, 2 NOEs between Lys-45 and Ala-362, Thr-46 and Phe-360, and Val-54 and Glu-364 and 1 NOE between Lys-45 and Asp-361 and between Val-44 and Ala-362 (supplemental Fig. S3). The proton resonances of VAP-AMSP Lys-43 δ and ϵ positions showed unusual upfield shifts. These shifts may reflect the electrostatic interaction between OSBPF Asp-361 and VAP-AMSP Lys-43. The side chain of Phe-359 of OSBPF binds in a hydrophobic pocket formed by residues Lys-45, Thr-47, Lys-87, and Met-89 of VAP-AMSP, and the side chain of Ala-362 of OSBPF binds in a hydrophobic pocket formed by residues Val-44, Thr-46, Val-54, and Asn-57 of VAP-AMSP. The side chain of Phe-360 of OSBPF interacts hydrophobically with Pro-49 of VAP-AMSP. The side chains of Pro-363, Glu-364, Ile-365, and Ile-366 of OSBPF also interact hydrophobically with VAP-AMSP. Three intermolecular backbone-backbone hydrogen bonds are observed between VAP-AMSP and OSBPF, where the amide nitrogens of Thr-46 and Val-54 of VAP-AMSP and Phe-360 of OSBPF form hydrogen bonds with the carbonyl oxygens of Phe-360 and Pro-362 of OSBPF and Thr-46 of VAP-AMSP domain, respectively. Amide proton resonances of Thr-46 and Val-54 of VAP-AMSP and Phe-360 of OSBPF shifted downfield upon complex formation, indicating formation of the respective hydrogen bonds.
FIGURE 3.
Details of the interaction. Residue numbers of OSBPF are written in italics. OSBPF is shown as a stick model. The surface of VAP-AMSP is represented with acidic (red), basic (blue), and hydrophobic (yellow) residues.
Intermolecular interactions observed in the structure were further investigated by mutational studies, and a series of OSBPF mutants was generated to achieve this end. For the quantitative analysis, ITC experiments were performed using OSBPF mutants with VAP-AMSP. The dissociation constants obtained from the ITC experiments are summarized in Table 3, and other thermodynamic parameters obtained from the ITC experiments are summarized in supplemental Table S1. The E356K/E364K double mutant showed the most significant loss of binding to VAP-AMSP. The F360A, D361A, and E364K mutants also showed significant loss of binding, although the F360Q mutant showed moderate loss of binding, and the E356K and E358A mutants showed only slight loss of binding.
TABLE 3.
The dissociation constants (Kd) obtained from the ITC experiments
VAP-AMSP (0.9 mm for E356K, 1.2 mm for E364K, and 1.0 mm for the others) was titrated into OSBPF (63–70 μm) at 293 K.
| OSBP | Kd |
|---|---|
| m | |
| E356K | 6.9 ± 0.7 × 10−6 |
| E358A | 4.1 ± 0.1 × 10−6 |
| F360A | 2.1 ± 0.1 × 10−5 |
| F360Q | 1.1 ± 0.1 × 10−5 |
| D361A | 2.8 ± 0.1 × 10−5 |
| E364K | 3.5 ± 0.3 × 10−5 |
| E356K/E364K | 1.1 ± 0.1 × 10−4 |
| WT | 2.1 ± 0.1 × 10−6 |
Disorder Property of Free OSBP
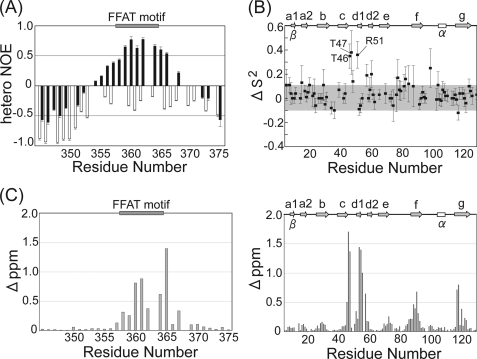
The disorder property of OSBP was examined using the IUPred algorithm (50, 51). The extensive region of OSBP (Q295-R406), including the FFAT motif (E358-E364), was estimated to be disordered. For experimental investigation of the disordered property of OSBP, 1H,15N hetero-NOE was applied to OSBPF. This 1H,15N hetero-NOE value is a good indicator of local environment dynamics; negative and large positive NOE values indicate flexible and rigid regions, respectively. All residues of OSBPF showed negative NOE values in the unbound state (Fig. 4A), suggesting that free OSBPF is flexible. Structure analysis of free OSBPF using the TALOS program (43) with the assigned 1HN, 15N, 13Cα, Cβ, and 13C′ chemical shifts also showed no ordered structure.
