Abstract
Early-life organophosphate (OP) exposures elicit neurobehavioral deficits through mechanisms other than inhibiting cholinesterase. Cell signaling cascades are postulated as critical noncholinesterase targets that mediate both the initial alterations in neurodevelopment as well as subsequent abnormalities of synaptic function. We exposed PC12 cells to chlorpyrifos, diazinon or parathion in the undifferentiated state and during neurodifferentiation; we then assessed the function of the adenylyl cyclase (AC) signaling cascade, measuring basal AC activity as well as responses to stimulants acting at G-proteins or on the AC molecule itself. In undifferentiated cells, a 2 day exposure to the OPs had no significant effect on AC signaling but the same treatment in differentiating cells produced deficits in all AC measures when exposure commenced at the initiation of differentiation. However, when exposure of the differentiating cells was continued for 6 days, AC activities then became supranormal. The same increase was obtained if cells were exposed only for the first two days of differentiation, followed by four subsequent days without the OPs. Further, the OP effects on cell signaling were entirely distinct from those on indices of cell number and neurite outgrowth. These results indicate that OP exposure reprograms the AC pathway during a discrete developmental stage at the commencement of neurodifferentiation, with effects that continue to emerge after OP exposure is discontinued. Importantly, the same sequence is seen with OP exposures in neonatal rats, indicating that direct effects of these agents to reprogram cell signaling provide a major mechanism for functional effects unrelated to cholinesterase inhibition.
Classification terms: Section: (2) Nervous System Development, Regeneration and Aging
Key words: Adenylyl cyclase, Chlorpyrifos, Diazinon, Organophosphate insecticides, Parathion
INTRODUCTION
The systemic toxicity of organophosphate pesticides (OPs) reflects their ability to inhibit cholinesterase (Mileson et al., 1998; Pope, 1999), leading to accumulation of acetylcholine and associated signs of excessive cholinergic stimulation. Nevertheless, it is increasingly clear that the developmental neurotoxicity of these agents involves mechanisms unrelated to cholinesterase inhibition (Casida and Quistad, 2004; Colborn, 2006; Gupta, 2004; Perera et al., 2005; Slotkin, 2005). Signal transduction cascades that regulate cell replication, differentiation and function are among the most sensitive targets for noncholinesterase actions of OPs and in particular, these agents affect the synthesis and utilization of the second messenger, cyclic AMP (Schuh et al., 2002; Slotkin, 2005; Wardle et al., 2008; Yanai et al., 2002, 2004). We recently showed how early life exposures to chlorpyrifos (CPF), diazinon (DZN) or parathion (PRT) all evoke lasting effects on components of the adenylyl cyclase (AC) cascade, the pathway that transduces signals from the vast array of G-protein coupled receptors to the generation of cyclic AMP (Adigun et al., 2010a; Meyer et al., 2004a, b; Song et al., 1997). Notably, these effects extend outside the central nervous system; indeed, neonatal OP exposures produce subsequent gain-of-function of hepatic AC signaling that contributes to the emergence of metabolic dysregulation akin to prediabetes (Adigun et al., 2010b; Auman et al., 2000; Lassiter et al., 2010; Meyer et al., 2004b; Slotkin et al., 2005).
These findings raise the possibility that, during a critical developmental period, OP exposures directly reprogram the functioning of the AC signaling pathway, an hypothesis that would be difficult to evaluate in vivo, given the myriad systemic changes elicited when these agents are given to animals. In the current study, we used an in vitro model to examine the effects of different OPs on AC signaling under conditions spanning different developmental stages from cell replication through early and later stages of differentiation. We had three specific objectives: first, to determine if OPs affect AC signaling during a discrete stage of cell development; second, to establish whether the effects persist so long as OP exposure continues or rather whether effects emerge beyond the exposure period; and third, to evaluate whether the effects on AC signaling are separable from effects on general aspects of cell growth. We conducted our evaluations in PC12 cells, a well-characterized neurodevelopmental model (Teng and Greene, 1994) that reproduces many of the key mechanisms and features of the adverse effects of OPs in vivo (Bagchi et al., 1995, 1996; Crumpton et al., 2000a, b; Das and Barone, 1999; Flaskos et al., 1994; Jameson et al., 2006a; Li and Casida, 1998; Nagata et al., 1997; Qiao et al., 2001, 2005; Slotkin, 1999, 2004, 2005; Song et al., 1998; Tuler et al., 1989; Yanai et al., 2002). When nerve growth factor (NGF) is introduced, PC12 cells exit the mitotic cycle and undergo neurodifferentiation (Fujita et al., 1989; Song et al., 1998; Teng and Greene, 1994). Here, we used these features to examine the effects of CPF, DZN and PRT on AC signaling in the undifferentiated state, at the initiation of differentiation, and after a more prolonged period of differentiation. Our AC assessments focused on measures that evaluate pathway function at sequential steps: basal enzymatic activity, the response to global stimulation of G-proteins by fluoride, and the responses to two direct AC stimulants, forskolin and Mn2+. Because the two stimulants act at different epitopes on the AC molecule, the preferential effects for one versus the other defines shifts in the expression and catalytic activities of different AC isoforms (Auman et al., 2000; Zeiders et al., 1997, 1999a). We then compared the effects on AC signaling to those on cell growth, focusing on measures of cell number and neurite formation. Each neural cell contains a single nucleus, so that measuring the DNA content evaluates the number of cells (Winick and Noble, 1965), whereas the expansion of the membrane surface area that accompanies the formation of neurites during neurodifferentiation leads to an increase in the membrane protein/DNA ratio (Abreu-Villaça et al., 2005; Jameson et al., 2006b; Slotkin et al., 2007b; Song et al., 1998).
