Abstract
OBJECTIVE
Study the effects of exenatide (EXE) plus rosiglitazone (ROSI) on β-cell function and insulin sensitivity using hyperglycemic and euglycemic insulin clamp techniques in participants with type 2 diabetes on metformin.
RESEARCH DESIGN AND METHODS
In this 20-week, randomized, open-label, multicenter study, participants (mean age, 56 ± 10 years; weight, 93 ± 16 kg; A1C, 7.8 ± 0.7%) continued their metformin regimen and received either EXE 10 μg b.i.d. (n = 45), ROSI 4 mg b.i.d. (n = 45), or EXE 10 μg b.i.d. + ROSI 4 mg b.i.d. (n = 47). Seventy-three participants underwent clamp procedures to quantitate insulin secretion and insulin sensitivity.
RESULTS
A1C declined in all groups (P < 0.05), but decreased most with EXE+ROSI (EXE+ROSI, −1.3 ± 0.1%; ROSI, −1.0 ± 0.1%, EXE, −0.9 ± 0.1%; EXE+ROSI vs. EXE or ROSI, P < 0.05). ROSI resulted in weight gain, while EXE and EXE+ROSI resulted in weight loss (EXE, −2.8 ± 0.5 kg; EXE+ROSI, −1.2 ± 0.5 kg; ROSI, + 1.5 ± 0.5 kg; P < 0.05 between and within all groups). At week 20, 1st and 2nd phase insulin secretion was significantly higher in EXE and EXE+ROSI versus ROSI (both P < 0.05). Insulin sensitivity (M value) was significantly higher in EXE+ROSI versus EXE (P = 0.014).
CONCLUSIONS
Therapy with EXE+ROSI offset the weight gain observed with ROSI and elicited an additive effect on glycemic control with significant improvements in β-cell function and insulin sensitivity.
Hyperglycemia in type 2 diabetes is caused by decreased insulin secretion due to progressive β-cell dysfunction, insulin resistance in peripheral tissues, and increased hepatic glucose output (1,2). Clinical questions focus on treatment approaches that may address these multiple defects and delay the progression of the disease. Although thiazolidinediones (TZDs) have been shown to improve β-cell function (1–5), their primary effect is to decrease peripheral insulin resistance (6–10), while biguanides decrease hepatic glucose output (11). Exenatide (EXE), a glucagon-like peptide-1 (GLP-1) receptor agonist, enhances glucose-dependent insulin secretion and suppresses elevated glucagon levels resulting in a decline in hepatic glucose output (12–15). Since biguanides, TZDs, and GLP-1 agonists exert their effects on different pathophysiologic defects, it seems reasonable to combine these agents in the treatment strategy.
To improve our understanding of the metabolic effects of combination therapy targeted at pathophysiologic defects in type 2 diabetes, we designed the present study to quantitate insulin secretion and insulin sensitivity when combining EXE and rosiglitazone (ROSI) versus each therapy alone in patients already on metformin.
RESEARCH DESIGN AND METHODS
Seventeen sites in the U.S. recruited participants with type 2 diabetes from 2006 to 2008. Inclusion criteria included age 18–75 years, BMI 25–40 kg/m2, stable body weight for at least 6 months prior to screening, A1C 6.8–10.0%, stable dose of metformin for at least 6 weeks prior to screening and no treatment with any other antidiabetic medication, and absence of islet cell autoantibodies. The study was approved at each site by a local institutional review board in accordance with the principles described in the Declaration of Helsinki. All participants gave informed written consent before participation.
