Abstract
The addictive potential of opioids may be related to their differential ability to induce G protein signaling and endocytosis. We compared the ability of 20 ligands (sampled from the main chemical classes of opioids) to promote the association of μ and δ receptors with G protein or β-arrestin 2. Receptor-arrestin binding was monitored by bioluminescence resonance energy transfer (BRET) in intact cells, where pertussis toxin experiments indicated that the interaction was minimally affected by receptor signaling. To assess receptor-G protein coupling without competition from arrestins, we employed a cell-free BRET assay using membranes isolated from cells expressing luminescent receptors and fluorescent Gβ1. In this system, the agonist-induced enhancement of BRET (indicating shortening of distance between the two proteins) was Gα-mediated (as shown by sensitivity to pertussis toxin and guanine nucleotides) and yielded data consistent with the known pharmacology of the ligands. We found marked differences of efficacy for G protein and arrestin, with a pattern suggesting more restrictive structural requirements for arrestin efficacy. The analysis of such differences identified a subset of structures showing a marked discrepancy between efficacies for G protein and arrestin. Addictive opiates like morphine and oxymorphone exhibited large differences both at δ and μ receptors. Thus, they were effective agonists for G protein coupling but acted as competitive enkephalins antagonists (δ) or partial agonists (μ) for arrestin. This arrestin-selective antagonism resulted in inhibition of short and long term events mediated by arrestin, such as rapid receptor internalization and down-regulation.
Keywords: Endocytosis, G Protein-coupled Receptors (GPCR), G Proteins, Receptor Desensitization, Receptor Structure-Function, β-Arrestin, Bioluminescence Resonance Energy Transfer, Ligand Efficacy, Opioid Receptors
Introduction
Physiological agonists are usually equally efficient in promoting the interaction of receptors with G protein and arrestin, but manmade analogues can show divergent molecular efficacies for the two transducers (1, 2). This phenomenon, often addressed with a pictorial terminology (3–5), has attracted great interest and if better understood might lead to new types of drugs.
The differential efficacy of opioids for G protein and arrestin interactions is also important in the mechanism of opiate addiction. As reported earlier, the addictive opiate morphine cannot induce and actually blocks desensitization and G protein uncoupling of δ-opioid receptors (DOPR)2 in neuroblastoma or in transfected cells (6, 7). Subsequent work shows that morphine is a poor inducer of rapid arrestin-dependent endocytosis for both δ and μ (MOPR) receptors (8–11), although one exception is in striatum neurons with high levels of G protein receptor kinases (12).
Two theories predict a relation between lack of endocytosis and the addiction liability of opioids, but the proposed explanations are radically different. One sees rapid endocytosis as a means to quench receptor signaling. Thus, the abnormally sustained signaling pattern produced by a drug that cannot internalize the receptor would promote post-receptor compensatory mechanisms, which may be responsible for the process of tolerance and withdrawal in vivo (13). The other regards endocytosis and the rapid recycling process that follows (more relevant in μ than in δ receptors (14)) as a tool for receptor recovery (15). Thus, a ligand promoting negligible endocytosis (e.g. morphine), even if interacts with arrestin weakly, would cause progressive accumulation of arrestin-bound desensitized receptors on prolonged exposure, as no significant receptor recovery would occur. That might cause tolerance and dependence in vivo (16, 17).
Recent knock-in mice models have established that the loss of signaling due to receptor endocytosis is related to tolerance in vivo. In a transgenic line expressing a fluorescent replacement of DOPR, the loss of analgesic effect upon repeated dosing of an agonist was clearly related to the ability of the agonist to promote receptor endocytosis (18). Likewise, reduced tolerance to morphine was found in mice bearing a MOP/DOPR chimeric receptor that can be internalized by morphine (19). However, because DOPR and the MOP/DOPR chimera undergo lysosomal down-regulation (20, 21) more than recycling (13, 14), the data do not dismiss the suspicion that the outcome in vivo of endocytosis in MOPR might be different in DOPRs (18).
The role of arrestin in morphine antinociception and tolerance needs clarification. Evidence that β-arrestin 2 plays a role in vivo despite the weak interactions observed in vitro comes from knock-out animals. Targeted deletion of the β-arrestin 2 gene results in an enhanced analgesic effect (22) and reduced tolerance to morphine but not other opioids (23). Similarly, delayed tolerance to morphine occurs in rats after antisense targeting of the β-arrestin 2 gene in the spinal cord (24). To explain this “morphine paradox,” it was proposed that the weak interaction that morphine-bound MOPR establishes with arrestin is the key factor, as it may lead to a progressive build-up of desensitized receptors that cannot be restored by endocytic recycling (16).
Given such background, we thought it useful to measure the differential efficacy for G protein and arrestin of μ and δ receptors, which are the main receptor subtypes involved in tolerance and addiction (25). We monitored the direct binding interaction between receptors and the two transducers using resonance energy transfer (RET) techniques (26) to obtain estimates of ligand efficacies unbiased by nonlinear amplification factors and cross-transducer antagonism that are inherent in indirect determinations from second messenger and protein kinase assays. We show that morphine-like ligands are mixed agonist-antagonists for the two transducers; i.e. they can activate G protein but block competitively arrestin.
EXPERIMENTAL PROCEDURES
Reagents and Drugs
Cell culture media, reagents, and fetal calf serum were from Invitrogen; restriction enzymes were from New England Biolabs; pertussis toxin was from List Biologicals; coelenterazine and bisdeoxycoelenterazine (sold as coelenterazine 400a) were from Biotium Inc.; EnduRen Live Cell luciferase substrate was from Promega. Radiolabeled opioid ligands and [35S]GTPγS were obtained from PerkinElmer Life Sciences. All others biochemicals and nucleotide analogues were purchased from Sigma. Opioid peptides were obtained from Bachem, except ICI 174,864 (from Tocris) and UFP-512 and N,N(CH3)2-Dmt-Tic-NH2 (both generous gifts from Dr. Remo Guerrini, University of Ferrara, Italy). All restricted drugs, such as morphine, oxymorphone, fentanyl, etc., were obtained from the restricted substances repository of the Istituto Superiore di Sanità (ISS) (Dr. Dora Macchia, ISS, Rome). All other opioid agonists and antagonists were purchased from Tocris, with the exclusion of lofentanyl (Janssen) and etorphine (Reckitt & Colman) kindly donated by Prof. Albert Herz.
Plasmids
Human MOPR and DOPR Rluc-tagged fusion proteins were made by replacing stop codons with a sequence encoding a 10-mer linker peptide (GPGIPPARAT) and cloned into pRluc-N1 (PerkinElmer). MOPR-Rluc and DOPR-Rluc inserts were then transferred into the retroviral expression vector pQIXN (Clontech). Bovine Gβ1 and Gγ2 N-terminal-tagged with RGFP (Prolume) were built by linking the RGFP sequence without its stop codon to Ser2 of Gβ1 or Ala2 of Gγ2 through a 21-mer linker peptide (EEQKLISEEDLGILDGGSGSG) and cloned into the retroviral expression vector pQIXH. The N termini of human β-arrestin 1 and β-arrestin 2 after removal of the start codon were tethered to the C terminus of RGFP through a 13-mer linker peptide (EEQKLISEEDLRT) and subcloned in pQIXH (26). The membrane-targeted form of RGFP (mt-RGFP) was obtained by fusing the C terminus with a short peptide containing the consensus sequence for farnesylation and palmitoylation signals of HRAS (underlined in the sequence) through a seven-mer linker peptide (RTSGLRSKLNPPDESGPGCMSCKCVLS). The encoding sequence was generated by annealing sense and antisense oligonucleotides and ligated to RGFP without stop codon. The mt-RGFP sequence was subcloned into pQIXH retroviral expression vector.
Cells and Membrane Preparation
HEK293 cells were grown in Dulbecco's modified Eagle's medium supplemented with 10%(v/v) fetal calf serum, 100 units/ml penicillin G, and 100 μg/ml streptomycin sulfate in a humidified atmosphere of 5% CO2 at 37 °C. SH-5YSY human neuroblastoma cells were grown under identical conditions in a 1:1 mixture of Dulbecco's modified Eagle's medium and F-12. Cell lines permanently co-expressing the different pairs of fusion proteins were prepared using the pantropic retroviral expression system by Clontech as described previously (26). When necessary, pertussis toxin was added to the medium (100 ng/ml) and incubated for 18 h. Enriched plasma membrane aliquots from transfected cells were prepared by differential centrifugation as described (6) and stored in aliquots at −80 °C before use.
