Summary
The formation of epithelial cell barriers results from the defined spatiotemporal differentiation of stem cells into a specialized and polarized epithelium, a process termed mesenchymal-epithelial transition. The reverse process, epithelial-mesenchymal transition (EMT), is a metastable process that enables polarized epithelial cells acquire a motile fibroblastoid phenotype. Physiological EMT also plays an essential role in promoting tissue healing, remodeling, or repair in response to a variety of pathological insults. On the other hand, pathophysiological EMT is a critical step in mediating the acquisition of metastatic phenotypes by localized carcinomas. Although metastasis clearly is the most lethal aspect of cancer, our knowledge of the molecular events that govern its development, including those underlying EMT, remain relatively undefined. Transforming growth factor-β (TGF-β) is a multifunctional cytokine that oversees and directs all aspects of cell development, differentiation, and homeostasis, as well as suppresses their uncontrolled proliferation and transformation. Quite dichotomously, tumorigenesis subverts the tumor suppressing function of TGF-β, and in doing so, converts TGF-β to a tumor promoter that stimulates pathophysiological EMT and metastasis. It therefore stands to reason that determining how TGF-β induces EMT in developing neoplasms will enable science and medicine to produce novel pharmacological agents capable of preventing its ability to do so, thereby improving the clinical course of cancer patients. Here we review the cellular, molecular, and microenvironmental mechanisms used by TGF-β to mediate its stimulation of EMT in normal and malignant cells.
Keywords: Epithelial-mesenchymal Transition, Metastasis, Signal Transduction, Transforming growth factor-β, Tumor Microenvironment
1. INTRODUCTION
The epithelium is comprised of highly specialized and diverse cells that play critical roles in nearly all biological processes [1, 2]. Indeed, epithelial cells serve as protective barriers that line both the outer (i.e., skin) and inner (i.e., airways, gastrointestinal tract, etc.) body cavities, as well as behave as secretory and glandular tissues. In addition, epithelial cell function varies widely between tissues and ranges from nutrient absorption in the intestines to gaseous exchange in the lungs to lactogenesis in the mammary gland. Equally important is the role of the epithelium in providing the first line of defense against exterior insults and infections, while simultaneously enabling the exchange of vital nutrients needed to maintain tissue homeostasis. The fidelity and function of the epithelium is maintained through its continual renewal and repair, and as such, it perhaps is not surprising to learn that the majority (i.e., ~90%; [3]) of cancers arise in cells derived from epithelial origins. Thus, it is imperative that science and medicine uncover the sequence of events that enable specialized and polarized epithelial cells to dedifferentiate along a tumorigenic pathway that terminates in their acquisition of metastatic phenotypes.
Recent evidence has linked the development of tissue fibrosis and cancer metastasis to the inappropriate reactivation of epithelial-mesenchymal transition (EMT), which is the process whereby immotile, polarized epithelial cells transition into highly motile, apolar fibroblastoid-like cells (Figure 1; [1, 2, 4-6]). Indeed, EMT is a normal physiological process essential for proper embryogenesis and tissue morphogenesis, particularly for the formation of the mesoderm, neural crest, cardiac valve, and secondary palate [1, 2, 7]. With respect to adult tissues, EMT also is engaged in wounded epithelia to facilitate their healing, remodeling, and repair in response to tissue damage. Thus, fully differentiated epithelial cells harbor a dormant embryonic transcriptional EMT program that can be reinitiated in response to a variety of specific environmental cues and signals, one of which is the pleiotropic cytokine, transforming growth factor-β (TGF-β). Interestingly, these same cellular and morphological features are observed in cells undergoing pathophysiological EMT, which underlies the development of several human pathologies, such as chronic inflammation, rheumatoid arthritis, and chronic fibrotic degenerative disorders of the lung, liver, and kidney [1, 2, 4-6, 8, 9]. Along these lines, aberrant reinitiation of EMT also engenders the acquisition of invasive and metastatic phenotypes in developing and progressing carcinomas, leading to their dissemination and colonization of distant organ sites suitable to support their metastatic growth. A commonality of physiological and pathophysiological EMT is their ability to be induced by TGF-β, which now is recognized as a master regulator of this transdifferentiation process.
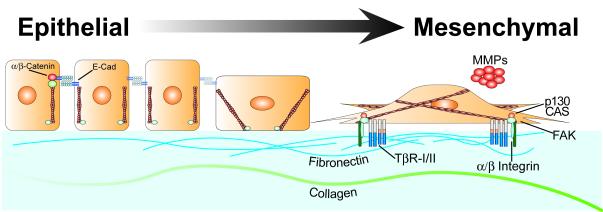
Figure 1. Epithelial Cells Transition to Mesenchymal-like Cells in Response to TGF-β.
This schematic depicts polarized epithelial cells and their cuboidal structure that is maintained via cell-cell junctions comprised of homotypic E-cadherin molecules that are linked to the cortical actin cytoskeleton by α- and β-catenins. TGF-β stimulation of EMT during wound healing or tumor invasive migration results in the delocalization, degradation, and/or downregulation of cell-cell junctions and, consequently, a loss of epithelial integrity. In addition, the morphologic transition of epithelial cells also is supported by the simultaneous formation of actin stress fibers, the upregulation of integrins, and the activation of focal adhesion complexes. Moreover, the increased production and section of ECM proteins, such as fibronectin and collagen, coupled with the elevated expression and activation of MMPs enables transitioned fibroblastoid-like cells to exhibit invasive and motile phenotypes.
TGF-β is a ubiquitously expressed and multifunctional cytokine that not only regulates EMT, but also oversees the development, differentiation, and survival of essentially all cell types and tissues [10-13]. TGF-β also is a powerful suppressor of cell growth and proliferation, particularly in cells of epithelial, endothelial, and hematopoietic origins [10-13]. Quite dichotomously, aberrations in the TGF-β signaling system regularly take place during tumorigenesis and elicit resistance to its anti-proliferative activities, contributing to the formation of human neoplasms. Upon being liberated from the cytostatic activities of TGF-β, cancer cells proliferate, invade, and metastasize beyond their tissue of origin when stimulated by TGF-β. How TGF-β suppresses these processes in normal epithelial cells is unclear, as is how TGF-β promotes these processes in their malignant counterparts. Despite the continued uncertainty of the molecular events associated with the diametric activities of TGF-β, it is absolutely clear that this cytokine stimulates the two deadliest aspects of cancer, namely cell invasion and metastasis. Moreover, recent studies indicate that acquisition of metastatic phenotypes by carcinoma cells is critically dependent upon their ability to undergo EMT [4-6, 8, 14]. Indeed, TGF-β stimulation of EMT was demonstrated originally by Miettinen et al [15] who observed normal mammary epithelial cells (MECs) to acquire fibroblastoid phenotypes in response to TGF-β. In addition, TGF-β3-deficient mice develop cleft palate due to defective palatogenesis associated with aberrant EMT [16]. Similar inactivation of TGF-β2 function impairs endocardial cushion development in chick hearts due to their absence of Slug expression and its ability to activate EMT [17]. Finally, Smad3-deficiency affords protection against EMT-driven retinal [18, 19] and renal [20] fibrosis in mice. Thus, these and other seminal studies have clearly established TGF-β as a master regulator of EMT. This review focuses on the myriad of evidence supporting this designation for TGF-β, particularly the cellular, molecular, and microenvironmental mechanisms that underlie the ability of TGF-β to induce EMT in normal and malignant cells.
2. TGF-β SIGNALING & EMT
The general mechanisms whereby TGF-β activates responsive cells and regulates their behavior is depicted in Figures 2 and 3. As shown, transmembrane signaling by TGF-β commences via its binding to three high-affinity receptors, namely the TGF-β type I (TβR-I), type II (TβR-II), and type III (TβR-III or betaglycan). When and where it is expressed, TβR-III clearly is the most abundant TGF-β receptor on the cell surface where it functions as an accessory receptor that binds and presents TGF-β to its signaling receptors, TβR-I and TβR-II, both of which possess intrinsic Ser/Thr protein kinase activity in their cytoplasmic domains [11, 12, 21-23]. The binding of TGF-β to TβR-II enables the recruitment and activation of TβR-I, leading to its induction of canonical Smad2/3-dependent signaling. Once activated, Smad2/3 form heterocomplexes with Smad4 and translocate into the nucleus where they regulate the cell type-specific expression of TGF-β-responsive genes [11, 12, 21-23]. It is interesting to note that the variety of cell responses exhibited in response to TGF-β are governed primarily by the cell type-specific expression of various Smad2/3-interacting transcription factors (e.g., AP-1 and Forkhead family members, Stats, etc. [11, 22]), as well as their association with additional transcriptional activators or repressors [11, 12, 21-23]. Moreover, the amplitude and duration of Smad2/3 signaling is modulated by several mechanisms, including the expression of (i) adapter and/or anchoring proteins SARA [24], Hgs [25], and Dab2 [26] that enable Smad2/3 phosphorylation by TβR-I, and (ii) the inhibitory Smad, Smad7, which prevents the phosphorylation of Smad2/3 [27-29] and induces the degradation of TGF-β receptors [30, 31]. In addition, the inhibitory functions of Smad7 are regulated by its interaction with STRAP [32], which potentiates the anti-TGF-β activity of Smad7, and by its association either with AMSH2 [33] or Arkadia [34-36], both of which negate the anti-TGF-β activity of Smad7. As alluded to above, the activation of Smad2/3 by TGF-β represents the canonical TGF-β signaling system, which is shown diagrammatically in Figure 3.
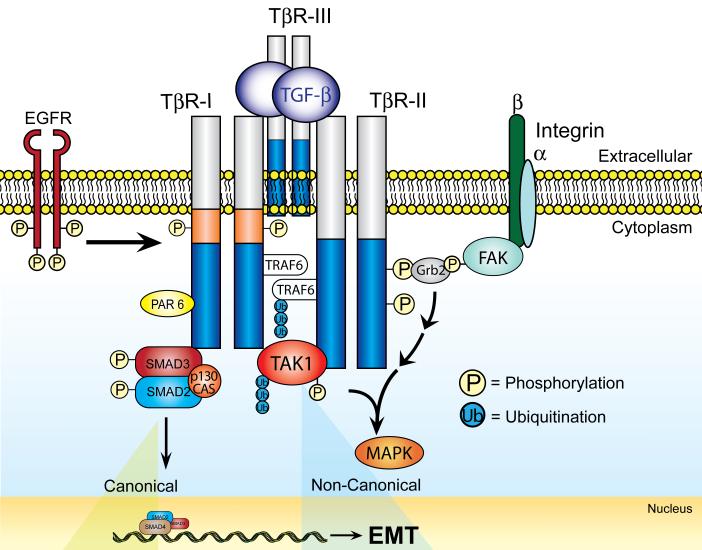
Figure 2. Differential Interactions of TGF-β Receptors with Transmembrane and Membrane Proximal Proteins Complexes Facilitate the Diversity TGF-β Signaling.
β1 and β3 integrins interact physically with TβR-II [43-45]. The association of TβR-II with β3 integrin is mediated by FAK, which facilitates the binding of TβR-II to the SH2-binding protein, Grb2. In addition, TβR-II also interacts physically with EGFR (M.K. Wendt and W.P. Schiemann, unpublished observation), which also is activated indirectly by TGF-β through its increased synthesis and secretion of EGFR ligands. The cytoplasmic tails of both TβR-I and TβR-II interact with TRAF6, which ubiquitinates itself and the MAPKKK, TAK1. Additional interactions include the binding of p130Cas to Smad3, as well as that of PAR6 with TβR-I. Importantly, the differential composition of TGF-β receptor and scaffolding complexes directs the coupling of TGF-β to canonical and noncanonical effector activation, as well as underlies the pathophysiological conversion of TGF-β signaling and EMT in malignant epithelial cells. The biological outcomes of these various protein-protein interactions are discussed in the text.
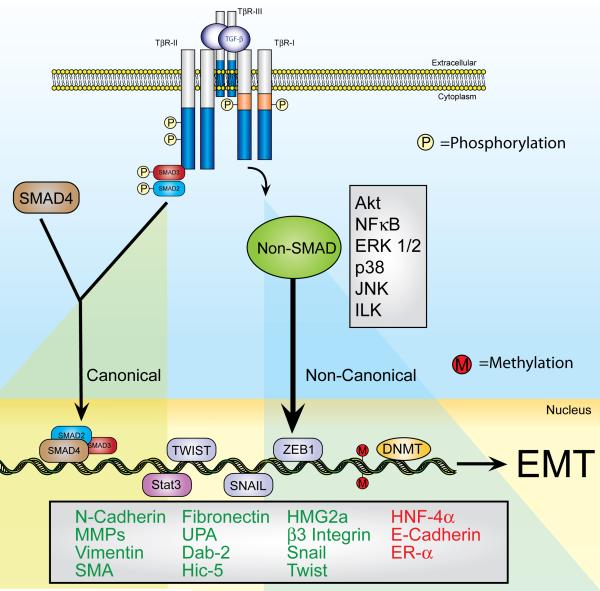
Figure 3. Diverse TGF-β Signaling Pathways Support a Complex Transcriptional Response During EMT.
TGF-β stimulates epithelial cells by binding and activating two transmembrane Ser/Thr protein kinase receptors, namely TGF-β type I (TβR-I) and type II (TβR-II). Activation of these ligand:receptor ternary complexes requires TβR-II to transphosphorylate TβR-I, which phosphorylates and activates Smad2/3. Once activated, Smad2/3 form heterocomplexes with Smad4, which collectively translocate to the nucleus to mediate canonical signaling events by TGF-β (left panel). Noncanonical (right panel) TGF-β signaling takes place through its ability to stimulate various alternate signaling pathways discussed in detail herein. Activation of canonical Smad2/3 signaling results in their nuclear translocation with Smad4 and subsequent regulation of gene expression through their numerous interactions with additional transcriptional activators and repressors. Alternatively, activation of noncanonical TGF-β signaling, such as MAP kinases, small GTPases, PI3K/AKT, and NF-κB, also couples TGF-β to its regulation of gene expression profiles operant in mediating EMT. Finally, activation of the transcription factors belonging to the Snail family (e.g., Snail, Twist, or ZEB1), or of Stat3 elicit EMT-gene expression, which ultimately promotes the prolonged induction of EMT and fibroblastoid-like phenotypes of carcinoma cells via DNA methylation-mediated silencing of E-cadherin expression. Altered coupling of TGF-β to its canonical and noncanonical effector pathways leads to differential gene expression patterns that ultimately contribute to the development of oncogenic signaling by TGF-β. Indeed, the initiation of oncogenic signaling by TGF-β converts its regulation of physiological EMT in normal epithelial cells to one of pathophysiologic EMT in their malignant counterparts.
Also depicted in Figure 3 is the coupling of TGF-β to a variety of noncanonical signaling systems, including (i) the MAP kinases ERK1/ERK2, p38 MAPK, and JNK; (ii) the growth and survival kinases PI3K, AKT/PKB, and mTOR; and (iii) the small GTP-binding proteins Ras, RhoA, Rac1, and Cdc42 [37-45]. In addition, TGF-β typically represses NF-κB activity in normal epithelial cells [46, 47], but readily activates this transcription factor in their malignant counterparts [47-51]. More recently, TGF-β has been shown to activate a number of protein tyrosine kinases (PTKs), including FAK [52, 53], Src [43-45, 54], and Abl [55, 56], which results in the inappropriate amplification of noncanonical TGF-β signaling in mesenchymal or dedifferentiated epithelial cells. Moreover, imbalances in the activation status of canonical and noncanonical TGF-β signaling systems may very well underlie the ability of TGF-β to induce EMT in normal and malignant cells. The importance of canonical and noncanonical TGF-β signaling systems to promote physiological and pathophysiological EMT is presented in greater detail below.
3. DEFINING EMT
The phenomenon of EMT is defined by the morphologic and genetic transition of epithelial cells to fibroblastoid- or mesenchymal-like cells. An inherent characteristic or hallmark of EMT, including that stimulated by TGF-β, is the dramatic phenotypic change in epithelial cell morphology [4-6, 8, 14]. Typically, fully differentiated epithelium manifests as a single layer of polarized epithelial cells comprised of well-defined apical and basolateral surfaces, as well as a clearly demarcated actin cytoskeleton arranged into discrete “cobblestones” that reflect regions of concentrated actin fibers at cell-cell junctions. In response to the initiation of EMT, cell-cell junctions disassemble and filamentous actin undergoes a dramatic redistribution to form prominent stress fibers, which is tracked experimentally via the use of a fluorescently-labeled mushroom toxin, phalloidin. The combined effect of these various cell biological activities is a loss of epithelial cell polarity (Figure 1).
