Abstract
Background
In vitro proliferative and differentiation potential of mesenchymal stromal cells generated from CD271+ bone marrow mononuclear cells (CD271-mesenchymal stromal cells) has been demonstrated in several earlier and recent reports. In the present study we focused, in addition to proliferative and differentiation potential, on in vitro and in vivo immunosuppressive and lymphohematopoietic engraftment-promoting potential of these mesenchymal stromal cells compared to bone marrow-derived mesenchymal stromal cells generated by plastic adherence (plastic adherence-mesenchymal stromal cells).
Design and Methods
We set up a series of experimental protocols in order to determine the phenotype of CD271-mesenchymal stromal cells, and their clonogenic, proliferative, differentiation and immunosuppressive potential. The potential of CD271-mesenchymal stromal cells to improve the engraftment of CD133+ hematopoietic stem cells at co-transplantation was evaluated in immunodeficient NOD/SCID-IL2Rγnull mice.
Results
In vitro studies demonstrated that CD271-mesenchymal stromal cells differentiate along adipogenic, osteogenic and chondrogenic lineages (trilineage potential), produce significantly higher levels of cytokines than plastic adherence-mesenchymal stromal cells, and significantly inhibit the proliferation of allogeneic T-lymphocytes in mixed lymphocyte reaction assays. Elevated levels of prostaglandin E2, but not nitric monoxide, mediated the majority of this immunosuppressive effect. In vivo studies showed that CD271-mesenchymal stromal cells promoted significantly greater lymphoid engraftment than did plastic adherence-mesenchymal stromal cells when co-transplanted with CD133+ hematopoietic stem cells at a ratio of 8:1 in immunodeficient NOD/SCID-IL2Rγnull mice. They induced a 10.4-fold increase in the number of T cells, a 2.5-fold increase in the number of NK cells, and a 3.6-fold increase in the number of B cells, indicating a major qualitative difference between these two mesenchymal stromal cell populations.
Conclusions
Our results indicate that CD271 antigen provides a versatile marker for prospective isolation and expansion of multipotent mesenchymal stromal cells with immunosuppressive and lymphohematopoietic engraftment-promoting properties. The co-transplantation of such cells together with hematopoietic stem cells in patients with hematologic malignancies may prove valuable in the prevention of impaired/delayed T-cell recovery and graft-versus-host disease.
Keywords: T-cell recovery, graft-versus-host disease, MSC
Introduction
Mesenchymal stromal cells (MSC) are non-hematopoietic multipotent cells that can be derived from bone marrow mononuclear cells (BM-MNC).1 However, cells with MSC-like characteristics can also be generated from adipose tissue,2,3 or other tissues such as fetal liver, lungs, spleen,4,5 amniotic fluid,6 cord blood,7 cord,8,9 placenta,10,11 human endometrium,12 and dental pulp.13 MSC demonstrate an immunomodulatory role both in vitro14–17 and in vivo,18,19 and are, therefore, able to improve the symptoms of graft-versus-host disease,20–23 autoimmune diseases,24 and degenerative diseases of the locomotor system.25 MSC can be generated either by a plastic adherence method, whereby the unknown progenitor cells adhere to plastic (referred to as PA-MSC), or by positive selection with antibodies against cell surface antigens expressed by MSC-progenitor cells.26–31
CD271, also known as low affinity nerve growth factor receptor (LNGFR) or p75NTR, belongs to the low affinity neurotrophin receptor and tumor necrosis factor receptor superfamily.32 This cell surface marker potentially defines an MSC precursor subpopulation, and may be used for the enrichment of non-hematopoietic stem cells from bone marrow aspirates27,33 and lipoaspirates.34 Colony-forming unit-fibroblast (CFU-F) activity was found only in the CD271+ cell fraction, whereas no CFU-F have been observed in the CD271– population. In addition, MSC generated from CD271+ BM-MNC showed a 1 to 3 log greater proliferative capacity than PA-MSC.27 In a comparative study, Jones et al.35 demonstrated that CD271 antigen (followed by CD146, CD106, D7-FIB, CD13, and CD166) remains one of the most selective markers for enriching progenitor cells for MSC from human bone marrow. However, no study has thus far demonstrated the overall in vitro and in vivo potential of bone marrow stromal cells derived from bone marrow CD271 (LNGFR)+mononuclear cells (referred to as CD271-MSC). The aim of the current study was, therefore, to compare the phenotype, proliferative and differentiation potential, cytokine and gene expression pattern, in vitro immunomodulatory potential, in vivo potential in long-term engraftment of hematopoietic stem cells, and multilineage differentiation of this cell type.
Design and Methods
Isolation of mobilized CD133+ cells from human peripheral blood
CD133+ cells were obtained, with informed consent, from healthy donors who were administered granulocyte colony-stimulating factor (G-CSF) to mobilize cells from the bone marrow into the peripheral blood. Low-density mononuclear cells were collected after centrifugation on a Ficoll-Paque density gradient (Biochrom, Berlin, Germany) and washed in phosphate-buffered saline (PBS). CD133 cells were isolated using the MACS cell isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany), according to the manufacturer’s instructions.
Immunomagnetic selection of CD271+ bone marrow mononuclear cells and generation of CD271-mesenchymal stromal cells
CD271+ MSC progenitor cells were isolated from bone marrow aspirates of healthy donors using a protocol approved by the University of Frankfurt Institutional Review Board. The cells were positively selected using the MSC Research Tool Box–CD271 (LNGFR)-APC (Miltenyi Biotec GmbH), according to the manufacturer’s instructions. CD271+ cells were cultured at a density of 5,000 cells/cm2 in DMEM low-glucose supplemented with 10% MSC-qualified fetal bovine serum (FBS) (Invitrogen, Karlsruhe, Germany) for roughly 1 week. Once the MSC had appeared and had grow to a confluence of 60–70%, they were trypsinized with TrypLE (Invitrogen) and further cultured at a density of 2×103 MSC/cm2 for four to five passages. PA-MSC were generated as described elsewhere,1,14 and were cultured in the same medium and at the same cell concentrations as the CD271-MSC for use as a valid control.
Colony forming unit-fibroblast assay and expansion potential of CD271-mesenchymal stromal cells
To assess the clonogenic potential of positively selected CD271+ cells and BM-MNC, the CFU-F assay was performed in 25 cm2 tissue culture flasks. To do this, 4×104 BM-MNC/cm2, 4×105 cells from the CD271-negative fraction/cm2, and 8×103 cells from the CD271-positive fraction/cm2 were cultured for 14 days. Colonies were stained with Giemsa solution (Merck, Darmstadt, Germany) and counted. In order to estimate the expansion potential of CD271-MSC, the population doubling (PD) and cumulative population doubling (CPD) levels were determined over 41 days of cell culture using the following equations:
where NH is the number of cells harvested and NI is the number of inoculated cells; CPD for 41 days of culture: ∑(PD).
