Abstract
In response to DNA double strand breaks, the histone variant H2AX at the break site is phosphorylated at serine 139 by DNA damage sensor kinases such as ataxia telangiectasia-mutated, forming γ-H2AX. This phosphorylation event is critical for sustained recruitment of other proteins to repair the break. After repair, restoration of the cell to a prestress state is associated with γ-H2AX dephosphorylation and dissolution of γ-H2AX-associated damage foci. The phosphatases PP2A and PP4 have previously been shown to dephosphorylate γ-H2AX. Here, we demonstrate that the wild-type p53-induced phosphatase 1 (WIP1) also dephosphorylates γ-H2AX at serine 139 in vitro and in vivo. Overexpression of WIP1 reduces formation of γ-H2AX foci in response to ionizing and ultraviolet radiation and blocks recruitment of MDC1 (mediator of DNA damage checkpoint 1) and 53BP1 (p53 binding protein 1) to DNA damage foci. Finally, these inhibitory effects of WIP1 on γ-H2AX are accompanied by WIP1 suppression of DNA double strand break repair. Thus, WIP1 has a homeostatic role in reversing the effects of ataxia telangiectasia-mutated phosphorylation of H2AX.
Keywords: DNA/Damage, Phosphorylation/Phosphatases/Serine-Threonine, Signal Transduction/Phosphoprotein Phosphatases, Signal Transduction/Phosphoprotein Phosphatases/Serine/Threonine, DNA Repair, 53BP1, H2AX, MDC1, PPM1D, WIP1
Introduction
The cellular genome is constantly exposed to endogenous and exogenous DNA damage. A particularly serious lesion is the DNA double strand break (DSB),4 which can lead to genomic instability or cancer if not repaired in a timely manner. In mammalian cells the DNA damage response (DDR) is initiated by sensor kinases of the phosphatidylinositol 3-kinase-like protein kinase (PIKK) family, including ataxia telangiectasia-mutated (ATM), ataxia telangiectasia mutated and Rad3-related (ATR) and DNA-dependent protein kinase (DNA-PKcs) (1–3). These kinases, which are activated by various types of DNA damage such as ionizing radiation (IR)-induced DSBs, UV-induced DNA damage, and replicative stress, phosphorylate multiple target proteins, eventually leading to cell cycle checkpoint responses and DNA repair. Whereas activation of the DDR through protein phosphorylation cascades has been well characterized, the mechanism of DDR deactivation remains underexplored. It is likely that protein phosphatases have a major role in the deactivation of the DDR (4).
One such DDR-inactivating phosphatase may be the wild-type p53-induced phosphatase 1 (WIP1, or PPM1D; protein phosphatase 1D Mg2+-dependent, delta isoform), a serine/threonine phosphatase and a member of the type 2C protein phosphatase family (PP2C) (5, 6). Like other PP2C members, WIP1 depends on divalent cations (mainly Mg2+ or Mn2+) for its activity. It functions as a monomer, and is insensitive to okadaic acid, unlike PP1 and PP2A (5, 7, 8). WIP1 transcription is up-regulated in response to various types of DNA damage in a p53-dependent manner (5, 9). Once up-regulated, it appears to dephosphorylate a number of protein targets associated with the ATM/ATR/DNA-PKcs-initiated DDR, including ATM itself (Ser-367 and Ser-1981), Chk1 (Ser-345), Chk2 (Thr-68), p53 (Ser-15), and Mdm2 (Ser-395) (10–13). For most of these targets, dephosphorylation by WIP1 is accompanied by reduced function relative to the phosphorylated form. Thus, we have proposed that the primary role of WIP1 may be the dephosphorylation and deactivation of DDR-activated proteins (6, 14).
The down-regulation of p53 and the DDR by WIP1 has significant cancer implications, particularly because the DDR has recently been shown to play a crucial role as an early anti-cancer barrier (15–17). In fact, accumulating evidence indicates that WIP1 is an oncogene (6). WIP1 gene amplification and/or overexpression have been observed in a variety of human tumors, including breast adenocarcinoma, ovarian clear cell adenocarcinoma, neuroblastoma, pancreatic adenocarcinoma, gastric carcinoma, and medulloblastoma (6). In vitro transformation assays showed that WIP1 promotes transformation of primary rodent fibroblasts in cooperation with other oncogenes (18, 19). Obviously, the mechanisms by which WIP1 contributes to tumorigenesis can be better understood by identifying its substrate targets. One critical early target of the DDR is the histone H2A variant H2AX (20). H2AX is rapidly phosphorylated at the C-terminal serine 139 by ATM, ATR, or DNA-PKcs (21–24). The Ser-139-phosphorylated form of H2AX, referred to as γ-H2AX, is readily visualized by immunofluorescence microscopy in DNA damage foci using an antibody specific for it (25). Following ionizing radiation, the γ-H2AX phosphorylation occurs first at DSBs and spreads up to several megabases around the breaks (25). It is one of the earliest DSB-associated events and is followed by recruitment of other DDR factors such as MDC1, 53BP1, BRCA1, and Mre11-Rad50-Nbs1 complex (23, 26, 27). H2AX-deficient mouse embryonic fibroblasts show normal initial recruitment of repair proteins after irradiation, but fail to sustain the recruitment, indicating a requirement for γ-H2AX for sustained damage signaling (28). H2AX-deficient mice display genomic instability phenotypes and enhanced cancer incidence (29, 30).
Following repair of DSBs, γ-H2AX foci gradually disappear, due in part to dephosphorylation of H2AX at serine 139 by multiple phosphatases (20). The Lieberman laboratory was the first to show in mammalian cells that the phosphatase PP2A dephosphorylates γ-H2AX and is involved in removing γ-H2AX damage foci (31). Inhibition of the catalytic subunit of PP2A, PP2A(C), resulted in persistence of γ-H2AX foci and less efficient DNA repair. In addition, it was recently shown that PP4 specifically dephosphorylates ATR-mediated γ-H2AX generated during DNA replication (32, 33). Another study suggests that the phosphatase PP2Cγ may play a modest role in H2AX dephosphorylation (34).
Here, we show that γ-H2AX is also a substrate target of the WIP1 phosphatase. WIP1 dephosphorylates γ-H2AX at serine 139 in both in vitro and in vivo contexts and facilitates the clearance of irradiation-induced foci. Overexpression of WIP1 inhibits formation of damage foci in IR and UV-irradiated cells and also suppresses recruitment of the checkpoint and DNA repair-associated proteins MDC1 and 53BP1 to damage foci. Moreover, we present evidence that WIP1 suppresses efficient DSB repair. Thus, WIP1 may be one of several phosphatases that dephosphorylate γ-H2AX, contributing to a homeostatic inactivation of the DDR after DNA repair is completed.
EXPERIMENTAL PROCEDURES
Cell Lines, Cell Culture, and DNA Damaging Agents
HeLa, MCF7, and 293T cell lines were obtained from the American Type Culture Collection (ATCC). The A-T cell line GM09607 was purchased from Coriell Cell Repositories (Camden, NJ). To establish a cell line stably expressing HA-ER-I-PpoI, retrovirus was generated as previously described (35) and used to infect MCF7 cells, then selected with 1 μg/ml puromycin for 1 week. All cell lines were cultured in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum and maintained at 37 °C in a humidified incubator at 5% CO2. IR-induced DNA damage was produced with a Cs-147 irradiator with a standard IR dose of 5 Gy. For UV-induced damage, the Stratalinker 1800 (Stratagene, La Jolla, CA) was utilized at 30 or 50 J/m2. Other agents used to induce DNA damage were bleomycin (Sigma, catalogue no. B2434) at 20 μg/ml, hydroxyurea (Sigma-Aldrich, catalogue no. H8627) at 10 mm, neocarzinostatin (Sigma-Aldrich) at 200 ng/ml, and KU55933 (EMD Biosciences, San Diego, CA) at 10 μm.
Mice
The Wip1 and Atm null mice have been previously described (36, 37). Genotyping of mice for Wip1 mutant alleles was performed by tail DNA PCR (35 cycles) using primers synthesized by Integrated DNA Technologies (Coralville, IA). Wip1 mutant allele-specific primers were 5′-ACAAGCTTGCAGGGCTGTTTGTGG-3′ (Wip1 intron 3) and 5′-CTTCCCAGCCTCTGAGCCCAGAAAGC-3′ (PGK-neo insert) and generate a 500-bp fragment after PCR. Wip1 wild-type allele-specific primers were 5′-GTGGAGCTATGATTTCTTCAGTGG-3′ (Wip1 exon 4F) and 5′-GATACGACACAAGACAAACCTCC-3′ (Wip1 Exon 4R) and generate a 300-bp fragment following PCR. Genotyping of mice for Atm mutant alleles was performed by tail DNA PCR. Atm mutant allele-specific primers were 5′-GCTGGACGTAAACTCCTCTTCAGAC-3′ and 5′-GTAGTAACTATTAGTTTCGTGCA-3′ and produce a 282-bp fragment. Atm wild-type allele-specific primers were 5′-TAGGGTGTACTAGTGGAGGA-3′ and 5′-GTAGTAACTATTAGTTTCGTGCA-3′ and generate a 473-bp fragment.