FIGURE 4.
A, backbone 1H,15N hetero-NOE values of OSBPF in the unbound (white bar) and bound (black bar) state are shown. Uncertainties were obtained using Monte Carlo simulations. B, shown are changes of S2 values of VAP-AMSP upon complex formation with OSBPF. ΔS2 = S2(complex) − S2(free). The region between ΔS2 = −0.1 ∼ 0.1 is shadowed. C, composite chemical shift changes of VAP-AMSP and OSBPF upon complex formation are shown. Composite chemical shift changes were calculated using the equation Δppm = {(ΔδH)2 + (ΔδN/5)2}1/2, where ΔδH and ΔδN represent the chemical shift changes of 1H and 15N, respectively.
Proteins containing native, unfolded functional regions (>30–40 residues) under physiological conditions are referred to as intrinsically disordered proteins (52). Larger interaction surfaces and structural plasticity in the native state is thought to be advantageous for intrinsically disordered proteins in recognizing various targets with sufficient specificity but without huge loss of affinity. A region of more than 100 consecutive residues in OSBP, including 33 residues characterized in this study, seem not to adopt an ordered structure. Thus OSBP belongs to the intrinsically disordered protein family.
Changes in Dynamics and Structure upon Complex Formation
1H,15N hetero-NOE values for the backbone amides of OSBPF in the bound state are shown in Fig. 4A. Upon binding to VAP-AMSP, 1H,15N hetero-NOE values of the residues around the FFAT motif of OSBPF changed from negative to positive, indicating that OSBPF formed a stable structure upon binding to VAP-AMSP. Fig. 4B shows the change in S2 for the backbone amides of VAP-AMSP upon complex formation. For Tyr-46, Tyr-47, and Arg-51, which are localized at a loop region involved in the binding to OSBPF, S2 values increased by about 0.35 upon complex formation, indicating that the ps∼ns motion of these residues was restricted upon binding to OSBPF and contributed to formation of a stable complex structure.
Fig. 4C shows backbone amide chemical shift changes of OSBPF and VAP-AMSP upon complex formation. Perturbed residues of OSBPF were observed for the FFAT motif and the C-terminal side of the FFAT motif, which interact with VAP-AMSP in our structure. Perturbed residues of VAP-AMSP were primarily located within or near the binding site. Two free VAP-AMSP structures have been previously determined (PDB IDs 2CRI3 and 1Z9L (21)). Positional root mean square deviations of superimposed Cα atoms of our structure with these free VAP-AMSP structures were both 1.3 Å, except for the loop regions (Phe-76—Lys-85). Judging from the chemical shift perturbation and the similarities between our structure and the free structures, the structure of VAP-AMSP does not change markedly upon complex formation.
DISCUSSION
Comparison with the Crystal Structure
A crystal structure of a complex between the rat VAP-AMSP and the rat ORP1 fragment containing the FFAT motif (SEDEFYDALS) (ORP1F) has been determined by Kaiser et al. (21). The overall structures of our structure and the crystal structure (PDB ID 1Z9O) are similar, and the positional root mean square deviation of superimposed Cα atoms of VAP-AMSP and the FFAT motif are 1.2 Å, although the crystal structure is composed of two VAP-AMSP and two ORP1F molecules. Clear differences were observed for the intermolecular interactions in that side chains of the third Phe/Tyr and fourth Asp residues of the FFAT motif (the consensus sequence EFFDAXE) interact with VAP-AMSP in the solution structure (supplemental Fig. S5). In the crystal structure, these residues do not interact with VAP-AMSP but interact with the second ORP1F molecule to stabilize the 2:2 complex (21). Substitution of the fourth Asp of the FFAT motif with Ala disrupts ER localization of Osh1, a yeast protein containing the FFAT motif (5), and disrupts the interaction of CERT, a ceramide transport protein containing the FFAT motif, with VAP-A in vivo (8). These results are consistent with our solution structure as the side chain of the fourth Asp residue of the FFAT motif interacts directly with VAP-AMSP. Thus, we assume that formation of the solution structure, comprising a 1:1 complex between VAP-AMSP and a FFAT protein, is essential for binding of the FFAT protein to VAP-A.