Immunophenotyping of expanded CD271-mesenchymal stromal cells and their differentiation capacity
CD271-MSC and PA-MSC from passage 4 were stained with monoclonal antibodies conjugated to fluorescein isothiocyanate, phycoerythrin, peridinin chlorophyll protein, or allophycocyanin. The antibodies CD14, CD29, CD34, CD44, CD45, CD73, CD90, HLA-A, HLA-B, HLA-C, HLA-DR, CD146, and CD166 were purchased from BD Pharmingen (Heidelberg, Germany), CD105 was purchased from Caltag-Invitrogen, and CD133-2 and CD271 were purchased from Miltenyi Biotec. Fluorochrome-conjugated mouse immunoglobulins were used as isotype controls. The stained cells were then analyzed on a FACSCalibur (Becton-Dickinson) equipped with Macintosh software for data analysis (CellQuest).
In order to study the differentiation of adipogenic, osteogenic, and chondrogenic lineages, MSC of passage 4 were cultured in tissue-specific media according to the manufacturer’s instructions (Miltenyi Biotec GmbH). Stained slides were examined on an Olympus IX71 microscope (Olympus, Hamburg, Germany) equipped with a Soft Imaging System F-View II camera and Cell^P imaging software.
Genetic profiling of CD271-mesenchymal stromal cells
The genetic profile of CR271-MSC was determined by reverse transcription polymerase chain reaction (RT-PCR) analysis. The RT-PCR conditions and primers for typical lineage-specific genes were used as previously described.36
Mitogenic stimulation of peripheral blood mononuclear cells
Peripheral blood mononuclear cells (PB-MNC) from healthy donors were isolated by gradient centrifugation, plated in triplicate in 96-well plates at a concentration of 105 cells/100 μL in RPMI 1640 with 10% FBS (Invitrogen), and stimulated with the following mitogens: phytohemagglutinin-P (10 μg/mL), concanavalin A (5 μg/mL), pokeweed mitogen (10 μg/mL), and staphylococcal enterotoxin B (1 μg/mL). All mitogens were purchased from Sigma (Sigma-Aldrich, Munich, Germany), with the exception of the cytokine interleukin-2 (IL-2) (500 IU/mL), which was purchased from PeproTech (PeproTech GmbH, Hamburg, Germany). Non-proliferative, lethally irradiated MSC (30 Gy) were resuspended in 100 μL of RPMI 1640 with 10% FBS, and were added to the total number of responder MNC at a ratio of 1:1. Cultures were incubated at 37°C in 5% CO2 for 5 days, and then labeled with BrdU (Roche Diagnostics GmbH, Mannheim, Germany) for 24 h. Relative light units (RLU/sec) were measured the following day using the luminometer 1420 Multilabel Counter Victor3 (Perkin Elmer, Rodgau–Jügesheim, Germany). The inhibitory effect of MSC on the proliferation of PB-MNC was calculated using the formula:
Prostaglandin E2 determination in the supernatants of mixed lymphocyte reactions
To test the immunosuppressive effect of CD271-MSC on the allogeneic reaction, PB-MNC from two unrelated donors were cultured for 5 days either alone (control group) or mixed with third party, lethally-irradiated MSC at a ratio of 1:1 (105 PB-MNC: 105 MSC). The experiment was performed with four unrelated MSC – PB-MNC donor pairs. The level of proliferation of PB-MNC was determined on day 6 by means of the BrdU assay, as described above. For the prostaglandin E2 inhibition experiments, MSC and allogeneic PB-MNC were cultured in the presence or absence of the prostaglandin E2 inhibitor, indomethacin (5 μM; Sigma). The levels of prostaglandin E2 in the cell culture supernatant were determined with a fluorescent FPIA kit, according to the manufacturer’s instructions (Biomol GmbH, Hamburg, Germany).
Cytokine profile determination
For quantitative analysis of IL-1β, IL-2, IL-3, IL-4, IL-6, IL-8, IL-10, IL-12, G-CSF, granulocyte-monocyte colony-stimulating factor (GM-CSF), monocyte chemoattractant protein-1 (MCP-1), interferon- gamma (IFN-γ), and tumor necrosis-alpha (TNF-α), the BD Cytometric Bead Array Flex system (CBA-Flex system) from BD Biosciences (Heidelberg, Germany) was used. The supernatants from passage 4 of both types of MSC were collected and analyzed with BD FACSArray equipment (BD Heidelberg, Germany), according to the manufacturer’s recommendations.
Co-transplantation of hematopoietic CD133+ cells and CD271-mesenchymal stromal cells in immunodeficient NOD/SCID knock-out mice
Immunodeficient NOD/SCID knock-out mice for the gamma chain of the IL-2 receptor (NOD/LtSz-scid IL2Rγnull) were purchased from Jackson Laboratories (MA, USA) and were maintained in microisolator cages under specific pathogen-free conditions. They were sublethally irradiated (300 cGy) 24 h before transplantation. To assist in the identification of human cells after transplantation into mice, green fluorescent protein (GFP) was introduced into passage 4 CD271-MSC and PA-MSC via lentiviral vectors.37 Transduced MSC, together with highly enriched mobilized peripheral blood CD133+ cells, were administered to the sublethally irradiated immunodeficient mice via an intravenous injection. The first group of mice was given CD133+ cells (1×105) only, whereas the other groups were co-transplanted with either CD271-MSC or PA-MSC at a ratio of 1:1 (105 CD133+ cells and 105 MSC) or 1:8 (105 CD133+ cells and 7×105 MSC). All procedures were approved by the Animal Care Committee of Frankfurt am Main University and the Regierungspräsidium Darmstadt (Gen. Nr. F. 133/06).
Engraftment analysis of human CD133+ cells and CD271-msenechymal stromal cells in different organs of NOD/SCID mice
Multiparameter flow cytometric analysis
Mice were sacrificed 14 weeks after co-transplantation of hematopoietic and mesenchymal cells. Engraftment of human CD133+ hematopoietic stem cells in the bone marrow was analyzed as previously described.38 Briefly, bone marrow cells were collected separately from each tibia and femur, mouse erythrocytes were lysed in lysis buffer (Mouse Erythrocyte Lysing Kit, R&D Systems, Wiesbaden, Germany), and washed twice with RPMI medium supplemented with 5% FBS. Cell aliquots were used to examine the percentages of human CD45, CD3, CD19, CD56, CD41a, and CD33-expressing cells by four-color flow cytometric analysis. The proportion of each lineage was calculated from at least 5×105 to 1×106 events acquired using CellQuest software (Becton Dickinson, San Jose, CA, USA).
In situ analysis of mouse organs to detect resident human cells
In order to track in vivo human GFP-positive MSC in different organs, frozen tissue sections were washed twice with PBS, fixed with 4% paraformaldehyde for 10 min, and permeabilized with 0.1% Triton X-100 in PBS containing 3% BSA. After 30 min of pre-incubation at room temperature, the slides were stained with an anti-GFP primary antibody (1:400, Molecular Probes, Invitrogen, Germany) for 60 min at room temperature. Unbound anti-GFP antibodies were removed by washing the slides three times with PBS. The samples were incubated with anti-rabbit Alexa Fluor 488-conjugated secondary antibodies (1:1000, Molecular Probes) and phycoerythrin-labeled anti-human HLA-A, HLA-B, and HLA-C antibodies (1:100) (BD Biosciences) for another 60 min at room temperature in the dark. After washing with PBS and mounting on coverslips in VectaShield mounting medium containing 4,6-diamino-2-phenylindole (DAPI) (Biozol Diagnostica Vertrieb GmbH, Eching, Germany), the results were evaluated using an Olympus IX71 microscope.