Experimental design
This was a 20-week, randomized, open-label, comparator-controlled, three-arm, multicenter study. Participants continued their metformin regimen and were randomized and stratified based on study site by computer-generated random sequence to one of three treatment groups: 1) EXE injection 5 μg b.i.d. for the first month and then 10 μg b.i.d. thereafter; 2) ROSI 2 mg b.i.d. for the first month and then 4 mg b.i.d. thereafter; and 3) combination of EXE+ROSI dosed as above. Efficacy measurements included A1C, glucose, insulin, C-peptide, lipids, and body weight. Safety measurements included adverse events, vital signs, hematology, and chemistries. The study was powered to detect a significant difference in the primary and the secondary end points between the EXE +ROSI and ROSI groups. The primary end point of the study was the measurement of glucose-potentiated arginine-stimulated incremental insulin area under the curve (ASI-iAUC) during the hyperglycemic clamp test for which a sample size of 39 would provide 80% power to detect a significant difference of 0.6 in the log-transformed ratio of ASI-iAUC at 20 weeks over baseline. The secondary end point was the glucose area under the curve (AUC) from 15 to 180 min during the meal challenge. An additional 51 participants (N = 90) who underwent only meal challenges would provide 80% power to detect a significant difference of 380 mmol/l per min between EXE+ROSI and ROSI. Eight of the 17 sites recruited subjects to undergo the hyperglycemic and hyperinsulinemic-euglycemic clamp test in addition to the meal tolerance test. Subjects recruited at these clamp sites could participate only by consenting to all procedures.
Standardized meal challenge
Participants underwent a standardized meal challenge test after an overnight fast at baseline and end point. The test consisted of eight ounces of a liquid meal supplement (240 kcal, four g fat, 40 g carbohydrate, 10 g protein) (Boost, Mead Johnson Nutritionals). Plasma glucose, insulin, and C-peptide were measured at −15, 0, 15, 30, 60, 90, 120, 150, and 180 min. At study end, ROSI and/or EXE were administered 15 min prior to meal ingestion.
Hyperglycemic clamp
The hyperglycemic clamp was performed at baseline and end point as described previously (16). Medications were withheld the morning of the procedure. At end point, participants administered study medication 15 min prior to the clamp. At time 0, body weight-adjusted intravenous (IV) bolus of 20% glucose was administered over 10 min to raise the plasma glucose concentration to ∼8.3 mmol/l (150 mg/dl) above baseline. A variable glucose infusion was then adjusted to maintain the targeted glucose level. At 80 min, an IV bolus of 5 g arginine (dissolved in 50 ml) was given over 45 s, and the glucose level was maintained at 8.3 mmol/l (150 mg/dl) above baseline for 30 min.
Hyperinsulinemic-euglycemic clamp
Participants returned within one week after the hyperglycemic clamp (16). Medications were withheld the morning of the procedure. Insulin was given as an IV bolus (0.1 units × kg body weight × desired plasma insulin concentration of 100 mU/l) over 10 min followed by a continuous infusion at 80 mU/min per m2 for 120 min. Plasma glucose concentration was maintained at 5 mmol/l by a variable infusion of 20% glucose.
Statistical analyses
The absolute and incremental AUCs (iAUC) for glucose, insulin, and C-peptide concentrations during the meal challenge and hyperglycemic clamp were calculated by the trapezoid method. For the meal test, the insulinogenic index (I/G) was calculated by the insulin AUC divided by the glucose AUC, the Matsuda whole-body insulin sensitivity index, and the disposition index (I/G × Matsuda was calculated as described by Matsuda and DeFronzo [17]). The M value, measured by insulin-stimulated glucose disposal during the euglycemic clamp, was used to quantify whole-body insulin sensitivity (16). The insulin secretion/insulin resistance (disposition) index from the clamp tests was calculated by the insulin iAUC multiplied by M/I, where “M” is the M value, “I” is the steady-state plasma insulin concentration during the euglycemic clamp, and iAUC is the incremental area under the curve (17).
