Abstract
Acid-sensing ion channels (ASICs) are voltage-independent Na+ channels activated by extracellular protons. ASIC1a is expressed in neurons in mammalian brain and is implicated in long term potentiation of synaptic transmission that contributes to learning and memory. In ischemic brain injury, however, activation of this Ca2+-permeable channel plays a critical role in acidosis-mediated, glutamate-independent, Ca2+ toxicity. We report here the identification of insulin as a regulator of ASIC1a surface expression. In modeled ischemia using Chinese hamster ovary cells, serum depletion caused a significant increase in ASIC1a surface expression that resulted in the potentiation of ASIC1a activity. Among the components of serum, insulin was identified as the key factor that maintains a low level of ASIC1a on the plasma membrane. Neurons subjected to insulin depletion increased surface expression of ASIC1a with resultant potentiation of ASIC1a currents. Intracellularly, ASIC1a is predominantly localized to the endoplasmic reticulum in Chinese hamster ovary cells, and this intracellular localization is also observed in neurons. Under conditions of serum or insulin depletion, the intracellular ASIC1a is translocated to the cell surface, increasing the surface expression level. These results reveal an important trafficking mechanism of ASIC1a that is relevant to both the normal physiology and the pathological activity of this channel.
Keywords: Acid-sensing Ion Channels (ASIC), Brain, Exocytosis, Insulin, Ion Channels, Ischemia, Trafficking
Introduction
Acid-sensing ion channels belong to the epithelial sodium channel and degenerin family of ion channels and primarily transport Na+ into cells. ASICs2 are activated by the presence of extracellular protons, which serve as ligands for these channels. So far six isoforms of ASICs (ASIC1a, 1b, 2a, 2b, 3, and 4) have been found in the mammalian central and peripheral nervous system. ASIC1a is expressed in various regions of brain including hippocampus, cerebral cortex, cerebellum, and amygdala (1–3). The role of ASIC1a in brain function is well characterized, in particular by electrophysiological and behavioral studies of ASIC1a knock-out (ASIC1a−/−) mice. ASIC1a H+-evoked currents are involved in synaptic transmission that contributes to important normal brain functions such as learning and memory in hippocampus and fear-related behaviors in the amygdala (3–5). Like other ASIC isoforms, the amino acid sequence of ASIC1a reveals a structure highly conserved among the epithelial sodium channel family (6). The crystal structure of a truncated chicken ASIC1a channel determined that this two-transmembrane protein is assembled as a trimer (7). Cerebral neurons express native ASIC1a as an assembly of homomultimers as well as heteromultimers in association with ASIC2a (8). Although ASIC1a and ASIC2a share high homology in their amino acid sequences, these proton-activated channels exhibit distinct sensitivity to extracellular pH. ASIC1a is more sensitive to changes in extracellular proton levels than ASIC2a and thus activates at a higher pH (pH of half-maximal channel activation pH0.5 = 6.2), whereas ASIC2a activates at a lower pH (pH0.5 = 4.4). Additional differences are found in cation selectivity, because homomultimeric ASIC1a channels, but not ASIC2a, permeate Ca2+ in addition to Na+. ASIC1a is activated during tissue acidosis following cerebral ischemia and seizures and such activation, and the ensuing Ca2+-influx causes neuronal injury (9, 10). Although N-methyl-d-aspartate-type glutamate receptors have been considered the foremost cause of intracellular Ca2+ overload and related toxicity following ischemic insults, recent observations have established the importance of ASIC1a to glutamate receptor-independent Ca2+ toxicity (9). In a mouse model of focal ischemia, the penumbral cortex undergoes tissue acidification to ∼pH 6.5 prior to the development of infarction (11, 12). Activation of ASIC1a during tissue acidification is directly responsible for ischemic infarction, because blockage of either tissue acidification by bicarbonate or ASIC1a activity by an antagonist psalmotoxin 1 efficiently reduced infarct volume (9, 12). The time window for acidosis and ASIC1a activation is longer than that for activation of glutamate receptors and extends many hours after the onset of stroke. This time window coincides with progression of ischemic infarction and thus underscores the crucial role of ASIC1a in this regard.
In this study we sought to identify the mechanism and regulatory components activating ASIC1a under ischemic stress. ASIC1a currents are significantly potentiated in cell culture models of ischemia, i.e. deprivation of oxygen and glucose and extracellular acidification, which consequently led to cell injury (9). The ischemic model in cell culture was previously established for neurons as well as heterologous cells expressing ASIC1a (9). Numerous studies have shown that ASIC1a, like ASIC2a, forms functional channels in heterologous cells, and the biophysical properties are indistinguishable from that of the native neuronal channels. Here we employed CHO cells stably expressing ASIC1a to investigate whether ASIC1a potentiation occurs through an increase in surface levels of ASIC1a under ischemic conditions. To our surprise, deprivation of serum, specifically insulin in serum, caused substantial accumulation of ASIC1a channels in the plasma membrane. Concurring with these results, serum depletion potentiated ASIC1a currents in CHO cells, as did insulin depletion in neuronal cells. Although ASIC1a mainly functions on the cell surface, it is predominantly localized in the ER and the outer nuclear membrane in CHO cells, and such intracellular localization was also observed in cortical neurons. The ER localization of ASIC1a signifies both regulation of surface expression by an ER retention mechanism and the presence of an intracellular reservoir for surface delivery and accumulation. It is striking that pathological settings causing ASIC1a potentiation have unveiled an important regulatory mechanism for surface expression of this channel that has been largely unknown so far.
EXPERIMENTAL PROCEDURES
Plasmids, Cell Culture, and Transfection
The rat cDNA clone of ASIC1a and ASIC2a in pCDNA3, and GFP fusion to both ASIC1a and ASIC2a at the C terminus (pCDNA-ASIC1a-EGFP and pCDNA-ASIC2a-EGFP) were described previously (13). ASIC1a and ASIC2a tagged with epitope FLAG (YKDDDDK) at the C terminus were constructed in plasmid pCDNA3. The N-terminal fusion of epitope HA (YPYDVPDYA) to ASIC1a was constructed in pCDNAJM1. CHO cells were cultured routinely in Dulbecco's modified Eagle's medium with high glucose (Invitrogen) supplemented with 10% fetal bovine serum in humidified 5% CO2 incubator. Cortical neurons of prenatal E16 Swiss mice were cultured in Neurobasal medium supplemented with B-27 and GlutaMax (Invitrogen). The neurons were cultured 4 days prior to transfection, 10 days prior to immunocytochemistry, and 12 days prior to electrophysiological recordings. CHO cells and neurons were transfected using FuGENE 6 (Roche Applied Science) and NeuroFect (Genlantis) transfection reagent, respectively.