Pertussis Toxin-catalyzed ADP-ribosylation
Labeling of the α-subunit was performed in 50-μl reactions using 25 μg of membranes, as described previously (27).
Luminescence Recording of Receptor-Transducer Interactions
Luminescence was recorded in sterile 96-well white plastic plates (Packard View-plate) using a plate luminometer (Victor light, PerkinElmer) equipped with two independent automatic injectors. For the determination of receptor/β-arrestin interactions on attached monolayers, cells were plated 24 h before the experiment (1 × 105 cells/well). The assay was started by replacing medium with 50 μl of Dulbecco's phosphate buffer saline (PBS) containing the luciferase substrate analogue bisdeoxycoelenterazine (bDOC, 5 μm). After 3 min, 50 μl of PBS containing different concentrations of ligands was rapidly added to the wells using a multichannel pipette and incubated for 3 min before counting. For the determination of receptor/Gβ interactions, membranes (2–5 μg of proteins) prepared from cells co-expressing DOPR/Rluc or MOPR/Rluc with RGFP/Gβ1 were added to wells in 50 μl of PBS containing coelenterazine (2–5 μm) for 10 min. Next, different concentrations of ligands in 50 μl of PBS were added and incubated for an additional 3 min before reading luminescence. For kinetics, wells containing plated cells or membranes (pretreated with substrates as described above in 100/200 μl of PBS) were first counted in the luminometer for 1–2 min to record a RET basal signal. Next, injections of ligands or guanine nucleotides (5–10 μl in PBS) were made via software-controlled automatic syringe injectors under continuous reading (integration, 0.5 s).
Internalization of Opioid Receptor
To monitor agonist-induced endocytosis of the opioid receptors we adapted to bioluminescence RET detection an assay previously used to study internalization of adrenergic receptors via fluorescence RET (28). Tethering the farnesyl-palmitoyl signal sequence of v-HA-ras to the RGFP C terminus targets the protein to membrane microdomains where small ras-like GTPases and heterotrimeric G proteins are concentrated (29). As agonists induce the transfer of receptors into endocytic vesicles, leaving on the surface the “spectator” mt-RGFP reporter, the RET signal proportionally decreases at a rate that follows the rate of receptor internalization (28). HEK293 or human neuroblastoma SH-SY5Y cells stably expressing DOPR-Rluc or MOPR-Rluc with mt-RGFP were seeded on 24-well white plastic plates in Dulbecco's modified Eagle's medium supplemented with 5% (v/v) fetal calf serum. The cells were serum-starved in Dulbecco's modified Eagle's medium without phenol red (Invitrogen) containing 5 μm EnduRen for 24 h. This substrate produces a stable and long-lived light emission in intact cells, thus allowing monitoring long-lasting biological events. Kinetics assay were started by adding the ligands to the wells, and the luminescence was immediately read with an integration time of 0.5 s at 2-min intervals for 1–2 h. To assess the ligand effects on the maximal level of internalization, cells were incubated with different ligands for 1 h before counting luminescence emission.
Expression Stoichiometry of Chimeric Proteins and Bioactivity
The levels of fusion proteins expressed in transfected cells were determined by measuring the intrinsic luminescence (DOPR-Rluc or MOPR-Rluc) or fluorescence (RGFP-Gβ1 or RGFP-βArr2) in lysed cell extracts or membrane preparations. For bioluminescence, 6 duplicate serial dilutions of each cell extract (1–20 μg of protein) or membrane preparation (0.2–6 μg) in PBS were counted in the luminometer using an automated protocol; to each sample 5 μm (final) of coelenterazine was automatically injected, and after a delay of 2 s total light emission was counted at 0.5-s intervals for 5 s. Integrated photon counts were plotted as a function of protein concentration, and the luminescence (counts/μg of protein) of the membrane/extract was computed by linear regression of the data. To record fluorescence, corresponding dilutions of the samples were measured in a Packard FluoroCount plate fluorometer using 460(20)- and 510(20)-nm filter sets for excitation and emission, respectively. Intrinsic fluorescence (RFU/μg) was computed by linear regression of the data after subtraction of background. The functional activity of chimeric and wild-type DOP and MOP receptors was compared in cell membranes prepared from transfected cells using a [35S]GTPγS radioligand binding assay as described (27).
Radioreceptor Binding Assays
To assess receptor expression levels of the cell lines and the extent of agonist-induced receptor down-regulation, radioreceptor binding assays using [3H]naltrindole (for DOPR) and [3H]diprenorphine (for DOPR and MOPR) were performed as described previously (27). Receptor density was obtained from the Bmax of [3H]diprenorphine binding isotherms (30).
Evaluation of Direct Effects of Opioids on Luciferase Activity
Because several chemical structures can affect the luciferase activity of Rluc either by directly binding to the enzymatic site or by unspecific optical effects (31), it was necessary to evaluate if the opioid ligands used in this study influenced Rluc activity. All the ligands used were assayed at 0.1 and 1 mm concentrations employing a cytosolic extract of COS-7 cells transfected with Rluc cDNA. No ligand produced significant stimulation, and at 100 μm only a few compounds produced modest (≤30%) inhibition of luciferase activity, with negligible effects on RET ratio determination. However, at 1 mm, three compounds (SNC-121, etorphine, and ethylketocyclazocine (EKC)) were found to inhibit Rluc activity by 50–60%. Although ratio measurements should not in principle be affected by the decrease in light intensity, we avoided concentrations of such compounds greater than 100 μm in RET assays, particularly in determinations based on membrane preparations. Guanine nucleotide (100 μm) had no detectable effects on the activity of Rluc.
RET Analysis and Data Calculations
The change in RET was determined from the ratios of light intensity from donor and acceptor after reading each well sequentially through different filters. Two different protocols were used depending on the luciferase substrate employed in the experiment. For native coelenterazine, the donor/acceptor emission maxima are only 30 nm apart. To correct for spectral overlap, we exploited a property of the Rluc/coelenterazine spectrum, which shows almost identical light emission at 450 and 510 nm (λ ratio 510:450 ≈ 1.06). Samples were counted using two band pass filters (blue, 450/20 nm, and green, 510/20 nm, 3rd Millenium, Omega Optical, Brattleboro, VT). Because the emission from RGFP at 450 nm is negligible, direct subtraction of blue filter counts from the green counts is sufficient to eliminate the fraction of light due to donor emission. Thus, the RET ratio corrected for filter cross bleeding was calculated using the relationship,
 |
where cpsG and cpsB indicate, respectively, photon counts per second recorded through the green and blue filter, and TG and TB are the relative transmittance of the filters, as reported by the manufacturer (0.86 and 0.77, respectively). This ratio was ≈0 ± 0.05 in samples containing only luciferase. For the bisdeoxy analogue of coelenterazine (λmax 398 nm), spectral overlap is negligible (acceptor-donor λmax difference ≈ 110 nm). Thus, samples were counted using blue short pass (cut-off 450 nm) and green long pass (cut-off 490 nm) filters (3rd Millenium, Omega Optical), and RET was quantified as a simple ratio of cps between the two filters (RET ratiobDOC = cpsG/cpsB). We call this ratiobDOC, to emphasize that it has a different scale, and it cannot be directly compared with the ratios determined using the native substrate.
Concentration-response curves for ligand-induced enhancement of RET were constructed using 11 half-log spaced concentrations of each ligand determined in duplicate wells. Four different ligands were simultaneously assayed on a single 96-well plate, and in each experiment the enkephalins analogue DADLE was included to account for interassay variability of the estimated parameters. Each experimental curve was analyzed by nonlinear curve fitting to the general logistic function y = (a − d)/(1 + (x/c)b) + d (where y and x are RET ratio and ligand concentration, a and d are the upper and lower asymptotes, c is the ligand concentration yielding half-maximal RET change, and b is the slope factor at c). From best-fitting parameters, the EC50 (c) and Emax (a–d) of each ligand were obtained. To measure competitive antagonism for the receptor-arrestin interaction, families of agonist concentration-response curves, generated in the absence or presence of increasing concentrations of antagonists, were fitted simultaneously. To evaluate if the antagonist had significant effect on the agonist Emax, the curves were first fitted with no constraints and then refitted by imposing a shared parameter a. The effect of the constraint was tested according to the extra sum of squares principle by comparing residual mean squares from the two fits with F statistics (32).
Kinetics data were normalized by setting the 0 point at the time of the first injection (i.e. the time point that triggered the syringe injector was subtracted from all time points). Data obtained by averaging the tracings of three different wells were analyzed by nonlinear fitting to the following exponential function,
 |
where, Y0 is the baseline, t is time, and Yj and kj are the amplitude and time constant of n exponential components. Data were sequentially fitted to models with increasing numbers of exponential components (up to n = 3), and the best-fitting model was chosen according to the extra sum of squares principle (32).