Examining the biochemical and molecular alterations in cell-cell junction formation and dissolution has enabled science and medicine to garner a more complete assessment of the events underlying EMT. Indeed, a number of recent examinations have elucidated a variety of molecular complexes and scaffolds that govern the development of cell-cell junctions, including tight junctions, adherens junctions, and desmosomes [5]. Not surprisingly, a series of coordinated and dynamic processes underlie formation of these macromolecular complexes during the development and maintenance of the epithelium, while changes in the expression and localization of junctional proteins constitute useful measures to track the progression of EMT. For instance, tight junctions are formed by the actions of the transmembrane proteins, claudins, occludins, and JAMs (Junctional Adhesion Molecules), which are linked to the actin cytoskeleton via the scaffold proteins ZO-1, −2, −3 [57, 58]. Moreover, following their formation, tight junctions and their constituents play essential roles in regulating the biology, homeostasis, and architecture of epithelial cells, and in preventing the initiation of EMT and tumorigenesis [59]. In contrast, the initiation of EMT induces a drastic modulation of tight junction localization in epithelial cells [15, 38]. For instance, the function of Par6 (partitioning-defective 6), which governs the formation of tight junctions, the establishment of apical-basolateral polarity, and the initiation of polarized cell migration [60], is compromised by its physical interaction with TβR-I and subsequent phosphorylation by TβR-II in epithelial cells stimulated with TGF-β [61]. Once phosphorylated, Par6 recruits and interacts with E3 ubiquitin ligase, Smurf1, which ubiquitinates the small GTPase, RhoA, leading to its degradation and subsequent dissolution of tight junctions during EMT stimulated by TGF-β [62]. The importance of Par6 to EMT induced by TGF-β is highlighted by the ability of TβR-II-resistant Par6 mutants (i.e., S345A-Par6) to prevent MECs from undergoing EMT in response to TGF-β [61].
Unlike tight junctions, adherens junctions consist of transmembrane E-cadherin (Epithelial-cadherin) proteins that are linked to the actin cytoskeleton by α- and β-catenins [63]. TGF-β stimulation of EMT represses E-cadherin transcription (see below), as well as disrupts its localization at the plasma membrane in part via diminished activation of the small GTPase, Rac1 [62]. The net effect of altered E-cadherin function during EMT is the dissolution of adherens junctions. In addition, the loss of cell-cell contacts parallels the development of prominent actin filaments and the appearance of fibroblastoid-like phenotypes in transitioning epithelial cells, processes requiring the activation of RhoA by TGF-β [64, 65]. The mechanisms underlying TGF-β regulation of adherens junction expression and function are discussed below.
4. EMT, TGF-β, & CELL MICROENVIRONMENTS
Maintaining homeostasis within cell microenvironments is essential to alleviating disease development in humans, particularly cancer. Tumor development has been likened to that of dysfunctional miniature organs that house a mixture of malignant and normal cells, including fibroblasts, endothelial, and immune cells [66]. It also is important to remember that the growth and progression of tumors are not inherent properties of the cancer cells themselves, but instead are dictated in large part by a delicate balance between positive and negative proliferative signals produced by diverse cell types within tumor microenvironments. Indeed, alterations within tumor microenvironments can either suppress or promote cancer progression in a manner that mirrors the acquisition of oncogenic signaling by TGF-β in developing neoplasms. Biologically, TGF-β is a master inhibitor of cell cycle progression; however, this cytokine also functions as a master regulator of ECM production, deposition, and remodeling, all of which are essential processes during EMT. Along these lines, recent evidence has shown that TGF-β stimulation of cancer progression proceeds in part via its reprogramming of cell microenvironments, particularly by its ability to target the behaviors of neighboring endothelial cells (ECs) and fibroblasts. Moreover, ECs and fibroblasts typically respond to TGF-β by synthesizing and secreting numerous cytokines, growth factors, and ECM components capable of driving the progression of tumors from indolent to aggressive states [67, 68]. A vital component of normal and malignant cell microenvironments is the ECM, which functions as (i) a gel-like structural scaffold for cells comprised of polysaccharides and fibrous proteins, including collagen, fibronectin, and elastin; and (ii) a molecular sensor that monitors, detects, and responds rapidly to physiological and pathophysiological changes within cell microenvironments. Indeed, under physiological conditions, the ECM serves as a storage reservoir that sequesters numerous growth factors and cytokines that can be rapidly released in response to ECM perturbations or insults, thereby circumventing the need for de novo protein synthesis to elicit biological behaviors [69]. Thus, the microenvironment of epithelial cells plays a critical role in maintaining their polarization and differentiation, processes that are disrupted temporarily during physiological EMT and its modification of epithelial cell microenvironments. In contrast, chronic disruptions within carcinoma cell microenvironments elicits pathologic EMT and its ability to support cancer cell invasion and metastasis. Table 1 identifies numerous EMT-associated genes whose expression is regulated by TGF-β, and readers desiring more in depth discussions of the activities and functions of these genes in governing EMT and epithelial cell biology are directed to several recent reviews [1, 2, 4-6]. In the following sections, we highlight many of the mechanisms that underlie the ability of TGF-β to induce EMT and its associated alterations within the microenvironments of transdifferentiating cells.
Table 1.
Expression of EMT-associated Genes Targeted by TGF-β
| Gene Name | Expression Change |
Reference |
|---|---|---|
| E-cadherin | Decrease | Miettinen et al [15] |
| β3 integrin | Increase | Galliher et al [43] |
| N-cadherin | Increase | Hazan et al [112] |
| NCAM | Increase | Lehembre et al [85] |
| MMP-2 | Increase | Duivenvoorden et al [72] |
| MMP-3 | Increase | Farina et al [235]; Radisky et al [79] |
| MMP-9 | Increase | Farina et al [235]; Kim et al [73] |
| Vimentin | Increase | Grunert et al [117] |
| α-Smooth Muscle Actin | Increase | Masszi et al [118] |
| Fibronectin | Increase | Ignotz et al [102] |
| Estrogen Receptor-α | Decrease | Dhasarathy et al [202] |
| Urokinase Plasminogen Activator |
Increase | Farina et al [235] |
| Dab2 | Increase | Hocevar et al [26] |
| Hic5 | Increase | Tumbarello et al [156] |
| HMG2A | Increase | Thuault et al [111] |
4.1. Matrix Metalloproteinases
Matrix metalloproteinases (MMPs) comprise a large family of proteases that regulate essential steps of embryogenesis and tissue morphogenesis, and of wound healing and cell growth. MMPs also possess the ability to degrade nearly all ECM and basement membrane components, as well as the ability to promote the development and progression human malignancies [70, 71]. Along these lines, TGF-β enhances the tumorigenicity and invasiveness of breast cancer cells by inducing their expression of MMPs 2 and 9 [72, 73], which is consistent with the general importance of upregulated MMP expression in mediating the acquisition invasive phenotypes in several cancers [74]. Indeed, aberrant MMP expression (e.g., MMP-7 or matrilysin) facilitates the development of mammary fibrosis and desmoplasia, which increase tumor rigidity and the selection, expansion, and dissemination of metastatic cells [75, 76]. Similarly, upregulated MMP-3 expression is sufficient to induce lung and mammary fibrosis [77, 78], and to stimulate EMT in carcinomas [79]. Thus, elucidating the connections between aberrant MMP expression and the development of fibrosis and/or EMT will offer important clues as to how EMT promotes cancer progression. For instance, does pathophysiologic EMT solely mediate the acquisition of invasive phenotypes by developing carcinomas, or does this event simply reflect the transdifferentiation of a subset of carcinoma cells into tumor supporting stroma cells (e.g., myofibroblasts) [80]? Indeed, tumor-associated myofibroblasts upregulate their production and secretion of TGF-β, which may serve in establishing a positive feedback loop that drives the selection and expansion of metastatic carcinoma cells [81-83]. Collectively, these findings point to the need for additional studies to fully address these questions, particularly since the expression and activity of MMPs alters the expression of E-cadherin, Snail, vimentin, and TGF-β in a manner consistent with the induction of EMT [79].
4.2. Neuronal Cell Adhesion Molecule
Neuronal cell adhesion molecule (NCAM) is a member of the immunoglobulin superfamily and has been implicated as a mediator of tumor progression and metastasis [84]. Recently, TGF-β stimulation of EMT was observed to induce NCAM expression in a manner correlated with downregulated expression E-cadherin [85]. Functionally, upregulated expression of NCAM during EMT facilitates the formation of β1 integrin-containing focal adhesion complexes [85]. Interestingly, the extracellular domain of NCAM is cleaved proteolytically by MMP-28 (epilysin), which also induces EMT through its ability activate latent TGF-β complexes from inactive ECM depots [86]. In addition, MMP-28 expression also is upregulated in a EMT-dependent manner in wounded epithelial cells, and in metastatic breast cancer cells [87]. Thus, future studies need to determine the physiological and pathophysiological connections between NCAM, MMP-28, and TGF-β during the initiation of EMT in normal and malignant epithelial cells.
4.3. Urokinase Plasminogen Activator
Urokinase plasminogen activator (uPA) is a serine protease whose elevated expression in human cancer correlates with advanced disease sates and poor clinical outcomes, presumably through its ability to promote cancer cell invasion and metastasis [88, 89]. Accordingly, uPA expression is essential for breast and ovarian cancer metastasis in mice [90, 91], and for hypoxia-induced EMT in breast cancer cells via uPA receptor-mediated activation of AKT and Rac1 [92]. TGF-β is a potent inducer of uPA expression, yet the role of this event in mediating EMT and metastasis stimulated by TGF-β remains to be elucidated fully. Recently, the activation of JNK1/2 was shown to be essential for TGF-β stimulation of uPA expression and EMT [93], which is consistent with the notion that noncanonical TGF-β signaling promotes its oncogenic activities in epithelial cells.
4.4. Plasminogen Activator Inhibitor-1
Plasminogen activator inhibitor-1 (PAI-1) is an antagonist of tissue-type plasminogen activator (tPA) and uPA, as well as a physical interactor of the ECM ligand, vitronectin [94, 95]. tPA and uPA both activate the serine protease activity of plasminogens (or plasmins), resulting in the degradation of blood plasma proteins, such as fibrin and von Willebrand factor, and of ECM proteins, such as fibronectin, thrombospondin, and laminin [95]. Through its ability to inhibit tPA and uPA, PAI-1 prevents the activation of intravascular and cell-associated plasminogen, and as such, impedes the breakdown of blood clots and ECM proteins necessary to enable carcinoma cells to undergo invasion and extravasation reactions during metastasis [95].
TGF-β is principal player involved in stimulating PAI-1 transcription in part via activation of p53, which binds and stabilizes PAI-1 transcripts [96, 97]. Quite dichotomously, overexpression of PAI-1 has been observed to reduce the migration and invasion of breast and ovarian cancers [94, 98]; however, PAI-1 polymorphisms or its aberrantly elevated expression also have been associated with a poor prognosis and the increased risk of metastasis in breast cancer patients [99]. Thus, the precise mechanisms underlying the dynamic relationship between PAI-1, plasminogen, and TGF-β regulatory loops, as well as their impact on cancer cell motility, remain an active and important topic of investigation.
4.5. Collagen
Collagen is an abundant ECM molecule that assembles into tensilely strong fibers that provide mechanical support to tissues. The major types of collagen, types I-IV, are distributed differentially in specific tissues of the body. For instance, collagen IV is a major component of the basal lamina, a specialized component of the basement membrane in the mammary gland. Invading breast cancer cells must degrade collagen IV to migrate into surrounding tissue. Interestingly, Endo180 is a cell surface receptor that promotes the uptake of collagen for its degradation intracellularly. Moreover, Endo180 expression is (i) elevated significantly in highly invasive breast cancer cells; (ii) induced transcriptionally by TGF-β stimulation in breast cancer cells; and (iii) reduced the collagen content and enhanced the growth of mammary tumors produced in mice [100]. In addition, TGF-β also governs collagen function by upregulating the expression of MMP-2 and other collagenases in normal and malignant MECs, leading to their enhanced migration and invasion [72, 73, 101].
4.6. Fibronectin
Fibronectin is a large and critical ECM glycoprotein whose elevated production by cancer cells classically is associated with the acquisition of EMT, and more recently, with the development of the metastatic niche [67]. TGF-β is a potent inducer of fibronectin production and deposition into the ECM [102], where it binds integrins and regulates cell adhesion and motility. The synthesis and secretion of fibronectin into the ECM is primarily mediated by fibroblasts, and by epithelial cells induced to undergo EMT (Table 1; [103]). With respect to the latter, nontumorigenic EpH4 MECs engineered to express oncogenic Ras (i.e., EpRas cells) significantly upregulate their expression of fibronectin and its receptor, α5β1 integrin when stimulated with TGF-β [104]. More importantly, administration of neutralizing α5 integrin antibodies to TGF-β treated EpRas cells inhibited their migration and induced a significant apoptotic response [104]. Thus, the synthesis and deposition of fibronectin, coupled with changes in expression and activation of integrins (see below), clearly represent an important mechanism that enables TGF-β to stimulate invasive migration during EMT.
4.7. Cadherin Switching
A phenotypic hallmark of EMT is its ability to downregulate the expression and function of E-cadherin, which is critical in mediating epithelial cell integrity and cell-cell adhesion [105]. Reduced E-cadherin expression in developing and progressing carcinomas takes places through several mechanisms that function en masse to promote cancer cell invasion [5]. For instance, E-cadherin can be inactivated by genetic mutations, and humans harboring these E-cadherin mutations have significantly increased risk of developing cancer [106]. In addition, epigenetic silencing of the E-cadherin (CDH1) promoter via hypermethylation of its 5′ CpG island also enhances the development of carcinomas [107]. Along these lines, TGF-β stimulation of EMT also represses the synthesis of E-cadherin transcripts in large part via its ability to induce the expression of the Snail/ZEB family of basic helix-loop-helix transcription factors, including that of Snail1, ZEB1, Snail2/Slug, Twist, and ZEB2/SIP1 [105, 108-110]. Although the relative contribution of canonical and noncanonical TGF-β signaling in mediating transcriptional activation of these E-cadherin repressors remains to be determined definitely, recent evidence suggests that these events do take place in a cell type-specific manner in response to TGF-β. For example, activation of Smad2/3 by TGF-β in MECs induces their expression of the nuclear high mobility group A2 (HMGA2), which promotes EMT by stimulating the expression of Snail1, Snail2/Slug, and Twist, and by inhibiting the expression of ID2 (inhibitor of differentiation 2) [111]. In addition, while the functional consequences of diminished E-cadherin expression on the behavior of transitioning epithelial cells is well established, recent studies have determined that these same cells also exhibit upregulated expression of N-cadherin (i.e., Neuronal-cadherin) [65], an event linked to elevated cell motility and poor clinical outcomes in cancer patients [112-114]. At present, the necessity of increased N-cadherin expression in mediating EMT, particularly that stimulated by TGF-β, remains to be clarified. Indeed, we [45] and others [115, 116] recently established murine 4T1 breast cancer cells as a model of advanced stage breast cancer whose increased malignancy is governed by TGF-β. Interestingly, while 4T1 cells undergo EMT and downregulate E-cadherin when stimulated by TGF-β [44, 45], these cells fail to express and/or elevate their expression of N-cadherin during EMT initiated by TGF-β (M.K. Wendt and W.P. Schiemann, unpublished observation). Thus, future studies aimed at determining the exact nature of N-cadherin in promoting the acquisition of EMT and metastatic phenotypes clearly are warranted.
4.8. Vimentin
The intermediate filament protein vimentin is expressed in all primitive cell types, but not in their differentiated counterparts. In light of its role as a master regulator of EMT, it perhaps is not surprising to learn that TGF-β stimulation of EMT reactivates vimentin expression in dedifferentiating epithelial cells, an event that serves as a canonical marker for detecting fully transitioned epithelial cells and their acquisition of fibroblastoid-like phenotype [117].
4.9. α-Smooth Muscle Actin
A major component of contractile microfilaments is α-smooth muscle actin (α-SMA), which also serves as a canonical marker for detecting fibroblasts/mesenchymal cells, particularly myofibroblasts. Indeed, during its induction of EMT, TGF-β stimulates α-SMA expression in transitioning epithelial cells [118], an event associated with increased tumor invasion and decreased patient survival rates [119].
