Abstract
Introduction
Adverse life events occurring in early development can result in long-term effects on behavioural, physiological and cognitive processes. In particular, perinatal stressors impair neurogenesis in the hippocampus which consequently impairs memory formation. Exercise has previously been shown to have antidepressant effects and to increase cognitive functioning by increasing neurogenesis and neurotrophins in the hippocampus. The current study examined the effects of maternal separation, which has been shown to model anxiety in animals, and the effects of exercise on learning and memory.
Methods
Forty-five male Sprague-Dawley rats were divided into 4 groups, maternally separated / non-runners, maternally separated / runners, non-separated / runners and non-separated / non-runners. Maternal separation occurred from postnatal day 2 (P2) to 14 (P14) for 3 hours per day. Exercised rats were given voluntary access to individual running wheels attached to their cages from P29 to P49. Behavioural testing (Morris water maze (MWM) and object recognition tests) took place from P49 to P63.
Results
Maternally separated rats showed no significant difference in anxiety levels in the elevated plus maze and the open field compared to the normally reared controls. However, rats that were allowed voluntary access to running wheels showed increased levels of anxiety in the elevated plus maze and in the open field. Maternal separation did not have any effect on memory performance in the MWM or the object recognition tasks. Exercise increased spatial learning and memory in the MWM with the exercised rats displaying a decreased latency in locating the hidden platform than the non-exercised rats. The exercised rats spent significantly less time exploring the most recently encountered object in the temporal order task in comparison to the non-exercised controls, therefore showing improved temporal recognition memory. All groups performed the same on the other recognition tasks, with all rats showing intact memory performance.
Conclusion
Results indicate that maternal separation had little effect on the rats whereas exercise enhanced both spatial and recognition memory.
Keywords: Maternal separation, stress, exercise, learning and memory
1. INTRODUCTION
The neonatal period in both humans and rodents is a fundamental phase in the normal developmental process. Adverse life events during this critical period can result in long term effects on behavioural, physiological and cognitive processes (Gareau et al. 2007; Horvath et al. 2004). One of the characteristic biochemical features of the pathological outcome of these early adverse life-events is dysregulation of the hypothalamic-pituitary-adrenal axis (HPA axis) (Mesquita et al. 2007; Tang et al. 2003). The HPA axis is a neuroendocrine system that responds to environmental challenges (Kandel et al. 2006). Stress stimulates the release of corticotrophin-releasing hormone (CRH) which in turn causes the release of adrenocorticotropic hormone (ACTH). ACTH stimulates the release of glucocorticoids from the adrenal glands (Kandel et al. 2006). The interplay between the elements of the HPA axis act to maintain a balanced homeostatic environment. In humans alterations to the HPA axis have accompanied certain psychiatric diseases such as depression and anxiety (Kathol et al. 1989).
Disturbances in mother-infant interaction have been shown to be a natural stressor which may lead to maladaptive development (Daniels et al. 2004). Maternal separation (MS) has widely been used to model the effects of adverse early life-experiences because it has been shown to produce the clinical features of depression and anxiety such as the abnormal HPA axis which is accompanied by the associated behavioural effects (Marais et al. 2008). Other neurochemical changes that occur in response to stress include decreased levels of brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) (Griesbach et al. 2004, Roceri et al. 2004).
Down-regulation of hippocampal BDNF was observed in maternally deprived rats in accordance with impaired memory formation and studies showing increased anxiety-like behaviour even in adult rats (Knuth & Etgen 2007; Roceri et al. 2002). The hippocampus is a brain region that is especially vulnerable to stress in early development due to the high density of glucocorticoid receptors (Pickering et al. 2006). Stress may reduce the proliferation of synapses in the hippocampus seen in the first 3 weeks of a pup’s life and contribute to impaired cognition in adulthood (Andersen & Teicher 2004).
Voluntary exercise has been shown to reduce anxiety levels and counteract the cognitive and mood effects of stress and depression (Clark et al. 2008). Running has also been shown to enhance neurogenesis and improve cognitive performance (Duman et al. 2008; Stranahan et al. 2007). One reason may be due to the increase in hippocampal BDNF levels seen in exercised rats (Vaynman et al. 2004). BDNF has previously been shown to be positively correlated with nerve terminal proteins (Ying et al. 2008).
The purpose of this study was firstly to determine whether maternal separation impairs certain aspects of memory formation (spatial memory in the Morris Water Maze and object recognition memory). Secondly, the effects of voluntary exercise were examined to determine if rats given voluntary access to running wheels displayed lower levels of anxiety (caused by maternal separation) and better memory performance than non-exercised rats.
2. MATERIALS AND METHODS
2.1 Animals
Forty-five male Sprague-Dawley rats were obtained from the University of Cape Town Animal Unit. On post-natal day two (P2) all litters were sexed (selecting males first) and then culled to 8 in order to standardize litter size. Rats were divided into 4 groups: the maternally separated/exercised (MSR) group (n=10), the maternally separated/non-exercised (MSnR) group (n=11), the non-separated/exercised (nMSR) group (n=11) and the non-separated/non-exercised (nMSnR) group (n=13). Dams and pups were housed in a 12-hour light/dark cycle, lights on at 06h00. On P21 rats were transferred to a new 12-hour light/dark cycle with the lights off at 11h00. Temperatures were maintained at 23-25°C. Food and water was available ad libitum. Rats were weighed on P49, P52, P55, P63 and P65. All experiments were approved by the Research Ethics Committee of the University of Cape Town.
