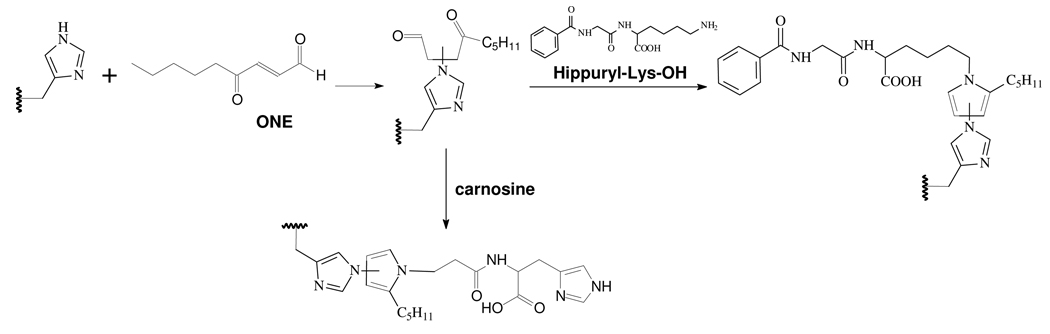
Abstract
The lipid oxidation product 4-oxo-2-nonenal (ONE) derived from peroxidation of polyunsaturated fatty acids is a highly reactive protein cross-linking reagent. The major family of cross-links reflects conjugate addition of side-chain nucleophiles such as sulfhydryl or imidazole groups to the C=C of ONE to give either a 2- or 3-substituted 4-ketoaldehyde, which then undergoes Paal-Knorr condensation with the primary amine of protein lysine side-chains. If ONE is intercepted in biological fluids by antielectrophiles such as glutathione (GSH) or β-alanylhistidine (carnosine), this would lead to circulating 4-ketoaldehydes that could then bind covalently to the protein Lys residues. This phenomenon was investigated by SDS–PAGE and mass spectrometry (MALDI-TOF and LC-ESI-MS/MS with both tryptic and chymotryptic digestion). Under the reaction conditions of 0.25 mM to 2 mM ONE, 1 mM GSH or carnosine, 0.25 mM bovine β-lactoglobulin (β-LG), 100 mM phosphate buffer (pH 7.4, 10% ethanol), 24 h, 37 °C, virtually every Lys of β-LG was found to be fractionally cross-linked to GSH. Cross-linking of Lys to carnosine was slightly less efficient. Using cytochrome c and RNase A, we showed that ONE becomes more protein-reactive in the presence of GSH, whereas protein modification by 4-hydroxy-2-nonenal is inhibited by GSH. Stable antielectrophile–ONE–protein cross-links may serve as biomarkers of oxidative stress and may represent a novel mechanism of irreversible protein glutathionylation.
Introduction
Oxidative stress is considered to contribute to the pathogenesis of many age-related and neurodegenerative diseases, such as Alzheimer’s disease and Parkinson’s disease (1–3). Lipid peroxidation during oxidative stress releases reactive bifunctional aldehydes such as malondialdehyde, acrolein, 4-hydroxy-2(E)-nonenal (HNE) and the more recently reported 4-oxo-2(E)-nonenal (ONE) (4–7). ONE has been found to be more reactive towards protein nucleophiles as well as more neurotoxic than HNE (8–10). It was reported that ONE can react with Cys and His through Michael addition (8, 11). ONE and Lys can form a readily reversible Schiff base adduct after short exposure time (9) which can be oxidized to a stable 4-ketoamide adduct at longer times (12). ONE is also capable of cross-linking proteins. Incubation of protein with ONE has resulted in fluorescent Lys–Lys cross-links (13, 14) and Cys–Lys or His–Lys pyrrole cross-links (11, 15, 16). The latter is very abundant in ONE-modified bovine β-lactoglobulin (β-LG) (17).
Glutathione (GSH) is the principal cellular nucleophile that functions by reaction with electrophiles generated as a consequence of both xenobiotic activation and normal physiologic side-reactions of oxidative metabolism. Other endogenous “antielectrophiles” without reducing capacity include the dipeptide carnosine (β-alanyl-L-histidine) (18, 19). The conjugation of GSH or carnosine with α,β-unsaturated aldehydes formed from lipid peroxidation is an important mechanism for their detoxification. GSH can react with HNE through Michael addition to form a GSH S-conjugate (20–23), which is a biomarker of oxidative stress (23). GSH can also trap ONE to finally form a very stable thiadiazabicyclo-ONE-GSH adduct (TOG) (24). According to their ability to capture lipoxidation products, these antielectrophiles are expected also to prevent protein cross-linking by bifunctional lipoxidation products. However, in the latter case, the antielectrophiles may instead become cross-linked to the proteins. Exploring this unusual mechanism of cross-linking was the key aim of the present investigation.
Materials and Methods
General
All solvents and reagents were commercially available in analytical grade unless stated specifically. All water was purified by a Type D4700 NANOpure Bioresearch Deionization system. GSH, β-LG, ribonuclease A (RNase A) and α-chymotrypsin were purchased from Sigma. L-Carnosine and cytochrome c were purchased from Acros Organics. Hippuryl-Lys-OH was purchased from Bachem. Sequencing grade modified trypsin was purchased from Promega. ZipTips were purchased from Millipore. PD-10 columns (Sephadex G-25 M) were purchased from GE Healthcare. GelCode blue stain reagent was purchased from Pierce. HNE (25) and ONE (11) were prepared as in our previous work. 6,6,7,7,8,8,9,9,9-d9-4-oxo-2(E)-nonenal (d9-ONE) was prepared by a method to be published separately. The β-LG X-ray crystal structure PDB file (ID: 1BSQ) (26) was downloaded from www.pdb.org (27) and viewed with ViewLite (Accelrys Inc.). Protein solutions were concentrated through centrifugal evaporation under reduced pressure with a Savant Speed Vac SC110 system (Forma Scientific, Inc.). Electrophoresis was performed with a Mini-PROTEAN 3 system (Bio-Rad Laboratories, Inc.).
HPLC-ESI-MS/MS
Reversed-phase HPLC was performed with a Surveyor LC system equipped with a 5 µm 2.1 × 250 mm Grace Vydac C18 column with a gradient elution program at a flow rate of 200 µL/min. Eluent A was a mixture of 95% H2O, 5% MeOH and 0.1% formic acid. Eluent B was a mixture of 95% MeOH, 5% H2O and 0.1% formic acid. The gradient program was from 80% A to 20% A over 70 min, 20% A to 80% A over 5 min, then 80% A for 5 min. ESI-MS was performed with a Thermo Finnigan LCQ Advantage instrument in the positive ion mode using nitrogen as the sheath and auxiliary gas. The capillary temperature was 300 °C, the capillary voltage was 35.00 V, and the source voltage was 4.50 kV. Two scan events were used: (1) m/z 300–2000 full scan MS; (2) data dependent scan MS/MS on the most intense ion from (1) or from the parent mass list. The spectra were recorded using dynamic exclusion of previously analyzed ions for 0.5 min with three repeats and a repeat duration of 0.5 min. The MS/MS normalized collision energy was set to 35%. All data were processed with the Qual browser module of Xcalibur (Thermo Electron).
Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry (MALDI-TOF-MS)
Protein spotting: 2 µL of protein sample was mixed with 23 µL of H2O and 25 µL of 3,5-dimethoxy-4-hydroxycinnamic acid (20 mg/mL in 70% aqueous acetonitrile and 0.1% trifluoroacetic acid (TFA)). 1 µL of this mixture was deposited directly onto the MALDI stainless steel target. Proteolytic digest spotting: 10 µL of digested sample was acidified with 2 µL of 2.5% TFA. A ZipTip was wet by 50% aqueous acetonitrile and then equilibrated with 0.1% TFA solution. The peptide sample was extracted with the ZipTip and washed with 0.1% TFA. Finally, the sample was eluted with 2 µL of α-cyano-4-hydroxycinnamic acid (20 mg/mL in 70% aqueous acetonitrile and 0.1% TFA) onto the MALDI stainless steel target. The MALDI-TOF mass spectra were acquired with a Bruker BiFlex III MALDI-TOF mass spectrometer (Bruker Daltonics) equipped with a pulsed nitrogen laser (3 ns pulse at 337 nm) after the samples were dried at 25 °C. All spectra were collected in the positive ion and linear mode (for the protein) or reflectron mode (for the proteolytic digest) with an average of 250 laser shots. All data were processed with XMass (Bruker Daltonics) and m over z (Proteometrics, LLC).
Proteolytic Digestion
Modified β-LG (1 mg) was dissolved in 100 µL of pH 8.0 buffer containing 6 M guanidine hydrochloride and 50 mM Tris. Dithiothreitol (200 mM, 2.5 µL) was added, vortexed, and incubated at 37 °C for 1 h. Then iodoacetamide (400 mM, 5.0 µL) was added, vortexed, and allowed to stand at 25 °C in a dark place for 1 h. Dithiothreitol (200 mM, 7.5 µL) was added again, vortexed, and allowed to consume the unreacted iodoacetamide at 25 °C for another 1 h. To 25 µL of this denatured sample was added 50 µL of trypsin (0.1 µg/µL) or 10 µL of α-chymotrypsin (2.0 µg/µL) solution. Then the digestion mixture was diluted to 200 µL with pH 7.8 NH4HCO3 solution (50mM), and the mixture was incubated at 37 °C for 24 h. The solution was stored at −20 °C for LC-MS or MALDI-TOF-MS analysis.
SDS–PAGE
13% resolving gel and 5% stacking gel were prepared by the Laemmli method (28). 20 µL of the protein sample was added to 40 µL of the denaturing buffer, vortexed and heated in a boiling water bath for 4 min. Aliquots (15 µL) of the denatured protein solution were injected into the wells of the stacking gel. After being electrophoresed at a constant current of 20 mA, the gel was stained with GelCode Blue stain reagent.
Preparation of Nα-Acetyl-Glutathione (AcGSH)
GSH (200 mg, 0.65 mmol) was dissolved in 1.65 mL of water, and 4.0 mL of acetic anhydride/acetic acid (v/v = 1/1) was added to the solution dropwise over 1 h. Then the reaction mixture was put in a 0 °C bath for 30 min. Another 1.0 mL acetic anhydride was added. After being stirred at 0 °C for 3 h, it was warmed up to 25 °C overnight. The supernatant was decanted and the solid was washed with ethyl ether 5 times using a spatula to scratch the inner side of the flask. The resulting solid was dissolved in 2.0 mL of water, adjusted to pH 12 with 0.2 N NaOH, stirred for 3 h at 25 °C, and then acidified to pH 6 with 1 N HCl. The neutralized mixture was concentrated, purified by semi-preparative HPLC and the solvent from the pooled peak fractions removed, affording 43 mg of white solid. 1H NMR (400 MHz, D2O) δ 4.37 (dd, 1H, J = 6.8, 5.6 Hz), δ 4.16 (dd, 1H, J = 9.6, 5.2 Hz), δ 3.82 (s, 2H), δ 2.73 (dd, 1H, J = 18.0, 14.0 Hz), δ 2.72 (dd, 1H, J = 19.6, 14.0 Hz), δ 2.28 (t, 2H, 7.2 Hz), δ 2.04 (m, 1H), δ 1.85 (s, 3H), δ 1.81 (m, 1H); HRMS (FAB) calcd for C12H20N3O7S (MH+) 350.1017, found, 350.1023.