Immunocytochemistry
CHO cell lines stably transfected with pCDNA-ASIC1a-EGFP, pCDNA-ASIC2a-EGFP, pCDNA3-ASIC1a, and pCDNA3-ASIC1a-FLAG were used. To visualize the expression pattern of HA-ASIC1a, the neurons were cultured for 4 days after transfection with pCDNA-HA-ASIC1a. CHO cells and neurons were fixed in 4% paraformaldehyde/PBS and permeablized and blocked in blocking buffer containing 2% normal goat serum, 2% bovine serum albumin, and 1% Triton X-100 in PBS. Frozen sections of brain slices were fixed in methanol, boiled in 10 mm sodium citrate buffer (pH 6) for 1 min for antigen retrieval, and blocked and permeablized in blocking buffer containing 10% normal goat serum, 2% bovine serum albumin, and 1% Triton X-100 in PBS. Primary and secondary antibodies were diluted in blocking buffer. The antibodies used were rabbit anti-ASIC1a (Sigma), mouse anti-FLAG (Sigma), rabbit anti-GRP78 BiP (abcam), rabbit anti-HA Y-11 (Santa Cruz), rabbit anti-lamin B (abcam), mouse anti-NeuN (Chemicon), and goat anti-mouse IgG-fluorescein isothiocyanate and goat anti-mouse IgG-Cy3 (Jackson Immuno Research). The nuclei were stained with 4′,6′-diamidino-2-phenylindole dihydrochloride (Jackson Immuno Research). Brain slices and cells were analyzed by epifluorescence microscopy (Leica DM LB) and confocal microscopy (Olympus; Molecular Microbiology and Immunology Core Facility at the Oregon Health and Science University).
Serum Deprivation Assays, Cell Surface Biotinylation, and Western Blot
CHO cell lines stably transfected with pCDNA-ASIC1a-FLAG or pCDNA-ASIC2a-FLAG were used. The cells were grown to confluency in 100-mm culture dishes in Dulbecco's modified Eagle's medium with 10% fetal bovine serum and 25 mm glucose for 2 days. For serum and/or glucose deprivation, the cells were extensively washed in PBS and incubated in Dulbecco's modified Eagle's medium with 25 mm glucose or without glucose. In experiments with cycloheximide and brefeldin A, the cells were treated with each drug for 1 h in serum containing medium prior to and during serum deprivation for indicated time period. Cycloheximide are used at 50 μm and brefeldin A at 1 μg/ml. B-27 serum-free supplement and the supplements B27 minus antioxidant, B27 minus insulin, and insulin-transferrin-selenium were purchased from Invitrogen. Bovine insulin and cycloheximide were purchased from Sigma, and brefeldin A was from Epicenter. For biotinylation, the cells in 100-mm culture dishes were washed in ice-cold PBS and incubated with 0.3 mg/ml EZ-Link sulfo-NHS-LC-Biotin (Thermo Scientific Pierce) at 4 °C on a rocker for 30 min. The cells were collected and lysed with 1% Triton X-100/PBS, and an aliquot of lysates was collected for measurement of protein concentration. Biotinylated proteins were separated from the intracellular protein fraction using 40 μl of agarose resin linked to NeutrAvidin (Thermo Scientific Pierce) by incubation overnight at 4 °C and subsequent centrifugation. After extensive washing in 0.5% Triton X-100/PBS, biotinylated proteins bound to avidine-agarose resins were resuspended in SDS-PAGE sample buffer in a volume according to the total protein concentration and eluted from avidin-agarose by heating at 95 °C. Equal amounts of biotinylated ASIC1a-FLAG and ASIC2a-FLAG were analyzed by Western blot to compare the changes in surface-expressed protein levels. 50 μg of intracellular proteins were loaded in each lane for comparison with intracellular ASICs and β-actin as loading control with. Following SDS-PAGE, the proteins were transferred to polyvinylidene difluoride membrane, which were then blocked in PBS containing 5% milk (blocking buffer) and probed with the primary antibodies mouse anti-FLAG (Sigma) and/or rabbit anti-β-actin (abcam). The membranes were probed with secondary antibody-horseradish peroxidase conjugates, goat anti-mouse IgG, and/or goat anti-rabbit IgG (Bio-Rad). Horseradish peroxidase bound to immunocomplex was visualized with ECL (Amersham Biosciences) and Kodak BioMax Chemiluminescence film. The intensity of the protein bands detected was scanned and quantified by the National Institutes of Health ImageJ gel analysis program.
Electrophysiology
ASIC currents were recorded with whole cell patch-clamp method from stably transfected CHO cells with pCDNA-ASIC1a-EGFP and primary culture of cortical neurons from mice. In CHO cells, GFP-positive cells were selected for recording of ASIC1a currents. For serum and B27 deprivation, the cells were incubated in either serum-free or B27-free medium for 1 h prior to current measurements. In general, the cells were voltage-clamped at a holding potential of −60 mV. The data were acquired using an AXOPATCH 200B amplifier with pCLAMP 8.2 software (Axon Instruments, CA). The data were filtered at 2 KHz and digitized at 5 KHz using Digidata 1322A (Axon Instruments). For changes of extracellular solutions, a multibarrel perfusion system (SF-77; Warner Instruments, Hamden, CT) was used. Low pH extracellular solutions were applied at 2-min intervals. The normal extracellular solution contained 140 mm NaCl, 5.4 mm KCl, 2 mm CaCl2, 1 mm MgCl2, 20 mm HEPES, and 10 mm glucose, 320–335 mOsm at pH 7.4. For the acidic extracellular solutions, the pH was adjusted with NaOH/HCl. Patch pipettes were pulled from borosilicate glass (1.5-mm diameter; WPI, Sarasota, FL) on a two-stage puller (PP83; Narishige, Tokyo, Japan). Pipettes had a resistance of 2–4 MΩ when filled with the intracellular solution, which contained 140 mm CsF, 2 mm TEACl, 5 mm EGTA, 10 mm HEPES, 1 mm CaCl2, 4 mm MgCl2 in pH 7.3 adjusted with CsOH/HCl, 290–300 mOsm. All of the experiments were carried out at room temperature. The recordings with an access resistance of less than 10 mΩ and a leak current less than 100 pA at −60 mV were included for analysis. The data are presented as the means ± S.E. The statistical significance was determined using two-way ANOVA and Student's t test where appropriate. The differences were considered significant when p < 0.05.