RESULTS
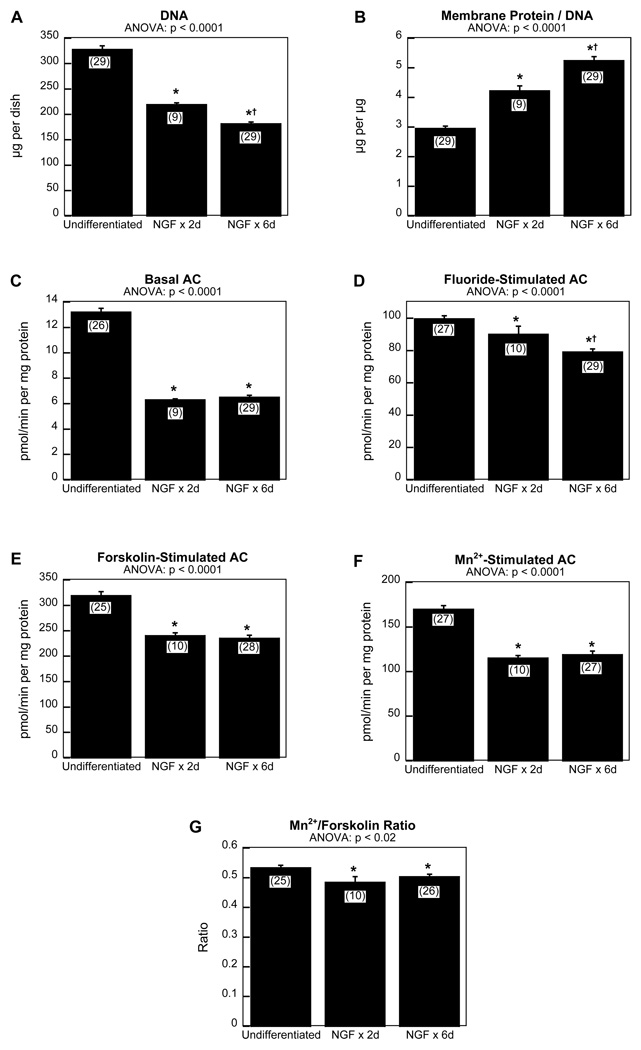
In control cells, NGF treatment elicited the expected switch from cell replication to neurodifferentiation, as evidenced by significantly lower numbers of cells (Fig. 1A) and greater membrane surface area (Fig. 1B) as compared to undifferentiated cells cultured for the same amount of time. NGF also elicited a significant overall reduction in AC activities relative to membrane protein (p < 0.0001 for the main effect of NGF), with selective effects on the responses to the various AC stimulants (p < 0.0001 for the interaction of NGF × stimulant measure). For basal activity (Fig. 1C), the net reduction was completed within 2 days of NGF treatment and showed no further reduction by the 6 day time point. In contrast, the response to fluoride showed a progressive loss over time (Fig. 1D). The decline in responses to forskolin (Fig. 1E) and Mn2+ (Fig. 1F) resembled that of basal activity, with a complete effect evident after 2 days of NGF; further, with the onset of differentiation, there was a small but significant decline in the Mn2+/forskolin response ratio (Fig. 1G). The patterns for basal AC and the forskolin and Mn2+-mediated responses were thus entirely distinct from the changes in cell number and membrane protein/DNA ratio, which showed distinct progression between 2 and 6 days of NGF exposure. Differentiation also altered the relative response to each of the stimulants. For fluoride, the increase over basal activity was 8-fold in undifferentiated cells, rising to 13-fold after NGF treatment; for forskolin the increase was from 25-fold to nearly 40-fold, and for Mn2+ the values were 12-fold and 18-fold, respectively.
Figure 1. Effects of NGF on parameters of cell growth and AC signaling.
(A) DNA, (B) membrane protein/DNA ratio, (C) basal AC, (D) fluoride-stimulated AC, (E) forskolin-stimulated AC, (F) Mn2+-stimulated AC, and (G) Mn2+/forskolin ratio. Cells were cultured for a total of 7 days. NGF was introduced after either 5 days in culture (2 days of NGF treatment, NGF × 2d) or after 1 day in culture (6 days of NGF treatment, NGF × 6d). Data represent means and standard errors of the number of determinations shown in parentheses. ANOVA appears above each panel; asterisks denote values for differentiating cells that differ from the undifferentiated state and daggers denote differences between 2 days and 6 days of NGF exposure. The values shown here were normalized and pooled from the control groups across all experiments; however organophosphate treatment effects in the remaining figures were assessed against only the matched contemporaneous controls for each study.
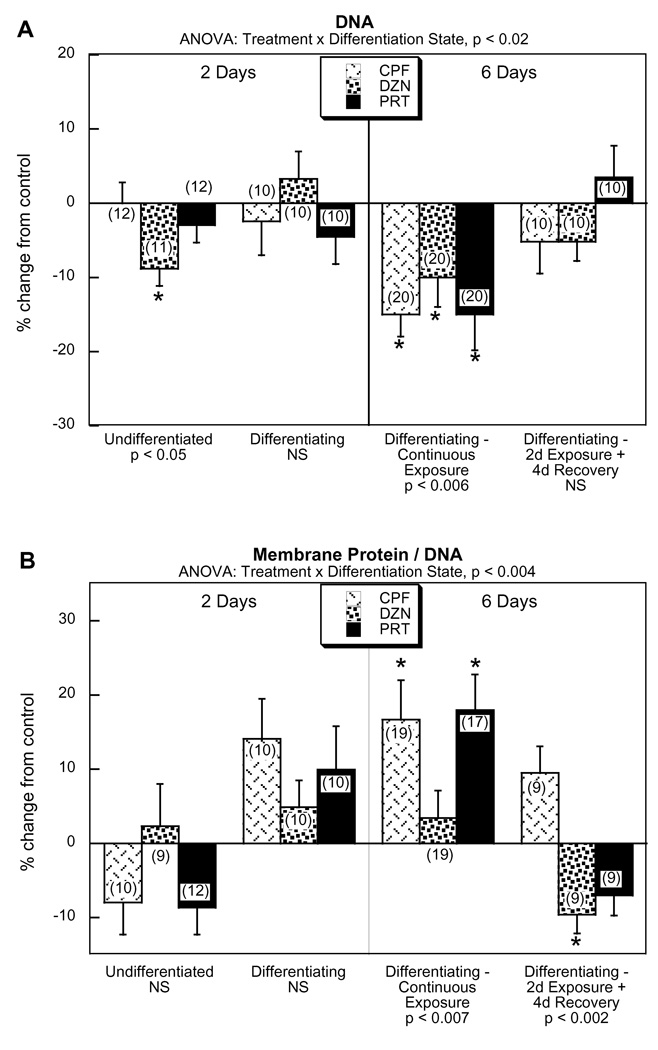
In undifferentiated cells, a 2 day exposure to CPF or PRT had little or no effect on DNA content but DZN produced a significant, albeit small, decrement (Fig. 2A). In differentiating cells, the 2 day OP treatment had no discernible effect on DNA but extending the exposure to 6 days produced a significant decline with all three agents. In contrast, when OP exposure was limited to the first 2 days of differentiation, followed by a 4 day recovery period, there were no DNA deficits. For the membrane protein/DNA ratio, undifferentiated cells showed no significant effects after a 2 day OP exposure (Fig. 2B). Differentiating cells showed a trend toward increases at 2 days that became statistically significant for CPF and PRT after 6 days. Again, limiting the exposure of differentiating cells to the first 2 days, followed by a 4 day recovery period, completely obtunded the increases and instead, there was a slight but significant decline seen for DZN.