2.2 Maternal Separation Paradigm
Starting on P2 the dams of the maternally separated pups were physically removed from the home cage and the pups were transferred in their home cage to a different room in order to prevent communication via ultra-sound vocalizations. Pups were separated for 3 hours per day, until P14 after which normal interaction with the dam was resumed. The control pups (non-maternally separated runners, nMSR, and non-runners, nMSnR) were not separated from the dams. On P21 all rats were weaned. Males were housed communally until P29.
2.3 Voluntary Exercise
On P29 rats were given free access to individual running wheels attached to their cages. The number of revolutions was mechanically recorded on a counter and the distance run was calculated from the revolution count and circumference of the wheel where one revolution was equal to 1 m. Recordings were taken daily 1 hour before the dark cycle began (when the rats became active) in order to get an accurate reading of daily running distances. Non-exercised rats (maternally separated non-runners, MSnR, and nMSnR) were housed singly in standard plastic cages from P29 – P49.
On P49 rats were removed from running wheels and all groups were then housed in standard plastic cages in two’s or three’s, until termination of the experiment.
2.4 Behavioural Testing
Rats were placed in separate cages, taken to the behavioural room and were allowed to habituate for 1 hour prior to behavioural testing. The behavioural rooms used were ventilated and maintained at a temperature of 21-23°C with a light intensity of 58 lux. Behavioural testing occurred during the dark phase of the rats light/dark cycle.
All arena and objects (except for the Morris Water Maze) used in the behavioural experiments were cleaned using 70% alcohol after each trial. The apparatus was allowed to dry to allow alcohol vapor to dissipate before further testing resumed. Faeces and other debris were removed from the Morris Water Maze between trials. All tests were recorded using a video camera and videos were analyzed using Noldus Ethovision version 5.
2.4.1 Open Field
At P49, rats were tested in the open field for anxiety-like behaviour and locomotor activity. Rats were placed in the corner of a wooden box measuring 100 cm × 100 cm × 50cm and allowed to freely explore for 5 minutes. The box was divided into an inner and outer zone demarcated by white tape on the floor 10 cm from the outer wall of the box. Time spent and distance covered in the inner and outer zones of the box as well as the frequency of transition from the outer to inner zones were analyzed. Significantly more time spent in the inner zone indicates increased exploration and therefore reduced anxiety (Prut & Belzung 2003). Distance covered is an indication of the locomotor activity; an increase indicates a desire to explore and therefore reduced anxiety (Prut & Belzung 2003).
2.4.2 Elevated Plus Maze
At P49, immediately after being tested in the open field, the rats were placed in the elevated plus maze, facing the open arm, for 5 minutes. The protocol of Walf and Frye (2007) was followed. The elevated plus maze is used to test for anxiety-like behaviour. Time spent in each arm, time spent in the centre zone and the frequency of entry into each arm were analyzed. Significantly more time spent in the open arm compared to the closed arm indicates reduced anxiety levels, as the closed arm provides shelter for the rat (Walf & Frye 2007).
2.4.3 Morris Water Maze (MWM)
The Morris Water Maze (MWM) protocol of Vorhees and Willams (2006) was used. The MWM consisted of a white circular tank with a diameter of 175 cm and height of 63 cm. The tank was filled with water maintained between 21°C – 24°C and made opaque with non-toxic tempera paint. The pool was divided into four quadrants and arbitrarily divided into southeast, northeast, northwest and southwest quadrants. A circular Perspex escape platform of 15cm diameter was submerged 30cm from the wall in the southeast quadrant of the pool (the target quadrant). The position of the platform was kept constant for the duration of the 5-day acquisition period. Four A3 sized pictures (black, white and blue circles, triangles, stripes and squares) were permanently fixed on the surrounding walls and served as distal navigation cues to enable location of the platform.
The MWM spatial learning test took place over a period of six days, from P50 to P55. The first five days (P50 – P54) were the acquisition or training days and on the sixth day (P55) the probe or retention trial was performed. The training period consisted of four trials per day, starting the rat at four different positions in the water maze, to avoid left and right navigation to the platform. This is a response strategy, described by Clements et al. (2007) where the rat remembers which direction their body turned instead of using visuospatial cues to guide them. Instead of hippocampal memory used in a place strategy where the rat uses spatial cues to guide them, the response strategy relies on striatal memory. Each trial began with the rat in the pool facing the sidewalls and ended when the rat found the platform or after 120 seconds; in both cases the rat was allowed to stay on the platform for 10 seconds.
On the 6th day a probe trial of 60 seconds was performed after the escape platform had been removed. The release position (northeast) for the probe was different from the three positions used for the spatial trials on the previous five days of testing. For the training trials, time taken to reach the platform; time spent in the target quadrant, mean swimming velocity and distance to platform were analyzed. For the probe trial, time spent in the peripheral and target quadrants (southeast quadrant), frequency of entry into the target and peripheral quadrant (northeast, northwest and southwest quadrants), mean velocity and distance covered were analyzed.