RESULTS
Surface Expression of ASIC1a Increases in the Absence of Serum
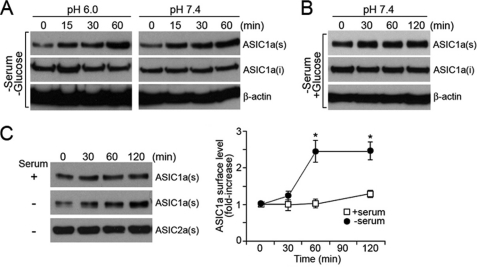
In both neurons and CHO cells, acid-activated currents by ASIC1a increase in response to ischemic conditions leading to cell injury (9). In those studies, in vitro conditions such as oxygen and glucose deprivation and acid treatment at pH 6 have been applied to the cultured cells to mimic conditions occurring in brain ischemia. Accordingly, we studied the cause of potentiation of ASIC1a currents under ischemic conditions induced in cell culture models and examined whether the pathological settings cause ASIC1a activation by increasing the surface expression levels of the channel. We used CHO cells stably expressing ASIC1a epitope-tagged with FLAG (ASIC1a-FLAG) to measure the surface expression level by biotinylation of surface proteins and Western blot analysis. We first tested the effect of serum and glucose deprivation at low extracellular pH (pH 6) on the surface trafficking of ASIC1a. Interestingly, surface-expressed ASIC1a markedly increased under these conditions. The increase was observed already by 15 min after the treatment and was significant at the 60-min time point (Fig. 1A). We further examined whether either of the conditions, deprivation of serum and glucose or treatment at pH 6, was responsible for the increase in the surface expression of ASIC1a. When CHO cells were exposed to serum and glucose withdrawal at pH 7.4, the surface expression level of ASIC1a increased significantly and was almost comparable with the amount observed under both serum and glucose deprivation at pH 6 (Fig. 1A). The changes in the surface levels of ASIC1a were insignificant when cells were treated only to low pH (pH 6) in serum- and glucose-containing medium, suggesting that acid activation is not involved in serum-responsive recruitment of ASIC1a to the cell surface (data not shown). We further determined the effect of serum on ASIC1a surface expression by exposing cells to medium deprived of serum but containing glucose. Western blot analysis of the surface-expressed ASIC1a showed that depletion of serum alone was sufficient to increase the expression level of ASIC1a on the cell surface (Fig. 1C). Quantification of the surface ASIC1a detected by the Western blot analysis showed an increase of about 2.5-fold during 60 min of serum depletion, and this increased amount was sustained for at least 120 min (Fig. 1C). To find out whether surface trafficking of ion channels triggered by serum depletion is specific to ASIC1a, we examined surface trafficking of ASIC2a epitope-tagged with FLAG (ASIC2a-FLAG) in CHO cells. ASIC2a is an isoform of ASIC1a sharing a high degree of homology in amino acid sequence and found to form heteromultimeric channels with ASIC1a (8). Unlike ASIC1a, the surface expression level of ASIC2a was not responsive to serum depletion at pH 7.4 and maintained the same expression levels in the presence and absence of serum. This result suggests that surface trafficking mediated by serum depletion is not a common phenomenon for all ASICs but is specific to ASIC1a.
FIGURE 1.
Surface biotinylation and Western blot analyses of surface-expressed ASIC1a. A, serum and glucose deprivation increases surface expression level of ASIC1a-FLAG in CHO cells both at pH 7.4 and pH 6. B, deprivation of serum alone increases surface expression levels of ASIC1a in the absence of serum. C, effect of serum on ASIC1a surface expression at pH 7.4. Surface levels detected by Western blot (left panel) and quantification of the Western blot results are shown as relative fold increases (right panel). The data represent the means ± S.E. of protein levels from at least four independent experiments, where an asterisk indicates p < 0.05 at the 60- and 120-min time points (Student's t test). Surface expression levels of ASIC2a-FLAG were shown as control. Anti-FLAG antibody was used to detect ASIC1a-FLAG and ASIC2a-FLAG. ASIC1a(s), surface located ASIC1a; ASIC1a(i), intracellular ASIC1a; β-actin: loading control.
To visualize the effect of serum depletion on ASIC1a expression, CHO cells expressing ASIC1a-GFP were examined by epifluorescence microscopy. ASIC1a-GFP fusion protein has been previously demonstrated to target correctly, to function as an ion channel, and to be regulated in the same way as native ASIC1a (13). In the presence of serum, ASIC1a-GFP was predominantly expressed in intracellular organelles including regions proximal to the nucleus and, to a much lesser extent, in the plasma membrane (supplemental Fig. S1). The intracellular localization of ASIC1a-GFP was not the result of altered protein trafficking caused by fusion of GFP at the C terminus, because a similar subcellular distribution was observed for ASIC1a without an epitope (see Fig. 5B), and the N-terminal fusion of HA to ASIC1a (13) expressed in CHO cells. Supporting the findings from the biotinylation assays, microscopic images showed translocation of ASIC1a-GFP to the plasma membrane (supplemental Fig. S1). The intense localization of ASIC1a proximal to the nucleus was less evident during 120 min of serum depletion. Instead, the channel exhibited a higher intensity of GFP in the plasma membrane. Different from ASIC1a, ASIC2a-GFP was primarily localized to the plasma membrane in CHO cells in the presence of serum. As shown by biotinylation assays, subcellular localization of ASIC2a-GFP did not show any apparent changes and maintained the same expression pattern in the absence of serum (supplemental Fig. S1). Taken together, these results suggested that serum depletion causes a significant increase in surface-expressed ASIC1a in CHO cells and that serum-responsive surface trafficking is specific to ASIC1a. These results further imply that potentiation of ASIC1a during ischemic conditions in vitro is primarily caused by an increase in surface-localized ASIC1a in response to serum depletion.
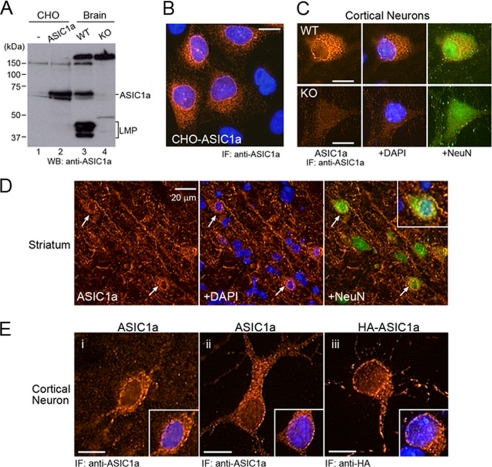
FIGURE 5.
ASIC1a is localized in intracellular organelles in neurons. A, Western blot analyses of ASIC1a expressed in transfected CHO cells and in brain of the wild-type (WT) and knock-out (KO) mice. Anti-ASIC1a antibody detects ASIC1a and nonspecific protein bands of >150 and ∼50 kDa in lysates of CHO cells and brain. B, immunostaining of transfected CHO cells with ASIC1a antibody. C, immunostaining of cultured cortical neurons from the wild-type and knock-out mice with ASIC1a antibody. D, immunostaining of ASIC1a in brain slices from the wild-type mice. ASIC1a is localized proximal to nuclei, soma, and dendrites of neurons. Inset shows a neuron in a higher magnification. E, intracellular localization of ASIC1a in cortical neurons. Endogenous ASIC1a in neurons in brain slices (panel i) and in primary culture (panel ii), and HA-tagged ASIC1a (HA-ASIC1a) in transfected culture neurons (panel iii) are shown. LMP, low molecular mass proteins. B–E, nuclei were stained with 4′,6′-diamidino-2-phenylindole dihydrochloride (DAPI), and neurons were stained with NeuN. Scale bars in B, C, and E, 10 μm. WB, Western blot; IF, immunofluorescence.