All data were replicated in at least three independent experiments and are reported as averages (±S.E.) of the indicated number of experiments. Representative concentration-response curves are shown after normalizing the data to the maximal effect of DADLE.
RESULTS
RET Detection of Receptor-Arrestin Interactions in Intact Cells
The photo proteins Rluc and RGFP are a donor-acceptor pair for a highly efficient RET mechanism that generates green luminescence in Renilla and can be used as fusion reporters to monitor the interaction between several GPCRs and arrestins (26).
We prepared HEK293 cell lines expressing either β-arrestin 1 or β-arrestin 2 (βArr1 and βArr2) tagged on the N terminus with the sequence of the RGFP acceptor. Both lines were further transduced with vectors encoding MOPR or DOPR tethered at the C termini to the Rluc donor. Agonist-mediated enhancement of RET signals was readily detectable in cells co-expressing receptors and βArr2 (DOP/βArr2 and MOP/βArr2) but not βArr1. Thus, only work describing the interaction of receptors with β-arrestin 2 will be presented in this paper. The Bmax of the cell lines used for this study are reported in supplemental Table S1.
The change of RET signal generated at maximal agonist concentration was roughly similar in DOP/βArr2 and MOP/βArr2 cells, consistent with the comparable levels of luminescent receptors and fluorescent arrestin expressed in the two lines (supplemental Table S2). However, the signal measured with native substrate was small (0.2–0.3 units) and not optimal for the detection of ligands with low intrinsic activity for the receptor-arrestin interaction. As shown previously, the enhancement of the apparent quantum yield resulting from the interaction between Rluc and RGFP when using the analog bDOC can provide a striking improvement of the ratio between stimulated versus basal signal (26). This was confirmed here (supplemental Fig. S1). Consequently, all further determinations of ligand-induced arrestin interaction were accomplished using bDOC as substrate.
Enkephalin-induced receptor-arrestin binding was rapid (supplemental Fig. S1) and could be satisfactorily described by a monoexponential kinetic process (although in some experiments a second component of opposite sign afforded a significantly better fit). The half-times (60–130 s across different experiments) were found to be not significantly different between DOPR and MOPR cells.
Disruption of functional Gαi/o subunits with pertussis toxin in MOPR/βArr cells had no effect on the EC50 and depressed slightly the maximal effect for DADLE but not for morphine (supplemental Fig. S2). A similar pattern was observed in DOPR cells (data not shown). This is consistent with previous observations showing that ligand-induced internalization does not depend on nor requires the efficient interaction of opioid receptors with the G protein α-subunits (33).
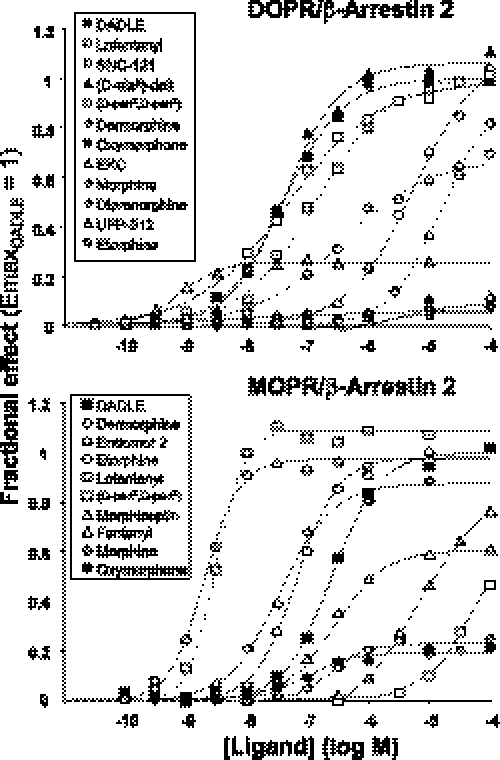
Efficacy of Ligands for Receptor-Arrestin Interactions
Concentration-response curves for the enhancement of RET RatiobDOC were fitted to a logistic function (see “Experimental Procedures”) to compute the intrinsic efficacy (Emax) and the potency (EC50) of ligands. The entire data set is listed in Table 1. Some representative concentration response curves of ligands acting as full or partial agonists for either DOP or MOP receptor-arrestin interactions are shown in Fig. 1. Ten opioid receptor antagonists, including the inverse agonists 174,864 and N,N(CH3)2-Dmt-Tic-NH2, were also tested at a single saturating concentration (100 μm). None produced significant effects in MOPR or DOPR cells (supplemental Fig. S3).
TABLE 1.
Intrinsic activities (Emax) and potencies (log EC50, m) of opioids for receptor-arrestin interactions
Concentration-response curves were analyzed as described under “Experimental Procedures.” Emax values were expressed and averaged as fraction of the Emax of DADLE, which was present in every experiment. Data are the means (±S.E.) of the indicated number of experiments (n). NM, not measurable; enk, enkephalins; DAGO, [d-Ala2,N-Me-Phe4, glycinol5]-enkephalins.
| Ligands | DOPR/βarr2 |
MOPR/βarr2 |
||||
|---|---|---|---|---|---|---|
| Emax (±S.E.) | Log EC50 (±S.E.) | n | Emax (±S.E.) | Log EC50 (±S.E.) | n | |
| Bremazocine | 0.01 (0.02) | NM | 3 | <0.01 | NM | 2 |
| Buprenorphine | 0.013 (0.06) | NM | 3 | 0.023 (0.01) | −7.2 (0.14) | 3 |
| DAGO | 0.8 (0.1)a | −3.2 (0.3)a | 3 | 0.89 (0.06) | −6.7 (0.08) | 13 |
| [d-Ala2]-deltorphin | 1.07 (0.02) | −7.5 (0.32) | 3 | 0.95 (0.05)a | −4.1 (0.2)a | 3 |
| Dermorphin | 0.97 (0.02) | −4.2 (0.10) | 3 | 0.93 (0.02) | −7.5 (0.06) | 6 |
| Diprenorphine | 0.14 (0.09) | −7.9 (0.19) | 5 | 0.012 (0.002) | NM | 3 |
| [d-Pen2,d-Pen5]-enk | 0.97 (0.04) | −7.6 (0.27) | 3 | 0.71a | −3.1 (0.6)a | 3 |
| Ethylketocyclazocine | 0.12 (0.03) | −6.0 (0.17) | 3 | 0.05 (0.02) | −7.8 (0.14) | 3 |
| Endomorphine 2 | NM | >−3 | 2 | 0.94 (0.04) | −7.3 (0.33) | 8 |
| Etorphine | 0.74 (0.07) | −6.8 (0.25) | 4 | 0.95 (0.02) | −8.5 (0.11) | 5 |
| Fentanyl | 0.01 (0.06) | NM | 3 | 0.77 (0.10) | −6.6 (0.18) | 3 |
| Lofentanyl | 0.78 (0.02) | −7.0 (0.13) | 3 | 1.06 (0.04) | −8.4 (0.08) | 7 |
| Meperidine | 0.013 | NM | 2 | 0.41 (0.2)a | −3.2 (0.31)a | 3 |
| Met-enkephalins | 1.05 | −7.1 | 1 | 1.08 | −6.4 | 1 |
| Morphiceptin | <0.01 | NM | 2 | 0.68 (0.07) | −5.0 (0.21) | 5 |
| Morphine | 0.08 (0.04) | NM | 3 | 0.24 (0.02) | −6.4 (0.08) | 6 |
| Oxymorphone | 0.09 (0.03) | −5.3 (0.46) | 3 | 0.20 (0.02) | −6.8 (0.09) | 8 |
| SNC-121 | 0.96 (0.09) | −5.3 (0.29) | 6 | NM | >−3 | 2 |
| UFP-512 | 0.27 (0.02) | −9.0 (0.15) | 3 | <0.01 | NM | 2 |
| [d-Ala2,d-Leu5]-enk | 1 | −7.6 (0.1) | 17 | 1 | −6.5 (0.08) | 21 |
a Values were extrapolated from data fitting but were not well defined experimentally because the curve did not reach a full upper asymptote.
FIGURE 1.