2.4.4 Object recognition tasks
No behavioural testing took place between P55 and P62. Memory testing (for object recognition/location) resumed at P63. All object recognition/location memory tasks were performed sequentially at the same time of day. All memory testing protocols were according to Barker et al. (2007) using a box (65 cm × 65 cm × 60 cm box in dimension) described by Bertsina-Anglade et al. (2006). Objects used were wooden shapes ranging in size from 5 cm × 5 cm to 5 cm × 10 cm in diameter and height. The objects were placed in the corners of the box, 3 cm from each adjacent wall and taped down to prevent the object from shifting. If an object was used in the following days, it was not placed in the same position. Positions of the novel and familiar objects were changed around from one rat to the next to rule out left/right preference.
On P62 all rats were allowed to explore the empty box for 2 minutes in order to familiarize the rat with the testing environment (Bevins & Besheer 2006). On P63, the novel object recognition task was performed, followed, approximately 2 hours later, by the object location task (Barker et al. 2007). On P64, the temporal order task was performed followed 2 hours later by the object-in-place task. The rats’ behaviour was recorded using a video camera. Time spent exploring each object was determined manually, using a stopwatch to record time spent with each object.
Exploratory behaviour for all rats was defined as the nose pointing toward the object at a distance of < 2 cm for > 2 seconds. Using the object to rear upwards was not counted as exploration.
2.4.4.1 Novel Object Recognition Task (NORT)
The Novel Object Recognition Task (NORT) is based on differential exploration of new objects. The task consisted of a trial phase and a test phase. Rats were placed at the mid-point of the wall opposite to the objects, with its nose pointing away from the objects. In the trial phase, rats were allowed to freely explore two identical objects for 5 minutes. Rats were removed from the box and the test phase took place 5 minutes later where one object was replaced by a different, novel object.
During the test, the rats were allowed to explore the novel object and previously experienced object for 3 minutes. During the test phase the exploratory behaviour towards each object was recorded using a stopwatch. More time spent investigating the novel object was considered to be indicative of working memory being intact (Barker et al. 2007).
2.4.4.2 Object Location task (OL)
The object location (OL) task measures recognition memory of object location. The task consisted of 2 phases the trial and test phase. Rats were placed at the mid-point of the wall opposite to the objects, with its nose pointing away from the objects. During the trial phase the rats were allowed to explore two identical objects for 3 minutes, after which the rats were removed from the box. In the test phase, performed 5 minutes after the sample phase, one object was placed in a different location to its original position and rats were then allowed to explore the objects for 3 minutes. Rats that showed superior recognition memory were expected to spend more time exploring the object that had been moved (Barker et al. 2007).
2.4.4.3 Temporal order task (TO)
The temporal order (TO) task consists of 3 phases; 2 trial phases and a test phase. Rats were placed at the mid-point of the wall opposite to the objects, with their nose pointing away from the objects. During phase 1, the rat was allowed to explore 2 identical objects for 4 minutes. The second phase took place 1 hour later when the rat was allowed to explore 2 new identical objects for another 4 minutes. A 3-minutes test trial was performed 3 hours later where 1 object from phase 1 and 2 were used. Between trial phases the rats remained in the behavioural room in their home cages. In the TO task rats with intact temporal memory were expected to spend more time exploring the objects used in the first phase relative to the object used in the second phase (Barker et al. 2007).
2.4.4.4 Object-in-place task (OIP)
The object-in-place task consisted of a 3 minutes sample phase and a 3 minutes test phase. In the sample phase the rats were allowed to explore 4 different objects (A, B, C, D), two of these objects were swapped around for the test phase which took place 5 minutes after completion of the sample phase. For both the trial and test phase, the rat was placed in the centre of the box facing the same wall from trial to test phase. Time spent exploring the 2 objects that had changed position and those that remained in the same position was recorded. If object-in-place memory was intact, the rats were expected to spend more time exploring the objects that had changed position (Barker et al. 2007).
2.6 Statistical Analysis
All statistical analyses were performed using Statistica version 8. Graphs were plotted using Graph Pad Prism 5. Data are presented as the means ± SEM. Statistically significant differences between groups and factorial effects were tested by 2-way ANOVA. When the interaction was shown to be significant, post-hoc analysis was performed using Tukey’s HSD test. Differences between two groups were assessed using the Students t-test for independent groups. Two-sided P < 0.05 was considered significant.
3. RESULTS
3.1 Voluntary exercise and weight
No significant difference was found between the maternally separated rats and the non-separated rats when the mean distance run daily, as well as the mean distance run over the 21 days of exercise, were compared.
Factorial ANOVA revealed that the non-separated rats (nMSnR and nMSR) had significantly lower body weights in comparison to the maternally separated rats (MSR and MSnR). This was noted on P21 (F(1,41) =7, p < 0.01), P50 (F(1,11) =8.7, p < 0.01), P55 (F(1,35) = 5.6, p < 0.05), P63 (F(1,41) = 11, p < 0.001), and P65 (F(1,41) = 6, p < 0.05).
3.2 The Open Field
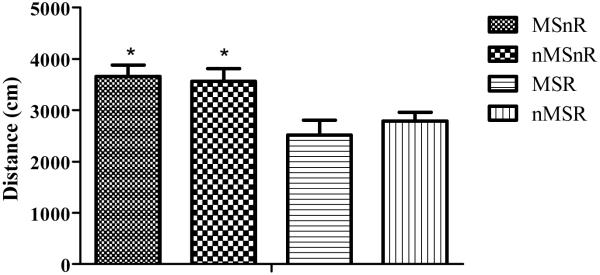
No significant difference (F(1,41) = 0.02, p > 0.05) was observed between the groups when comparing time spent in the inner and outer zones of the open field. Although the maternally separated rats seemed to spend more time in the outer zone, it did not reach a significant level. However, rats that were exercised (n = 21, 10 MSR and 11 nMSR) showed a significant reduction in locomotor activity when compared to the non-exercised (n = 24, 11 MSnR and 13 nMSnR) rats (F(1,41) = 16.03, p < 0.001, Figure 1).