Recognition Mechanism of Intrinsically Disordered OSBPF by VAP-AMSP
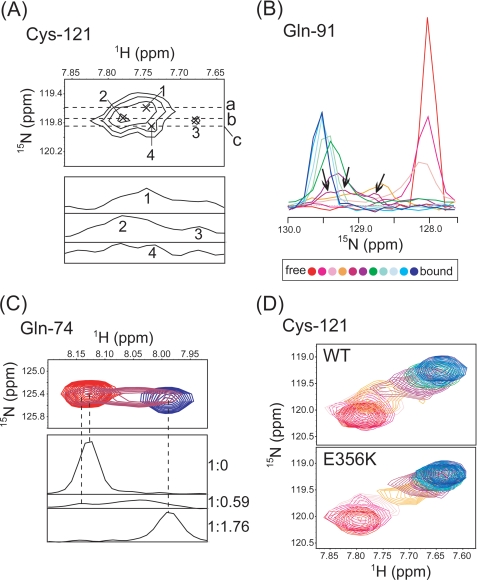
In an effort to further understand the recognition mechanism of OSBPF by VAP-AMSP, we inspected the perturbed chemical shift patterns in titration experiments that were carried out using 15N-labeled VAP-AMSP or OSBPF. Nonlinear behavior was found for eight residues of VAP-AMSP, whereas the other peaks show fast/intermediate line shapes with linear patterns (supplemental Fig. S6). The line shape simulation using the LineShapeKin software package could only account for the behavior of four residues, although the other four residues, Ser-58, Gln-74, Gln-91, and Cys-121, remained elusive using the two-site exchange model (supplemental Fig. S6). The complicated features of these residues were revealed by inspecting the line shapes of these residues. For instance, Cys-121 consisted of 4 peaks at a 1:0.44 molar ratio of VAP-AMSP to OSBPF as shown in Fig. 5A. The overlaid one-dimensional titration curve of Gln-91 also displayed 3 peak tops at a molar ratio of 1:0.59 (Fig. 5B). Glu-74 showed two peaks at a molar ratio of 1:0.59 (Fig. 5C). One is found at 8.03 ppm 1H between free (8.13 ppm) and complex (7.99 ppm) peak positions, and the other (8.14 ppm) is out of range. A convincing explanation for these observations is to assume formation of intermediate states. The intermediate complex is thought to be maintained by nonspecific interactions considering the observations that the peaks in the middle of the titration have several peak tops and there is no clear intermolecular NOE.
FIGURE 5.
A, shown are cross-peaks of Cys-121 at a molar ratio of OSBPF to VAP-AMSP of 1:0. 44. In the upper panel, peaks are indicated by a cross and are labeled. The lower panel shows 1H cross-sections corresponding to lines a, b, and c in the upper panel. Numbers indicate corresponding peaks in the upper panel. B, shown is an overlay of 15N cross-sections of Gln-91 in a titration of VAP-AMSP with OSBPF. Molar ratios (VAP-AMSP:OSBPF) ranged from 1:0 (red) to 1:1.76 (blue). Peak tops at a molar ratio of 1:0.59 are shown by arrows. C, the upper panel shows an overlay of cross-peaks for Gln-74 at molar ratios of OSBPF of 0 (red), 0.59 (purple), and 1.76 (blue). The lower panel shows 1H cross-sections of peaks at each molar ratio. Corresponding peaks in the upper and lower panels are connected by dashed lines. D, shown is a comparison of cross-peaks for Cys-121 in titrations with OSBPF (WT) and the E356K mutant.