Wild-type, Wip1−/−, Atm−/−, and Wip1−/−Atm−/− double knock-out mice were treated with 5-Gy ionizing radiation and euthanized 6 h later, and spleen lysates were immunoblotted with antibodies to γ-H2AX and H2AX as previously described (19). All animals were handled in strict accordance with good animal practice as defined by the Institutional Animal Care and Use Committee for Baylor College of Medicine and Affiliates (Animal Protocol AN336) and by the Association for the Assessment and Accreditation of Laboratory Animal Care, Guide for the Care and Use of laboratory Animals (NRC1996).
In Vitro Phosphatase Assays
The in vitro phosphatase assays have been performed as previously described (38). For substrates, γ-H2AX phosphopeptide (Ac-GKKATQApSQEY-amide), negative control phosphopeptide UNG2 pT31 (Ac-AVQGpTGVAGV-amide), and positive control peptide p38 pT180 (Ac-TDDEMpTGpYVAT-amide) were synthesized by New England Peptide (Gardner, MA). To obtain intact γ-H2AX proteins, 293T cells were transfected with myc-H2AX expression plasmid and treated with 20 μg/ml bleomycin for 1 h. Then, Myc-tagged H2AX proteins were immunopurified using anti-Myc antibody (Clontech, Mountain View, CA, catalogue no. 631206). For purified phosphatases in the assays, the recombinant human WIP1 proteins were purified from bacteria as described (39). PP2Cα (Calbiochem, catalogue no. 539569) and PP2A (Millipore, Billerica, MA, catalogue no. 14-111) were purchased.
Cell Transfection with Plasmids or siRNAs
The human wild-type WIP1 expression plasmid has been previously described (40). The phosphatase-dead (D314A) mutation was introduced using site-directed mutagenesis (Stratagene). Cell transfection with plasmid DNA was performed using Lipofectamine and PLUS reagent (Invitrogen, catalogue no. 18324–012 and #11514–015). For siRNA transfection, Oligofectamine reagent (Invitrogen, catalogue no. 12252–011) was used. WIP1 siRNAs from Dharmacon (Lafayette, CO, J-004554–05 and J-004554–06) and negative control siRNA from Ambion (Austin, TX, AM4635) were purchased.
Antibodies, Western Blotting, and Immunoprecipitation
The antibodies used for Western blotting and immunoprecipitation were anti-γ-H2AX (Millipore, catalogue no. 07-164), anti-H2AX (Cell Signaling Technology, Danvers, MA, catalogue no. 2595), anti-H2AX (Millipore, catalogue no. 07-627), anti-TBP (Affinity Bioreagents, Huntsville, AL, catalogue no. MA1-21516), anti-α-Tubulin (Santa Cruz Biotechnology, Santa Cruz, CA, catalogue no. sc5286), anti-WIP1 (Abgent, San Diego, CA, catalogue no. AP8437b), anti-Myc (Clontech, catalogue no. 631206), anti-FLAG (Sigma-Aldrich, catalogue no. F3165), and EZview Red anti-FLAG M2 affinity Gel (Sigma-Aldrich, catalogue no. F2426). Western blotting and immunoprecipitation were performed as described previously (38).
Immunofluorescence Studies
Immunofluorescence staining was performed by standard methods. Cells were fixed with chilled 4% formaldehyde in phosphate-buffered saline. The antibodies used for immunofluorescence staining were anti-γ-H2AX (Cell Signaling Technology, catalogue no. 2577), anti-γ-H2AX (Millipore, catalogue no. 05-636), anti-MDC1 (Bethyl Laboratories, Montgomery, TX, catalogue no. A300-051A), anti-FLAG (Sigma, catalogue no. F1804), anti-53BP1 (Novus, catalogue no. NB100-304), anti-FLAG (BioLegend, San Diego, CA, catalogue no. 637301), anti-WIP1 (Santa Cruz Biotechnology, catalogue no. sc20712), goat anti-mouse and rabbit IgG conjugated to Alexa Fluor 488 or Alexa Fluor 594 (Invitrogen), or goat anti-rat IgG conjugated to Alexa Fluor 568 (Invitrogen). Images were acquired with the Applied Precision SoftWoRx image restoration deconvolution microscope. The brightness/contrast adjustment and channel overlay were done with NIH ImageJ and Adobe Photoshop CS2 software. For high throughput cell analysis, images were acquired with the Cell Lab IC-100 Image Cytometer (Beckman Coulter, IC100) and analyzed with CytoShop software. The acquired fluorescence intensity values were evaluated with the unpaired Student's t test (two-tailed).
Chromatin Immunoprecipitation and Repair Assay Using the I-PpoI System
The I-PpoI system has been described previously (35). After I-PpoI-mediated DSBs were induced by addition of 4-hydroxytamoxifen (4-OHT, Sigma-Aldrich, catalogue no. H7904), cells were cross-linked for 10 min with 1% formaldehyde, and the cross-linking was stopped by addition of 0.125 m glycine. Chromatin was purified and sonicated to an average size of ∼500 bp. For the repair defect assay, real-time PCR was performed with primers flanking the I-PpoI cut site in chromosome 1 and GAPDH as an internal control: I-PpoI site (5′-TCACTGAAGACTTGGTGGGA-3′ and 5′-AAACCATACGTGGCAGAGTG-3′) and GAPDH site (5′-GCTTGCCCTGTCCAGTTAAT-3′ and 5′-TAGCTCAGCTGCACCCTTA-3′). Amplification of the PCR product was analyzed with the ΔΔCT method (as explained on-line by Applied Biosystems). The antibodies used for ChIP were anti-γ-H2AX (Millipore, catalogue no. 07-164, anti-H2B (Millipore, catalogue no. 07-371), and rabbit IgG (Jackson ImmunoResearch Laboratories, West Grove, PA, catalogue no. 011-000-002). Immunoprecipitated DNA fragments were PCR-analyzed with primers: region adjacent to the I-PpoI site in chromosome 1 (5′-TGCTGCTTTTTCTTCTTCTCC-3′ and 5′-CTTCTTTCCCACCAAGTCTTC-3′) and GAPDH site (5′-TCGGTTCTTGCCTCTTGTC-3′ and 5′-CTTCCATTCTGTCTTCCACTC-3′).
Short Hairpin RNA Lentivirus
To make lentivirus expressing shRNA against WIP1, DNA oligonucleotides were synthesized based on the WIP1 siRNA (Dharmacon, catalogue no. J-004554-05 and J-004554) and inserted into the FG12 lentiviral vector (41). Viruses were obtained by co-transfection of 293T cells with FG12-shWIP1 lentiviral vector plasmid, packaging constructs (pRSV-REV and pMDLg/pRRE), and the vesicular stomatitis virus G expression plasmid (pHCMV-VSVG). Virus production was verified by using a green fluorescent protein expression marker. WIP1 knockdown following the lentivirus infection for 24 h was confirmed in MCF7 cells by immunoblotting.
DNA Repair Assays Using LB4 and TNeo99-7 Cells
To measure the frequency of DSB repair by homologous recombination (HR) and non-homologous end-joining (NHEJ), two cell lines derived from the normal ATM-proficient human fibroblast cell line GM00637 were used, LB4 and pTNeo99-7 (Fig. 8A). Briefly, for each trial in Fig. 8 (B and C), 5 × 106 cells from each cell line were suspended in phosphate-buffered saline containing 10 μg of an I-SceI expression vector and 0–10 μg of wild-type/mutant WIP1 or control expression vector. Cells were electroporated, plated into 150-mm flasks, grown for 2 days without selection, and then plated into medium supplemented with G418 to screen for cells that repaired the I-SceI-induced DSB. After 10–14 days, G418-resistant colonies were counted, propagated, and validated by DNA sequencing. For the WIP1 inhibition studies in Fig. 8D, WIP1 shRNA lentiviral vector RHS3979-9571555 from Open Biosystems was used to transduce both LB4 and TNeo99-7 cells prior to I-SceI introduction. In addition, WIP1 inhibitors arsenic trioxide (catalogue no. 17971) and CCT007093 (catalogue no. 9369) from Sigma-Aldrich at 10 μm and 25 μm, respectively, were used to treat LB4 and TNeo99-7 cells prior to I-SceI introduction.
