Abstract
14C has long been used as a tracer for quantifying the in vivo human metabolism of food components, biopharmaceuticals, and nutrients. Minute amounts (≤1 × 10 −18 mol) of 14C can be measured with high-throughput 14C-accelerator mass spectrometry (HT 14C-AMS) in isolated chemical extracts of biological, biomedical, and environmental samples. Availability of in vivo human data sets using a 14C tracer would enable current concepts of the metabolic behavior of food components, biopharmaceuticals, or nutrients to be organized into models suitable for quantitative hypothesis testing and determination of metabolic parameters. In vivo models are important for specification of intake levels for food components, biopharmaceuticals, and nutrients. Accurate estimation of the radiation exposure from ingested 14C is an essential component of the experimental design. Therefore, this paper illustrates the calculation involved in determining the radiation exposure from a minute dose of orally administered 14C-β-carotene, 14C-α-tocopherol, 14C-lutein, and 14C-folic acid from four prior experiments. The administered doses ranged from 36 to 100 nCi, and radiation exposure ranged from 0.12 to 5.2 μSv to whole body and from 0.2 to 3.4 μSv to liver with consideration of tissue weighting factor and fractional nutrient. In comparison, radiation exposure experienced during a 4 h airline flight across the United States at 37000 ft was 20 μSv.
Keywords: 14C tracer, radiation exposure calculation, in vivo in human, accelerator mass spectrometry (AMS)
Introduction
Carbon-14 (14C) is a radioactive isotope of carbon that is produced by nitrogen-14 (14N) capture of thermal neutrons generated from cosmic rays (1n + 14N → 14C + 1H) (1, 2). The natural abundance of 14C is 1.2 × 10−10% (2, 3). 14C has long half-life (t1/2 = 5730 years), and it decays to nonradioactive 14N through radioactive β-decay, which emits an electron and an antineutrino (2−6). Due to very low natural abundance and long half-life, 14C is an ideal tracer for environmental as well as in vivo human or animal studies.
Traditionally, 14C has been measured using decay counters such as a liquid scintillation counter (LSC). LSC usually requires a gram-sized carbon sample and long measuring time (>48 h) to obtain a statistical precision of ≤1% (4, 5). Conventional mass spectrometry (MS) was also an alternative technique to detect various isotopes. However, isotopes such as 14C with rare natural abundance cannot be detected for lack of sensitivity of conventional MS (detection limit of 14C/12C ratio using MS, ≥1 × 10−7, vs ambient level of 14C/12C ratio, 1 × 10−12) and for isobar interference (14C vs 14N, 12CH2−, 12CD, 13CH1−) (5−9).
Accelerator mass spectrometry (AMS) is the ultimate technique to measure long-lived isotopes such as 14C due to extremely high selectivity and sensitivity. AMS was first developed in the early 1930s (10), but it was not used for 14C measurement until 1977 due to atomic isobar interference with 14N (11). Development of the cesium sputter (Cs+) method to generate 14C− negative ions enabled AMS to be used for radiocarbon dating (5, 8, 12, 13). 14N does not form stable 14N− ions.
AMS methods for various radioisotopes were developed to advance specific research objectives: radiocarbon dating (14C), biological/biomedical study (3H, 14C, 26Al, 41Ca, 129I), nuclear weapon testing (41Ca), monitoring migration of nuclear waste from nuclear power plant (36Cl, 129I), hydrogeological study (10He, 36Cl), and exposure dating (10Be, 26Al, 36Cl) (5). 14C was the most popular radioisotope measured with AMS (3, 5). AMS measured the 14C/12C or 14C/13C ratio (rather than 14C decay) in samples of interest and thereby increased sensitivity to the attomole (10−18) level, making AMS 1000 times more sensitive than LSC (3, 5, 14). AMS required a milligram or less of carbon (≤mg of C). Sample mass for AMS was 1000 times less than that for LSC (3−5, 8, 14). Furthermore, AMS achieved a precision of ≈1% in 5−10 min (3−6). The great efficiency and sensitivity made AMS the ultimate tool for quantifying metabolic behaviors of food components, nutrients, and biopharmaceuticals using a 14C tracer.
Biological/biomedical applications using AMS were first suggested ≈30 years ago (15), and these applications began in earnest in the early 1990s (16). In vivo in human or in vivo in animal dynamic and kinetic behaviors, absorption, distribution, metabolism, elimination (ADME) of food component, nutrients (17−21), or environmental chemicals (22−24) using 14C-AMS have been reported during the past two decades.