Incubation of β-LG with ONE or HNE for MALDI-TOF Analysis
A solution of β-LG (2.5 mM, 25 µL) added to a pH 7.4 phosphate buffer (100 mM, 200 µL) was incubated with a solution of various concentrations of ONE in EtOH (0, 2.5, 5.0, 10.0 or 20.0 mM; 25 µL) or HNE in EtOH (5.0 mM, 25 µL) at 37 °C. After 24 h, the solution was mixed with the matrix solution and deposited onto the MALDI target for analysis.
Incubation of β-LG and GSH, Carnosine or AcGSH with ONE or HNE for MALDI-TOF Analysis
A solution of β-LG (2.5 mM, 25 µL) added to a pH 7.4 phosphate buffer (100 mM, 197.5 µL) was incubated with GSH, carnosine or AcGSH aqueous solution (100 mM, 2.5 µL) and a solution of various concentrations of ONE in EtOH (0, 2.5, 5.0, 10.0 or 20.0 mM; 25 µL) or HNE in EtOH (5.0 mM, 25 µL) at 37 °C. After 24 h, the solution was mixed with the matrix solution and deposited onto the MALDI target for analysis.
Incubation of β-LG and GSH, Carnosine or Hippuryl-Lys-OH with ONE for SDS–PAGE and Proteolytic Digestion
A solution of β-LG (2.5 mM, 50 µL) added to a pH 7.4 phosphate buffer (100 mM, 395 µL) was incubated with GSH, carnosine or hippuryl-Lys-OH aqueous solution (100 mM, 5.0 µL) and a solution of various concentrations of d0-ONE or d9-ONE in EtOH (5.0, 10.0 or 20.0 mM; 50 µL) at 37 °C. After 24 h, d0-ONE and d9-ONE reaction mixtures with the same ONE concentration were mixed together (v/v = 1/1). 20 µL of each was used for SDS–PAGE except for the hippuryl-Lys-OH case. The others were diluted to 2.5 mL with water and applied to a PD-10 column eluting with 3.5 mL water to remove the unbound ONE and the buffer salts. The eluted protein solution was concentrated by centrifugal evaporation prior to proteolytic digestion.
Incubation of Cytochrome c or RNase A and ONE with or without GSH
A solution of cytochrome c or RNase A (2.5 mM, 50 µL) added to a pH 7.4 phosphate buffer (100 mM, 400 µL or 390 µL if GSH was added) was incubated with a solution of ONE (10 mM, 50 µL) only or ONE (10 mM, 50 µL) and GSH aqueous solution (50 mM, 10 µL) at 37 °C. After 5, 20 min, 1, 6 and 24 h, aliquots of the solution were mixed with the matrix solution and deposited onto the MALDI target for analysis.
Preparation of β-LG-SSG
GSSG (20 mM, final) was added to a solution containing β-LG (0.2 mM, final) and GSH (0.8 mM, final) in PBS. The mixture was incubated for 30 min in a 30 °C water bath.
Western Blot Analysis of β-LG Adducts
Following incubation of β-LG (0.25 mM) with GSH (1 mM) and/or ONE (1 mM), mixtures were purged with argon, and N-ethylmaleimide was added (20 mM, final) to react with any remaining reduced sulfhydryl groups. To these mixtures, as well as to β-LG-SSG, was added Laemmli’s sample buffer +/− DTT (25 mM), and volumes corresponding to 1 µg β-LG (or 75 µg β-LG-SSG) were loaded on 12.5% SDS-polyacrylamide gels, then transferred to PVDF membranes. Membranes were blocked with 5% milk in TBS (1% Tween) for 1 h, then incubated with α-GSH primary antibody (Virogen, 1:500) for 1 h, washed in TBST (Tris-Buffered Saline Tween-20), incubated with α-mouse secondary antibody (Jackson Laboratories, 1:10,000) for 1 h, washed in TBST, and developed using the Super Signal West Pico Chemiluminescent Detection Reagent (ThermoScientific).
Results and Discussion
Inhibition of ONE-based β-LG Cross-Linking by GSH and Carnosine
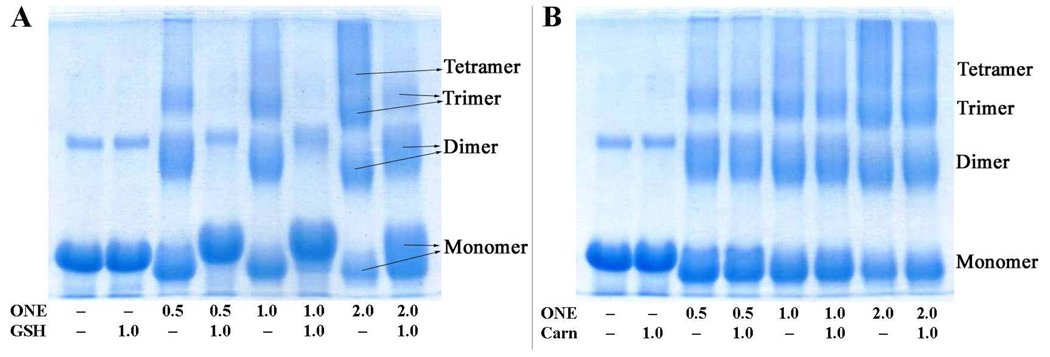
Our previous study showed that ONE has a higher potential to cross-link proteins than acrolein, 2,4-decadienal (DDE), HNE or malondialdehyde (29). ONE has been shown to cross-link proteins principally through its ability to undergo conjugate addition of Cys and His side-chains to the C=C (at either C2 or preferentially at C3(11)), resulting in a protein-bound 4-ketoaldehyde that can subsequently undergo Paal-Knorr condensation with the ε-amino group of Lys side-chains to give Cys–Lys and His–Lys pyrrole cross-links (11, 15). This type of cross-linking is one of the predominant modifications observed for ONE-modified β-LG (17). Although it was anticipated that formation of a Cys or His Michael adduct (MA) following the Lys–ONE Schiff base adduct would lead to ONE-based protein cross-links (30), no mass spectrometric evidence from our study showed that this cross-linking existed after incubation of ONE and β-LG for 10 min, 5 h or 24 h with or without NaBH4 reduction treatment. Small molecules which contain reactive sulfhydryl or imidazole nucleophiles can react with ONE through Michael addition. The resulting 4-ketoaldehyde products are also able to condense with Lys residues of the protein, thus forming pseudo-cross-links. GSH has a free sulfhydryl, and carnosine has an imidazole group. Both nucleophiles were found by SDS–PAGE to inhibit ONE-induced protein cross-linking, dependent on the ONE concentration (Figure 1). GSH proved to be the more effective cross-linking inhibitor. When the ONE concentration is lower than or equal to the GSH concentration (Figure 1A, lanes 4 and 6), only minimal protein cross-linking is observed. When the ONE concentration is higher than the GSH concentration (Figure 1A, lane 8), some ONE-induced protein cross-linking is observed, presumably due to the ONE not consumed by GSH.
Figure 1.
SDS–PAGE of 0.25 mM of β-LG incubated with ONE (d0:d9 = 1:1) at various concentrations (mM) with or without 1.0 mM of GSH (A) or carnosine (B).
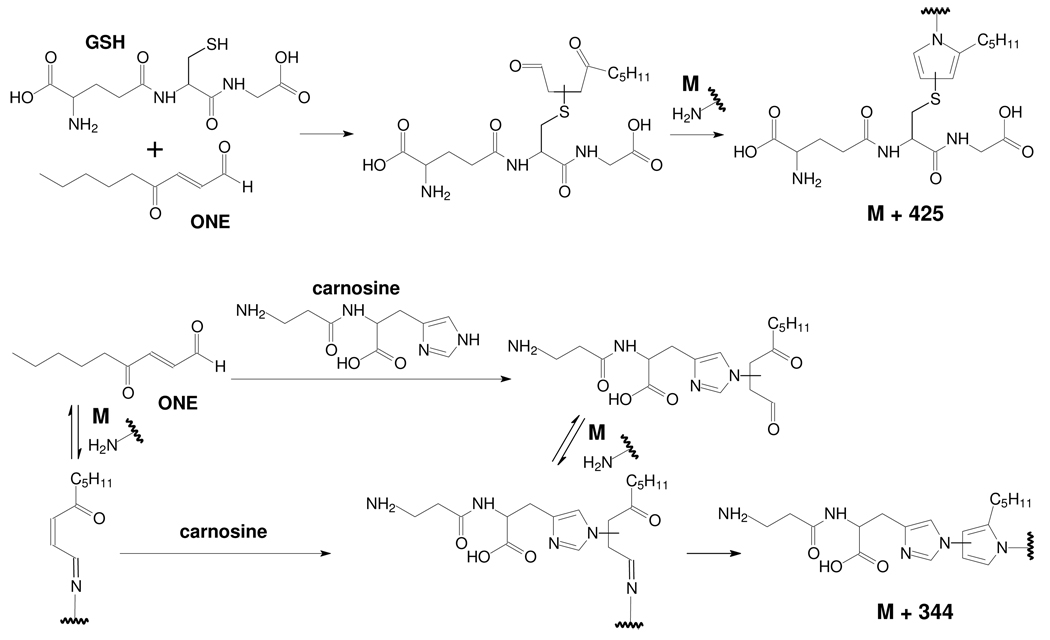
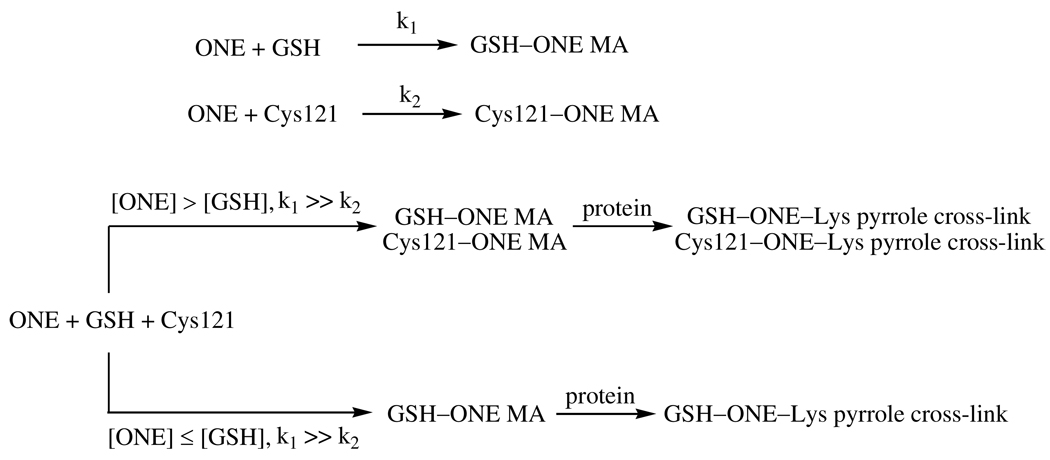
The rate constants for ONE reacting with GSH or Cys are much faster (> 1000 times) than with Lys and His (8, 9). Therefore, ONE is quickly trapped by GSH through–sulfhydryl.ONE Michael addition after they are mixed together with β-LG, resulting in the formation of GSH-adducted 4-ketoaldehydes, which can subsequently react with primary amines, leading to ONE-derived adduction of GSH to the protein (Scheme 1). However, the rate constants for the reaction of ONE with His and Lys are comparable (8, 9). Therefore, when β-LG is incubated with ONE and carnosine, ONE-induced carnosine-protein cross-links could be formed by two pathways (Scheme 1). One path is the initial reaction of ONE with carnosine to form the MA, which then reacts with a protein-bound amine to form a Schiff base-MA intermediate which evolves into the ONE-derived adduction of carnosine to the protein via a pyrrole. The second path is the initial reaction of ONE with a protein-bound amine to form a Schiff base, which then reacts with carnosine through Michael addition to produce a common intermediate, which cyclizes into a pyrrole cross-link. As Cys reacts with ONE much faster than His and Lys, the GSH–ONE MA is more readily formed than the carnosine–ONE MA or amine–ONE Schiff base adduct, which can explain the greater efficiency of GSH as an inhibitor of ONE-based protein cross-linking.
