Abstract
Polymorphisms in the transcription factor interferon regulatory factor 5 (IRF5) are strongly associated in human genetic studies with an increased risk of developing the autoimmune disease systemic lupus erythematosus. However, the biological role of IRF5 in lupus pathogenesis has not previously been tested in an animal model. In this study we show that IRF5 is absolutely required for disease development in the FcγRIIB−/−Yaa and FcγRIIB−/− lupus models. In contrast to IRF5-sufficient FcγRIIB−/−Yaa mice, IRF5-deficient FcγRIIB−/−Yaa mice do not develop lupus manifestations and have a phenotype comparable to wildtype mice. Strikingly, full expression of IRF5 is required for the development of autoimmunity as IRF5-heterozygotes had dramatically reduced disease. One effect of IRF5 is to induce the production of the type I interferon IFN-α, a cytokine implicated in lupus pathogenesis. To address the mechanism by which IRF5 promotes disease, we evaluated FcγRIIB−/−Yaa mice lacking the type I interferon receptor IFNAR1. Unlike the IRF5-deficient and IRF5-heterozygous FcγRIIB−/−Yaa mice, IFNAR1-deficient FcγRIIB−/−Yaa mice maintained a substantial level of residual disease. Furthermore, in FcγRIIB−/− mice lacking Yaa, IRF5-deficiency also markedly reduced disease manifestations indicating that the beneficial effects of IRF5 deficiency in FcγRIIB−/−Yaa mice are not due only to inhibition of the enhanced TLR7 signaling associated with the Yaa mutation. Overall, we demonstrate that IRF5 plays an essential role in lupus pathogenesis in murine models and that this is mediated through pathways beyond that of type I interferon production.
Introduction
SLE is a systemic inflammatory autoimmune disease characterized by the production of autoantibodies and the involvement of various organ systems resulting in appreciable morbidity and mortality. The etiology of SLE is poorly understood with disease resulting from a complex interaction between environmental and genetic factors (1-3). A large number of distinct chromosomal loci show evidence for linkage with disease or disease-related traits in human genetic studies, although it is not yet clear how each contributes to disease pathogenesis (1, 4, 5). Recently, polymorphisms in the transcription factor interferon regulatory factor 5 (IRF5) have been strongly associated in multiple studies with an increased risk of developing SLE (6-9). These polymorphisms are thought to cause the expression of novel IRF5 isoforms (6, 7) and/or an increased level of IRF5 expression by promoting the stability of the IRF5 mRNA or protein (10-12). Individuals possessing particular combinations of these polymorphisms have a greater risk of developing SLE and have higher serum IFNα activity than individuals not possessing these combinations (11, 13).
The precise role of IRF5 in lupus pathogenesis however, still remains incompletely defined. In addition, it is not known to what extent the level of IRF5 expression per se, as opposed to the functional effects of novel IRF5 isoforms, might contribute to disease pathogenesis. One way to address these issues is through the use of animal models where expression levels can be manipulated.
IRF5 is a member of the IRF family that collectively are involved in the regulation of innate immune responses, immune cell development and oncogenesis (14). It is one of a number of transcription factors that participate in signaling cascades downstream of Toll-like receptors TLR3, TLR4, TLR5, TLR7 and TLR9 (15-18). Given that dysregulated TLR7 and TLR9 activation is linked to lupus pathogenesis (19), any effects of IRF5 in lupus could potentially be mediated, at least in part, through modulation of TLR triggered events. IRF5 has also recently been linked to pathways downstream of the retinoic acid inducible gene I (RIG-I) family, a family of proteins that recognize cytoplasmic viral RNA (20).
IRF5 is involved in the production of type I interferon (IFN-α and IFN-β) in response to TLR activation and viral infection (17, 18, 20-22). Given the potential role of type I IFN in SLE pathogenesis (23-25), it has been suggested that the induction of these interferons might be the most important function of IRF5 in the context of SLE (6, 13). However, IRF5 is also involved in the production of pro-inflammatory cytokines such as IL-6 (15, 17, 18), that further contribute to lupus pathogenesis (26). Importantly, the extent of the IRF5 contribution to type I IFN and pro-inflammatory cytokine production is both cell-type and stimulus specific (15-18, 20, 22). IRF5 is also associated with apoptotic pathways in response to viral infection, DNA damage, and Fas-ligand or TRAIL-induced apoptosis and has also been shown to promote cell-cycle arrest (14, 20, 27, 28). Therefore the effects of IRF5 on the pathogenesis of SLE could involve type I IFN induction or IFN-independent pathways (29).
To examine the role of IRF5 in the development of SLE, and its potential functions beyond regulation of type I IFN expression, we have now compared the impact of deficiency of IRF5 and the type I IFN receptor IFNAR1 in the C57BL/6 FcγRIIB−/−Yaa and FcγRIIB−/− models of SLE. FcγRIIB deficiency interacts with a number of C57BL/6-specific genes to induce a spontaneous SLE-like disease, characterized by the presence of autoantibodies against chromatin and the development of lethal glomerulonephritis (30, 31). It has been proposed that this epistatic property of the FcγRIIB−/− B6 model mimics the multigenic nature of human SLE (31). Addition of the Yaa locus to the FcγRIIB−/− B6 model results in a marked increase in severity of the autoimmune disease (31), due to the duplication of TLR7 and other uncharacterized disease-promoting gene(s) (32-35). Therefore we have investigated both the FcγRIIB−/ and the FcγRIIB−/−Yaa models. We found that IRF5 deficiency had a much stronger influence on disease manifestations than IFNAR1 deficiency. Importantly, IRF5 heterozygotes were substantially protected from disease development, thereby demonstrating the pivotal effect of IRF5 expression levels in these lupus models.
Materials and Methods
Mice
IRF5-deficient mice backcrossed eight generations to C57BL/6 were obtained from T. Tanaguchi (University of Tokyo) and T. Mak (University of Toronto) (15). FcγRIIB −/− Yaa mice on a C57BL/6 background were obtained from S. Bolland (National Institute of Allergy and Infectious Diseases) (32). IFNAR1-deficient mice on a C57BL/6 background were obtained from J. Sprent (Garvan Institute of Medical Research) (36). C57BL/6 mice were purchased from Jackson Laboratories. Animal experiments were approved by the Institutional Animal Care and Use Committee at Boston University.
Serological assays
IgG isotypes and anti-ribonucleoprotein (SmRNP) autoantibodies were measured by enzyme-linked immunosorbent assays (ELISA) established using commercially available reagents. Antinuclear autoantibody (ANA) titer was measured by immunofluorescence using HEp-2-coated-slides (Antibodies Inc.). Anti-dsDNA autoantibodies were measured by immunofluorescence analysis of Crithidia lucillae kinetoplast staining (The Binding Site). Serum cytokine levels other than IFN-α were measured by multiplex cytokine analysis (Luminex) at the Baylor Institute for Immunology Research Luminex Core Facility. Serum IFN-α was measured by ELISA (PBL). This ELISA has a sensitivity of 12.5 pg/ml and samples were tested at a 1:4 dilution. Blood urea nitrogen (BUN) levels were measured using a QuantiChrom Urea Assay kit (BioAssay Systems).
Autoantigen arrays
Autoantigen arrays were performed and analyzed as described previously, using a panel of recombinant or native proteins (37). Arrays were probed with sera, and bound antibodies revealed using IgG/IgM-specific secondary antibodies conjugated to fluorophores. The signal intensities obtained were hierarchically clustered by sample based on Pearson correlation with average linkage (38). Significance Analysis of Microarrays (SAM) was performed to identify statistically significant differences between autoantigen reactivities in the experimental groups (39). q values < 0.05 were considered significant. Antigens were ordered by the SAM observed score in descending order. The microarray data has been deposited in the GEO database, accession number GSE17926 (http://www.ncbi.nlm.nih.gov/geo/).
Histology
Hematoxylin and eosin-stained kidney sections were evaluated in a blinded manner. Randomly selected areas of cortex were digitally photographed using a RT color spot camera (Diagnostic Instruments) and the images were recorded using Spot Advanced software version 4.0.9 (Diagnostic Instruments). Crescents were identified by their characteristic appearance and 100 glomeruli from each animal were examined to determine the percentage of glomeruli with crescents. Interstitial disease was semi-quantitatively scored on a scale of 0 to 3 (40). Mean glomerular cell count was determined by computer-assisted image analysis (Adobe Photoshop CS3) of at least 25 equatorially sectioned glomeruli from each mouse.
Immunohistochemistry
Kidneys were snap-frozen in OCT (Tissue-Tek) and stored at −80°C. Eight micrometer cryosections were cut and blocked with 1% donkey serum and then stained with Cy3-conjugated donkey anti-mouse IgG (Jackson) followed by FITC-conjugated goat anti-mouse C3 (Cappel). Stained sections were coded, and then digitally photographed and analyzed in a blinded manner using a fluorescent stereo microscope (Nikon) fitted with a RT color spot camera (Diagnostic Instruments). Fluorescence intensity, representing IgG and C3 deposition, was measured as the mean luminosity in 7-10 glomeruli per mouse (Adobe Photoshop CS3). To obtain the representative glomerular images shown in Fig. 5E, stained sections were digitally photographed using the Nikon TE-2000 inverted epifluorescence microscope fitted with a CoolSnap HQ camera (Photometrics). Z-stack images were deconvolved using NIS Elements (Nikon) with Media Cybernetics deconvolution plugin.