For biological/biomedical 14C-AMS applications, very small amounts of 14C-labeled compound were administered to humans and commonly called microdosing in drug development studies. The U.S. Food and Drug Administration (25) and European Medicines Agency (26) defined microdosing as only 1% of a therapeutic dose or ≤100 μg/person. Because pharmacokinetics (PK) with microdosing was ≈70% equivalent of a PK with therapeutic dose (27), microdosing combined with AMS enabled physiological-based (steady-state) kinetic/dynamic behavior study of food components or nutrients to be conducted. Thus, AMS methods led to better understanding of in vivo in human metabolism of food components or nutrients (14). The combination of microdosing and AMS also minimized the risk of toxicity, cost, time, and labor, especially for new drug development in the pharmaceutical industry (4).
The typical radiation exposure experienced during a 4 h airplane ride at 37000 feet altitude was 20 μSv (28). The radiation exposure of 20 μSv was considered to be an acceptable level of exposure in the United States, so 20 μSv is used as a reference level. Human radiation exposure calculation from the administered 14C-labeled food components or nutrients is an integral part of protocols for biological/biomedical AMS applications. Therefore, the present study reported a practical and complete method for calculating the 14C radiation exposure during the conduct of our four prior studies (18−21). The method took into account 14C dose level, body mass, biological half-life of 14C-labeled nutrients, tissue weighting factor (radiation exposure in an organ and/or tissue relative to that of the whole body mass in dimensionless multiplicative factor), and fractional nutrient content in each organ or tissue.
Materials and Methods
Subject Selection
Human subjects who were healthy nonsmoking adults aged 20−60 years with a BMI of 20−37.5 kg/m2 were recruited (Table 1). Written informed consent was obtained from volunteers per University of California—Davis (UCD) and Lawrence Livermore National Laboratory (LLNL) Human Subjects Review Committees. All studies were performed with Good Clinical Practice Guidelines (ver. 1989) and the ethical guidelines of the 1975 Declaration of Helsinki (18−21).
Table 1. Summary of Our Prior 14C-Labeled Nutrient Studies (18−21) and Daily Reference Nutrient Amounts.
| α-tocopherol (20) |
||||||
|---|---|---|---|---|---|---|
| unit | β-carotene (18) | lutein (19) | RRRa | all-raca | folic acid (21) | |
| molecular weight | g/mol | 536.873 | 568.9 | 430.71 | 430.71 | 441.4 |
| specific activity, SA | Ci/mol | 98.8(18) | 0.2888 | 56 | 60 | 1.24b |
| gender | male (n = 4) | female (n = 1) | male (n = 1) | male (n = 1) | male (n = 7) | |
| female (n = 4) | female (n = 6) | |||||
| subject | kg | 76.7 ± 23.2 | 64.1 | 79.5 | 79.5 | 78.3 ± 0.28 |
| body mass index | kg/m2 | 25.0 ± 4.2 | 27.6 | 23.8 | 23.8 | 26.5 ± 5.9 |
| age | year | 35 ± 8.2 | 45 | 36 | 36 | 32 ± 0.38 |
| 14C dose (18−21) | nmol [nCi] | 1.01 [100] | 125 [36] | 1.821 [101.5] | 1.667 [99.98] | 0.5 [100] |
| ratio (mol/mol) between 14C dose (18−21) and Recommended Daily Allowance (RDA) or average intake (AI) in the U.S. | 1/6544 | 1/3 through 1/28 | 1/19124 | 1/20891 | 1/1812 (>19 years) 1/2719 (pregnancy) |
|
| reference nutrient amount/day | ||||||
| RDA or AI in the U.S. | mmol/day | 0.0066 (AI) (17) | 0.0003−0.0035 (AI) (38) | 0.0348 (>19 years) (RDA) (39) | 0.0009 (>19 years) 0.0014 (pregnancy) (RDA) (40) |
|
| therapeutic level | mmol/day | ≈0.32 (39) | 0.011−0.018 (38) | 0.12−7.43 (41) | 0.0005−0.022 (41) | |
| tolerable upper intake level/day | mmol/day | ndc(39) | nd (39) | 2.32 (>19 years) (39) | 0.0023 (>19 years) (40) | |
The study design was a test and retest in one subject.
SA was determined by using a mix of 0.5 nmol of 14C-folic acid and 79.5 nmol of nonlabeled cold folic acid.
The Institute of Medicine at the National Academy of Sciences did not establish a Tolerable Upper Intake Level (UL) for carotenoids when it reviewed these compounds in 2000.