FIGURE 8.
WIP1 inhibits repair of DNA DSBs. A, recombination substrates pLB4 and pTNeo99-7 in stable cell lines, LB4 and TNeo99-7. Each substrate contains a functional hygromycin gene (hyg), used to select for stably transfected cells, and a tk-neo fusion gene that is disrupted by a 22-bp oligonucleotide containing the recognition site for endonuclease I-SceI. Substrate pLB4 contains a 2.5-kb HindIII fragment containing a complete HSV-1 tk gene that serves as a donor for the homologous recombination with the disrupted tk-neo gene. B, WIP1, but not phosphatase-dead WIP1 inhibits DSB repair. I-SceI and wild-type/mutant WIP1 or control expression vector DNA were transfected into cells, and the frequency of recovery of G418R clones was measured. C, WIP1 has dose-dependent inhibitory effects on DSB repair. As indicated, varying amounts of WIP1 vector DNA or control vector DNA were introduced into cells, and the frequency of recovery of G418R clones was measured. D, suppression of WIP1 activity by WIP1 shRNA and the WIP1 inhibitors arsenic trioxide (ATO) and CCT007093 enhances DSB repair. LB4 and TNeo99-7 cells were transduced with a lentiviral WIP1 shRNA vector or were incubated in 10 μm ATO or 25 μm CCT007093 prior to I-SceI introduction. Formation of G418R clones was then measured. E, the effects of WIP1 on DSB repair are not solely dependent on ATM. LB4 and TNeo99-7 cells were transfected with control or WIP1 expression vector DNA, treated with neocarzinostatin (200 ng/ml) and with or without KU55933 (10 μm). Proteins were detected by their specific antibodies in immunoblotting (left panel). The effects of WIP1 on DSB repair were also examined in LB4 and TNeo99-7 cells in the presence or absence of KU55933 (right panel).
RESULTS
WIP1 Dephosphorylates γ-H2AX in Vitro
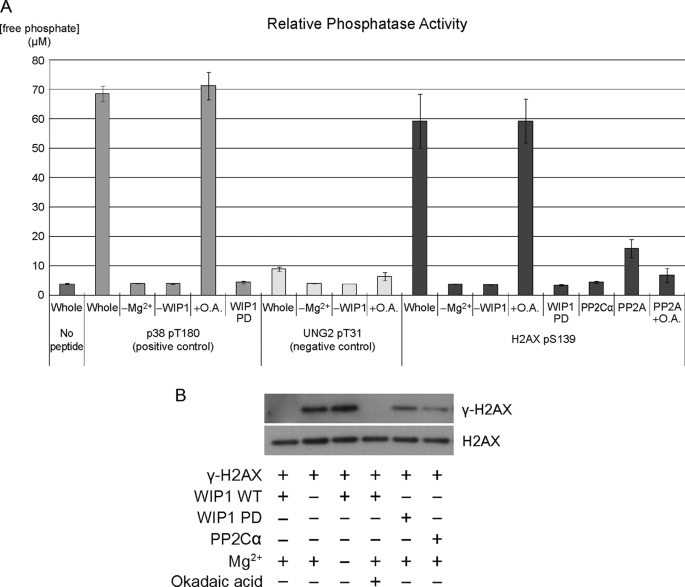
Having identified several ATM/ATR targets dephosphorylated by WIP1, we performed a candidate search to identify new WIP1 targets. We obtained synthesized pSQ or pTQ containing phosphopeptides derived from ATM/ATR target proteins. Candidate phosphopeptides were incubated in the presence of Mg2+ and bacterially purified WIP1. Phosphatase activity was measured by release of free phosphate from the phosphopeptide. The in vitro phosphatase assay revealed that the H2AX pS139 phosphopeptide is robustly dephosphorylated by WIP1 in the presence of Mg2+, but not in its absence (Fig. 1A). Appella and colleagues had also reported WIP1 activity on a similar H2AX phosphopeptide in in vitro phosphatase assays (42). WIP1 activity on the H2AX phosphopeptide was not inhibited by okadaic acid, as expected (5). Incubation of WIP1 with the positive control p38 pT180 phosphopeptide and negative control UNG2 pT31 resulted in high and low activities, respectively. The WIP1 D314A phosphatase-dead mutant, as well as the closely related PP2Cα phosphatase, showed no activity. Finally, PP2A did display activity on the γ-H2AX phosphopeptide and was inhibited by okadaic acid, consistent with previous findings that PP2A dephosphorylates this site (31).
FIGURE 1.
γ-H2AX is dephosphorylated by WIP1 in vitro. A, H2AX pS139 phosphopeptide was incubated with human recombinant WIP1 protein. p38 pT180 and UNG2 pT31 phosphopeptides were used as a positive and negative control, respectively. Free phosphate released from the phosphopeptide was measured by malachite green phosphate assay to determine relative phosphatase activities on each phosphopeptide. Error bars correspond to standard error (n = 3). O.A., okadaic acid. WIP1 PD is a phosphatase-dead point mutant used as a control. B, 293T cells were transfected with myc-H2AX expression plasmid and treated with bleomycin for 1 h to induce γ-H2AX phosphorylation. Myc-tagged full-length H2AX protein was immunopurified using anti-Myc antibody and incubated with human recombinant WIP1 protein, WIP1 phosphatase-dead protein, or PP2C alpha in vitro. γ-H2AX phosphorylation levels were determined by immunoblotting using a γ-H2AX-specific antibody. The key components of each assay are indicated below each lane.
To confirm that full-length γ-H2AX is a direct target of WIP1 in vitro, we immunopurified full-length H2AX protein from bleomycin-treated 293T cells and incubated it with purified recombinant WIP1 in an in vitro phosphatase assay. After immunoblotting the reaction components with antibodies specific for γ-H2AX and H2AX protein, we found that wild-type WIP1, but not D314A mutant WIP1 or PP2Cα, dephosphorylated γ-H2AX in a magnesium-dependent and okadaic acid-insensitive manner, indicating that WIP1 directly targets γ-H2AX for dephosphorylation (Fig. 1B).
WIP1 Impairs γ-H2AX Phosphorylation in Cells
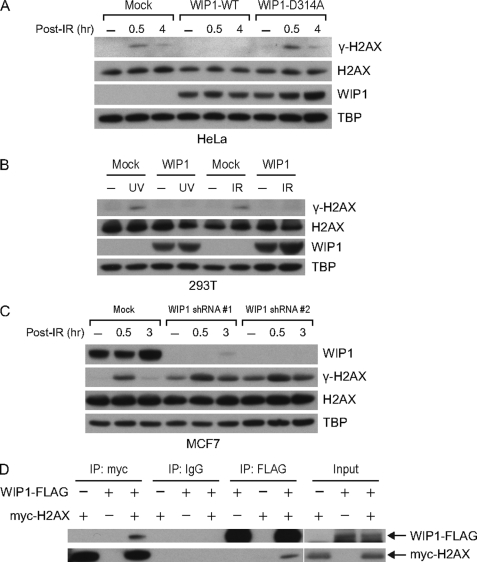
To evaluate the effect of WIP1 on γ-H2AX phosphorylation in cells, the levels of γ-H2AX in radiation-treated cells following WIP1 overexpression or siRNA knockdown were examined. Immunoblotting of HeLa cell nuclear lysates after 5-Gy IR treatment demonstrated that cells transfected with a wild-type WIP1 expression vector displayed reduced γ-H2AX levels compared with mock transfected cells and cells transfected with a phosphatase-dead WIP1 expression vector (Fig. 2A). Because p53 is inactivated in HeLa cells and WIP1 expression is largely p53-dependent, WIP1 protein induction following DNA damage was not detectable (43). When wild-type WIP1 was overexpressed, γ-H2AX phosphorylation was significantly impaired up to 8 h after IR (Fig. 2A and data not shown). Because the total levels of H2AX protein remained constant, these results indicate that the γ-H2AX levels are reduced mostly by protein dephosphorylation. γ-H2AX phosphorylation was also impaired by WIP1 overexpression in 293T cells in response to both IR and UV irradiation, indicating that WIP1 inhibition of γ-H2AX was not cell type-specific or specific to IR-induced damage (Fig. 2B).
FIGURE 2.