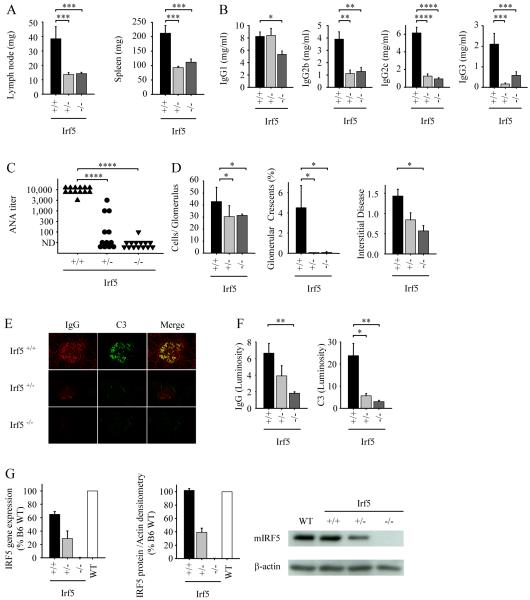
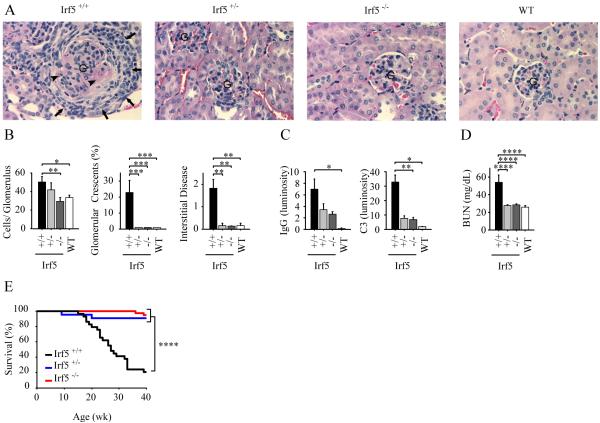
FIGURE 5.
IRF5 deficiency reduces disease manifestations in RII mice lacking Yaa. A-F, All analyses were performed at 8 months of age. A, Lymph node and spleen weights from Irf5+/+ (n = 16), Irf5+/− (n = 16), and Irf5−/− (n=23) RII female mice. B and C, Serum IgG isotype concentrations (B) and serum ANA titers (C) from Irf5+/+ (n = 13), Irf5+/− (n = 12), and Irf5−/− (n=14) RII female mice. D, Quantitation of renal disease in Irf5+/+ (n = 14), Irf5+/− (n = 19), and Irf5−/− (n=21) RII female mice as shown by cell number per glomerulus, percentage of glomeruli with crescents, and interstitial disease score. E and F, Representative examples (E) and quantitation (F) of glomerular IgG and complement C3 deposition measured by fluorescence intensity (luminosity) in Irf5+/+ (n = 6), Irf5+/− (n = 6), and Irf5−/− (n=6) RII female mice. G, IRF5 mRNA (left panel, RT-PCR) and protein (middle panel, Western blot) expression in B220+ splenocytes from 19 wk old Irf5+/+ (n = 3), Irf5+/− (n = 3), and Irf5−/− (n=3) RII female mice and 13 wk old C57BL/6 wildtype mice (WT; n=3). A representative Western blot is shown in the right panel. Data are presented as mean ± SEM. *, p < 0.05; **, p< 0.01; ***, p < 0.001; ****, p < 0.0001 by Mann-Whitney U test.
Flow cytometry
Splenocytes were labeled with monoclonal antibodies (BD Biosciences) specific for CD4, CD8 and pan-Vβ to identify T cells, CD19 and B220 to identify B cells, and CD69 and CD44 to identify activation markers. Immunofluorescence was measured with a FACScan flow cytometer (BD Biosciences) and the data analyzed with FlowJo software (Tree Star). For T cell activation marker expression, where 2 distinct cell populations were observed, the data is expressed as percent of cells positive, with the threshold for positivity set at the trough between the 2 separate populations. For B cell activation marker expression where only a single population was observed, the data is expressed as mean fluorescence intensity (MFI).
Western blot analysis
B220+ cells were purified from the spleens of 19 wk old Irf5+/+, Irf5+/−, and Irf5−/− FcγRIIB−/− female mice and 13 wk old C57BL/6 wildtype female mice using anti-mouse CD45R/B220 magnetic particles (BD Biosciences). Cells were lysed in RIPA buffer (Boston BioProducts) containing protease inhibitors (Calbiochem). Protein concentration was measured using Pierce BCA Protein Assay Kit (Thermo Scientific). 4X sample buffer (Boston BioProducts) was added to the samples which were then denatured at 95°C for 5 minutes. Samples were separated by 10% SDS-PAGE, electroblotted onto a PVDF membrane (Millipore), and IRF5 and β-actin detected using rabbit anti-mouse IRF5 and rabbit anti-β-actin antibodies (both from Cell Signaling). IRF5 and β actin levels were quantitated using Image J (NIH).
Quantification of IRF5, IFIT1 and MX2 gene expression
For IRF5 gene expression, B220+ cells were purified from the spleens of 19 wk old Irf5+/+, Irf5+/−, and Irf5−/− FcγRIIB −/− female mice and 13 wk old C57BL/6 wildtype female mice using anti-mouse CD45R/B220 magnetic particles (BD Biosciences), and total RNA obtained using an RNeasy Micro kit (Qiagen). For IFIT1 and MX2 gene expression, kidneys were isolated from 4-5 month old Irf5+/+ and Irf5−/− FcγRIIB −/− Yaa mice and 4 month old C57BL/6 wildtype mice, homogenized using a Brinkmann Polytron Homogenizer (Brinkmann Instruments), and total RNA obtained using an RNeasy Micro kit (Qiagen). 150 ng of RNA was reverse transcribed into cDNA using SuperScript II Reverse Transcriptase (Invitrogen) and quantitative real time PCR (Applied Biosystems StepOnePlus Instrument and software) using TaqMan probes and primers (Applied Biosystems) was performed to determine the expression levels of IRF5, IFIT1 and MX2 target genes. The Δ-Δ Ct threshold cycle method was used for analysis. All genes of interest were normalized against the house keeping gene GAPDH and changes were expressed as fold change relative to the C57BL/6 wildtype samples (C57BL/6 B220+ splenocytes for IRF5, and C57BL/6 kidney for IFIT1 and MX2).
Statistical analysis
The Kaplan-Meier method was used to analyze the survival studies, and the log-rank test was used for statistical analysis. Two-tailed Mann-Whitney U tests were used for all other analyses. Bonferroni correction for multiple comparisons was performed. p values < 0.05 were considered significant.
Results
IRF5-deficiency abrogates disease in the FcγRIIB −/− Yaa lupus model
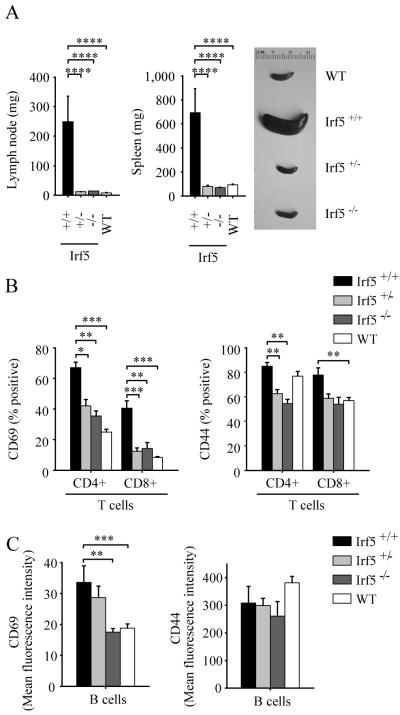
To test the role of IRF5 in the pathogenesis of SLE, we intercrossed IRF5-deficient mice with FcγRIIB −/− Yaa mice to generate the following experimental groups: FcγRIIB −/− Yaa IRF5-sufficient male mice (Irf5+/+ RII.Yaa mice); FcγRIIB −/− Yaa IRF5-heterozygous male mice (Irf5+/− RII.Yaa mice); and FcγRIIB −/− Yaa IRF5-deficient male mice (Irf5−/− RII.Yaa mice). At 5 months of age, we compared disease manifestations in these cohorts, using age- and sex-matched C57BL/6 wildtype mice as controls. Consistent with the previously observed phenotype of FcγRIIB −/− Yaa B6 mice (31), the Irf5+/+ RII.Yaa mice developed massive lymphadenopathy and splenomegaly. This was not observed in the Irf5−/− RII.Yaa mice, which had lymph node and spleen weights similar to those of wildtype mice (Fig. 1A). Expression of the activation markers CD69 and CD44 on splenic T cells was markedly reduced in Irf5−/− RII.Yaa mice compared to Irf5+/+ RII.Yaa mice, due predominantly to a decreased percentage of T cells expressing these activation markers (Fig. 1B). B cell expression of CD69 was also reduced in Irf5−/− RII.Yaa mice, due predominantly to a overall reduction of expression in the total B cell population (Fig. 1C). All four IgG isotypes were elevated in Irf5+/+ RII.Yaa mice compared with wildtype mice, consistent with global B cell activation (Fig. 2A). However, antibody titers were much lower in the Irf5−/− cohort compared to Irf5+/+ RII.Yaa mice. The reduction was particularly striking for IgG2b and IgG2c, where serum concentrations in the Irf5−/− mice were similar to those found in wildtype C57BL/6 mice. Thus, IRF5 expression has the most dramatic effect on those isotypes associated with pathogenic autoantibodies (41).
FIGURE 1.
Lymphadenopathy, splenomegaly and lymphocyte activation is reduced in IRF5-deficient RII.Yaa mice. A, Lymph node and spleen weights from Irf5+/+ (n = 12), Irf5+/− (n = 12), and Irf5−/− (n=14) RII.Yaa mice and wildtype (WT) mice (n=12) were measured at 5 months of age. Representative spleens are shown in panel on right. B and C, CD69 and CD44 expression on splenic T cells (B) and B cells (C) from 5 month old Irf5+/+ (n = 6), Irf5+/− (n = 10), and Irf5−/− (n=9) RII.Yaa mice and wildtype (WT) mice (n=11). Data are presented as mean ± SEM. *, p < 0.05; **, p< 0.01; ***, p < 0.001; ****, p < 0.0001 by Mann-Whitney U test.
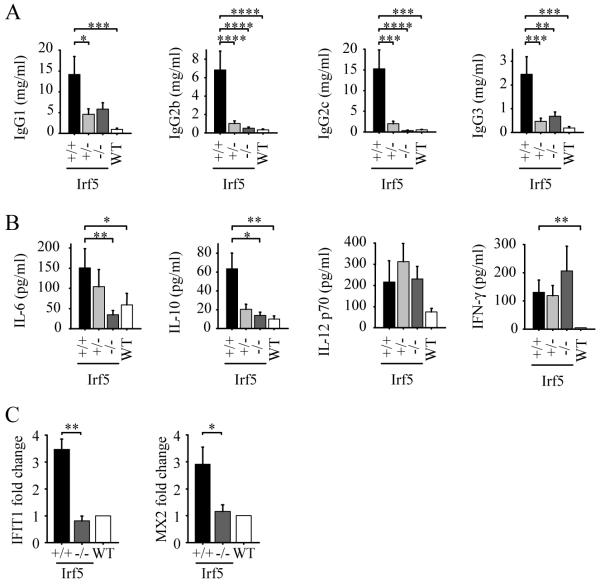
FIGURE 2.