Figure 2. Effects of OP exposure on cell growth parameters.
(A) DNA, (B) membrane protein/DNA ratio. Data represent means and standard errors of the number of determinations shown in parentheses, given as the percentage change from control values. ANOVA appears above each panel and lower-order tests for each differentiation state appear at the bottom. Asterisks denote individual values that differ significantly from the corresponding control. Abbreviation: NS, not significant.
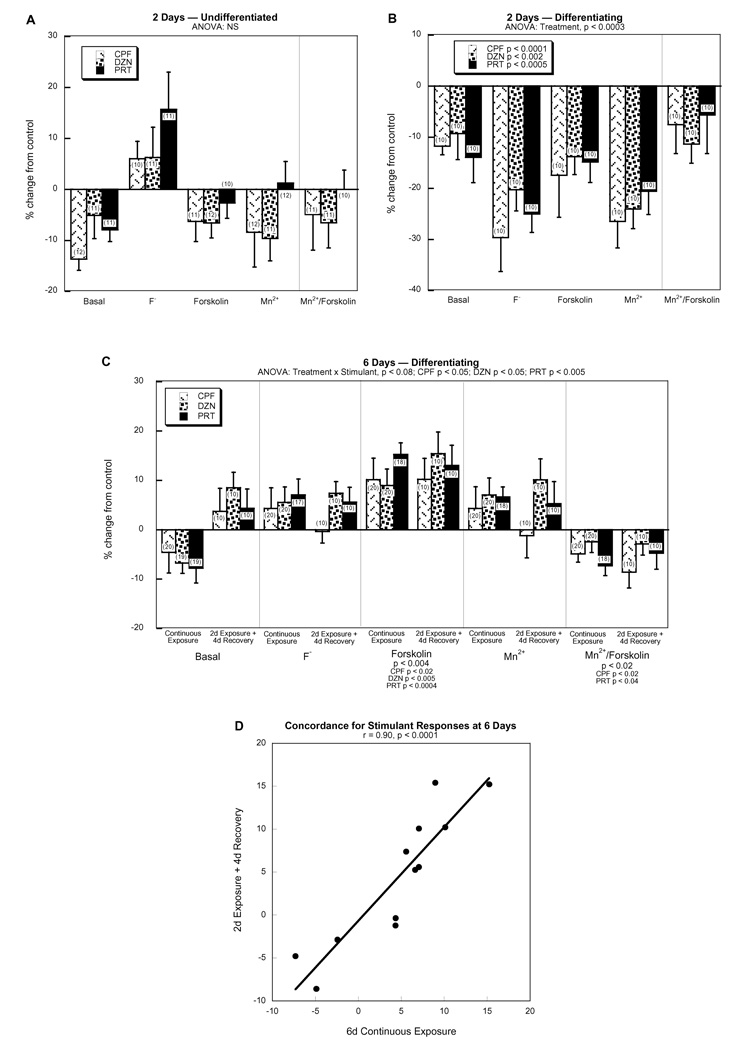
Exposure of undifferentiated cells to the three OPs for a period of 2 days did not have any statistically significant overall effects on AC signaling parameters (Fig. 3A) but in differentiating cells there was a robust suppression of activity regardless of stimulant condition, an effect that was statistically significant overall as well as individually for CPF, DZN and PRT (Fig. 3B). With continued exposure of differentiating cells for 6 days, there was a complete reversal of the inhibitory effect of the OPs on AC signaling parameters (Fig. 3C). Indeed, the response to forskolin became significantly elevated for all three agents, and similar but nonsignificant trends were present for fluoride and Mn2+; the nonsignificant increases for the latter two were statistically indistinguishable from the significant increase in the forskolin response. Importantly, the same effect was noted in cells that had been exposed for only the first 2 days of differentiation and that were then carried out for an additional 4 days in the absence of the OPs. The OP exposure in differentiating cells also reduced the Mn2+/forskolin response ratio. The effect on the ratio was statistically significant for either of the 6 day regimens but not for the 2 day exposure; however, the effect at 2 days was in the same direction as at 6 days and was not statistically distinguishable from the significant reduction at the later time points, and in fact, a comparison of all three regimens for differentiating cells identified only a main treatment effect (p < 0.04) without a treatment × regimen interaction.
Figure 3. Effects of OP exposure on AC activities.