WIP1 impairs γ-H2AX phosphorylation in cells. A, overexpression of WIP1 suppresses γ-H2AX in IR-treated HeLa cells. HeLa cells were either mock transfected or transfected with WIP1-FLAG expression plasmid. At 24 h after transfection, cells were irradiated with 5-Gy IR and harvested. Nuclear lysates were immunoblotted to assess γ-H2AX and total H2AX protein, and WIP1 protein. TBP (TATA-binding protein) served as a loading control. B, overexpression of WIP1 suppresses γ-H2AX in IR and UV-treated 293T cells. 293T cells were transfected as in A. After 24 h, cells were irradiated with either 5-Gy IR or 50 J/m2 UV and harvested 30 min after IR or 2 h after UV treatment. Nuclear lysates were immunoblotted as indicated. C, WIP1 knockdown increased γ-H2AX levels in IR-treated MCF7 cells. Cells were transduced with lentivirus expressing WIP1 shRNA (#1 and #2). After 48 h, cells were irradiated with 5-Gy IR, harvested, and then analyzed by immunoblot with the indicated antibodies. D, WIP1 and H2AX physically interact in 293T cells. 293T cells were co-transfected with myc-H2AX and WIP1-FLAG expression plasmids. Interaction between H2AX and WIP1 was examined by immunoprecipitation with anti-FLAG antibody followed by immunoblotting with anti-Myc antibody and vice versa.
To complement the WIP1 overexpression experiments, we investigated whether γ-H2AX phosphorylation is increased in WIP1-depleted cells. Using two distinct WIP1 shRNA vectors, we silenced WIP1 expression in MCF7 cells where WIP1 gene expression is highly up-regulated due to gene amplification (18, 44). Both WIP1 shRNAs were tested and showed greater than 90% knockdown efficiency (Fig. 2C). Compared with control cells, MCF7 cells transduced with both WIP1 shRNAs prior to IR treatment displayed higher γ-H2AX levels before and from 0.5–3 h post-IR.
The ability of WIP1 to efficiently dephosphorylate γ-H2AX in vitro and in cells suggests at least a transient interaction between the two proteins. We tested this possibility by performing co-immunoprecipitation experiments following transfection of 293T cells with Myc-tagged H2AX and FLAG-tagged WIP1 expression constructs. In the immunoprecipitation-Western blots shown in Fig. 2D we did identify reciprocal physical interactions between WIP1 and H2AX. However, attempts to detect interactions between endogenous WIP1 and H2AX were unsuccessful, suggesting a transient interaction between enzyme (WIP1) and substrate (γ-H2AX). This could be similar in nature to the transient interaction we have observed between WIP1 and p53, which is efficiently dephosphorylated at serine 15 by WIP1 (11).
WIP1 Dephosphorylates γ-H2AX in an ATM-independent Manner
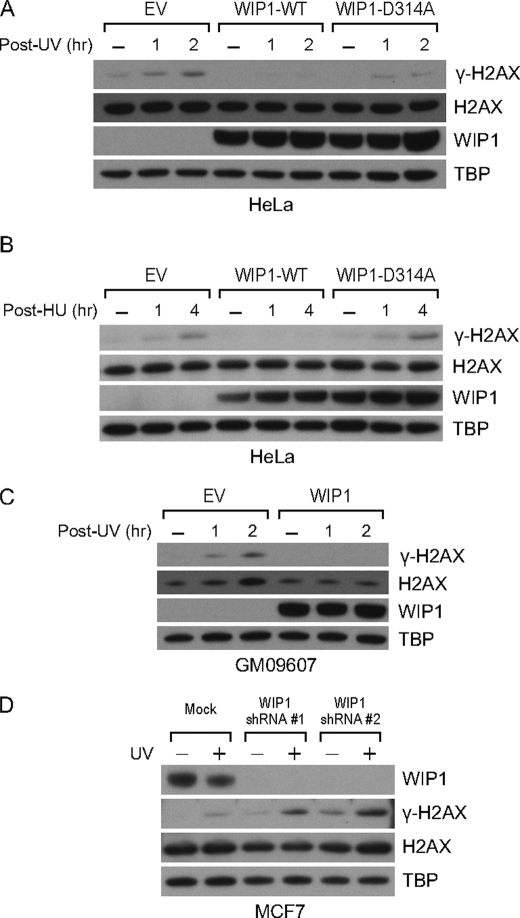
Because ATM has been known to be a target of WIP1 (10), the impairment of γ-H2AX phosphorylation by WIP1 following IR could be an indirect result of WIP1-mediated ATM inactivation. To investigate whether WIP1 is capable of directly targeting γ-H2AX independently of ATM, we first checked γ-H2AX phosphorylation in UV-irradiated or hydroxyurea-treated cells. In response to UV or hydroxyurea, ATR, rather than ATM, is the primary mediator of γ-H2AX phosphorylation (45). After exposure to UV or hydroxyurea, the γ-H2AX levels in empty vector-transfected cells and phosphatase-dead WIP1-expressing cells increased over time (Fig. 3, A and B). However, in WIP1-overexpressing cells, γ-H2AX phosphorylation was suppressed. Next, we repeated the experiment in GM09607 cells, an ATM null cell line, and observed similar effects. γ-H2AX phosphorylation following DNA damage still occurs in ATM null cells, because DNA-PKcs and ATR likely compensate for ATM deficiency. WIP1 overexpression in GM09607 cells strongly suppressed γ-H2AX phosphorylation after UV irradiation (Fig. 3C). To test whether down-regulation of WIP1 could enhance UV-mediated γ-H2AX phosphorylation, we transduced MCF-7 cells with two different WIP1 shRNA lentiviral vectors and examined γ-H2AX levels before and after UV treatment. In fact, >90% reduction of WIP1 levels resulted in greatly increased γ-H2AX levels following UV treatment in comparison to control MCF-7 cells with high levels of WIP1 (Fig. 3D).
FIGURE 3.
WIP1 reverses ATR-mediated γ-H2AX phosphorylation. A and B, WIP1 suppresses γ-H2AX phosphorylation after UV (A) and hydroxyurea treatment (B). HeLa cells were transfected with empty vector or WIP1-FLAG expression plasmid. At 24 h after transfection, cells were irradiated with 50 J/m2 of UV (A) or treated with 10 mm hydroxyurea (HU) (B) and harvested at the indicated time points. Nuclear lysates were immunoblotted as indicated. C, WIP1 suppresses γ-H2AX phosphorylation in the absence of ATM. GM09607 (ATM null) fibroblasts were transfected with either empty vector or WIP1-FLAG expression plasmid. After 24 h, cells were irradiated with 50 J/m2 of UV and harvested. Nuclear lysates were immunoblotted as indicated. D, suppression of WIP1 in MCF-7 cells results in enhanced γ-H2AX phosphorylation following UV irradiation. MCF-7 cells were transduced with one of two different shRNA lentiviral vectors prior to 10 J/m2 UV irradiation. Nuclear lysates were harvested 1 h post-UV treatment along with un-irradiated control cells and mock transduced controls. Immunoblot analysis for WIP1 protein levels, H2AX protein levels, and γ-H2AX levels was performed.
γ-H2AX Phosphorylation Is Increased in Wip1 Null Mice
To show that WIP1 can also promote γ-H2AX dephosphorylation in normal cells in a live mammal, we examined γ-H2AX phosphorylation status in spleen tissue of Wip1−/− and Wip1+/+ mice. We had previously shown that Wip1 null mice are phenotypically normal for the most part, but are highly cancer resistant (19, 36, 46). Mock treatment or treatment of Wip1+/+ and Wip1−/− mice with 5-Gy whole body irradiation was followed by euthanasia 6 h later and preparation of spleen tissue lysates and immunoblotting with γ-H2AX and H2AX protein antibodies. As shown in Fig. 4A, basal levels of γ-H2AX were very low in un-irradiated Wip1+/+ spleen tissue, but induced to moderately high levels 6 h after IR treatment. Interestingly, in un-irradiated Wip1−/− tissue, γ-H2AX basal levels were higher than those in un-irradiated Wip1+/+ tissue (Fig. 4, A and B). Importantly, irradiated Wip1−/− tissue exhibited higher γ-H2AX than did irradiated Wip1+/+ tissue.
FIGURE 4.
Wip1 null mice exhibit increased γ-H2AX phosphorylation after IR treatment. A, absence of Wip1 in irradiated mouse tissue increases γ-H2AX phosphorylation. Wild-type, Wip1−/−, Atm−/−, and Atm−/− Wip1−/− double knock-out mice were exposed to 5 Gy of whole body ionizing irradiation. Six hours after irradiation, spleens were harvested from each mouse and tissue lysates were immunoblotted with the indicated antibodies. B, quantitative analysis of γ-H2AX phosphorylation levels in A. Immunoblot bands were quantitated with NIH ImageJ software. γ-H2AX bands were normalized to the total H2AX bands. The γ-H2AX level in irradiated wild-type tissue (lane 3 in A) was set to 1.0.