Concentration response curves of several opioid ligands for stimulation of receptor-β-arrestin2 coupling recorded in cells coexpressing RGFP-βArr2 with DOPR-Rluc (top) or MOPR-Rluc (bottom). All data are expressed as the fraction of the Emax value computed for DADLE (which was tested in every experiment) and are representative of several experiments (see Table 1). Best fitting theoretical curves (solid lines) were computed as described under “Experimental Procedures.” d-Pen2,d-Pen5), [d-Pen2,d-Pen5]enkephalins; d-Ala2]-Delt, [d-Ala2]deltorphin.
Partial agonism was not due to a major change in reaction kinetics between agonist-bound receptor and arrestin but primarily to a reduced maximal extent of the interaction, both in MOPR (Fig. 2, top) and DOPR (Fig. 2, bottom). Thus, the abundance rather than the speed of GPCR-arrestin complex formation accounts for the differential strength of agonism in this system.
FIGURE 2.
Kinetics of partial agonists on RET recorded in cells expressing MOPR-Rluc or DOPR-Rluc and RGFP-βArr2 (as indicated). Data were fitted to an exponential function (“Experimental Procedures”). Calculated half-times for MOPR-Rluc were: DADLE, 125 s; etorphine, 115 s; fentanyl, 210 s; oxymorphone, 132 s. Half-times for DOPR-Rluc were: DADLE, 123 s; etorphine, 99 s; UFP-512, 86 s; EKC, 112 s. Data were averaged from tracings recorded in three different wells and are representative of several experiments that gave similar results.
RET Detection of the Receptor-G Protein Interaction in Membrane Preparations
To minimize the inhibitory effect of arrestins on the interaction between receptor and G protein, we investigated the reaction in isolated membrane preparations, where neither arrestin recruiting nor receptor internalization can occur. In fact, in membranes prepared from either DOPR/βArr2 or MOPR/βArr2 cells there was very little if any detectable change of RET signals induced by agonists (data not shown).
We prepared cells co expressing each of the two luminescent receptors with either Gβ1 or Gγ2 bearing a RGFP sequence tethered on the N terminus. Clear enhancements of RET upon the addition of agonist were recorded in membranes containing either fluorescent Gβ1 or Gγ2. The net increase of ratio was similar, but the basal levels were smaller in the presence of Gβ. Thus, all subsequent experiments were performed using the receptor/Gβ1 system. To verify that C-terminal fusion to the Rluc sequence did not impair receptor-G protein interactions, we used a [35S]GTPγS/GDP radioligand exchange assay. The biological activities of a panel of agonists in activating Rluc-fused and wild-type proteins were compared. Both at MOP and DOP receptors we found a good correlation between the intrinsic activities of agonists for enhancing nucleotide binding via the native receptor and the corresponding Rluc fusion chimera (supplemental Fig. S0). Furthermore, the binding affinities of DADLE for luminescent receptors (measured as competition for [3H]diprenorphine binding) were identical to those determined in wild-type receptors (data not shown).
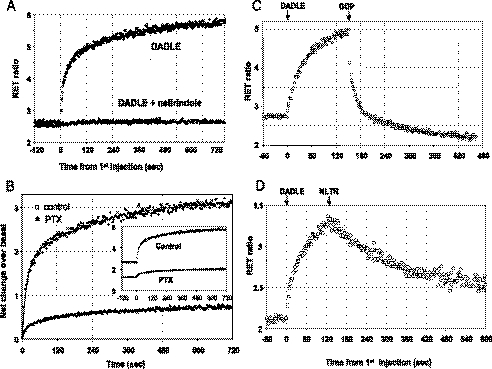
Upon the addition of DADLE, the RET ratio increased first rapidly and then more slowly to reach a plateau (Fig. 3), indicating the establishment of a state of closer molecular proximity between the N terminus of the Gβ subunit and the C terminus of the receptor. The kinetics were multiexponential, requiring the sum of two (or occasionally three) rate components for a satisfactory fit of the data. The half-time of the fastest component (6–12 s across different experiments) was similar in membranes from cells expressing MOPR/Gβ1 or DOPR/Gβ1.
FIGURE 3.
Kinetics of the enhancement of RET ratios in membranes obtained from cells expressing DOPR-Rluc and RGFP-Gβ1. A, membranes incubated with or without the antagonist naltrindole (10 μm) were injected with DADLE (1 μm, final concentration) at time point 0. Note that the response is abolished in the presence of antagonist. B, membranes were prepared from cells untreated (control) or treated with pertussis toxin (PTX, 100 ng/ml 18 h). DADLE (1 μm) was injected at time 0. The data are expressed as net change of RET by subtracting the average of each base line to illustrate the effect of the toxin treatment on the agonist (raw traces are shown in the inset). Note that a small enhancement of RET signal is still detectable in toxin-treated membranes. C, GDP reversal of RET enhancement induced by agonist is shown. Membranes were first injected with DADLE (time 0, 1 μm), and after 150 s GDP (100 μm) was injected from the second syringe. D, antagonist reversal is shown. The experiment was performed as in C, except that in the first injection DADLE was 100 nm, and in the second injection naltrindole (NLTR, 10 μm) was added (similar experiments were performed using membranes from MOPR/Gβ cells not shown here, with essentially identical results).
Although such complex kinetics demand additional investigation, the membrane interaction was clearly dependent on the agonist-bound and -activated receptor, and it was abolished in the presence of antagonist (Fig. 3A). Moreover, the following experiments shows that the enhancement of molecular proximity between GPCR and Gβ1 is mediated by endogenous Gα subunits.
First, the signal was sharply reduced in membranes of cells treated with pertussis toxin, where a small agonist-induced change remained detectable (Fig. 3B). Second, the enhancement was abolished by guanine nucleotides. In fact, DADLE addition to membranes in the presence of GTP, GDP (both 100 μm), or GTPγS (10 μm) produced no effect (data not shown), and the injection of GDP after triggering the interaction with agonist, produced a rapid reversal of RET ratio (Fig. 3C). Interestingly, the reversion induced by GDP was faster than that caused by the addition of antagonist to displace bound agonist from the receptor (Fig. 3D). Both di- or trisphosphate guanine nucleotides were active, but neither GMP nor ATP produced a significant effect (supplemental Fig. S4), indicating that the nucleotide specificity of the response is typical of the recognition properties of the Gα binding site. The EC50 (200–400 nm) measured for GTP inhibition (supplemental Fig. S4) is similar to the Km for agonist stimulation of GTPase. Note that GTP (or GDP) inhibited agonist-stimulated activity to a level lower than basal in DOPR/Gβ but not in MOPR/Gβ membranes (supplemental Fig. S4 and Fig. 3C). This suggests that there might be some extent of ligand-independent activity greater in the DOPR than in the MOPR system.
To further investigate α subunit requirement, we exposed intact cells to increasing concentrations of pertussis toxin and compared the extent of Gα ADP-ribosylation with the level of agonist-stimulated RET response that survived in membranes. There was no proportional loss of the two activities, so that at the highest toxin concentration (where at least 95% of Gα subunits had been ADP-ribosylated in vivo), 15–20% of agonist response was still detectable (supplemental Fig. S5). One possible, albeit not univocal, interpretation of such results is that an additional pertussis-resistant Gα type might be coupled to opioid receptors in this cellular system.
Efficacy and Receptor Selectivity of Opioids for G Protein Coupling
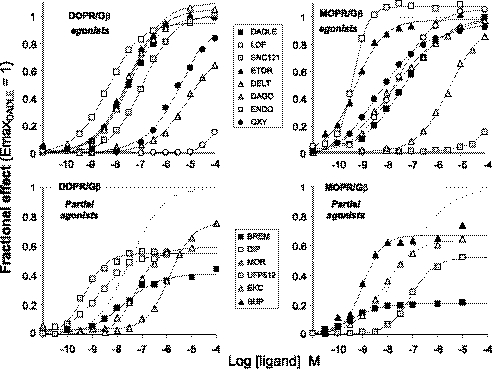
Concentration response curve for ligand-induced enhancement of RET ratios in membranes prepared from DOPR/Gβ1 and MOPR/Gβ1 cells were analyzed to compute the potency (EC50) and the intrinsic efficacy (Emax) of each ligand. The data are summarized in Table 2, whereas samples of representative curves for ligands that act as full or partial agonists at DOP and MOP receptors are shown in Fig. 4.
TABLE 2.