Statistical analyses were performed by SAS Drug Development (SAS, Cary, NC). Tests were performed with α = 0.05 without adjustment for multiplicities. Unless specified otherwise, all analyses were performed based on the intent-to-treat principle and included participants with a baseline and at least one postbaseline value. The least squares mean (LS mean) ± SE is reported for all continuous variables except for baseline characteristics where mean ± SD is reported. Fisher exact test was used to compare categorical variables. An ANCOVA model with treatment group as a factor and baseline value of the dependent variable as a covariate was used to compare continuous variables without repeated measurements after randomization. A mixed model repeated-measures approach was used to analyze continuous variables with repeated measurements.
RESULTS
Participant characteristics
One hundred and thirty-seven participants were randomized and received EXE (45 randomized, 33 completed), EXE+ROSI (47 randomized, 34 completed), ROSI (45 randomized, 34 completed). Seventy-three participants participated in the clamp studies (EXE, 23 with seven withdrawals; EXE+ROSI, 24 with six withdrawals; ROSI, 23 with seven withdrawals) (supplemental Fig. 1, available in an online appendix at http://care.diabetesjournals.org/cgi/content/full/dc09-1521/DC1). Four participants withdrew before receiving study medication. For the entire population, baseline characteristics were similar between the three groups (mean ± SD): A1C = 7.8 ± 0.7%; age = 56 ± 10 years; BMI = 32.5 ± 4.3 kg/m2; diabetes duration = 4.7 ± 3.7 years; number (%) female = 67 (49%); Caucasian = 84 (61%); Hispanic = 32 (23%); African American = 16 (12%); others = 5 (4%). Corresponding values in the clamp subset were: A1C = 8.0 ± 0.8%; age = 52 ± 9 years; BMI = 32.4 ± 4.2 kg/m2; diabetes duration = 4.7 ± 4.6 years; number (%) female = 27 (54%); Caucasian = 19 (38%); Hispanic = 21 (42%); African American = 8 (16%); others = 2 (4%).
Metabolic parameters
A1C decreased in all groups, but the decrement in EXE+ROSI was significantly greater than with EXE or ROSI alone (Table 1). After 20 weeks, fasting plasma glucose was significantly reduced to a similar extent in all groups; fasting insulin decreased significantly from baseline with ROSI and EXE+ROSI (P < 0.001) but did not change with EXE (Table 1).
Table 1.
Metabolic parameters
| LS Mean ± SEM |
P |
|||||
|---|---|---|---|---|---|---|
| EXE | EXE+ROSI | ROSI | EXE vs. EXE+ROSI | EXE vs. ROSI | EXE+ROSI vs. ROSI | |
| n | 45 | 47 | 45 | |||
| HbA1C (%) | ||||||
| Baseline | 7.8 ± 0.1 | 7.8 ± 0.1 | 7.9 ± 0.1 | |||
| 20 weeks | 7.0 ± 0.1* | 6.6 ± 0.1* | 6.9 ± 0.1* | |||
| Change | −0.9 ± 0.1 | −1.3 ± 0.1 | −1.0 ± 0.1 | 0.016 | 0.720 | 0.039 |
| Weight (kg) | ||||||
| Baseline | 93.0 ± 2.4 | 93.8 ± 2.4 | 91.8 ± 2.4 | |||
| 20 weeks | 89.7 ± 0.5* | 91.3 ± 0.5* | 94.0 ± 0.5* | |||
| Change | −2.8 ± 0.5 | −1.2 ± 0.5 | 1.5 ± 0.5 | 0.038 | <0.001 | <0.001 |
| Fasting glucose (mmol/l) | ||||||
| Baseline | 8.42 ± 0.28 | 8.43 ± 0.27 | 8.48 ± 0.27 | |||
| 20 weeks | 6.98 ± 0.25* | 6.84 ± 0.24* | 6.63 ± 0.25* | |||