(A) undifferentiated cells with a 2 day exposure, (B) differentiating cells with a 2 day exposure, (C) differentiating cells with a 6 day continuous exposure or with a 2 day exposure followed by a 4 day recovery period. Data represent means and standard errors of the number of determinations shown in parentheses, given as the percentage change from control values. A global ANOVA incorporating all variables and dependent measures in a single test identified a main treatment effect (p < 0.02) and interactions of treatment × differentiation state (p < 0.0007) and treatment × state × stimulant (p < 0.03), necessitating subdivision into the individual differentiation states (A,B,C). Accordingly, separate ANOVAs for each differentiation state appear above the panels. Lower order tests were not carried out for (A) and (B) because of the absence of treatment interactions with other variables; in (B), the main effects for each OP are shown within the legend box. In (C), the treatment × stimulant interaction necessitated separate examinations for each stimulant condition and the corresponding ANOVAs are shown below the panel; main effects for each OP are shown without conducting separate tests for each exposure regimen because of the absence of a treatment × regimen interaction. Panel (D) shows the concordance (least squares fit and linear correlation coefficient) between OP effects on stimulant responses (fluoride, forskolin, Mn2+, Mn2+/forskolin) for differentiating cells with 6 days of continuous OP exposure vs. 2 days of exposure + 4 days of recovery. Abbreviation: NS, not significant.
To reinforce the similarities in the patterns of OP effects on AC stimulant responses seen with 6 days of continuous exposure and with 2 days of exposure + 4 days of recovery, we replotted the data to examine the correlation between the two regimens and found high concordance (Fig. 3D); thus, the similar outcomes did not depend solely on the significant difference in the forskolin response but rather was reflected in the overall pattern for all the AC stimulants.
DISCUSSION
The results obtained in this study indicate that OP exposure reprograms the AC signaling pathway during a discrete developmental stage at the commencement of neurodifferentiation, with effects that continue to emerge after OP exposure is discontinued, actions that are distinct from the impact on cell growth. In control cells, we noted that the first two days after the start of NGF-induced neurodifferentiation had a critical effect on AC activity profiles. Although there was a drop in total activity, stimulants acting at the level of G-proteins or AC itself then elicited larger proportional increases over basal activity than in the undifferentiated state. Further, differentiation produced a drop in the Mn2+/forskolin response ratio, indicative of a shift in AC isoform expression (Zeiders et al., 1999b). Over the ensuing four days, most of the effects of NGF on AC signaling leveled off, whereas those on cell growth parameters (DNA, membrane protein/DNA) showed a clear progression over time. This suggested to us that the initial stages of neurodifferentiation might indeed be especially sensitive for disruption of AC signaling by OPs and we designed our studies to test that hypothesis.
If OPs act on AC signaling during a specific stage of neurodifferentiation, we would expect to see a distinct difference in the effects on undifferentiated PC12 cells as compared to those exposed to NGF. After a 2 day exposure to CPF, DZN or PRT, there were no significant changes in AC signaling in the undifferentiated state but there was a robust, global decrement in the differentiating cells. This resembles our earlier finding of overall decreases in AC activities in differentiating PC12 cells with a more prolonged exposure to lower concentrations of CPF (Slotkin et al., 2007a). More importantly, though, the same effects are seen in the developing brain after CPF exposure in neonatal rats (Song et al., 1997), reinforcing the view that the effects of OPs on AC signaling are mediated directly on the differentiating neurons themselves. These conclusions are bolstered by studies at the transcriptional level, which show a relative lack of concordance between undifferentiated and differentiating cells in the impact of CPF on the mRNAs encoding AC isoforms and G-proteins (Adigun et al., 2010a).
Given the OP-induced reductions in AC signaling in differentiating cells at the 2-day point, we were surprised to find that continuing the exposure to 6 days resulted not only in disappearance of the deficits, but actual increases in responses; again, in contrast to the findings for AC, the more general indices of cell numbers and neurite formation showed progressively greater effects of the OPs at 6 days than at 2 days. The findings thus indicate either that the cells adapt to the continued presence of the OPs, or alternatively, that exposure during the critical first 2 days of neurodifferentiation reprograms the function of the pathway. To distinguish between these two possibilities, we exposed the cells to the OPs for the first 2 days and then discontinued exposure for the ensuing 4 days. If the biphasic response represents an adaptation to continuous OP exposure, then at the end of the 4 day recovery period, we would expect to see either a small residual deficit or restoration of normal AC activities. If on the other hand, the elevations seen after 6 days of continuous exposure represented a reprogramming elicited by exposure in the first 2 days, then the pattern at the end of the recovery period should be indistinguishable from that seen with continuous exposure. In fact, the latter turned out to be true: the entire pattern of initial AC deficits, subsequent elevations and a decrement in the Mn2+/forskolin ratio was recapitulated with just an initial 2 day exposure conducted at the onset of neurodifferentiation. Indeed, these findings with an in vitro model reproduce exactly the pattern seen for exposure of neonatal rats to OPs, namely initial deficits in AC signaling, followed by persistent upregulation and supersensitivity emerging well after the end of OP exposure (Adigun et al., 2010b; Meyer et al., 2004a); indeed the in vivo outcomes are similar for AC signaling in peripheral tissues (Adigun et al., 2010b; Meyer et al., 2004b), reinforcing the concept of a direct effect of early OP exposure that reprograms the function of this signal transduction pathway, unrelated to cytotoxicity or generalized actions on cell growth.
Separable from their impact on AC signaling, the OPs showed selective effects on indices of cell growth that are of additional interest. Given their shared antimitotic and proapoptotic properties (Slotkin, 2005; Slotkin et al., 2007b; Slotkin and Seidler, 2007; Yousefpour et al., 2006; Yu et al., 2008), it was not surprising that all three OPs elicited a decline in the number of cells after 6 days of exposure, as evidenced by a deficit in the DNA content. Unlike the AC effects, the cell loss required the continuous presence of the OPs over the 6 day span: when we limited exposure to the first 2 days and then allowed 4 days for recovery, cell loss was no longer evident. This points out that the sensitivity of the AC pathway to reprogramming by OP exposure is far more sensitive than are general effects on cell acquisition or loss. The membrane protein/DNA ratio displayed a dichotomy, with a significant increase evident for CPF and PRT but not DZN. These findings support earlier conclusions about differential effects of the various OPs on the formation of neuritic projections. CPF blunts the development of long axons while favoring the creation of short dendritic branches (Howard et al., 2005). In contrast, DZN shows greater overall inhibitory effects on neurite outgrowth, whereas PRT is more like CPF (Axelrad et al., 2003; Slotkin et al., 2006). Again, though, these growth-related effects of CPF and PRT were dependent on continuous exposure over the 6 day span; interestingly, the 2 day exposure appeared to augment the inhibitory effects of DZN, an unexpected finding that should be followed up with structural measures.