Decreased serum IgG and cytokine levels in IRF5-deficient RII.Yaa mice. A and B, Irf5+/+ (n = 12), Irf5+/− (n = 12), and Irf5−/− (n=14) RII.Yaa mice and wildtype (WT) mice (n=12) were analyzed at 5 months of age. A, Serum IgG isotype concentrations. B, Serum cytokine levels. C, IFIT1 and MX2 mRNA expression in kidneys of 4-5 month old Irf5+/+ (n = 5) and Irf5−/− (n=5) RII.Yaa mice shown as fold change relative to expression in kidneys of 4 month old C57BL/6 wildtype mice. Data are presented as mean ± SEM. *, p < 0.05; **, p< 0.01; ***, p < 0.001; ****, p < 0.0001 by Mann-Whitney U test.
Because IRF5 has been linked to pro-inflammatory cytokine and type I IFN production (15, 21), serum cytokine levels were measured. This analysis revealed a decrease in serum levels of IL-6 and IL-10 from Irf5−/− RII.Yaa mice compared to Irf5+/+ RII.Yaa mice, whereas there were no differences in serum levels of IL-12 p70 and IFN-γ (Fig. 2B). IFN-γ concentrations remained elevated in all FcγRIIB −/− Yaa groups compared to controls. Hence, despite a marked overall reduction in immune cell activation, IRF5-deficiency does not abrogate all components of the autoimmune phenotype. The reduction in IL-6 and IL-10 may be relevant for lupus pathogenesis, as both cytokines contribute to B cell activation and autoantibody production and correlate with disease activity in human studies (26). IFN-α was not detected in sera using an ELISA with a level of sensitivity of 50 pg/ml (data not shown).
Measurement of serum IFN-α by ELISA is, however, not sufficiently quantitative and there is often evidence of induction of type I IFN-induced genes in peripheral blood mononuclear cells of lupus patients in situations where no serum type I IFN is detected by ELISA (42). We therefore measured mRNA expression of the type I IFN-regulated genes IFIT1 and MX2 (42, 43) in B220+ splenocytes and kidney from additional cohorts of Irf5+/+ RII.Yaa, Irf5−/− RII.Yaa and C57BL/6 wildtype mice. No increase in IFIT1 or MX2 expression was seen in B220+ splenocytes from Irf5+/+ or Irf5−/− RII.Yaa mice compared to wildtype mice (data not shown). However, an approximate 3-fold induction of both IFIT1 and MX2 was seen in kidneys from Irf5+/+ RII.Yaa mice compared to wildtype mice, whereas no induction was seen in kidneys from Irf5−/− RII.Yaa mice (Fig. 2C). Thus, there is evidence for IRF5-dependent type I IFN expression in FcγRIIB −/− Yaa mice, albeit at low level and only at a site of severe inflammation.
Autoantibodies directed against nuclear components, in particular DNA/protein or RNA/protein macromolecular complexes, are a diagnostic feature of SLE and contribute to disease pathogenesis (3). As expected, Irf5+/+ RII.Yaa mice produced high titers of anti-nuclear autoantibodies (ANA) as measured by immunofluorescence on HEp2 cells (Fig. 3A). However, ANA were almost totally absent from the sera of Irf5−/− RII.Yaa mice (Fig. 3A) as were antibodies to ribonucleoprotein (SmRNP) (Fig. 3B) and double-stranded DNA (Fig. 3C).
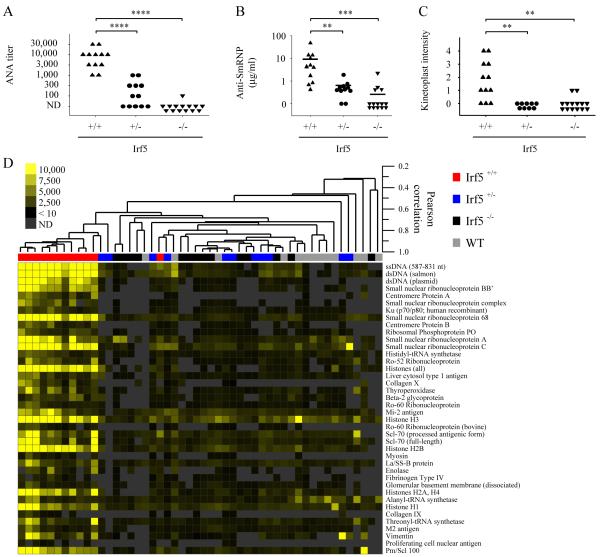
FIGURE 3.
Decreased autoantibody production in IRF5-deficient RII.Yaa mice. Sera from Irf5+/+ (n = 11-12), Irf5+/− (n = 9-12), and Irf5−/− (n = 14) RII.Yaa mice were analyzed at 5 months of age. A, Anti-nuclear autoantibody (ANA) titers; ND, not detected. B, Anti-ribonucleoprotein (Sm/RNP) autoantibody levels. C, Anti-double-stranded DNA autoantibodies determined by kinetoplast staining intensity. Bars represent median values (B). **, p< 0.01; ***, p < 0.001; ****, p < 0.0001 by Mann-Whitney U test. D, Autoantigen array analysis was performed on sera from 5 month old Irf5+/+, Irf5+/− and Irf5−/− RII.Yaa mice and from wildtype (WT) mice. Samples are arranged by hierarchical clustering and displayed as a heat map. Significance Analysis of Microarrays (SAM) identified significant differences between Irf5+/+ lupus mice and the other experimental groups (q < 0.0001, false discovery rate = 0 for all 40 antigens shown). Antigens are ordered by the SAM observed score in descending order.
To extend our analysis to a more comprehensive panel of autoantigens, we analyzed sera from all cohorts with multiplexed autoantigen microarrays composed of a broad panel of autoantigens found in various autoimmune conditions (37). This demonstrated highly significant differences between sera from the Irf5+/+ and Irf5−/− RII.Yaa mice (Fig. 3D). The analysis confirmed the marked reduction in anti-SmRNP and anti-dsDNA autoantibodies in the Irf5−/− RII.Yaa mice shown by ELISA and Crithidia immunofluorescence respectively (Fig. 3, B and C). The analysis further demonstrated a reduction in autoantibodies directed against a variety of other autoantigen targets such as centromere proteins A and B, histones, liver cytosol type 1 antigen, collagen, thyroperoxidase, β-2 glycoprotein I, Mi-2 antigen, and Pm/Scl 100 (Fig. 3D). Despite the previous association of TLR9 and TLR7 with the generation of autoantibodies reactive with DNA- and RNA-associated autoantigens respectively (44), many of these autoantibodies identified by microarray do not bind DNA or RNA macromolecules and are not known to be regulated by TLR7 or TLR9. This indicates either that TLR7 and TLR9 control the production of a greater range of autoantibodies than is currently appreciated, or that the IRF5 regulation of autoantibody production is not simply through its involvement in TLR7 and TLR9 signaling pathways.
Renal disease in human lupus as well as in animal models of the disease is characterized by immune complex deposition and complement activation, with a proliferative glomerulonephritis leading to an increase in glomerular cell number (45). Glomerular crescents and interstitial disease are indicators of more severe renal injury (45). All these features were strongly evident in the Irf5+/+ RII.Yaa mice as expected (31, 34). In contrast, Irf5−/− RII.Yaa mice exhibited a renal phenotype indistinguishable from that of wildtype mice, apart from small amounts of glomerular IgG and complement C3 deposition (Fig. 4, A-C). To evaluate whether these abnormalities in renal pathology were sufficiently severe to cause renal failure, we measured serum levels of blood urea nitrogen (BUN). Normal serum BUN in C57BL/6 mice is less than 30 mg/dl with elevated levels indicating a decrease in renal function (46, 47). Irf5+/+ RII.Yaa mice had high BUN levels (Fig. 4D), similar to those seen in another severe mouse model of lupus, MRL-lpr (48). In contrast, Irf5−/− RII.Yaa mice had BUN levels similar to wildtype C57BL/6 mice (Fig. 4D).
FIGURE 4.
Decreased renal disease and enhanced survival in IRF5-deficient RII.Yaa mice. A and B, Irf5+/+ (n = 12), Irf5+/− (n = 12), and Irf5−/− (n=14) RII.Yaa mice and wildtype (WT) mice (n=12) were analyzed at 5 months of age. A, Representative renal histology. G indicates glomerulus. Arrows indicate cellular crescent. Arrowheads indicate necrotic areas within glomerulus. B, Quantitation of renal disease as shown by cell number per glomerulus, percentage of glomeruli with crescents, and interstitial disease score. C, Glomerular IgG and complement C3 deposition measured by fluorescence intensity (luminosity) in Irf5+/+ (n = 6), Irf5+/− (n = 3), Irf5−/− (n=6) RII.Yaa mice and WT mice (n = 4). D, Serum blood urea nitrogen (BUN) levels in Irf5+/+ (n = 10), Irf5+/− (n = 11), Irf5−/− (n=14) RII.Yaa mice and WT mice (n = 10). Data are presented as mean ± SEM. *, p < 0.05; **, p< 0.01; ***, p < 0.001; ****, p < 0.0001 by Mann-Whitney U test. E, Irf5+/+ (black line, n = 29), Irf5+/− (blue line, n = 22), and Irf5−/− (red line, n=38) RII.Yaa mice were observed until the time of death. ****, p < 0.0001, logrank test.
To determine whether the decrease in disease severity would translate into differences in survival, we bred new cohorts of Irf5+/+ and Irf5−/− RII.Yaa mice and monitored them until the time of death or until they met pre-determined criteria for euthanasia. Irf5+/+ RII.Yaa mice had a median survival of 27 weeks, consistent with previous reports (31) (Fig. 4E). In contrast, more than 90% of mice in the Irf5−/− cohort were alive at the conclusion of the experiment at 40 weeks of age.