ASIC1a Is Predominantly Localized in the ER, and Serum Depletion Causes Translocation from the ER to the Plasma Membrane
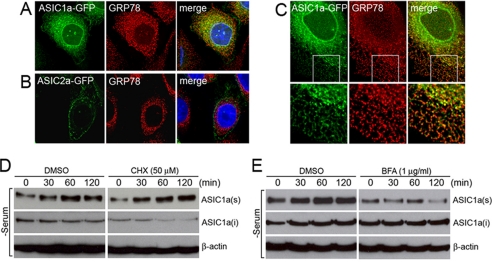
To determine the intracellular localization of ASIC1a, cells expressing ASIC1a-GFP were immunostained with an antibody recognizing an ER protein GRP78 (BiP) or lamin B located in the inner nuclear membrane and examined by confocal microscopy. Immunostaining with anti-GRP78 revealed localization of ASIC1a in the ER with a distribution pattern overlapping with that of GRP78 (Fig. 2, A and C). Immunostaining with lamin B and analysis of reconstructed images of three-dimensional stacks showed that ASIC1a expressed adjacent to the nucleus in a ring-shaped pattern does not colocalize with lamin B but is localized to the outer nuclear membrane that is an extension of the ER (supplemental Fig. S2). Unlike ASIC1a, ASIC2a is not colocalized with GRP78 but is largely expressed on the cell surface (Fig. 2B). The differences between ASIC1a and ASIC2a subcellular localization imply that these two channels have distinctive surface trafficking mechanisms despite having a high level of amino acid sequence identity. Further, these data imply that the retention of ASIC1a in the ER might be a regulatory mechanism for surface trafficking of this channel whereby extracellular signals including serum depletion cause translocation of the channel from the ER to the plasma membrane.
FIGURE 2.
ASIC1a is localized in the ER and accumulates in the plasma membrane upon serum depletion. A and B, subcellular distribution of ASIC1a-GFP and ASIC2a-GFP in CHO cells shown by confocal microscopy. GRP78 was immunostained for Colocalization with ASIC1a (top row) or ASIC2a (bottom row). C, colocalization of ASIC1a-GFP with GRP78 in the ER in CHO cells. Insets in the top row are shown in higher magnification in the bottom row. D, increase in surface-expressed ASIC1a during serum depletion does not require de novo protein synthesis. Inhibition of protein synthesis by 50 μm cycloheximide (CHX) does not prevent surface accumulation of ASIC1a during serum depletion. E, inhibition of the forward protein transport from the ER to the Golgi by 1 μg/ml brefeldin A (BFA) prevents surface accumulation of ASIC1a during serum depletion. D and E show cell surface biotinylation assay, and anti-FLAG antibody was used to detect ASIC1a-FLAG by Western blot analysis. In control cells, dimethyl sulfoxide (DMSO) was applied instead of cycloheximide or brefeldin A as vehicle. The cells were treated with dimethyl sulfoxide, cycloheximide, or brefeldin A 1 h prior to and during the serum depletion by incubation in Dulbecco's modified Eagle's medium without serum for indicated time periods (0, 30, 60, and 120 min). ASIC1a(s), surface located ASIC1a; ASIC1a(i), intracellular ASIC1a; β-actin, loading control. Representative blots of three independent experiments are shown.
We next tested the possibility that serum depletion induces translocation of the ER-resident ASIC1a to the cell surface and that this process does not require de novo protein synthesis under serum depletion. In this assay, the cells expressing ASIC1a-FLAG were treated with 50 μm cycloheximide, an inhibitor of protein synthesis, 1 h prior to and during serum depletion. Cell surface biotinylation followed by Western blot analysis with anti-FLAG antibody showed that inhibition of protein synthesis does not change the effect of serum-responsive trafficking of ASIC1a (Fig. 2D). The release of ASIC1a from the ER to the plasma membrane was further tested by blocking the forward protein transport from the ER to the Golgi in the presence of brefeldin A. When cells were treated with 1 μg/ml brefeldin A 1 h prior to and during serum depletion, no increased accumulation of ASIC1a was observed in the plasma membrane (Fig. 2E). These results suggested that the increased surface levels of ASIC1a do not depend on new protein synthesis but rather result from exocytosis of the pre-existing proteins from the ER.
Serum Depletion Increases Acid-activated Currents in CHO Cells without Modification of Channel Properties
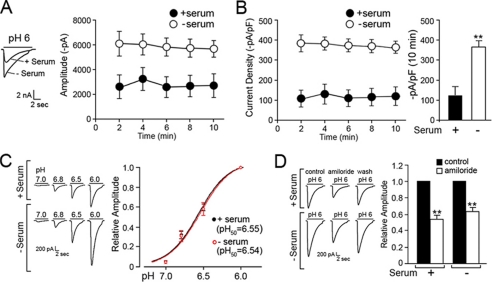
One important question is whether the newly recruited channels are functionally active and therefore contribute to the increase in ASIC currents observed under ischemic conditions. To examine the activity of the channels, acid-induced currents were measured from CHO cells stably expressing ASIC1a GFP by the whole cell patch-clamp method. It has been demonstrated that GFP fusion to ASIC1a does not change the electrophysiological properties of the channel (13). Upon activation by acidic extracellular solution (pH 6), ASIC1a showed typical transient inward currents at a holding potential of −60 mV. As shown in Fig. 3A, the amplitude of acid-evoked inward currents was significantly higher in cells incubated in serum-depleted medium for 1 h prior to the current recordings compared with control cells without serum depletion. The peak amplitude of the serum-deprived cells (5688 ± 672 pA, n = 6) was 2-fold higher than that of the control cells (2755 ± 672 pA, n = 5) at the 10-min time point. Analysis of current densities, measured by normalizing the peak current amplitude to the cell capacitance, revealed a significantly larger difference (3-fold) between the cell groups (119 ± 45 pA/pF in control group and 364 ± 31 pA/pF in serum-depleted group, p < 0.01, two-way ANOVA; Fig. 3B). These data demonstrate that surface ASIC1a channels under serum depletion conditions are functionally active. Furthermore, the increase in current density suggests an increase in the number of channels in the plasma membrane, in agreement with the data shown by biotinylation assay of surface proteins. Nevertheless, we further tested whether the surface ASIC1a under serum depletion conditions exhibit the same properties as the channels without serum stress to rule out any question of ASIC1a potentiation by biophysical property changes. Measurement of the pH of half-maximum activation (pH0.5) for ASIC1a showed that depletion of serum did not change pH sensitivity of this channel (pH0.5 6.55 ± 0.05 in the control cells and pH0.5 6.54 ± 0.07 in the serum-depleted cells; Fig. 3C). Next, pharmacological property was examined by the application of amiloride, a nonspecific blocker of ASICs. Serum depletion did not change dose-responsive inhibition of the channel or the recovery of the channel activity after removal of the channel blocker. The addition of 100 μm amiloride in the extracellular solution decreased the channel activity to 54.16 ± 0.05% in untreated cells and to 61.89 ± 0.05% in cells treated with serum depletion (Fig. 3D). These data suggest that serum depletion does not change channel function, and channels newly recruited during serum depletion are functionally indistinguishable from the channels expressed in the presence of serum. It is therefore likely that ASIC1a channels are present in the ER as functional channels, not as a single subunit, and are retained by a mechanism specific to this channel.