| Change | −1.46 ± 0.25 | −1.60 ± 0.24 | −1.80 ± 0.25 | 0.693 | 0.331 | 0.555 |
| Fasting insulin (μIU/ml) | ||||||
| Baseline | 17.9 ± 2.0 | 13.8 ± 2.0 | 16.2 ± 2.0 | |||
| 20 weeks | 16.3 ± 1.2 | 10.2 ± 1.2* | 11.9 ± 1.2* | |||
| Change | 0.2 ± 1.2 | −5.9 ± 1.2 | −4.2 ± 1.2 | <0.001 | 0.011 | 0.316 |
| Total fasting cholesterol (mmol/l) | ||||||
| Baseline | 4.42 ± 0.15 | 4.41 ± 0.14 | 4.62 ± 0.15 | |||
| 20 weeks | 4.33 ± 0.12 | 4.71 ± 0.11* | 4.89 ± 0.12* | |||
| Change | −0.13 ± 0.12 | 0.26 ± 0.11 | 0.44 ± 0.12 | 0.020 | <0.001 | 0.276 |
| Fasting HDL (mmol/l) | ||||||
| Baseline | 1.13 ± 0.05 | 1.17 ± 0.05 | 1.17 ± 0.05 | |||
| 20 weeks | 1.16 ± 0.03 | 1.19 ± 0.03 | 1.20 ± 0.03 | |||
| Change | 0.02 ± 0.03 | 0.05 ± 0.03 | 0.06 ± 0.03 | 0.566 | 0.445 | 0.840 |
| Fasting LDL (mmol/l) | ||||||
| Baseline | 2.59 ± 0.13 | 2.57 ± 0.13 | 2.71 ± 0.13 | |||
| 20 weeks | 2.55 ± 0.10 | 2.69 ± 0.10 | 2.93 ± 0.10* | |||
| Change | −0.05 ± 0.10 | 0.10 ± 0.10 | 0.33 ± 0.10 | 0.308 | 0.008 | 0.096 |
| Fasting triglycerides (mmol/l) | ||||||
| Baseline | 1.77 ± 0.19 | 1.82 ± 0.18 | 2.14 ± 0.18 | |||
| 20 weeks | 1.59 ± 0.17* | 1.94 ± 0.16 | 2.01 ± 0.17 | |||
| Change | −0.34 ± 0.17 | 0.00 ± 0.16 | 0.07 ± 0.17 | 0.140 | 0.079 | 0.752 |
*P < 0.05 from baseline.
Weight increased significantly in the ROSI group and decreased significantly in the EXE and EXE+ROSI groups (EXE and EXE+ROSI vs. ROSI, P < 0.001) (Table 1).
Total cholesterol increased significantly in ROSI and EXE+ROSI and did not change significantly in the EXE group (Table 1). Fasting HDL cholesterol did not change significantly from baseline in any group. Fasting LDL cholesterol increased significantly from baseline with ROSI (P = 0.001). At end point, ROSI had significantly greater fasting LDL than EXE (P = 0.008). Fasting triglycerides declined significantly from baseline with EXE, but the change was not significantly different from other groups (Table 1).
Standardized meal challenge
At 20 weeks, the AUC for glucose (AUCG), insulin (AUCI), and C-peptide (AUCCP) significantly decreased for all treatment groups during the meal challenge (Table 2) (Fig. 1). The AUCG was lower in EXE+ROSI (P = 0.004) and tended to be lower with EXE (P = 0.065) versus ROSI. AUCI was reduced to a greater extent with ROSI versus EXE (P = 0.047), but this did not reach statistical significance for AUCCP. The I/G increased from baseline by 0.82 μIU-min/ml/mmol-min/l in EXE (P = 0.003), by 0.03 μIU-min/ml/mmol-min/l in EXE+ROSI, and decreased from baseline by 0.53 μIU-min/ml/mmol-min/l in ROSI, but these changes were not statistically significant (P = 0.926 and 0.061 for EXE+ROSI and ROSI, respectively) (Table 2). The Matsuda whole-body insulin sensitivity index (17) during the meal challenge was similar in all groups at baseline and increased significantly in all groups at end point (all P < 0.05) (Table 2). At end point the increase in the Matsuda index was greater in EXE+ROSI versus EXE (P = 0.015) (Table 2). The disposition index (I/G × Matsuda) was significantly improved in all groups, but there were no significant differences between groups at end point (Table 2).