IRF5 heterozygote FcγRIIB −/− Yaa mice also develop minimal disease manifestations
Human IRF5 polymorphisms are predicted to modulate expression levels, and therefore Irf5+/− mice were included in our study in order to evaluate the effect of gene dosage. Remarkably, the Irf5+/− RII.Yaa mice exhibited only minimal evidence of disease as documented by the absence of splenomegaly, lymphadenopathy, and lymphocyte activation (Fig. 1, A and B), IgG titers comparable to Irf5−/− RII.Yaa mice (Fig. 2A), and greatly reduced autoantibody production (Fig. 3, A-D). The Irf5+/− RII.Yaa mice developed limited renal disease as detected by increased glomerular cell number, but there was no detectable increase in glomerular crescents, or interstitial disease (Fig. 4, A and B). Moreover, the extent of complement deposition in the Irf5+/− RII.Yaa mice was not significantly greater than that observed in the Irf5−/− mice (Fig. 4C), and Irf5+/− RII.Yaa mice had normal serum BUN levels (Fig. 4D). Notably, the survival rate of the Irf5+/− mice was comparable to that of the Irf5−/− mice at 40 weeks (Fig. 4E). Thus, IRF5 heterozygosity was sufficient to prevent the development of any major clinical phenotype.
IRF5 deficiency also abrogates disease in FcγRIIB −/− mice lacking Yaa
Mice bearing the Yaa mutation have duplication of approximately 17 X-chromosome-specific genes, a number of which may contribute to autoimmunity on the appropriate genetic background (32-35). As IRF5 is known to be involved in signaling cascades downstream of at least one of these genes, TLR7 (16, 17), it was important to determine whether the observed beneficial effects of IRF5 deficiency were mediated predominantly through downregulation of the enhanced function of genes associated with the Yaa mutation. Therefore we evaluated the effect of IRF5 deficiency in female FcγRIIB −/− (RII) mice that lack Yaa but nevertheless develop severe autoimmune disease, albeit at an older age than FcγRIIB −/− Yaa mice (30).
At 8 months of age, Irf5+/+ RII mice exhibited lymphadenopathy and splenomegaly (Fig. 5A), whereas Irf5+/− and Irf5−/− RII mice had lymph node and spleen weights (Fig. 5A) comparable to those of B6 wildtype mice (Fig. 1A). Effects on IgG isotype were similar to those observed in the FcγRIIB −/− Yaa (RII.Yaa) model (Fig. 2A), with Irf5+/− and Irf5−/− RII mice having marked reductions in serum levels of IgG2b, IgG2c and IgG3 as compared with Irf5+/+ RII mice (Fig. 5B). Serum IgG1 levels were only modestly reduced in Irf5−/− RII mice, and no difference in IgG1 levels was seen between the Irf5+/− and Irf5+/+ RII mice, indicating that the effects of IRF5 on IgG production are not due simply to a global inhibition of B cell activation. Strikingly, ANA production was almost completely abolished in Irf5−/− RII mice, and markedly reduced or absent in the Irf5+/− RII mice (Fig. 5C). The greater than 100-fold reduction in ANA titer (Fig. 5C) as compared to the 2 -7 fold reduction in IgG titer (Fig. 5B) in Irf5−/− RII mice suggests that the effect on autoantibody production is at least partly specific and is not purely due to effects on IgG levels. Development of renal disease was also substantially IRF5-dependent, with marked reductions in glomerular hypercellularity, crescent formation, interstitial disease and glomerular IgG and complement deposition observed in the Irf5+/− and Irf5−/− RII mice, although the extent of reduction in renal disease was less complete in the IRF5 heterozygotes (Fig. 5, D-F). Overall, these results demonstrate that IRF5 deficiency markedly abrogates disease in FcγRIIB −/− mice lacking Yaa. This indicates that the beneficial effects of IRF5 deficiency in the FcγRIIB −/− Yaa model are not mediated solely through effects on the enhanced TLR7 signaling resulting from the Yaa mutation.
Given the surprising finding that the IRF5 heterozygous RII and RII.Yaa mice were largely protected from disease development, it was important to measure IRF5 expression levels. We measured IRF5 mRNA and protein in B220+ splenocytes from Irf5+/+, Irf5+/− and Irf5−/− RII mice and wildtype C57BL/6 mice. This demonstrated that IRF5 expression in Irf5+/− RII mice is approximately 40% of that in Irf5+/+ RII mice, with no expression being seen in Irf5−/− RII mice (Fig. 5G). IRF5 protein expression in Irf5+/+ RII mice is similar to that in wildtype C57BL/6 mice. Thus, normal levels of IRF5 are sufficient to promote disease in RII mice, whereas a 60% reduction in IRF5 expression is protective.
IFNAR1 deficiency does not affect autoantibody levels but partially reduces end-organ disease in the FcγRIIB −/− Yaa lupus model
All type I IFNs act through a single cell surface type I IFN receptor, termed IFNAR (49-51). To determine the extent to which the protective effect of IRF5 deficiency was linked to its effects on type I IFN expression, we examined the disease phenotype of FcγRIIB −/− Yaa mice that lacked the IFNAR1 chain of the IFNAR and were therefore unable to respond to type I IFN (36, 52). A similar approach to assessing the role of type I IFN in lupus pathogenesis has been used by other investigators in a number of different mouse lupus models, with variable effects on disease outcome (25).
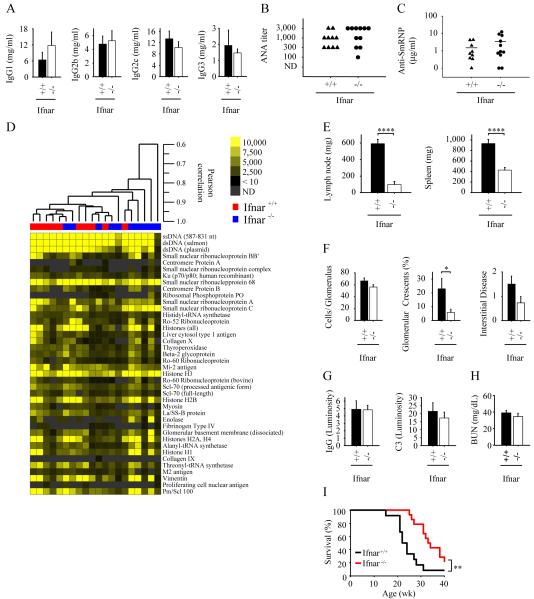
In contrast to the Irf5−/− mice, there were no significant differences in serum levels of IgG isotypes between Ifnar1+/+ and Ifnar1−/− RII.Yaa mice (Fig. 6A). There were also no significant differences in serum ANA titer, anti-SmRNP antibody levels, or autoantigen microarray profiles (Fig. 6, B-D). Nevertheless, both lymph node and spleen sizes were smaller in the Ifnar1−/− RII.Yaa mice relative to the Ifnar1+/+ RII.Yaa mice (Fig. 6E), although spleen size in the Ifnar1−/− RII.Yaa mice (426 ± 50 mg) was larger than in the Irf5−/− RII.Yaa mice (70 ± 3 mg; Fig. 1A; p < 0.0001 for comparison of Ifnar1−/− and Irf5−/−). Renal disease was also less severe in the Ifnar1−/− than in the Ifnar1+/+ RII.Yaa mice as shown by a reduction in glomerular crescent formation (p = 0.04) and a trend toward a reduction in cell number per glomerulus (p = 0.10) and interstitial disease (p = 0.07) (Fig. 6F). Nevertheless, substantial residual renal disease remained in the Ifnar1−/− RII.Yaa mice, with an increase in all these measures of renal injury compared to Irf5−/− RII.Yaa or wildtype C57BL/6 mice (Fig. 4B). Furthermore, the amount of glomerular IgG and complement deposition and the degree of serum BUN elevation was similar in Ifnar1−/− and Ifnar1+/+ RII.Yaa mice (Fig. 6, G and H)
FIGURE 6.
Autommune disease parameters in IFNAR1-deficient RII.Yaa mice. Ifnar1+/+ (n = 10) and Ifnar1−/− (n=11) RII.Yaa mice were analyzed at 5 months of age. A, Serum IgG isotype concentrations. B, Anti-nuclear autoantibody (ANA) titers in serum; ND, not detected. C, Anti-ribonucleoprotein (SmRNP) autoantibody levels in serum. D, Autoantigen array analysis was performed on sera. Samples are arranged with hierarchical clustering and displayed as a heat map. Antigens are ordered using the order defined by the SAM observed score in Fig. 3D. Significance Analysis of Microarrays identified no significant differences between the Ifnar1+/+ and Ifnar1−/− groups. E, Lymph node and spleen weights. F, Quantitation of renal disease as shown by cell number per glomerulus, percentage of glomeruli with crescents, and interstitial disease score. G, Glomerular IgG and complement C3 deposition in Ifnar1+/+ (n = 6) and Ifnar1−/− (n=6) RII.Yaa mice. H, Serum blood urea nitrogen (BUN) levels in Ifnar+/+ (n = 8) and Ifnar−/− (n=10) RII.Yaa mice. Bars represent median values (C). Data are presented as mean ± SEM *, p < 0.05; **, p< 0.01; ****, p < 0.0001 by Mann-Whitney U test. H, Ifnar1+/+ (black line, n = 12) and Ifnar1−/− (red line, n=14) RII.Yaa mice were observed until the time of death. **, p = 0.0043, logrank test.
The effects of IFNAR1 deficiency on survival were also determined. Ifnar1−/− RII.Yaa mice did survive longer than Ifnar1+/+ RII.Yaa mice (Fig. 6I), confirming that the FcγRIIB −/− Yaa model is at least in part type I IFN-dependent. However, Ifnar1−/− RII.Yaa mice (Fig. 6I) did not survive as long as either the Irf5−/− or Irf5+/− RII.Yaa mice (Fig. 4E) (p < 0.0001 and p = 0.00014 respectively). Thus overall, in contrast to IRF5 deficiency or heterozygosity, IFNAR1 deficiency did not affect autoantibody production, and only partially ameliorated end-organ disease.