FIGURE 3.
Serum depletion increases ASIC1a current amplitudes in CHO cells without changing electrophysiological property of the channel. A, current amplitude of ASIC1a in the serum-replete and -depleted cells. Representatives of ASIC1a current traces with and without serum depletion were superimposed (left panel). The peak current amplitudes were measured for 10 min, and the value is plotted (right panel). B, analysis of peak current amplitudes in cell density. The current densities from untreated and serum-depleted cells are plotted as a function of time, where the significant differences (p < 0.01) were measured at all time points. The value at the 10-min time point is shown. C, pH responsive activity of ASIC1a in serum-replete and -depleted cells. ASIC1a was activated by exposure to pH ranging from 7 to 6. The peak current amplitudes were plotted as function of pH, and the pH of half-maximum activation (pH0.5) was measured for ASIC1a in untreated (black circles) and serum-depleted (red circles) cells. D, inhibition of ASIC1a activity in response to 100 μm amiloride. ASIC1a current amplitudes were recorded from the serum-replete and -depleted cells before (control), during (amiloride), and after (wash) the drug treatment. Relative amplitudes are shown to compare the changes in ASIC1a channel activity upon amiloride treatment. Statistical analysis was carried out using two-way ANOVA. The values are the means ± S.E. (n = 5–6), and ** indicates p < 0.01.
Insulin Depletion Causes Accumulation of ASIC1a in the Plasma Membrane
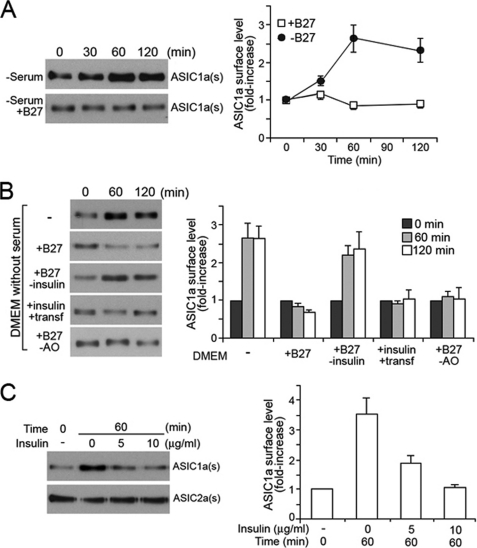
Increased surface expression of ASIC1a in the absence of serum suggests a role for serum in maintaining low levels of the ASIC1a in the plasma membrane. To identify the serum component(s) crucial for the ASIC1a surface regulation, we tested a number of cell culture components in next experiments. A serum supplement known as B27 contains 20 compounds that are essential for neuronal cell culture in serum-free medium in the absence of a feeder layer of glial cells (14). The components of B27 are derived from serum and consist of growth factors, hormones, and a variety of antioxidants, among others, with well defined concentrations (14). To investigate whether B27 could restore the effect of serum, retaining surface ASIC1a at a low level, serum depletion experiments were performed in medium supplemented with B27 but without serum. In this setting, B27 was able to fully restore the effect of serum, which is suppression of ASIC1a accumulation (Fig. 4A). This result suggested that the regulatory factor(s) are present in B27. Accordingly, the following serum depletion assays were performed with B27 components individually or as mixtures. Among those tested, the most conclusive effect was observed with a combined solution of insulin and transferrin and B27 without insulin (Fig. 4B). The mixture of insulin and transferrin was able to prevent the accumulation of ASIC1a on the cell surface, whereas accumulation of surface ASIC1a still occurred in the presence of B27 without insulin during serum depletion, indicating that B27 without insulin no longer restores the effect of serum or the complete B27 complex on ASIC1a trafficking. In contrast, other components of B27 such as antioxidants (Fig. 4B), vitamin mixtures, corticosterone, or lipid complex (data not shown) had no significant effect on ASIC1a trafficking. Based on these results, we examined the effect of insulin in the serum-depleted medium. The addition of 10 μg/ml insulin, the amount present in B27, completely restored the effect of serum on ASIC1a trafficking, maintaining low expression of ASIC1a on the surface during serum depletion (Fig. 4C). In agreement with the results obtained using serum, the insulin effect was specific to ASIC1a trafficking, because the surface expression of ASIC2a was not responsive to insulin in the absence of serum (Fig. 4C).
FIGURE 4.
Insulin modulates surface expression of ASIC1a in CHO cells. A, serum supplement B27 maintains a low level of surface-expressed ASIC1a in the serum-depleted but B27-replete medium. B, effect of B27 components on surface expression of ASIC1a. The cells were treated in serum-depleted medium but supplemented with variants of B27. DMEM, Dulbecco's modified Eagle's medium. C, effect of insulin alone on surface expression of ASIC1a and ASIC2a in the serum-depleted medium. The data are from the Western blot analyses of the biotinylated surface ASIC1a. Representative blots are shown in the left panels, and quantification is shown in the right panels. The data represent the means ± S.E. of protein levels from at least four independent experiments. ASIC1a(s) and ASIC2a(s), surface located ASIC1a and ASIC2a, respectively. B27-insulin, B27 without insulin; B27-AO, B27 without antioxidants.