In conclusion, results of this study show that OP exposure during early neurodifferentiation reprograms the development of the AC signaling cascade, with initial deficits replaced by subsequent upregulation resulting in a net gain-of-function. The effects in PC12 cells mimic those seen after OP exposure in neonatal rats both in the brain and peripheral tissues, effects that are thus likely to contribute to neurobehavioral deficits and metabolic dysfunction. Accordingly, the lasting impact of early-life OP exposure on cell signaling cascades is likely to represent one of the most critical noncholinesterase targets for these common pesticides.
EXPERIMENTAL PROCEDURE
Cell cultures
Because of the clonal instability of the PC12 cell line (Fujita et al., 1989), the experiments were performed on cells that had undergone fewer than five passages and all studies were repeated several times with different batches of cells. As described previously (Crumpton et al., 2000a; Qiao et al., 2003; Song et al., 1998), 3 × 106 PC12 cells (American Type Culture Collection, 1721-CRL; Duke Comprehensive Cancer Center, Durham, NC) were seeded onto 100 mm poly-D-lysine-coated plates in RPMI-1640 medium (Sigma Chemical Co., St. Louis, MO) supplemented with 10% horse serum (Sigma), 5% fetal bovine serum (Sigma), and 50 µg/ml penicillin streptomycin (Invitrogen, Carlsbad, CA); cells were incubated with 5% CO2 at 37°C. All cultures were evaluated 7 days after plating to ensure a comparable basis for measurement regardless of treatment time or differentiation state. The medium was changed 24 hours after plating and at intervals of 48 hours thereafter. For studies in the undifferentiated state, OPs were introduced after 5 days in culture, 50 µM CPF, DZN or PRT (Chem Service, West Chester, PA), and cells were then examined after 2 days of exposure. Because of their poor water solubility, the toxicants were dissolved in dimethylsulfoxide (Sigma), achieving a final concentration of 0.1% in the culture medium. The control cultures also included the vehicle, which has no effect on replication or differentiation of PC12 cells (Qiao et al., 2001, 2003; Song et al., 1998).
For studies in differentiating cells with 2 days of exposure to the OPs, the cells were cultured in the undifferentiated state for 5 days, as already described. At that point, the medium was changed to include 50 ng/ml of 2.5 S murine NGF (Invitrogen) along with the test agents and culturing continued for the ensuing 2 days. For treatment effects after 6 days of exposure, NGF and test agents were added 24 hours after plating, with subsequent media changes at 2 day intervals, including replacement of the test agents. In some experiments, toxicant exposure was continued throughout the 6 day period, whereas in others, exposure was limited to the initial 2 day period, followed by 4 days in culture without the toxicants. We chose the 50 µM test concentration because it elicits robust oxidative stress, inhibition of DNA synthesis and interference with cell acquisition, without producing outright cytotoxicity (Crumpton et al., 2000b; Das and Barone, 1999; Jameson et al., 2006a; Qiao et al., 2001, 2003, 2005; Slotkin et al., 2007b; Song et al., 1998). Each culture was examined under a microscope to verify the outgrowth of neurites after NGF treatment.
DNA and protein assays
For determinations of DNA content and membrane protein, the medium was aspirated and the culture was rinsed with a buffer consisting of 154 mM NaCl and 10 mM sodium phosphate (pH 7.4). Cells were harvested in ice-cold buffer, homogenized (Polytron, Brinkmann Instruments, Westbury, NY) and an aliquot was withdrawn for measurements of DNA (Trauth et al., 2000). The cell membrane fraction was prepared by sedimentation at 40,000 × g for 15 min. The pellets were washed twice and then resuspended in 250 mM sucrose, 2 mM MgCl2, and 50 mM Tris and aliquots were withdrawn for determination of membrane protein (Smith et al., 1985) and for AC assays.
Adenylyl cyclase activity
The assay procedures and stimulant concentration profiles have been described in detail previously (Auman et al., 2000; Meyer et al., 2005; Slotkin et al., 2007a; Zeiders et al., 1999a). Aliquots of the membrane preparation were incubated for 30 min at 30°C with final concentrations of 100 mM Tris-HCl (pH 7.4), 10 mM theophylline, 1 mM ATP, 2 mM MgCl2, 10 µM GTP, 1 mg/ml bovine serum albumin, and a creatine phosphokinase–ATP–regenerating system consisting of 10 mM sodium phosphocreatine and 8 IU/ml phosphocreatine kinase (all reagents from Sigma). The enzymatic reaction was stopped by heating and sedimentation, and the supernatant solution was then assayed for cyclic AMP using commercial immunoassay kits (GE Healthcare Biosciences, Piscataway, NJ). In addition to assessing basal AC activity, we evaluated responses to 10 mM NaF, 100 µM forskolin and 10 mM MnCl2 (all reagents from Sigma). These concentrations produce maximal responses to each stimulant as assessed in earlier studies (Auman et al., 2000; Zeiders et al., 1997, 1999a). Activities were determined as the amount of cyclic AMP formed per minute per mg of membrane protein.
Data analysis
All studies were performed in multiple batches of cells, with several independent cultures for each treatment in each batch. Data are presented as means and standard errors, with treatment comparisons carried out by analysis of variance (ANOVA, data log-transformed because of heterogeneous variance across the various AC stimulants). Comparisons entailed multiple factors: cell batch, treatment, time in culture, differentiation state (undifferentiated vs. differentiating) and the multiple dependent measures (basal AC, fluoride-stimulated AC, forskolin-stimulated AC and Mn2+-stimulated AC); the latter was considered to be a repeated measure because the same membrane preparation was used for each of the multiple assay conditions. In the initial test, we found that the treatment effects were the same across the different batches of cells, although the absolute values differed from batch to batch; accordingly, we normalized the results across batches prior to combining them for presentation. As justified by significant interactions of treatment with the other variables, data were then subdivided to permit lower-order ANOVAs to determine treatments and AC stimulant responses that differed from control values, followed where appropriate by Fisher’s Protected Least Significant Difference Test to identify pairwise differences. For all tests, significance for main treatment effects was assumed at the level of p < 0.05. However, for interactions at 0.05 < p < 0.1, we also examined whether lower-order main effects were detectable after subdivision of the interactive variables (Snedecor and Cochran, 1967). The criterion for interaction terms was not used to assign significance to the effects but rather to identify interactive variables requiring subdivision for lower-order tests of main effects of toxicant exposure, the variable of chief interest.