Discussion
A large number of genes have been associated with SLE in human genetic studies (1, 4, 5), however their biological roles in disease pathogenesis are incompletely understood. In this report we demonstrate that deficiency of a single gene, IRF5, robustly associated with an increased risk of developing human lupus, abrogates disease in the FcγRIIB−/−Yaa and FcγRIIB−/− mouse models of SLE.
The initial reports of the strong association of IRF5 polymorphisms with SLE (6, 7) have now been confirmed in multiple studies in different population groups (9, 11, 12, 53-55). It is not yet clear exactly how these polymorphisms affect IRF5 protein production and function although the polymorphisms are predicted to result in an increased level of IRF5 expression or activity (1, 29). In addition to the polymorphisms that confer risk, there appear to be IRF5 variants that confer protection (11, 12). Human IRF5, unlike mouse IRF5, is expressed in multiple spliced variants and some of these are transcriptionally inactive and may function as dominant negative mutants (18, 56). A genetic model has been proposed of an SLE risk haplotype carrying multiple mutations of IRF5 (1, 11).
We found an unexpectedly strong requirement for IRF5 gene dose in disease pathogenesis. In animal models of SLE, it is common for gene targeted heterozygotes to express a phenotype similar to the wildtype controls, or to express an intermediate phenotype. For example, Tlr9+/− MRL/lpr mice have a survival rate similar to Tlr9+/+ mice, and do not resemble Tlr9−/− mice (44). Similarly, MyD88+/− mice on both the MRL-lpr and 56R+FcγRIIB −/− backgrounds have phenotypes comparable to MyD88+/+ mice, and quite different from their MyD88−/− counterparts (41, 57). In contrast, Irf5+/− mice on both the FcγRIIB −/− Yaa and FcγRIIB −/− backgrounds display a phenotype comparable to Irf5−/− mice and develop only very limited disease manifestations. This demonstrates that IRF5 expression levels are important in regulating disease activity and may help explain how IRF5 polymorphisms that modulate expression levels could increase the risk of developing SLE.
Our findings are consistent with IRF5 contributing to lupus pathogenesis at least in part through its role in TLR signaling. Autoantibodies in SLE are thought to be pathogenic with the predominant autoantigenic targets being protein-nucleic acid complexes, either chromatin or small nuclear ribonucleoproteins (3). In animal models, TLR9 contributes to the development of anti-chromatin autoantibodies and TLR7 to the development of anti-ribonucleoprotein autoantibodies, although they have opposing effects on disease severity with TLR9 deficiency unexpectedly aggravating disease in most models and TLR7 deficiency partially ameliorating disease (41, 44, 58-62). The ultimate effects of TLR7 and TLR9 engagement are mediated through the downstream activation of a number of transcription factors including IRF5, interferon regulatory factor 7 (IRF7), nuclear factor κB (NF-κB) and activator protein 1 (AP-1) (63).
In our study, IRF5-deficient mice did not develop autoantibodies against either chromatin or ribonucleoprotein. The absolute requirement for IRF5 in autoantibody production and overall disease development was unexpected and suggests that IRF5 plays a critical and non-redundant role in TLR7 and TLR9 signaling in SLE. Alternatively, it may be that IRF5 contributes to autoantibody production and disease development through TLR7- and TLR9-independent pathways. This latter possibility would be consistent with our microarray data showing that IRF5-deficiency greatly reduces the production of a wide range of autoantibodies in addition to those directed against RNA- or DNA-associated autoantigens. It will be necessary to explore these alternatives in future studies by, for example, comparing the phenotype of TLR7/9-deficient lupus models with the phenotype of lupus models deficient both in TLR7/9 and IRF5.
IRF5 plays an important role in proinflammatory cytokine production following TLR activation and viral infection (15-18, 20-22). In our study, serum levels of IL-6 and IL-10 were greatly reduced in the IRF5-deficient FcγRIIB −/− Yaa mice. IL-6 has been linked to lupus pathogenesis in both animal models and in human disease (64). Serum levels of IL-10 correlate with disease activity in human lupus (65, 66), and may contribute to pathogenesis through enhancement of B cell autoantibody production (67). In a preliminary study, treatment of lupus patients with an anti-IL-10 monoclonal antibody reduced disease activity (68). Thus IRF5 could contribute to lupus pathogenesis in part through promoting the production of both IL-6 and IL-10.
Type I IFN is thought to play an important role in SLE pathogenesis with the major source of type I IFN derived from plasmacytoid dendritic cells activated through TLR9 and TLR7 by DNA- or RNA-containing immune complexes respectively (23, 25, 69-71). IRF5 was originally identified as a regulator of type I IFN expression in human cell lines (21, 72), a finding confirmed in subsequent human cell line studies (16). IRF5 has also been shown to participate in type I IFN production in murine experimental systems both in vitro and in vivo (17, 18, 20, 73, 74). In addition, high serum IFN-α is a heritable risk factor for human lupus and the IRF5 lupus risk haplotype is associated with higher serum IFNα activity in lupus patients (13, 75). A central issue regarding the mechanism of IRF5 action in SLE is the extent to which induction of type I IFN by IRF5 is responsible for disease pathogenesis.
In our lupus model, we observed a modest protective effect of IFNAR1-deficiency on survival as has been seen in certain other (76-78), but not all (79), lupus mouse models. IRF5 played a role in mediating the effects of type I IFN as the low level expression of the type I IFN-induced genes IFIT1 and MX2 seen in the kidneys of IRF5-sufficient FcγRIIB −/− Yaa mice was not evident in the IRF5-deficient FcγRIIB −/− Yaa mice. However, we observed a far more profound effect of IRF5 deficiency on disease manifestations compared with IFNAR1 deficiency. This does not exclude an important role for IRF5 in the induction of type I IFN in SLE, but it clearly demonstrates the involvement of IRF5 in additional pathogenic signaling cascades independent of type I IFN production, at least in these models. The extent to which these IRF5-mediated type I IFN-independent pathogenic pathways are involved in human lupus remains to be determined, and it is certainly possible that the relative contribution of the type I IFN-dependent pathway may be greater in human lupus than in certain mouse models. Determining the relative contributions of these IRF5-mediated pathways in human lupus will be important, not only in terms of understanding disease pathogenesis but also because it relates directly to therapeutic approaches to treat the disease. If the major role of IRF5 in SLE pathogenesis is through type I IFN production, then inhibition of IRF5 as a therapy would likely not be more efficacious than type I IFN inhibition. However, if the IRF5-mediated type I IFN-independent pathway(s) does play a substantial role, then targeting IRF5 may confer additional therapeutic benefit. Another unresolved issue relating to heritable risk factors for lupus such as high serum IFN-α levels or enhanced IRF5 function, is whether they are involved primarily in disease initiation or whether they also play a role in ongoing disease activity (25). Clinical trials of type I IFN inhibition in SLE are currently in progress and may help to resolve this question as it relates to IFN-α. Our current study examined the role of IRF5 in disease initiation and development, but the role of IRF5 in ongoing disease activity could be addressed in future studies in FcγRIIB −/− Yaa mice by inhibiting IRF5 expression after disease onset.
All type I IFNs act through a single cell surface type I IFN receptor, termed IFNAR (49-51). The IFNAR is comprised of 2 chains designated IFNAR1 and IFNAR2 (51, 80). Ligand-induced cross-linking of IFNAR1 and IFNAR2 induces a pleiotropic cellular response (81). IFNAR1 is necessary for signaling and also participates in ligand binding (52, 80, 81). Studies using IFNAR1-deficient mice have demonstrated that IFNAR1 is essential for responses to multiple IFNα subtypes as well as IFNβ (36, 52). We used IFNAR1-deficient mice to evaluate the contribution of type I IFN to disease development in our model. While it is difficult to definitively exclude the possibility of residual type I IFN signaling in IFNAR1-deficient mice, there is no evidence at present in the literature as far as we are aware that such residual signaling, if present, has a biologically important effect in vivo.
In addition to its role in cytokine production, IRF5 is also associated with apoptosis. IRF5 expression can be induced by the tumor suppressor p53, suggesting a connection between IRF5 and p53-induced pro-apoptotic pathways (82). Like p53, IRF5 stimulates the cyclin-dependent kinase inhibitor p21 while repressing cyclin B1, and stimulates the expression of the proapoptotic genes Bak 1, Bax, caspase 8 and DAP kinase 2 (72, 83). IRF5 promotes cell cycle arrest and apoptosis independently of p53 (84). IRF5 is required for Fas-induced apoptosis in hepatocytes and dendritic cells but not in thymocytes (27), and is required for DNA damage-induced apoptosis in embryonic fibroblasts (20). Given the strong association between dysregulated apoptosis and apoptotic material clearance and SLE (85-87), it is certainly conceivable that IRF5 regulation of apoptotic pathways could contribute to disease pathogenesis in SLE.
In summary, we have shown that IRF5 plays an essential role in disease pathogenesis in the FcγRIIB−/−Yaa and FcγRIIB−/− mouse lupus models. Although IRF5 contributes to type I IFN production in FcγRIIB−/−Yaa mice, it is likely that in this model the major effects of IRF5 are mediated through type I IFN-independent pathways, possibly through inhibition of the production of IL-6 and IL-10. In addition, even IRF5 heterozygous mice are substantially protected from disease development, indicating that a certain threshold level of IRF5 is required for disease development. It will be important to evaluate whether IRF5 deficiency has similar effects in other mouse models of SLE. It will also be particularly important to determine in future studies whether IRF5 is involved in disease onset and/or disease progression and whether manipulation of IRF5 levels can reverse established disease. If IRF5 is involved in disease progression in multiple models and if inhibiting IRF5 can reverse established disease, this would suggest that IRF5 might be a key therapeutic target in lupus, particularly since a partial reduction in the level of IRF5 could have a meaningful effect on disease severity.
Acknowledgments
We thank Mark Shlomchik for helpful discussions and careful reading of the manuscript. We thank Tadatsugu Tanaguchi and Tak Mak for providing the IRF5-deficient mice, Silvia Bolland for providing the FcγRIIB −/− Yaa mice, and Jonathan Sprent for providing the IFNAR1-deficient mice. We thank John Connolly for help with the Luminex assays.