ASIC1a Is Localized in Intracellular Organelles in Neurons
Although previous studies have demonstrated the localization of ASIC1a in dendrites, dendritic spines, and the soma with a distinctive expression pattern of clusters in neuronal cells, the intracellular expression of ASIC1a has been overlooked so far (3, 5, 13, 15). Because ASIC1a is predominantly localized in the ER in CHO cells, which is the source of surface-expressed channels in the absence of insulin, we examined the possibility of intracellular localization of ASIC1a in neuronal cells. For immunostaining of endogenous ASIC1a in neurons, the anti-ASIC1a antibody was first tested by Western blot. In transfected CHO cells, the antibody detected primarily ASIC1a plus two minor bands of ∼65 and 150 kDa (Fig. 5A). By comparison, brain lysates of wild-type (ASIC1a +/+) mice showed a protein band specific to ASIC1a at the expected molecular mass (∼65 kDa) and several additional protein bands of between 37 and 50 kDa, and 150 kDa (Fig. 5A). The lower molecular mass proteins (LMP) were specific to ASIC1a, because these proteins were absent in brain lysates of the ASIC1a knock-out mouse (ASIC1a−/−). Although these proteins are yet to be identified, it is likely that they are degradation products from the full-length ASIC1a proteins. The high molecular mass protein (>150 kDa) represented a nonspecific cross-reacting protein, because this band was also detected in knock-out mice. Although Western blot showed detection of one nonspecific protein, this antibody was used for immunostaining of brain slices and cultured neurons because of the lack of antibody specific only for ASIC1a. Although the immunoreactivity was highly specific to ASIC1a in CHO cells (Fig. 5B), neuronal culture from the knock-out mouse showed some background staining, possibly from the nonspecific protein detected by Western blot (Fig. 5C). However, neurons from the knock-out mouse did not exhibit any clusters in dendrites and the soma, a characteristic of ASIC1a expression pattern. In contrast, neurons from the wild-type mice revealed a strong immunoreactivity of ASIC1a with the distinctive cluster pattern (Fig. 5C). In these cells, staining was also observed in the region adjoining to the nucleus, which is similar to the pattern observed in CHO cells. To rule out the possibility of artificial effects of growth in culture on the ASIC1a expression pattern, brain slices from wild-type mice were immunostained. ASIC1a was abundantly expressed in striatal neurons in brain slice (Fig. 5D). Colabeling with a neuronal marker NeuN confirmed that high expression of ASIC1a occurred in neurons and that a significant proportion of the staining localized to clusters adjacent to the nucleus. Furthermore, cortical neurons in the brain slices and from the primary culture also exhibited ASIC1a localization adjacent to the nucleus, probably in the outer nuclear membrane (Fig. 5E). To verify the intracellular localization of ASIC1a, we transfected cultured cortical neurons with HA-tagged ASIC1a (HA-ASIC1a) cDNA. Immunostaining with anti-HA antibody showed ASIC1a subcellular localization that was indistinguishable from the endogenous ASIC1a localization detected by anti-ASIC1a antibody (Fig. 5E).
Insulin Withdrawal Potentiates ASIC Currents in Neurons
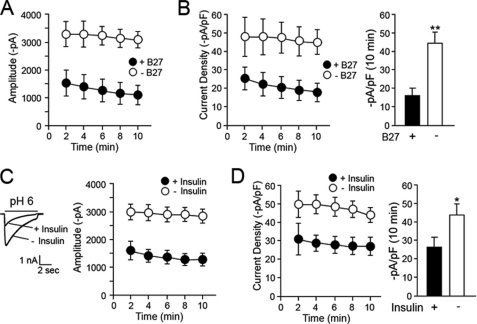
The effect of the serum supplement B27 and insulin on ASIC current was assessed in cultured cortical neurons, to examine whether depletion of these components increased acid-evoked currents. Electrophysiological measurements were chosen instead of biotinylation of surface-expressed ASIC1a in neurons because of the low abundance of endogenous ASIC1a in these cells. In neuronal cultures, B27 serum supplement replaced serum because of the cytoxicity of serum toward neuronal cultures. Neurons express ASIC1a and ASIC2a as homo- and heteromultimeric channels. To activate ASIC1a channels in neurons, but not ASIC2a, the extracellular pH was lowered to pH 6.0. This strategy was based on previous studies demonstrating that neurons from ASIC1a knock-out mice did not generate any currents at pH 6.0 (3, 9). Treatment of neurons in medium without B27 for 1 h prior to recordings significantly increased the amplitude of acid-induced currents compared with the control neurons maintained in the B27-containing medium (Fig. 6A). The peak amplitude of the neurons in the absence of B27 (3097 ± 258 pA, n = 5, p < 0.01) was 3.1-fold higher than that of the control neurons (993 ± 293 pA, n = 5) at the 10-min time point following the establishment of whole cell configuration. Analysis of current density also showed a significant increase (2.8-fold) in the B27-depleted cells (16 ± 4 pA/pF in control cells and 45 ± 6 pA/pF in B27-depleted cells; p < 0.01, two-way ANOVA; Fig. 6B). To examine whether insulin is the key component of B27 preventing potentiation of ASIC1a currents, the cells were incubated either in medium without B27 but supplemented with insulin (control group) or in medium depleted of both B27 and insulin for 1 h. In agreement with results obtained using B27, 10 μg/ml insulin prevented potentiation of ASIC currents (Fig. 6C). Neurons incubated in insulin-depleted medium exhibited a significant increase in currents (2804 ± 266 pA, n = 13, p < 0.01) compared with the control groups (1379 ± 246 pA, n = 10). A significant difference was also observed in current densities (42 ± 4 pA/pF in control groups and 29 ± 6 pA/pF in insulin-depleted cells, p < 0.05; Fig. 6D). These results suggest that withdrawal of insulin significantly potentiates ASIC currents in neurons. We further tested whether the addition of insulin to the B27-deleted cells reverses the potentiation of ASIC1a currents. The neurons were first incubated in B27-depleted medium for 1 h and subsequently in medium supplemented with insulin for 1 h at 37 °C. Treatment of the cells with insulin following B27 depletion showed ASIC currents significantly lower than the cells without the additional incubation with insulin (1371 ± 411 pA, n = 9, for insulin-supplemented cells and 2804 ± 266 pA, n = 10 for B27-depleted cells, p < 0.05 at 14-min time point; supplemental Fig. S3). The currents of the insulin-treated cells were almost comparable with the mock treated control cells.
FIGURE 6.
Depletion of B27 or insulin increases ASIC1a currents in cortical neurons. A and B, depletion of B27 from the culture media increases acid (pH 6)-induced current amplitude and current density. The current densities of B-27 replete (+B-27) and deplete (−B-27) are plotted as a function of time (B, left panel), and the value at the 10-min time point is shown (B, right panel). C and D, insulin depletion increases acid-induced current amplitude and current density in neurons. Representatives of ASIC1a current traces with and without insulin depletion were superimposed (left panel in C). The current densities are plotted as a function of time (D, left panel), and the value at the 10-min time point is shown (D, right panel). Statistical analysis was carried out using two-way ANOVA and Student's t test. * and ** indicate p < 0.05 and p < 0.01, respectively. The values are the means ± S.E. (n = 5 for A and B, n = 10–13 for C and D).
Considering the intracellular localization of ASIC1a in neurons, it is plausible that the increased ASIC currents observed in the absence of insulin are mediated by increased trafficking of the intracellular ASIC1a to the plasma membrane, as demonstrated in the CHO model system. The observed reduction in ASIC1a levels following the addition of insulin to insulin-deprived neurons provides evidence for the reversibility of the trafficking effect.