Quantification of human cells in the organs of NOD/SCID mice
Fourteen weeks after transplantation, tissues were harvested, digested with collagenase or dispase, and processed for DNA polymerase chain reaction. Genomic DNA of organs was extracted using the QIAamp blood and tissue kit (Qiagen, Hilden, Germany). The presence of human-specific DNA in the organs of transplanted mice was confirmed by real-time polymerase chain reaction amplification of the human albumin gene.39 The specificity of primers was assessed by amplifying chromosomal mouse DNA.
Statistics
Statistical significance was analyzed using GraphPad Prism 5 software (GraphPad Software, San Diego, CA, USA). Significance was assessed with a Mann-Whitney rank sum or Student’s t test. A P value less than 0.05 was considered statistically significant.
Results
Phenotypic characterization of CD271+ bone marrow mononuclear cells, and clonogenic and proliferative potential of CD271-mesenchymal stromal cells
To determine the phenotype of CD271+ MSC progenitor cells, BM-MNC were stained with monoclonal anti-bodies against typical MSC antigens. Gating on all CD271+ BM-MNC (Online Supplementary Data, Figure S1A), we found that most of these cells (60–95%) expressed CD29, HLA-A, HLA-B, HLA-C, HLA-DR, and CD166. A considerable proportion of cells (20–30%) expressed CD105, CD15, CD13, SSEA-1, CD184, CD56, CD34, and CD133. However, only a small proportion of cells (10–20%) expressed CD146, CD73, CD90, and early stage-specific embryonic antigen-4 (SSEA-4) (Online Supplementary Data, Figure S1B). As assessed by flow cytometry, the frequency of CD271+ cells in our analyzed sample of BM-MNC fractions was 0.94±0.2% (range, 0.2% to 2.5%; median 0.7%). Using a CD271 MicroBead Kit (APC) (Miltenyi Biotec), we positively selected for CD271+ BM-MNC with an average purity of 77.6±6.6% (range, 40% to 97.3%) (Online Supplementary Data, Figure S1C). Colony-forming efficiency assays were conducted for enriched CD271+ BM-MNC. An average of 0.3 ± 0.2 CFU-F/102 cells was observed in the CD271+ fraction, translating into an enrichment of 1.5×102 compared to BM-MNC and a recovery of approximately 90% of the total CFU-F found in unfractionated bone marrow. No colonies were formed by CD271− cells plated at the same density (Online Supplementary Data, Figure S1D). Estimation of population doublings (n=3) over a period of 41 days revealed an approximately one log higher proliferative potential of CD271-MSC compared to PA-MSC (Online Supplementary Data, Figure S1E).
Phenotype and differentiation potential of the expanded CD271-mesenchymal stromal cells
Passage 4 CD271-MSC expressed typical mesenchymal cell surface markers, such as CD73, CD90, CD105, CD146, CD166, and HLA class I molecules. However, they were negative for the hematopoietic cell markers CD14, CD45, CD34, and CD133, as well as CD184, HLA-DR, and SSEA-4 (Figure 1A). Culture of the expanded CD271-MSC in tissue-specific media demonstrated that their differentiation potential along adipogenic, osteogenic, and chondrogenic lineages (Figure 1Bi–iii) was as effective as that of PA-MSC (Figure 1Ci–iii). RT-PCR expression analysis of the genes involved in trilineage differentiation confirmed the multi-potentiality of CD271-MSC (Online Supplementary Data, Figure S2A).
Figure 1.
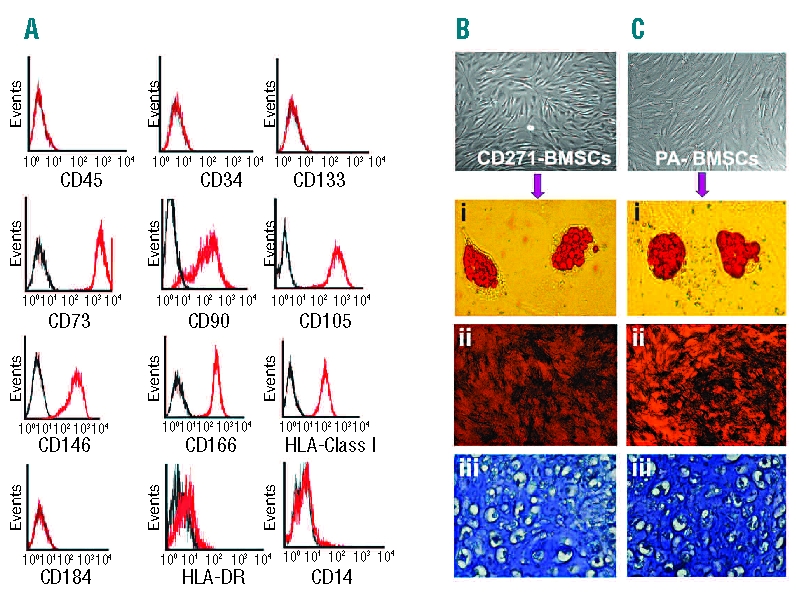
Phenotype of ex vivo expanded CD271- MSC and their in vitro differentiation potential. (A) CD271-MSC express high levels of all typical MSC markers such as CD73, CD90, CD146, CD166 and CD105. They also express HLA- class I antigens but do not express HLA-DR, CD45, CD14, CD184, or hematopoietic stem cells markers (CD34 and CD133). (B) Differentiation potential of CD271-MSC and PA-MSC (C). Like PA-MSC (Ci-iii) these MSC differentiate into adipocytes (Bi), osteoblasts (Bii) and chondrocytes (Biii). Accumulation of intracellular lipid vacuoles was shown by oil red-O staining for 18 days in culture with NH AdipoDiff medium, while osteoblast differentiation was detected by alkaline phosphatase activity after 10 days in NH OsteoDiff medium. Cartilage matrix deposition along with chondrocytes in lacunae was demonstrated by metachromatic toluidine blue staining after 24 days in NH ChondroDiff medium. Magnification for microphotographs of MSC and osteoblasts was 20x; the magnification for adipocytes was 200x and for chondrocytes 400x.
Cytokine profile of CD271-mesenchymal stromal cells
Comparison of the steady state levels of cytokines that are involved in hematopoietic cell growth (IL-2, G-CSF, GM-CSF), pro-inflammatory cytokines (IFN-γ, TNF-α, IL-1β, IL-8, IL-12p70, MCP-1) which can mediate the immunosuppressive effect of MSC (e.g. IFN-γ through induction of IDO enzyme) and cytokines with anti-inflammatory activity (IL-4, IL-10) in the supernatants of passage 4 MSC demonstrated that CD271-MSC secreted significantly higher levels (P<0.05) of 11 out of the 12 cytokines than did PA-MSC; the only exception was for the secretion of IL-6 (Online Supplementary Data, Figure S2B).