5. TRANSMEMBERANE & MEMBRANE PROXIMAL PROTEIN COMPLEXES THAT IMPACT TGF-β SIGNALING & EMT
Recent evidence suggests that cell surface signaling receptors, such as receptor tyrosine kinases (RTKs) and G-protein-coupled receptors, do not function in isolation and instead require accessory signaling inputs that arise from interacting receptors and signaling modules. As shown in Figure 3, the function and behavior of TGF-β receptors also are modulated via their association with an ever expanding array of receptor-interacting molecules and scaffolding proteins. Included in this growing list of TGF-β receptor regulators are members of the integrin superfamily of heterodimeric transmembrane adhesion receptors, which function as direct physical conduits that link the ECM to the cytoskeleton of the cell [120, 121]. Integrin signaling commences upon their clustering and subsequent stimulation of the Ser/Thr protein kinase ILK (integrin-linked kinase), as well as members of the Src family of PTKs and FAK (focal adhesion kinase), leading to the activation of a vast array of downstream effectors, including members of the MAP kinase family of protein kinases, members of the Ras/Rho family of small GTPases, and members of the PI3K and AKT signaling axes [120-124]. Integrins also regulate cell behavior through their ability to form complexes with RTKs [125, 126]. For instance, β1 integrins form FAK-dependent complexes with the receptors for EGF, PDGF, and HGF [126, 127], and in doing so, enables growth factor-mediated induction of cell migration and invasion [126]. Interestingly, the scaffolding function of FAK is independent of its PTK activity, but does require its N-terminal FERM (FAK Ezrin Radixin Moesin) domain and C-terminal FAT (Focal Adhesion Targeting) domain to bind RTKs and β1 integrins, respectively [126]. Lastly, the establishment of EMT phenotypes in cultured cells, as well as the development of late stage cancers and their acquisition of invasive and metastatic phenotypes both have been linked to dramatic changes in the expression and localization of integrins in epithelial cells [104, 128].
In addition to its regulation of cell cycle progression, TGF-β also figures prominently in mediating ECM remodeling and repair via its ability to regulate integrin expression [129-131]. Moreover, αvβ6 and αvβ8 integrin ligation promotes the activation of TGF-β1 and TGF-β3 from inactive ECM depots [132-137], which regulates alveolar development, wound closure, fibrosis, and EMT [130, 132, 138-140]. In addition, epidermal transgenic expression of α6β4 integrin also elicits elevated development of metastatic papillomas and carcinomas in a chemical carcinogenesis model of skin cancer. Importantly, the tumorigenicity associated with α6β4 integrin expression was linked to its ability to uncouple TGF-β from activating Smad2/3 and preventing cell cycle progression [141]. Similar reciprocity between integrins and TGF-β is observed in cancers of the prostate, whose metastasis to bone is stimulated by TGF-β and its induction of α2β1 integrin, which binds to bone-derived type I collagen [142]. Thus, the ability of TGF-β to stimulate cancer progression and metastasis requires an intricate interplay between signals arising from TGF-β receptors and those initiated by integrins. Accordingly, integrins have been found to associate with TGF-β receptors and play a critical function in coupling TGF-β to activation of its noncanonical effectors, and to its induction of EMT. For instance, neutralizing β1 integrin antibodies abrogated the ability of TGF-β to activate p38 MAPK and induce EMT in MECs [39]. Similarly, hepatocellular carcinoma cells elevate their expression of α3β1 integrin in response to TGF-β, an event that enhances their motility and invasiveness [143]. Moreover, administering laminin-5 together with TGF-β stimulated hepatocellular carcinoma cells to undergo EMT in an α3 integrin-dependent manner [144], further demonstrating the necessity of integrins to cooperate with TGF-β to induce EMT and invasion in transitioning cells.
We also described the functional cooperation between integrins and TGF-β in promoting EMT, as well as in stimulating the development and progression of mammary tumors. For instance, we found the expression and activity of αvβ3 integrin and its downstream effector Src to be essential for TGF-β stimulation of MEC proliferation, invasion, and EMT [43-45]. In addition, transgenic expression of αvβ3 integrin not only negated the cytostatic response of normal MECs to TGF-β, but also enhanced its stimulation of MEC invasion and p38 MAPK activation. Importantly, inactivation of either αvβ3 integrin or Src function abolished the ability of TGF-β to stimulate EMT and invasion in normal and malignant MECs [43, 44]. Mechanistically, β3 integrin interacts physically with TβR-II, leading to its (i) phosphorylation on Y284 by Src; (ii) interaction with Grb2 and Shc at phosphorylated Y284; (iii) activation of p38 MAPK; and (iv) stimulation of EMT and invasive migration in normal and malignant MECs [44]. Along these lines, the growth and metastasis of breast cancer cells in mice absolutely required TβR-II to be phosphorylated on Y284, a phosphotransferase reaction that disrupts the delicate balance between canonical and noncanonical TGF-β signaling inputs activated during mammary tumorigenesis [45]. In addition to its ability to promote pulmonary metastasis stimulated by TGF-β [45], αvβ3 integrin expression also directs breast cancer cell metastasis to bone [145, 146] and lung [146] in part through a TGF-β-dependent pathway. Collectively, these findings suggest that pharmacological targeting of noncanonical TGF-β effectors, particularly αvβ3 integrin, Src, and p38 MAPK, may prove efficacious in treating metastatic breast cancers.
Besides integrins, a growing number of intracellular proteins also have been shown to interact with and regulate the activity of TGF-β receptors. For instance, two members of the focal adhesion complex, namely FAK and its downstream effector p130Cas (p130Crk-associated substrate), both influence the cellular response to TGF-β through dramatically different mechanisms. Indeed, TGF-β stimulates FAK and its relative PYK2 during EMT [147], leading to the activation of JNK and the subsequent upregulation α-SMA in fibroblasts [148]. In addition, FAK activation in hepatocytes is necessary for the transcription of mesenchymal and invasive gene expression profiles, as well as for the delocalization of E-cadherin from the plasma membrane [149]. Finally, we recently established FAK as a molecular scaffold that facilitates the formation of oncogenic β3 integrin:TβR-II complexes and their activation of Src and interaction with Grb2 (M.K. Wendt and W.P. Schiemann, unpublished observation). Moreover, the ability of FAK to form these signaling complexes is essential for TGF-β stimulation of p38 MAPK in breast cancer cells, as well as for their induction of EMT and metastasis stimulated by TGF-β (M.K. Wendt and W.P. Schiemann, unpublished observation; [45]). Thus, the aberrant recruitment of FAK to TGF-β receptors readily influences the oncogenic conversion of TGF-β from a tumor suppressor to a tumor promoter, including its stimulation of pathophysiological EMT in carcinoma cells.
In stark contrast to FAK, the incorporation of p130Cas into active TGF-β receptor complexes alters the coupling of TGF-β to the canonical Smad2/3 pathway. Indeed, the activation and phosphorylation of p130Cas following cellular adhesion to ECM matrices led to its association and inactivation of Smad3, and to diminished cytostatic activity of TGF-β [150]. Similarly, we find that rendering malignant, metastatic MECs deficient in p130Cas enhances Smad2/3 activation by TGF-β, but fails to alter its coupling to p38 MAPK; however, this same cellular condition selectively inhibited breast cancer metastasis only in cells that possessed heightened TGF-β signaling (M.K. Wendt and W.P. Schiemann, unpublished observation), suggesting that p130Cas acts as a molecular integrator of canonical Smad2/3 signaling when confronted with elevated oncogenic behavior mediated by the receptors for TGF-β or EGF [151].
Recently, the regulation of TGF-β signaling has been shown to be modulated by two additional adapter proteins that localize to focal adhesions, namely Hic5 and Disabled-2 (Dab2). Indeed, Hic5 is a member of the paxillin superfamily and, like paxillin, functions as an adapter protein at focal adhesions [152], as well as resides in the nucleus where functions as a transcriptional coactivator in regulating gene expression induced by the androgen [153] and glucocorticoid [154, 155]. Moreover, Hic5 expression is low in quiescent MECs, but is induced rapidly via a RhoA/ROCK-dependent pathway following administration of TGF-β [152]. In addition, uncoupling Hic5 from TGF-β regulation prevents its induction of EMT in normal MECs [156]. Thus, Hic5 plays an essential role in coupling TGF-β receptors to activation of RhoA/ROCK and, consequently, to the induction of EMT. Along these lines, Dab2 was identified originally as an ovarian tumor suppressor gene [157, 158] that regulates the actin cytoskeletal architecture during cell migration and adhesion [159]. More recently, Prunier et al [160] established Dab2 as a novel gene target of TGF-β in MECs undergoing EMT in part via its ability to (i) associate with TGF-β receptor complexes [26]; (ii) promote Smad2/3 activation by TGF-β receptors [26]; and (iii) stimulate the activation of TAK1 and JNK, which induced fibronectin expression and enhanced cell motility [161]. Along these lines, TGF-β stimulates Dab2 expression in MECs undergoing EMT, which promotes the formation of Dab2:β1 integrin complexes and their activation of FAK [160]. Importantly, measures capable of disrupting Dab2 function prevents EMT stimulated by TGF-β, as well as promotes its ability to induce apoptosis in MECs. Although the molecular mechanisms underlying the ability of TGF-β to stimulate Dab2 expression remains to be defined, these studies do provide interesting insights into the connections that govern alterations in cell survival and morphology regulated by TGF-β.
Finally, two laboratories recently identified a novel collaboration between signaling molecules activated by TNF-α and those activated by TGF-β. Indeed, both studies demonstrated the ability of TGF-β to induce the physical association of its receptors with that of TRAF6 (TNF receptor-associated factor 6) [162, 163], leading to K63-linked polyubiquitination and activation of TAK1 and its subsequent stimulation of p38 MAPK and JNK. Moreover, whereas TRAF6-deficiency had no effect on the coupling of TGF-β to Smad2/3, this same cellular condition uncoupled TGF-β from activation of MAP kinases and prevented this cytokine from inducing EMT in normal MECs [163]. Taken together, these studies reinforce the notion that imbalances in the TGF-β signaling system that favor its activation of noncanonical effectors over that of its canonical Smads are crucial to its induction of EMT in normal and malignant epithelial cells. These findings also point to the need for additional studies to define precisely how these aberrant protein complexes and modules impact the epithelial cell response to TGF-β, and how science and medicine can better target these effector molecules that promote oncogenic signaling and EMT initiation by TGF-β.
6. SIGNALING SYSTEMS INVOLVED IN EMT STIMULATED BY TGF-β
Transmembrane signaling by TGF-β traditionally is associated with its activation of Smad2/3 and their ability to alter the transcription of TGF-β-responsive genes, which clearly play an important role in mediating the ability of TGF-β to induce EMT, tumor formation, and cancer cell metastasis [164]. The necessity of Smads 2 and 3 for TGF-β stimulation of EMT has been reviewed extensively in the scientific literature, and readers desiring a more in depth description of Smad2/3 function in regulating EMT in normal and malignant cells are directed to several recent reviews [4, 5, 11]. As alluded to above, the enhanced coupling of TGF-β to its noncanonical effectors figures prominently in mediating its biological and pathological behaviors, particularly its ability to induce EMT and cancer cell metastasis. Table 2 lists a variety of noncanonical effectors targeted by TGF-β during its activation of EMT, while the role of these signaling molecules during epithelial cell EMT induced by TGF-β is discussed below.
Table 2.
Signaling Pathways Activated During EMT Stimulated by TGF-β
| Pathway | Reference |
|---|---|
| Smad2/3 | Piek et al [236] |
| Rho family of small GTPases |
Bhowmick et al [65] |
| PI3K and AKT | Bakin et al [38] |
| NF-κB | Huber et al [179] |
| ERK1/2 | Xie et al [183] |
| p38 MAPK | Bhowmick et al [39]; Galliher and Schiemann [43-45] |
| JNK | Hocevar et al [185] |
| Integrin-linked kinase | Lee et al [177]; Lin et al [91] |
6.1. Rho Family of Small GTPases
The Rho family of small GTPases is comprised of RhoA, Rac1, and Cdc42, which regulate the formation of stress fibers, lamellipodia, or filopodia, respectively [165, 166]. Indeed, Rac1 is an established inducer of cell-cell adhesions in epithelial cells [167], which contrasts sharply with the ability of RhoA to dissolve these adhesive complexes to facilitate times of cell migration [62]. Given the importance of these small GTPases in overseeing cell adhesion, morphology, and migration, it is fitting to find that these effectors are intimately involved in EMT stimulated by TGF-β. For instance, the activation of RhoA by TGF-β enables MECs to undergo EMT, while measures capable of inhibiting RhoA function or that of its downstream effector, p160ROCK, uncouple TGF-β from EMT in MECs [65]. Moreover, RhoA activation also is essential for TGF-β stimulation of α-SMA expression in renal epithelial cells undergoing EMT [118]; however, completion of this same cellular event in lens epithelial cells requires signaling inputs from both RhoA/ROCK and Smad2/3 [168]. Taken together, these studies point to the overall importance of noncanonical TGF-β signaling, particularly that induced by RhoA/ROCK, to induce EMT in epithelial cells.
6.2. PI3K/AKT
The tumor suppressing activity of TGF-β not only reflects its ability to induce cytostasis, but also its propensity to activate apoptosis in epithelial cells [10, 11, 13, 169]. Interestingly, the ability of TGF-β to stimulate apoptosis frequently is subverted during tumorigenesis, leading to enhanced cancer cell survival via activation of the PI3K and AKT signaling systems by TGF-β. Indeed, administration of PI3K inhibitors to MECs inhibits their activation of AKT and ability to undergo EMT in response to TGF-β [38]. The activation of AKT by TGF-β can transpire directly via TGF-β receptors or indirectly via the transactivation of EGF [170] and PDGF [171] receptors, which induces the expression of genes operant in mediating cancer cell EMT, metastasis, and survival. In addition to altering gene expression profiles, AKT also regulates mRNA translation when impacting the response of epithelial cells to TGF-β. For instance, TGF-β stimulation of EMT in MECs is accompanied by an increase in cell size and protein content, both of which correlate with the rapid activation of mTOR (mammalian target of rapamycin) in transitioning MECs [40]. Somewhat unexpectedly, administering the mTOR inhibitor, rapamycin, to MECs failed to affect their acquisition of an EMT morphology in response to TGF-β; however, this same cellular condition completely prevented the ability of TGF-β to increase MEC size and protein production, as well as inhibited their migration and invasion [40]. Taken together, these findings highlight an important bifurcation in the TGF-β signaling system that dissociates the ability of TGF-β to alter cell morphology from its ability to elevate cell motility. Future studies need to identify the transcriptional and translational objectives targeted by TGF-β, as well as determine their relative contribution to oncogenic signaling stimulated by TGF-β in normal and malignant cells.
6.3. Integrin-linked Kinase (ILK)
In addition to their stimulation of PTKs, the ECM engagement of β1 and β3 integrins also activates the Ser/Thr protein kinase ILK and its ability to mediate the (i) stimulation of MAP kinases, PI3K/AKT, and small GTPases; and (ii) the inhibition of GSK3β [172-174]. Accordingly, targeting ILK expression to mouse mammary glands elicited a hyperplastic reaction that progressed to full-blown breast cancer in part via constitutive activation of ERK1/2 and AKT, which inactivated GSK3β [175]. Elevated ILK expression is associated with the acquisition of EMT phenotypes by MECs, including reductions in their expression of E-cadherin and adhesion, as well as increases in their formation of actin stress fibers and invasion [176]. ILK also participates in EMT stimulated by TGF-β by coupling this cytokine to its activation of AKT [177], and to its elevated expression of MMP-2 and uPA [91]. Collectively, these findings suggest that ILK may function analogously to FAK in mediating oncogenic signaling by TGF-β, and as such, suggest that ILK interfaces integrin signaling with that stimulated by TGF-β in epithelial cells undergoing EMT.
6.4. NF-κB
NF-κB is a principal player involved in regulating the production of proinflammatory cytokines [178], and in stimulating tumor growth, vascularization, survival, and invasion [178]. In addition, NF-κB activity was observed to be essential in mediating the ability of Ras-transformed breast cancer cells to undergo EMT and colonize the lung when stimulated by TGF-β [48, 179]. Along these lines, NF-κB activity also associates with several hallmarks of EMT, including downregulated E-cadherin expression and upregulated expression of vimentin [180]. It is interesting to note that TGF-β typically represses NF-κB activity in normal epithelial cells [47, 181, 182], but readily induces the activation of this transcription factor in their malignant counterparts [47, 182]. Recently, we demonstrated that the activation of NF-κB by TGF-β transpires via the aberrant formation of a TAB1:xIAP:TAK1:IKKβ signaling module that only materializes in malignant MECs, or in normal MECs following their induction of EMT by TGF-β [47]. Functionally, the formation of TAB1:xIAP:TAK1:IKKβ complexes is essential for TGF-β stimulation of (i) Cox-2 expression and its induction of EMT and invasion in normal and malignant MECs [47, 182]; and (ii) mammary tumor growth in immunocompetent and immunocompromised mice [47], suggesting a potentially important role of NF-κB in regulating innate immunity by TGF-β. Collectively, these findings demonstrate the role of NF-κB in supporting the development of oncogenic signaling by TGF-β in normal and malignant cells, particularly its ability to drive the growth, metastasis, and EMT of tumors in response to TGF-β.