DISCUSSION
Tight control of ASIC1a activity is vital for normal physiological function of the brain, because the synaptic activity of this channel plays a crucial role in the formation of learning and memory (3, 5). However, during cerebral ischemia and in vitro models of acidosis-related ischemic conditions, ASIC1a activity exceeds the normal physiological levels, leading to neuronal death (9, 12). Depletion of oxygen and glucose are accepted experimental ischemic conditions modeling ischemia, replicating the detrimental metabolic characteristics occurring in brain during blockage in blood flow. The present study demonstrated that the absence of insulin causes a significant accumulation of ASIC1a on the cell surface. In CHO cells, depletion of glucose or low extracellular pH did not impact the surface expression of ASIC1a at the level that serum depletion did, because a high accumulation occurred regardless of glucose levels or pH during serum depletion. By contrast, insulin was able to fully restore the effect of serum by maintaining surface ASIC1a at low levels when present and by promoting increased surface accumulation of ASIC1a when absent. Supporting these data, electrophysiological measurements showed that the absence of serum and insulin significantly increased ASIC1a activity in CHO cells and cultured cortical neurons, respectively. Furthermore, patch-clamp recordings showed that serum depletion increased ASIC1a currents without changing channel properties, as demonstrated by pH of half-maximum activation and response to the channel blocker amiloride. These results demonstrate that potentiation of ASIC1a activity under ischemic conditions is caused by increased surface expression of this channel during depletion of serum and in particular of insulin. Thus, this study suggests that insulin, in addition to oxygen and glucose, may be a crucial component of serum affecting the outcome of cerebral ischemia. Because cerebral ischemia results in reduced blood flow to the affected region of the brain, stroke will result in decreased delivery of insulin to the affected tissue. Hence the effect of insulin on ASIC1a expression observed in vitro is likely to be relevant to the pathogenesis of the stroke.
Insulin is present throughout the brain. Although insulin generally functions in glucose uptake in various organs, brain insulin is involved in neuronal functions such as growth and maturation and modulation of surface expression of various ion channels and neurotransmitter receptors (16–18). In kidney tissue, insulin increases surface expression of epithelial sodium channel subunits via serum and the glucocorticoid-regulated kinase SGK1 pathway (19). Interestingly, a recent study by Arteaga et al. (20) reported that SGK1.1, a brain-specific splice isoform of SGK1, decreases expression of ASIC1a at the cell surface. It would be speculative yet interesting to find out whether insulin-mediated ASIC1a regulation occurs through the SGK1.1 signaling pathway in neuronal cells, because SGK1.1 is not regulated by glucocorticoid in the brain (20).
Analyses by immunostaining revealed intracellular localization of ASIC1a, especially in the ER and in the outer nuclear membrane in CHO cells. Inhibition of protein transport from the ER to the Golgi by brefeldin A, but not the inhibition of de novo protein synthesis by cycloheximide, prevented ASIC1a surface accumulation, suggesting that the intracellular pool of ASIC1a in the ER is the source for the surface accumulation during serum and insulin depletion. These data support ER retention as a trafficking mechanism for ASIC1a. We rule out the interpretation of intracellular localization of ASIC1a as trafficking failure caused by expression in heterologous cells, because proteins defective in trafficking would not move to the plasma membrane in response to depletion of serum or insulin. Moreover, intracellular localization of ASIC1a is also evident in neuronal cells both in the primary culture and brain slices. Endogenous as well as transfected ASIC1a in neuronal cells exhibited a distinctive localization proximal to the nucleus, which is most likely the outer nuclear membrane as shown in CHO cells. These findings lead to the question of what mechanisms underlie the retention and retrieval of ASIC1a in and out of the ER modulating the surface expression of this channel. ER retention mechanisms are considered to exert quality control for trafficking of proteins to the plasma membrane (21). The delivery of properly folded and assembled ion channels to the plasma membrane is a crucial process for ion homeostasis in the cell, and more delivery of channels to the synaptic sites in excitable cells is of particular importance for synaptic transmission as shown by the GluR2 subunit of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor and the NR1 subunit of N-methyl-d-aspartate receptors (22, 23). Previous studies localized ASIC1a to the dendrites, postsynaptic sites in dendritic spines, and the soma (3, 13, 15, 24, 25). However, the delivery mechanism of ASIC1a to the synaptic sites and the soma is still unknown. ASIC1a and ASIC2a share 67% identity in amino acid sequence (2, 26). Nevertheless these isoforms differ from each other in subcellular localization in CHO cells; whereas ASIC1a is largely retained in intracellular locations, the majority of ASIC2a is located in the plasma membrane. This difference in subcellular localization suggests an ER retention mechanism embedded in the ASIC1a sequence. Numerous studies have identified protein sequence motifs for ER retention in extracellular, cytoplasmic, or transmembrane regions in various surface proteins. ASIC1a does not contain any of the known motifs such as KDEL, KKXX, and RXR in the protein sequence (reviewed in Ref. 27), implying that the ER retention signal in ASIC1a might operate via a mechanism different from the ones identified so far.
A number of factors are involved in potentiation of ASIC1a activity. Among them are extracellular factors changing the redox state of the extracellular loop domain or intracellular regulatory proteins that change the phosphorylation state of the cytoplasmic domains (13, 28–33, 34). Although these factors addressed potentiation of ASIC1a caused by changes at the level of electrophysiological property, a cytoplasmic protein annexin II light chain p11 has been shown to potentiate ASIC1a activity by promoting surface trafficking of this channel (35). p11 is also involved in surface trafficking of TASK-1 K+ channel (36). The interaction between TASK-1 and p11 is intriguing because p11 binding causes release of the channel from the ER by masking the retention signal. An ER membrane protein sigma-1 receptor (Sig-1R) has also been identified as a modulator of ASIC1a activity, by which inhibition of this protein activated ASIC1a channel (37). Although the underlying mechanisms are unknown, association of p11 and Sig-1R with ASIC1a activity points to regulation of this channel at the ER level. Thus, ER retention of ASIC1a together with regulation by Sig-1R and p11 appear to constitute significant regulatory mechanisms for ASIC1a that may operate at the level of channel trafficking.
Under normal conditions, neuronal cells show ASIC1a in distinctive subcellular distributions including synaptic localizations, suggesting a specific targeting mechanism for this channel. It is yet to be characterized whether channel targeting and localization is affected when an excess of ASIC1a is present in the plasma membrane in the absence of insulin. As seen with potassium channel Kv2.1 (38), uncontrolled targeting and distribution under ischemic conditions could result in changes in electrophysiological properties that contribute to channel functions that are detrimental for the neurons.
Supplementary Material
Acknowledgments
We thank Aurelie Snyder (Molecular Microbiology and Immunology Core Imaging Facility, Oregon Health and Sciences University) for confocal microscopy and Jing-Quan Lan and Tao Yang for preparation of rat brain slices.
This work was supported, in whole or in part, by National Institutes of Health Grants R01NS050610 (to R. P. S.) and R01NS047506 (to Z. G. X.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3.
- ASIC
- acid-sensing ion channel
- ER
- endoplasmic reticulum
- CHO
- Chinese hamster ovary
- GFP
- green fluorescent protein
- EGFP
- enhanced GFP
- HA
- hemagglutinin
- PBS
- phosphate-buffered saline
- ANOVA
- analysis of variance.