In vitro immunoregulatory potential of CD271-mesenchymal stromal cells
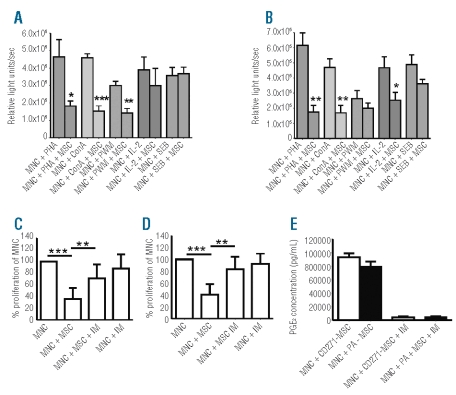
To determine whether CD271-MSC may affect the proliferative response of PB-MNC to mitogenic stimulation, both types of lethally irradiated MSC were cultured with these cells at a 1:1 ratio (Figure 2A). Third-party CD271-MSC markedly inhibited the proliferation of PB-MNC after mitogenic stimulation with phytohemagglutinin (41.3±2.9% of control, P<0.03) and concanavalin-A (34.3±8.0% of control, P<0.0001) as specific mitogens for the stimulation of T cells and pokeweed mitogen (48.1±9.1% of control, P<0.002) as a mitogenic stimulator of both T and B cells. In contrast, they were unable to inhibit the proliferation of PB-MNC after stimulation with the superantigen staphylococcal enterotoxin B (108.8±15.6% of control, P<0.9) like PA-MSC (Figure 2B).
Figure 2.
The effect of CD271-MSC and PA-MSC on proliferation of mitogen-activated lymphocytes and allogeneic reaction. The figure shows the inhibitory effect of the CD271-MSC population (A) and PA-MSC population at passage 4 at the ratio 1:1 (B). In each experiment, 100,000 peripheral blood lymphocytes were stimulated in triplicate with phytohemagglutin (PHA), concanavalin A (con-A), pokeweed mitogen (PWM), interleukin- 2 (IL-2) and staphylococcal enterotoxin B (SEB) in the presence or absence (control group) of lethally irradiated mesenchymal stromal cells. The values represent the mean of the triplicate experiments ± standard error of mean (SEM) (n=5). Statistically significant differences between groups were assessed with a Student’s ttest. P values of less than 0.05 were considered to be statistically significant: *P<0.02; **P<0.003; P<0.0001. Inhibition of allogeneic reaction through CD271- MSC (C) and PA-MSC (D). PB-MNC of two unrelated donors were cultured for 5 days either alone (control group) or mixed with lethally irradiated third-party MSC at the ratio of 1:1 (105 PB-MNC: 105 MSC). For the prostaglandine E2 (PGE2) inhibition experiments, the MSC were cultured with MNC in the presence or absence of the PGE2 inhibitor, indomethacin (IM, final concentration: 5 μM). Proliferation levels of PB-MNC were determined on day 6 by means of the BrdU assay. The significance of inhibition of proliferation of allogeneic cells by MSC and abrogation of this effect by indomethacin was assessed with a Student’s t test. P values of less than 0.05 were considered to be statistically significant: **P<0.005; P<0.0001. The experiment was performed with four unrelated MSC-PBMC donor pairs. The inhibitory effect of CD271-MSC or PA-MSC correlated very well with the elevated levels of PGE2 in the supernatants of mixed lymphocyte reactions containing MNC and CD271-MSC or MNC and PA-MSC (E).
Next, we asked whether there were any differences between CD271-MSC and PA-MSC in the suppression of proliferation of PB-MNC as a result of an allogeneic reaction in mixed lymphocyte reactions. Our results demonstrate that both CD271-MSC and PA-MSC significantly inhibited this proliferation (35.6±6.6% of control proliferation, P<0.0001 and 41.5±5.5%, P<0.006, respectively) (Figure 2C and D). As prostaglandin E2 has been shown to modulate a wide variety of immune functions in vitro,14 we examined whether inhibiting the production of prostaglandin E2 leads to reversal of this MSC-mediated inhibition. The data depicted in Figure 2C demonstrate that indomethacin (an inhibitor of prostaglandin E2 synthesis) abrogates roughly 40% of both CD271-MSCmediated inhibition (P<0.005) and PA-MSC-mediated inhibition (P<0.003) (Figure 2D). Measurement of prostaglandin E2 in the supernatants demonstrated elevated levels of this molecule in the wells in which CD271- MSC and PA-MSC were in contact with unrelated PB-MNC and inhibited their proliferation (Figure 2E).
Multilineage engraftment of hematopoietic CD133+ stem cells after co-transplantation with CD271-mesenchymal stromal cells
Several lines of evidence suggest that PA-MSC induce improved hematopoietic engraftment. However, thus far there is no evidence regarding the capacity of CD271- MSC on multilineage engraftment of hematopoietic stem cells or their differentiation potential in vivo. Flow cytometric analysis using an anti-human CD45 antibody demonstrated significantly improved engraftment of hematopoietic CD133+ cells in the bone marrow of NOD/SCID mice co-transplanted with either CD271- MSC (2.9±0.9%, P<0.01) or PA-MSC (2.0±0.5%, P<0.02) at a ratio of 1:1 in comparison to that in a control group of mice transplanted with CD133+ cells only (0.3±0.02%) (Figure 3A). Both types of MSC at this ratio showed similar effects; the number of myeloid (CD33+) cells increased between 2- and 3-fold, whereas the number of megakaryocytes (CD41a+) remained at levels comparable to those present in the control group. It is noteworthy that, at this ratio, both types of co-transplanted MSC increased the number of cells with a B-cell phenotype (CD19+) about 8- to 9-fold (15.4±8.1% when CD271-MSC were co-transplanted and 13.3±4.2% when PA-MSC were co-transplanted) compared with the number in the control group (1.7±0.7%). Furthermore, neither type of MSC at this ratio showed an effect on the development of cells with a T-cell (CD3+), NK-cell (CD56+), or NKT-cell (CD3+CD56+) phenotype from engrafted CD133+ cells. In contrast, co-transplantation of CD133+ cells with PA-MSC at a ratio of 1:8 (Figure 3C) induced greater overall engraftment of the hematopoietic cells in the bone marrow compared to CD271-MSC (Figure 3D) and to the control group (Figure 3B). Both types of MSC transplanted at this ratio doubled the number of myeloid cells (CD33+) and megakaryocytes (CD41a+) in comparison to the group transplanted with CD133+ cells alone. However, co-transplantation of CD133+ hematopoietic cells with CD271-MSC at a ratio of 1:8 induced their multilineage differentiation by giving rise to cells with a lymphoid lineage phenotype. The numbers of cells with a T-cell and B-cell phenotype were roughly 7-fold (P<0.01) and 4-fold higher than those in the control group (Figure 3E). The number of NK cells decreased significantly (Figure 3F) to the normal levels compared to the control group, thereby demonstrating the expansion (P<0.0005) and suggesting that CD271-MSC may control the in vivo proliferation of these cells. In contrast, PA-MSC failed to give rise to the lymphoid lineage to the same extent as CD271-MSC, indicating a major qualitative difference between these two MSC populations.
Figure 3.