6.5. MAP Kinases
Members of the MAP kinase family of protein kinases, which includes ERK1/2, JNKs, and p38 MAPKs, all have been implicated in mediating EMT and metastasis stimulated by TGF-β [39, 183, 184]. For instance, pharmacological inhibition of ERK1/2 in MECs uncouples TGF-β from inducing EMT and its associated formation of stress fibers and delocalization of ZO-1 and E-cadherin [183]. Similarly, inactivation of JNK also prevents the ability of TGF-β to stimulate the morphological and transcriptional changes that drive EMT in epithelial cells [93, 161]. Indeed, the activation of JNK by TGF-β induces fibronectin expression during EMT, and during fibroproliferative disorders that may progress to carcinoma [185]. Along these lines, collagen I and other ECM proteins can promote EMT via their activation of PI3K, Rac1, and JNK [186]; however, while it remains to be determined whether TGF-β is intimately involved in this ECM-dependent induction of EMT, it seems likely that the ability of TGF-β to stimulate the synthesis and secretion of ECM components is reciprocated by the ability of the ECM to establish paracrine and autocrine TGF-β signaling loops that perpetuate EMT in normal and malignant epithelial cells.
Besides its ability to activate ERK1/2 and JNK, TGF-β also stimulates p38 MAPK during its induction of EMT in normal and malignant cells [39]. Interestingly, the activation of p38 MAPK by TGF-β requires the expression and activity of either β1 or β3 integrins [39, 43, 44]. Indeed, we established the necessity of β3 integrin to form oncogenic signaling complexes with TβR-II, resulting in its phosphorylation on Y284 by Src [43, 44]. Once phosphorylated, Y284 functions as a SH2-binding site that coordinates the recruitment of either ShcA or Grb2, as well as their subsequent activation of p38 MAPK [44]. Most importantly, pharmacologic or genetic inactivation of this oncogenic signaling axis prevented TGF-β from stimulating the growth and pulmonary metastasis of breast cancers produced in mice [45]. Finally, the activation of p38 MAPK not only induces EMT, but it also stimulates the expression of prometastatic genes, particularly TβR-II and MMPs 2 and 9 [187, 188], which collectively points to the importance of inappropriate p38 MAPK activation in mediating the conversion of TGF-β from a tumor suppressor to a tumor promoter.
7. MECHANISMS OF GENE REGULATION BY TGF-β
The importance of aberrant genetic and epigenetic events in promoting tumorigenesis is highlighted by the consistent and repeated finding that cancer cells that have lost their ability to regulate various rate-limiting steps that normally suppress malignant development. These untoward events typically are associated with the (i) mutational activation of oncogenes, (ii) mutational inactivation of tumor suppressor genes, or (iii) amplified or silenced expression of genes coupled to the development of cancer hallmarks [189]. Although many of the signaling systems and genes targeted by TGF-β during its activation of EMT have been discussed above, the succeeding sections focus on the transcriptional mechanisms that orchestrate its transitioning of epithelial cells into their mesenchymal counterparts (Figure 3).
7.1. Nuclear Factors
The Snail family of transcription factors are master regulators of EMT and include (i) SNAI1 (Snail) and SNAI2 (Slug); (ii) two ZEB factors, ZEB1 and ZEB2 (SIP1); and (iii) FOXC2 [110, 190]. Indeed, the binding of Snail to conserved E-box sequences present in E-cadherin promoter is classically associated with EMT and the repression of E-cadherin expression, as well as that of the aforementioned cell polarity genes, occludin and claudin [191]. The essential function of various Snail family members in mediating EMT and cancer metastasis have been extensively reviewed, and as such, readers desiring a more in-depth description of their functions and behaviors in governing EMT are directed several recent reviews [109, 190]. Besides Snail family members, emerging evidence also implicates dysregulated Myc expression in promoting the ability of epithelial cells to undergo EMT in response to TGF-β. Indeed, the tumor suppressing activity of TGF-β is intimately linked to its ability to rapidly repress Myc expression in epithelial cells [11, 13]. Accordingly, uncoupling TGF-β from regulation of Myc expression is a common occurrence in developing carcinomas, resulting in their insensitivity to cytostasis mediated by TGF-β [192, 193]. Somewhat unexpectedly, Myc recently was observed to function cooperatively with Smad4 to induce Snail expression during TGF-β stimulation of EMT in MECs [194]. Taken together, these findings suggest the Myc functions as a molecular detector that enables epithelial cells to sense TGF-β as a mediator of cytostasis or EMT.
7.2. STAT3
Signal transducer and activator of transcription 3 (STAT3) is a critical component of cell survival and proliferative responses, and its inappropriate activation can endow this transcription factor with oncogene-like properties in developing and progressing neoplasms [195]. A recent study has suggested that TGF-β couples to STAT3 phosphorylation and activation via a PKA-dependent mechanism [196]. Moreover, STAT3 activation by TGF-β is necessary for its ability to induce apoptosis and EMT [196], and to stimulate the invasion and metastasis of Smad4-deficient pancreatic cancer cells [197]. In addition, carcinoma cells that overexpressed EGFR readily acquired EMT phenotypes when stimulated with EGF, a cellular reaction that required EGF/EGFR to activate STAT3 and its subsequent upregulation of TWIST [198]. Thus, while several studies have shown EGF to cooperate with TGF-β in mediating tumorigenesis, the extent to which this tumor-and EMT-promoting alliance requires STAT3 remains to be determined definitively.
7.3. Estrogen Receptor-α
Aberrant repression of the nuclear hormone receptor, estrogen receptor-α ER-α) has long been recognized as a major event that promotes the development and progression of mammary tumors, as well as significantly worsens the clinical prognosis of patients with metastatic breast cancer [199, 200]. In addition to its prominent role in regulating mammary gland development and homeostasis, ER-α also prevents the ability of malignant MECs to acquire EMT and metastatic phenotypes, doing so via its stimulation of MTA3 (metastasis tumor antigen 3) expression, which in turn represses the expression of Snail [201]. Thus, inactivation or loss of ER-α in MECs promotes their EMT and invasion by allowing for their expression of Snail. Somewhat surprisingly, constitutive Snail expression in breast cancer cells was observed to inhibit ER-α expression [202], leading to enhanced invasion of these ER-α-deficient breast cancer cells. It is interesting to note that physiological actions of estrogen in mammary tissues typically oppose those of TGF-β. Accordingly, inactivation of ER-α signaling led to elevated expression of components of the TGF-β signaling system and, presumably, to enhanced EMT in breast cancer cells [202]. Thus, Snail appears to function as a novel molecular sensor that integrates the opposing cellular functions of ER-α and TGF-β, particularly their ability to inhibit and stimulate EMT, respectively.
7.4. TGF-β, microRNAs, & EMT
A number of recent studies have established microRNAs as important players that participate in cell and tissue development, as well as control cell proliferation and motility through their ability to repress mRNA translation, or to induce mRNA degradation [203-206]. These studies also have shown that a single microRNA can repress the translation of multiple transcripts, and as such, dysregulated expression of a single microRNA, either positively or negatively, could initiate a cascade of gene silencing events capable of eliciting disease development in humans, including cancer. Accordingly, aberrant regulation of several microRNAs (or miRs) is observed in human cancers (see [207]), especially in those of the breast, which can in fact be subtyped based on their differential expression of various microRNAs [205, 208]. Along these lines, microRNAs also play a prominent role in regulating the expression of EMT-related genes. For instance, members of the miR-200 family suppress EMT by downregulating the expression of ZEB1 and ZEB2 (SIP1), which as mentioned above function in repressing the expression of E-cadherin [209-211]. Indeed, miR-200 family member expression marks epithelial cells that express E-cadherin and not vimentin, as well as identifies cancer cells that are poorly motile [212]. With respect to EMT and its regulation by TGF-β, a recent study established that this cytokine downregulates the expression of microRNA-200 family members and miR-205, which promotes ZEB1 and ZEB2 expression and their initiation of EMT [211]. In addition, these same microRNAs are frequently downregulated in invasive human breast cancer cells that exhibit a mesenchymal-like morphology [211]. Somewhat surprisingly, elevated ZEB1 expression also was found to repress that of miR-41 and miR-200c, both of which belong to the miR-200 family and whose absence establishes a negative feedback loop that stabilizes the acquisition of EMT phenotypes in epithelial cells [213].
In contrast to the miR-200 family of microRNAs, metastatic breast cancers were found to preferentially upregulate their expression of miR-10b, which promotes the invasion and metastasis of malignant MECs both in vitro and in vivo [214]. Mechanistically, Twist was observed to induce miR-10b expression that results in the (i) diminished translation of HoxD10 transcripts, and (ii) induction of the prometastatic gene, RhoC [214]. More recently, administration of TGF-β to normal MECs induced miR-155 expression via a Smad4-dependent mechanism, an event that elicited EMT in cytokine-stimulated MECs [215]. Once expressed, miR-155 abrogated MEC expression of RhoA and prevented their ability to undergo EMT in response to TGF-β [215]. Similar overexpression of miR-21 also is observed in human cancers and results in the repression of the tumor suppressor, tropomyosin-1 [216, 217]. The net effect of these events is the enhanced ability of breast cancer cells to grow in an anchorage-independent fashion [218], and to resist apoptotic stimuli in part via upregulated expression of the survival factor, Bcl-2 [216-218]. As above, the ability of TGF-β to induce EMT has been linked to its induction of miR-21 [219], which enhances cancer cell motility and invasive migration by downregulating tropomyosin expression [220-222].
Taken together, these findings suggest that the ability of TGF-β to govern microRNA expression plays an important role in dictating whether this cytokine propagates tumor suppressing or promoting signals to responsive cells; they also suggest that the development of chemotherapeutic agents capable of targeting microRNAs may function in “normalizing” carcinoma cells and, consequently, rendering them insensitive to the oncogenic activities of TGF-β.
7.5. DNA Hypermethylation
DNA hypermethylation is well established in its ability to aberrantly silence the expression of tumor suppressor genes in developing and progressing carcinomas [107]. Importantly, epigenetic silencing of the E-cadherin promoter via hypermethylation leads to morphological and differential gene expression profiles indicative of EMT phenotypes [107, 223]. Besides silencing of the E-cadherin promoter, EMT and mammary tumorigenesis usurp the inactivation of p16INK4a as a means to promote expanded DNA hypermethylation. Indeed, Roberts et al [224] observed the loss of p16INH4a expression to depress that of the polycomb genes, EZH2 and SUZ12, which collectively enhance DNA hypermethylation and the generation of MECs locked into a perpetual plastic state. Interestingly, the repression of E-cadherin expression during EMT appears to function as a prerequisite for directed gene hypermethylation during the development and progression of mammary tumorigenesis [225]. Moreover, hypermethylation of the E-cadherin promoter served to mark stable EMT in Ras-transformed MECs that was induced by serum versus a transient EMT induced in these same MECs by TGF-β [225]. Clearly, additional investigations are warranted to further our understanding of the linkages between TGF-β and DNA hypermethylation in mediating EMT in normal and malignant cells. Indeed, upregulated ZEB1 expression and its ability to induce EMT is tightly correlated with the loss of E-cadherin expression in cultured epithelial cells, and in metastatic carcinoma cells in vivo [226]. Based on these findings, it is tempting to speculate that initiation of EMT results in (i) the expression of Snail family members that collectively function in repressing that of E-cadherin, and (ii) the subsequent recruitment of DNA methyltransferases that potentiate and stabilize the EMT phenotype.
8. FUTURE PERSPECTIVE
Embryogenesis and its associated EMT creates progenitor cells that ultimately give rise to every cell- and tissue-type within mature organisms. For instance, EMT underlying gastrulation results in the generation of the mesoderm, which subsequently develops along distinct differentiation pathways that elicit the production of muscle, bone, and connective tissues [7]. Similarly, a single mammary stem cell can give rise to both the outer myoepithelial and inner luminal layers that comprise the branched structure of these glands[227-229]. These and other studies suggest an important link between physiologic EMT and the generation and maintenance of stem cells, of which both phenomena require signaling inputs elicited by the TGF-β signaling system [230]. Given the parallels between physiologic and pathophysiologic EMT, it is fitting to find that the inappropriate reactivation of EMT in malignant tissues also promotes the selection and expansion of cancer stem cells. For instance, aggressive and poorly differentiated breast cancer and glioma cells exhibit gene signatures characteristic of stem cells [231]. In addition, TGF-β stimulation of EMT in human and mouse MECs established a population of transitioning cells that possessed stem cell-like properties [232, 233], suggesting that EMT induced by TGF-β promotes “stemness.” Along these lines, inactivation of TGF-β signaling in cancer stem cells induced a mesenchymal-epithelial transition that reestablished a more epithelial-like morphology in aggressive cancer cells [234]. Thus, these intriguing findings suggest that the ability of TGF-β to stimulate the selection and expansion of stem cell-like progenitors in post-EMT epithelial cells may represent the molecular crux that endows TGF-β with oncogenic activity. Clinically, these findings also suggest that the development of chemoresistance may reflect the induction of EMT and its expansion of cancer stem cells by TGF-β. If correct, then the studies reviewed herein offer important insights into how science and medicine may one day target the TGF-β signaling system and its coupling to EMT in order to (i) regulate the behaviors and activities of normal and cancer stem cells, and (ii) alleviate the devastating effects of TGF-β in promoting the acquisition of invasive and metastatic phenotypes in human cancers.
Executive Summary
Defining EMT
EMT is defined by the morphologic and genetic transition of epithelial cells to fibroblastoid- or mesenchymal-like cells.
The major cell-cell junctions include tight junctions, adherens junctions, and desmosomes.
Tight junctions are composed of claudins, occludins, and JAMs, which are linked to the actin cytoskeleton via ZO-1, −2, and −3.
During EMT, Par6 recruits the E3 ubiquitin ligase, Smurf1, which ubiquitinates RhoA, leading to its degradation and subsequent dissolution of tight junctions.
Adherens junctions consist E-cadherin that is linked to the actin cytoskeleton by α- and β-catenins.
EMT represses E-cadherin transcription and disrupts its localization at the plasma membrane.
EMT, TGF-β, and Cell Microenvironments
Tumors house a mixture of malignant and normal cells, including fibroblasts, endothelial, and infiltrating immune cells, which collectively comprise the tumor microenvironment.
Alterations within tumor microenvironments can either suppress or promote cancer progression in a manner that mirrors the acquisition of oncogenic signaling by TGF-β.
Transient disruption of the ECM and epithelial cell microenvironments are characteristic of physiological EMT. In contrast, chronic disruptions within carcinoma cell microenvironments elicits pathologic EMT and its ability to support cancer cell invasion and metastasis.
MMPs comprise a large family of proteases that regulate essential steps of embryogenesis and tissue morphogenesis, wound healing, and cell growth. MMPs also degrade nearly all ECM and basement membrane components, leading to the development and progression human malignancies.
NCAM is a member of the immunoglobulin superfamily whose expression is increased during EMT.
The extracellular portion of NCAM is cleaved by MMP-28.
uPA is a serine protease whose expression is elevated during EMT and associates with advanced disease states and poor clinical outcomes.
PAI-1 antagonizes tPA and uPA; its expression also is increased during EMT and associates with advanced disease states and poor clinical outcomes.
EMT leads to the upregulation of collagen and fibronectin, whose expression drastically alters cell microenvironments.
Vimentin is an intermediate filament protein, while α-smooth muscle actin is a component of contractile microfilaments. Upregulated expression of both proteins are considered to be markers of fully transitioned cells.
Transmembrane and Membrane Proximal Protein Complexes the Impact TGF-β Signaling and EMT
α and β integrin heterodimers function in linking the ECM to intracellular signaling pathways, and to the cellular cytoskeletal system.
Integrins interact with several intracellular kinases, as well as several transmembrane RTKs.
Integrin β1 and β3 interact with TβR-II and profoundly affect downstream signaling events stimulated by TGF-β.
β3 integrin is upregulated dramatically during EMT induced by TGF-β.
Interaction between αvβ3 integrin and TβR-II leads to Src-mediated phosphorylation of TβR-II at Tyr284, which binds Grb2 and promotes the activation of downstream MAP kinases.