Scheme 1.
Trapping of ONE by GSH or carnosine results in a 4-ketoaldehyde capable of modifying protein-based lysines
β-LG modified directly by ONE and modified by the GSH–ONE MA both show alterations of migration in SDS–PAGE compared to untreated β-LG. ONE-modified β-LG migrates faster than the native protein. ONE modification can reduce the charge on β-LG as converting a Lys ε-amine to a pyrrole reduces the pKa. The hydrophobic alkyl chain may also enhance the binding of SDS. While the GSH–ONE MA modified protein migrates more slowly as expected due to the addition of mass to the protein after modification.
Cross-Linking of GSH or Carnosine to β-LG by ONE
The SDS–PAGE experiments (Figure 1) demonstrated that GSH inhibited ONE-based protein cross-linking, leading to an alternative protein modification. Therefore, we investigated the structural nature of ONE-dependent cross-linking of GSH to protein by mass spectrometry, with both MALDI-TOF-MS and LC-ESI-MS/MS. MALDI-TOF-MS can straightforwardly provide some information about modifications of the protein or peptide by mass difference, and LC-ESI-MS/MS can identify the modified sites of the tryptic or chymotryptic peptides based on the fragmentation patterns.
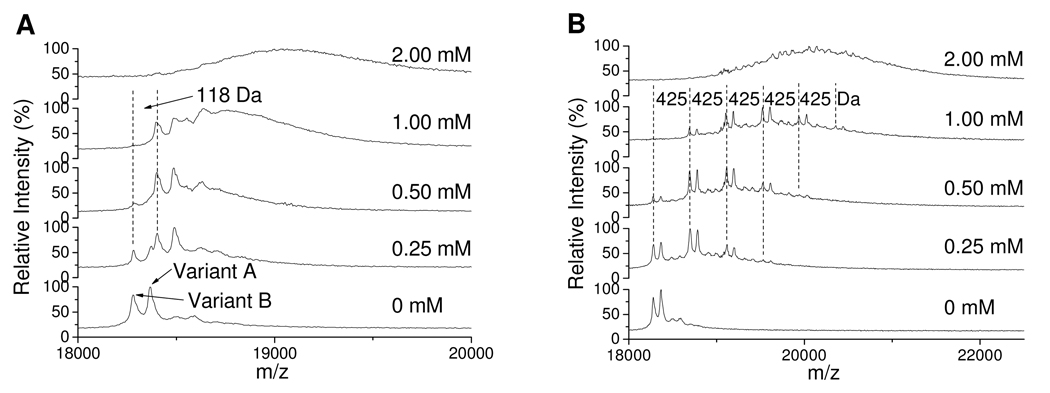
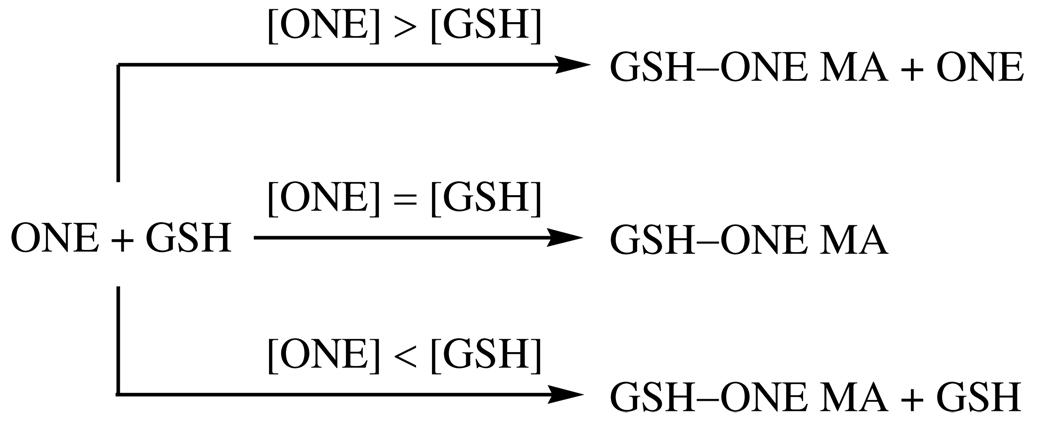
β-LG is composed of two variants, a heavier A chain and a lighter B chain. Variant B is effectively the D64G/V118A double mutant of variant A (31). There are 15 Lys, 2 His and 1 free Cys residues in both variants of β-LG. Incubation of β-LG with a slight stoichiometric excess of ONE results in a selective increase of 118 Da in both variants due to generation of an internal Cys–ONE–Lys pyrrole cross-link (Figure 2A). Higher concentrations of ONE result in generation of a more diffuse mixture of multiply-modified protein chains, which makes the monomer peaks broad and indiscernible. In contrast, incubation of β-LG with ONE in the presence of equal or excess GSH results in a mass shift of 425 Da (Figure 2B) which is consistent with the modification of Lys residues by the GSH–ONE MA (Scheme 1). When the [ONE] is twice the [GSH], besides the modification from GSH–ONE, the protein is also modified by excess ONE alone, so the observed peak is broadened (see the [ONE] = 2.00 mM of Figure 2B). Importantly, the spectra show that when [ONE] ≤ [GSH] ONE-alone modification is barely detectable, and this condition is more typical of the intracellular milieu. In this study, in order to observe obvious mass changes and readily detect GSH–ONE MA modified peptides in the mass spectrum of the protein after the modification, up to millimolar ONE was used. This ONE concentration may be much higher than the in vivo concentration during oxidative stress. At physiologic conditions, where [ONE] is much lower than [GSH] and [proteins], it is expected that ONE will be predominantly trapped by excess GSH to form the GSH–ONE MA, which has the potential to form a pyrrole cross-link with protein Lys residues. The low concentration of [GSH–ONE MA] will result in a small fraction of many different protein Lys residues being modified. The low abundance of any individual one of these GSH-ONE MA cross-linked peptides may be difficult to be detected by LC-MS.
Figure 2.
MALDI-TOF spectra of 0.25 mM β-LG modified by ONE at various concentrations (shown in figures) without GSH (A) or with 1 mM GSH (B). The mass difference of 118 Da in A is due to the Cys–ONE–Lys pyrrole cross-link, and 425 Da is due to the cross-link of GSH to the protein by ONE.
As the GSH–d0-ONE MA and GSH–d9-ONE MA modified peptides will introduce mass shifts of 425 and 434 Da (Scheme 1), respectively, the mass of all possible modified peptides can be predicted. Scheme 1 presumes that only Lys containing peptides can be modified by the GSH–ONE MA. Therefore, any Lys or amine-containing peptide from tryptic or chymotryptic digests is a potential target of this modification, and their modified masses (m+425 and m+434) were calculated and included in a parent mass list during the data dependent scan, allowing their preferential fragmentation during elution by LC-MS/MS. With the aid of the double peaks of the same peptide modified by GSH–d0-ONE MA and GSH–d9-ONE MA, respectively, the modified peptides were searched for and validated manually. For a peptide to be identified as containing a GSH–ONE MA modification, it had to meet two requirements. First, it appeared as a predicted doublet (m+425:m+434), where the m+425 and m+434 peptides had similar retention times and intensities with the peak m+434 eluting a little earlier. Second, the mass spectra of both peptides had to exhibit similar fragmentation patterns with y and b ions consistent with the predicted sequence. In addition, the GSH–ONE adducted peptides always undergo a facile neutral loss of m/z 129 and 64.5 for singly and doubly charged peptides, respectively (32). However, if the MS/MS spectrum of each peak of the doublet had too little information to be interpreted, such as the peaks marked with “$” in Table 1, the doublet peaks were manually searched in the spectrum of the control which was the incubation mixture of β-LG and ONE (d0:d9 = 1:1) without GSH. If these doublet peaks were absent in the control, they were interpreted as GSH–ONE MA modified peptides.
Table 1.
^. GSH–ONE-modified β-LG peptides detected by HPLC-ESI-MS/MS in the tryptic digest.
| Peptide sequence | Position | Mass of GSH–ONE-modified peptide | Modified residue |
|
|---|---|---|---|---|
| Theoretical | Observed | |||
| LIVTQTMK | 1–8 | 1358.71/1367.76 | 1358.57/1367.58 | L1 |
| LIVTQTMKGLDIQK | 1–14 | 2013.08/2022.13 | 1007.25/1011.87#$ | L1 or K8 |
| VYVEELKPTPEGDLEILLQK | 41–60 | 2738.42/2747.48 | 1369.95/1374.48# | K47 |
| WENDEC*AQKK | 61–70 | 1732.73/1741.79 | 1732.46/1741.53 | K69 |
| WENGEC*AQKK | 61–70 | 1674.73/1683.78 | 1674.44/1683.47 | K69 |
| KIIAEK | 70–75 | 1126.62/1135.67 | 1126.55/1135.55 | K70 |
| IIAEKTK | 71–77 | 1227.67/1236.72 | 1227.54/1236.61 | K75 |
| TKIPAVFK | 76–83 | 1328.73/1337.78 | 1328.50/1337.65 | K77 |
| IPAVFKIDALNENK | 78–91 | 1997.04/2006.10 | 999.22/1003.53#$ | K83 |
| IDALNENKVLVLDTDYK | 84–100 | 2388.20/2397.26 | 1194.80/1199.32# | K91 |
| VLVLDTDYKK | 92–101 | 1618.84/1627.90 | 1618.61/1627.60 | K100 |
| TPEVDDEALEKFDK | 125–138 | 2060.94/2070.00 | 1031.14/1035.64#$ | K135 |
| FDKALK | 136–141 | 1146.59/1155.64 | 1146.50/1155.55 | K138 |
| ALKALPMHIR | 139–148 | 1574.86/1583.91 | 1574.64/1583.68 | K141 |
SICs and tandem mass spectra of adducted peptides were presented in the Supporting Information, Figures S1–S14
alkylated with iodoacetamide
double charged peak
Both tandem mass spectra of GSH-d0-ONE and GSH-d9-ONE modified peptides had too little information to be interpreted, but their masses, which matched the modification, and double LC peaks with similar retention times and intensities, which were not found in the control, implied the right assignment.