Figure 1.
Graph showing the mean distance covered ±SEM in the open field as an indication of locomotor activity. Factorial ANOVA revealed that the non-exercised rats covered a greater distance in the open field (* p < 0.01) than exercised rats. MSnR = maternally separated non-runners, n = 11; nMSnR = non-separated non-runners, n = 13; MSR = maternally separated runners, n = 10, nMSR = non-separated runners, n = 11.
3.3 The Elevated Plus Maze
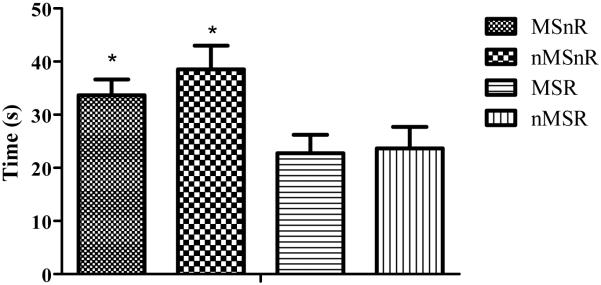
Rats that were exercised (MSR and nMSR) spent significantly less time in the open arms of the elevated plus maze and made significantly fewer entries into the open arms compared to the non-exercised rats (F(1,41) =10.8, p < 0.01, F(1,41) = 7.1, p < 0.01, Figure 2).
Figure 2.
Graph showing mean time spent ±SEM in the open arms of the elevated plus maze. Non-exercised rats spent a significantly greater amount of time (* p < 0.01) in the open arms in comparison to exercised rats. MSnR = maternally separated non-runners, n = 11; nMSnR = non-separated non-runners, n = 13; MSR = maternally separated runners, n = 10, nMSR = non-separated runners, n = 11.
3.4 The Morris Water Maze
Six rats were excluded from the MWM data (4 from nMSnR group and 2 from the nMSR group). The exclusion criteria were as follows: If by day three the rats did not remain on the platform when guided to it, or placed on the platform, it was assumed that no learning had taken place and therefore the rats were excluded from the study.
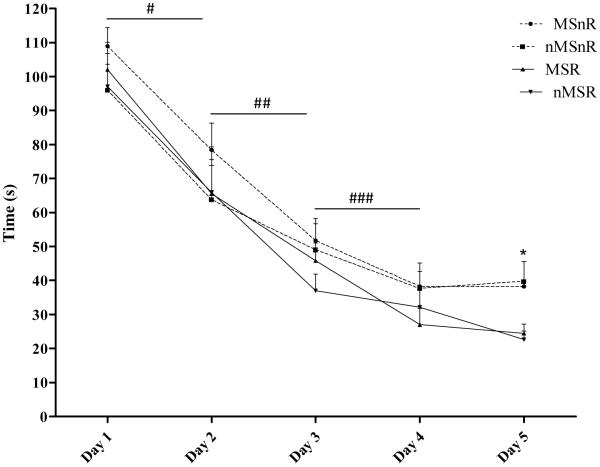
Performance in the MWM for each of the 5 trial days was determined by the average latency to locate the platform. Rats quickly learned the task, showing asymptotic levels of performance (Figure 3). Analysis of the day-to-day recordings showed a significant improvement from day 1 through to day 4 (n = 39; p < 0.01). From day 3 the exercised rats displayed a lower latency to locate the platform, this decrease reached a significant level on day 5 when the exercised rats showed a significant decrease in the time taken to locate the platform compared to the non-exercised rats (F(1,35) = 8.5, p < 0.01). The MSnR group took longer to locate the platform than other rats during the 5 day trial period, however, this difference did not achieve significance.
Figure 3.
Graph showing the mean time ±SEM taken to reach the platform for the 5 trial days. A significant decrease in the latency to reach the platform was found on the following days: day 1 compared to day 2, # p < 0.01; day 2 compared to day 3, ## p < 0.01; day 3 compared to day 4, ### p < 0.01. Factorial ANOVA showed that exercised rats had a significantly lower latency to locate the platform on day 5 than non-exercised rats, *p < 0.01 MSnR and nMSnR compared to MSR and nMSR.
No correlation was found between the total distance run over the 21 days of exercise by each of the exercising rats and the latency to locate the platform on day 5.
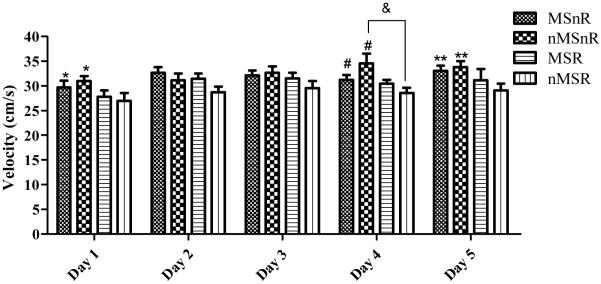
The mean velocity was analyzed using Noldus Ethovision version 5 in order to determine whether the differences in latency to locate the platform were due to differences in learning or due to differences in physical abilities. The mean velocity for the 4 trials of each day was calculated for each rat; thereafter the group means were calculated. Factorial ANOVA revealed a significant difference in velocity on day 1 between the exercised (MSR and nMSR) and the non-exercised rats (MSnR and nMSnR), where the exercised rats (runners) swam more slowly than the non-exercised rats (non-runners, F(1,35) = 5, p < 0.01, Figure 4). This was replicated on day 4 (F(1,35) = 7.2, p < 0.01) and day 5 (F(1,28) = 5, p < 0.05), where exercised rats swam more slowly than non-exercised rats.