To enable ready visualization of treatment effects across different OPs, treatment regimens and stimulants, some of the results are given as the percent change from control values but statistical procedures were always conducted on the original data. For reference, the normalized control values are shown in Figure 1; however, statistically significant differences for each study were computed by comparing treated groups only to the contemporaneous control group.
Acknowledgment
Research was supported by NIH ES10356.
Abbreviations
- AC
adenylyl cyclase
- ANOVA
analysis of variance
- CPF
chlorpyrifos
- DZN
diazinon
- PRT
parathion
- NGF
nerve growth factor
- OP
organophosphate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: TAS has provided expert witness testimony in the past three years at the behest of the following law firms: The Calwell Practice (Charleston WV), Frost Brown Todd (Charleston WV), Weltchek Mallahan & Weltchek (Lutherville MD), Finnegan Henderson Farabow Garrett & Dunner (Washington DC), Frommer Lawrence Haug (Washington DC), Carter Law (Peoria IL), Corneille Law (Madison WI), Angelos Law (Baltimore MD), Kopff, Nardelli & Dopf (New York NY), Gutglass Erickson Bonville & Larson (Madison WI) and Pardieck Law (Seymour IN).
REFERENCES
- Abreu-Villaça Y, Seidler FJ, Qiao D, Slotkin TA. Modeling the developmental neurotoxicity of nicotine in vitro: cell acquisition, growth and viability in PC12 cells. Dev. Brain Res. 2005;154:239–246. doi: 10.1016/j.devbrainres.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Adigun AA, Seidler FJ, Slotkin TA. Disparate developmental neurotoxicants converge on the cyclic AMP signaling cascade, revealed by transcriptional profiles in vitro and in vivo. Brain Res. 2010a doi: 10.1016/j.brainres.2009.12.025. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adigun AA, Wrench N, Seidler FJ, Slotkin TA. Neonatal organophosphorus pesticide exposure alters the developmental trajectory of cell signaling cascades controlling metabolism: differential effects of diazinon and parathion. Environ. Health Perspect. 2010b doi: 10.1289/ehp.0901237. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auman JT, Seidler FJ, Slotkin TA. Neonatal chlorpyrifos exposure targets multiple proteins governing the hepatic adenylyl cyclase signaling cascade: implications for neurotoxicity. Dev. Brain Res. 2000;121:19–27. doi: 10.1016/s0165-3806(00)00021-3. [DOI] [PubMed] [Google Scholar]
- Axelrad JC, Howard CV, McLean WG. The effects of acute pesticide exposure on neuroblastoma cells chronically exposed to diazinon. Toxicology. 2003;185:67–78. doi: 10.1016/s0300-483x(02)00592-9. [DOI] [PubMed] [Google Scholar]
- Bagchi D, Bagchi M, Hassoun EA, Stohs SJ. In vitro and in vivo generation of reactive oxygen species, DNA damage and lactate dehydrogenase leakage by selected pesticides. Toxicology. 1995;104:129–140. doi: 10.1016/0300-483x(95)03156-a. [DOI] [PubMed] [Google Scholar]
- Bagchi D, Bhattacharya G, Stohs SJ. In vitro and in vivo induction of heat shock (stress) protein (Hsp) gene expression by selected pesticides. Toxicology. 1996;112:57–68. doi: 10.1016/0300-483x(96)03350-1. [DOI] [PubMed] [Google Scholar]
- Casida JE, Quistad GB. Organophosphate toxicology: safety aspects of nonacetylcholinesterase secondary targets. Chem. Res. Toxicol. 2004;17:983–998. doi: 10.1021/tx0499259. [DOI] [PubMed] [Google Scholar]
- Colborn T. A case for revisiting the safety of pesticides: a closer look at neurodevelopment. Environ. Health Perspect. 2006;114:10–17. doi: 10.1289/ehp.7940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crumpton TL, Seidler FJ, Slotkin TA. Developmental neurotoxicity of chlorpyrifos in vivo and in vitro: effects on nuclear transcription factor involved in cell replication and differentiation. Brain Res. 2000a;857:87–98. doi: 10.1016/s0006-8993(99)02357-4. [DOI] [PubMed] [Google Scholar]
- Crumpton TL, Seidler FJ, Slotkin TA. Is oxidative stress involved in the developmental neurotoxicity of chlorpyrifos? Dev. Brain Res. 2000b;121:189–195. doi: 10.1016/s0165-3806(00)00045-6. [DOI] [PubMed] [Google Scholar]
- Das KP, Barone S. Neuronal differentiation in PC12 cells is inhibited by chlorpyrifos and its metabolites: is acetylcholinesterase inhibition the site of action? Toxicol. Appl. Pharmacol. 1999;160:217–230. doi: 10.1006/taap.1999.8767. [DOI] [PubMed] [Google Scholar]
- Flaskos J, McLean WG, Hargreaves AJ. The toxicity of organophosphate compounds towards cultured PC12 cells. Toxicol. Lett. 1994;70:71–76. doi: 10.1016/0378-4274(94)90146-5. [DOI] [PubMed] [Google Scholar]
- Fujita K, Lazarovici P, Guroff G. Regulation of the differentiation of PC12 pheochromocytoma cells. Environ. Health Perspect. 1989;80:127–142. doi: 10.1289/ehp.8980127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RC. Brain regional heterogeneity and toxicological mechanisms of organophosphates and carbamates. Toxicol. Mech. Meth. 2004;14:103–143. doi: 10.1080/15376520490429175. [DOI] [PubMed] [Google Scholar]