We hypothesized that electrostatic interactions between OSBPF and VAP-AMSP play a role in forming the intermediate, as initial screening of sample conditions showed that the titration under 500 mm KCl does not reach saturation even at a 10:1 ratio of OSBPF to VAP-AMSP (data not shown). Additionally, VAP-AMSP has a large basic area at the same surface in the region, which OSBPF binds to (Fig. 2C), and a plethora of acidic residues are found in OSBPF as well as its homologous sequences. In addition to the three acidic residues that are within the FFAT motif, there is an acidic patch of 5 residues comprising Asp-352, Glu-353, Asp-354, Asp-355, and Glu-356 at a region preceding the FFAT motif. This acidic patch region does not converge in the final ensemble structures due to the absence of medium to long range intra- and intermolecular NOE restraints, suggesting that there is no stable interaction between the acidic patch and VAP-AMSP. This is also indicated by small differences in chemical shifts before and after binding to VAP-AMSP and the lower hetero-NOE values of these residues in the complex (Fig. 4, A and B). We examined the role of the acidic patch by preparing the charge reversal E356K mutant of OSBPF and conducting titration experiments using 15N-labeled VAP-AMSP. Remarkably in this case, with the exception of Gln-74 and Gln-91, the nonlinear patterns that were found in titrations with WT disappeared, thereby allowing for simulation using a two-site model (Fig. 5D and supplemental Fig. S7). It should be noted that the chemical shifts of VAP-AMSP in the saturated state are similar even in the complex with the E356K mutant, implying that the final structures are similar. Nonetheless, the ITC experiment showed that the E356K mutant has reduced binding affinity for VAP-AMSP (Table 3). In a three-site exchange consisting of free, intermediate, and binding complex, the exchange between the intermediate and final product is expected to be much faster than that between the free and intermediate states. The E356K mutant probably possesses an increased off-rate from the intermediate to free conformation, which in turn leads to higher values of Kd and a faster apparent exchange rate. These results suggest the possibility that the acidic patch of OSBPF, including Glu-356 of OSBPF, takes part in increasing the apparent binding affinity by contributing to the formation of an intermediate complex with VAP-AMSP through electrostatic interactions.
Effects of P56S Mutation on VAPMSP
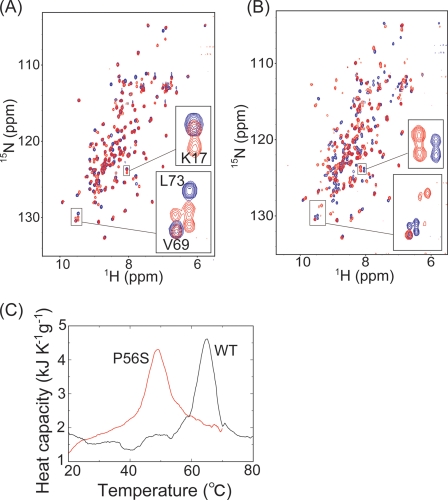
The P56S mutation in human VAP-B causes familial autosomal dominant motoneuronal diseases, ALS8, which exhibits a typical ALS phenotype with rapid progression or late onset spinal muscular atrophy (22). The P56S mutation in VAP-B induces insolubility and aggregate formation of VAP-B (23). Pro-56 is conserved not only in the VAP family of proteins but also in the MSP family of proteins (53). To investigate the effects of the P56S mutation on VAPMSP, we prepared the P56S mutant of VAP-AMSP. Fig. 6A shows the 1H,15N HSQC spectrum of P56S VAP-AMSP superimposed on that of WT VAP-AMSP. The number of peaks in the 1H,15N HSQC spectrum of P56S VAP-AMSP was greater than that expected from the amino acid sequence of P56S VAP-AMSP. Some residues of P56S VAP-AMSP probably gave double peaks, and the intensities of these double peaks were similar without obvious line-broadenings. These residues did not map to a specific region, although the assignments were not completed. Hence, it is concluded that P56S VAP-AMSP has two conformations with similar abundance.
FIGURE 6.
A, 1H,15N HSQC spectra of P56S (red) and WT (blue) VAP-AMSP are shown. In the boxed region, assignments of peaks of WT VAP-AMSP are indicated. B, 1H,15N HSQC spectra of P56S VAP-AMSP in the presence of OSBPF with a molar ratio of 1:2 (red) and in the absence of OSBPF (blue) are shown. C, DSC profiles of P56S (red) and WT (black) VAP-AMSP are shown.
We then investigated the ability of P56S VAP-AMSP to bind the FFAT motif. Fig. 6B shows the 1H,15N HSQC spectrum of P56S VAP-AMSP in the presence of OSBPF superimposed on that in the absence of OSBPF. Double peaks in both were perturbed by the addition of OSBPF. Therefore, both conformations of P56S VAP-AMSP can bind to OSBPF. In the presence of OSBPF, some double peaks have similar intensity (Fig. 6B, upper inset), whereas others have different intensities (Fig. 6B, lower inset). It seems that the binding modes for OSBPF differ between the two conformations.