Table 2.
Meal challenge and hyperglycemic clamp results
| LS Mean ± SEM |
P |
|||||
|---|---|---|---|---|---|---|
| EXE | EXE+ROSI | ROSI | P value EXE vs. EXE+ROSI | P value EXE vs. ROSI | P value EXE+ROSI vs. ROSI | |
| Meal challenge | ||||||
| n | 33 | 34 | 34 | |||
| Glucose AUC (mmol-min/l) | ||||||
| Baseline | 1,783 ± 60 | 1,800 ± 60 | 1,742 ± 60 | |||
| End point | 1,215 ± 51* | 1,140 ± 50* | 1,349 ± 51* | |||
| Change | −560 ± 51 | −635 ± 50 | −426 ± 51 | 0.296 | 0.065 | 0.004 |
| Insulin AUC (μIU-min/ml) | ||||||
| Baseline | 6,116 ± 723 | 5,203 ± 712 | 6,797 ± 734 | |||
| End point | 5,024 ± 339* | 4,152 ± 336* | 4,050 ± 346* | |||
| Change | −999 ± 339 | −1,871 ± 336 | −1,973 ± 346 | 0.071 | 0.047 | 0.833 |
| C-peptide AUC (nmol-min/l) | ||||||
| Baseline | 333 ± 18 | 330 ± 18 | 342 ± 18 | |||
| End point | 310 ± 13 | 277 ± 13* | 282 ± 13* | |||
| Change | −24 ± 13 | −58 ± 13 | −53 ± 13 | 0.067 | 0.118 | 0.805 |
| I/G Index (AUC) (μIU-min/ml)/(mmol-min/l) | ||||||
| Baseline | 3.64 ± 0.48 | 3.05 ± 0.47 | 4.10 ± 0.48 | |||
| 20 weeks | 4.41 ± 0.27* | 3.61 ± 0.27 | 3.06 ± 0.28 | |||
| Change | 0.82 ± 0.27 | 0.03 ± 0.27 | −0.53 ± 0.28 | 0.041 | 0.001 | 0.160 |
| Matsuda index | ||||||
| Baseline | 4.0 ± 0.6 | 4.4 ± 0.6 | 3.4 ± 0.6 | |||
| 20 weeks | 5.6 ± 0.8* | 8.4 ± 0.8* | 7.1 ± 0.8* | |||
| Change | 1.6 ± 0.8 | 4.4 ± 0.8 | 3.1 ± 0.8 | 0.015 | 0.205 | 0.258 |
| I/G (AUC) × Matsuda | ||||||
| Baseline | 10.8 ± 1.0 | 8.8 ± 1.0 | 10.9 ± 1.0 | |||
| 20 weeks | 17.1 ± 1.4* | 20.4 ± 1.4* | 18.4 ± 1.5* | |||
| Change | 7.0 ± 1.4 | 10.3 ± 1.4 | 8.2 ± 1.5 | 0.111 | 0.550 | 0.325 |
| Hyperglycemic Clamp (μIU-min/ml) | ||||||
| n | 16 | 18 | 16 | |||
| ASI-iAUC | ||||||
| Baseline | 643 ± 107 | 686 ± 104 | 786 ± 114 | |||
| 20 weeks | 1,449 ± 187* | 896 ± 182 | 602 ± 200 | |||
| Change | 747 ± 187 | 195 ± 182 | −100 ± 200 | 0.039 | 0.004 | 0.282 |
| 1st phase iAUC (0–10 min) | ||||||
| Baseline | 6 ± 14 | −10 ± 14 | 23 ± 15 | |||
| 20 weeks | 105 ± 24* | 59 ± 24* | 17 ± 26 | |||
| Change | 99 ± 24 | 54 ± 25 | 12 ± 26 | 0.195 | 0.018 | 0.252 |
| 2nd phase iAUC (10–70 min) | ||||||
| Baseline | 937 ± 291 | 740 ± 282 | 1,125 ± 309 | |||
| 20 weeks | 5,436 ± 833* | 3,422 ± 813* | 487 ± 891 | |||
| Change | 4,513 ± 833 | 2,500 ± 813 | −435 ± 891 | 0.09 | <0.001 | 0.019 |
| 1st and 2nd phase iAUC (0–70 min) | ||||||
| Baseline | 955 ± 306 | 742 ± 297 | 1,162 ± 326 | |||
| 20 weeks | 5,611 ± 862* | 3,527 ± 842* | 503 ± 922 | |||
| Change | 4,671 ± 862 | 2,587 ± 842 | −437 ± 922 | 0.09 | <0.001 | 0.02 |
Data are LS means ± SEM.