Figure 4.
Graph showing the mean velocity ±SEM for each day. Exercised rats had a lower velocity than non- exercised rats (MSnR and nMSnR compared to MSR and nMSR) on day 1, * p < 0.01; day 4, # p < 0.01 and day 5, ** p < 0.05. On day 4 there was a significant interaction between maternal separation and exercise with nMSnR swimming faster than nMSR, & p < 0.05.
No significant (F(1,41) = 0.1 p > 0.05) difference was observed in time spent in the target quadrant between any of the groups during the probe trial. This result was replicated with no significant difference (F(1,41) = 1.7 p > 0.05) between groups with regard to frequency of entry into the target quadrant.
3.5. Object Recognition Tasks
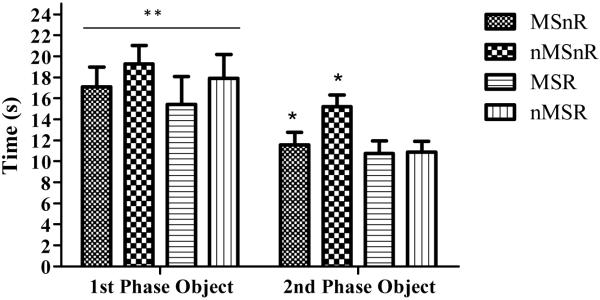
Factorial ANOVA was performed and results showed that all rats displayed intact memory. They spent significantly more time with the novel object, less with the recent object and the object that had changed position. There were no significant group differences in the object-in-place test, novel object recognition task or object location task. This was also true when discrimination ratios were calculated (time spent exploring novel or original object/total time spent exploring). In the temporal order task, factorial ANOVA revealed that the exercised rats (MSR and nMSR) had spent significantly less time exploring the object from the second phase in comparison to the non-exercised rats (MSnR and nMSnR) (F(1,41) = 5, p < 0.05, Figure 5).
Figure 5.
Graph showing mean time spent ±SEM with the object from the 1st phase and the object from the 2nd phase of the temporal order task. Factorial ANOVA revealed that non-exercised rats spent a greater amount of time with the object from the 2nd phase in comparison to the exercised rats, * p < 0.05 (MSnR and nMSnR compared to MSR and nMSR). All rats spent significantly more time with the object from the 1st phase in comparison to the object from the 2nd phase, ** p < 0.01.
4. DISCUSSION
Animals were tested in the open field and elevated plus maze to examine the effects of maternal separation and exercise on locomotor activity and anxiety respectively. Although maternally separated rats spent less time in the open arms of the elevated plus maze and inner zone of the open field, the difference failed to reach significant levels. Using the same MS paradigm as the current study, Kalinichev et al. (2006) also found that the maternally separated group did not spend significantly less time in the open arms of the elevated plus maze in comparison to the normally reared group. Faure et al. (2007) showed that even with an additional triple stressor, maternally separated rats displayed no significant difference in behaviour in the elevated plus maze and open field when compared to controls.
The failure of the maternally separated rats to display anxiety-like behaviour in various studies, including the current study, may be due to the dam-pup interaction when the pup is returned to the cage (Marci et al. 2008). Dams have previously been shown to care more actively for maternally separated pups after separation in comparison to handled and normally reared controls (Marci et al. 2008, Marmendal et al. 2006). It has been suggested that dams counteract the previously deprived environment through compensatory up-regulation of maternal care (Marci et al. 2008). One critique of the study of Macri et al. (2008) however is that pups were not placed in a separate room from the dam during the separation period therefore potentially allowing communication between the dam and the pups, thereby influencing the subsequent dam-pup interaction when reunited. Up-regulation in maternal care like high levels of licking, grooming and arched-back nursing have been shown to be positively correlated with levels of synaptophysin and increased learning in the MWM (Liu et al. 2000) which would counteract the expected decrease in neurogenesis seen with stress (Roceri et al. 2002). On the contrary, many other studies also show that MS dams are slower to initiate pup retrieval when compared to dams of the normally reared litters (Hunsaker et al. 2008). Madruga et al. (2006) suggest that the smell of the dam on the saw-dust may influence the stressful nature of the separation. When Wigger et al. (1999) removed the pups from the home cage and placed the pups in a different cage during the separation period they found that the MS pups displayed less exploration of the open arms of the elevated plus maze, this however may have been due to the stress of handling.