Dose Administration
Table 1 summarizes the 14C dose, nutrient dose, etc. in each study (18−21). Nutrients were chemically (β-carotene, α-tocopherol) or biologically (lutein, folic acid) labeled with 14C. 14C-Nutrients (36−100 nCi) were orally administered to human subjects with breakfast. Oral dose administration was also approved by the UCD and LLNL Human Subjects Review Committees prior to 14C-nutrient studies (18−21). A small ratio (mol/mol) of 14C-nutrient dose to Recommend Dietary Allowances (RDA) was necessary for the physiological-based (steady-state) 14C-tracer study. The RDAs of β-carotene and lutein were not decided yet, so U.S. average intake (AI) was considered for steady-state 14C-tracer study.
Sample Collections
Prior to 14C-nutrient dose, fasting plasma, urine, and feces were collected for 14C baselines of each human subject. Serial plasmas, urines, and feces were collected over time since 14C-nutrient dose (18−21). A predose baseline blood sample was collected. Additional blood samples were usually collected at every 0.5 h interval from 0 to 12 h, hourly from 12 to 16 h, and then at 24 and 36 h after 14C-nutrient dose. Subsequent blood samples were collected daily from 2 to 7 days, every other day from 7 to 14 days, then weekly, biweekly, and/or monthly for the remainder of the study. In addition, prior to dosing 14C-nutrient in each study, a complete collection of feces and urine was also taken to establish baseline values. Serial collections of feces and urine usually continued for 14 and 21 days, respectively, after the day of 14C-nutrient dosing. Durations of β-carotene, lutein, α-tocopherol, and folate studies were 5, 3, 3, and 6 months, respectively. When 100 nCi of 14C was dosed to the 70 kg human subject, collected samples had 0.0014 nCi of 14C/g, a level considered to be nonradioactive according to the U.S. Code of Federal Regulations, Title 10, Section 20.2005, 1991 (cutoff, ≤50 nCi/g or 1 μCi/year), enabling waste to be disposed of as nonradioactive waste.
Graphitization
All reagents and materials for sample preparation (18−21) and graphitization/14C-AMS measurement (29−31) were previously described and are summarized below. High-throughput (HT) 14C-AMS applications usually required 1 mg of C sample, so carbon content in the sample of interest was measured (32). Prior to 14C-AMS measurement, samples of interest were converted to elemental carbon, usually graphite-like materials. Sample preparation (often called graphitization) consisted of oxidation (combustion: sample → CO2) and reduction (graphitization: CO2 → Cgraphite-like materials) steps. We have previously used three different graphitization methods for β-carotene (31), lutein (31), α-tocopherol (29, 30), and folic acid (29) studies. Recently, we optimized our graphitization method (maximum 270 samples/day) for accurate and precise HT-14C-AMS measurement (33). More information about graphitization is included in the Supporting Information (Figure 1; Table 1).
14C-AMS Measurement
One million voltage AMS at the Center for AMS at LLNL (see Supporting Information, Figure 2) measured 14C/13C ratios from graphitized solid samples. Measured 14C/13C ratios of samples were normalized with one of the popular AMS standards such as oxalic acid, NIST SRM 4990B/4990C, or sucrose, IAEA-C6 (34, 35). Finally, 14C level in samples was defined as “Modern or Fraction Modern (Fm)”. A current/natural 14C level in living organisms is about 1.1 Fm (= 107.6 amol of 14C/mg of C, 6.72 fCi/mg of C, or 14.92 dpm/g of C) (30, 36).
Human Radiation Exposure Calculation from 14C Dose
Radiation exposure from 14C-labeled nutrient was calculated (3) as
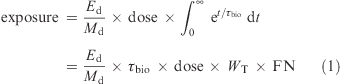 |
where Ed is energy deposition per decay as joules (8.3 fJ/decay), Md is the affected mass (kg), dose is the amount of radioactivity in dpm, τbio is the biological mean-life of 14C-labeled compound in minutes or seconds, WT is tissue weighting factor (radiation exposure in an organ and/or tissue relative to that of the whole body (WB) in dimensionless multiplicative factor (see Supporting Information, Table 2) (37), and FN is the fractional nutrient content in each organ or tissue (see Supporting Information, Table 2).