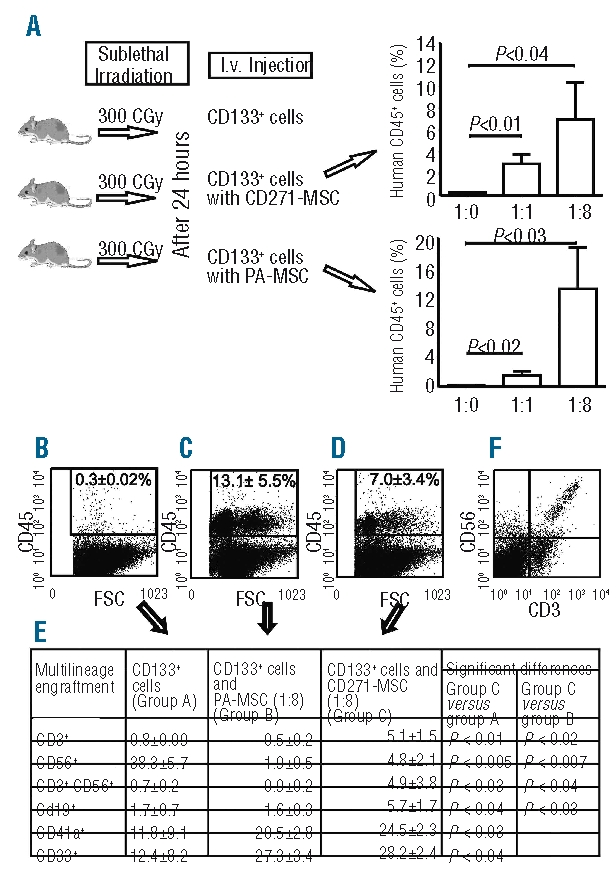
Hematopoietic engraftment of human CD133+ cells in bone marrow of NOD/SCID IL-2Rγnull mice. (A) Experimental design of the in vivo studies. Five groups of NOD/SCID IL-2Rγnull mice (5 mice each) were sublethaly irradiated and on the next day transplanted with 100,000 human CD133+ cells only (control group), 100,000 CD133+ cells and 100,000 human GFP-transduced CD271- MSC (ratio 1:1) and the other group with 100,000 CD133+ cells and 700,000 human GFP-transfected CD271-MSC (ratio 1:8). At the same ratios 100,000 CD133+ cells were transplanted with GFP-transduced PA-MSC. The overall human cell engraftment in the bone marrow (as measured by anti-human CD45 immunostaining) 14 weeks post-transplantation is shown in the right panel. The top right panel shows the graph for co-transplantation of CD133+ cells with CD271-MSC; the bottom right panel shows co-transplantation of CD133+ cells with PA-MSC. (B) A representative dot plot of the group transplanted with 100,000 mobilized peripheral blood CD133+ cells only, the group co-transplantated with PA-MSC at the ratio 1:8 (C) and the group co-transplanted with CD271-MSC at the ratio 1:8 (D). (E) Multilineage differentiation of CD133+ without or with co-transplantation of CD271-MSC or PA-MSC, respectively. The percentage of positive cells for each lineage marker was determined by gating on human CD45+ cells as shown in panels B, C and D. Differences in the engraftment level of each cell population between the groups were tested by Student’s t-test, A P value of less than 0.05 was considered to be statistically significant. (F) Representative flow cytometric data on the engraftment of human T, NK and NKT cells in the group transplanted with 105 CD133+ cells and 7x105 CD271-MSC (1:8 ratio).
Distribution of human cells in the organs of co-transplanted NOD/SCID mice
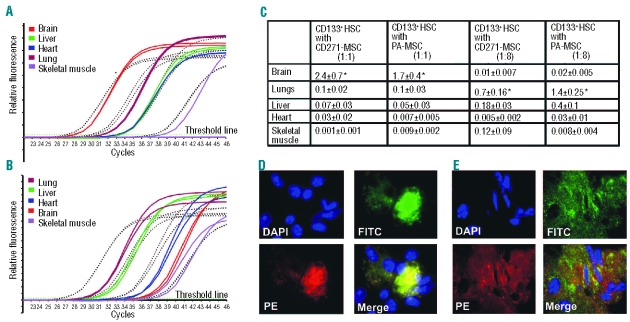
Real-time polymerase chain reaction analysis of organs from the mice co-transplanted with CD133+ hematopoietic stem cells and CD271-MSC or PA-MSC demonstrated a detectable presence of human DNA in all organs (Figure 4A–C). When both types of MSC were transplanted with hematopoietic stem cells at a ratio of 1:1, most of the human DNA was detected in the brain and, to a lesser extent, in the lungs, liver, heart, and skeletal muscle. Approximately 2% of the human DNA in immunodeficient mice was detected in the brain. In contrast to this finding, co-transplantation of CD133+ hematopoietic stem cells with MSC at a ratio of 1:8 was associated with an increase in human DNA in the lungs, suggesting the possible entrapment of human cells in this organ. When hematopoietic stem cells were co-transplanted with CD271-MSC or PA-MSC at a ratio of 1:8, the amount of human DNA in the lungs represented about 0.7±0.16% or 1.4±0.25%, respectively. In addition to the lungs, the second organ in which human DNA was detected at significant quantities was the liver; very low amounts of human DNA were detected in the brain, heart, and skeletal muscle (Figure 4C). In contrast, real-time polymerase chain reaction analysis of mouse organs transplanted with different doses of CD271-MSC or PA-MSC separately revealed consistently higher amounts of human DNA in the lungs, and very low levels in the liver, heart, and skeletal muscle. No human DNA was detected in the brain of the mice (Online Supplementary Data, Table S1), indicating a typical migration pattern of MSC with lung entrapment of these cells.
Figure 4.
Distribution of human cells in the tissues of NOD/SCID mice 14 weeks after transplantation. (A) Real-time PCR amplification of human albumin gene in tissue samples of mice co-transplanted with human CD133+ cells and CD271-MSC at a ratio of 1:1. (B) Real-time PCR amplification of human albumin gene in tissue samples of mice co-transplanted with human CD133+ cells and CD271-MSC at a ratio 1:8. Standard curve achieved from serial dilutions of human DNA in mouse DNA (10−1 to 10−4) is depicted with dashed lines. (C) Organ distribution of human cells in mouse tissues. Fourteen weeks after transplantation genomic DNA was extracted and the presence of human-specific DNA in the organs of transplanted mice was confirmed by real-time polymerase chain reaction (RQ-PCR) for human albumin gene. The values represent calculated mean percentages of human DNA ± SD of four experiments. Significant differences were observed in the brain of groups co-transplanted with HSC and MSC at the 1:1 ratio as compared with the content of human DNA in the brain of groups co-transplanted with HSC and MSC at a 1:8 ratio (*P<0.04 when HSC were co-transplanted with CD271-MSC and *P<0.01 when HSC were co-transplanted with PA-MSC). In contrast, when HSC were co-transplanted with MSC at a 1:8 ratio a significantly higher amount of human DNA was observed in the lungs as compared to the content of human DNA in the same organ of mice co-transplanted with HSC and MSC at a 1:1 ratio (*P<0.01 when HSC were co-transplanted with CD271-MSC and *P<0.04 when HSC were co-transplanted with PA-MSC) (D) and (E) A representative immunostaining of GFP-transduced CD271-MSC in the tissue sections with phycoerythrin (PE)-conjugated mouse anti-human HLA-class I and Alexa 488-conjugated anti-GFP antibody. Localization of MSC-derived cells in the lungs (D) of mice co-transplanted with 7x105 CD271-MSC and 105 CD133+ HSC and brain (E) when CD271-MSC were co-transplanted with CD133+ HSC at a ratio of 1:1 (magnification 600x).