*P <0.05 from baseline. Matsuda Index = , where FPG and FPI = fasting plasma glucose and insulin and G̅ = average glucose during the meal challenge.
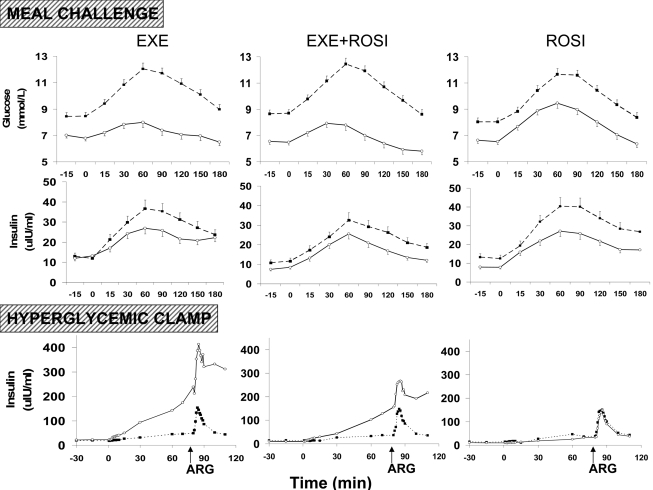
Figure 1.
Glucose and insulin concentrations during the meal challenge test and insulin concentrations during the hyperglycemic clamp before (■, broken line) and after (○, solid line) treatment with EXE, EXE+ROSI, or ROSI. Arrows indicate time of arginine stimulation (ARG) during the hyperglycemic clamp. Data are presented as LS means ± SE for the meal challenge test and LS means for the hyperglycemic clamp.
Insulin secretion, hyperglycemic clamp
In the 50 participants who completed the baseline and end point hyperglycemic clamps (Fig. 1), 1st phase (0–10 min) and 2nd phase (10–70 min) insulin iAUC were increased from baseline with both EXE and EXE+ROSI (both P < 0.05) but not with ROSI (Table 2) (Fig. 1), and the increase in insulin iAUC tended to be greater in EXE versus EXE+ROSI (P = 0.09). ASI-iAUC, a measure of β-cell secretory capacity, was significantly increased with EXE (Table 2). At 20 weeks, ASI-iAUC was significantly higher with EXE versus EXE+ROSI and ROSI (both P < 0.05). C-peptide iAUC results paralleled the insulin iAUC results (data not shown).
Insulin sensitivity, euglycemic insulin clamp
Forty-seven participants completed both baseline and end point euglycemic insulin clamps. EXE+ROSI and ROSI significantly improved the M value at 20 weeks (P < 0.05), while EXE had no significant effect on insulin-stimulated glucose disposal. When M was adjusted for the steady-state plasma insulin concentration during the clamp (M/I), similar results were observed (Fig. 2).
Figure 2.