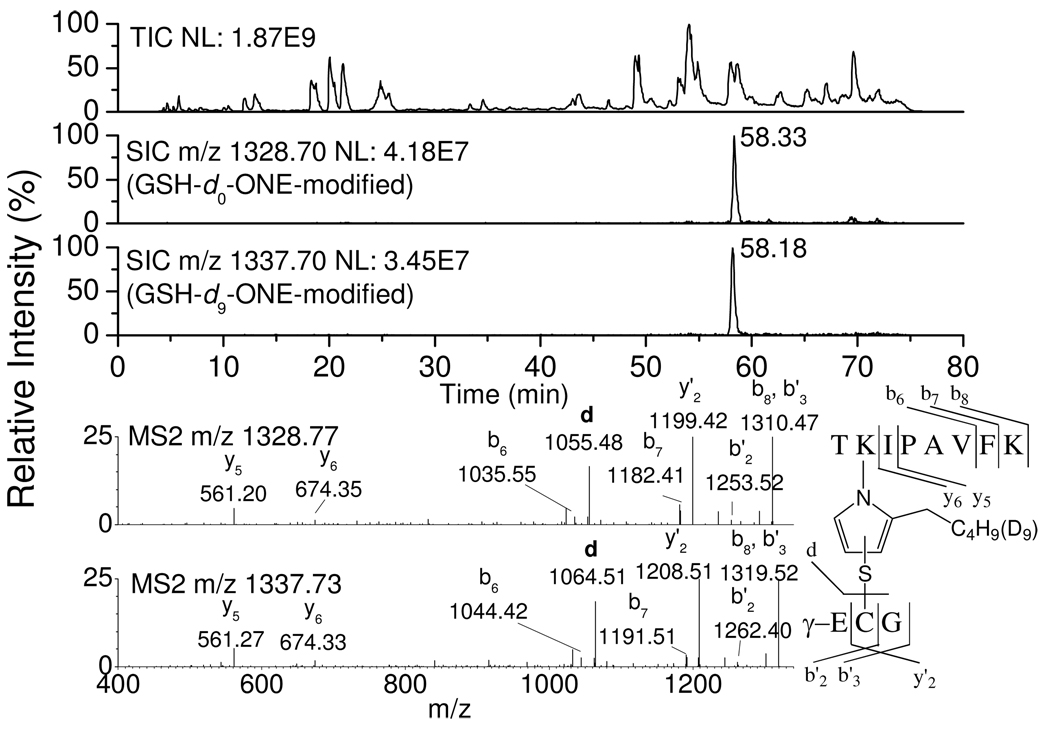
LC-ESI-MS/MS analysis of tryptic (Table 1, selected ion chromatograms (SICs) and tandem mass spectra of adducted peptides are present in the Supporting Information, Figures S1–S14) or chymotryptic (Table S1, Supporting Information) digests of β-LG incubated with 1 mM ONE (d0:d9 = 1:1) and 1 mM GSH shows that essentially every primary amine, viz. each Lys and the N-terminal amine, is modified to some extent by ONE cross-linked with GSH. All of the GSH–ONE-modified peptides have two LC peaks with similar intensity, i.e. m/z m+425 (for GSH–d0-ONE modification) and m/z m+434 (for GSH–d9-ONE modification), and their sequences could be confirmed by MS/MS spectra. Figure 3 shows an example of LC chromatograms, tandem mass spectra of the GSH–ONE-modified peptide (76TKIPAVF83K) and the fragmentation and sequence assignment of the peptide. The top trace is the smoothed total ion chromatogram (TIC) of the tryptic digest of modified β-LG. The second and third traces are the SICs of the GSH–d0-ONE-modified (m/z 1328.70) and GSH–d9-ONE-modified (m/z 1337.70) peptide 76TKIPAVF83K, respectively, each modified at K77. The retention time of the peak containing d9-ONE (e.g. 58.18 min for m/z 1337.70) is always a little earlier than the corresponding d0-ONE one (e.g. 58.33 min for m/z 1328.70). The bottom two MS/MS spectra of these two peaks, which have similar fragmentation patterns, clearly show that GSH is covalently attached by d0-or d9-ONE to the peptide 76TKIPAVF83K at K77. The abundant y'2 and d ions indicate that Cys of the GSH is directly connected to ONE, excluding the alternative possibility of modification of the α-amino group of glutamic acid of the GSH. The y5–y6 and b6–b7 ions show that K77 of 76TKIPAVF83K, and not K83, is modified.
Figure 3.
TIC (1st trace) of the tryptic digest of modified β-LG by ONE (d0:d9 = 1:1) in the presence of GSH and SIC and tandem mass spectrum of modified 76TKIPAVF83K by GSH.d0-ONE MA (2nd trace and 1st spectrum) or GSH.d9-ONE MA (3rd trace and 2nd spectrum).
The proteolytic digest of modified β-LG was also subjected to MALDI analysis. MALDI-TOF spectra not only reveal the ONE-modified peptides easily, since the d0-ONE and d9-ONE-modified peptides appear as doublets, but also further confirm that GSH can inhibit the ONE-based protein cross-linking.
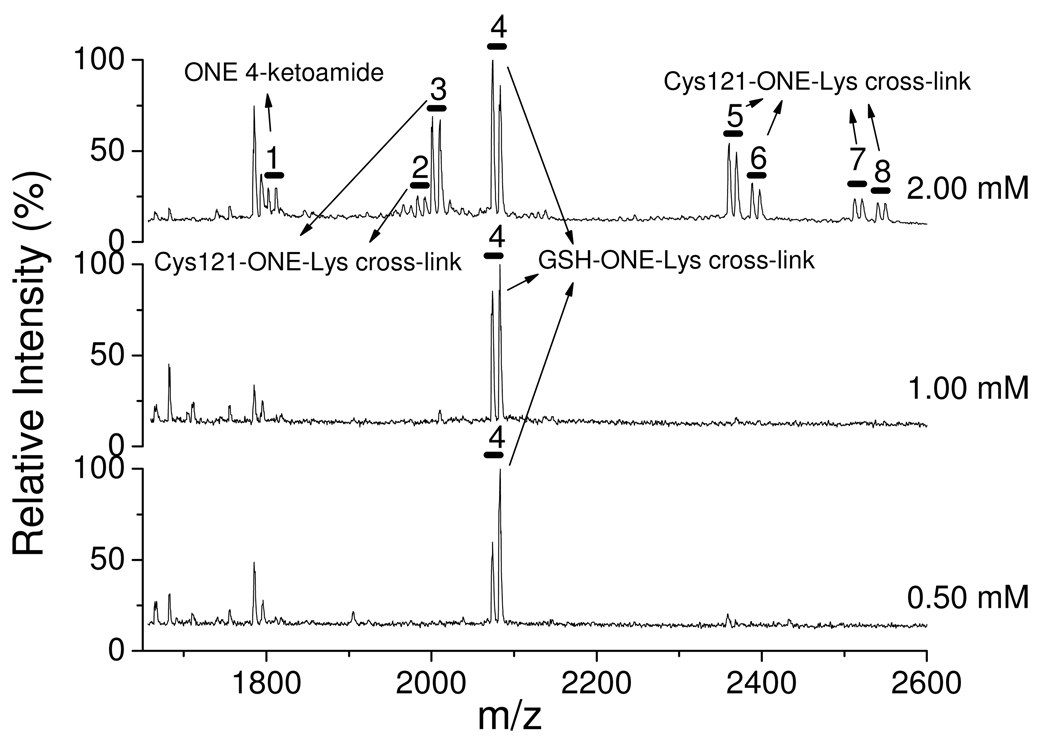
Figure 4 indicates that when the [ONE] is twice the [GSH] (see the top trace), 8 doublets (m and m+9) are observed in the range shown. They were identified and sequenced by LC-MS/MS (Table 2). Doublet 1 is the 4-ketoamide product of K135 of 123VRTPEVDDEALEK136F. Doublets 2, 3, 5, 6, 7 and 8 are Cys–ONE–Lys pyrrole cross-links. Doublet 4 is GSH–ONE MA modified K135 of 123VRTPEVDDEALEK136F. When the [ONE] is equal to or lower than the [GSH] (see the middle and bottom traces, respectively), doublet 4 is still observed, while all of the other ONE-based modifications, including the pyrrole cross-links, are not detectable. These observations support the conclusion that GSH–ONE cross-linking to the protein inhibits ONE-induced protein-protein cross-linking as well as other ONE-based modifications by stoichiometric reaction of ONE and GSH followed by adduction of the ONE-GSH MA to the protein.
Figure 4.
MALDI-TOF spectra of the chymotryptic digest of β-LG incubated with 1 mM GSH and ONE (d0:d9 = 1:1, 0.50, 1.00 and 2.00 mM).
Table 2.