Abbreviations used in this paper
- ANA
anti-nuclear autoantibodies
- IFNAR1
type 1 interferon receptor
- IRF5
interferon regulatory factor 5
- SLE
systemic lupus erythematosus
- Sm/RNP
Smith/ribonucleoprotein
Footnotes
This work was supported by grants from the National Institutes of Health (P01 AR050256 to A.M-R and I.R.R, and R01 AR35230 to A.M-R). C. R. was supported by grants from Société Française de Rhumatologie, CHU de Bordeaux, and Réseau Rhumatologie. T.A. was supported by a Research Training in Nephrology T32 Grant DK07053 from the National Institutes of Health. P.J.U. was supported by NHLBI Proteomics Contract N01-HV-28183, a grant from the Northern California Chapter of the Arthritis Foundation, and a gift from the Floren Family Trust. C.L.L.was supported by NIAMS T32 grants AI07290-23 and AR050942-04 from the National Institutes of Health.
Publisher's Disclaimer: “This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This version of the manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence, it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the U.S. National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org.”
References
- 1.Rhodes B, Vyse TJ. The genetics of SLE: an update in the light of genome-wide association studies. Rheumatology (Oxford) 2008;47:1603–1611. doi: 10.1093/rheumatology/ken247. [DOI] [PubMed] [Google Scholar]
- 2.Simard JF, Costenbader KH. What can epidemiology tell us about systemic lupus erythematosus? Int J Clin Pract. 2007;61:1170–1180. doi: 10.1111/j.1742-1241.2007.01434.x. [DOI] [PubMed] [Google Scholar]
- 3.Rahman A, Isenberg DA. Systemic lupus erythematosus. N Engl J Med. 2008;358:929–939. doi: 10.1056/NEJMra071297. [DOI] [PubMed] [Google Scholar]
- 4.Lauwerys BR, Wakeland EK. Genetics of lupus nephritis. Lupus. 2005;14:2–12. doi: 10.1191/0961203305lu2052oa. [DOI] [PubMed] [Google Scholar]
- 5.Harley IT, Kaufman KM, Langefeld CD, Harley JB, Kelly JA. Genetic susceptibility to SLE: new insights from fine mapping and genome-wide association studies. Nat Rev Genet. 2009;10:285–290. doi: 10.1038/nrg2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sigurdsson S, Nordmark G, Goring HH, Lindroos K, Wiman AC, Sturfelt G, Jonsen A, Rantapaa-Dahlqvist S, Moller B, Kere J, Koskenmies S, Widen E, Eloranta ML, Julkunen H, Kristjansdottir H, Steinsson K, Alm G, Ronnblom L, Syvanen AC. Polymorphisms in the tyrosine kinase 2 and interferon regulatory factor 5 genes are associated with systemic lupus erythematosus. Am J Hum Genet. 2005;76:528–537. doi: 10.1086/428480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graham RR, Kozyrev SV, Baechler EC, Reddy MV, Plenge RM, Bauer JW, Ortmann WA, Koeuth T, Gonzalez Escribano MF, Pons-Estel B, Petri M, Daly M, Gregersen PK, Martin J, Altshuler D, Behrens TW, Alarcon-Riquelme ME. A common haplotype of interferon regulatory factor 5 (IRF5) regulates splicing and expression and is associated with increased risk of systemic lupus erythematosus. Nat Genet. 2006;38:550–555. doi: 10.1038/ng1782. [DOI] [PubMed] [Google Scholar]
- 8.Kelly JA, Kelley JM, Kaufman KM, Kilpatrick J, Bruner GR, Merrill JT, James JA, Frank SG, Reams E, Brown EE, Gibson AW, Marion MC, Langefeld CD, Li QZ, Karp DR, Wakeland EK, Petri M, Ramsey-Goldman R, Reveille JD, Vila LM, Alarcon GS, Kimberly RP, Harley JB, Edberg JC. Interferon regulatory factor-5 is genetically associated with systemic lupus erythematosus in African Americans. Genes Immun. 2008;9:187–194. doi: 10.1038/gene.2008.4. [DOI] [PubMed] [Google Scholar]
- 9.Harley JB, Alarcon-Riquelme ME, Criswell LA, Jacob CO, Kimberly RP, Moser KL, Tsao BP, Vyse TJ, Langefeld CD, Nath SK, Guthridge JM, Cobb BL, Mirel DB, Marion MC, Williams AH, Divers J, Wang W, Frank SG, Namjou B, Gabriel SB, Lee AT, Gregersen PK, Behrens TW, Taylor KE, Fernando M, Zidovetzki R, Gaffney PM, Edberg JC, Rioux JD, Ojwang JO, James JA, Merrill JT, Gilkeson GS, Seldin MF, Yin H, Baechler EC, Li QZ, Wakeland EK, Bruner GR, Kaufman KM, Kelly JA. Genome-wide association scan in women with systemic lupus erythematosus identifies susceptibility variants in ITGAM, PXK, KIAA1542 and other loci. Nat Genet. 2008;40:204–210. doi: 10.1038/ng.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graham DS, Manku H, Wagner S, Reid J, Timms K, Gutin A, Lanchbury JS, Vyse TJ. Association of IRF5 in UK SLE families identifies a variant involved in polyadenylation. Hum. Mol. Genet. 2007;16:579–591. doi: 10.1093/hmg/ddl469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graham RR, Kyogoku C, Sigurdsson S, Vlasova IA, Davies LR, Baechler EC, Plenge RM, Koeuth T, Ortmann WA, Hom G, Bauer JW, Gillett C, Burtt N, Cunninghame Graham DS, Onofrio R, Petri M, Gunnarsson I, Svenungsson E, Ronnblom L, Nordmark G, Gregersen PK, Moser K, Gaffney PM, Criswell LA, Vyse TJ, Syvanen AC, Bohjanen PR, Daly MJ, Behrens TW, Altshuler D. Three functional variants of IFN regulatory factor 5 (IRF5) define risk and protective haplotypes for human lupus. Proc Natl Acad Sci U S A. 2007;104:6758–6763. doi: 10.1073/pnas.0701266104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferreiro-Neira I, Calaza M, Alonso-Perez E, Marchini M, Scorza R, Sebastiani GD, Blanco FJ, Rego I, Pullmann R, Jr., Pullmann R, Kallenberg CG, Bijl M, Skopouli FN, Mavromati M, Migliaresi S, Barizzone N, Ruzickova S, Dostal C, Schmidt RE, Witte T, Papasteriades C, Kappou-Rigatou I, Endreffy E, Kovacs A, Ordi-Ros J, Balada E, Carreira P, Gomez-Reino JJ, Gonzalez A. Opposed independent effects and epistasis in the complex association of IRF5 to SLE. Genes Immun. 2007;8:429–438. doi: 10.1038/sj.gene.6364407. [DOI] [PubMed] [Google Scholar]
- 13.Niewold TB, Kelly JA, Flesch MH, Espinoza LR, Harley JB, Crow MK. Association of the IRF5 risk haplotype with high serum interferon-alpha activity in systemic lupus erythematosus patients. Arthritis Rheum. 2008;58:2481–2487. doi: 10.1002/art.23613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tamura T, Yanai H, Savitsky D, Taniguchi T. The IRF family transcription factors in immunity and oncogenesis. Annu Rev Immunol. 2008;26:535–584. doi: 10.1146/annurev.immunol.26.021607.090400. [DOI] [PubMed] [Google Scholar]
- 15.Takaoka A, Yanai H, Kondo S, Duncan G, Negishi H, Mizutani T, Kano S, Honda K, Ohba Y, Mak TW, Taniguchi T. Integral role of IRF-5 in the gene induction programme activated by Toll-like receptors. Nature. 2005;434:243–249. doi: 10.1038/nature03308. [DOI] [PubMed] [Google Scholar]
- 16.Schoenemeyer A, Barnes BJ, Mancl ME, Latz E, Goutagny N, Pitha PM, Fitzgerald KA, Golenbock DT. The interferon regulatory factor, IRF5, is a central mediator of toll-like receptor 7 signaling. J Biol Chem. 2005;280:17005–17012. doi: 10.1074/jbc.M412584200. [DOI] [PubMed] [Google Scholar]
- 17.Yasuda K, Richez C, Maciaszek JW, Agrawal N, Akira S, Marshak-Rothstein A, Rifkin IR. Murine dendritic cell type I IFN production induced by human IgG-RNA immune complexes is IFN regulatory factor (IRF)5 and IRF7 dependent and is required for IL-6 production. J Immunol. 2007;178:6876–6885. doi: 10.4049/jimmunol.178.11.6876. [DOI] [PubMed] [Google Scholar]
- 18.Paun A, Reinert JT, Jiang Z, Medin C, Balkhi MY, Fitzgerald KA, Pitha PM. Functional characterization of murine interferon regulatory factor 5 (IRF-5) and its role in the innate antiviral response. J Biol Chem. 2008;283:14295–14308. doi: 10.1074/jbc.M800501200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marshak-Rothstein A, Rifkin IR. Immunologically active autoantigens: the role of toll-like receptors in the development of chronic inflammatory disease. Annu Rev Immunol. 2007;25:419–441. doi: 10.1146/annurev.immunol.22.012703.104514. [DOI] [PubMed] [Google Scholar]
- 20.Yanai H, Chen HM, Inuzuka T, Kondo S, Mak TW, Takaoka A, Honda K, Taniguchi T. Role of IFN regulatory factor 5 transcription factor in antiviral immunity and tumor suppression. Proc Natl Acad Sci U S A. 2007;104:3402–3407. doi: 10.1073/pnas.0611559104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barnes BJ, Moore PA, Pitha PM. Virus-specific activation of a novel interferon regulatory factor, IRF-5, results in the induction of distinct interferon alpha genes. J Biol Chem. 2001;276:23382–23390. doi: 10.1074/jbc.M101216200. [DOI] [PubMed] [Google Scholar]