In this experiment, we also included Atm−/− and Atm−/− Wip1−/− double knock-out mice to examine Atm-independent γ-H2AX phosphorylation (Fig. 4, A and B). Note that Atm deficiency alone did not impair IR-induced γ-H2AX phosphorylation, presumably as a result of compensatory phosphorylation by other PIKKs. Either in the presence or absence of IR damage, Atm null and Atm/Wip1 double knock-out tissue exhibited comparable γ-H2AX phosphorylation with wild-type and Wip1 null tissue, respectively. These results indicate that Wip1 suppresses γ-H2AX phosphorylation in vivo in an Atm-independent manner.
WIP1 Inhibits Damage Focus Formation
Following IR, γ-H2AX phosphorylation in the vicinity of DSBs leads to the recruitment of DDR factors such as MDC1 and 53BP1 and formation of subnuclear foci called ionizing radiation-induced foci. To test whether WIP1 affects the formation of γ-H2AX-associated DNA damage foci, we performed immunofluorescence experiments using antibodies to detect γ-H2AX and WIP1 in IR-treated cells with low or overexpressed levels of wild-type or mutant WIP1. As shown in Fig. 5A, mock transfection of HeLa cells followed by 5-Gy ionizing radiation treatment and subsequent immunofluorescence with γ-H2AX antibodies resulted in numerous discrete intensely staining damage foci within the nucleus, indicating an effective DNA damage response. However, when the HeLa cells were transfected with a FLAG-tagged WIP1 expression construct prior to irradiation, lower numbers of weakly fluorescent nuclear foci appeared. This WIP1-mediated foci inhibition was clearly dependent on WIP1 phosphatase function, because HeLa cells transfected with the WIP1 D314A (phosphatase-dead) expression construct exhibited high numbers of intense nuclear foci after IR treatment (Fig. 5A). Quantitative analysis of γ-H2AX and WIP1 fluorescence in large numbers of cells by high throughput fluorescence microscopy imaging revealed a highly significant inverse correlation between WIP1 and γ-H2AX fluorescence, indicating higher WIP1 levels suppressed γ-H2AX-associated damage foci (Fig. 5, B and C). Similar results were obtained for UV-treated HeLa cells mock transfected or transfected with WIP1 expression constructs (supplemental Fig. S1).
FIGURE 5.
WIP1 inhibits damage-induced focus formation. A, overexpression of WIP1 inhibits IR-induced damage foci in HeLa cells. HeLa cells were mock-transfected or transfected with either wild-type or D314A mutant WIP1-FLAG expression plasmid. At 24 h after transfection, cells were irradiated with 5-Gy IR. After 30 min, cells were fixed and immunostained with anti-γ-H2AX (red fluorescence) and anti-FLAG antibodies (green fluorescence) to assess damage-induced foci formation. 4′,6-Diamidino-2-phenylindole staining (DAPI, blue fluorescence) was used to identify nuclei. B, quantitative analysis of γ-H2AX-asociated damage foci in IR-treated HeLa cells with or without overexpressed WIP1. The fluorescence intensity values of γ-H2AX from >1300 independent cells from the mock-transfected and WIP1-transfected cells were measured by high throughput microscopic imaging and plotted. Error bars represent the standard error. C, fluorescence intensity of γ-H2AX-associated damage foci is inversely correlated with WIP1 fluorescence intensity. At 30-min post-IR, individual WIP1-transfected HeLa cells were subdivided into low (n = 138), medium (n = 406), or high (n = 181) WIP1-expressing cells based on high throughput quantitative fluorescence imaging. The fluorescence intensity values for γ-H2AX for each cell were also measured, and mean γ-H2AX fluorescence for each of the three WIP1 expression categories was plotted. Error bars represent the standard error (Student's t test; p < 10−10 between Low and Med and p < 10−40 between Med and High). D, knockdown of WIP1 in MCF7 cells results in increased γ-H2AX-associated damage foci. MCF7 cells were transfected with negative control siRNA or WIP1 siRNA. At 24 h after transfection, cells were irradiated with 5-Gy IR, fixed at 3 h after irradiation, and immunostained with anti-γ-H2AX (red fluorescence) and anti-WIP1 antibodies (green fluorescence). E, WIP1 inhibition γ-H2AX-associated damage foci is ATM-independent. GM09607 (ATM null) fibroblasts were transfected with WIP1-FLAG expression plasmid. After 24 h, cells were irradiated with 5-Gy IR, fixed 30 min after irradiation and then immunostained with the indicated antibodies.
The effect of WIP1 depletion on γ-H2AX focus formation was also examined. We transfected WIP1 siRNA into MCF7 cells and immunostained IR-irradiated cells with WIP1 and γ-H2AX antibodies 3 h after 5 Gy of IR treatment. WIP1 expression is high in MCF7 cells as a result of WIP1 amplification (18, 44) but was considerably reduced by WIP1 siRNA transfection (Fig. 5D and supplemental Fig. S2). IR treatment resulted in larger numbers of more intense γ-H2AX staining foci in the WIP1 siRNA-transfected cells compared with the control siRNA-transfected MCF7 cells, providing further evidence that WIP1 suppresses damage foci.
We also examined whether WIP1 suppression of γ-H2AX damage foci was ATM-independent by examining IR-induced focus formation in the presence and absence of high WIP1 expression in A-T cells. GM09607 ATM null human fibroblasts were IR- or UV-irradiated after mock transfection or WIP1-FLAG expression construct transfection. Fluorescence microscopy using FLAG or γ-H2AX antibodies revealed an inverse correlation between WIP1 overexpression and γ-H2AX-associated damage foci (Fig. 5E and supplemental Fig. S3), indicating that WIP1 can effectively suppress damage focus formation in an ATM-independent manner.
WIP1 Inhibits Recruitment of MDC1 and 53BP1 to Damage Foci
MDC1 (mediator of DNA damage checkpoint 1) binds to the phosphorylated C-terminal tail of γ-H2AX through a tandem set of BRCT domains and then recruits Nbs1 and other components of the repair complex to initiate DNA DSB repair (26, 47, 48). Phosphorylation of H2AX at serine 139 is critical for the H2AX-MDC1 interaction (26). To test whether WIP1 affects the recruitment of MDC1 to γ-H2AX-associated damage foci, HeLa cells were IR-treated after mock transfection or transfection with WIP1-FLAG expression constructs and then examined by fluorescence microscopy using antibodies to FLAG epitope and MDC1. As shown in Fig. 6A and supplemental Fig. S4, MDC1 staining in mock transfected cells is intense and co-localizes with γ-H2AX to discrete foci, indicative of damage foci. In contrast, WIP1-overexpressing cells exhibit primarily diffuse nuclear staining of MDC1, indicative of an inability to localize to damage foci (Fig. 6A and supplemental Fig. S4). Thus, WIP1 appears to inhibit migration of MDC1 to IR-induced damage foci, presumably due to an inability to interact with WIP1-dephosphorylated H2AX. 53BP1 (p53-binding protein 1) is another mediator/adaptor protein that rapidly migrates to damage foci following DNA DSBs (49). Efficient recruitment of 53BP1 to damage foci is dependent on γ-H2AX phosphorylation and MDC1 recruitment (28, 50). We examined the effects of WIP1 overexpression on 53BP1 recruitment to damage foci by irradiating HeLa cells with 5-Gy IR following mock transfection or transfection with a FLAG-WIP1 expression construct. Immunofluorescence microscopy of the irradiated and transfected cells after fixing and incubating with FLAG antibody (for WIP1 protein) and 53BP1 protein revealed that overexpression of WIP1 suppressed association of 53BP1 with damage foci, whereas cells not overexpressing WIP1 showed strong association of 53BP1 with damage foci (Fig. 6B and supplemental Fig. S5). Thus, WIP1 strongly suppresses association of both MDC1 and 53BP1 with DNA damage foci.
FIGURE 6.
WIP1 inhibits recruitment of repair complex proteins MDC1 and 53BP1 to DNA damage foci. A, WIP1 inhibits recruitment of MDC1 to IR-induced damage foci. HeLa cells were transfected with WIP1-FLAG expression plasmid. At 24 h after transfection, cells were irradiated with 5-Gy IR. Thirty minutes later, cells were fixed and immunostained with anti-MDC1 (far-red fluorescence), anti-γ-H2AX (green fluorescence), and anti-FLAG (red fluorescence) antibodies. B, Wip1 inhibits recruitment of 53BP1 to IR-induced damage foci. HeLa cells were mock transfected or transfected with WIP1-FLAG expression plasmid, treated with 5-Gy IR, fixed, and immunostained with anti-53BP1 and anti-FLAG antibodies as in A.