Intrinsic activities and potencies of opioids for receptor-G protein coupling
Data are derived from concentration response curve measured in membranes and are calculated and presented as in Table 1. enk, enkephalins; DAGO, [d-Ala2,N-Me-Phe4, glycinol5]-enkephalins.
| Ligands | DOPR/Gβ1 |
MOPR/Gβ1 |
||||
|---|---|---|---|---|---|---|
| Emax (±S.E.) | Log EC50 (±S.E.) | n | Emax (±S.E.) | Log EC50 (±S.E.) | n | |
| Bremazocine | 0.51 (0.07) | −7.9 (0.08) | 3 | 0.22 (0.01) | −9.4 (0.15) | 5 |
| Buprenorphine | 0.14 (0.10) | −8.8 (0.21) | 3 | 0.65 (0.06) | −8.8 (0.14) | 4 |
| DAGO | 0.72a (0.04) | −5.3a (0.13) | 3 | 0.91 (0.07) | −7.5 (0.07) | 5 |
| [d-Ala2]-deltorphin | 1.03 (0.03) | −7.7 (0.07) | 3 | 1.02a (0.02) | −5.3a (0.14) | 4 |
| Dermorphin | 0.98a (0.06) | −5.5a (0.34) | 3 | 0.97 (0.06) | −8.2 (0.15) | 3 |
| Diprenorphine | 0.60 (0.07) | −8.6 (0.08) | 4 | 0.18 (0.04) | −8.7 (0.26) | 6 |
| [d-Pen2,d-Pen5]-enk | 1.01 (0.02) | −7.5 (0.14) | 3 | 0.98a (0.02) | −5.7a (0.05) | 3 |
| Ethylketocyclazocine | 0.75 (0.10) | −7.5 (0.05) | 3 | 0.65 (0.01) | −8.5 (0.12) | 3 |
| Endomorphine 2 | NM | >−3 | 2 | 1.05 (0.04) | −7.3 (0.11) | 5 |
| Etorphine | 0.94 (0.07) | −7.7 (0.10) | 3 | 0.97 (0.01) | −9.4 (0.10) | 4 |
| Fentanyl | 0.99 (0.06) | −5.5 (0.12) | 3 | 0.98 (0.03) | −8.0 (0.06) | 3 |
| Lofentanyl | 0.99 (0.03) | −8.3 (0.03) | 4 | 1.11 (0.02) | −9.5 (0.13) | 4 |
| Meperidine | 1 | −3.5 (0.1)(b) | 2 | 0.86 (0.03) | −5.6 (0.17) | 3 |
| Met-enkephalins | 1.03 (0.12) | −7.4 (0.10) | 3 | 1.04 (0.08) | −7.4 (0.12) | 3 |
| Morphiceptin | 0.95a (0.05) | −3.0a (0.36) | 2 | 0.93 (0.042) | −5.9 (0.02) | 3 |
| Morphine | 0.75 (0.05) | −5.7 (0.17) | 3 | 0.73 (0.10) | −7.8 (0.23) | 3 |
| Oxymorphone | 0.87 (0.03) | −5.7 (0.05) | 3 | 0.94 (0.02) | −8.1 (0.11) | 4 |
| SNC-121 | 1.10 (0.05) | −6.9 (0.14) | 3 | NM | >−3 | 2 |
| UFP-512 | 0.60 (0.05) | −8.9 (0.13) | 4 | 0.59 (0.05) | −7.4 (0.05) | 5 |
| [d-Ala2,d-leu5]-enk | 1 | −7.5 (0.04) | 19 | 1 | −7.3 (0.06) | 24 |
a Values were extrapolated from data fitting but were not well defined experimentally because the curve did not reach a full upper asymptote.
FIGURE 4.
Concentration response curves of opioids for stimulation of receptor-Gβ coupling recorded in membranes prepared from cells co expressing RGFP-Gβ1 with DOPR-Rluc or MOPR-Rluc (as indicated). Full agonists (top) and partial agonists (bottom) are shown in separated graphs, and the best fitting curve for the full agonist DADLE is replotted as a dashed line. Data are normalized as in Fig. 1. LOF, lofentanyl; ETOR, etorphine; DELT, [d-Ala2]deltorphin; ENDO, endomorphine 2; OXY, oxymorphone; BREM, bremazocine; DIP, diprenorphine; MOR, morphine; BUP, buprenorphine.
It is interesting to examine how μ/δ receptor selectivity shows up when observed from the perspective of receptor-G protein coupling. There are two different ways for a ligand to produce subtype selective activation. One depends on the difference in potency for induction of G protein coupling at the two receptors and is expressed as a ratio of EC50 (supplemental Fig. S6 top). Endomorphine and SNC-121 show virtually absolute selectivity for MOPR and DOPR, respectively (although not studied with a comparable number of experiments, SNC-80 showed a similar behavior). The other mode of selectivity regards agonists with different levels of efficacy in activating MOP or DOP receptors and is expressed as the net difference of relative efficacies for the two receptor-G protein systems. Two oripavines, buprenorphine for MOPR and diprenorphine for DOPR, displayed the greatest efficacy-based selectivity (supplemental Fig. S6, bottom). Taken collectively, the selectivity of opioids measured here by direct visualization of receptor-G protein interactions in isolated membranes is in good agreement with the data obtained from conventional pharmacological assays.
Differential Efficacy of Opioids for Arrestin and G Protein Interactions
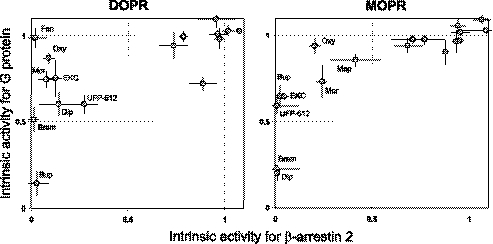
We compared the relative efficacies of ligands for promoting the interaction of DOPR and MOPR with arrestin and G protein. The data (Tables 1 and 2) clearly show that many ligands with full or significant efficacy for G protein display low or null efficacy for arrestin. In contrast, there was no example of a ligand with efficacy for arrestin greater than for G protein. Focusing on partial agonists, it appears that a level of efficacy for receptor-arrestin interaction becomes significant only when G protein efficacy ≥0.5/0.6. Some ligands, however, display very low arrestin efficacy even if their G protein efficacy surmounts such apparent threshold value.
This is best shown when relative intrinsic activities for G protein are plotted as a function of those for arrestin for each receptor (Fig. 5). It is clear that there is no simple linear correlation between the efficacies for the two transducers, although, particularly in case of MOPR, the data may suggest a very strong hyperbolic relation. Regardless of the nature of such relation, these plots highlight a number of agonists that have a remarkably low efficacy for arrestin despite a considerable extent of G protein efficacy. Oxymorphone, morphine, and EKC are among the ligands with a high level of discrepancy both at DOPR and MOPR, whereas others, such as fentanyl or meperidine, show efficacy divergences that are more receptor-selective (Fig. 5).
FIGURE 5.
Relationships between transducers efficacy of ligands at DOPR and MOPR. The relative intrinsic activities (Emax ligand versus Emax DADLE) computed from Tables 1 and 2 for G protein and arrestin are plotted against each other. Standard errors were calculated from the sum of variances. To improve readability, only ligands showing greater discrepancy between intrinsic activities at the two transducers were labeled in the plot (fen, fentanyl; Mep, meperidine; Oxy, oxymorphone; Brem, bremazocine; Dip, diprenorphine; Mor, morphine; Bup, buprenorphine). Regressions computed for the two sets of data yielded low correlations coefficients (0.66 ± 0.19 and 0.83 ± 0.15 for DOPR and MOPR, respectively) consistent with the lack of a linear relationship. However, both plots appear to suggest a strong hyperbolic relation between the intrinsic activities for the two transducers. Note that not all ligands of Table 1 and 2 are present in the graphs, because for some ligands intrinsic activities were not determined.
Selective Antagonists of Receptor-Arrestin Interaction
Opioids with high efficacy for G protein interaction, but poor or null for arrestin interaction, should constitute a special class of competitive antagonists. Upon binding to the receptor they can promote G protein coupling but block the interaction with arrestin. We verified this hypothesis in further experiments.