Doublets assignment of the Figure 4 by LC-MS/MS
| No. | Observed mass (MALDI-TOF) |
Assigned peptide sequence |
Position | Theoretical mass |
Modified residue |
Modified type |
|---|---|---|---|---|---|---|
| 1 | 1801.9/1811.0 | VRTPEVDDEALEKF | 123–136 | 1801.9/1811.0 | K135 | 4-ketoamide |
| 2 | 1982.1/1991.1 | CLVRTPEVDDEALE KF |
121–136 | 1982.0/1991.1 | C121 + K135 |
Pyrrole cross-link |
| 3 | 2000.1/2009.1 | CL+ VRTPEVDDEALEKF |
121–122 + 123–136 |
2000.0/2009.1 | C121 + K135 |
Pyrrole cross-link |
| 4 | 2073.1/2082.1 | Glutathione + VRTPEVDDEALEKF |
GSH + 123–136 |
2073.0/2082.0 | GSH + K135 |
Pyrrole cross-link |
| 5 | 2359.3/2368.3 | AC*QCL + VRTPEVDDEALEKF |
118–122 + 123–136 |
2359.1/2368.2 | C121 + K135 |
Pyrrole cross-link |
| 6 | 2387.3/2396.3 | VC*QCL + VRTPEVDDEALEKF |
118–122 + 123–136 |
2387.2/2396.2 | C121 + K135 |
Pyrrole cross-link |
| 7 | 2511.3/2520.4 | AC@QCL + VRTPEVDDEALEKF |
118–122 + 123–136 |
2511.1/2520.2 | C121 + K135 |
Pyrrole cross-link |
| 8 | 2539.3/2548.4 | VC@QCL + VRTPEVDDEALEKF |
118–122 + 123–136 |
2539.2/2548.2 | C121 + K135 |
Pyrrole cross-link |
alkylated with iodoacetamide
connected with disulfide bond to dithiothreitol in which one sulfhydryl group is alkylated with iodoacetamide
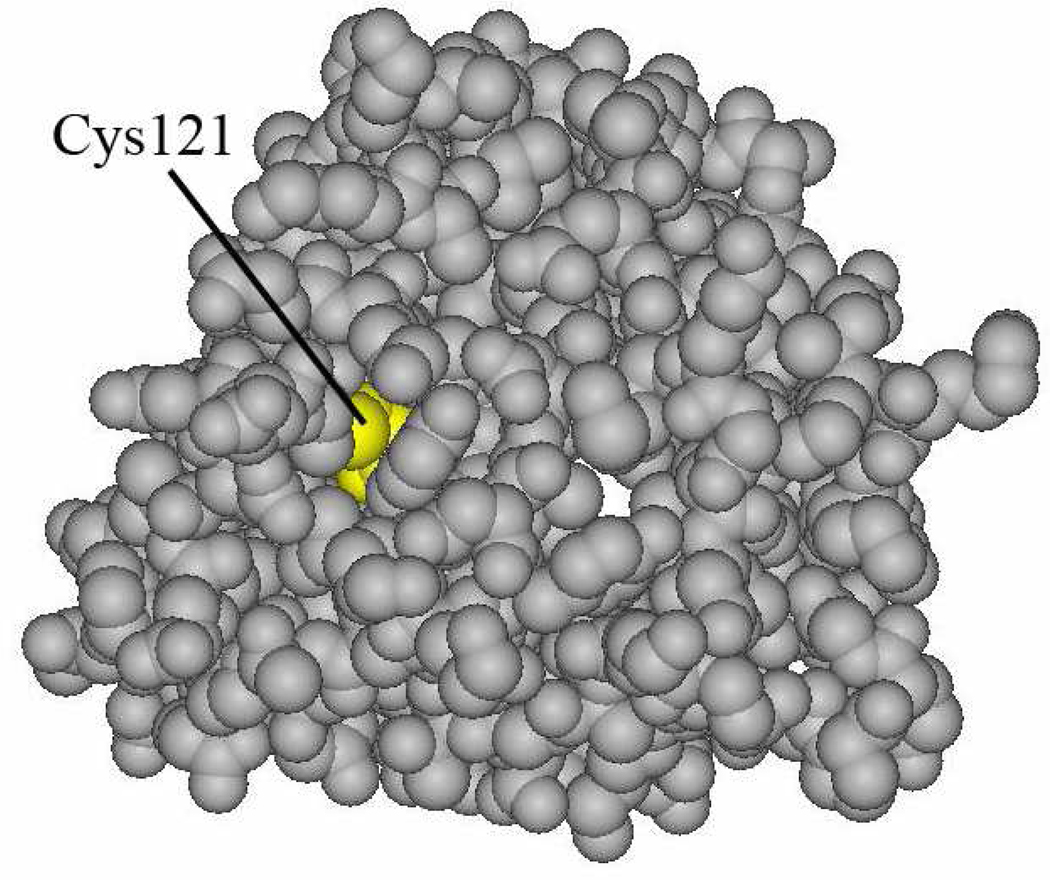
In the presence of ONE in excess of GSH, except for the Lys–ONE 4-ketoamide, only Cys121–ONE–Lys pyrrole cross-links were detected (Figure 4) by MALDI-TOF-MS, although we did find peptides consistent with formation of a His–ONE–Lys pyrrole cross-link with much lower intensity by LC-MS/MS. It is quite interesting that all protein Cys121–ONE–Lys135 cross-links disappear while the GSH–ONE–Lys135 cross-link remains when the [ONE] is equal to or lower than the [GSH]. Possible explanations for these observed phenomena are shown in Scheme 2, which indicates that k1 >> k2 in the formation of GSH– or Cys121–ONE–Lys pyrrole cross-links. Three reasons k2 may be slower are that ONE cannot access Cys121 easily, the pKa of Cys 121 is elevated relative to that of GSH and that a noncovalent complex of ONE and protein is formed before the reaction. Figure 5 is the X-ray structure of variant B of the β-LG. It shows that Cys121 is deeply buried in the protein, so the exogenous molecule could not reach it readily. Burial in a hydrophobic pocket disfavors charge formation and raising the thiol pKa. In addition, like ref (33), the reaction between Cys121 and ONE may be driven in part by noncovalent association of ONE in a hydrophobic pocket. The burial of Cys121 is also a feature of the structure of variant A (structure not shown). In contrast, the Cys thiol of GSH is completely solvent accessible. Therefore, the reaction of Cys121 and ONE may have a much smaller rate constant (k2) than that of GSH and ONE (k1), and effectively all of the ONE will be depleted by GSH before ONE could react with Cys121.
Scheme 2.
Formation of GSH– or Cys121–ONE–Lys pyrrole cross-link
Figure 5.
X-ray structure of β-LG B chain.
As the imidazole-containing peptide carnosine is less reactive than the sulfhydryl-containing GSH, it will compete less well with Cys121 and His residues of β-LG. Figure 1 shows that carnosine only slightly inhibited the ONE-based protein cross-linking, suggesting that at the concentrations employed, few carnosine moieties would be cross-linked to the protein. In addition, comparison of the MALDI-TOF spectra of ONE-modified β-LG in the presence and absence of carnosine did not reveal any significant differences (data not shown). However, LC-ESI-MS/MS analysis of the proteolytic digests did detect carnosine cross-linked to 13 out of 15 lysines of β-LG as well as the N-terminal amine (Table3, SICs and tandem mass spectra of adducted peptides are present in the Supporting Information, Figures S15–S21, and Table S2, Supporting Information), although the peak intensities are much lower than those obtained with GSH (data not shown).
Table 3.
^. Carnosine–ONE-modified β-LG peptides detected by HPLC-ESI-MS/MS in the tryptic digest
| Peptide sequence | Position | Mass of Carnosine–ONE-modified peptide | Modified residue |
|
|---|---|---|---|---|
| Theoretical | Observed | |||
| LIVTQTMK | 1–8 | 1277.73/1286.76 | 1277.78/1286.67 | L1 |
| VYVEELKPTPEGDLEILLQK | 41–60 | 2657.44/2666.47 | 1329.36/1334.00# | K47 |
| IIAEKTK | 71–77 | 1146.69/1155.71 | 1146.63/1155.71 | K57 |
| KIIAEK | 70–75 | 1045.64/1054.67 | 1045.58/1054.65 | K70 |
| TKIPAVFK | 76–83 | 1247.75/1256.78 | 1247.65/1256.68 | K77 |
| FDKALK | 136–141 | 1065.61/1074.64 | 1065.53/1074.58 | K138 |
| ALPMHIR | 142–148 | 1181.66/1190.69 | 1181.68/1190.59 | H146 |
SICs and tandem mass spectra of adducted peptides were presented in the Supporting Information, Figures S15–S21
double charged peak
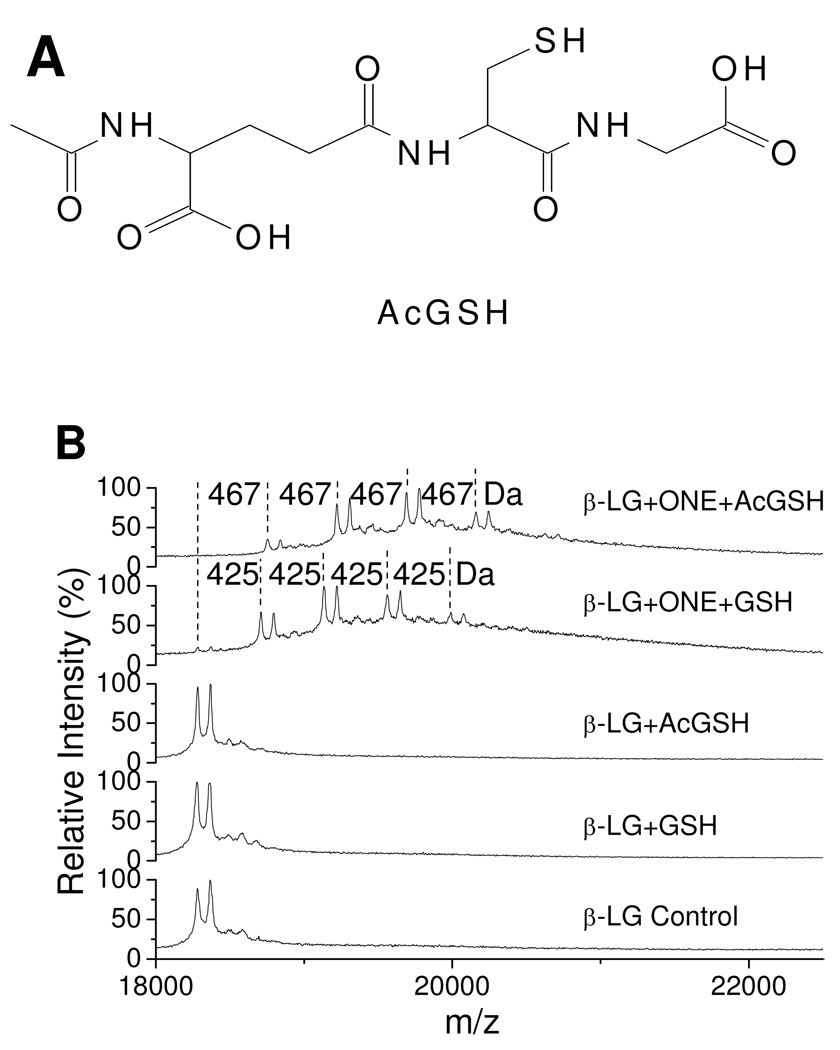
GSH has two nucleophiles, namely an amino group and a sulfhydryl group, both of which can react with ONE. Consistent with previous studies showing that the rate constant for the reaction of Cys and ONE is much faster (> 1000 times) than that of Lys and ONE (8, 9), our tandem mass spectra (Figure 3) demonstrate that the sulfhydryl, and not the α-amino group of GSH, is responsible for the GSH–ONE-protein cross-linking. We still could not unequivocally exclude the amino group from playing a role in this reaction course, since Jian and Blair reported that the α-amino group of GSH was involved in the formation of TOG (24), an intramolecular pyrrole cross-linking product of GSH by ONE. Therefore, we incubated β-LG and ONE with AcGSH (Figure 6A) as a surrogate of GSH. The AcGSH–ONE MA condensed with Lys results in a mass shift of 467 Da instead of 425 Da for GSH. MALDI-TOF spectra show that AcGSH could also be cross-linked to the protein, which has the same chemistry as that of GSH (Figure 6B). The use of AcGSH resulted in slightly greater ONE-dependent modification of β-LG than with GSH (Figure 6B top two traces). This enhanced cross-linking is attributed to AcGSH’s lack of an amino-terminus which can “self-consume” some of the GSH–ONE MAs through the formation of TOG and other adducts (24), and thus a fraction of the GSH–ONE MA is prevented from forming adducts to the protein. The small difference between GSH and AcGSH reactivity suggests that the sulfhydryl group of GSH is the major nucleophile that promotes GSH–ONE cross-linking to the protein. On the other hand, as the His and Cys residues of β-LG can also first react with ONE to form an unconjugated 4-ketoaldehyde, which can be condensed with an amino group either intramolecularly from the protein (15) or from an exogenous reagent such as GSH or carnosine to form a 3-substituted pyrrole cross-link. We did not find evidence for this kind of cross-link in the proteolytic digests of β-LG in the presence of GSH. However, carnosine (Table 3 and S2, Supporting Information) or hippuryl-Lys-OH (data not shown) were cross-linked to His146 through the β or ε-amino group (Scheme 3) when ONE, carnosine or hippuryl-Lys-OH and β-LG were incubated under the same conditions used for the GSH incubations. These different results should not be attributed to the difference between α-amino group of GSH and β-amino group of carnosine or ε-amino group of hippuryl-Lys-OH since both α and ε-amino groups can be condensed with the GSH–ONE MA to form the pyrrole (Table 1 and S1, Supporting Information). The likely explanation is that when ONE is in excess of GSH, almost all of the GSH reacts quickly with ONE through sulfhydryl–ONE Michael addition (Scheme 4), minimizing the GSH available to be condensed to the protein-bound 4-ketoaldehydes formed from the extra ONE with a much slower rate constant than the GSH–ONE MA formation. When the ONE is equal to or lower than the GSH, almost all of the ONE is rapidly trapped by GSH through sulfhydryl–ONE Michael addition (Scheme 4), eliminating the potential for ONE to form the protein-bound 4-ketoaldehydes even if some free GSH were left. Therefore, it is understandable why cross-linking of the α-amino group of GSH to β-LG-bound 4-ketoaldehydes could not be observed under any conditions.