The exercised rats displayed a significant reduction in locomotor activity in the open field in comparison to the non-exercised rats. This result of increased levels of anxiety was reinforced in the elevated plus maze where exercised rats spent significantly less time in the open arms as well as a decreased frequency of entry into the open arm of the elevated plus maze. Similar findings were shown by Binder et al. (2004) where non-exercised controls explored the centre zone more frequently and had a decreased latency to enter the centre of the open field in comparison to the exercised rats. The unexpected result of increased anxiety in the exercised rats in the current study may be due to the stress of being deprived of habitual running (Howells et al. 2005). Rats were removed from their cages with attached running wheels on the day of the open field and elevated plus maze testing; the testing time corresponded with the dark cycle when the rats would be physically active in the running wheel. In support of this suggestion, Howells et al. (2005) used immobilized running wheels for one hour per day as a stressor in their study. In addition, Widenfalk et al. (1999), showed that interruption of exercise decreased BDNF and TrkB levels in rats that had been allowed to run for 5 weeks, demonstrating that deprivation of habitual running caused changes in central brain function (Widenfolk et al. 1999).
Running has also been shown to activate the HPA axis (Coleman et al. 1998). Stranahan et al. (2006) found that running increased the levels of glucocorticoids, which were significantly higher in the rats running in isolation compared to the socially grouped rats. It was therefore suggested by Stranahan et al. (2006) that social interaction buffers the exercised rats from negative actions of high glucocorticoid levels. The increase in glucocorticoid levels seen in the isolated exercised rats could account for the increased anxiety shown in this study.
The MWM is a test of spatial learning and reference memory. Maternal separation had no effect on spatial learning and memory. This was in accordance with previous literature (Vallee et al. 1997, Lee 2005, Aisa et al. 2007, Hunsaker et al. 2008). The exercised rats showed enhanced spatial learning and memory in the MWM behavioural testing. The exercised rats had a lower latency to reach the hidden platform from day 3 onwards; the decrease reached a significant level on day 5. This was in accordance with previous studies (Vaynman et al. 2004 and 2007). The latency to locate the platform on day 5 was not correlated with the total distance run by each of the exercising rats. This suggests that the amount of exercise was not related to spatial memory. The probe test showed no significant difference in memory retention, with all groups spending similar amounts of time in the target quadrant. This was unexpected as it has previously been shown that exercised rats spend more time in the target quadrant when compared to controls (Vaynman et al. 2004 and 2007).
A negative correlation was found between latency to locate the platform and weight. Nutritional factors have been suggested to influence the BDNF system and therefore affect hippocampal development (Gomez-Pinilla et al. 2005). The effects of MS-induced stress may have been masked by the significantly lower weight of the non-separated rats in comparison to the maternally separated rats.
Other memory tasks investigated the effects of exercise and maternal separation on different components of recognition memory which included the discrimination of individual objects, object-location and the order of presented objects (Barker et al. 2007). Exercise had an effect on memory of the temporal order of objects placed in the arena, with exercised rats spending less time exploring the more recently encountered object from the second phase compared to non-exercised rats. All groups however did not show impaired temporal order memory as time spent exploring the second phase object was significantly lower than the time spent exploring the object from the first phase; exercise therefore improved temporal order memory. Exercise and maternal separation had no effect on novel object recognition, object location and object-in-place memory. Vallee et al. (1997) reported similar results using handling as a form of neonatal stress. They showed that the number of visits into the previously visited arms in a two-trial memory test on the Y-Maze were similar for developmentally stressed and non-stressed animals therefore showing intact responses to novelty. Binder et al. (2004) showed that exercise had no effect on object recognition memory, which agreed with results found by Mello et al. (2008), where chronic exercise (8 weeks of treadmill running) did not affect long or short term memory in object recognition tasks. However, Aisa et al. (2007) found that maternally separated rats had a significantly lower discrimination index in the novel object recognition tests compared to normally reared controls. Maternal separation has also been shown to prevent long-term potentiation in rat hippocampal slices using electrophysiological recordings (Gruss et al. 2008).
Conclusion
No effect of MS was seen on behaviour whereas running enhanced learning and memory in the MWM task and improved object recognition memory in the temporal order task.