Radiation exposure was calculated previously without considering WT and the FN (3). The formula (eq 1) for radiation exposure estimation was modified by considering WT for each organ as well as different FN. The formula (eq 1) can more completely calculate radiation exposure over the biological mean-life. For example, if 100 nCi of 14C-β-carotene, which had the τbio of 36.1 days (equals to 51984 min), was administered to a human subject of 61.2 kg (see Supporting Information, Table 2), the radiation exposures to WB and liver (≈1.5 kg) were calculated by considering WT and FN as follows:
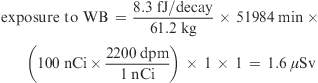 |
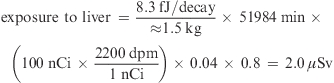 |
All radiation exposure calculations from 14C-food component and 14C-nutrient studies are available as Supporting Information (Table 2).
14C Dosing Calculation for Detecting Optimal 14C Concentration with 14C-AMS
A 14C dose estimation was required for determining the optimal 14C range for the 14C-AMS, which ranged from 0.01 to 100 Modern with resolution of ≤0.05 Modern to the ambient Modern (baseline). The 14C dose level was calculated as follows: (3):
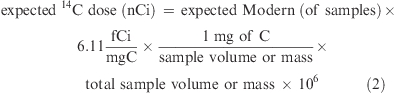 |
In general, biological/biomedical AMS easily measured 10 Modern in sample containing 1 mg of C. For example, if 10 Modern was from 25 μL of plasma from a 70 kg human subject, if the 14C-compound was water-soluble, which can be distributed to WB water (maximum 42 L), and if absorption is 100%, then the 14C dose level can be calculated as
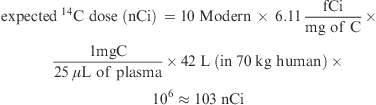 |
Therefore, when the water-soluble 14C compound of ≈100 nCi was dosed to a 70 kg human subject, a 25 μL plasma sample would be about 10 Modern. The formula (eq 2) can be used to estimate the amount of 14C dose. For more complete estimation, the bioavailability and body distribution of the food component or nutrient should be considered (3).
Results and Discussion
Calculating Human Radiation Exposure from 14C-Nutrients
The dose of 14C-nutrients and radiation exposures were estimated over the biological mean-life (τbiological) (Table 2). The 14C-nutrients containing 36−101.5 nCi were orally ingested at breakfast by human subjects weighing 52−116 kg. Biological half-lives (t1/2) of these nutrients ranged from 2.5 to 100 days.
Table 2. Estimated Radiation Exposure from 14C-Labeled Nutrients over the Biological Mean-Life (τbiological) as a Function of Time since Dose.
| α-tocopherol (20) |
||||||
|---|---|---|---|---|---|---|
| unit | β-carotene (18) | lutein (19) | RRR | all-rac | folic acid (21) | |
| subject body weight | kg | 76.8 ± 23.2 (n = 8) | 64.1 (n = 1) | 79.5 (n = 1) | 79.5 (n = 1) | 78.3 ± 22.3 (n = 13) |
| dose | nCi | 100 | 36 | 101.5 | 99.95 | 100 |
| half-life (t1/2) | day | 25 | 14 | 2.5 | 2.5 | 100 |
| mean-life (τbiological = t1/2/Ln 2) | day | 36.1 | 20.2 | 3.6 | 3.6 | 144.3 |
| tissue weighting factor, WT, liver | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | |
| fractional nutrient, FN, liver | 0.8 (as carotene) 0.9 (as retinol) |
0.5 | 0.8 | 0.8 | 0.333 | |
| radiation exposurea | ||||||
| whole body, WB | μSv | 1.3 ± 0.4 | 0.3 | 0.12 | 0.12 | 5.2 ± 1.5 |
| liver, 1.5 kgb | μSv | 63.3 (as carotene) 354 (as retinol) |
12.7 | 6.4 | 6.3 | 253 |
| liver, 1.5 kgc | μSv | 2.0 (as carotene) 12.8 (as retinol) |
0.3 | 0.2 | 0.2 | 3.4 |
Nutrient absorption is assumed 100%.
Radiation exposure regardless of WT and FN.
Radiation exposure regard of WT (ICRP 103, 2008) and FN. The dimensionless multiplicative factors (WT and FN) are 1 for WB.
Radiation exposure to WB ranged from 0.12 to 5.2 μSv, which was 4−170 times lower than that experienced during a 4 h flight (20 μSv) (28). In general, radiation exposure increased as the biological mean-life of nutrient increased and as the organ or tissue mass decreased. Assuming 100% of the body’s stored 14C-nutrients was housed in liver regardless of the WT and FN, then radiation exposure to a 1.5 kg liver was calculated to be ≈50 times higher than that to a 70 kg WB. If all of a 100 nCi 14C-β-carotene dose was converted to 14C-retinol and stored in the liver, the radiation exposure to liver was increased to a maximum 354 μSv (Table 2). Sequestration of 100% of the 14C label in the liver represents the “worst case” scenario.