WIP1 Inhibits DNA DSB Repair
Because γ-H2AX is of central importance in efficiently recruiting DNA repair proteins to the site of DSBs and facilitating break repair, we attempted to determine whether WIP1 inhibition of γ-H2AX phosphorylation would be accompanied by deficiencies in DSB repair. Two systems for analyzing specific DSBs were employed. The first system, developed by Kastan and colleagues, allows analysis of protein recruitment to specific DSBs in human cells as well as DSB repair efficiency (35). DSBs are initiated by transducing a vector expressing the eukaryotic homing endonuclease I-PpoI fused to a mutant estrogen receptor hormone-binding domain that is responsive to the estrogen analogue 4-OHT. Treatment of the vector-transduced cells with 4-OHT activates the endonuclease, which cleaves at a limited number of sites in the human genome. ChIP with an anti-γ-H2AX antibody and PCR primers specific for a site adjacent to one of the defined DSBs and PCR across the same DSB produced by I-PpoI were performed to monitor the γ-H2AX recruitment to the site-specific DSB and the efficiency of DSB repair (Fig. 7A).
FIGURE 7.
WIP1 inhibits DSB repair and γ-H2AX DSB association. A, strategy employed to measure γ-H2AX binding adjacent to a DSB (B) and the efficiency of DNA DSB repair (C) using the I-PpoI system. Real-time PCR with primers flanking the I-PpoI cut site allow measurement of DSB break repair efficiency. γ-H2AX association was monitored with ChIP followed by PCR with primers 50 and 280 bp downstream of the I-PpoI cleavage site. B, ChIP assay for γ-H2AX association at I-PpoI cleavage site shows increased γ-H2AX when WIP1 levels are decreased. MCF7 cells expressing HA-ER-I-PpoI were infected with either empty or WIP1 shRNA lentivirus. After 4-OHT treatment, cells were fixed at the indicated time points and subjected to the ChIP assay with primers adjacent to the single I-PpoI cut site in chromosome 1. A ChIP assay with primers from the GAPDH region not near an I-PpoI cut site (GAPDH) serves as a negative control. C, DNA DSB repair is enhanced when WIP1 levels are reduced. I-PpoI was activated in MCF7 cells by 4-OHT treatment for 12 h. Genomic DNA was obtained and analyzed with real-time PCR to measure the efficiency of DSB repair. The relative amounts of the trans-DSB PCR fragment were assessed by the ΔΔCT method. Error bars represent the standard error (n = 3).
MCF7 cells stably expressing the I-PpoI fusion endonuclease were infected with a lentiviral vector expressing a control shRNA or WIP1 shRNA followed by activation of the endonuclease by 4-OHT addition. Cells were then fixed at various time points following endonuclease activation, and ChIP analysis was performed with anti-γ-H2AX antibody and PCR primers specific for a site next to a defined I-PpoI DSBs. As shown in Fig. 7B, at most time points after introduction of the defined DSB, relative association of γ-H2AX near the DSB is higher in the MCF7 cells transduced with WIP1 shRNA compared with cells transduced with control shRNA. This result suggests that reduction of WIP1 enhances retention of γ-H2AX near the break site and thus would facilitate more efficient recruitment of DNA repair factors to the DSB. One unexpected result was that, prior to 4-OHT addition, there was γ-H2AX at the defined DSB site (Fig. 7B). This may be due to leakiness of the I-PpoI vector. A control ChIP experiment performed on GAPDH DNA away from any known I-Ppol cleavage site showed minimal levels of γ-H2AX association.
A second assay with the I-PpoI system utilized real-time PCR across the defined DSB to measure the relative efficiency of DSB repair. At 12 h after I-PpoI activation, virtually all of the DSBs were repaired in the WIP1 shRNA-treated MCF7 cells based on relative amounts of the trans-DSB PCR fragment (Fig. 7C). In contrast, the MCF7 cells transduced with the control vector exhibited a high level of unrepaired DSBs relative to the WIP1-silenced MCF7 cells, indicating more efficient repair of DSBs when WIP1 levels are reduced. Thus, WIP1 is likely to suppress the efficiency of DSB repair.
A second system utilized two cell lines to measure DSB repair and allowed differentiation between HR- and NHEJ-mediated DSB repair. Derived from the normal human fibroblast cell line GM00637, the cell line LB4 contains a single stably integrated copy of construct pLB4, whereas the cell line TNeo99-7 also contains a single stably integrated copy of pTNeo99-7 (Fig. 8A) (51, 52). Both substrates contain a tk-neo fusion gene disrupted by the insertion of the recognition site for endonuclease I-SceI. pLB4 also contains a “donor” tk sequence to serve as a recombination partner for the fusion gene, so cell line LB4 is useful for studying both HR and NHEJ, while cell line TNeo99-7 is suitable for studying NHEJ only. In the absence of I-SceI, the background frequencies of G418-resistant clones are extremely low in both cell lines (<10−6 in LB4 cells and <10−7 in TNeo99-7 cells) (52). Introduction of endonuclease I-SceI will generate a DSB within the integrated substrate. DSB repair events are recovered by selecting for clones that become resistant to the neomycin analog G418 due to restoration of the tk-neo fusion gene.
Overexpression of WIP1 reduced the frequency of recovery of G418-resistant clones in LB4 cells and TNeo99-7 cells by 55 and 42%, respectively, compared with that of control cells, following I-SceI cleavage (Fig. 8B). Phosphatase-dead WIP1 mutant (D314A) had no significant effects in both cell lines. The results suggest that WIP1 inhibited both HR- and NHEJ-mediated DSB repair pathways, and that WIP1 inhibition is dependent on its phosphatase activity. When increasing amounts of WIP1 were expressed, the activities of DNA repair were decreased in a dose-dependent manner in both cell lines (Fig. 8C).
We also assessed HR- and NHEJ-mediated DSB repair in LB4 and TNeo99-7 cells in which WIP1 levels were inhibited by WIP1 shRNA vectors and two known WIP1 inhibitors, arsenic trioxide and CCCT007093 (53, 54). Introduction of I-SceI into all three categories of WIP1-depleted LB4 and TNeo99-7 cells consistently resulted in increased recombination frequency compared with I-SceI-containing control cells with normal WIP1 levels (Fig. 8D).
We then examined whether the effects of WIP1 are dependent on ATM in cells treated with the ATM inhibitor KU55933. H2AX was phosphorylated at serine 139 in both LB4 and TNeo99-7 cell lines in the presence of DSB caused by neocarzinostatin, whereas the total H2AX levels were unchanged (Fig. 8E, left panel). KU55933 treatment significantly reduced γ-H2AX phosphorylation, and overexpression of WIP1 had minimal additional inhibitory effects, consistent with previous reports that H2AX is mainly phosphorylated by ATM in this context (Fig. 8E, left panel). We next examined the effects of the ATM inhibitor on DSB repair in the same cells. As expected, KU55933 had marked inhibition on DSB repair, including HR and NHEJ in LB4 cells, but only had slight effects on the frequency of NHEJ in TNeo99-7 cells, suggesting ATM is a key regulator for global DSB repair, but not NHEJ repair (Fig. 8E, right panel). In KU55933-treated TNeo99-7 cells, overexpressed WIP1 further suppressed NHEJ-mediated break repair, suggesting that WIP1 may regulate NHEJ through DNA-PK pathways. A number of studies have shown that DNA-PKcs plays a key role in NHEJ (55).
DISCUSSION
The phosphorylation of H2AX at serine 139 by ATM/ATR/DNA-PKcs in response to DNA DSBs plays a critical role in the recruitment of repair factors to the site of DNA damage and the amplification of the initial damage signal (20). Once the DSB is repaired, however, the DDR must be reversed, and the repair complexes, kinases, and checkpoint proteins that enforce the DDR must be deactivated so that the cell can return to the prestress state. Obvious candidates for reversal of the ATM/ATR/DNA-PKcs-initiated kinase cascades include phosphatases. Those phosphatases that dephosphorylate the same sites phosphorylated by activated PIKKs could be important for homeostatic regulation of the DDR.
Here, we show that WIP1 phosphorylates H2AX at serine 139 in vitro and in cells, a key site targeted by ATM/ATR/DNA-PKcs in the DDR. The activity of WIP1 on this site is consistent with previous studies showing that WIP1 targets other ATM/ATR-phosphorylated DDR proteins containing the canonical pSQ or pTQ site (56). WIP1 has already been shown to dephosphorylate ATM, CHK1, CHK2, p53, and MDM2 at ATM/ATR-phosphorylated sites (10–13). In all cases so far examined, WIP1 acts to suppress activities associated with the DDR, which include kinase activities associated with engagement of the DDR (ATM, CHK, and CHK2), damage-initiated cell cycle checkpoint activities (p53, CHK1, and CHK2), and p53 anti-proliferative functions (p53 and MDM2). Thus, we have proposed that WIP1 functions as a key homeostatic regulator of the ATM/ATR/DNA-PKcs-initiated DDR (6, 14).