Disposition index, M value, and M/I index before (□) and after (■) treatment with EXE, EXE+ROSI, and ROSI. *P < 0.05 when compared with baseline, †P < 0.05 between EXE+ROSI and ROSI at baseline. Data are LS means ± SE. BL, baseline; E, exenatide; E+R, exenatide plus rosiglitazone; EP, end point; I, steady-state plasma insulin concentration during the euglycemic clamp; M, insulin-stimulated glucose disposal during the euglycemic clamp; R, rosiglitazone.
β-Cell function
The disposition index, derived from the hyperglycemic and euglycemic insulin clamps, provides the gold standard measure of β-cell function (17). The disposition index from 80–90 min of the hyperglycemic clamp increased significantly and similarly with EXE and EXE+ROSI (both P < 0.001) but not with ROSI (Fig. 2). The disposition index from 0–70 min during the hyperglycemic clamp increased with EXE and EXE+ROSI (both P < 0.001) but not with ROSI.
Safety
The most common adverse events were nausea (EXE 47; EXE+ROSI 47; ROSI 4%), vomiting (EXE 22; EXE+ROSI 19; ROSI 0%), and diarrhea (EXE 7; EXE+ROSI 21; ROSI 4%). Two participants in EXE discontinued due to nausea; two in EXE+ROSI discontinued due to nausea, one due to vomiting, and one due to breast cancer; and one participant in ROSI discontinued due to peripheral edema. Pedal edema occurred in 21 (47%) of ROSI participants versus eight (18%) treated with EXE (P = 0.007). Fourteen participants (30%) in EXE+ROSI developed pedal edema (not significant vs. EXE and vs. ROSI). The occurrence of hypoglycemia (defined as signs or symptoms associated with hypoglycemia and with a glucose meter reading of <3.0 mmol/l) was not significantly different among EXE (n = 2), EXE+ROSI (n = 2), and ROSI (n = 0). One participant treated with EXE+ROSI reported severe hypoglycemia, defined as requiring the assistance of another person and associated with a glucose meter reading of <2.84 mmol/l.
CONCLUSIONS
Abnormalities in both insulin action and insulin secretion occur early in the pathogenesis of diabetes (1,2,18–24). Therefore, treatment of type 2 diabetes should be initiated early and target these pathogenic mechanisms in order to improve β-cell function and ameliorate the underlying insulin resistance.
This is the first study to examine the metabolic effects of combined TZD (ROSI) and GLP-1 (EXE) therapy in inadequately controlled (mean A1C = 7.8 ± 0.7%) metformin-treated patients. Limitations include the open label design and, despite randomization, a slightly lower baseline M value in the EXE+ROSI group. As per study design, study medications were given prior to the procedures and although this prevented discrimination between the acute and chronic effects of these therapies, the primary aim was to study the effects of these therapies as used in general practice.
The incidence of gastrointestinal side effects was higher in subjects treated with ESE and pedal edema was more common in those on ROSI. While the overall percentage of subjects withdrawing from the study due to adverse events was higher in the EXE+ROSI group, there were no statistically significant differences between treatments in withdrawal rates due to adverse events (EXE, two [4%]; EXE+ROSI, five [11%]; ROSI, one [2%], P > 0.05 between all groups).
The study employs the gold standard measurements of insulin resistance (euglycemic insulin clamp) and β-cell function (disposition index) and demonstrates that EXE has a major effect to improve β-cell function but does not exert any significant insulin–sensitizing action as determined by the M value during the insulin clamp. Consistent with recently published results (25), EXE treatment markedly improved both 1st and 2nd phase insulin secretion (Table 2) (Fig. 1); glucose-potentiated, arginine-stimulated insulin secretion increased more than twofold following EXE therapy. The disposition index during the hyperglycemic clamp (0–10, 10–70, and response to arginine stimulation) also increased dramatically. These favorable effects of EXE on β-cell function also were observed during the meal tolerance test (I/G × Matsuda index), demonstrating the physiologic relevance of the observations. Further, EXE significantly reduced weight, A1C (−0.9%), and plasma triglyceride concentrations (−0.34 mmol/l).