By considering the WT and FN, radiation exposures to liver (Table 2) were calculated to range from 0.2 to 12.8 μSv, which was lower than that experienced during a 4 h flight at 37000 ft altitude (20 μSv). Although 14C-retinol had a longer half-life (140 days), radiation exposure to liver from 14C-retinol was calculated to be 12.8 μSv when the WT (0.04 in liver) and FN (0.9 in liver) were considered. Consequently, radiation exposure from dose with 100 nCi 14C was equal to or less than that experienced from common/natural radiation (see Supporting Information, Table 3).
Radioactive isotopes emit energy depending on α-, β-, or γ-decay. People are exposed to various radiation sources during their lifetime, and the limit of annual radiation exposure differed between WB, organ, or tissue (see Supporting Information Table 3). 14C emitted an electron for each β-decay, the average energy of which was 8.3 fJ/decay (3). Thus, the reference human (70 kg, ≈ 1.1 Modern, assumed 23% carbon content) was naturally/constantly exposed to ≈1.7 nSv/h at the ambient 14C level
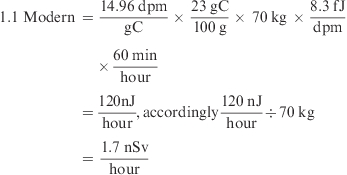 |
where J/kg = Sv) (3).
Deposition of the radiation energy from the administered 14C-nutrient was neither complete nor constant throughout the human body during the study because the 14C-nutrient was metabolized, stored, and eventually excreted. The radiation exposure from ingested 14C-nutrients or food components can be calculated using an exponential elimination model as a function of time since dose, as long as the biological mean-lives of the 14C-labeled nutrients were shorter than the expected human life span (3). Therefore, full dynamic/kinetic modeling was required for the more exact calculation of human radiation exposure. Furthermore, correction of radiation exposure due to radioisotope decay was not necessary when τisotope was much longer than τbio. In addition, considering WT and FN enabled more complete calculation than a prior calculation (3) of radiation exposure to individual organs and tissues. In the present study, radiation exposure to liver was 30−75 times lower when the WT and FN of each nutrient were considered (Table 2). Finally, radiation exposures to WB and liver from all 14C-nutrients (Table 2) were lower than natural/human-made radiation exposure.
Dose Calculation for Optimal 14C-AMS Measurement of 1 mg of C Size Samples
Table 3 summarizes the calculated and measured maximum 14C levels in human plasma, feces, or urine samples that contained 1 mg of C. The physiological-based 14C-nutrient study required a dose with the same or smaller amount of 14C-nutrient relative to its RDA or AI. The ratio (mol/mol) of 14C-nutrient to the RDA or AI was ≤1/1812 (17, 18, 20, 21), which was even smaller than the microdosing level, 1/100 (25, 26). The ratio (mol/mol) of 14C-lutein to its AI ranged from 1/3 to 1/28 (19), due to the low specific activity of 14C-lutein ingested (Table 1), and even this ratio (1/3 to 1/28) was suitable for physiological-based pharmacokinetics and ADME measurements (Table 1).
Table 3. Estimated and Measured 14C Levels from 14C-Labeled Nutrients as a Function of Time since Dose.
| α-tocopherol (20) |
||||||
|---|---|---|---|---|---|---|
| unit | β-carotene (18) | lutein (19) | RRR | all-rac | folic acid (21) | |
| dose | nCi | 100 | 36 | 101.5 | 99.95 | 100 |
| estimated maximum 14C level/mg of C (in 25 μL of plasma)a | ||||||
| distributed in plasma of 3 L | Modern | 137 | 49 | 139 | 137 | 137 |
| distributed in interstitial water of 18 L | Modern | 23 | 8 | 23 | 23 | 23 |
| distributed in WB water of 42 L | Modern | 10 | 4 | 10 | 10 | 10 |
| measured maximum14C level/mg of C | ||||||
| plasma | Modern | 13 | 3.8 | 21 | 14 | 4.7 ± 1.6 |
| fecesb | Modern | ≥35 | 54 | 18 | 11 | 53 ± 50 |
| urineb | Modern | ≈ 53 | 1.0 | 0.6 | 1.8 | 66 ± 22 |
Peak 14C levels in plasma, urine, and feces occurred within the first 5 days as a function of time since dosing. For human subjects dosed with 36−100 nCi of 14C, the calculated peak of 14C in plasma (mg of C) ranged from 49 to 137 Modern for a 3 L plasma pool, from 8 to 23 Modern for an 18 L interstitial water pool, and from 4 to 10 Modern for a 42 L WB water pool. The measured level of 14C in plasma (mg of C), however, varied over a 10−30-fold lower range, because 14C-nutrients were distributed, metabolized, and excreted (Table 3). The measured peak range (3.8−21 Modern) of 14C in plasma was within the optimal 14C-AMS range of 0.01−100 Modern.