Consistent with this model, overexpression of WIP1 is associated with reduced formation of γ-H2AX damage foci after IR and UV irradiation, whereas WIP1 silencing is associated with enhanced formation of such foci (Fig. 5). Moreover, WIP1 suppresses migration of DSB repair complex components MDC1 and 53BP1 to damage foci (Fig. 6), which would negatively impact recruitment of other repair proteins to the damage site (26, 47, 48). Whether WIP1 directly migrates to the damage foci and dephosphorylates γ-H2AX could not be determined, because WIP1 fluorescence both before and after irradiation is pan-nuclear and inversely correlated with γ-H2AX fluorescence (Fig. 5). We can say that alteration of WIP1 levels profoundly affects the cellular pools of γ-H2AX (Fig. 2). The effects of WIP1 on H2AX are likely to be due largely to direct dephosphorylation of serine 139 by WIP1 and not to indirect effects of WIP1 dephosphorylation of ATM (10). Alteration of WIP1 levels affects γ-H2AX dephosphorylation and γ-H2AX-associated damage focus formation equally well in the absence of ATM.
Because γ-H2AX has been shown to be an early facilitator of DNA DSB repair, we examined whether DSB repair is affected by WIP1. In two assay systems that specifically measure repair of DNA DSBs, alteration of WIP1 levels strongly affects DSB repair efficiency. Reduction of WIP1 levels in MCF7 cells improved repair efficiency of a defined DSB site at 12 h after initiation of the break (Fig. 7C). Moreover, levels of γ-H2AX near the specific DSB were increased (Fig. 7B). A second assay system allowed us to examine the contributions of HR- and NHEJ-mediated events to the DSB repair process. Overexpression of WIP1 clearly inhibited both processes in DSB repair (Fig. 8). Moreover, WIP1 overexpression continued to suppress NHEJ-mediated DSB repair even when ATM signaling was inhibited, suggesting that WIP1 may also directly suppress DNA-PKcs, an important mediator of the NHEJ process (55). The inhibition of DSB repair by WIP1 is consistent with its presumptive homeostatic role in the DDR. Once the DSB is repaired, there is a need to dephosphorylate γ-H2AX and disassemble the repair complexes formed around the repaired DSB. We recognize that the role of WIP1 in DSB repair inhibition is unlikely to be solely dependent on its effects on γ-H2AX and probably results from combined effects of dephosphorylation of a number of DNA repair proteins and DDR proteins. We postulate that WIP1 dephosphorylation of γ-H2AX is merely a contributing factor in the overall homeostatic response following successful DNA repair.
With respect to phosphatases that specifically dephosphorylate γ-H2AX, it now appears that as many as four have been identified (31–34). The first such phosphatase was PP2A, which was shown to interact with H2AX and directly dephosphorylate it after treatment with DNA-damaging agents (31). Similar to the results we observed with WIP1, down-regulation of PP2A resulted in persistence of γ-H2AX damage foci. Interestingly, inhibition of PP2A resulted in more inefficient DNA repair, contrasting to the enhanced DSB repair observed when WIP1 was inhibited. The differing repair outcomes resulting from PP2A and WIP1 down-regulation argue that, aside from the fact that both phosphatases target H2AX serine 139 for dephosphorylation, each enzyme may have a different set of target proteins that result in different biological outcomes.
Recently, a three-protein PP4 phosphatase complex in mammalian cells was shown to dephosphorylate ATR-mediated γ-H2AX generated during DNA replication (32, 33). Silencing of the PP4 complex also resulted in more inefficient repair of DNA replication-mediated breaks in one study (32) and little effect on IR-damage-induced in another study (33). Again, the differing effects of PP4 and WIP1 on DNA repair are likely due to differences in target specificities and differing functional roles of the two phosphatases. WIP1 may exist primarily as a homeostatic regulator to reverse the DDR and DNA repair, whereas PP2A and PP4 more likely function to selectively facilitate DNA repair or replication.
We have shown here that WIP1 dephosphorylates γ-H2AX at serine 139 in vitro and in cells and inhibits formation of γ-H2AX damage foci. This is accompanied by suppression of DSB repair, due in part to inhibition of γ-H2AX functions. It is highly likely that, in addition to γ-H2AX, WIP1 will be shown to dephosphorylate a large number of cellular proteins involved not only in the DDR, but also a significant fraction of the proteins necessary for DSB repair.
Supplementary Material
Acknowledgments
We thank Michael Kastan for providing the I-PpoI vector system. We thank Hiroshi Yamaguchi and Ettore Appella for providing the WIP1 bacterial expression plasmid. We also thank Ivan Uray at the Baylor College of Medicine Integrated Microscopy Core for help with immunofluorescence analyses.
This work was supported, in whole or in part, by National Institutes of Health Grant CA100420 from the NCI (to L. A. D.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S5.
- DSB
- double strand break
- WIP1
- wild-type p53-induced phosphatase 1
- PPM1D
- protein phosphatase 1D magnesium-dependent, delta isoform
- ATM
- ataxia telangiectasia mutated
- ATR
- ataxia telangiectasia mutated and Rad3-related
- H2AX
- H2A histone family, member X
- MDC1
- mediator of DNA-damage checkpoint 1
- 53BP1
- tumor protein p53-binding protein 1
- DDR
- DNA damage response
- PIKK
- phosphatidylinositol 3-kinase-like protein kinase
- DNA-PKcs
- DNA-dependent protein kinase, catalytic subunit
- IR
- ionizing radiation
- BRCT
- breast cancer suppressor protein (BRCA1), C-terminal domain
- 4-OHT
- 4-hydroxytamoxifen
- HR
- homologous recombination
- NHEJ
- non-homologous end joining
- PP2A
- protein phosphatase 2, alpha isoform
- PP4
- protein phosphatase 4
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- ChIP
- chromatin immunoprecipitation
- siRNA
- small interference RNA.
REFERENCES
- 1.Abraham R. T. (2001) Genes Dev. 15, 2177–2196 [DOI] [PubMed] [Google Scholar]
- 2.Shiloh Y. (2003) Nat. Rev. Cancer 3, 155–168 [DOI] [PubMed] [Google Scholar]
- 3.Lovejoy C. A., Cortez D. (2009) DNA Repair 8, 1004–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakkenist C. J., Kastan M. B. (2004) Trends Cell Biol. 14, 339–341 [DOI] [PubMed] [Google Scholar]
- 5.Fiscella M., Zhang H., Fan S., Sakaguchi K., Shen S., Mercer W. E., Vande Woude G. F., O'Connor P. M., Appella E. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 6048–6053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu X., Nguyen T. A., Moon S. H., Darlington Y., Sommer M., Donehower L. A. (2008) Cancer Metastasis Rev. 27, 123–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barford D. (1996) Trends Biochem. Sci. 21, 407–412 [DOI] [PubMed] [Google Scholar]
- 8.Jackson M. D., Denu J. M. (2001) Chem. Rev. 101, 2313–2340 [DOI] [PubMed] [Google Scholar]
- 9.Takekawa M., Adachi M., Nakahata A., Nakayama I., Itoh F., Tsukuda H., Taya Y., Imai K. (2000) EMBO J. 19, 6517–6526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shreeram S., Demidov O. N., Hee W. K., Yamaguchi H., Onishi N., Kek C., Timofeev O. N., Dudgeon C., Fornace A. J., Anderson C. W., Minami Y., Appella E., Bulavin D. V. (2006) Mol. Cell 23, 757–764 [DOI] [PubMed] [Google Scholar]