The ability of TGF-β to stimulate cancer progression and metastasis requires an intricate interplay between signals arising from TGF-β receptors and those initiated by integrins.
FAK is required for EMT stimulated by TGF-β.
p130Cas inhibits Smad3 activity and alters cytostasis induced by TGF-β.
Hic5 is a member of the paxillin superfamily that is induced by and required for EMT stimulated by TGF-β.
TRAF6 interacts physically with both TβR-I and TβR-II, leading to TAK1 activation and the stimulation of p38 MAPK and JNK.
Signaling Systems Involved in EMT Stimulated by TGF-β
Transmembrane signaling by TGF-β activates Smad2/3 and their ability to alter the transcription of TGF-β-responsive genes, which play important roles during TGF-β stimulation of cancer cell EMT and metastasis.
Small GTPases RhoA, Rac1, and Cdc42 regulate the formation of stress fibers, lamellipodia, or filopodia, respectively, and are intimately involved in EMT stimulated by TGF-β.
The ability of TGF-β to stimulate apoptosis frequently is subverted during tumorigenesis, leading to enhanced cancer cell survival via activation of the PI3K and AKT signaling systems.
ECM engagement of β1 and β3 integrins activates the Ser/Thr protein kinase ILK and its ability to mediate the (i) stimulation of MAP kinases, and (ii) the inhibition of GSK3β, PI3K/AKT, and small GTPases.
ILK participates in EMT stimulated by TGF-β by coupling this cytokine the activation of AKT, and to the elevated expression of MMP-2 and uPA.
NF-κB activity enables Ras-transformed breast cancer cells to undergo EMT and colonize the lung when stimulated by TGF-β.
Activation of NF-κB also associates with several hallmarks of EMT, including the downregulated expression of E-cadherin and upregulated expression of vimentin.
MAP kinase family members, including ERK1/2, JNK, and p38 MAPK, have all been implicated in mediating EMT and metastasis stimulated by TGF-β.
Mechanisms of Gene Regulation by TGF-β
Aberrant genetic and epigenetic events promote tumorigenesis by circumventing various rate-limiting cellular steps that normally suppress neoplastic development. These key steps are known as the “Hallmarks of Cancer” and include (i) oncogene activation; (ii) tumor suppressor inactivation; (iii) apoptosis resistance; (iv) angiogenesis activation; (v) invasion and metastasis initiation; and (vi) immortality acquisition.
Snail transcription factor family members are master regulators of EMT and include (i) SNAI1 (Snail) and SNAI2 (Slug); (ii) ZEB1 and ZEB2 (SIP1); and (iii) FOXC2.
Evidence implicates dysregulated Myc expression in promoting the ability of epithelial cells to undergo EMT in response to TGF-β. The tumor suppressing activity of TGF-β is intimately linked to its repression of Myc expression in epithelial cells.
STAT3 mediates cell survival and proliferative signals, and serves as an oncogene in several human cancers.
TGF-β activates STAT3 via a PKA-dependent mechanism, leading to the induction of EMT.
ER-α promotes mammary gland develop and homeostasis, and suppresses EMT by inducing the expression of MTA3 (metastasis tumor antigen 3), which represses the expression of Snail.
microRNAs are essential mediators of all facets of cell and tissue development, and of cell proliferation, motility, and survival.
Members of the miR-200 family suppress EMT by downregulating the expression of ZEB1 and ZEB2.
Epigenetic silencing of the E-cadherin promoter via hypermethylation promotes the acquisition of EMT phenotypes and gene expression profiles.
EMT and mammary tumorigenesis usurp the inactivation of p16INK4a as a means to expand aberrant DNA hypermethylation.
Redefining EMT Induced by TGF-β
Inappropriate reactivation of EMT by TGF-β in malignant tissues promotes the selection and expansion of cancer stem and progenitor cells.
Targeting the molecular links between TGF-β, EMT, and stemness reduces breast cancer tumorigenicity.
The development of pharmacological agents that inhibit EMT stimulated by TGF-β may provide new avenues to manipulate the behaviors of normal and cancer stem cells, and to alleviate the acquisition of cancer metastasis.
ACKNOWLEDGEMENTS
We thank members of the Schiemann Laboratory for critical comments and reading of the manuscript. W.P.S. was supported by grants from the National Institutes of Health (CA114039 and CA129359), the Komen Foundation (BCTR0706967), and the Department of Defense (BC084651); M.K.W. was supported by the American Cancer Society (PF-09-120-01-CS); and T.M.A was supported by the Department of Defense (BC083323).
REFERENCES
- 1.Baum B, Settleman J, Quinlan MP. Transitions between epithelial and mesenchymal states in development and disease. Semin. Cell Dev. Biol. 2008;19:294–308. doi: 10.1016/j.semcdb.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev. Cell. 2008;14:818–829. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 3.Elenbaas B, Weinberg RA. Heterotypic signaling between epithelial tumor cells and fibroblasts in carcinoma formation. Exp. Cell Res. 2001;264:169–184. doi: 10.1006/excr.2000.5133. [DOI] [PubMed] [Google Scholar]
- 4.Moustakas A, Heldin CH. Signaling networks guiding epithelial-mesenchymal transitions during embryogenesis and cancer progression. Cancer Sci. 2007;98:1512–1520. doi: 10.1111/j.1349-7006.2007.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zavadil J, Bottinger EP. TGF-β and epithelial-to-mesenchymal transitions. Oncogene. 2005;24:5764–5774. doi: 10.1038/sj.onc.1208927. [DOI] [PubMed] [Google Scholar]
- 6.Thiery JP. Epithelial-mesenchymal transitions in development and pathologies. Curr. Opin. Cell Biol. 2003;15:740–746. doi: 10.1016/j.ceb.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Shook D, Keller R. Mechanisms, mechanics and function of epithelial-mesenchymal transitions in early development. Mech, Dev. 2003;120:1351–1383. doi: 10.1016/j.mod.2003.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat. Rev. Cance. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 9.Willis BC, Borok Z. TGF-β-induced EMT: mechanisms and implications for fibrotic lung disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007;293:L525–534. doi: 10.1152/ajplung.00163.2007. [DOI] [PubMed] [Google Scholar]
- 10.Blobe GC, Schiemann WP, Lodish HF. Role of TGF-β in human disease. N. Engl. J. Med. 2000;342:1350–1358. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- 11.Galliher AJ, Neil JR, Schiemann WP. Role of TGF-β in cancer progression. Future Oncol. 2006;2:743–763. doi: 10.2217/14796694.2.6.743. [DOI] [PubMed] [Google Scholar]
- 12.Massague J, Gomis RR. The logic of TGF-β signaling. FEBS Lett. 2006;580:2811–2820. doi: 10.1016/j.febslet.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 13.Siegel PM, Massague J. Cytostatic and apoptotic actions of TGF-β in homeostasis and cancer. Nat. Rev. Cancer. 2003;3:807–821. doi: 10.1038/nrc1208. [DOI] [PubMed] [Google Scholar]
- 14.Savagner P. Leaving the neighborhood: molecular mechanisms involved during epithelial-mesenchymal transition. Bioessays. 2001;23:912–923. doi: 10.1002/bies.1132. [DOI] [PubMed] [Google Scholar]
- 15.Miettinen PJ, Ebner R, Lopez AR, Derynck R. TGF-β induced transdifferentiation of mammary epithelial cells to mesenchymal cells: involvement of type I receptors. J. Cell Biol. 1994;127:2021–2036. doi: 10.1083/jcb.127.6.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *16.Kaartinen V, Voncken JW, Shuler C, et al. Abnormal lung development and cleft palate in mice lacking TGF-β3 indicates defects of epithelial-mesenchymal interaction. Nature Genetics. 1995;11:415–421. doi: 10.1038/ng1295-415. Study established the critical role for TGF-β3 in regulating developmental EMT
- 17.Romano LA, Runyan RB. Slug is an essential target of TGF-β2 signaling in the developing chicken heart. Dev. Biol. 2000;223:91–102. doi: 10.1006/dbio.2000.9750. [DOI] [PubMed] [Google Scholar]
- 18.Saika S, Kono-Saika S, Ohnishi Y, et al. Smad3 signaling is required for epithelial-mesenchymal transition of lens epithelium after injury. Am. J. Pathol. 2004;164:651–663. doi: 10.1016/S0002-9440(10)63153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saika S, Kono-Saika S, Tanaka T, et al. Smad3 is required for dedifferentiation of retinal pigment epithelium following retinal detachment in mice. Lab. Invest. 2004;84:1245–1258. doi: 10.1038/labinvest.3700156. [DOI] [PubMed] [Google Scholar]
- 20.Sato M, Muragaki Y, Saika S, Roberts AB, Ooshima A. Targeted disruption of TGF-β 1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. J. Clin. Invest. 2003;112:1486–1494. doi: 10.1172/JCI19270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng XH, Derynck R. Specificity and versatility in TGF-β signaling through Smads. Annu. Rev. Cell Dev. Biol. 2005;21:659–693. doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- 22.Moustakas A, Heldin CH. Non-Smad TGF-β signals. J. Cell Sci. 2005;118:3573–3584. doi: 10.1242/jcs.02554. [DOI] [PubMed] [Google Scholar]
- 23.Shi Y, Massague J. Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- *24.Tsukazaki T, Chiang TA, Davison AF, Attisano L, Wrana JL. SARA, a FYVE domain protein that recruits Smad2 to the TGF-β receptor. Cell. 1998;95:779–791. doi: 10.1016/s0092-8674(00)81701-8. Identified SARA as a novel adapter molecule that facilitates Smad2/3 recruitment and activation by TGF-β receptors
- 25.Miura S, Takeshita T, Asao H, et al. Hgs (Hrs), a FYVE domain protein, is involved in Smad signaling through cooperation with SARA. Mol. Cell Biol. 2000;20:9346–9355. doi: 10.1128/mcb.20.24.9346-9355.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hocevar BA, Smine A, Xu XX, Howe PH. The adaptor molecule Disabled-2 links the TGF-β receptors to the Smad pathway. EMBO J. 2001;20:2789–2801. doi: 10.1093/emboj/20.11.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *27.Hayashi H, Abdollah S, Qiu Y, et al. The MAD-related protein Smad7 associates with the TGF-β receptor and functions as an antagonist of TGF-β signaling. Cell. 1997;89:1165–1173. doi: 10.1016/s0092-8674(00)80303-7. [DOI] [PubMed] [Google Scholar]
- *28.Nakao A, Afrakht M, Moren A, et al. Identification of Smad7, a TGF-β-inducible antagonist of TGF-β signalling. Nature. 1997;389:631–635. doi: 10.1038/39369. Together with Ref 27, identified Smad7 as an inhibitory molecule of the TGF-β signaling system
- 29.Souchelnytskyi S, Nakayama T, Nakao A, et al. Physical and functional interaction of murine and Xenopus Smad7 with bone morphogenetic protein receptors and TGF-β receptors. J. Biol. Chem. 1998;273:25364–25370. doi: 10.1074/jbc.273.39.25364. [DOI] [PubMed] [Google Scholar]
- 30.Ebisawa T, Fukuchi M, Murakami G, et al. Smurf1 interacts with TGF-β type I receptor through Smad7 and induces receptor degradation. J. Biol. Chem. 2001;276:12477–12480. doi: 10.1074/jbc.C100008200. [DOI] [PubMed] [Google Scholar]
- 31.Kavsak P, Rasmussen RK, Causing CG, et al. Smad7 binds to Smurf2 to form an E3 ubiquitin ligase that targets the TGF-β receptor for degradation. Mol. Cell. 2000;6:1365–1375. doi: 10.1016/s1097-2765(00)00134-9. [DOI] [PubMed] [Google Scholar]
- 32.Datta PK, Moses HL. STRAP and Smad7 synergize in the inhibition of TGF-β signaling. Mol. Cell. Biol. 2000;20:3157–3167. doi: 10.1128/mcb.20.9.3157-3167.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ibarrola N, Kratchmarova I, Nakajima D, et al. Cloning of a novel signaling molecule, AMSH-2, that potentiates TGF-β signaling. BMC Cell Biol. 2004;5:2. doi: 10.1186/1471-2121-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koinuma D, Shinozaki M, Komuro A, et al. Arkadia amplifies TGF-β superfamily signalling through degradation of Smad7. EMBO J. 2003;22:6458–6470. doi: 10.1093/emboj/cdg632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu FY, Li XZ, Peng YM, Liu H, Liu YH. Arkadia-Smad7-mediated positive regulation of TGF-β signaling in a rat model of tubulointerstitial fibrosis. Am. J. Nephrol. 2007;27:176–183. doi: 10.1159/000100518. [DOI] [PubMed] [Google Scholar]
- 36.Liu W, Rui H, Wang J, et al. Axin is a scaffold protein in TGF-β signaling that promotes degradation of Smad7 by Arkadia. EMBO J. 2006;25:1646–1658. doi: 10.1038/sj.emboj.7601057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *37.Bakin AV, Rinehart C, Tomlinson AK, Arteaga CL. p38 mitogen-activated protein kinase is required for TGF-β-mediated fibroblastic transdifferentiation and cell migration. J. Cell Sci. 2002;115:3193–3206. doi: 10.1242/jcs.115.15.3193. [DOI] [PubMed] [Google Scholar]
- *38.Bakin AV, Tomlinson AK, Bhowmick NA, Moses HL, Arteaga CL. Phosphatidylinositol 3-kinase function is required for TGF-β-mediated epithelial to mesenchymal transition and cell migration. J. Biol. Chem. 2000;275:36803–36810. doi: 10.1074/jbc.M005912200. [DOI] [PubMed] [Google Scholar]
- *39.Bhowmick NA, Zent R, Ghiassi M, McDonnell M, Moses HL. Integrin β1 signaling is necessary for TGF-β activation of p38MAPK and epithelial plasticity. J. Biol. Chem. 2001;276:46707–46713. doi: 10.1074/jbc.M106176200. [DOI] [PubMed] [Google Scholar]
- *40.Lamouille S, Derynck R. Cell size and invasion in TGF-β induced epithelial to mesenchymal transition is regulated by activation of the mTOR pathway. J. Cell Biol. 2007;178:437–451. doi: 10.1083/jcb.200611146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *41.Perlman R, Schiemann WP, Brooks MW, Lodish HF, Weinberg RA. TGF-β-induced apoptosis is mediated by the adapter protein Daxx that facilitates JNK activation. Nat. Cell Biol. 2001;3:708–714. doi: 10.1038/35087019. [DOI] [PubMed] [Google Scholar]
- *42.Zavadil J, Bitzer M, Liang D, et al. Genetic programs of epithelial cell plasticity directed by TGF-β. Proc. Natl. Acad. Sci. USA. 2001;98:6686–6691. doi: 10.1073/pnas.111614398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *43.Galliher AJ, Schiemann WP. β3 integrin and Src facilitate TGF-β mediated induction of epithelial-mesenchymal transition in mammary epithelial cells. Breast Cancer Res. 2006;8:R42. doi: 10.1186/bcr1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Galliher AJ, Schiemann WP. Src phosphorylates Tyr284 in TGF-β type II receptor and regulates TGF-β stimulation of p38 MAPK during breast cancer cell proliferation and invasion. Cancer Res. 2007;67:3752–3758. doi: 10.1158/0008-5472.CAN-06-3851. [DOI] [PubMed] [Google Scholar]
- 45.Galliher-Beckley AJ, Schiemann WP. Grb2 binding to Tyr284 in TGF-β is essential for mammary tumor growth and metastasis stimulated by TGF-β. Carcinogenesis. 2008;29:244–251. doi: 10.1093/carcin/bgm245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Azuma M, Motegi K, Aota K, Yamashita T, Yoshida H, Sato M. TGF-β1 inhibits NF-κB activity through induction of IκBα expression in human salivary gland cells: a possible mechanism of growth suppression by TGF-β1. Exp. Cell Res. 1999;250:213–222. doi: 10.1006/excr.1999.4503. [DOI] [PubMed] [Google Scholar]
- *47.Neil JR, Schiemann WP. Altered TAB1:IκB kinase interaction promotes TGF-β-mediated NF-κB activation during breast cancer progression. Cancer Res. 2008;68:1462–1470. doi: 10.1158/0008-5472.CAN-07-3094. Together with References 37-42, these studies established various noncanonical TGF-β effectors as critical mediators of EMT and oncogenic signaling stimulated by TGF-β.