We then investigated the thermodynamic stability of P56S VAP-AMSP by DSC (Fig. 6C). The temperature of maximum heat capacity of WT VAP-AMSP is 64.6 °C and that of P56S VAP-AMSP is 49.0 °C, indicating that P56S VAP-AMSP is thermally unstable. The heat capacity peak of P56S VAP-AMSP is broader than that of WT, indicating that the change in enthalpy (ΔH) of protein unfolding of P56S VAP-AMSP is smaller than that of WT. In fact, DSC curve analyses showed that ΔH of protein unfolding of WT and P56S VAP-AMSP is 529 and 416 (436 at 64.6 °C) kJ/mol, respectively. The decrease in ΔH of protein unfolding associated with the P56S mutation indicates that the thermal instability of P56S VAP-AMSP results from losses of hydrogen bonds and/or van der Waals interactions in the protein. As judged by the 1H,15N HSQC spectra of P56S and WT VAP-AMSP, the structure of P56S VAP-AMSP does not differ greatly from that of WT. However, results of the DSC experiments showed that P56S VAP-AMSP is more thermally unstable than WT. Because the MSP domains of VAP-A and VAP-B share 82% amino acid sequence identity, their structures are probably similar. It is likely that the effects of the P56S mutation on VAP-BMSP are similar to the effects of the P56S mutation on VAP-AMSP. Thus, it is reasonable to assume that the P56S mutation in VAP-B decreases the thermal stability of VAP-B and facilitates aggregation.
Concluding Remarks
In this report, we have determined the solution structure of the complex between VAP-AMSP and OSBPF and found that most residues in the FFAT motif interact with VAP-AMSP. This solution structure explained the roles of five of six conserved residues in the FFAT motif, and three of five were not explained before. Furthermore, we found that 1) VAP-AMSP and OSBPF form a complex with a molar ratio of 1:1 in solution, 2) the central region of OSBP including the FFAT motif is intrinsically disordered, 3) VAP-AMSP and OSBPF form an intermediate complex before forming a stable 1:1 complex, 4) electrostatic interactions are important for binding, and 5) at least one acidic residue in an acidic patch preceding the FFAT motif is involved in intermediate complex formation and enhances the binding. These findings suggest that disordered OSBP initially binds VAP-A through nonspecific charge interactions involving acidic residues at the N-terminal side of the FFAT motif and finally forms a stable complex structure through a “fly-casting”-like process (54, 55).
Supplementary Material
Acknowledgments
We thank M. Yoneyama, H. Kinoshita, and A. Yashuba for technical assistance.
This work was supported by grants from the Ministry of Education, Culture, Sports and Technology (Japan) through Target Proteins Research Program, Global Centers of Excellence Program, and grants-in-aid for scientific research.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table S1 and Figs. S1–S7.
The atomic coordinates and structure factors (code 2rr3) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
H. Endo, F. Hayashi, M. Yoshida, and S. Yokoyama, unpublished information.
- ER
- endoplasmic reticulum
- OSBP
- oxysterol-binding protein
- ORP
- OSBP-related protein
- VAP-A
- VAMP-associated protein
- MSP
- major sperm protein
- ALS8
- amyotrophic lateral sclerosis 8
- ITC
- isothermal titration calorimetry
- DSC
- differential scanning calorimetry
- HSQC
- heteronuclear single quantum correlation
- DTT
- dithiothreitol
- WT
- wild type
- NOESY
- nuclear Overhauser effect (NOE) spectroscopy.