- 11.Lu X., Nannenga B., Donehower L. A. (2005) Genes Dev. 19, 1162–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujimoto H., Onishi N., Kato N., Takekawa M., Xu X. Z., Kosugi A., Kondo T., Imamura M., Oishi I., Yoda A., Minami Y. (2006) Cell Death Differ. 13, 1170–1180 [DOI] [PubMed] [Google Scholar]
- 13.Lu X., Ma O., Nguyen T. A., Jones S. N., Oren M., Donehower L. A. (2007) Cancer Cell 12, 342–354 [DOI] [PubMed] [Google Scholar]
- 14.Lu X., Nguyen T. A., Donehower L. A. (2005) Cell Cycle 4, 1060–1064 [PubMed] [Google Scholar]
- 15.Bartkova J., Horejsí Z., Koed K., Krämer A., Tort F., Zieger K., Guldberg P., Sehested M., Nesland J. M., Lukas C., Ørntoft T., Lukas J., Bartek J. (2005) Nature 434, 864–870 [DOI] [PubMed] [Google Scholar]
- 16.Gorgoulis V. G., Vassiliou L. V., Karakaidos P., Zacharatos P., Kotsinas A., Liloglou T., Venere M., Ditullio R. A., Jr., Kastrinakis N. G., Levy B., Kletsas D., Yoneta A., Herlyn M., Kittas C., Halazonetis T. D. (2005) Nature 434, 907–913 [DOI] [PubMed] [Google Scholar]
- 17.Halazonetis T. D., Gorgoulis V. G., Bartek J. (2008) Science 319, 1352–1355 [DOI] [PubMed] [Google Scholar]
- 18.Bulavin D. V., Demidov O. N., Saito S., Kauraniemi P., Phillips C., Amundson S. A., Ambrosino C., Sauter G., Nebreda A. R., Anderson C. W., Kallioniemi A., Fornace A. J., Jr., Appella E. (2002) Nat. Genet. 31, 210–215 [DOI] [PubMed] [Google Scholar]
- 19.Nannenga B., Lu X., Dumble M., Van Maanen M., Nguyen T. A., Sutton R., Kumar T. R., Donehower L. A. (2006) Mol. Carcinog. 45, 594–604 [DOI] [PubMed] [Google Scholar]
- 20.Kinner A., Wu W., Staudt C., Iliakis G. (2008) Nucleic Acids Res. 36, 5678–5694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Durocher D., Jackson S. P. (2001) Curr. Opin. Cell Biol. 13, 225–231 [DOI] [PubMed] [Google Scholar]
- 22.Modesti M., Kanaar R. (2001) Curr. Biol. 11, R229–R232 [DOI] [PubMed] [Google Scholar]
- 23.Paull T. T., Rogakou E. P., Yamazaki V., Kirchgessner C. U., Gellert M., Bonner W. M. (2000) Curr. Biol. 10, 886–895 [DOI] [PubMed] [Google Scholar]
- 24.van Gent D. C., Hoeijmakers J. H., Kanaar R. (2001) Nat. Rev. Genet. 2, 196–206 [DOI] [PubMed] [Google Scholar]
- 25.Rogakou E. P., Boon C., Redon C., Bonner W. M. (1999) J. Cell Biol. 146, 905–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stewart G. S., Wang B., Bignell C. R., Taylor A. M., Elledge S. J. (2003) Nature 421, 961–966 [DOI] [PubMed] [Google Scholar]
- 27.Lou Z., Minter-Dykhouse K., Franco S., Gostissa M., Rivera M. A., Celeste A., Manis J. P., van Deursen J., Nussenzweig A., Paull T. T., Alt F. W., Chen J. (2006) Mol. Cell 21, 187–200 [DOI] [PubMed] [Google Scholar]
- 28.Celeste A., Fernandez-Capetillo O., Kruhlak M. J., Pilch D. R., Staudt D. W., Lee A., Bonner R. F., Bonner W. M., Nussenzweig A. (2003) Nat. Cell Biol. 5, 675–679 [DOI] [PubMed] [Google Scholar]
- 29.Celeste A., Petersen S., Romanienko P. J., Fernandez-Capetillo O., Chen H. T., Sedelnikova O. A., Reina-San-Martin B., Coppola V., Meffre E., Difilippantonio M. J., Redon C., Pilch D. R., Olaru A., Eckhaus M., Camerini-Otero R. D., Tessarollo L., Livak F., Manova K., Bonner W. M., Nussenzweig M. C., Nussenzweig A. (2002) Science 296, 922–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Celeste A., Difilippantonio S., Difilippantonio M. J., Fernandez-Capetillo O., Pilch D. R., Sedelnikova O. A., Eckhaus M., Ried T., Bonner W. M., Nussenzweig A. (2003) Cell 114, 371–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chowdhury D., Keogh M. C., Ishii H., Peterson C. L., Buratowski S., Lieberman J. (2005) Mol. Cell 20, 801–809 [DOI] [PubMed] [Google Scholar]
- 32.Chowdhury D., Xu X., Zhong X., Ahmed F., Zhong J., Liao J., Dykxhoorn D. M., Weinstock D. M., Pfeifer G. P., Lieberman J. (2008) Mol. Cell 31, 33–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakada S., Chen G. I., Gingras A. C., Durocher D. (2008) EMBO Rep. 9, 1019–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kimura H., Takizawa N., Allemand E., Hori T., Iborra F. J., Nozaki N., Muraki M., Hagiwara M., Krainer A. R., Fukagawa T., Okawa K. (2006) J. Cell Biol. 175, 389–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berkovich E., Monnat R. J., Jr., Kastan M. B. (2007) Nat. Cell Biol. 9, 683–690 [DOI] [PubMed] [Google Scholar]
- 36.Choi J., Nannenga B., Demidov O. N., Bulavin D. V., Cooney A., Brayton C., Zhang Y., Mbawuike I. N., Bradley A., Appella E., Donehower L. A. (2002) Mol. Cell Biol. 22, 1094–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borghesani P. R., Alt F. W., Bottaro A., Davidson L., Aksoy S., Rathbun G. A., Roberts T. M., Swat W., Segal R. A., Gu Y. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 3336–3341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu X., Bocangel D., Nannenga B., Yamaguchi H., Appella E., Donehower L. A. (2004) Mol. Cell 15, 621–634 [DOI] [PubMed] [Google Scholar]
- 39.Yamaguchi H., Minopoli G., Demidov O. N., Chatterjee D. K., Anderson C. W., Durell S. R., Appella E. (2005) Biochemistry 44, 5285–5294 [DOI] [PubMed] [Google Scholar]
- 40.Lu X., Nguyen T. A., Appella E., Donehower L. A. (2004) Cell Cycle 3, 1363–1366 [DOI] [PubMed] [Google Scholar]
- 41.Qin X. F., An D. S., Chen I. S., Baltimore D. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 183–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamaguchi H., Durell S. R., Chatterjee D. K., Anderson C. W., Appella E. (2007) Biochemistry 46, 12594–12603 [DOI] [PubMed] [Google Scholar]
- 43.Yaginuma Y., Westphal H. (1991) Cancer Res. 51, 6506–6509 [PubMed] [Google Scholar]
- 44.Li J., Yang Y., Peng Y., Austin R. J., van Eyndhoven W. G., Nguyen K. C., Gabriele T., McCurrach M. E., Marks J. R., Hoey T., Lowe S. W., Powers S. (2002) Nat. Genet. 31, 133–134 [DOI] [PubMed] [Google Scholar]
- 45.Cimprich K. A., Cortez D. (2008) Nat. Rev. Mol. Cell Biol. 9, 616–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bulavin D. V., Phillips C., Nannenga B., Timofeev O., Donehower L. A., Anderson C. W., Appella E., Fornace A. J., Jr. (2004) Nat. Genet. 36, 343–350 [DOI] [PubMed] [Google Scholar]
- 47.Stucki M., Clapperton J. A., Mohammad D., Yaffe M. B., Smerdon S. J., Jackson S. P. (2005) Cell 123, 1213–1226 [DOI] [PubMed] [Google Scholar]
- 48.Stucki M., Jackson S. P. (2006) DNA Repair 5, 534–543 [DOI] [PubMed] [Google Scholar]
- 49.FitzGerald J. E., Grenon M., Lowndes N. F. (2009) Biochem. Soc. Trans. 37, 897–904 [DOI] [PubMed] [Google Scholar]
- 50.Bekker-Jensen S., Lukas C., Melander F., Bartek J., Lukas J. (2005) J. Cell Biol. 170, 201–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bannister L. A., Waldman B. C., Waldman A. S. (2004) DNA Repair 3, 465–474 [DOI] [PubMed] [Google Scholar]
- 52.Smith J. A., Bannister L. A., Bhattacharjee V., Wang Y., Waldman B. C., Waldman A. S. (2007) Mol. Cell Biol. 27, 7816–7827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yoda A., Toyoshima K., Watanabe Y., Onishi N., Hazaka Y., Tsukuda Y., Tsukada J., Kondo T., Tanaka Y., Minami Y. (2008) J. Biol. Chem. 283, 18969–18979 [DOI] [PubMed] [Google Scholar]
- 54.Rayter S., Elliott R., Travers J., Rowlands M. G., Richardson T. B., Boxall K., Jones K., Linardopoulos S., Workman P., Aherne W., Lord C. J., Ashworth A. (2008) Oncogene 27, 1036–1044 [DOI] [PubMed] [Google Scholar]
- 55.Collis S. J., DeWeese T. L., Jeggo P. A., Parker A. R. (2005) Oncogene 24, 949–961 [DOI] [PubMed] [Google Scholar]
- 56.Kim S. T., Lim D. S., Canman C. E., Kastan M. B. (1999) J. Biol. Chem. 274, 37538–37543 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.