- 48.Arsura M, Panta GR, Bilyeu JD, et al. Transient activation of NF-κB through a TAK1//IKK kinase pathway by TGF-β1 inhibits AP-1//SMAD signaling and apoptosis: implications in liver tumor formation. Oncogene. 2003;22:412–425. doi: 10.1038/sj.onc.1206132. [DOI] [PubMed] [Google Scholar]
- 49.Kim DW, Sovak MA, Zanieski G, et al. Activation of NF-κB/Rel occurs early during neoplastic transformation of mammary cells. Carcinogenesis. 2000;21:871–879. doi: 10.1093/carcin/21.5.871. [DOI] [PubMed] [Google Scholar]
- 50.Park J-I, Lee M-G, Cho K, et al. TGF-β1 activates interleukin-6 expression in prostate cancer cells through the synergistic collaboration of the Smad2, p38-NF-κB, JNK, and Ras signaling pathways. Oncogene. 2003;22:4314–4332. doi: 10.1038/sj.onc.1206478. [DOI] [PubMed] [Google Scholar]
- 51.Rayet B, Gelinas C. Aberrant Rel/NF-κB genes and activity in human cancer. Oncogene. 1999;18:6938–6947. doi: 10.1038/sj.onc.1203221. [DOI] [PubMed] [Google Scholar]
- 52.Horowitz JC, Rogers DS, Sharma V, et al. Combinatorial activation of FAK and AKT by TGF-β1 confers an anoikis-resistant phenotype to myofibroblasts. Cell Signal. 2007;19:761–771. doi: 10.1016/j.cellsig.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thannickal VJ, Lee DY, White ES, et al. Myofibroblast differentiation by TGF-β1 is dependent on cell adhesion and integrin signaling via focal adhesion kinase. J. Biol. Chem. 2003;278:12384–12389. doi: 10.1074/jbc.M208544200. [DOI] [PubMed] [Google Scholar]
- 54.Park SS, Eom YW, Kim EH, et al. Involvement of c-Src kinase in the regulation of TGF-β1-induced apoptosis. Oncogene. 2004;23:6272–6281. doi: 10.1038/sj.onc.1207856. [DOI] [PubMed] [Google Scholar]
- 55.Wang S, Wilkes MC, Leof EB, Hirschberg R. Imatinib mesylate blocks a non-Smad TGF-β pathway and reduces renal fibrogenesis in vivo. FASEB J. 2005;19:1–11. doi: 10.1096/fj.04-2370com. [DOI] [PubMed] [Google Scholar]
- 56.Wilkes MC, Leof EB. TGF-β activation of c-Abl is independent of receptor internalization and regulated by phosphatidylinositol 3-kinase and PAK2 in mesenchymal cultures. J. Biol. Chem. 2006;281:27846–27854. doi: 10.1074/jbc.M603721200. [DOI] [PubMed] [Google Scholar]
- 57.Ebnet K, Suzuki A, Ohno S, Vestweber D. Junctional adhesion molecules (JAMs): more molecules with dual functions? J. Cell Sci. 2004;117:19–29. doi: 10.1242/jcs.00930. [DOI] [PubMed] [Google Scholar]
- 58.Schneeberger EE, Lynch RD. The tight junction: a multifunctional complex. Am. J. Physiol. Cell. Physiol. 2004;286:C1213–1228. doi: 10.1152/ajpcell.00558.2003. [DOI] [PubMed] [Google Scholar]
- 59.Itoh M, Bissell MJ. The organization of tight junctions in epithelia: implications for mammary gland biology and breast tumorigenesis. J. Mammary Gland Biol. Neoplasia. 2003;8:449–462. doi: 10.1023/B:JOMG.0000017431.45314.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bose R, Wrana JL. Regulation of Par6 by extracellular signals. Curr. Opin. Cell Biol. 2006;18:206–212. doi: 10.1016/j.ceb.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 61.Ozdamar B, Bose R, Barrios-Rodiles M, Wang HR, Zhang Y, Wrana JL. Regulation of the polarity protein Par6 by TGF-β receptors controls epithelial cell plasticity. Science. 2005;307:1603–1609. doi: 10.1126/science.1105718. Identified the novel interaction between Par6 and TGF-β receptors, which promote EMT via the ubiquitination and degradation of RhoA
- 62.Takaishi K, Sasaki T, Kotani H, Nishioka H, Takai Y. Regulation of cell-cell adhesion by Rac and Rho small G proteins in MDCK Cells. J. Cell Biol. 1997;139:1047–1059. doi: 10.1083/jcb.139.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Niessen CM. Tight junctions/adherens junctions: basic structure and function. J. Invest. Dermatol. 2007;127:2525–2532. doi: 10.1038/sj.jid.5700865. [DOI] [PubMed] [Google Scholar]
- 64.Ridley AJ, Hall A. The small GTP-binding protein Rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 65.Bhowmick NA, Ghiassi M, Bakin A, et al. TGF-β1 mediates epithelial to mesenchymal transdifferentiation through a RhoA-dependent mechanism. Mol. Biol. Cell. 2001;12:27–36. doi: 10.1091/mbc.12.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mueller MM, Fusenig NE. Friends or foes - bipolar effects of the tumour stroma in cancer. Nat. Rev. Cancer. 2004;4:839–849. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- 67.Kaplan RN, Rafii S, Lyden D. Preparing the “soil”: the premetastatic niche. Cancer Res. 2006;66:11089–11093. doi: 10.1158/0008-5472.CAN-06-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tlsty TD, Coussens LM. Tumor stroma and regulation of cancer development. Annu. Rev. Pathol. 2006;1:119–150. doi: 10.1146/annurev.pathol.1.110304.100224. [DOI] [PubMed] [Google Scholar]
- 69.Park CC, Bissell MJ, Barcellos-Hoff MH. The influence of the microenvironment on the malignant phenotype. Mol. Med. Today. 2000;6:324–329. doi: 10.1016/s1357-4310(00)01756-1. [DOI] [PubMed] [Google Scholar]
- 70.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 71.Mott JD, Werb Z. Regulation of matrix biology by matrix metalloproteinases. Curr. Opin. Cell Biol. 2004;16:558–564. doi: 10.1016/j.ceb.2004.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Duivenvoorden WC, Hirte HW, Singh G. TGF-β1 acts as an inducer of matrix metalloproteinase expression and activity in human bone-metastasizing cancer cells. Clin. Exp. Metastasis. 1999;17:27–34. doi: 10.1023/a:1026404227624. [DOI] [PubMed] [Google Scholar]
- 73.Kim ES, Sohn YW, Moon A. TGF-β-induced transcriptional activation of MMP-2 is mediated by activating transcription factor (ATF)2 in human breast epithelial cells. Cancer Lett. 2007;252:147–156. doi: 10.1016/j.canlet.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 74.Coussens LM, Tinkle CL, Hanahan D, Werb Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell. 2000;103:481–490. doi: 10.1016/s0092-8674(00)00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Paszek MJ, Weaver VM. The tension mounts: mechanics meets morphogenesis and malignancy. J. Mammary Gland Biol Neoplasia. 2004;9:325–342. doi: 10.1007/s10911-004-1404-x. [DOI] [PubMed] [Google Scholar]
- *76.Anderson AR, Weaver AM, Cummings PT, Quaranta V. Tumor morphology and phenotypic evolution driven by selective pressure from the microenvironment. Cell. 2006;127:905–915. doi: 10.1016/j.cell.2006.09.042. Very interesting study that used a multiscale mathematical modeling approach to predict how altered microenvironmental factors and conditions impact tumor development and progression.
- 77.Sternlicht MD, Bissell MJ, Werb Z. The matrix metalloproteinase stromelysin-1 acts as a natural mammary tumor promoter. Oncogene. 2000;19:1102–1113. doi: 10.1038/sj.onc.1203347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *78.Sternlicht MD, Lochter A, Sympson CJ, et al. The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis. Cell. 1999;98:137–146. doi: 10.1016/s0092-8674(00)81009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *79.Radisky DC, Levy DD, Littlepage LE, et al. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature. 2005;436:123–127. doi: 10.1038/nature03688. Together with Reference 78, established the essential role of MMPs in regulating the interactions between cells and their microenvironments, and in stimulating EMT.
- 80.Radisky DC, Kenny PA, Bissell MJ. Fibrosis and cancer: do myofibroblasts come also from epithelial cells via EMT? J. Cell Biochem. 2007;101:830–839. doi: 10.1002/jcb.21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr. Opin. Cell Biol. 2005;17:548–558. doi: 10.1016/j.ceb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 82.Gotzmann J, Mikula M, Eger A, et al. Molecular aspects of epithelial cell plasticity: implications for local tumor invasion and metastasis. Mutat. Res. 2004;566:9–20. doi: 10.1016/s1383-5742(03)00033-4. [DOI] [PubMed] [Google Scholar]
- 83.Jechlinger M, Grunert S, Beug H. Mechanisms in epithelial plasticity and metastasis: insights from 3D cultures and expression profiling. J. Mammary Gland Biol. Neoplasia. 2002;7:415–432. doi: 10.1023/a:1024090116451. [DOI] [PubMed] [Google Scholar]
- 84.Cavallaro U, Niedermeyer J, Fuxa M, Christofori G. N-CAM modulates tumour-cell adhesion to matrix by inducing FGF-receptor signalling. Nat. Cell Biol. 2001;3:650–657. doi: 10.1038/35083041. [DOI] [PubMed] [Google Scholar]
- 85.Lehembre F, Yilmaz M, Wicki A, et al. NCAM-induced focal adhesion assembly: a functional switch upon loss of E-cadherin. EMBO J. 2008;27:2603–2615. doi: 10.1038/emboj.2008.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Illman SA, Lehti K, Keski-Oja J, Lohi J. Epilysin (MMP-28) induces TGF-β mediated epithelial to mesenchymal transition in lung carcinoma cells. J. Cell Sci. 2006;119:3856–3865. doi: 10.1242/jcs.03157. [DOI] [PubMed] [Google Scholar]
- 87.Illman SA, Lohi J, Keski-Oja J. Epilysin (MMP-28)--structure, expression and potential functions. Exp. Dermatol. 2008;17:897–907. doi: 10.1111/j.1600-0625.2008.00782.x. [DOI] [PubMed] [Google Scholar]
- 88.Harbeck N, Kates RE, Schmitt M, et al. Urokinase-type plasminogen activator and its inhibitor type 1 predict disease outcome and therapy response in primary breast cancer. Clin. Breast Cancer. 2004;5:348–352. doi: 10.3816/cbc.2004.n.040. [DOI] [PubMed] [Google Scholar]
- 89.Duffy MJ, Duggan C. The urokinase plasminogen activator system: a rich source of tumour markers for the individualised management of patients with cancer. Clin. Biochem. 2004;37:541–548. doi: 10.1016/j.clinbiochem.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 90.Mitra SK, Lim ST, Chi A, Schlaepfer DD. Intrinsic focal adhesion kinase activity controls orthotopic breast carcinoma metastasis via the regulation of urokinase plasminogen activator expression in a syngeneic tumor model. Oncogene. 2006;25:4429–4440. doi: 10.1038/sj.onc.1209482. [DOI] [PubMed] [Google Scholar]
- 91.Lin SW, Ke FC, Hsiao PW, Lee PP, Lee MT, Hwang JJ. Critical involvement of ILK in TGF-β1-stimulated invasion/migration of human ovarian cancer cells is associated with urokinase plasminogen activator system. Exp. Cell Res. 2007;313:602–613. doi: 10.1016/j.yexcr.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 92.Lester RD, Jo M, Montel V, Takimoto S, Gonias SL. uPAR induces epithelial mesenchymal transition in hypoxic breast cancer cells. J. Cell Biol. 2007;178:425–436. doi: 10.1083/jcb.200701092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Santibanez JF. JNK mediates TGF-β1-induced epithelial mesenchymal transdifferentiation of mouse transformed keratinocytes. FEBS Lett. 2006;580:5385–5391. doi: 10.1016/j.febslet.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 94.Whitley BR, Church FC. Wound-induced migration of MDA-MB-435 and SKOV-3 cancer cells is regulated by plasminogen activator inhibitor-1. Int. J. Oncol. 2005;27:749–757. [PubMed] [Google Scholar]
- 95.Binder BR, Christ G, Gruber F, et al. Plasminogen activator inhibitor 1: physiological and pathophysiological roles. News Physiol. Sci. 2002;17:56–61. doi: 10.1152/nips.01369.2001. [DOI] [PubMed] [Google Scholar]
- 96.Shetty S, Shetty P, Idell S, Velusamy T, Bhandary YP, Shetty RS. Regulation of plasminogen activator inhibitor-1 expression by tumor suppressor protein p53. J. Biol. Chem. 2008;283:19570–19580. doi: 10.1074/jbc.M710268200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kortlever RM, Bernards R. Senescence, wound healing and cancer: the PAI-1 connection. Cell Cycle. 2006;5:2697–2703. doi: 10.4161/cc.5.23.3510. [DOI] [PubMed] [Google Scholar]
- 98.Whitley BR, Palmieri D, Twerdi CD, Church FC. Expression of active plasminogen activator inhibitor-1 reduces cell migration and invasion in breast and gynecological cancer cells. Exp. Cell Res. 2004;296:151–162. doi: 10.1016/j.yexcr.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 99.Descotes F, Riche B, Saez S, et al. Plasminogen activator inhibitor type 1 is the most significant of the usual tissue prognostic factors in node-negative breast ductal adenocarcinoma independent of urokinase-type plasminogen activator. Clin. Breast Cancer. 2008;8:168–177. doi: 10.3816/CBC.2008.n.018. [DOI] [PubMed] [Google Scholar]
- 100.Wienke D, Davies GC, Johnson DA, et al. The collagen receptor Endo180 (CD280) Is expressed on basal-like breast tumor cells and promotes tumor growth in vivo. Cancer Res. 2007;67:10230–10240. doi: 10.1158/0008-5472.CAN-06-3496. [DOI] [PubMed] [Google Scholar]
- 101.Kim ES, Kim MS, Moon A. TGF-β in conjunction with H-Ras activation promotes malignant progression of MCF10A breast epithelial cells. Cytokine. 2005;29:84–91. doi: 10.1016/j.cyto.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 102.Ignotz RA, Massague J. TGF-β stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J. Biol. Chem. 1986;261:4337–4345. [PubMed] [Google Scholar]
- 103.Xie L, Law B, Aakre M, et al. TGF-β-regulated gene expression in a mouse mammary gland epithelial cell line. Breast Cancer Res. 2003;5:R187–R198. doi: 10.1186/bcr640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Maschler S, Wirl G, Spring H, et al. Tumor cell invasiveness correlates with changes in integrin expression and localization. Oncogene. 2005;24:2032–2041. doi: 10.1038/sj.onc.1208423. [DOI] [PubMed] [Google Scholar]
- 105.Kang Y, Massague J. Epithelial-mesenchymal transitions: Twist in development and metastasis. Cell. 2004;118:277–279. doi: 10.1016/j.cell.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 106.Bremnes RM, Veve R, Hirsch FR, Franklin WA. The E-cadherin cell-cell adhesion complex and lung cancer invasion, metastasis, and prognosis. Lung Cancer. 2002;36:115–124. doi: 10.1016/s0169-5002(01)00471-8. [DOI] [PubMed] [Google Scholar]
- 107.Graff JR, Greenberg VE, Herman JG, et al. Distinct patterns of E-cadherin CpG island methylation in papillary, follicular, Hurthle’s cell, and poorly differentiated human thyroid carcinoma. Cancer Res. 1998;58:2063–2066. [PubMed] [Google Scholar]
- 108.Comijn J, Berx G, Vermassen P, et al. The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion. Mol. Cell. 2001;7:1267–1278. doi: 10.1016/s1097-2765(01)00260-x. [DOI] [PubMed] [Google Scholar]
- 109.Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat. Rev. Cancer. 2007;7:415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 110.Cano A, Perez-Moreno MA, Rodrigo I, et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat. Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 111.Thuault S, Valcourt U, Petersen M, Manfioletti G, Heldin CH, Moustakas A. TGF-β employs HMGA2 to elicit epithelial-mesenchymal transition. J. Cell Biol. 2006;174:175–183. doi: 10.1083/jcb.200512110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hazan RB, Phillips GR, Qiao RF, Norton L, Aaronson SA. Exogenous Expression of N-Cadherin in breast cancer cells induces cell migration, invasion, and metastasis. J. Cell Biol. 2000;148:779–790. doi: 10.1083/jcb.148.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gravdal K, Halvorsen OJ, Haukaas SA, Akslen LA. A switch from E-cadherin to N-cadherin expression indicates epithelial to mesenchymal transition and is of strong and independent importance for the progress of prostate cancer. Clin. Cancer Res. 2007;13:7003–7011. doi: 10.1158/1078-0432.CCR-07-1263. [DOI] [PubMed] [Google Scholar]
- 114.Pyo SW, Hashimoto M, Kim YS, et al. Expression of E-cadherin, P-cadherin and N-cadherin in oral squamous cell carcinoma: correlation with the clinicopathologic features and patient outcome. J. Craniomaxillofac. Surg. 2007;35:1–9. doi: 10.1016/j.jcms.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 115.Yang L, Huang J, Ren X, et al. Abrogation of TGF-β signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell. 2008;13:23–35. doi: 10.1016/j.ccr.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nam JS, Terabe M, Mamura M, et al. An anti-TGF-β antibody suppresses metastasis via cooperative effects on multiple cell compartments. Cancer Res. 2008;68:3835–3843. doi: 10.1158/0008-5472.CAN-08-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Grunert S, Jechlinger M, Beug H. Diverse cellular and molecular mechanisms contribute to epithelial plasticity and metastasis. Nat. Rev. Mol. Cell Biol. 2003;4:657. doi: 10.1038/nrm1175. [DOI] [PubMed] [Google Scholar]
- 118.Masszi A, Di Ciano C, Sirokmany G, et al. Central role for Rho in TGF-β1-induced α-smooth muscle actin expression during epithelial-mesenchymal transition. Am. J. Physiol. Renal Physiol. 2003;284:F911–924. doi: 10.1152/ajprenal.00183.2002. [DOI] [PubMed] [Google Scholar]
- 119.Yazhou C, Wenlv S, Weidong Z, Licun W. Clinicopathological significance of stromal myofibroblasts in invasive ductal carcinoma of the breast. Tumour Biol. 2004;25:290–295. doi: 10.1159/000081394. [DOI] [PubMed] [Google Scholar]
- 120.Cary LA, Guan JL. Focal adhesion kinase in integrin-mediated signaling. Front. Biosci. 1999;4:D102–113. doi: 10.2741/cary. [DOI] [PubMed] [Google Scholar]
- 121.Cary LA, Han DC, Guan JL. Integrin-mediated signal transduction pathways. Histol. Histopathol. 1999;14:1001–1009. doi: 10.14670/HH-14.1001. [DOI] [PubMed] [Google Scholar]
- 122.Schwartz MA, Ginsberg MH. Networks and crosstalk: integrin signalling spreads. Nat. Cell Biol. 2002;4:E65–68. doi: 10.1038/ncb0402-e65. [DOI] [PubMed] [Google Scholar]
- 123.Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat. Rev Cancer. 2002;2:91–100. doi: 10.1038/nrc727. [DOI] [PubMed] [Google Scholar]
- 124.Ginsberg MH, Partridge A, Shattil SJ. Integrin regulation. Curr. Opin. Cell Biol. 2005;17:509–516. doi: 10.1016/j.ceb.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 125.Guo W, Giancotti FG. Integrin signalling during tumour progression. Nat. Rev. Mol Cell Biol. 2004;5:816–826. doi: 10.1038/nrm1490. [DOI] [PubMed] [Google Scholar]
- 126.Sieg DJ, Hauck CR, Ilic D, et al. FAK integrates growth-factor and integrin signals to promote cell migration. Nat. Cell Biol. 2000;2:249–256. doi: 10.1038/35010517. [DOI] [PubMed] [Google Scholar]
- 127.Chen SY, Chen HC. Direct interaction of focal adhesion kinase (FAK) with Met is required for FAK to promote hepatocyte growth factor-induced cell invasion. Mol. Cell. Biol. 2006;26:5155–5167. doi: 10.1128/MCB.02186-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mizejewski GJ. Role of integrins in cancer: survey of expression patterns. Proc. Soc. Exp. Biol. Med. 1999;222:124–138. doi: 10.1177/153537029922200203. [DOI] [PubMed] [Google Scholar]
- 129.Sheppard D, Cohen DS, Wang A, Busk M. TGF-β differentially regulates expression of integrin subunits in guinea pig airway epithelial cells. J. Biol. Chem. 1992;267:17409–17414. [PubMed] [Google Scholar]
- 130.Kumar NM, Sigurdson SL, Sheppard D, Lwebuga-Mukasa JS. Differential modulation of integrin receptors and extracellular matrix laminin by TGF-β1 in rat alveolar epithelial cells. Exp. Cell Res. 1995;221:385–394. doi: 10.1006/excr.1995.1389. [DOI] [PubMed] [Google Scholar]
- 131.Wang A, Yokosaki Y, Ferrando R, Balmes J, Sheppard D. Differential regulation of airway epithelial integrins by growth factors. Am. J. Respir. Cell Mol. Biol. 1996;15:664–672. doi: 10.1165/ajrcmb.15.5.8918373. [DOI] [PubMed] [Google Scholar]
- *132.Munger JS, Huang X, Kawakatsu H, et al. The integrin αvβ6 binds and activates latent TGF-β1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. First established the importance of integrins in promoting the activation of latent TGF-β complexes.