Acknowledgments
This material is based upon work supported financially by the National Research Foundation (NRF), the National Institutes of Health (NIH) Fogarty International Center grant R01TW008040 to Michael J. Zigmond, and the University of Cape Town. The authors would also like to thank Mr Letlogonolo Selaledi and Ms Nuraan Ismail for assistance with animal care. Any opinion, findings and conclusions, or recommendations expressed in this material are those of the authors and therefore the NRF does not accept any liability in regard thereto
References
- Aisa B, Tordera R, Lasheras B, Del Rio J, Ramirez MJ. Cognitive impairment associated to HPA axis hyperactivity after maternal separation. Psychoneuroendocrinology. 2007;32:256–266. doi: 10.1016/j.psyneuen.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Delayed effects of early stress on hippocampal development. Neuropsychopharmacology. 2004;29:1988–1993. doi: 10.1038/sj.npp.1300528. [DOI] [PubMed] [Google Scholar]
- Barker GRI, Bird F, Alexander V, Warburton EC. Recognition memory for objects, place, and temporal order: A disconnection analysis of the role of the medial prefrontal cortex and perirhinal cortex. The Journal of Neuroscience. 2007;27:2948–2957. doi: 10.1523/JNEUROSCI.5289-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertaina-Anglade V, Enjuanes E, Morillon D, la Rochelle D. The object recognition task in rats and mice: A simple and rapid model in safety pharmacology to detect amnesic properties of a new chemical entity. Journal of Pharmacological and Toxicological Methods. 2006;54:99–105. doi: 10.1016/j.vascn.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Besheer J. Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study ‘recognition memory’. Nature Protocols. 2006;1:1306–1311. doi: 10.1038/nprot.2006.205. [DOI] [PubMed] [Google Scholar]
- Binder E, Droste SK, Ohl F, Reul JM. Regular voluntary exercise reduces anxiety-related behaviour and impulsiveness in mice. Behavioural Brain Research. 2004;155:197–206. doi: 10.1016/j.bbr.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Clark PJ, Brzezinska WJ, Thomas MW, Ryzhenko NA, Toshkov SA, Rhodes JS. Intact neurogenesis is required for benefits of exercise on spatial memory but not motor performance or contextual fear conditioning in C57BL/6J mice. Neuroscience. 2008;155:1048–1058. doi: 10.1016/j.neuroscience.2008.06.051. [DOI] [PubMed] [Google Scholar]
- Clements KM, Saunders AJ, Robertson BA, Wainwright PE. Spontaneously hypertensive, Wistar Kyoto and Sprague–Dawley rats differ in their use of place and response strategies in the water radial arm maze. Neurobiology of Learning and Memory. 2007;87:285–294. doi: 10.1016/j.nlm.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Coleman MA, Garland T, Marler CA, Newton SS, Swallow JG, Carter PA. Glucocorticoid response to forced exercise in laboratory house mice (Mus domesticus) Physiology and Behavior. 1998;63:279–285. doi: 10.1016/s0031-9384(97)00441-1. [DOI] [PubMed] [Google Scholar]
- Daniels WMU, Pietersen CY, Carstens ME, Stein DJ. Maternal separation in rats leads to anxiety-like behavior and a blunted ACTH response and altered neurotransmitter levels in response to a subsequent stressor. Metabolic Brain Disease. 2004;19:1–2. doi: 10.1023/b:mebr.0000027412.19664.b3. [DOI] [PubMed] [Google Scholar]
- Duman CH, Schlesinger L, Russell DS, Duman RS. Voluntary exercise produces antidepressant and anxiolytic behavioral effects in mice. Brain Research. 2008;1199:148–158. doi: 10.1016/j.brainres.2007.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure J, Uys JDK, Marais L, Stein DJ, Daniels WMU. Early maternal separation alters the response to traumitization: resulting in increased levels of hippocampal neurotrophic factors. Metabolic Brain Disease. 2007;22:183–195. doi: 10.1007/s11011-007-9048-3. [DOI] [PubMed] [Google Scholar]
- Gareau MG, Jury J, Perdue MH. Neonatal maternal separation of rat pups results in abnormal cholinergic regulation of epithelial permeability. Am J Physiol Gastrointest Liver Physiol. 2007;293:198–203. doi: 10.1152/ajpgi.00392.2006. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Vaynman S. A “deficient environment” in prenatal life may compromise systems important for cognitive function by affecting BDNF in the hippocampus. Experimental Neurology. 2005;192:235–243. doi: 10.1016/j.expneurol.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Griesbach GS, Hovda DA, Molteni R, Wu A, Gomez-Pinnilla F. Voluntary exercise following traumatic brain injury: Brain-derived neurotrophic factor upregulation and recovery of function. Neuroscience. 2004;125:129–139. doi: 10.1016/j.neuroscience.2004.01.030. [DOI] [PubMed] [Google Scholar]
- Gruss M, Braun K, Frey JU, Korz V. Maternal separation during a specific postnatal time window prevents reinforcement of the hippocampal long-term potentiation in adolescent rats. Neuroscience. 2008;152:1–7. doi: 10.1016/j.neuroscience.2007.12.033. [DOI] [PubMed] [Google Scholar]
- Horvath KM, Harkany T, Mulder J, Koolhaas JM, Luiten PG, Meerlo P. Neonatal handling increases sensitivity to acute neurodegeneration in adult rats. Journal of Neurobiol. 2004;60:463–472. doi: 10.1002/neu.20037. [DOI] [PubMed] [Google Scholar]
- Howells FM, Russell VA, Mabandla MV, Kellaway LA. Stress reduces the neuroprotective effect of exercise in a rat model for Parkinson’s disease. Behavioural Brain Research. 2005;165:210–220. doi: 10.1016/j.bbr.2005.06.044. [DOI] [PubMed] [Google Scholar]
- Hunsaker MR, Lee B, Kesner RP. Evaluating the temporal context of episodic memory: The role of CA3 and CA1. Behavioural Brain Research. 2008;188:310–315. doi: 10.1016/j.bbr.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinichev M, Easterling KW, Plotsky PM, Holtzman SG. Long-lasting changes in stress-induced corticosterone response and anxiety-like behaviors as a consequence of neonatal maternal separation in Long-Evans rats. Pharmacology, Biochemistry and Behavior. 2002;73:131–140. doi: 10.1016/s0091-3057(02)00781-5. [DOI] [PubMed] [Google Scholar]