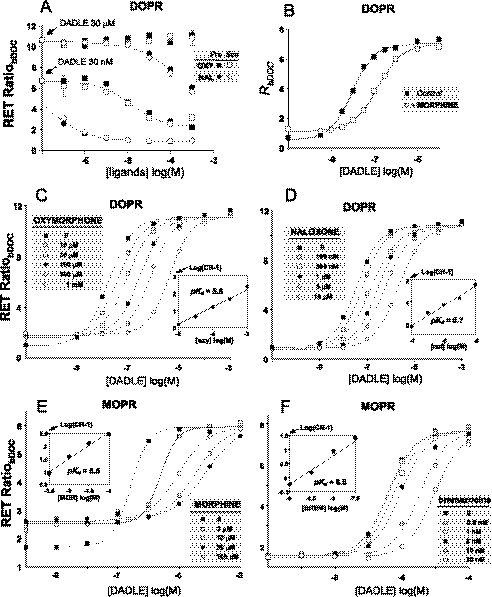
Because the interaction of agonist-bound receptor with arrestin leads to segregation of receptors into internalizing vesicles, we wondered whether this “irreversible” agonist sequestering step might be significant enough during the capture of RET signals to interfere with the ability to detect competitive antagonist behavior. We compared in DOPR/βArr2 cells the RET signals triggered by half-maximal (30 nm) and supramaximal (30 μm) concentrations of DADLE in the presence of increasing concentrations of either oxymorphone (which lacks arrestin- but not G protein efficacy) or naloxone (which lacks both efficacies). Both ligands inhibited receptor-arrestin interaction with potencies that were dramatically shifted by the maximal agonist concentration. The effects were identical regardless of whether inhibitors were added simultaneously with the peptide or 3 min before the peptide (Fig. 6A). Therefore, ligand inhibition of receptor-arrestin interaction appears to conform to a simple competitive receptor occupation model, within the time frame of our RET determinations.
FIGURE 6.
Competitive antagonism of opioids for receptor-β-arrestin 2 interactions. A, DOPR/βArr2 cells were incubated with two concentrations of DADLE in the absence or presence of increasing concentrations of naloxone (NAL) or oxymorphone (OXY). The inhibitors were added either simultaneously (○, □) or 3 min before (●, ■) the addition of DADLE. B, shown is a concentration response curve of DADLE in the absence or presence of morphine (100 μm) recorded in DOPR/βArr2 cells. C, shown are concentration-response curves of DADLE in the presence of increasing concentrations (as indicated) of oxymorphone. The inset is a Schild plot of the data (CR, concentration ratio, i.e. ratio of EC50 in the presence versus absence of antagonist; pKd, negative logarithm of the antagonist dissociation constant). Data were recorded in DOPR/βArr2 cells. D, the experiment was the same as in C but using naloxone as antagonist. E and F, the same experiments were performed in MOPR/βArr2 cells using morphine and bremazocine as antagonists.
Morphine (Fig. 6B) and oxymorphone (Fig. 6C) behaved as typical competitive antagonists for the DOPR/arrestin interaction. They caused a rightward shift in the concentration-response curves of DADLE with no significant changes of maximal effect. Schild analysis of the data yielded plots with unity slopes (Fig. 6C) like those of naloxone (Fig. 6D). Moreover, although both ligands displayed partial efficacy for the MOPR/arrestin interaction, morphine (Fig. 6E) and oxymorphone (not shown) caused an evident concentration-dependent shift of agonist EC50 also in this system (Fig. 6E).
Also, other compounds with partial efficacy for G protein and almost null efficacy for arrestin behaved as arrestin antagonists. Bremazocine (Fig. 6F) and EKC (data not shown) were competitive arrestin antagonists at MOPR. Diprenorphine had a similar role at DOPR, although the slope of the Schild plot obtained with this ligand was significantly greater than 1 (data not shown). This may suggest a more complex interaction of the ligand with the receptor, which was not, however, further investigated in this study.
Antagonism of Ligand-induced Receptor Endocytosis
To verify whether the selective antagonism of receptor-arrestin interaction exerted by some opioid alkaloids also results in blockade of endocytosis, we used a spectator reporter assay to monitor real-time receptor internalization (see “Experimental Procedures”). In HEK293 cells expressing DOPR-Rluc receptor and mt-RGFP, the addition of DADLE produced a decrease of the basal RET signal. This effect was totally abolished in the presence of 450 mm sucrose, a well known inhibitor of clathrin-mediated endocytosis (data not shown).
The rate of decay was mono-exponential (Fig. 7A) and somewhat slower (t½ 10–12 min) than the rates measured for arrestin-receptor interaction. Similar results were observed in SH-SY5Y neuroblastoma cells, which were similarly engineered with luminescent receptors and mt-RGFP to study the process in a host with closer properties to a physiological neuronal background. Different concentrations of agonist enhanced the extent, but not the rate of internalization (Fig. 7B). Oxymorphone at 100 μm blocked the internalization induced by the peptide, but it did not produce any detectable internalization of DOP receptors in either cell lines (Fig. 7, A and B). In contrast, in neuroblastoma cells expressing MOP receptors, both morphine and oxymorphone induced detectable levels of endocytosis. However, the effect was clearly smaller than those induced by full agonists of arrestin-receptor interaction, such as enkephalins or lofentanyl (Fig. 7C). Thus, on MOPR, both ligands are partial agonists for receptor internalization just like they are for receptor-arrestin interactions.
FIGURE 7.
Antagonism by opiates of enkephalin-induced receptor internalization and down-regulation. A, HEK cells expressing DOPR-Rluc and mt-RGFP, serum-starved, and equilibrated for 24 h with EnduRen substrate were incubated in the absence (control) or presence of 100 μm oxymorphone (oxy), 100 nm DADLE (DDL) or both for the indicated times. The decay of RET ratio indicates receptor endocytosis. The rate of decay induced by the peptide was estimated by fitting the data (solid line) to a single exponential function (t½ = 11.4 min). Note that oxymorphone does not induce any detectable change of RET but blocks the decrease triggered by DADLE. Data points are the means ± S.E. of three different tracings. B, data are the same as in A, generated using neuroblastoma SH-5YSY cells co-expressing DOPR-Rluc and mt-RGFP. The t½ for internalization computed by exponential fitting were 5.6 and 7.1 min with 10 and 100 nm DADLE, respectively. C, neuroblastoma SH-5YSY cells co-expressing MOPR-Rluc and mt-RGFP, serum-starved, and equilibrated with EnduRen for 24 h were incubated for 1 h with ligands at the indicated concentrations. The plate luminescence was counted repeatedly five times to verify signal stability. Data are the means of three determinations. D, HEK293 cells expressing wild-type DOPR were incubated in the absence (Control) or presence of DADLE (100 nm), morphine (100 μm), or both ligands before membrane preparation. Binding parameters (Kd and Bmax) were computed by nonlinear fitting of 12-point [3H]diprenorphine binding isotherms to a single-site mass-action-law binding model. Kd values (range 2–3 nm) were not significantly affected by the treatment. Bmax values are plotted with the S.E. obtained from the computer fit.
Finally, to verify that the antagonism for arrestin also results in inhibition of delayed arrestin-mediated responses, we studied the effect of oxymorphone and DADLE on down-regulation of wild-type DOP receptors. As shown in Fig. 7D, a 48-h pretreatment of cells with DADLE but not oxymorphone produced a sharp decrease in receptor density. This peptide-induced down-regulation was effectively antagonized in the presence of the alkaloid.
DISCUSSION
We have used two assays based on RET technology to measure the efficacy of δ- and μ-opioids in driving the interaction of the receptor with β-arrestin 2 and G protein. Efficacy data were derived from direct measurements of protein-protein interactions, rather than indirectly from biochemical events resulting from those interactions (e.g. second messenger levels, ERK phosphorylation, or receptor internalization). Thus, they should closely reflect the intrinsic property of each ligand structure to stabilize a receptor conformation that can favor the interaction with either arrestin or G protein.
The interaction receptor-arrestin was only marginally affected by treatment of cells with pertussis toxin, suggesting that the assessment of ligand efficacy for this interaction is not altered by the concurrent interaction of the receptor with G proteins and the consequent signaling. To achieve a similar situation for the determination of receptor-G protein interaction, we developed a RET assay of G protein coupling in membrane particles. This application was not described previously. Therefore, although not the focus of the study, some experiments were directed to better characterize the GPCR/Gβ response in membranes.
Receptor-Gβ interactions have been described previously in cell suspensions using different bioluminescence RET acceptor/donors (34) or in single cells by epifluorescence fluorescence RET microscopy (35). The kinetics reported in those studies (subsecond halftimes) is faster than what we measure in membranes. However, considering the strong effect of nucleotides on the interaction plus the diversity in reaction environment and membrane integrity between the experimental systems, a difference in kinetics properties might be not surprising.
The enhancement of RET in membranes was both receptor- and Gα-mediated, indicating that the change that brings the N terminus of Gβ closer to the receptor C terminus requires the α-subunit. This increased proximity might either result from the motion of one (or both) reacting partners inside the membrane layer to form a complex with the “dark” Gα subunit, or equally likely, it might result from intermolecular rearrangement among the three partners within a preassembled complex. Whatever the underlying mechanism, the rapid reversal by guanine nucleotide suggests that the driving force behind is release of bound GDP from the Gα subunit. Our working hypothesis is that the RET signal observed in membranes may gauge the power of activated receptor to induce the “empty” state of Gαβγ.