- 133.Jenkins RG, Su X, Su G, et al. Ligation of protease-activated receptor 1 enhances αvβ6 integrin-dependent TGF-β activation and promotes acute lung injury. J. Clin. Invest. 2006;116:1606–1614. doi: 10.1172/JCI27183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Neurohr C, Nishimura SL, Sheppard D. Activation of TGF-β by the integrin αvβ8 delays epithelial wound closure. Am. J. Respir. Cell Mol. Biol. 2006;35:252–259. doi: 10.1165/rcmb.2006-0013OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Morris DG, Huang X, Kaminski N, et al. Loss of integrin αvβ6-mediated TGF-β activation causes MMP12-dependent emphysema. Nature. 2003;422:169–173. doi: 10.1038/nature01413. [DOI] [PubMed] [Google Scholar]
- 136.Mu D, Cambier S, Fjellbirkeland L, et al. The integrin αvβ8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-β1. J Cell Biol. 2002;157:493–507. doi: 10.1083/jcb.200109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Knight PA, Wright SH, Brown JK, Huang X, Sheppard D, Miller HR. Enteric expression of the integrin αvβ6 is essential for nematode-induced mucosal mast cell hyperplasia and expression of the granule chymase, mouse mast cell protease-1. Am. J. Pathol. 2002;161:771–779. doi: 10.1016/s0002-9440(10)64236-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kim KK, Kugler MC, Wolters PJ, et al. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc. Natl. Acad. Sci. USA. 2006;103:13180–13185. doi: 10.1073/pnas.0605669103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bates RC, Bellovin DI, Brown C, et al. Transcriptional activation of integrin β6 during the epithelial-mesenchymal transition defines a novel prognostic indicator of aggressive colon carcinoma. J. Clin. Invest. 2005;115:339–347. doi: 10.1172/JCI23183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ma LJ, Yang H, Gaspert A, et al. TGF-β-dependent and -independent pathways of induction of tubulointerstitial fibrosis in β6(-/-) mice. Am. J. Pathol. 2003;163:1261–1273. doi: 10.1016/s0002-9440(10)63486-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Owens DM, Broad S, Yan X, Benitah SA, Watt FM. Suprabasal α5β1 integrin expression stimulates formation of epidermal squamous cell carcinomas without disrupting TGF-β signaling or inducing spindle cell tumors. Mol. Carcinog. 2005;44:60–66. doi: 10.1002/mc.20118. [DOI] [PubMed] [Google Scholar]
- 142.Kostenuik PJ, Singh G, Orr FW. TGF-β upregulates the integrin-mediated adhesion of human prostatic carcinoma cells to type I collagen. Clin. Exp. Metastasis. 1997;15:41–52. doi: 10.1023/a:1018484323210. [DOI] [PubMed] [Google Scholar]
- 143.Giannelli G, Fransvea E, Marinosci F, et al. TGF-β1 triggers hepatocellular carcinoma invasiveness via α3β1 integrin. Am. J. Pathol. 2002;161:183–193. doi: 10.1016/s0002-9440(10)64170-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Giannelli G, Bergamini C, Fransvea E, Sgarra C, Antonaci S. Laminin-5 with TGF-β1 induces epithelial to mesenchymal transition in hepatocellular carcinoma. Gastroenterology. 2005;129:1375–1383. doi: 10.1053/j.gastro.2005.09.055. [DOI] [PubMed] [Google Scholar]
- 145.Sloan EK, Pouliot N, Stanley KL, et al. Tumor-specific expression of αvβ3 integrin promotes spontaneous metastasis of breast cancer to bone. Breast Cancer Res. 2006;8:R20. doi: 10.1186/bcr1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bandyopadhyay A, Agyin JK, Wang L, et al. Inhibition of pulmonary and skeletal metastasis by a TGF-β type I receptor kinase inhibitor. Cancer Res. 2006;66:6714–6721. doi: 10.1158/0008-5472.CAN-05-3565. [DOI] [PubMed] [Google Scholar]
- 147.Nakamura K, Yano H, Schaefer E, Sabe H. Different modes and qualities of tyrosine phosphorylation of Fak and Pyk2 during epithelial-mesenchymal transdifferentiation and cell migration: analysis of specific phosphorylation events using site-directed antibodies. Oncogene. 2001;20:2626–2635. doi: 10.1038/sj.onc.1204359. [DOI] [PubMed] [Google Scholar]
- 148.Liu S, Shi-wen X, Kennedy L, et al. FAK is required for TGF-β-induced JNK phosphorylation in fibroblasts: implications for acquisition of a matrix-remodeling phenotype. Mol. Biol. Cell. 2007;18:2169–2178. doi: 10.1091/mbc.E06-12-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cicchini C, Laudadio I, Citarella F, et al. TGF-β-induced EMT requires focal adhesion kinase (FAK) signaling. Exp. Cell Res. 2008;314:143. doi: 10.1016/j.yexcr.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 150.Kim W, Kang Y Seok, Kim J Soo, Shin N-Y, Hanks SK, Song WK. The integrin-coupled signaling adaptor p130Cas suppresses Smad3 function in TGF-β signaling. Mol. Biol. Cell. 2008;19:2135–2146. doi: 10.1091/mbc.E07-10-0991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cabodi S, Tinnirello A, Di Stefano P, et al. p130Cas as a new regulator of mammary epithelial cell proliferation, survival, and HER2-Neu oncogene-dependent breast tumorigenesis. Cancer Res. 2006;66:4672–4680. doi: 10.1158/0008-5472.CAN-05-2909. [DOI] [PubMed] [Google Scholar]
- 152.Tumbarello DA, Brown MC, Hetey SE, Turner CE. Regulation of paxillin family members during epithelial-mesenchymal transformation: a putative role for paxillin δ. J. Cell Sci. 2005;118:4849–4863. doi: 10.1242/jcs.02615. [DOI] [PubMed] [Google Scholar]
- 153.Fujimoto N, Yeh S, Kang HY, et al. Cloning and characterization of androgen receptor coactivator, ARA55, in human prostate. J. Biol. Chem. 1999;274:8316–8321. doi: 10.1074/jbc.274.12.8316. [DOI] [PubMed] [Google Scholar]
- 154.Guerrero-Santoro J, Yang L, Stallcup MR, DeFranco DB. Distinct LIM domains of Hic-5/ARA55 are required for nuclear matrix targeting and glucocorticoid receptor binding and coactivation. J. Cell Biochem. 2004;92:810–819. doi: 10.1002/jcb.20109. [DOI] [PubMed] [Google Scholar]
- 155.Yang L, Guerrero J, Hong H, DeFranco DB, Stallcup MR. Interaction of the Tau2 transcriptional activation domain of glucocorticoid receptor with a novel steroid receptor coactivator, Hic-5, which localizes to both focal adhesions and the nuclear matrix. Mol. Biol. Cell. 2000;11:2007–2018. doi: 10.1091/mbc.11.6.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tumbarello DA, Turner CE. Hic-5 contributes to epithelial-mesenchymal transformation through a RhoA/ROCK-dependent pathway. J. Cell Physiol. 2007;211:736–747. doi: 10.1002/jcp.20991. [DOI] [PubMed] [Google Scholar]
- 157.Mok SC, Wong KK, Chan RK, et al. Molecular cloning of differentially expressed genes in human epithelial ovarian cancer. Gynecol. Oncol. 1994;52:247–252. doi: 10.1006/gyno.1994.1040. [DOI] [PubMed] [Google Scholar]
- 158.Mok SC, Chan WY, Wong KK, et al. DOC-2, a candidate tumor suppressor gene in human epithelial ovarian cancer. Oncogene. 1998;16:2381–2387. doi: 10.1038/sj.onc.1201769. [DOI] [PubMed] [Google Scholar]
- 159.Prunier C, Hocevar BA, Howe PH. Wnt signaling: physiology and pathology. Growth Factors. 2004;22:141–150. doi: 10.1080/08977190410001720860. [DOI] [PubMed] [Google Scholar]
- 160.Prunier C, Howe PH. Disabled-2 (Dab2) is required for TGF-β-induced epithelial to mesenchymal transition (EMT) J. Biol. Chem. 2005;280:17540–17548. doi: 10.1074/jbc.M500974200. [DOI] [PubMed] [Google Scholar]
- 161.Hocevar BA, Prunier C, Howe PH. Disabled-2 (Dab2) mediates TGF-β-stimulated fibronectin synthesis through TGF-β-activated kinase 1 and activation of the JNK pathway. J. Biol. Chem. 2005;280:25920–25927. doi: 10.1074/jbc.M501150200. [DOI] [PubMed] [Google Scholar]
- *162.Sorrentino A, Thakur N, Grimsby S, et al. The type I TGF-β receptor engages TRAF6 to activate TAK1 in a receptor kinase-independent manner. Nat. Cell Biol. 2008;10:1199–1207. doi: 10.1038/ncb1780. [DOI] [PubMed] [Google Scholar]
- *163.Yamashita M, Fatyol K, Jin C, Wang X, Liu Z, Zhang YE. TRAF6 mediates Smad-independent activation of JNK and p38 by TGF-β. Mol. Cell. 2008;31:918–924. doi: 10.1016/j.molcel.2008.09.002. Together with Reference 162, these studies demonstrated the importance of TRAF6 to interact physically with TGF-β receptors, leading to their activation of MAP kinases via ubiquitination of TAK1.