REFERENCES
- 1.Yang H. (2006) Trends Cell Biol. 16, 427–432 [DOI] [PubMed] [Google Scholar]
- 2.Lehto M., Olkkonen V. M. (2003) Biochim. Biophys. Acta 1631, 1–11 [DOI] [PubMed] [Google Scholar]
- 3.Wang P. Y., Weng J., Anderson R. G. (2005) Science 307, 1472–1476 [DOI] [PubMed] [Google Scholar]
- 4.Wyles J. P., McMaster C. R., Ridgway N. D. (2002) J. Biol. Chem. 277, 29908–29918 [DOI] [PubMed] [Google Scholar]
- 5.Loewen C. J., Roy A., Levine T. P. (2003) EMBO J. 22, 2025–2035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wyles J. P., Ridgway N. D. (2004) Exp. Cell Res. 297, 533–547 [DOI] [PubMed] [Google Scholar]
- 7.Amarilio R., Ramachandran S., Sabanay H., Lev S. (2005) J. Biol. Chem. 280, 5934–5944 [DOI] [PubMed] [Google Scholar]
- 8.Kawano M., Kumagai K., Nishijima M., Hanada K. (2006) J. Biol. Chem. 281, 30279–30288 [DOI] [PubMed] [Google Scholar]
- 9.Nishimura Y., Hayashi M., Inada H., Tanaka T. (1999) Biochem. Biophys. Res. Commun. 254, 21–26 [DOI] [PubMed] [Google Scholar]
- 10.Lapierre L. A., Tuma P. L., Navarre J., Goldenring J. R., Anderson J. M. (1999) J. Cell Sci. 112, 3723–3732 [DOI] [PubMed] [Google Scholar]
- 11.Skehel P. A., Fabian-Fine R., Kandel E. R. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 1101–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weir M. L., Xie H., Klip A., Trimble W. S. (2001) Biochem. Biophys. Res. Commun. 286, 616–621 [DOI] [PubMed] [Google Scholar]
- 13.Pennetta G., Hiesinger P. R., Fabian-Fine R., Meinertzhagen I. A., Bellen H. J. (2002) Neuron 35, 291–306 [DOI] [PubMed] [Google Scholar]
- 14.Brickner J. H., Walter P. (2004) PLoS Biol. 2, e342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao L., Aizaki H., He J. W., Lai M. M. (2004) J. Virol. 78, 3480–3488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weir M. L., Klip A., Trimble W. S. (1998) Biochem. J. 333, 247–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skehel P. A., Martin K. C., Kandel E. R., Bartsch D. (1995) Science 269, 1580–1583 [DOI] [PubMed] [Google Scholar]
- 18.Soussan L., Burakov D., Daniels M. P., Toister-Achituv M., Porat A., Yarden Y., Elazar Z. (1999) J. Cell Biol. 146, 301–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foster L. J., Weir M. L., Lim D. Y., Liu Z., Trimble W. S., Klip A. (2000) Traffic 1, 512–521 [DOI] [PubMed] [Google Scholar]
- 20.Loewen C. J., Gaspar M. L., Jesch S. A., Delon C., Ktistakis N. T., Henry S. A., Levine T. P. (2004) Science 304, 1644–1647 [DOI] [PubMed] [Google Scholar]
- 21.Kaiser S. E., Brickner J. H., Reilein A. R., Fenn T. D., Walter P., Brunger A. T. (2005) Structure 13, 1035–1045 [DOI] [PubMed] [Google Scholar]
- 22.Nishimura A. L., Mitne-Neto M., Silva H. C., Richieri-Costa A., Middleton S., Cascio D., Kok F., Oliveira J. R., Gillingwater T., Webb J., Skehel P., Zatz M. (2004) Am. J. Hum. Genet. 75, 822–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanekura K., Nishimoto I., Aiso S., Matsuoka M. (2006) J. Biol. Chem. 281, 30223–30233 [DOI] [PubMed] [Google Scholar]
- 24.Ridgway N. D., Dawson P. A., Ho Y. K., Brown M. S., Goldstein J. L. (1992) J. Cell Biol. 116, 307–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ridgway N. D. (1995) J. Lipid Res. 36, 1345–1358 [PubMed] [Google Scholar]
- 26.Perry R. J., Ridgway N. D. (2006) Mol. Biol. Cell 17, 2604–2616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olkkonen V. M., Johansson M., Suchanek M., Yan D., Hynynen R., Ehnholm C., Jauhiainen M., Thiele C., Lehto M. (2006) Biochem. Soc. Trans. 34, 389–391 [DOI] [PubMed] [Google Scholar]