To visualize human cells and to better discriminate between hematopoietic or non-hematopoietic cells (MSC) within mouse organs, a monoclonal anti-human HLA-class I antibody highly selective for human cells was used. Since co-transplanted MSC were transduced with GFP prior to transplantation, counterstaining was performed with a fluorescein isothiocyanate-conjugated anti-GFP antibody. Hence, cells stained with the anti-HLA-class I antibody alone were considered as hematopoietic, and cells stained with both antibodies were considered as nonhematopoietic (i.e., MSC, Figure 4D, E).
Discussion
Most of the researchers agree that one of the drawbacks of generating MSC through plastic adherence is the unknown MSC progenitor cell and the derivation of a very heterogeneous cell population with regard to proliferative and differentiation potentials. Since the origin of MSC still remains elusive, there is an increasing need for novel markers and methods of detection, enumeration, and isolation of MSC progenitor cells from the bone marrow and other tissues as a prerequisite for establishing stringent protocols for the clinical-scale generation of MSC without any hematopoietic contamination.26–31,40
In the present study, we investigated the proliferative, differentiation, and immunomodulatory potentials of CD271-MSC both in vitro and in vivo. In the CFU-F assay, only enriched CD271+ BM-MNC generated colonies, whereas no CFU-F were obtained from the CD271-negative cell fraction. This finding indicates that the CD271-positive cell fraction comprises the majority of MSC progenitors. In addition, polymerase chain reaction analysis demonstrated that CD271-MSC, like PA-MSC, express adipogenic, osteogenic, and chondrogenic genetic markers and differentiate in vitro into adipocytes, osteocytes and chondrocytes, indicating their trilineage potential.
There are, however, no data so far on whether CD271-MSC share at least the same in vitro and in vivo immunosuppressive and engraftment-promoting properties with PA-MSC14,15,18,19,41 Our in vitro data demonstrated that CD271-MSC strongly inhibited the mitogenic-induced proliferation of PB-MNC after stimulation with concanavalin-A (65.7±8.2% inhibition), phytohemagglutinin (58.7%±2.9%), or pokeweed mitogen (51.9±9.1%). In addition, third-party CD271-MSC, like PA-MSC, significantly inhibited the proliferation of PB-MNC as a result of an allogeneic reaction in the two-way mixed lymphocyte reaction. Addition of a specific inhibitor of prostaglandin E2 synthesis abrogated roughly 40% of MSC-mediated inhibition, suggesting that other molecules may be involved in the immunosuppressive effect of CD271-MSC and PA-MSC.
MSC play an important role in supporting hematopoiesis through their adhesion/interaction with hematopoietic stem cells and secretion of growth factors necessary for their differentiation.42 Our results demonstrate that CD271-MSC secreted a series of molecules with immunosuppressive (IL-10), pro-inflammatory (IFN-γ, TNF-α), chemoattractant (MCP-1, IL-8, IL-1β), and differentiation (G-CSF, GM-CSF) activities as well as T-cell growth factor (IL-2) at significantly higher levels than PA-MSC; the only cytokine investigated that was secreted at lower levels by CD271-MSC was IL-6. These molecules may orchestrate the improved in vivo engraftment and differentiation of hematopoietic CD133+ cells not only to myeloid but also to the lymphoid lineage after their co-transplantation with CD271-MSC.
An emerging body of evidence indicates that co-transplantation of PA-MSC with human CD34+ cells facilitates their engraftment and induces a shift in their differentiation from predominantly B lymphocytes to predominantly enhanced myelopoiesis (CD13+, CD14+, and CD33+ cells) and megakaryocytopoiesis.43 We asked whether CD271-MSC share the same properties with PA-MSC or may be more efficient in modulating hematopoietic engraftment. Our in vivo data revealed that co-transplantation of CD133+ cells with CD271-MSC significantly improved their multilineage engraftment in the bone marrow of immunodeficient NOD/SCID mice at both 1:1 (about 10-fold) and 1:8 (about 23-fold) ratios in comparison to transplantation of CD133+ cells alone. We found an approximately 2- to 3-fold increase in the number of myeloid cells (CD33+) and enhanced megakaryopoiesis (CD41a+), especially at the 1:8 ratio (about 2-fold more megakaryocytes); these findings indicate the differentiation of CD133+ cells towards more primitive CD45+CD41a+ megakaryocytes.44 In contrast to the above studies, however, co-transplantation of CD133+ hematopoietic stem cells with CD271-MSC at a ratio of 1:8 promoted their differentiation towards the lymphoid lineage, in addition to megakaryopoiesis and myelopoiesis. Remarkably, the number of CD3+ cells increased 10.4-fold (P<0.02), the number of NK cells increased 2.5-fold (P<0.0007) and the number of B cells increased 3.6-fold (P<0.03), compared to the group transplanted with CD133+ hematopoietic stem cells and PA-MSC at the same ratio. In addition, CD271-MSC decreased the number of CD56+ cells to approximately normal levels, whereas in the control group an expansion of these cells was observed. This finding is in agreement with data demonstrating that MSC may alter the phenotype of NK cells and suppress proliferation, cytokine secretion, and cytotoxicity against HLA class I-expressing targets.45 Taken together, our data demonstrate that, in contrast to PA-MSC, CD271-MSC are able not only to improve the engraftment of hematopoietic stem cells, but also to promote their differentiation into myeloid and lymphoid lineages. After co-transplantation with human CD34+ hematopoietic cells in children with hematologic malignancies, PA-MSC induced a sustained engraftment that, nonetheless, did not promote any significant T- or NK-cell recovery relative to the control group transplanted with hematopoietic stem cells alone.46 Based on our findings, we assume that CD271-MSC offer a better alternative to PA-MSC for post-transplant T-cell reconstitution, which substantially contributes to the successful outcome of hematopoietic stem cell transplantation.
Systemic delivery of MSC has been reported by several groups, who found that the majority of cells were entrapped in the lungs and a small amount engrafted in the heart, liver, and spleen.47,48 Data obtained from experiments in which CD271-MSC or PA-MSC were individually transplanted into NOD/SCID mice via an intravenous route revealed a similar organ distribution, with the majority of the cells being entrapped in the lungs. When expanded CD271-MSC or PA-MSC were co-transplanted with hematopoietic stem cells at a ratio of 1:1, however, quantitative polymerase chain reaction analysis for the human albumin gene demonstrated that the cells migrated more efficiently to brain than to the lungs, liver, heart, or skeletal muscle. In contrast, when co-transplanted with hematopoietic stem cells at a ratio of 8:1, CD271-MSC or PA-MSC migrated mainly to the lungs and, to a much lesser extent, to the liver, brain, heart, and skeletal muscle. Our data, therefore, suggest that not only can MSC influence hematopoietic stem cell engraftment but also that hematopoietic stem cells may affect the migration and homing pattern of MSC in vivo.