In general, the maximum 14C level in feces and in urine was higher than the maximum 14C level in plasma. Therefore, feces and urine samples containing >100 Modern usually needed to be diluted with tributyrin, which was devoid of 14C. Thus, the levels of 14C in the diluted feces and urine were within the optimal 14C-AMS working range. Masses or volumes of the feces and of urine collections were difficult to control compared to those of human blood. Furthermore, feces and urine collections varied widely in their carbon content compared to human blood. Consequently, the Modern values of feces and urine were higher and more variable than those of human blood, due to the variable concentrations of carbon in feces/urines and the first-pass elimination of 14C-nutrient in feces. Although maximum 14C levels in feces and urine were variable depending on ADME, nutrient hydrophobicity, subject traits, etc., dosing of ≤100 nCi was suitable for 14C-AMS, the optimal range of which was 0.01−100 Modern.
In fact, determination of 14C-dose amount for optimal 14C-AMS measurement was difficult because that determination needed to consider the hydrophilic, hydrophobic, and bioavailability characteristics of nutrients, human body weight, and sensitivity of AMS. Most nutrients would be transferred, partitioned, or distributed among body organs (liver, gut, etc.), intracellular (21 L), interstitial (18 L), intravascular fluids (3 L), and WB water (42 L). If water-soluble compounds were distributed in WB water of 42 L, ≈100 nCi of 14C would peak at about 10 Modern in 25 μL of plasma (which contained 1 mg of C), even though the bioavailability of nutrients was variable. On the other hand, very hydrophobic compounds or compounds with ≤20% bioavailability required a larger dose of ≈200 nCi to study 14C-nutrients with long biological half-life (3).
Even though the administered doses ranged from 36 to 100 nCi in our four prior studies, by considering tissue weighting factor and fractional nutrient factor, the radiation exposures to WB (0.12−5.2 μSv) or to liver (0.2−3.4 μSv) were much lower than that from a 4 h aircraft flight (20 μSv). Furthermore, 14C-doses of 36−100 nCi were measured in the range of 0.01−100 Modern in plasma, urine, and feces aliquots that contained 1 mg of C. All materials and samples from graphitization and 14C-AMS measurement can be considered nonradioactive waste materials, at significant cost saving for disposal.
Acknowledgments
We thank the reviewers for their perceptive and helpful comments.
Supporting Information Available
Two figures, three tables, and supporting text and references. This material is available free of charge via the Internet at http://pubs.acs.org.
This work was supported by NIH DK-078001, DK-081551, DK-45939, DK-48307, and the USDA Regional Research W-2002 from the California Agricultural Experiment Station. Aspects of this work were performed under the auspices of the U.S. Department of Energy by Lawrence Livermore National Laboratory and supported by the Office of Science, Office of Basic Energy Sciences, Division of Materials Science and Engineering under Contract DE-AC52-07NA27344 and NIH National Center for Research Resources Grant RR13461.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Kamen M. D. Early history of carbon-14: discovery of this supremely important tracer was expected in the physical sense but not in the chemical sense. Science 1963, 140, 584–590. [DOI] [PubMed] [Google Scholar]
- Pierson H. O.The element carbon. In Handbook of Carbon, Graphite, Diamond, and Fullerenes, Processing, Properties and Applications; Noyes Publications: Park Ridge, NY, 1993; pp 20−21. [Google Scholar]
- Vogel J. S.; Love A. H. Quantitating isotpic molecular labels with accelerator mass spectrometry. Methods Enzymol. 2005, 402, 402–422. [DOI] [PubMed] [Google Scholar]
- Salehpour M.; Possnert G.; Bryhni H. Subattomole sensitivity in biological accelerator mass spectrometry. Anal. Chem. 2008, 80, 3515–3521. [DOI] [PubMed] [Google Scholar]
- Hellborg R.; Skog G. Accelerator mass spectrometry. Mass Spectrom. Rev. 2008, 27, 398–427. [DOI] [PubMed] [Google Scholar]
- Tuniz C.; Bird J. R.; Fink D.; Herzog G. F.. Performance of AMS systems. In Accelerator Mass Spectrometry. Ultrasensitive Analysis for Global Science; CRC Press: New York, 1998; pp 21−39. [Google Scholar]