- Kandel ER, Shwartz JH, Jessell TM. Principals of neural science. McGraw-Hill; United States: 2006. [Google Scholar]
- Kathol RG, Jaeckle RS, Lopez JF, Meller WH. Pathophsiology of HPA axis abnormalities in patients with major depression: an update. Am J Psychiatry. 1989;146:311–317. doi: 10.1176/ajp.146.3.311. [DOI] [PubMed] [Google Scholar]
- Knuth ED, Etgen AM. Long-term behavioral consequences of brief, repeated neonatal isolation. Brain Research. 2007;1128:139–147. doi: 10.1016/j.brainres.2006.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I. The role of hippocampal subregions in detecting spatial novelty. Behavioral Neuroscience. 2005;119:145–153. doi: 10.1037/0735-7044.119.1.145. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Day JC, Francis DD, Meaney MJ. Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nature Neuroscience. 2000;3:799–806. doi: 10.1038/77702. [DOI] [PubMed] [Google Scholar]
- Macri S, Chiarotti F, Wurbel H. Maternal separation and maternal care act independently on the development of HPA responses in male rats. Behavioural Brain Research. 2008;191:227–234. doi: 10.1016/j.bbr.2008.03.031. [DOI] [PubMed] [Google Scholar]
- Madruga C, Xavier LL, Achaval M, Sanvitto GL, Lucion AB. Early handling, but not maternal separation, decreases emotional responses in two paradigms of fear without changes in mesolimbic dopamine. Behavioural Brain Research. 2006;166:241–246. doi: 10.1016/j.bbr.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Marais L, van Rensburg SJ, van Zyl JM, Stein DJ, Daniels WMU. Maternal separation of rat pups increases the risk of developing depressive-like behavior after subsequent chronic stress by altering corticosterone and neurotrophin levels in the hippocampus. Neuroscience Research. 2008;61:106–112. doi: 10.1016/j.neures.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Marmendal M, Erkisson CJP, Fahlke C. Early deprivation increases exploration and locomotion in adult male Wistar offspring. Pharmacology, Biochemistry and Behavior. 2006;85:535–544. doi: 10.1016/j.pbb.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Mello PB, Benetti F, Cammarota M, Izquierdo I. Effects of acute and chronic physical exercise and stress on different types of memory in rats. Anais da Academia Brasilera de Ciencias. 2008;80:301–309. doi: 10.1590/s0001-37652008000200008. [DOI] [PubMed] [Google Scholar]
- Mesquita AR, Pego JM, Summaveille T, Maciel P, Almeida OFX, Sousa N. Neurodevelopment milestone abnormalities in rats exposed to stress in early life. Neuroscience. 2007;147:1022–1033. doi: 10.1016/j.neuroscience.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Pickering C, Gustafsson L, Cebere A, Nylander I, Liljequist S. Repeated maternal separation of male Wistar rats alters glutamate receptor expression in the hippocampus but not the prefrontal cortex. Brain Research. 2006;1099:101–108. doi: 10.1016/j.brainres.2006.04.136. [DOI] [PubMed] [Google Scholar]
- Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. European Journal of Pharmacology. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- Roceri M, Hendricks W, Racagni G, Ellenbroek BA, Riva MA. Early maternal deprivation reduces the expression of BDNF and NMDA receptor subunits in rat hippocampus. Molecular Psychiatry. 2002;7:609–616. doi: 10.1038/sj.mp.4001036. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Khalil D, Gould E. Social isolation delays the positive effects of running on adult neurogenesis. Nature Neuroscience. 2006;9:526–533. doi: 10.1038/nn1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Khalil D, Gould E. Running induces widespread structural alterations in the hippocampus and the entorhinal cortex. Hippocampus. 2007;17:1017–1022. doi: 10.1002/hipo.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang AC, Reeb BC, Romeo RD, McEwen BS. Modification of social memory, hypothalamic-pituitary-adrenal axis, and brain asymmetry by neonatal novelty exposure. The Journal of Neuroscience. 2003;23:8254–8260. doi: 10.1523/JNEUROSCI.23-23-08254.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee M, Mayo W, Dellu F, Le Moal M, Simon H, Maccari S. Prenatal stress induces high anxiety and postnatal handling induces low anxiety in adult offspring: correlation with stress-induced corticosterone secretion. The Journal of Neuroscience. 1997;7:2626–2636. doi: 10.1523/JNEUROSCI.17-07-02626.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. Interplay between brain-derived neurotrophic factor and signal transduction modulators in the regulation of the effects of exercise on synaptic-plasticity. Neuroscience. 2003;122:647–657. doi: 10.1016/j.neuroscience.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. European Journal of Neuroscience. 2004;20:2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. The select action of hippocampal calcium calmodulin protein kinase II in mediating exercise-enhanced cognitive function. Neuroscience. 2007;144:825–833. doi: 10.1016/j.neuroscience.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nature Protocols. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nature Protocols. 2007;2:322–328. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widenfalk J, Olson L, Thoren P. Deprived of habitual running, rats down-regualte BDNF and TrkB messages in the brain. Neuroscience Research. 1999;34:125–132. doi: 10.1016/s0168-0102(99)00051-6. [DOI] [PubMed] [Google Scholar]
- Wigger A, Neumann ID. Gender-Dependent alterations in behavioral and neuroendocrine responses to emotional stress in adult rats. Physiology and Behavior. 1999;66:293–302. doi: 10.1016/s0031-9384(98)00300-x. [DOI] [PubMed] [Google Scholar]
- Ying Z, Roy RR, Zhong H, Zdunowski S, Edgerton VR, Gomez-Pinilla F. BDNF - exercise interactions in the recovery of symmetrical stepping after a cervical hemisection in rats. Neuroscience. 2008;155:1070–1078. doi: 10.1016/j.neuroscience.2008.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]