Another question is the direction of the change in RET. Agonists do not increase but shorten the distance between receptor and β subunit. This is in contrast with one fluorescence RET study of Gα-Gβγ interactions indicating heterotrimer dissociation by agonists (36), but it does agree with many other RET investigations of receptor-G protein coupling (34, 35, 37, 38). Such findings may challenge the idea that Gβγ dissociation is the primary step of G protein activation and, together with other lines of evidence (39), may suggest that in several GPCR/G protein systems activation would involve subunit rearrangements within a preformed complex without physical dissociation of the heterotrimer. Alternatively we may speculate that our results reflect a characteristic of reactions carried over in isolated membranes, where the absence of guanine nucleotides might favor the formation of a dead-end intermediate consisting of nucleotide-free Gα that may trap receptor and Gβγ in an undissociable complex.
Regardless of the underlying molecular mechanism, the best evidence that what we measured in membranes is a faithful indicator of ligand ability to promote receptor-mediated G protein activation comes from pharmacological data. Relative potencies, efficacies, and μ/δ selectivity of the panel of opioids measured via RET in this study are in broad agreement with results from a variety of in vivo and in vitro pharmacological assays. We, thus, conclude that the membrane assay of receptor-G protein coupling described in this paper can provide estimates of ligand intrinsic activities that are neither biased by nonlinear amplification factors of the signaling network nor by competitive inhibition from arrestin (40).
Ligand efficacy for G protein and arrestin interactions were compared in both μ and δ receptors. Although not exhaustive, the list of studied ligands represents a fairly broad exploration of the conformational space that can fit δ/μ-opioid receptor binding sites. At least one member of the major chemical classes of opioid was in fact tested, including peptides, natural or semi-synthetic alkaloids, benzomorphans, 4-phenyl- and 4-anilidopiperidines, benzhydrylpiperazines, and Dmt-Tic “peptoids.”
The arrestin and G protein efficacies of ligands are markedly different both at DOPR and MOPR. However, they are not randomly independent. No ligand displayed greater efficacy for arrestin than for G protein, and partial agonism at G protein was invariably associated with lesser or no efficacy for arrestin. This pattern suggests that the structural requirements for an agonist to trigger the interaction of the receptor with arrestin are more stringent than those sufficient to initiate G protein coupling. All tested antagonists were inactive on arrestin, including two inverse agonists. This finding is in contrast with observations made on different GPCRs (41) and tells that a positive efficacy for β-arrestin 2 is not a general property of inverse agonism. We cannot obviously rule out that an opioid with greater efficacy for arrestin than for G protein may actually exist. A high-throughput screening search might be necessary to discover a structure with such property.
The molecular mechanism underlying the differential agonism of ligands for arrestin and G protein deserves further study. Because two receptor domains (intracellular loops and the C terminus) are differentially involved in G protein and arrestin recognition (42), the balance of molecular perturbations transmitted from the ligand binding pocket to the cytosolic area may be a key factor. Structural requirements needed to propagate a change to the somewhat segregated C terminus might be more stringent than those sufficient to affect loops that are contiguous to the transmembrane bundle. That may explain why full agonists for both interactions are more frequently encountered in analogues of the endogenous enkephalins (which was “fine-tuned” by evolution to reach both targets) than when manipulating the structure of opium-derived phenanthrene alkaloids or other synthetic templates.
However, the existence of a subset of ligands with poor arrestin efficacy despite a close to full G protein efficacy suggests that in those structures molecular interactions that are optimal to allosterically induce the receptor interface for G protein may be detrimental to shape that for arrestin. Thus, there might be specific contacts with receptor residues that differentially channel conformational perturbations resulting in alternative three-dimensional configurations of the cytosolic area. If so, the shortening from N-allyl to the N-methyl group that converts naloxone (a dual G protein/arrestin antagonist) into oxymorphone (a mixed agonist/antagonist) is particularly interesting because it seems sufficient to restore full receptor efficacy for G protein but not for arrestin. Conversely, the double addition of one methyl and one carboxymethyl group to the piperidine ring of fentanyl (which makes lofentanyl) is sufficient to convert in DOPR an agonist/antagonist into a dual full agonist. More detailed structure-activity relationship analysis of the data might provide further insight into this issue.
It is interesting that the discrepancy between G protein and arrestin efficacy of partial agonists described here for opioids is much stronger than what reported for the β2-adrenergic receptor (28). This suggests that our observations may not be broadly applicable to other GPCR or ligand systems and that factors related to the cellular expression host may also play a key role. Particularly important in the case of arrestin-receptor interactions is the contribution of G protein receptor kinase-mediated receptor phosphorylation.
Although studies with radiolabeled arrestin indicate that agonists can regulate arrestin binding regardless of phosphorylation (43), recent findings on the CCR7 chemokine receptors show that the differential activation of G protein receptor kinase isotypes by distinct agonist can result in marked differences of arrestin recruitment (44). This implies the possibility that intrinsic differences in phosphorylation might play a key role in determining distinct relationships between transducers efficacy across different ligands and GPCR types.
The biological consequence of receptor occupation by a ligand that has strong or full efficacy for G protein but weak or null efficacy for arrestin is agonism for the first interaction and competitive antagonism for the second. This defines a new type of transducer-selective antagonism, where the conflicting efficacies reflect the interaction of the same receptor with two distinct transduction partners. As shown here, oxymorphone and morphine are indeed virtually pure arrestin antagonists at DOPR and weak partial agonists with strong antagonistic properties at MOPR.
Our data confirm the morphine paradox mentioned in the introduction; targeted deletion of the β-arrestin 2 gene affects the acute and chronic pharmacology of morphine more than other opiates (16). Yet, as shown here, morphine and oxymorphone are among the agonists with the worst efficiency to promote the interaction of the receptor with β-arrestin 2. The additional point emerging from our study is that morphine-like alkaloids are mixed agonist/antagonists of receptor-transducer interactions. In fact, as shown here, they can activate G protein-mediated signaling but also protect the receptor from internalization and down-regulation induced by endogenous enkephalins. This explains the antagonistic effects reported earlier in neuroblastoma cells (6). Whether such dual property might be related to the ability of morphine to up-regulate DOPR trafficking, often observed in brain (45, 46), or to the strong tolerance that such ligands exert in vivo remains to be established. Other ligands share similar although not identical profiles. Fentanyl at δ but not μ receptors is one example. Some benzomorphans and oripavines also have G protein agonism and arrestin antagonism, although they are weaker agonists for G proteins and have considerable activity for κ receptors.
The mix of agonism and antagonism that each ligand can exhibit not only across different receptor subtypes but through the same subtype also across different transducers draws a very complex maze of potential signaling outputs. This makes it really difficult to predict how such molecular interactions may be linked to the acute and chronic drug responses occurring in vivo. As recently shown by groundbreaking work in the knock-in mouse carrying a fluorescent replacement of the δ receptor (18, 47), animal models in which molecular interactions are engineered for in vivo detection may be a solution to the problem and are likely to provide the kind of physiological and clinical relevant insight that progress in molecular knowledge alone cannot deliver.
Supplementary Material
Acknowledgments
We are grateful to Dr. Remo Guerrini (University of Ferrara) and Prof. Albert Herz (Max Planck Institut für Psychiatrie, Munich, Germany) for the gift of opioid compounds. Dr. Antonio De Blasi (University of Rome) kindly provided the cDNAs encoding human β-arrestin 1 and 2 sequences. We also thank Dr. Girolamo Calò (University of Ferrara, Italy) for useful discussions.
This work was supported in part by FIRB Internationalization Program Grant RBIN04CKYN_001.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Tables S1 and S2 and Figs. S0–S6.
- DOPR
- δ-opioid receptor
- MOPR
- μ-opioid receptor
- RET
- resonance energy transfer
- HEK293
- human embryonic kidney 293 cells
- RGFP
- Renilla green fluorescent protein
- Rluc
- Renilla luciferase
- GPCR
- G-protein-coupled receptor
- cps
- counts/s
- βArr1 and βArr2
- β-arrestin 1 and 2
- bDOC
- bisdeoxy-coelenterazine
- DADLE
- [d-Ala2, d-Leu5]-enkephalins
- EKC
- ethylketocyclazocine
- GTPγS
- guanosine 5′-O-(thiotriphosphate)
- mt
- membrane-targeted
- Dmt
- 2′,6′-dimethyltyrosine
- Tic
- 1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid.