Figure 6.
Structure of AcGSH (A) and MALDI-TOF mass spectra of 0.25 mM of β-LG modified by 1.00 mM of ONE with or without 1.00 mM GSH or 1.00 mM AcGSH (B). The mass differences of 425 and 467 Da are due to the cross-link of GSH and AcGSH to the protein by ONE, respectively.
Scheme 3.
Protein-bound 4-ketoaldehyde is able to modify the exogenous amino group
Scheme 4.
Reaction of ONE and GSH
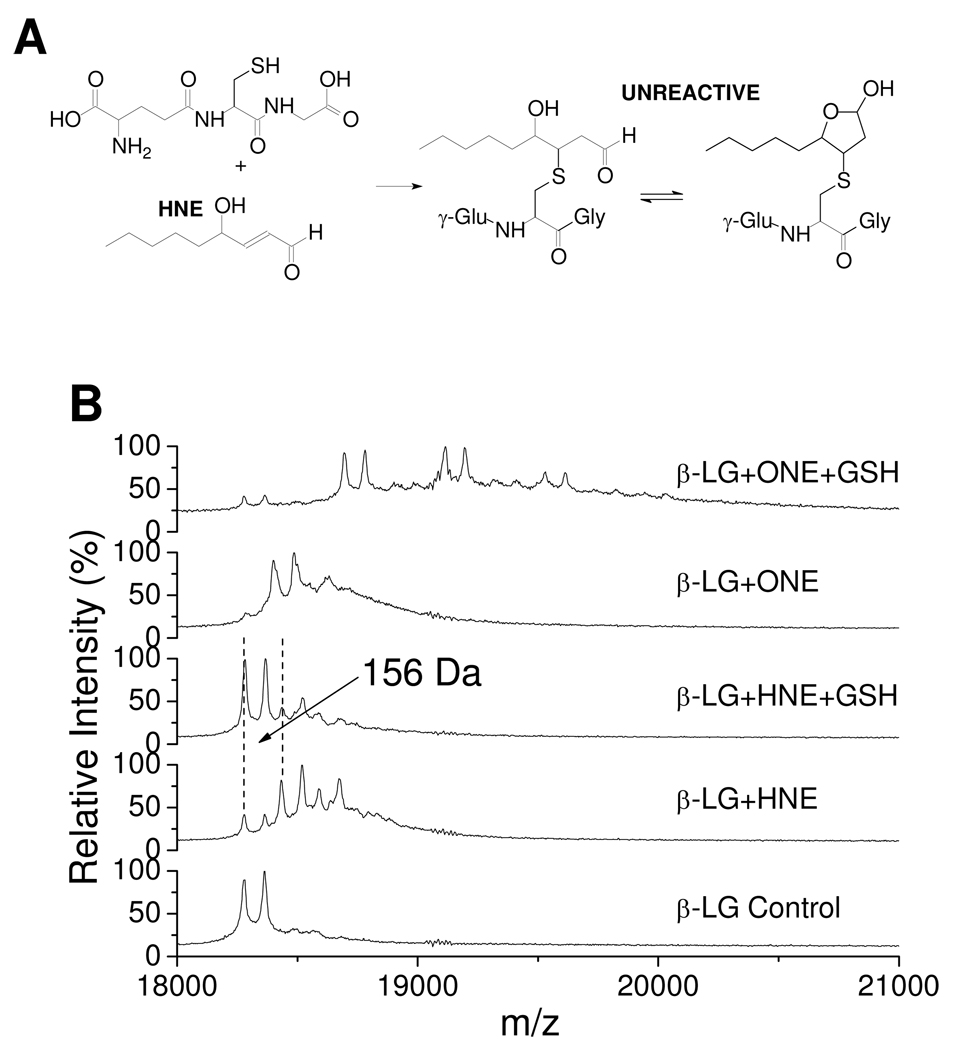
Relative reactivity of the GSH–ONE and GSH-HNE MAs with Proteins
Although ONE undergoes efficient conjugate addition by Cys and His residues, irreversible modification of Lys residues by ONE is slow (12). In contrast, the 4-ketoaldehyde resulting from trapping of ONE by GSH reacts rapidly with Lys residues (34, 35). HNE is also known to form a MA with GSH (Figure 7A) (21, 23, 36, 37). In order to compare the reactivity of the GSH–HNE MA with the GSH–ONE MA, β-LG modified by HNE in the presence of GSH was analyzed by MALDI. Figure 7B shows that HNE is adducted to β-LG in the absence of GSH with a mass shift of 156 Da (see the fourth trace), which is due to HNE MA formation. However, when GSH is present, the HNE MA is significantly reduced, and there is no GSH–HNE-based cross-link to β-LG observed (see the third trace). Therefore, GSH inhibits HNE-based β-LG modification, and the GSH–HNE conjugate is not protein-reactive. This dramatically contrasts with the behavior of the GSH–ONE conjugate which is quite reactive toward the protein (see the top trace). The difference must be attributed to the carbonyl present at C4 on ONE. In ONE, the initial Schiff base formed at either C4 or C1 will irreversibly form the pyrrole adduct, but in HNE where C4 is present at the more reduced hydroxyl level, the reversibly formed Schiff base at C1 is not trapped.
Figure 7.
Reaction of GSH and HNE (A) and MALDI-TOF mass spectra of 0.25 mM of β-LG modified by 0.50 mM of HNE or ONE with or without 1.00 mM GSH (B). The mass difference of 156 Da is due to the HNE MA.
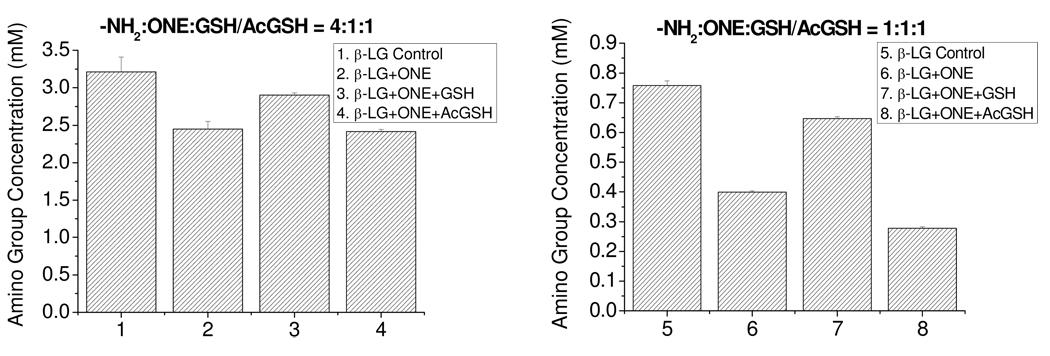
GSH cross-linking to the protein by ONE results in the loss of Lys residues of the protein. Similarly, ONE-based modification of the protein such as 4-ketoamide formation and Cys or His–ONE–Lys pyrrole cross-links consume Lys residues. In order to compare the Lys reactivity of ONE itself with the GSH–ONE MA, a colorimetric 2,4,6-trinitrobenzenesulfonic acid (TNBS) assay (38) was used to quantify the amino groups for the ONE-modified β-LG at two different concentrations of ONE in the presence or absence of GSH (Figure 8), after dialysis to remove the unreacted GSH. However, as GSH contains a free amino-terminal, even if the GSH–ONE MA is condensed with Lys to form the pyrrole, the GSH amino group would still be detected by TNBS (columns 3 and 7). However, the detected amino group concentrations for the treated β-LG samples were lower than that for the β-LG control (columns 1 and 5), probably because some Lys residues were still modified by ONE even in the presence of GSH, or the ε-amino group of the Lys side chain is a better nucleophile toward TNBS than the α-amino group. To remove this ambiguity, AcGSH, which lacks a compensatory amino group, was used as a surrogate. Both Figures 8A and 8B showed that more amino groups were lost in the presence of AcGSH (columns 4 and 8) than in its absence (columns 2 and 6), consistent with the higher Lys reactivity with the AcGSH–ONE MA in contrast to ONE itself.
Figure 8.
TNBS assay results of β-LG modified by ONE with or without GSH or AcGSH at different conditions. (A), 0.25 mM β-LG (4 mM amino groups), 1 mM ONE, 1 mM GSH/AcGSH, 24 h; Paired T-tests reveal columns 2 and 4 are each significantly different from columns 1 and 3 at p<0.05 (B), 0.0625 mM β-LG (1 mM amino groups), 1 mM ONE, 1 mM GSH/ AcGSH, 24 h. Paired T-tests indicate all four results are significantly different at p<0.05.