REFERENCES
- 1.García-Añoveros J., Derfler B., Neville-Golden J., Hyman B. T., Corey D. P. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 1459–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waldmann R., Champigny G., Bassilana F., Heurteaux C., Lazdunski M. (1997) Nature 386, 173–177 [DOI] [PubMed] [Google Scholar]
- 3.Wemmie J. A., Chen J., Askwith C. C., Hruska-Hageman A. M., Price M. P., Nolan B. C., Yoder P. G., Lamani E., Hoshi T., Freeman J. H., Jr., Welsh M. J. (2002) Neuron 34, 463–477 [DOI] [PubMed] [Google Scholar]
- 4.Wemmie J. A., Askwith C. C., Lamani E., Cassell M. D., Freeman J. H., Jr., Welsh M. J. (2003) J. Neurosci. 23, 5496–5502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wemmie J. A., Coryell M. W., Askwith C. C., Lamani E., Leonard A. S., Sigmund C. D., Welsh M. J. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 3621–3626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kellenberger S., Schild L. (2002) Physiol. Rev. 82, 735–767 [DOI] [PubMed] [Google Scholar]
- 7.Jasti J., Furukawa H., Gonzales E. B., Gouaux E. (2007) Nature 449, 316–323 [DOI] [PubMed] [Google Scholar]
- 8.Bassilana F., Champigny G., Waldmann R., de Weille J. R., Heurteaux C., Lazdunski M. (1997) J. Biol. Chem. 272, 28819–28822 [DOI] [PubMed] [Google Scholar]
- 9.Xiong Z. G., Zhu X. M., Chu X. P., Minami M., Hey J., Wei W. L., MacDonald J. F., Wemmie J. A., Price M. P., Welsh M. J., Simon R. P. (2004) Cell 118, 687–698 [DOI] [PubMed] [Google Scholar]
- 10.Yermolaieva O., Leonard A. S., Schnizler M. K., Abboud F. M., Welsh M. J. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 6752–6757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nedergaard M., Kraig R. P., Tanabe J., Pulsinelli W. A. (1991) Am. J. Physiol. 260, R581–R588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pignataro G., Simon R. P., Xiong Z. G. (2007) Brain 130, 151–158 [DOI] [PubMed] [Google Scholar]
- 13.Chai S., Li M., Lan J., Xiong Z. G., Saugstad J. A., Simon R. P. (2007) J. Biol. Chem. 282, 22668–22677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brewer G. J., Torricelli J. R., Evege E. K., Price P. J. (1993) J. Neurosci. Res. 35, 567–576 [DOI] [PubMed] [Google Scholar]
- 15.Zha X. M., Wemmie J. A., Green S. H., Welsh M. J. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 16556–16561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wan Q., Xiong Z. G., Man H. Y., Ackerley C. A., Braunton J., Lu W. Y., Becker L. E., MacDonald J. F., Wang Y. T. (1997) Nature 388, 686–690 [DOI] [PubMed] [Google Scholar]
- 17.Man H. Y., Lin J. W., Ju W. H., Ahmadian G., Liu L., Becker L. E., Sheng M., Wang Y. T. (2000) Neuron 25, 649–662 [DOI] [PubMed] [Google Scholar]
- 18.Skeberdis V. A., Lan J., Zheng X., Zukin R. S., Bennett M. V. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 3561–3566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tiwari S., Nordquist L., Halagappa V. K., Ecelbarger C. A. (2007) Am. J. Physiol. Renal. Physiol. 293, F178–F185 [DOI] [PubMed] [Google Scholar]
- 20.Arteaga M. F., Coric T., Straub C., Canessa C. M. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 4459–4464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ellgaard L., Molinari M., Helenius A. (1999) Science 286, 1882–1888 [DOI] [PubMed] [Google Scholar]
- 22.Greger I. H., Khatri L., Ziff E. B. (2002) Neuron 34, 759–772 [DOI] [PubMed] [Google Scholar]
- 23.Standley S., Roche K. W., McCallum J., Sans N., Wenthold R. J. (2000) Neuron 28, 887–898 [DOI] [PubMed] [Google Scholar]
- 24.Duggan A., Garcia-Anoveros J., Corey D. P. (2002) J. Biol. Chem. 277, 5203–5208 [DOI] [PubMed] [Google Scholar]
- 25.Hruska-Hageman A. M., Wemmie J. A., Price M. P., Welsh M. J. (2002) Biochem. J. 361, 443–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lingueglia E., de Weille J. R., Bassilana F., Heurteaux C., Sakai H., Waldmann R., Lazdunski M. (1997) J. Biol. Chem. 272, 29778–29783 [DOI] [PubMed] [Google Scholar]
- 27.Teasdale R. D., Jackson M. R. (1996) Annu. Rev. Cell Dev. Biol. 12, 27–54 [DOI] [PubMed] [Google Scholar]
- 28.Andrey F., Tsintsadze T., Volkova T., Lozovaya N., Krishtal O. (2005) Biochim. Biophys. Acta 1745, 1–6 [DOI] [PubMed] [Google Scholar]
- 29.Cadiou H., Studer M., Jones N. G., Smith E. S., Ballard A., McMahon S. B., McNaughton P. A. (2007) J. Neurosci. 27, 13251–13260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cho J. H., Askwith C. C. (2007) Am. J. Physiol. Cell Physiol. 292, C2161–C2174 [DOI] [PubMed] [Google Scholar]
- 31.Chu X. P., Close N., Saugstad J. A., Xiong Z. G. (2006) J. Neurosci. 26, 5329–5339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao J., Duan B., Wang D. G., Deng X. H., Zhang G. Y., Xu L., Xu T. L. (2005) Neuron 48, 635–646 [DOI] [PubMed] [Google Scholar]
- 33.Sherwood T. W., Askwith C. C. (2008) J. Biol. Chem. 283, 1818–1830 [DOI] [PubMed] [Google Scholar]
- 34.Xie J., Price M. P., Wemmie J. A., Askwith C. C., Welsh M. J. (2003) J. Neurophysiol. 89, 2459–2465 [DOI] [PubMed] [Google Scholar]
- 35.Donier E., Rugiero F., Okuse K., Wood J. N. (2005) J. Biol. Chem. 280, 38666–38672 [DOI] [PubMed] [Google Scholar]
- 36.Girard C., Tinel N., Terrenoire C., Romey G., Lazdunski M., Borsotto M. (2002) EMBO J. 21, 4439–4448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herrera Y., Katnik C., Rodriguez J. D., Hall A. A., Willing A., Pennypacker K. R., Cuevas J. (2008) J. Pharmacol. Exp. Ther. 327, 491–502 [DOI] [PubMed] [Google Scholar]
- 38.Misonou H., Mohapatra D. P., Park E. W., Leung V., Zhen D., Misonou K., Anderson A. E., Trimmer J. S. (2004) Nat. Neurosci. 7, 711–718 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.