- 22.Barnes BJ, Kellum MJ, Field AE, Pitha PM. Multiple regulatory domains of IRF-5 control activation, cellular localization, and induction of chemokines that mediate recruitment of T lymphocytes. Mol Cell Biol. 2002;22:5721–5740. doi: 10.1128/MCB.22.16.5721-5740.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baechler EC, Gregersen PK, Behrens TW. The emerging role of interferon in human systemic lupus erythematosus. Curr Opin Immunol. 2004;16:801–807. doi: 10.1016/j.coi.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 24.Banchereau J, Pascual V. Type I interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity. 2006;25:383–392. doi: 10.1016/j.immuni.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 25.Theofilopoulos AN, Baccala R, Beutler B, Kono DH. Type I interferons (alpha/beta) in immunity and autoimmunity. Annu Rev Immunol. 2005;23:307–336. doi: 10.1146/annurev.immunol.23.021704.115843. [DOI] [PubMed] [Google Scholar]
- 26.Illei GG, Tackey E, Lapteva L, Lipsky PE. Biomarkers in systemic lupus erythematosus: II. Markers of disease activity. Arthritis Rheum. 2004;50:2048–2065. doi: 10.1002/art.20345. [DOI] [PubMed] [Google Scholar]
- 27.Couzinet A, Tamura K, Chen HM, Nishimura K, Wang Z, Morishita Y, Takeda K, Yagita H, Yanai H, Taniguchi T, Tamura T. A cell-type-specific requirement for IFN regulatory factor 5 (IRF5) in Fas-induced apoptosis. Proc Natl Acad Sci U S A. 2008;105:2556–2561. doi: 10.1073/pnas.0712295105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu G, Barnes BJ. IRF-5 is a mediator of the death receptor-induced apoptotic signaling pathway. J Biol Chem. 2009;284:2767–2777. doi: 10.1074/jbc.M804744200. [DOI] [PubMed] [Google Scholar]
- 29.Kozyrev SV, Alarcon-Riquelme ME. The genetics and biology of Irf5-mediated signaling in lupus. Autoimmunity. 2007;40:591–601. doi: 10.1080/08916930701510905. [DOI] [PubMed] [Google Scholar]
- 30.Bolland S, Ravetch JV. Spontaneous autoimmune disease in Fc(gamma)RIIB-deficient mice results from strain-specific epistasis. Immunity. 2000;13:277–285. doi: 10.1016/s1074-7613(00)00027-3. [DOI] [PubMed] [Google Scholar]
- 31.Bolland S, Yim YS, Tus K, Wakeland EK, Ravetch JV. Genetic modifiers of systemic lupus erythematosus in FcgammaRIIB(−/−) mice. J Exp Med. 2002;195:1167–1174. doi: 10.1084/jem.20020165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pisitkun P, Deane JA, Difilippantonio MJ, Tarasenko T, Satterthwaite AB, Bolland S. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science. 2006;312:1669–1672. doi: 10.1126/science.1124978. [DOI] [PubMed] [Google Scholar]
- 33.Subramanian S, Tus K, Li QZ, Wang A, Tian XH, Zhou J, Liang C, Bartov G, McDaniel LD, Zhou XJ, Schultz RA, Wakeland EK. A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proc Natl Acad Sci U S A. 2006;103:9970–9975. doi: 10.1073/pnas.0603912103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deane JA, Pisitkun P, Barrett RS, Feigenbaum L, Town T, Ward JM, Flavell RA, Bolland S. Control of toll-like receptor 7 expression is essential to restrict autoimmunity and dendritic cell proliferation. Immunity. 2007;27:801–810. doi: 10.1016/j.immuni.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santiago-Raber ML, Kikuchi S, Borel P, Uematsu S, Akira S, Kotzin BL, Izui S. Evidence for genes in addition to Tlr7 in the Yaa translocation linked with acceleration of systemic lupus erythematosus. J Immunol. 2008;181:1556–1562. doi: 10.4049/jimmunol.181.2.1556. [DOI] [PubMed] [Google Scholar]
- 36.Muller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 37.Robinson WH, DiGennaro C, Hueber W, Haab BB, Kamachi M, Dean EJ, Fournel S, Fong D, Genovese MC, de Vegvar HE, Skriner K, Hirschberg DL, Morris RI, Muller S, Pruijn GJ, van Venrooij WJ, Smolen JS, Brown PO, Steinman L, Utz PJ. Autoantigen microarrays for multiplex characterization of autoantibody responses. Nat Med. 2002;8:295–301. doi: 10.1038/nm0302-295. [DOI] [PubMed] [Google Scholar]
- 38.Thibault DL, Chu AD, Graham KL, Balboni I, Lee LY, Kohlmoos C, Landrigan A, Higgins JP, Tibshirani R, Utz PJ. IRF9 and STAT1 are required for IgG autoantibody production and B cell expression of TLR7 in mice. J Clin Invest. 2008;118:1417–1426. doi: 10.1172/JCI30065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bonegio RG, Fuhro R, Wang Z, Valeri CR, Andry C, Salant DJ, Lieberthal W. Rapamycin ameliorates proteinuria-associated tubulointerstitial inflammation and fibrosis in experimental membranous nephropathy. J Am Soc Nephrol. 2005;16:2063–2072. doi: 10.1681/ASN.2004030180. [DOI] [PubMed] [Google Scholar]
- 41.Ehlers M, Fukuyama H, McGaha TL, Aderem A, Ravetch JV. TLR9/MyD88 signaling is required for class switching to pathogenic IgG2a and 2b autoantibodies in SLE. J Exp Med. 2006;203:553–561. doi: 10.1084/jem.20052438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hua J, Kirou K, Lee C, Crow MK. Functional assay of type I interferon in systemic lupus erythematosus plasma and association with anti-RNA binding protein autoantibodies. Arthritis Rheum. 2006;54:1906–1916. doi: 10.1002/art.21890. [DOI] [PubMed] [Google Scholar]
- 43.Seo YJ, Kim GH, Kwak HJ, Nam JS, Lee HJ, Suh SK, Baek KM, Sohn YW, Hong SH. Validation of a HeLa Mx2/Luc Reporter Cell Line for the Quantification of Human Type I Interferons. Pharmacology. 2009;84:135–144. doi: 10.1159/000235158. [DOI] [PubMed] [Google Scholar]
- 44.Christensen SR, Shupe J, Nickerson K, Kashgarian M, Flavell RA, Shlomchik MJ. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity. 2006;25:417–428. doi: 10.1016/j.immuni.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 45.Weening JJ, D’Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, Balow JE, Bruijn JA, Cook T, Ferrario F, Fogo AB, Ginzler EM, Hebert L, Hill G, Hill P, Jennette JC, Kong NC, Lesavre P, Lockshin M, Looi LM, Makino H, Moura LA, Nagata M. The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol. 2004;15:241–250. doi: 10.1097/01.asn.0000108969.21691.5d. [DOI] [PubMed] [Google Scholar]
- 46.Muhlfeld AS, Spencer MW, Hudkins KL, Kirk E, LeBoeuf RC, Alpers CE. Hyperlipidemia aggravates renal disease in B6.ROP Os/+ mice. Kidney Int. 2004;66:1393–1402. doi: 10.1111/j.1523-1755.2004.00854.x. [DOI] [PubMed] [Google Scholar]
- 47.Kimura A, Ishida Y, Wada T, Yokoyama H, Mukaida N, Kondo T. MRP-1 expression levels determine strain-specific susceptibility to sodium arsenic-induced renal injury between C57BL/6 and BALB/c mice. Toxicol Appl Pharmacol. 2005;203:53–61. doi: 10.1016/j.taap.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 48.Kevil CG, Hicks MJ, He X, Zhang J, Ballantyne CM, Raman C, Schoeb TR, Bullard DC. Loss of LFA-1, but not Mac-1, protects MRL/MpJ-Fas(lpr) mice from autoimmune disease. Am J Pathol. 2004;165:609–616. doi: 10.1016/S0002-9440(10)63325-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oritani K, Kincade PW, Zhang C, Tomiyama Y, Matsuzawa Y. Type I interferons and limitin: a comparison of structures, receptors, and functions. Cytokine Growth Factor Rev. 2001;12:337–348. doi: 10.1016/s1359-6101(01)00009-0. [DOI] [PubMed] [Google Scholar]
- 50.Fenner JE, Starr R, Cornish AL, Zhang JG, Metcalf D, Schreiber RD, Sheehan K, Hilton DJ, Alexander WS, Hertzog PJ. Suppressor of cytokine signaling 1 regulates the immune response to infection by a unique inhibition of type I interferon activity. Nat Immunol. 2006;7:33–39. doi: 10.1038/ni1287. [DOI] [PubMed] [Google Scholar]
- 51.Uze G, Schreiber G, Piehler J, Pellegrini S. The receptor of the type I interferon family. Curr Top Microbiol Immunol. 2007;316:71–95. doi: 10.1007/978-3-540-71329-6_5. [DOI] [PubMed] [Google Scholar]
- 52.Hwang SY, Hertzog PJ, Holland KA, Sumarsono SH, Tymms MJ, Hamilton JA, Whitty G, Bertoncello I, Kola I. A null mutation in the gene encoding a type I interferon receptor component eliminates antiproliferative and antiviral responses to interferons alpha and beta and alters macrophage responses. Proc Natl Acad Sci U S A. 1995;92:11284–11288. doi: 10.1073/pnas.92.24.11284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Demirci FY, Manzi S, Ramsey-Goldman R, Minster RL, Kenney M, Shaw PS, Dunlop-Thomas CM, Kao AH, Rhew E, Bontempo F, Kammerer C, Kamboh MI. Association of a common interferon regulatory factor 5 (IRF5) variant with increased risk of systemic lupus erythematosus (SLE) Ann Hum Genet. 2007;71:308–311. doi: 10.1111/j.1469-1809.2006.00336.x. [DOI] [PubMed] [Google Scholar]
- 54.Kozyrev SV, Lewen S, Reddy PM, Pons-Estel B, Witte T, Junker P, Laustrup H, Gutierrez C, Suarez A, Francisca Gonzalez-Escribano M, Martin J, Alarcon-Riquelme ME. Structural insertion/deletion variation in IRF5 is associated with a risk haplotype and defines the precise IRF5 isoforms expressed in systemic lupus erythematosus. Arthritis Rheum. 2007;56:1234–1241. doi: 10.1002/art.22497. [DOI] [PubMed] [Google Scholar]