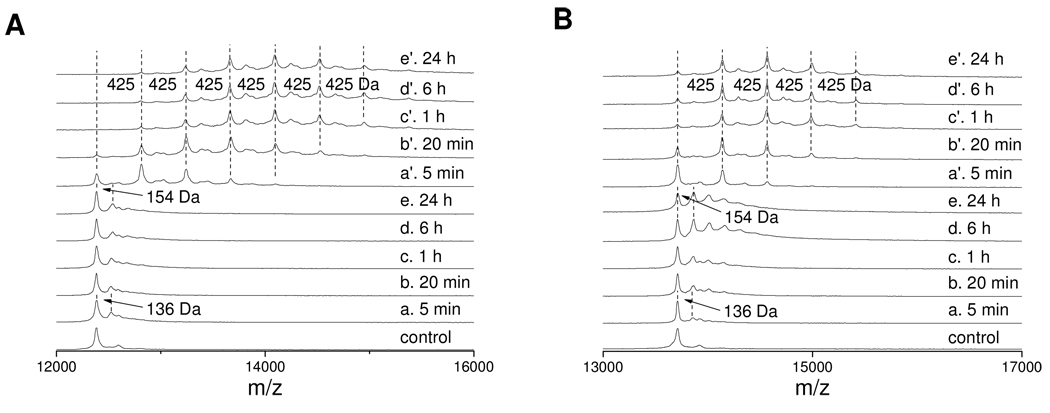
Furthermore, for Lys-rich proteins, the absolute reactivity of ONE with the proteins should be increased in the presence of GSH. Thus, cytochrome c and RNase A were incubated with ONE only, or ONE and GSH together at 37 °C. MALDI-TOF-MS analysis of the modified cytochrome c (Figure 9A) and RNase A (Figure 9B) as a function of time showed that the native proteins were consumed faster by ONE in the presence of GSH (Figure 9 a' –e') than in the absence of GSH (Figure 9 a–e). In addition, the GSH–ONE–protein cross-linking was totally finished within 1 h as traces d' and e' are almost identical to c' in both Figures 9A and 9B, indicating that the two reactions required to form this cross-link occurred rapidly (Scheme 1, GSH–ONE–Michael addition and subsequent Paal-Knorr condensation). For the ONE alone modification, trace a shows that the early ONE modification of these two proteins was the Schiff base formation which has a mass shift of 136 Da (9). But trace e indicates that the late stage of ONE modification was formation of Lys 4-ketoamide (12) and/or His Michael addition (8), both of which have isobaric mass shifts of 154 Da.
Figure 9.
MALDI-TOF mass spectra of 0.25 mM of cytochrome c (A) or 0.25 mM of RNase A (B) modified by 1.00 mM of ONE without (a–e) or with (a'–e') 1.00 mM of GSH at various time. The mass difference of 154 Da is due to the Lys–ONE 4-ketoamide adduct or His–ONE MA and 425 Da is due to the cross-link of GSH to proteins.
These results provide a potential chemical explanation for the enhanced toxicity of ONE relative to HNE. The reaction of ONE with GSH, an abundant cellular antioxidant, results not in a decrease in electrophilicity, but an enhancement in the specific ability to modify amino groups on proteins. The analogous reactions of HNE with GSH results in a reduced reactivity of the product.
Detection of GSH–ONE Crosslinks by α-GSH Antibodies
When ONE and GSH are incubated together with β-LG, a band approximating the Mr of β-LG is identified with a commercial GSH-specific antibody used routinely to identify glutathionylated proteins (i.e., disulfide adducted glutathione). This anti-GSH specific band was not observed in the absence of either ONE or GSH. A disulfide linked β-LG-SSG protein could be produced by incubation of β-LG with GSSG, and this glutathionyl disulfide adduct was similarly detected by the anti-GSH antibody. However, the disulfide linked β-LG-SSG protein could be differentiated from the GSH–ONE adducted protein by incubation with mercaptoethanol or dithiothreitol prior to electrophoresis, because these disulfide reducing agents remove the glutathionyl moiety from β-LG-SSG but not from GSH–ONE modified β-LG; hence anti-GSH immunoreactivity persisted with the latter. These results suggest that in vivo production of ONE by lipid peroxidation may lead to the irreversible incorporation of GSH into proteins during oxidative stress, in addition to reversible protein-SSG formation. Therefore only DTT-reversible anti-GSH immunoreactivity should be attributed to reversible protein-SSG formation, and further analysis is necessary to characterize glutathionyl-protein adducts that are not dissociable by thiol reducing agents.
Conclusions
When incubated with proteins in vitro, the bifunctional lipoxidation-derived aldehyde ONE can form cross-links through generation of Cys–Lys and His–Lys pyrrole cross-links. ONE reacts rapidly with the antioxidants GSH and carnosine, and thus ONE-based protein-protein cross-linking is inhibited. However, unlike HNE and presumably other α,β-unsaturated aldehydes including DDE and acrolein, the reaction of ONE and GSH or carnosine uniquely leads to the formation of a 4-ketoaldehyde, which is quite reactive toward primary amines through Paal-Knorr condensation. As a consequence, GSH or carnosine can be cross-linked to proteins by ONE.
SDS–PAGE showed that both GSH and carnosine could inhibit β-LG cross-linking by ONE, with the former being the more effective inhibitor. MALDI-TOF mass spectra showed that the GSH–ONE MA was rapidly adducted to model proteins such as β-LG, cytochrome c and RNase A. GSH was detected to be cross-linked to all 15 lysines and the N-terminus of β-LG through ONE with LC-ESI-MS/MS, while carnosine was found to be cross-linked to a lesser extent and with lower intensity. By observing the time course for disappearance of the native peak of Lys-rich proteins cytochrome c and RNase A, the GSH–ONE MA was found to be more reactive than ONE itself toward these two proteins. Consistently, more Lys residues were found to be lost when β-LG was incubated with ONE in the presence of AcGSH than its absence, which indicated that the AcGSH–ONE MA was more reactive with Lys than ONE. All of these observations lead to the conclusion that although GSH is considered to be a detoxification reagent for α,β-unsaturated aldehydes (39), instead it enhances the reactivity of ONE, since the GSH–ONE MA rapidly modifies protein Lys residues. In contrast, the GSH–HNE MA was unreactive with protein, therefore GSH will protect protein from modification by HNE. The toxic activation of ONE by GSH is reminiscent of the reaction of GSH with ethylene dibromide which becomes mutagenic and carcinogenic after conjugation with GSH (40–42).
Uchida and coworkers reported that the acrolein-modified bovine serum albumin could also incorporate GSH to form a C-glutathionylated protein (43). In their case, acrolein reacts with the protein first, and then the acrolein-derived Lys adduct reacts with GSH. Our study presents a novel mechanism for protein C-glutathionylation. Unlike reversible protein S-glutathionylation, which plays an important role in redox signal transduction and associated cellular functions (44–46), the potential impact of protein C-glutathionylation on cellular homeostasis is unknown. However, the known generation of ONE by oxidative stress in the presence of intracellular GSH strongly suggests that GSH–ONE modified proteins will occur in vivo. In addition, our observations provide a cautionary note that appropriate controls (+/− disulfide reducing agent) must be included when using anti-GSH antibodies to distinguish reversibly S-glutathionylated proteins from C-glutathionylated proteins that are irreversibly modified.
Supplementary Material
Acknowledgement
We thank Jianye Zhang for synthesizing d9-ONE. This work was supported by NIH grants HL 53315 (LMS) and AG 15885 (JJM, LMS, & VEA).
Abbreviations
- AcGSH
Nα-acetyl-glutathione
- DDE
2(E),4(E)-decadienal
- GSH
glutathione
- HNE
4-hydroxy-2(E)-nonenal
- β-LG
bovine β-lactoglobulin
- MA
Michael adduct
- MALDI-TOF-MS
matrix-assisted laser desorption/ionization time-of-flight mass spectrometry
- ONE
4-oxo-2(E)-nonenal
- d9-ONE
6,6,7,7,8,8,9,9,9-d9-4-oxo-2(E)-nonenal
- RNase A
ribonuclease A
- SIC
selected ion chromatogram
- TBST
Tris-Buffered Saline Tween-20
- TFA
trifluoroacetic acid
- TIC
total ion chromatogram
Footnotes
Presented in preliminary form: Sayre, L. M. and Zhu, X. (2007) Abstracts of Papers, 234th ACS National Meeting, Boston, MA, United States, August 19–23, American Chemical Society, Washington, D.C. (TOXI-059).
This work was mainly performed in Dr. Sayre’s laboratory; however, prior to submission he suffered an incapacitating stroke.