- Vogel J. S.; Turteltaub K. W.; Finkel R.; Nelson D. E. Accelerator mass spectrometry, isotope quantification at attomole sensitivity. Anal. Chem. 1995, 67, 353A–359A. [DOI] [PubMed] [Google Scholar]
- Vogel J. S.; Turteltaub K. W.. Accelerator mass spectrometry as a biological tool for nutritional research. In Mathematical Modeling in Experimental Nutrition AEMB; Clifford A. J., Muller H.-G., Eds.; Plenum Press:New York, 1998; Vol. 445, pp 397−410. [DOI] [PubMed] [Google Scholar]
- Aitken M. J. Archeological involvements of physics. Phys. Rep. Rev. Sect.: Phys. Lett. 1978, 40, 278–351. [Google Scholar]
- Wilson R. R.; Littauer R. In Accelerator: Machines of Nuclear Physics, 1st ed.; Anchor Books: New York, 1960; p 60. [Google Scholar]
- Muller R. A. Radioisotope dating with a cyclotron. Science 1977, 196, 189–494. [DOI] [PubMed] [Google Scholar]
- Nelson D. E.; Korteling R. G.; Stott W. R. Carbon-14: direct detection at natural concentration. Science 1977, 198, 508–510. [DOI] [PubMed] [Google Scholar]
- Bennett C. L.; Beukens R. P.; Clover M. R.; Gove H. E.; Liebert R. B.; Litherland A. E.; Purser K. H.; Sondheim W. E. Radiocarbon dating using electrostatic accelerator: negative-ions provide key. Science 1977, 198, 508–510. [DOI] [PubMed] [Google Scholar]
- de Moura F. F.; Burri B. J.; Clifford A. J.. Accelerator mass spectrometry in the study of vitamins ans mineral metabolism in humans. In Handbook of Vitamins; Zempleni J., Rucker R. B., McCormick D. B., Suttie J. W., Eds.; CRC Press/Taylor and Francis Group: Boca Raton, FL, 2007; pp 545−557. [Google Scholar]
- Keilson J.; Waterhouse C.. First Conference on Radiocarbon Dating with Accelerators; Grove H. E., Ed.; University of Rochester: Rochester, NY, 1978. [Google Scholar]
- Turteltaub K. W.; Felton J. S.; Gledhill B. L.; Vogel J. S.; Southon J. R.; Caffee M. W.; Finkle R. C.; Nelson D. E.; Proctor I. D.; Davis J. C. Accelerator mass spectrometry in biomedical dosimetry: relationship between low-level exposure and covalent binding of heterocyclic amine carcinogens to DNA. Proc. Natl. Acad. Sci. U.S.A. 1990, 87, 5288–5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho C. C.; de Moura F. F.; Kim S. H.; Clifford A. J. Excentral cleavage of β-carotene in vivo in a healthy man. Am. J. Clin. Nutr. 2007, 85, 770–777. [DOI] [PubMed] [Google Scholar]
- Ho C. C.; de Moura F. F.; Kim S.-H.; Burri B. J.; Clifford A. J. A minute dose of 14C-β-carotene is absorbed and converted to retinoids in humans. J. Nutr. 2009, 139 (8), 1480–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Moura F. F.; Ho C. C.; Getachew G.; Hickenbottom S.; Clifford A. J. Kinetics of 14C distribution after tracer dose of 14C-lutein in an adult woman. Lipids 2005, 40, 1069–1073. [DOI] [PubMed] [Google Scholar]
- Clifford A. J.; de Moura F. F.; Ho C. C.; Chuang J. C.; Follett J.; Fadel J. G.; Novotny J. A. A feasibility study quantifying in vivo human α-tocopherol metabolism. Am. J. Clin. Nutr. 2006, 84, 1430–1441. [DOI] [PubMed] [Google Scholar]
- Lin Y.; Dueker S. R.; Follett J. R.; Fadel J. G.; Arjomand A.; Schneider P. D.; Miller J. W.; Green R.; Buchholz B. A.; Vogel J. S.; Phair R. D.; Clifford A. J. Quantitation of in vivo human folate metabolism. Am. J. Clin. Nutr. 2004, 80, 680–691. [DOI] [PubMed] [Google Scholar]
- Turteltaub K. W.; Dingley K. H.; Curtis K. D.; Malfatti M. A.; Turesky R. J.; Colin Garner R.; Felton J. S.; Lang N. P. Macromolecular adduct formation and metabolism of heterocyclic amines in humans and rodents at low doses. Cancer Lett. 1999, 143, 149–155. [DOI] [PubMed] [Google Scholar]
- Dingley K. H.; Freeman S. P. H. T.; Nelson D. O.; Garner R. C.; Turteltaub K. W. Covalent binding of 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline to albumin and hemoglobin at environmentally relevant doses. Comparison of human subjects and F344 rats. Drug Metab. Dispos. 1998, 26, 825–828. [PubMed] [Google Scholar]
- Turteltaub K. W.; Mauthe R. J.; Dingley K. H.; Vogel J. S.; Frantz C. E.; Garner R. C.; Shen N. MeIQx-DNA adduct formation in rodent and human tissues at low doses. Mutat. Res. 1997, 376, 243–252. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration, U.S. Department of Health and Human Services Guidelines for Industry Investigators and Reviewers. Exploratory IND Studies, F. R., MD, USA, January 2006.