In conclusion, based on the immunosuppressive and engraftment-promoting properties toward the lymphoid lineage (i.e., T, NK, NKT, and B cells), we assume that cotransplantation of CD271-MSC with hematopoietic stem cells in patients with hematologic malignancies may prove valuable in improving both impaired/delayed T-cell recovery and graft-versus-host disease after transplantation.
Acknowledgments
the authors would like to thank Miriam Reising, Vida Meyer and Ralf Lieberz for their excellent technical assistance and Christian Brändel for his expert assistance with microscopy. Funding: this work was supported by Wilhelm Sander Stiftung (No 2006.067.1) (P.B. and S.K.) and “Hilfe für krebskranke Kinder Frankfurt e.V.“.
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
SK and ZK designed and performed research and wrote the paper, HK, ED, KP, SH, ChSA, SKo, ER, MG, and HLP performed research and analyzed data. UK, RH, TT and DvL provided reagents and analyzed data. TK and PB analyzed and discussed data and assisted with the preparation of the manuscript.
No potential conflicts of interests relevant to this article were reported.
References
- 1.Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3(4):393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 2.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13(12):4279–95. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dicker A, Le Blanc K, Aström G, van Harmelen V, Götherström C, Blomqvist L, et al. Functional studies of mesenchymal stem cells derived from adult human adipose tissue. Exp Cell Res. 2005;308(2):283–90. doi: 10.1016/j.yexcr.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 4.Campagnoli C, Roberts IA, Kumar S, Bennett PR, Bellantuono I, Fisk NM. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 2001;98(8):2396–402. doi: 10.1182/blood.v98.8.2396. [DOI] [PubMed] [Google Scholar]
- 5.in 't Anker PS, Noort WA, Scherjon SA, Kleijburg-van der Keur C, Kruisselbrink AB, van Bezooijen RL, et al. Mesenchymal stem cells in human second-trimester bone marrow, liver, lung, and spleen exhibit a similar immunophenotype but a heterogeneous multilineage differentiation potential. Haematologica. 2003;88(8):845–52. [PubMed] [Google Scholar]
- 6.In 't Anker PS, Scherjon SA, Kleijburg-van der Keur C, Noort WA, Claas FH, Willemze R, et al. Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood. 2003;102(4):1548–9. doi: 10.1182/blood-2003-04-1291. [DOI] [PubMed] [Google Scholar]
- 7.Bieback K, Kluter H. Mesenchymal stromal cells from umbilical cord blood. Curr Stem Cell Res Ther. 2007;2(4):310–23. doi: 10.2174/157488807782793763. [DOI] [PubMed] [Google Scholar]
- 8.Qiao C, Xu W, Zhu W, Hu J, Qian H, Yin Q, et al. Human mesenchymal stem cells isolated from the umbilical cord. Cell Biol Int. 2008;32(1):8–15. doi: 10.1016/j.cellbi.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Secco M, Zucconi E, Vieira NM, Fogaça LL, Cerqueira A, Carvalho MD, et al. Mesenchymal stem cells from umbilical cord: do not discard the cord! Neuromuscul Disord. 2008;18(1):17–8. doi: 10.1016/j.nmd.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Miao Z, Jin J, Chen L, Zhu J, Huang W, Zhao J, et al. Isolation of mesenchymal stem cells from human placenta: comparison with human bone marrow mesenchymal stem cells. Cell Biol Int. 2006;30(9):681–7. doi: 10.1016/j.cellbi.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 11.In 't Anker PS, Scherjon SA, Kleijburg-van der Keur C, de Groot-Swings GM, Claas FH, Fibbe WE, Kanhai HH. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells. 2004;22(7):1338–45. doi: 10.1634/stemcells.2004-0058. [DOI] [PubMed] [Google Scholar]
- 12.Schwab KE, Gargett CE. Co-expression of two perivascular cell markers isolates mesenchymal stem-like cells from human endometrium. Hum Reprod. 2007;22(11):2903–11. doi: 10.1093/humrep/dem265. [DOI] [PubMed] [Google Scholar]
- 13.Pierdomenico L, Bonsi L, Calvitti M, Rondelli D, Arpinati M, Chirumbolo G, et al. Multipotent mesenchymal stem cells with immunosuppressive activity can be easily isolated from dental pulp. Transplantation. 2005;80(6):836–42. doi: 10.1097/01.tp.0000173794.72151.88. [DOI] [PubMed] [Google Scholar]
- 14.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4):1815–22. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 15.Jones S, Horwood N, Cope A, Dazzi F. The antiproliferative effect of mesenchymal stem cells is a fundamental property shared by all stromal cells. J Immunol. 2007;179(5):2824–31. doi: 10.4049/jimmunol.179.5.2824. [DOI] [PubMed] [Google Scholar]
- 16.Karlsson H, Samarasinghe S, Ball LM, Sundberg B, Lankester AC, Dazzi F, et al. Mesenchymal stem cells exert differential effects on alloantigen and virus-specific T cell responses. Blood. 2008;112(3):532–41. doi: 10.1182/blood-2007-10-119370. [DOI] [PubMed] [Google Scholar]
- 17.Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, Dazzi F. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101(9):3722–9. doi: 10.1182/blood-2002-07-2104. [DOI] [PubMed] [Google Scholar]
- 18.Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30(1):42–8. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 19.Fibbe WE, Nauta AJ, Roelofs H. Modulation of immune responses by mesenchymal stem cells. Ann NY Acad Sci. 2007;1106:272–8. doi: 10.1196/annals.1392.025. [DOI] [PubMed] [Google Scholar]
- 20.Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M, Ringdén O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363(9419):1439–41. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 21.Ball L, Bredius R, Lankester A, Schweizer J, van den Heuvel-Eibrink M, Escher H, et al. Third party mesenchymal stromal cell infusions fail to induce tissue repair despite successful control of severe grade IV acute graft-versus-host disease in a child with juvenile myelo-monocytic leukemia. Leukemia. 2008;22(6):1256–7. doi: 10.1038/sj.leu.2405013. [DOI] [PubMed] [Google Scholar]
- 22.Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371(9624):1579–86. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 23.Dazzi F, Marelli-Berg FM. Mesenchymal stem cells for graft-versus-host disease: close encounters with T cells. Eur J Immunol. 2008;38(6):1479–82. doi: 10.1002/eji.200838433. [DOI] [PubMed] [Google Scholar]
- 24.Bocelli-Tyndall C, Bracci L, Spagnoli G, Braccini A, Bouchenaki M, Ceredig R, et al. Bone marrow mesenchymal stromal cells (BM-MSCs) from healthy donors and autoimmune disease patients reduce the proliferation of autologous- and allogeneic-stimulated lymphocytes in vitro. Rheumatology (Oxford) 2007;46(3):403–8. doi: 10.1093/rheumatology/kel267. [DOI] [PubMed] [Google Scholar]