- 164.Gal A, Sjoblom T, Fedorova L, Imreh S, Beug H, Moustakas A. Sustained TGF-β exposure suppresses Smad and non-Smad signalling in mammary epithelial cells, leading to EMT and inhibition of growth arrest and apoptosis. Oncogene. 2008;27:1218–1230. doi: 10.1038/sj.onc.1210741. [DOI] [PubMed] [Google Scholar]
- 165.Hall A. Rho GTPases and the control of cell behaviour. Biochem. Soc. Trans. 2005;33:891–895. doi: 10.1042/BST20050891. [DOI] [PubMed] [Google Scholar]
- 166.Hall A, Nobes CD. Rho GTPases: molecular switches that control the organization and dynamics of the actin cytoskeleton. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2000;355:965–970. doi: 10.1098/rstb.2000.0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sander EE, ten Klooster JP, van Delft S, van der Kammen RA, Collard JG. Rac downregulates Rho activity: reciprocal balance between both GTPases determines cellular morphology and migratory behavior. J. Cell Biol. 1999;147:1009–1022. doi: 10.1083/jcb.147.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Cho HJ, Yoo J. Rho activation is required for TGF-β-induced epithelial-mesenchymal transition in lens epithelial cells. Cell Biol. Int. 2007;31:1225–1230. doi: 10.1016/j.cellbi.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 169.Massague J. How cells read TGF-β signals. Nat. Rev. Mol. Cell. Biol. 2000;1:169–178. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- 170.Murillo MM, del Castillo G, Sanchez A, Fernandez M, Fabregat I. Involvement of EGF receptor and c-Src in the survival signals induced by TGF-β1 in hepatocytes. Oncogene. 2005;24:4580–4587. doi: 10.1038/sj.onc.1208664. [DOI] [PubMed] [Google Scholar]
- 171.Jechlinger M, Sommer A, Moriggl R, et al. Autocrine PDGFR signaling promotes mammary cancer metastasis. J. Clin. Invest. 2006;116:1561–1570. doi: 10.1172/JCI24652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Dedhar S, Williams B, Hannigan G. Integrin-linked kinase (ILK): a regulator of integrin and growth-factor signalling. Trends. Cell Biol. 1999;9:319–323. doi: 10.1016/s0962-8924(99)01612-8. [DOI] [PubMed] [Google Scholar]
- 173.Hannigan G, Troussard A, ADedhar S. Integrin-linked kinase: a cancer therapeutic target unique among its ILK. Nat. Rev. Cancer. 2005;5:51–63. doi: 10.1038/nrc1524. [DOI] [PubMed] [Google Scholar]
- 174.Hehlgans S, Haase M, Cordes N. Signalling via integrins: implications for cell survival and anticancer strategies. Biochim. Biophys. Acta. 2007;1775:163–180. doi: 10.1016/j.bbcan.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 175.White DE, Cardiff RD, Dedhar S, Muller WJ. Mammary epithelial-specific expression of the integrin-linked kinase (ILK) results in the induction of mammary gland hyperplasias and tumors in transgenic mice. Oncogene. 2001;20:7064–7072. doi: 10.1038/sj.onc.1204910. [DOI] [PubMed] [Google Scholar]
- 176.Somasiri A, Howarth A, Goswami D, Dedhar S, Roskelley CD. Overexpression of the integrin-linked kinase mesenchymally transforms mammary epithelial cells. J. Cell Sci. 2001;114:1125–1136. doi: 10.1242/jcs.114.6.1125. [DOI] [PubMed] [Google Scholar]
- 177.Lee YI, Kwon YJ, Joo CK. Integrin-linked kinase function is required for TGF-β-mediated epithelial to mesenchymal transition. Biochem. Biophys. Res. Commun. 2004;316:997–1001. doi: 10.1016/j.bbrc.2004.02.150. [DOI] [PubMed] [Google Scholar]
- 178.Karin M. NF-κB in cancer development and progression. Nature. 2006;441:431. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 179.Huber MA, Azoitei N, Baumann B, et al. NF-κB is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression. J. Clin. Invest. 2004;114:569–581. doi: 10.1172/JCI21358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Chua HL, Bhat-Nakshatri P, Clare SE, Morimiya A, Badve S, Nakshatri H. NF-κB represses E-cadherin expression and enhances epithelial to mesenchymal transition of mammary epithelial cells: potential involvement of ZEB-1 and ZEB-2. Oncogene. 2006;26:711. doi: 10.1038/sj.onc.1209808. [DOI] [PubMed] [Google Scholar]
- 181.Sovak MA, Arsura M, Zanieski G, Kavanagh KT, Sonenshein GE. The inhibitory effects of TGF-β1 on breast cancer cell proliferation are mediated through regulation of aberrant NF-κB/Rel expression. Cell Growth Differ. 1999;10:537–544. [PubMed] [Google Scholar]
- 182.Neil JR, Johnson KM, Nemenoff RA, Schiemann WP. Cox-2 inactivates Smad signaling and enhances EMT stimulated by TGF-β through a PGE2-dependent mechanisms. Carcinogenesis. 2008;29:2227–2235. doi: 10.1093/carcin/bgn202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Xie L, Law BK, Chytil AM, Brown KA, Aakre ME, Moses HL. Activation of the ERK pathway is required for TGF-β1-induced EMT in vitro. Neoplasia. 2004;6:603–610. doi: 10.1593/neo.04241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Atfi A, Djelloul S, Chastre E, Davis R, Gespach C. Evidence for a role of Rho-like GTPases and stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK) in TGF-β-mediated signaling. J. Biol. Chem. 1997;272:1429–1432. doi: 10.1074/jbc.272.3.1429. [DOI] [PubMed] [Google Scholar]
- 185.Hocevar BA, Brown TL, Howe PH. TGF-β induces fibronectin synthesis through a c-Jun N-terminal kinase-dependent, Smad4-independent pathway. EMBO J. 1999;18:1345–1356. doi: 10.1093/emboj/18.5.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Shintani Y, Wheelock MJ, Johnson KR. Phosphoinositide-3 kinase-Rac1-c-Jun NH2-terminal kinase signaling mediates collagen I-induced cell scattering and up-regulation of N-cadherin expression in mouse mammary epithelial cells. Mol. Biol. Cell. 2006;17:2963–2975. doi: 10.1091/mbc.E05-12-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ke Z, Lin H, Fan Z, et al. MMP-2 mediates ethanol-induced invasion of mammary epithelial cells over-expressing ErbB2. Int. J. Cancer. 2006;119:8–16. doi: 10.1002/ijc.21769. [DOI] [PubMed] [Google Scholar]
- 188.Buck MB, Knabbe C. TGF-β signaling in breast cancer. Ann. NY Acad. Sci. 2006;1089:119–126. doi: 10.1196/annals.1386.024. [DOI] [PubMed] [Google Scholar]
- 189.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 190.Moreno-Bueno G, Portillo F, Cano A. Transcriptional regulation of cell polarity in EMT and cancer. Oncogene. 2008;27:6958–6969. doi: 10.1038/onc.2008.346. [DOI] [PubMed] [Google Scholar]
- 191.Ikenouchi J, Matsuda M, Furuse M, Tsukita S. Regulation of tight junctions during the epithelium-mesenchyme transition: direct repression of the gene expression of claudins/occludin by Snail. J. Cell Sci. 2003;116:1959–1967. doi: 10.1242/jcs.00389. [DOI] [PubMed] [Google Scholar]
- *192.Alexandrow MG, Kawabata M, Aakre M, Moses HL. Overexpression of the c-Myc oncoprotein blocks the growth-inhibitory response but is required for the mitogenic effects of TGF-β. Proc. Natl. Acad. Sci. USA. 1995;92:3239–3243. doi: 10.1073/pnas.92.8.3239. Established c-Myc as an a molecule that possesses anti-TGF-β activity during tumorigenesis.
- 193.Chen CR, Kang Y, Massague J. Defective repression of c-Myc in breast cancer cells: A loss at the core of the TGF-β growth arrest program. Proc. Natl. Acad. Sci. USA. 2001;98:992–999. doi: 10.1073/pnas.98.3.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Smith AP, Verrecchia A, Faga G, et al. A positive role for Myc in TGF-β-induced Snail transcription and epithelial-to-mesenchymal transition. Oncogene. 2008;28:422–430. doi: 10.1038/onc.2008.395. [DOI] [PubMed] [Google Scholar]
- 195.Bromberg JF, Wrzeszczynska MH, Devgan G, et al. Stat3 as an oncogene. Cell. 1999;98:295–303. doi: 10.1016/s0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- 196.Yang Y, Pan X, Lei W, et al. Regulation of TGF-β1-induced apoptosis and epithelial-to-mesenchymal transition by protein kinase A and signal transducers and activators of transcription 3. Cancer Res. 2006;66:8617–8624. doi: 10.1158/0008-5472.CAN-06-1308. [DOI] [PubMed] [Google Scholar]
- 197.Zhao S, Venkatasubbarao K, Lazor JW, et al. Inhibition of STAT3Tyr705 phosphorylation by Smad4 suppresses TGF-β-mediated invasion and metastasis in pancreatic cancer cells. Cancer Res. 2008;68:4221–4228. doi: 10.1158/0008-5472.CAN-07-5123. [DOI] [PubMed] [Google Scholar]
- 198.Lo HW, Hsu SC, Xia W, et al. Epidermal growth factor receptor cooperates with signal transducer and activator of transcription 3 to induce epithelial-mesenchymal transition in cancer cells via up-regulation of TWIST gene expression. Cancer Res. 2007;67:9066–9076. doi: 10.1158/0008-5472.CAN-07-0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ali S, Coombes RC. Endocrine-responsive breast cancer and strategies for combating resistance. Nat. Rev. Cancer. 2002;2:101–112. doi: 10.1038/nrc721. [DOI] [PubMed] [Google Scholar]
- 200.Coombes RC, Gibson L, Hall E, Emson M, Bliss J. Aromatase inhibitors as adjuvant therapies in patients with breast cancer. J. Steroid Biochem. Mol. Biol. 2003;86:309–311. doi: 10.1016/s0960-0760(03)00372-8. [DOI] [PubMed] [Google Scholar]
- 201.Fujita N, Jaye DL, Kajita M, Geigerman C, Moreno CS, Wade PA. MTA3, a Mi-2/NuRD complex subunit, regulates an invasive growth pathway in breast cancer. Cell. 2003;113:207–219. doi: 10.1016/s0092-8674(03)00234-4. [DOI] [PubMed] [Google Scholar]
- 202.Dhasarathy A, Kajita M, Wade PA. The transcription factor snail mediates epithelial to mesenchymal transitions by repression of estrogen receptor-α. Mol. Endocrinol. 2007;21:2907–2918. doi: 10.1210/me.2007-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Silveri L, Tilly G, Vilotte JL, Le Provost F. MicroRNA involvement in mammary gland development and breast cancer. Reprod. Nutr. Dev. 2006;46:549–556. doi: 10.1051/rnd:2006026. [DOI] [PubMed] [Google Scholar]
- 204.Croce CM, Calin GA. miRNAs, cancer, and stem cell division. Cell. 2005;122:6–7. doi: 10.1016/j.cell.2005.06.036. [DOI] [PubMed] [Google Scholar]
- 205.Iorio MV, Ferracin M, Liu CG, et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005;65:7065–7070. doi: 10.1158/0008-5472.CAN-05-1783. [DOI] [PubMed] [Google Scholar]
- 206.Blenkiron C, Miska EA. miRNAs in cancer: approaches, aetiology, diagnostics and therapy. Hum. Mol. Genet. 2007;16:R106–113. doi: 10.1093/hmg/ddm056. [DOI] [PubMed] [Google Scholar]
- 207.Dalmay T, Edwards DR. MicroRNAs and the hallmarks of cancer. Oncogene. 2006;25:6170–6175. doi: 10.1038/sj.onc.1209911. [DOI] [PubMed] [Google Scholar]
- 208.Blenkiron C, Goldstein LD, Thorne NP, et al. MicroRNA expression profiling of human breast cancer identifies new markers of tumor subtype. Genome Biol. 2007;8:R214. doi: 10.1186/gb-2007-8-10-r214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Hurteau GJ, Carlson JA, Spivack SD, Brock GJ. Overexpression of the microRNA Hsa-miR-200c leads to reduced expression of transcription factor 8 and increased expression of E-cadherin. Cancer Res. 2007;67:7972–7976. doi: 10.1158/0008-5472.CAN-07-1058. [DOI] [PubMed] [Google Scholar]
- 210.Korpal M, Lee ES, Hu G, Kang Y. The miR-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2. J. Biol. Chem. 2008;283:14910–14914. doi: 10.1074/jbc.C800074200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *211.Gregory PA, Bert AG, Paterson EL, et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat. Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- *212.Park SM, Gaur AB, Lengyel E, Peter ME. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 2008;22:894–907. doi: 10.1101/gad.1640608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *213.Burk U, Schubert J, Wellner U, et al. A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep. 2008;9:582–589. doi: 10.1038/embor.2008.74. Together with References 211 and 212, these studies provide the first evidence linking altered microRNA expression to EMT stimulated by TGF-β.
- 214.Ma L, Teruya-Feldstein J, Weinberg RA. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature. 2007;449:682–688. doi: 10.1038/nature06174. [DOI] [PubMed] [Google Scholar]
- 215.Kong W, Yang H, He L, et al. MicroRNA-155 is regulated by the TGF-β/Smad pathway and contributes to epithelial cell plasticity by targeting RhoA. Mol. Cell. Biol. 2008;28:6773–6784. doi: 10.1128/MCB.00941-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Si ML, Zhu S, Wu H, Lu Z, Wu F, Mo YY. miR-21-mediated tumor growth. Oncogene. 2007;26:2799–2803. doi: 10.1038/sj.onc.1210083. [DOI] [PubMed] [Google Scholar]
- 217.Zhu S, Si ML, Wu H, Mo YY. MicroRNA-21 targets the tumor suppressor gene tropomyosin 1 (TPM1) J. Biol. Chem. 2007;282:14328–14336. doi: 10.1074/jbc.M611393200. [DOI] [PubMed] [Google Scholar]
- 218.Zhu S, Wu H, Wu F, Nie D, Sheng S, Mo YY. MicroRNA-21 targets tumor suppressor genes in invasion and metastasis. Cell Res. 2008;18:350–359. doi: 10.1038/cr.2008.24. [DOI] [PubMed] [Google Scholar]
- 219.Zavadil J, Narasimhan M, Blumenberg M, Schneider RJ. TGF-β and microRNA:mRNA regulatory networks in epithelial plasticity. Cells Tissues Organs. 2007;185:157–161. doi: 10.1159/000101316. [DOI] [PubMed] [Google Scholar]
- 220.Bakin AV, Safina A, Rinehart C, Daroqui C, Darbary H, Helfman DM. A critical role of tropomyosins in TGF-β regulation of the actin cytoskeleton and cell motility in epithelial cells. Mol. Biol. Cell. 2004;15:4682–4694. doi: 10.1091/mbc.E04-04-0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Varga AE, Stourman NV, Zheng Q, et al. Silencing of the tropomyosin-1 gene by DNA methylation alters tumor suppressor function of TGF-β. Oncogene. 2005;24:5043–5052. doi: 10.1038/sj.onc.1208688. [DOI] [PubMed] [Google Scholar]
- 222.Zheng Q, Safina A, Bakin AV. Role of high-molecular weight tropomyosins in TGF-β-mediated control of cell motility. Int. J. Cancer. 2008;122:78–90. doi: 10.1002/ijc.23025. [DOI] [PubMed] [Google Scholar]
- 223.Lombaerts M, van Wezel T, Philippo K, et al. E-cadherin transcriptional downregulation by promoter methylation but not mutation is related to epithelial-to-mesenchymal transition in breast cancer cell lines. Br. J. Cancer. 2006;94:661. doi: 10.1038/sj.bjc.6602996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Reynolds PA, Sigaroudinia M, Zardo G, et al. Tumor suppressor p16INK4A regulates polycomb-mediated DNA hypermethylation in human mammary epithelial cells. J. Biol. Chem. 2006;281:24790–24802. doi: 10.1074/jbc.M604175200. [DOI] [PubMed] [Google Scholar]
- 225.Dumont N, Wilson MB, Crawford YG, Reynolds PA, Sigaroudinia M, Tlsty TD. Sustained induction of epithelial to mesenchymal transition activates DNA methylation of genes silenced in basal-like breast cancers. Proc. Natl. Acad Sci. USA. 2008;105:14867–14872. doi: 10.1073/pnas.0807146105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Singh M, Spoelstra NS, Jean A, et al. ZEB1 expression in type I vs type II endometrial cancers: a marker of aggressive disease. Mod. Pathol. 2008;21:912. doi: 10.1038/modpathol.2008.82. [DOI] [PubMed] [Google Scholar]
- 227.Shackleton M, Vaillant F, Simpson KJ, et al. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- 228.Stingl J, Raouf A, Eirew P, Eaves CJ. Deciphering the mammary epithelial cell hierarchy. Cell Cycle. 2006;5:1519–1522. doi: 10.4161/cc.5.14.2983. [DOI] [PubMed] [Google Scholar]
- 229.Villadsen R, Fridriksdottir AJ, Ronnov-Jessen L, et al. Evidence for a stem cell hierarchy in the adult human breast. J Cell Biol. 2007;177:87–101. doi: 10.1083/jcb.200611114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Mishra L, Derynck R, Mishra B. TGF-β signaling in stem cells and cancer. Science. 2005;310:68–71. doi: 10.1126/science.1118389. [DOI] [PubMed] [Google Scholar]
- 231.Ben-Porath I, Thomson MW, Carey VJ, et al. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat. Genet. 2008;40:499–507. doi: 10.1038/ng.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *232.Mani SA, Guo W, Liao MJ, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *233.Morel AP, Lievre M, Thomas C, Hinkal G, Ansieau S, Puisieux A. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS ONE. 2008;3:e2888. doi: 10.1371/journal.pone.0002888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *234.Shipitsin M, Campbell LL, Argani P, et al. Molecular definition of breast tumor heterogeneity. Cancer Cell. 2007;11:259–273. doi: 10.1016/j.ccr.2007.01.013. Together with References 232 and 233, these studies provide the first evidence linking EMT to the acquisition of “stemness.” Importantly, TGF-β signaling plays a major role in overseeing EMT and the appearance of cancer stem cells.
- 235.Farina AR, Coppa A, Tiberio A, et al. TGF-β1 enhances the invasiveness of human MDA-MB-231 breast cancer cells by up-regulating urokinase activity. Int. J. Cancer. 1998;75:721–730. doi: 10.1002/(sici)1097-0215(19980302)75:5<721::aid-ijc10>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 236.Piek E, Ju WJ, Heyer J, et al. Functional characterization of TGF-β signaling in Smad2- and Smad3-deficient fibroblasts. J. Biol. Chem. 2001;276:19945–19953. doi: 10.1074/jbc.M102382200. [DOI] [PubMed] [Google Scholar]