REFERENCES
- 1.Violin J. D., Lefkowitz R. J. (2007) Trends Pharmacol. Sci. 28, 416–422 [DOI] [PubMed] [Google Scholar]
- 2.Masri B., Salahpour A., Didriksen M., Ghisi V., Beaulieu J. M., Gainetdinov R. R., Caron M. G. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 13656–13661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Urban J. D., Clarke W. P., von Zastrow M., Nichols D. E., Kobilka B., Weinstein H., Javitch J. A., Roth B. L., Christopoulos A., Sexton P. M., Miller K. J., Spedding M., Mailman R. B. (2007) J. Pharmacol. Exp. Ther. 320, 1–13 [DOI] [PubMed] [Google Scholar]
- 4.Kenakin T. (2007) Mol. Pharmacol. 72, 1393–1401 [DOI] [PubMed] [Google Scholar]
- 5.Kenakin T. P. (2008) Br. J. Pharmacol. 153, 432–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vachon L., Costa T., Herz A. (1987) Biochem. Pharmacol. 36, 2889–2897 [DOI] [PubMed] [Google Scholar]
- 7.Eisinger D. A., Ammer H., Schulz R. (2002) J. Neurosci. 22, 10192–10200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arttamangkul S., Quillinan N., Low M. J., von Zastrow M., Pintar J., Williams J. T. (2008) Mol. Pharmacol. 74, 972–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keith D. E., Anton B., Murray S. R., Zaki P. A., Chu P. C., Lissin D. V., Monteillet-Agius G., Stewart P. L., Evans C. J., von Zastrow M. (1998) Mol. Pharmacol. 53, 377–384 [PubMed] [Google Scholar]
- 10.Keith D. E., Murray S. R., Zaki P. A., Chu P. C., Lissin D. V., Kang L., Evans C. J., von Zastrow M. (1996) J. Biol. Chem. 271, 19021–19024 [DOI] [PubMed] [Google Scholar]
- 11.Whistler J. L., von Zastrow M. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 9914–9919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haberstock-Debic H., Kim K. A., Yu Y. J., von Zastrow M. (2005) J. Neurosci. 25, 7847–7857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finn A. K., Whistler J. L. (2001) Neuron 32, 829–839 [DOI] [PubMed] [Google Scholar]
- 14.Whistler J. L., Enquist J., Marley A., Fong J., Gladher F., Tsuruda P., Murray S. R., Von Zastrow M. (2002) Science 297, 615–620 [DOI] [PubMed] [Google Scholar]
- 15.Koch T., Widera A., Bartzsch K., Schulz S., Brandenburg L. O., Wundrack N., Beyer A., Grecksch G., Höllt V. (2005) Mol. Pharmacol. 67, 280–287 [DOI] [PubMed] [Google Scholar]
- 16.Bohn L. M., Dykstra L. A., Lefkowitz R. J., Caron M. G., Barak L. S. (2004) Mol. Pharmacol. 66, 106–112 [DOI] [PubMed] [Google Scholar]
- 17.Koch T., Höllt V. (2008) Pharmacol. Ther. 117, 199–206 [DOI] [PubMed] [Google Scholar]
- 18.Pradhan A. A., Becker J. A., Scherrer G., Tryoen-Toth P., Filliol D., Matifas A., Massotte D., Gavériaux-Ruff C., Kieffer B. L. (2009) PLoS One 4, e5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim J. A., Bartlett S., He L., Nielsen C. K., Chang A. M., Kharazia V., Waldhoer M., Ou C. J., Taylor S., Ferwerda M., Cado D., Whistler J. L. (2008) Curr. Biol. 18, 129–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marie N., Lecoq I., Jauzac P., Allouche S. (2003) J. Biol. Chem. 278, 22795–22804 [DOI] [PubMed] [Google Scholar]
- 21.Tsao P. I., von Zastrow M. (2000) J. Biol. Chem. 275, 11130–11140 [DOI] [PubMed] [Google Scholar]
- 22.Bohn L. M., Lefkowitz R. J., Gainetdinov R. R., Peppel K., Caron M. G., Lin F. T. (1999) Science 286, 2495–2498 [DOI] [PubMed] [Google Scholar]
- 23.Bohn L. M., Gainetdinov R. R., Lin F. T., Lefkowitz R. J., Caron M. G. (2000) Nature 408, 720–723 [DOI] [PubMed] [Google Scholar]
- 24.Przewlocka B., Sieja A., Starowicz K., Maj M., Bilecki W., Przewlocki R. (2002) Neurosci. Lett. 325, 107–110 [DOI] [PubMed] [Google Scholar]
- 25.Kieffer B. L. (1999) Trends Pharmacol. Sci. 20, 19–26 [DOI] [PubMed] [Google Scholar]
- 26.Molinari P., Casella I., Costa T. (2008) Biochem. J. 409, 251–261 [DOI] [PubMed] [Google Scholar]
- 27.Molinari P., Ambrosio C., Riitano D., Sbraccia M., Grò M. C., Costa T. (2003) J. Biol. Chem. 278, 15778–15788 [DOI] [PubMed] [Google Scholar]
- 28.Drake M. T., Violin J. D., Whalen E. J., Wisler J. W., Shenoy S. K., Lefkowitz R. J. (2008) J. Biol. Chem. 283, 5669–5676 [DOI] [PubMed] [Google Scholar]
- 29.Abankwa D., Vogel H. (2007) J. Cell Sci. 120, 2953–2962 [DOI] [PubMed] [Google Scholar]
- 30.Vachon L., Costa T., Herz A. (1987) Mol. Pharmacol. 31, 159–168 [PubMed] [Google Scholar]
- 31.Auld D. S., Southall N. T., Jadhav A., Johnson R. L., Diller D. J., Simeonov A., Austin C. P., Inglese J. (2008) J. Med. Chem. 51, 2372–2386 [DOI] [PubMed] [Google Scholar]
- 32.DeLean A., Munson P. J., Rodbard D. (1978) Am. J. Physiol. 235, E97–E102 [DOI] [PubMed] [Google Scholar]
- 33.Bradbury F. A., Zelnik J. C., Traynor J. R. (2009) J. Neurochem. 109, 1526–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galés C., Rebois R. V., Hogue M., Trieu P., Breit A., Hébert T. E., Bouvier M. (2005) Nat. Methods 2, 177–184 [DOI] [PubMed] [Google Scholar]
- 35.Hein P., Frank M., Hoffmann C., Lohse M. J., Bünemann M. (2005) EMBO J. 24, 4106–4114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gibson S. K., Gilman A. G. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 212–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ayoub M. A., Maurel D., Binet V., Fink M., Prézeau L., Ansanay H., Pin J. P. (2007) Mol. Pharmacol. 71, 1329–1340 [DOI] [PubMed] [Google Scholar]
- 38.Galés C., Van Durm J. J., Schaak S., Pontier S., Percherancier Y., Audet M., Paris H., Bouvier M. (2006) Nat. Struct. Mol. Biol. 13, 778–786 [DOI] [PubMed] [Google Scholar]
- 39.Klein S., Reuveni H., Levitzki A. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 3219–3223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Violin J. D., DiPilato L. M., Yildirim N., Elston T. C., Zhang J., Lefkowitz R. J. (2008) J. Biol. Chem. 283, 2949–2961 [DOI] [PubMed] [Google Scholar]
- 41.Azzi M., Charest P. G., Angers S., Rousseau G., Kohout T., Bouvier M., Piñeyro G. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 11406–11411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gurevich V. V., Gurevich E. V. (2006) Pharmacol. Ther. 110, 465–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gurevich V. V., Dion S. B., Onorato J. J., Ptasienski J., Kim C. M., Sterne-Marr R., Hosey M. M., Benovic J. L. (1995) J. Biol. Chem. 270, 720–731 [DOI] [PubMed] [Google Scholar]
- 44.Zidar D. A., Violin J. D., Whalen E. J., Lefkowitz R. J. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 9649–9654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hack S. P., Bagley E. E., Chieng B. C., Christie M. J. (2005) J. Neurosci. 25, 3192–3198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morinville A., Cahill C. M., Esdaile M. J., Aibak H., Collier B., Kieffer B. L., Beaudet A. (2003) J. Neurosci. 23, 4888–4898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scherrer G., Tryoen-Tóth P., Filliol D., Matifas A., Laustriat D., Cao Y. Q., Basbaum A. I., Dierich A., Vonesh J. L., Gavériaux-Ruff C., Kieffer B. L. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 9691–9696 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.