- 25.Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M, et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5(3):309–13. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- 26.Jones EA, Kinsey SE, English A, Jones RA, Straszynski L, Meredith DM, et al. Isolation and characterization of bone marrow multipotential mesenchymal progenitor cells. Arthritis Rheum. 2002;46(12):3349–60. doi: 10.1002/art.10696. [DOI] [PubMed] [Google Scholar]
- 27.Quirici N, Soligo D, Bossolasco P, Servida F, Lumini C, Deliliers GL. Isolation of bone marrow mesenchymal stem cells by anti-nerve growth factor receptor antibodies. Exp Hematol. 2002;30(7):783–91. doi: 10.1016/s0301-472x(02)00812-3. [DOI] [PubMed] [Google Scholar]
- 28.Gang EJ, Bosnakovski D, Figueiredo CA, Visser JW, Perlingeiro RCR. SSEA-4 identifies mesenchymal stem cells from bone marrow. Blood. 2007;109(4):1743–51. doi: 10.1182/blood-2005-11-010504. [DOI] [PubMed] [Google Scholar]
- 29.Martinez C, Hofmann TJ, Marino R, Dominici M, Horwitz EM. Human bone marrow mesenchymal stromal cells express the neural ganglioside GD2: a novel surface marker for the identification of MSCs. Blood. 2007;109(10):4245–8. doi: 10.1182/blood-2006-08-039347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Battula VL, Treml S, Abele H, Buhring HJ. Prospective isolation and characterization of mesenchymal stem cells from human placenta using a frizzled-9-specific monoclonal antibody. Differentiation. 2008;76(4):326–36. doi: 10.1111/j.1432-0436.2007.00225.x. [DOI] [PubMed] [Google Scholar]
- 31.Sorrentino A, Ferracin M, Castelli G, Biffoni M, Tomaselli G, Baiocchi M, et al. Isolation and characterization of CD146+ multipotent mesenchymal stromal cells. Exp Hematol. 2008;36(8):1035–46. doi: 10.1016/j.exphem.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 32.Thomson TM, Rettig WJ, Chesa PG, Green SH, Mena AC, Old LJ. Expression of human nerve growth factor receptor on cells derived from all three germ layers. Exp Cell Res. 1988;174(2):533–9. doi: 10.1016/0014-4827(88)90323-0. [DOI] [PubMed] [Google Scholar]
- 33.Jones EA, English A, Kinsey SE, Straszynski L, Emery P, Ponchel F, McGonagle D. Optimization of a flow cytometry-based protocol for detection and phenotypic characterization of multipotent mesenchymal stromal cells from human bone marrow. Cytometry B Clin Cytom. 2006;70(6):391–9. doi: 10.1002/cyto.b.20118. [DOI] [PubMed] [Google Scholar]
- 34.Meyerrose TE, De Ugarte DA, Hofling AA, Herrbrich PE, Cordonnier TD, Shultz LD, et al. In vivo distribution of human adipose-derived mesenchymal stem cells in novel xenotransplantation models. Stem Cells. 2007;25(1):220–7. doi: 10.1634/stemcells.2006-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones E, McGonagle D. Human bone marrow mesenchymal stem cells in vivo. Rheumatology (Oxford) 2008;47(2):126–31. doi: 10.1093/rheumatology/kem206. [DOI] [PubMed] [Google Scholar]
- 36.Song L, Tuan RS. Transdifferentiation potential of human mesenchymal stem cells derived from bone marrow. FASEB J. 2004;18(9):980–2. doi: 10.1096/fj.03-1100fje. [DOI] [PubMed] [Google Scholar]
- 37.Jaganathan BG, Ruester B, Dressel L, Stein S, Grez M, Seifried E, Henschler R. Rho inhibition induces migration of mesenchymal stromal cells. Stem Cells. 2007;25(8):1966–74. doi: 10.1634/stemcells.2007-0167. [DOI] [PubMed] [Google Scholar]
- 38.Kuçi S, Wessels JT, Bühring HJ, Schilbach K, Schumm M, Seitz G, et al. Identification of a novel class of human adherent CD34-stem cells that give rise to SCID-repopulating cells. Blood. 2003;101(3):869–76. doi: 10.1182/blood-2002-03-0711. [DOI] [PubMed] [Google Scholar]
- 39.Pongers-Willemse MJ, Verhagen OJ, Tibbe GJ, Wijkhuijs AJ, de Haas V, Roovers E, et al. Real-time quantitative PCR for the detection of minimal residual disease in acute lymphoblastic leukemia using junctional region specific TaqMan probes. Leukemia. 1998;12(12):2006–14. doi: 10.1038/sj.leu.2401246. [DOI] [PubMed] [Google Scholar]
- 40.Deschaseaux F, Gindraux F, Saadi R, Obert L, Chalmers D, Herve P. Direct selection of human bone marrow mesenchymal stem cells using an anti-CD49a antibody reveals their CD45med,low phenotype. Br J Haematol. 2003;122(3):506–17. doi: 10.1046/j.1365-2141.2003.04469.x. [DOI] [PubMed] [Google Scholar]
- 41.Le Blanc K, Tammik L, Sundberg B, Haynesworth SE, Ringdén O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003;57(1):11–20. doi: 10.1046/j.1365-3083.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- 42.Majumdar MK, Thiede MA, Haynesworth SE, Bruder SP, Gerson SL. Human marrow-derived mesenchymal stem cells (MSCs) express hematopoietic cytokines and support long-term hematopoiesis when differentiated toward stromal and osteogenic lineages. J Hematother Stem Cell Res. 2000;9(6):841–8. doi: 10.1089/152581600750062264. [DOI] [PubMed] [Google Scholar]
- 43.Angelopoulou M, Novelli E, Grove JE, Rinder HM, Civin C, Cheng L, Krause DS. Cotransplantation of human mesenchymal stem cells enhances human myelopoiesis and megakaryocytopoiesis in NOD/SCID mice. Exp Hematol. 2003;31(5):413–20. doi: 10.1016/s0301-472x(03)00042-0. [DOI] [PubMed] [Google Scholar]
- 44.Qiao X, Loudovaris M, Unverzagt K, Walker DE, Smith SL, Martinson J, et al. Immunocytochemistry and flow cytometry evaluation of human megakaryocytes in fresh samples and cultures of CD34+ cells. Cytometry. 1996;23(3):250–9. doi: 10.1002/(SICI)1097-0320(19960301)23:3<250::AID-CYTO8>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 45.Sotiropoulou PA, Perez SA, Gritzapis AD, Baxevanis CN, Papamichail M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006;24(1):74–85. doi: 10.1634/stemcells.2004-0359. [DOI] [PubMed] [Google Scholar]
- 46.Ball LM, Bernardo ME, Roelofs H, Lankester A, Cometa A, Egeler RM, et al. Cotransplantation of ex vivo expanded mesenchymal stem cells accelerates lymphocyte recovery and may reduce the risk of graft failure in haploidentical hematopoietic stem-cell transplantation. Blood. 2007;110(7):2764–7. doi: 10.1182/blood-2007-04-087056. [DOI] [PubMed] [Google Scholar]
- 47.Barbash IM, Chouraqui P, Baron J, Feinberg MS, Etzion S, Tessone A, et al. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation. 2003;108(7):863–8. doi: 10.1161/01.CIR.0000084828.50310.6A. [DOI] [PubMed] [Google Scholar]
- 48.Gao J, Dennis JE, Muzic RF, Lundberg M, Caplan AI. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs. 2001;169(1):12–20. doi: 10.1159/000047856. [DOI] [PubMed] [Google Scholar]