- Howard AS, Bucelli R, Jett DA, Bruun D, Yang DR. Chlorpyrifos exerts opposing effects on axonal and dendritic growth in primary neuronal cultures. Toxicol. Appl. Pharmacol. 2005;207:112–124. doi: 10.1016/j.taap.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Jameson RR, Seidler FJ, Qiao D, Slotkin TA. Chlorpyrifos affects phenotypic outcomes in a model of mammalian neurodevelopment: critical stages targeting differentiation in PC12 cells. Environ. Health Perspect. 2006a;114:667–672. doi: 10.1289/ehp.8750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson RR, Seidler FJ, Qiao D, Slotkin TA. Adverse neurodevelopmental effects of dexamethasone modeled in PC12 cells: identifying the critical stages and concentration thresholds for the targeting of cell acquisition, differentiation and viability. Neuropsychopharmacology. 2006b;31:1647–1658. doi: 10.1038/sj.npp.1300967. [DOI] [PubMed] [Google Scholar]
- Lassiter TL, Ryde IT, Levin ED, Seidler FJ, Slotkin TA. Neonatal exposure to parathion alters lipid metabolism in adulthood: interactions with dietary fat intake and implications for neurodevelopmental deficits. Brain Res. Bull. 2010;81:85–91. doi: 10.1016/j.brainresbull.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WW, Casida JE. Organophosphorus neuropathy target esterase inhibitors selectively block outgrowth of neurite-like and cell processes in cultured cells. Toxicol. Lett. 1998;98:139–146. doi: 10.1016/s0378-4274(98)00116-7. [DOI] [PubMed] [Google Scholar]
- Meyer A, Seidler FJ, Aldridge JE, Tate CA, Cousins MM, Slotkin TA. Critical periods for chlorpyrifos-induced developmental neurotoxicity: alterations in adenylyl cyclase signaling in adult rat brain regions after gestational or neonatal exposure. Environ. Health Perspect. 2004a;112:295–301. doi: 10.1289/ehp.6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Seidler FJ, Slotkin TA. Developmental effects of chlorpyrifos extend beyond neurotoxicity: critical periods for immediate and delayed-onset effects on cardiac and hepatic cell signaling. Environ. Health Perspect. 2004b;112:170–178. doi: 10.1289/ehp.6690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Seidler FJ, Aldridge JE, Slotkin TA. Developmental exposure to terbutaline alters cell signaling in mature rat brain regions and augments the effects of subsequent neonatal exposure to the organophosphorus insecticide, chlorpyrifos. Toxicol. Appl. Pharmacol. 2005;203:154–166. doi: 10.1016/j.taap.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Mileson BE, Chambers JE, Chen WL, Dettbarn W, Ehrich M, Eldefrawi AT, Gaylor DW, Hamernik K, Hodgson E, Karczmar AG, Padilla S, Pope CN, Richardson RJ, Saunders DR, Sheets LP, Sultatos LG, Wallace KB. Common mechanism of toxicity: a case study of organophosphorus pesticides. Toxicol. Sci. 1998;41:8–20. doi: 10.1006/toxs.1997.2431. [DOI] [PubMed] [Google Scholar]
- Nagata K, Huang CS, Song JH, Narahashi T. Direct actions of anticholinesterases on the neuronal nicotinic acetylcholine receptor channels. Brain Res. 1997;769:211–218. doi: 10.1016/s0006-8993(97)00707-5. [DOI] [PubMed] [Google Scholar]
- Perera FP, Rauh V, Whyatt RM, Tang D, Tsai WY, Bernert JT, Tu YH, Andrews H, Barr DB, Camann DE, Diaz D, Dietrich J, Reyes A, Kinney PL. A summary of recent findings on birth outcomes and developmental effects of prenatal ETS, PAH, and pesticide exposures. Neurotoxicology. 2005;26:573–587. doi: 10.1016/j.neuro.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Pope CN. Organophosphorus pesticides: do they all have the same mechanism of toxicity? J. Toxicol. Environ. Health. 1999;2:161–181. doi: 10.1080/109374099281205. [DOI] [PubMed] [Google Scholar]
- Qiao D, Seidler FJ, Slotkin TA. Developmental neurotoxicity of chlorpyrifos modeled in vitro: comparative effects of metabolites and other cholinesterase inhibitors on DNA synthesis in PC12 and C6 cells. Environ. Health Perspect. 2001;109:909–913. doi: 10.1289/ehp.01109909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao D, Seidler FJ, Violin JD, Slotkin TA. Nicotine is a developmental neurotoxicant and neuroprotectant: stage-selective inhibition of DNA synthesis coincident with shielding from effects of chlorpyrifos. Dev. Brain Res. 2003;147:183–190. doi: 10.1016/s0165-3806(03)00222-0. [DOI] [PubMed] [Google Scholar]
- Qiao D, Seidler FJ, Slotkin TA. Oxidative mechanisms contributing to the developmental neurotoxicity of nicotine and chlorpyrifos. Toxicol. Appl. Pharmacol. 2005;206:17–26. doi: 10.1016/j.taap.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Schuh RA, Lein PJ, Beckles RA, Jett DA. Noncholinesterase mechanisms of chlorpyrifos neurotoxicity: altered phosphorylation of Ca2+/cAMP response element binding protein in cultured neurons. Toxicol. Appl. Pharmacol. 2002;182:176–185. doi: 10.1006/taap.2002.9445. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. Developmental cholinotoxicants: nicotine and chlorpyrifos. Environ. Health Perspect. 1999;107 suppl 1:71–80. doi: 10.1289/ehp.99107s171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA. Cholinergic systems in brain development and disruption by neurotoxicants: nicotine, environmental tobacco smoke, organophosphates. Toxicol. Appl. Pharmacol. 2004;198:132–151. doi: 10.1016/j.taap.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. Developmental neurotoxicity of organophosphates: a case study of chlorpyrifos. In: Gupta RC, editor. Toxicity of Organophosphate and Carbamate Pesticides. San Diego: Elsevier Academic Press; 2005. pp. 293–314. [Google Scholar]