- 55.Hom G, Graham RR, Modrek B, Taylor KE, Ortmann W, Garnier S, Lee AT, Chung SA, Ferreira RC, Pant PV, Ballinger DG, Kosoy R, Demirci FY, Kamboh MI, Kao AH, Tian C, Gunnarsson I, Bengtsson AA, Rantapaa-Dahlqvist S, Petri M, Manzi S, Seldin MF, Ronnblom L, Syvanen AC, Criswell LA, Gregersen PK, Behrens TW. Association of systemic lupus erythematosus with C8orf13-BLK and ITGAM-ITGAX. N Engl J Med. 2008;358:900–909. doi: 10.1056/NEJMoa0707865. [DOI] [PubMed] [Google Scholar]
- 56.Mancl ME, Hu G, Sangster-Guity N, Olshalsky SL, Hoops K, Fitzgerald-Bocarsly P, Pitha PM, Pinder K, Barnes BJ. Two discrete promoters regulate the alternatively spliced human interferon regulatory factor-5 isoforms. Multiple isoforms with distinct cell type-specific expression, localization, regulation, and function. J Biol Chem. 2005;280:21078–21090. doi: 10.1074/jbc.M500543200. [DOI] [PubMed] [Google Scholar]
- 57.Sadanaga A, Nakashima H, Akahoshi M, Masutani K, Miyake K, Igawa T, Sugiyama N, Niiro H, Harada M. Protection against autoimmune nephritis in MyD88-deficient MRL/lpr mice. Arthritis Rheum. 2007;56:1618–1628. doi: 10.1002/art.22571. [DOI] [PubMed] [Google Scholar]
- 58.Christensen SR, Kashgarian M, Alexopoulou L, Flavell RA, Akira S, Shlomchik MJ. Toll-like receptor 9 controls anti-DNA autoantibody production in murine lupus. J Exp Med. 2005;202:321–331. doi: 10.1084/jem.20050338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lartigue A, Courville P, Auquit I, Francois A, Arnoult C, Tron F, Gilbert D, Musette P. Role of TLR9 in anti-nucleosome and anti-DNA antibody production in lpr mutation-induced murine lupus. J Immunol. 2006;177:1349–1354. doi: 10.4049/jimmunol.177.2.1349. [DOI] [PubMed] [Google Scholar]
- 60.Yu P, Wellmann U, Kunder S, Quintanilla-Martinez L, Jennen L, Dear N, Amann K, Bauer S, Winkler TH, Wagner H. Toll-like receptor 9-independent aggravation of glomerulonephritis in a novel model of SLE. Int Immunol. 2006;18:1211–1219. doi: 10.1093/intimm/dxl067. [DOI] [PubMed] [Google Scholar]
- 61.Berland R, Fernandez L, Kari E, Han JH, Lomakin I, Akira S, Wortis HH, Kearney JF, Ucci AA, Imanishi-Kari T. Toll-like receptor 7-dependent loss of B cell tolerance in pathogenic autoantibody knockin mice. Immunity. 2006;25:429–440. doi: 10.1016/j.immuni.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 62.Savarese E, Steinberg C, Pawar RD, Reindl W, Akira S, Anders HJ, Krug A. Requirement of Toll-like receptor 7 for pristane-induced production of autoantibodies and development of murine lupus nephritis. Arthritis Rheum. 2008;58:1107–1115. doi: 10.1002/art.23407. [DOI] [PubMed] [Google Scholar]
- 63.Kawai T, Akira S. TLR signaling. Semin Immunol. 2007;19:24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 64.Tackey E, Lipsky PE, Illei GG. Rationale for interleukin-6 blockade in systemic lupus erythematosus. Lupus. 2004;13:339–343. doi: 10.1191/0961203304lu1023oa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Houssiau FA, Lefebvre C, Vanden Berghe M, Lambert M, Devogelaer JP, Renauld JC. Serum interleukin 10 titers in systemic lupus erythematosus reflect disease activity. Lupus. 1995;4:393–395. doi: 10.1177/096120339500400510. [DOI] [PubMed] [Google Scholar]
- 66.Park YB, Lee SK, Kim DS, Lee J, Lee CH, Song CH. Elevated interleukin-10 levels correlated with disease activity in systemic lupus erythematosus. Clin Exp Rheumatol. 1998;16:283–288. [PubMed] [Google Scholar]
- 67.Llorente L, Zou W, Levy Y, Richaud-Patin Y, Wijdenes J, Alcocer-Varela J, Morel-Fourrier B, Brouet JC, Alarcon-Segovia D, Galanaud P, Emilie D. Role of interleukin 10 in the B lymphocyte hyperactivity and autoantibody production of human systemic lupus erythematosus. J Exp Med. 1995;181:839–844. doi: 10.1084/jem.181.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Llorente L, Richaud-Patin Y, Garcia-Padilla C, Claret E, Jakez-Ocampo J, Cardiel MH, Alcocer-Varela J, Grangeot-Keros L, Alarcon-Segovia D, Wijdenes J, Galanaud P, Emilie D. Clinical and biologic effects of anti-interleukin-10 monoclonal antibody administration in systemic lupus erythematosus. Arthritis Rheum. 2000;43:1790–1800. doi: 10.1002/1529-0131(200008)43:8<1790::AID-ANR15>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 69.Pascual V, Farkas L, Banchereau J. Systemic lupus erythematosus: all roads lead to type I interferons. Curr Opin Immunol. 2006;18:676–682. doi: 10.1016/j.coi.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 70.Crow MK. Type I interferon in systemic lupus erythematosus. Curr Top Microbiol Immunol. 2007;316:359–386. doi: 10.1007/978-3-540-71329-6_17. [DOI] [PubMed] [Google Scholar]
- 71.Ronnblom L, Alm GV, Eloranta ML. Type I interferon and lupus. Curr Opin Rheumatol. 2009;21:471–477. doi: 10.1097/BOR.0b013e32832e089e. [DOI] [PubMed] [Google Scholar]
- 72.Barnes BJ, Field AE, Pitha-Rowe PM. Virus-induced heterodimer formation between IRF-5 and IRF-7 modulates assembly of the IFNA enhanceosome in vivo and transcriptional activity of IFNA genes. J Biol Chem. 2003;278:16630–16641. doi: 10.1074/jbc.M212609200. [DOI] [PubMed] [Google Scholar]
- 73.Richez C, Yasuda K, Watkins AA, Akira S, Lafyatis R, van Seventer JM, Rifkin IR. TLR4 ligands induce IFN-alpha production by mouse conventional dendritic cells and human monocytes after IFN-beta priming. J Immunol. 2009;182:820–828. doi: 10.4049/jimmunol.182.2.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pandey AK, Yang Y, Jiang Z, Fortune SM, Coulombe F, Behr MA, Fitzgerald KA, Sassetti CM, Kelliher MA. NOD2, RIP2 and IRF5 play a critical role in the type I interferon response to Mycobacterium tuberculosis. PLoS Pathog. 2009;5:e1000500. doi: 10.1371/journal.ppat.1000500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Niewold TB, Hua J, Lehman TJ, Harley JB, Crow MK. High serum IFN-alpha activity is a heritable risk factor for systemic lupus erythematosus. Genes Immun. 2007;8:492–502. doi: 10.1038/sj.gene.6364408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Santiago-Raber ML, Baccala R, Haraldsson KM, Choubey D, Stewart TA, Kono DH, Theofilopoulos AN. Type-I interferon receptor deficiency reduces lupus-like disease in NZB mice. J Exp Med. 2003;197:777–788. doi: 10.1084/jem.20021996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Braun D, Geraldes P, Demengeot J. Type I Interferon controls the onset and severity of autoimmune manifestations in lpr mice. J Autoimmun. 2003;20:15–25. doi: 10.1016/s0896-8411(02)00109-9. [DOI] [PubMed] [Google Scholar]
- 78.Nacionales DC, Kelly-Scumpia KM, Lee PY, Weinstein JS, Lyons R, Sobel E, Satoh M, Reeves WH. Deficiency of the type I interferon receptor protects mice from experimental lupus. Arthritis Rheum. 2007;56:3770–3783. doi: 10.1002/art.23023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hron JD, Peng SL. Type I IFN protects against murine lupus. J Immunol. 2004;173:2134–2142. doi: 10.4049/jimmunol.173.3.2134. [DOI] [PubMed] [Google Scholar]
- 80.de Weerd NA, Samarajiwa SA, Hertzog PJ. Type I interferon receptors: biochemistry and biological functions. J Biol Chem. 2007;282:20053–20057. doi: 10.1074/jbc.R700006200. [DOI] [PubMed] [Google Scholar]
- 81.Lamken P, Gavutis M, Peters I, Van der Heyden J, Uze G, Piehler J. Functional cartography of the ectodomain of the type I interferon receptor subunit ifnar1. J Mol Biol. 2005;350:476–488. doi: 10.1016/j.jmb.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 82.Mori T, Anazawa Y, Iiizumi M, Fukuda S, Nakamura Y, Arakawa H. Identification of the interferon regulatory factor 5 gene (IRF-5) as a direct target for p53. Oncogene. 2002;21:2914–2918. doi: 10.1038/sj.onc.1205459. [DOI] [PubMed] [Google Scholar]
- 83.Paun A, Pitha PM. The IRF family, revisited. Biochimie. 2007;89:744–753. doi: 10.1016/j.biochi.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hu G, Barnes BJ. Interferon regulatory factor-5-regulated pathways as a target for colorectal cancer therapeutics. Expert Rev Anticancer Ther. 2006;6:775–784. doi: 10.1586/14737140.6.5.775. [DOI] [PubMed] [Google Scholar]
- 85.Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–975. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 86.Sheriff A, Gaipl US, Voll RE, Kalden JR, Herrmann M. Apoptosis and systemic lupus erythematosus. Rheum Dis Clin North Am. 2004;30:505–527. doi: 10.1016/j.rdc.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 87.Mahoney JA, Rosen A. Apoptosis and autoimmunity. Curr Opin Immunol. 2005;17:583–588. doi: 10.1016/j.coi.2005.09.018. [DOI] [PubMed] [Google Scholar]