Supporting Information Available: Tables S1–S2 and Figures S1–S21 are available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Nunomura A, Castellani RJ, Zhu X, Moreira PI, Perry G, Smith MA. Involvement of oxidative stress in Alzheimer's disease. Journal of Neuropathology & Experimental Neurology. 2006;65:631–641. doi: 10.1097/01.jnen.0000228136.58062.bf. [DOI] [PubMed] [Google Scholar]
- 2.Chauhan V, Chauhan A. Oxidative stress in Alzheimer's disease. Pathophysiology. 2006;13:195–208. doi: 10.1016/j.pathophys.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Jenner P. Oxidative stress in Parkinson's disease. Annals of Neurology. 2003;53:S26–S38. doi: 10.1002/ana.10483. [DOI] [PubMed] [Google Scholar]
- 4.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radical Biol. Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 5.Miyake T, Shibamoto T. Simultaneous determination of acrolein, malonaldehyde and 4-hydroxy-2-nonenal produced from lipids oxidized with Fenton's reagent. Food and Chemical Toxicology. 1996;34:1009–1011. doi: 10.1016/s0278-6915(96)00063-4. [DOI] [PubMed] [Google Scholar]
- 6.Lee SH, Blair IA. Characterization of 4-oxo-2-nonenal as a novel product of lipid peroxidation. Chem. Res. Toxicol. 2000;13:698–702. doi: 10.1021/tx000101a. [DOI] [PubMed] [Google Scholar]
- 7.Spiteller P, Kern W, Reiner J, Spiteller G. Aldehydic lipid peroxidation products derived from linoleic acid. Biochim. Biophys. Acta, Mol. Cell Biol. Lipids. 2001;1531:188–208. doi: 10.1016/s1388-1981(01)00100-7. [DOI] [PubMed] [Google Scholar]
- 8.Doorn JA, Petersen DR. Covalent modification of amino acid nucleophiles by the lipid peroxidation products 4-hydroxy-2-nonenal and 4-oxo-2-nonenal. Chem. Res. Toxicol. 2002;15:1445–1450. doi: 10.1021/tx025590o. [DOI] [PubMed] [Google Scholar]
- 9.Lin D, Lee H-g, Liu Q, Perry G, Smith MA, Sayre LM. 4-Oxo-2-nonenal is both more neurotoxic and more protein reactive than 4-hydroxy-2-nonenal. Chem. Res. Toxicol. 2005;18:1219–1231. doi: 10.1021/tx050080q. [DOI] [PubMed] [Google Scholar]
- 10.Sayre LM, Lin D, Yuan Q, Zhu X, Tang X. Protein adducts generated from products of lipid oxidation: focus on HNE and ONE. Drug Metab. Rev. 2006;38:651–675. doi: 10.1080/03602530600959508. [DOI] [PubMed] [Google Scholar]
- 11.Zhang W-H, Liu J, Xu G, Yuan Q, Sayre LM. Model studies on protein side chain modification by 4-oxo-2-nonenal. Chem. Res. Toxicol. 2003;16:512–523. doi: 10.1021/tx020105a. [DOI] [PubMed] [Google Scholar]
- 12.Zhu X, Sayre LM. Long-lived 4-oxo-2-enal-derived apparent lysine Michael adducts are actually the isomeric 4-ketoamides. Chem. Res. Toxicol. 2007;20:165–170. doi: 10.1021/tx600295j. [DOI] [PubMed] [Google Scholar]
- 13.Xu G, Sayre LM. Structural characterization of a 4-hydroxy-2-alkenal-derived fluorophore that contributes to lipoperoxidation-dependent protein crosslinking in aging and degenerative disease. Chem. Res. Toxicol. 1998;11:247–251. doi: 10.1021/tx980003d. [DOI] [PubMed] [Google Scholar]
- 14.Xu G, Liu Y, Sayre LM. Independent synthesis, solution behavior, and studies on the mechanism of formation of a primary amine-derived fluorophore representing cross-linking of proteins by (E)-4-hydroxy-2-nonenal. J. Org. Chem. 1999;64:5732–5745. [Google Scholar]
- 15.Liu Z, Minkler PE, Sayre LM. Mass spectroscopic characterization of protein modification by 4-hydroxy-2-(E)-nonenal and 4-oxo-2-(E)-nonenal. Chem. Res. Toxicol. 2003;16:901–911. doi: 10.1021/tx0300030. [DOI] [PubMed] [Google Scholar]
- 16.Oe T, Arora JS, Lee SH, Blair IA. A novel lipid hydroperoxide-derived cyclic covalent modification to Histone H4. J. Biol. Chem. 2003;278:42098–42105. doi: 10.1074/jbc.M308167200. [DOI] [PubMed] [Google Scholar]
- 17.Zhu X, Sayre LM. Mass spectrometric evidence for long-lived protein adducts of 4-oxo-2-nonenal. Redox Rep. 2007;12:45–49. doi: 10.1179/135100007X162176. [DOI] [PubMed] [Google Scholar]
- 18.Aldini G, Granata P, Carini M. Detoxification of cytotoxic alpha, beta -unsaturated aldehydes by carnosine: characterization of conjugated adducts by electrospray ionization tandem mass spectrometry and detection by liquid chromatography/mass spectrometry in rat skeletal muscle. Journal of Mass Spectrometry. 2002;37:1219–1228. doi: 10.1002/jms.381. [DOI] [PubMed] [Google Scholar]
- 19.Guiotto A, Calderan A, Ruzza P, Borin G. Carnosine and carnosine-related antioxidants: A review. Curr Med Chem. 2005;12:2293–2315. doi: 10.2174/0929867054864796. [DOI] [PubMed] [Google Scholar]
- 20.Ishikawa T, Esterbauer H, Sies H. Role of cardiac glutathione transferase and of the glutathione S-conjugate export system in biotransformation of 4-hydroxynonenal in the heart. J. Biol. Chem. 1986;261:1576–1581. [PubMed] [Google Scholar]
- 21.Boon PJM, Marinho HS, Oosting R, Mulder GJ. Glutathione conjugation of 4-hydroxy-trans-2,3-nonenal in the rat in vivo, the isolated perfused liver and erythrocytes. Toxicol. Appl. Pharmacol. 1999;159:214–223. doi: 10.1006/taap.1999.8742. [DOI] [PubMed] [Google Scholar]
- 22.Alary J, Bravais F, Cravedi J-P, Debrauwer L, Rao D, Bories G. Mercapturic acid conjugates as urinary end metabolites of the lipid peroxidation product 4-hydroxy-2-nonenal in the rat. Chem. Res. Toxicol. 1995;8:34–39. doi: 10.1021/tx00043a004. [DOI] [PubMed] [Google Scholar]
- 23.Volkel W, Alvarez-Sanchez R, Weick I, Mally A, Dekant W, Pahler A. Glutathione conjugates of 4-hydroxy-2(E)-nonenal as biomarkers of hepatic oxidative stress-induced lipid peroxidation in rats. Free Radical Biol. Med. 2005;38:1526–1536. doi: 10.1016/j.freeradbiomed.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 24.Jian W, Lee SH, Mesaros C, Oe T, Silva Elipe MV, Blair IA. A novel 4-oxo-2(E)-nonenal-derived endogenous thiadiazabicyclo glutathione adduct formed during cellular oxidative stress. Chem. Res. Toxicol. 2007;20:1008–1018. doi: 10.1021/tx700001t. [DOI] [PubMed] [Google Scholar]
- 25.Nadkarni DV, Sayre LM. Structural definition of early lysine and histidine adduction chemistry of 4-hydroxynonenal. Chem. Res. Toxicol. 1995;8:284–291. doi: 10.1021/tx00044a014. [DOI] [PubMed] [Google Scholar]
- 26.Qin BY, Bewley MC, Creamer LK, Baker EN, Jameson GB. Functional implications of structural differences between variants A and B of bovine beta -lactoglobulin. Protein Science. 1999;8:75–83. doi: 10.1110/ps.8.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE. The protein data bank. Nucleic Acids Research. 2000;28:235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London, United Kingdom) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 29.Yuan Q, Zhu X, Sayre LM. Chemical nature of stochastic generation of protein-based carbonyls: metal-catalyzed oxidation versus modification by products of lipid oxidation. Chem. Res. Toxicol. 2007;20:129–139. doi: 10.1021/tx600270f. [DOI] [PubMed] [Google Scholar]
- 30.Stewart BJ, Doorn JA, Petersen DR. Residue-specific adduction of tubulin by 4-hydroxynonenal and 4-oxononenal causes cross-linking and inhibits polymerization. Chem. Res. Toxicol. 2007;20:1111–1119. doi: 10.1021/tx700106v. [DOI] [PubMed] [Google Scholar]
- 31.Turula VE, Bishop RT, Ricker RD, de Haseth JA. Complete structure elucidation of a globular protein by particle beam liquid chromatography-Fourier transform infrared spectrometry and electrospray liquid chromatography-mass spectrometry. Sequence and conformation of beta-lactoglobulin. Journal of Chromatography, A. 1997;763:91–103. doi: 10.1016/s0021-9673(96)00960-0. [DOI] [PubMed] [Google Scholar]
- 32.Baillie TA, Davis MR. Mass spectrometry in the analysis of glutathione conjugates. Biological Mass Spectrometry. 1993;22:319–325. doi: 10.1002/bms.1200220602. [DOI] [PubMed] [Google Scholar]
- 33.Szapacs ME, Riggins JN, Zimmerman LJ, Liebler DC. Covalent adduction of human serum albumin by 4-hydroxy-2-nonenal: kinetic analysis of competing alkylation reactions. Biochemistry. 2006;45:10521–10528. doi: 10.1021/bi060535q. [DOI] [PubMed] [Google Scholar]
- 34.Amarnath V, Anthony DC, Amarnath K, Valentine WM, Wetterau LA, Graham DG. Intermediates in the Paal-Knorr synthesis of pyrroles. J. Org. Chem. 1991;56:6924–6931. [Google Scholar]
- 35.Amarnath V, Amarnath K, Valentine WM, Eng MA, Graham DG. Intermediates in the Paal-Knorr synthesis of pyrroles. 4-oxoaldehydes. Chem. Res. Toxicol. 1995;8:234–238. doi: 10.1021/tx00044a008. [DOI] [PubMed] [Google Scholar]
- 36.Enoiu M, Herber R, Wennig R, Marson C, Bodaud H, Leroy P, Mitrea N, Siest G, Wellman M. gamma-Glutamyltranspeptidase-dependent metabolism of 4-hydroxynonenal-glutathione conjugate. Arch. Biochem. Biophys. 2002;397:18–27. doi: 10.1006/abbi.2001.2633. [DOI] [PubMed] [Google Scholar]
- 37.Orioli M, Aldini G, Beretta G, Facino RM, Carini M. LC-ESI-MS/MS determination of 4-hydroxy-trans-2-nonenal Michael adducts with cysteine and histidine-containing peptides as early markers of oxidative stress in excitable tissues. Journal of Chromatography, B: Analytical Technologies in the Biomedical and Life Sciences. 2005;827:109–118. doi: 10.1016/j.jchromb.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 38.Habeeb AFSA. Determination of free amino groups in proteins by trinitrobenzenesulfonic acid. Anal. Biochem. 1966;14:328–336. doi: 10.1016/0003-2697(66)90275-2. [DOI] [PubMed] [Google Scholar]
- 39.Zhou S, Decker EA. Ability of carnosine and other skeletal muscle components to quench unsaturated aldehydic lipid oxidation products. Journal of Agricultural and Food Chemistry. 1999;47:51–55. doi: 10.1021/jf980780j. [DOI] [PubMed] [Google Scholar]
- 40.Anders MW. Glutathione-dependent bioactivation of haloalkanes and haloalkenes. Drug Metabolism Reviews. 2004;36:583–594. doi: 10.1081/dmr-200033451. [DOI] [PubMed] [Google Scholar]
- 41.Peterson LA, Harris TM, Guengerich FP. Evidence for an episulfonium ion intermediate in the formation of S-[2-(N7-guanyl)ethyl]glutathione in DNA. J. Am. Chem. Soc. 1988;110:3284–3291. [Google Scholar]
- 42.Webb WW, Elfarra AA, Webster KD, Thom RE, Anders MW. Role for an episulfonium ion in S-(2-chloroethyl)-DL-cysteine-induced cytotoxicity and its reaction with glutathione. Biochemistry. 1987;26:3017–3023. doi: 10.1021/bi00385a010. [DOI] [PubMed] [Google Scholar]
- 43.Furuhata A, Nakamura M, Osawa T, Uchida K. Thiolation of protein-bound carcinogenic aldehyde: an electrophilic acrolein-lysine adduct that covalently binds to thiols. J. Biol. Chem. 2002;277:27919–27926. doi: 10.1074/jbc.M202794200. [DOI] [PubMed] [Google Scholar]
- 44.Shelton MD, Chock PB, Mieyal JJ. Glutaredoxin: role in reversible protein S-glutathionylation and regulation of redox signal transduction and protein translocation. Antioxidants & Redox Signaling. 2005;7:348–366. doi: 10.1089/ars.2005.7.348. [DOI] [PubMed] [Google Scholar]
- 45.Shelton MD, Mieyal JJ. Regulation by reversible S-glutathionylation: molecular targets implicated in inflammatory diseases. Molecules and Cells. 2008;25:332–346. [PMC free article] [PubMed] [Google Scholar]
- 46.Gallogly MM, Mieyal JJ. Mechanisms of reversible protein glutathionylation in redox signaling and oxidative stress. Curr. Opin. Pharmacol. 2007;7:381–391. doi: 10.1016/j.coph.2007.06.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.