- Slotkin TA, Brown KK, Seidler FJ. Developmental exposure of rats to chlorpyrifos elicits sex-selective hyperlipidemia and hyperinsulinemia in adulthood. Environ. Health Perspect. 2005;113:1291–1294. doi: 10.1289/ehp.8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Levin ED, Seidler FJ. Comparative developmental neurotoxicity of organophosphate insecticides: effects on brain development are separable from systemic toxicity. Environ. Health Perspect. 2006;114:746–751. doi: 10.1289/ehp.8828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, MacKillop EA, Ryde IT, Seidler FJ. Ameliorating the developmental neurotoxicity of chlorpyrifos: a mechanisms-based approach in PC12 cells. Environ. Health Perspect. 2007a;115:1306–1313. doi: 10.1289/ehp.10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, MacKillop EA, Ryde IT, Tate CA, Seidler FJ. Screening for developmental neurotoxicity using PC12 cells: comparisons of organophosphates with a carbamate, an organochlorine and divalent nickel. Environ. Health Perspect. 2007b;115:93–101. doi: 10.1289/ehp.9527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. Comparative developmental neurotoxicity of organophosphates in vivo: transcriptional responses of pathways for brain cell development, cell signaling, cytotoxicity and neurotransmitter systems. Brain Res. Bull. 2007;72:232–274. doi: 10.1016/j.brainresbull.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Snedecor GW, Cochran WG. Statistical Methods. Ames, Iowa: Iowa State University Press; 1967. [Google Scholar]
- Song X, Seidler FJ, Saleh JL, Zhang J, Padilla S, Slotkin TA. Cellular mechanisms for developmental toxicity of chlorpyrifos: targeting the adenylyl cyclase signaling cascade. Toxicol. Appl. Pharmacol. 1997;145:158–174. doi: 10.1006/taap.1997.8171. [DOI] [PubMed] [Google Scholar]
- Song X, Violin JD, Seidler FJ, Slotkin TA. Modeling the developmental neurotoxicity of chlorpyrifos in vitro: macromolecule synthesis in PC12 cells. Toxicol. Appl. Pharmacol. 1998;151:182–191. doi: 10.1006/taap.1998.8424. [DOI] [PubMed] [Google Scholar]
- Teng KK, Greene LA. Cultured PC12 cells: a model for neuronal function and differentiation. In: Celis JE, editor. Cell Biology: A Laboratory Handbook. San Diego: Academic Press; 1994. pp. 218–224. [Google Scholar]
- Trauth JA, Seidler FJ, Slotkin TA. An animal model of adolescent nicotine exposure: effects on gene expression and macromolecular constituents in rat brain regions. Brain Res. 2000;867:29–39. doi: 10.1016/s0006-8993(00)02208-3. [DOI] [PubMed] [Google Scholar]
- Tuler SM, Hazen AA, Bowen JM. Release and metabolism of dopamine in a clonal line of pheochromocytoma (PC12) cells exposed to fenthion. Fund. Appl. Toxicol. 1989;13:484–492. doi: 10.1016/0272-0590(89)90284-4. [DOI] [PubMed] [Google Scholar]
- Wardle NJ, Bligh SW, Hudson HR. Organophosphorus compounds: intervention in mechanisms of signal transduction relevant to proliferative, immunological and circulatory disorders. Curr. Med. Chem. 2008;15:2230–2257. doi: 10.2174/092986708785747517. [DOI] [PubMed] [Google Scholar]
- Winick M, Noble A. Quantitative changes in DNA, RNA and protein during prenatal and postnatal growth in the rat. Dev. Biol. 1965;12:451–466. doi: 10.1016/0012-1606(65)90009-6. [DOI] [PubMed] [Google Scholar]
- Yanai J, Vatury O, Slotkin TA. Cell signaling as a target and underlying mechanism for neurobehavioral teratogenesis. Ann. N.Y. Acad. Sci. 2002;965:473–478. doi: 10.1111/j.1749-6632.2002.tb04188.x. [DOI] [PubMed] [Google Scholar]
- Yanai J, Beer A, Huleihel R, Izrael M, Katz S, Levi Y, Rozenboim I, Yaniv SP, Slotkin TA. Convergent effects on cell signaling mechanisms mediate the actions of different neurobehavioral teratogens: alterations in cholinergic regulation of PKC in chick and avian models. Ann. N.Y. Acad. Sci. 2004;1025:595–601. doi: 10.1196/annals.1316.074. [DOI] [PubMed] [Google Scholar]
- Yousefpour M, Bahrami F, Shahsavan Behboodi B, Khoshbaten A, Asgari A. Paraoxon-induced ultrastructural growth changes of rat cultured hippocampal cells in neurobasal/B27. Toxicology. 2006;217:221–227. doi: 10.1016/j.tox.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Yu F, Wang Z, Ju B, Wang Y, Wang J, Bai D. Apoptotic effect of organophosphorus insecticide chlorpyrifos on mouse retina in vivo via oxidative stress and protection of combination of vitamins C and E. Exp. Toxicol. Pathol. 2008;59:415–423. doi: 10.1016/j.etp.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Zeiders JL, Seidler FJ, Slotkin TA. Ontogeny of regulatory mechanisms for b-adrenoceptor control of rat cardiac adenylyl cyclase: targeting of G-proteins and the cyclase catalytic subunit. J. Mol. Cell. Cardiol. 1997;29:603–615. doi: 10.1006/jmcc.1996.0303. [DOI] [PubMed] [Google Scholar]
- Zeiders JL, Seidler FJ, Iaccarino G, Koch WJ, Slotkin TA. Ontogeny of cardiac b-adrenoceptor desensitization mechanisms: agonist treatment enhances receptor/G-protein transduction rather than eliciting uncoupling. J. Mol. Cell. Cardiol. 1999a;31:413–423. doi: 10.1006/jmcc.1998.0875. [DOI] [PubMed] [Google Scholar]
- Zeiders JL, Seidler FJ, Slotkin TA. Agonist-induced sensitization of b-adrenoceptor signaling in neonatal rat heart: expression and catalytic activity of adenylyl cyclase. J. Pharmacol. Exp. Ther. 1999b;291:503–510. [PubMed] [Google Scholar]