- 28.Lehto M., Laitinen S., Chinetti G., Johansson M., Ehnholm C., Staels B., Ikonen E., Olkkonen V. M. (2001) J. Lipid Res. 42, 1203–1213 [PubMed] [Google Scholar]
- 29.Xu Y., Liu Y., Ridgway N. D., McMaster C. R. (2001) J. Biol. Chem. 276, 18407–18414 [DOI] [PubMed] [Google Scholar]
- 30.Lehto M., Tienari J., Lehtonen S., Lehtonen E., Olkkonen V. M. (2004) Cell Tissue Res. 315, 39–57 [DOI] [PubMed] [Google Scholar]
- 31.Wang C., JeBailey L., Ridgway N. D. (2002) Biochem. J. 361, 461–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Furuita K., Mishima M., Kojima C. (2006) J. Biomol. NMR 36, 69. [DOI] [PubMed] [Google Scholar]
- 33.Delaglio F., Grzesiek S., Vuister G. W., Zhu G., Pfeifer J., Bax A. (1995) J. Biomol. NMR 6, 277–293 [DOI] [PubMed] [Google Scholar]
- 34.Sattler M. S., Schleucher J., Griesinger C. (1999) Prog. Nucl. Magn. Reson. Spectrosc. 34, 93–158 [Google Scholar]
- 35.Cavanagh J., Fairbrother W. J., Palmer A. G., Rance M., Skelton N. J. (2007) Protein NMR Spectroscopy, 2nd Ed., pp. 613–655, 686,–692, 782–796, Academic Press, Inc., San Diego, CA [Google Scholar]
- 36.Archer S. J., Ikura M., Torchia D. A., Bax A. (1991) J. Magn. Reson. 95, 636–641 [Google Scholar]
- 37.Grzesiek S., Ikura M., Clore G. M., Gronenborn A. M., Bax A. (1992) J. Magn. Reson. 96, 215–221 [Google Scholar]
- 38.Hu J. S., Bax A. (1997) J. Biomol. NMR 9, 323–328 [DOI] [PubMed] [Google Scholar]
- 39.Kovrigin E. L., Loria J. P. (2006) Biochemistry 45, 2636–3647 [DOI] [PubMed] [Google Scholar]
- 40.Palmer A. G., Wright P. E., Rance M. (1991) Chem. Phys. Lett. 185, 41–46 [Google Scholar]
- 41.Dosset P., Hus J. C., Blackledge M., Marion D. (2000) J. Biomol. NMR 16, 23–28 [DOI] [PubMed] [Google Scholar]
- 42.Herrmann T., Güntert P., Wüthrich K. (2002) J. Mol. Biol. 319, 209–227 [DOI] [PubMed] [Google Scholar]
- 43.Cornilescu G., Delaglio F., Bax A. (1999) J. Biomol. NMR 13, 289–302 [DOI] [PubMed] [Google Scholar]
- 44.Güntert P., Mumenthaler C., Wüthrich K. (1997) J. Mol. Biol. 273, 283–298 [DOI] [PubMed] [Google Scholar]
- 45.Güntert P. (2004) Methods Mol. Biol. 278, 353–378 [DOI] [PubMed] [Google Scholar]
- 46.Pearlman D. A., Case D. A., Caldwell J. W., Ross W. S., Cheatham T. E., III, DeBolt S., Ferguson D., Seibel G., Kollman P. (1995) Comput. Phys. Commun. 91, 1–41 [Google Scholar]
- 47.Onufriev A., Bashford D., Case D. A. (2004) Proteins 55, 383–394 [DOI] [PubMed] [Google Scholar]
- 48.Laskowski R. A., Rullmannn J. A., MacArthur M. W., Kaptein R., Thornton J. M. (1996) J. Biomol. NMR 8, 477–486 [DOI] [PubMed] [Google Scholar]
- 49.Salerno W. J., Seaver S. M., Armstrong B. R., Radhakrishnan I. (2004) Nucleic Acids Res. 32, W566–W568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dosztányi Z., Csizmók V., Tompa P., Simon I. (2005) J. Mol. Biol. 347, 827–839 [DOI] [PubMed] [Google Scholar]
- 51.Dosztányi Z., Csizmok V., Tompa P., Simon I. (2005) Bioinformatics 21, 3433–3434 [DOI] [PubMed] [Google Scholar]
- 52.Fink A. L. (2005) Curr. Opin. Struct. Biol. 15, 35–41 [DOI] [PubMed] [Google Scholar]
- 53.Tarr D. E., Scott A. L. (2005) Trends Parasitol. 21, 224–231 [DOI] [PubMed] [Google Scholar]
- 54.Shoemaker B. A., Portman J. J., Wolynes P. G. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 8868–8873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sugase K., Dyson H. J., Wright P. E. (2007) Nature 447, 1021–1025 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.