- Position Paper on Nonclinical Safety Studies to Support Clinical Trails with a Single Microdose. Position paper CPMP/SWP/2599, E. L., UK, 23 June 2004.
- Garner R. C.; Lappin G. Commentary: The phase 0 microdosing concept. Br. J. Clin. Pharmacol. 2006, 61, 367–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg W.; Copeland K.; Duke F. E.; O’Brien K.; Darden E. B. Radiation exposure during air travel: guideline provided by the Federal Aviation Administarion for air carrier crews. Health Phys. 2000, 79, 591–595. [DOI] [PubMed] [Google Scholar]
- Vogel J. S. Rapid production of graphite without contamination for biomedical AMS. Radiocarbon 1992, 34, 344–350. [Google Scholar]
- Ognibene T. J.; Bench G.; Vogel J. S.; Peaslee G. F.; Murov S. A high-throughput method for the conversion of CO2 obtained from biochemical samples to graphite in septa-sealed vials for quantification of 14C via accelerator mass spectrometry. Anal. Chem. 2003, 75, 2192–2196. [DOI] [PubMed] [Google Scholar]
- Getachew G.; Kim S. H.; Burri B. J.; Kelly P. B.; Haack K. W.; Ognibene T. J.; Buchholz B. A.; Vogel J. S.; Modrow J.; Clifford A. J. How to convert biological carbon into graphite for AMS. Radiocarbon 2006, 48, 325–336. [Google Scholar]
- Pella E. Elemental organic analysis. Am. Lab. 1990, 22, 116–125. [Google Scholar]
- Kim S. H.; Kelly P. B.; Clifford A. J. Biological/biomedical accelerator mass spectrometry targets. 1. Optimizing the CO2 reduction step using zinc dust. Anal. Chem. 2008, 80, 7651–7660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. H.; Kelly P. B.; Clifford A. J. Accelerator mass spectrometry targets of submilligrams carbonaceous samples using high-throughput Zn reduction method. Anal. Chem. 2009, 81, 5949–5954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue D. J.; Linick T. W.; Jull A. J. T. Isotope-ratio and background corrections for accelerator mass spectrometry radiocarbon measurements. Radiocarbon 1990, 32, 135–142. [Google Scholar]
- Stuiver M.; Polach H. A. Discussion reporting of 14C data. Radiocarbon 1977, 19, 355–363. [Google Scholar]
- ICRP Publishing, Icrp, C.R.P.I.C.R.P. In ICRP 103: The 2007 Recommendation of the International Commission on Radiological Protection; Valentin J., Ed.; Pergamon Press: New York, 2007; Vol. 37, p 56. [Google Scholar]
- Lewis B.Promoting eye and skin health through intake of the natural carotenoid lutein. In Wild-Type Food in Health Promotion and Disease Prevention; De Meester F., Watson R. R., Eds.; Humana Press: Totowa, NJ, 2008; pp 331−342. [Google Scholar]
- Board, N. A. o. S. I. o. M. F. a. N. Vitamin E and β-caroetene and other carotenoids. In Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; National Academy Press: Washington, DC, 2000; pp 240, 250,, 366. [Google Scholar]
- Board, N. A. o. S. I. o. M. F. a. N. Folate. In Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline; National Academy Press: Washington, DC, 1998; pp 228, 233,, 238,, 281. [PubMed] [Google Scholar]
- Gerber J. M.Vitamins. In Handbook of Preventive and Therapeutic Nutrition; Jones and Bartlett Publishers: Sudbury, MA, 1993; pp 261−279. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