ROSI treatment for 20 weeks reduced A1C (−1.0%) similarly to EXE, but did so by different mechanisms. TZD caused a twofold increase in insulin sensitivity, measured as M/I during the euglycemic insulin clamp or the Matsuda index during the meal tolerance test. The improvement in insulin sensitivity was associated with the significant reduction in the insulin response during the meal tolerance test. Despite the reductions in insulin response, the disposition index during the meal tolerance test increased significantly (Table 2). Thus, in addition to its insulin sensitizing effect, ROSI also improved β-cell function despite modest weight gain, which was consistent with previously published results (9).
In metformin-treated patients, combination EXE-ROSI therapy reduced A1C (▵ = −1.3%) to a greater extent than either EXE alone (▵ = −0.9%) or ROSI alone (▵ = −1.0%). This greater reduction in A1C primarily was accounted for by a greater reduction in postprandial plasma glucose excursion after the meal (Table 2) with a similar decrement in fasting plasma glucose (Table 1). The beneficial effect of EXE+ROSI on A1C was due to two factors 1) a significantly greater improvement in insulin sensitivity (M/I during the insulin clamp) (Fig. 2) and 2) a significant improvement in β-cell function as measured by the disposition index. Although the amount of insulin secreted in response to glucose alone or with arginine during the hyperglycemic clamp was markedly reduced in the group receiving combination therapy compared with EXE alone, the disposition index of β-cell function was similar in the EXE and EXE+ROSI groups. This indicates that combination therapy improves insulin secretion as a function of insulin sensitivity. These improvements in glucose metabolism were accompanied by a decrease in weight in contrast to the weight gain observed with ROSI alone.
Although studies of longer duration and with a larger number of subjects will be necessary to examine the long-term effects of combination therapy with EXE plus a TZD in type 2 diabetic patients inadequately controlled on metformin, the present results indicate that this combination improves both insulin resistance and the defect in insulin secretion.
Supplementary Material
Acknowledgments
R.A.D. has received grants from Bristol Myers Squibb, Amylin, Eli Lilly, Novartis, Pfizer, Takeda, Roche, and Merck; participates in the speaker's bureau for Eli Lilly, Novartis, and Takeda; and is on the advisory board for Bristol Myers Squibb, Amylin, Eli Lilly, Novartis, Pfizer, Takeda, Roche, Merck, and Johnson & Johnson. C.T. participates in the speaker's bureau for Amylin and Eli Lilly and is on the advisory board for Amylin. Y.Q. and L.C.G. are employees of Lilly. M.S.L. is an employee of Lilly USA. D.M. is an employee of Amylin. No other potential conflicts of interest relevant to this article were reported.
Parts of this study were presented in poster form at the 69th Scientific Sessions of the American Diabetes Association, New Orleans, Louisiana, 5–9 June 2009.
We thank Dr. Mark Hartman and Dr. John Holcombe for their careful scientific review of the manuscript, Rebecca Wolfe and Eric Meskimen for coordination of the study, Ying Guo for statistical analysis support, and Lorrie Albarado for administrative support.
Footnotes
Clinical trial reg. no. NCT00135330, clinicaltrials.gov.
A complete list of participating investigators is available in an online appendix at http://care.diabetesjournals.org/cgi/content/full/dc09-1521/DC1.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Reaven GM: Banting Lecture 1988: Role of insulin resistance in human disease. Diabetes 1988; 37: 1595– 1607 [DOI] [PubMed] [Google Scholar]
- 2.Wajchenberg BL: Beta-cell failure in diabetes and preservation by clinical treatment. Endocr Rev 2007; 28: 187– 218 [DOI] [PubMed] [Google Scholar]
- 3.Buchanan TA, Xiang AH, Peters RK, Kjos SL, Marroquin A, Goico J, Ochoa C, Tan S, Berkowitz K, Hodis HN, Azen SP: Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk Hispanic women. Diabetes 2002; 51: 2796– 2803 [DOI] [PubMed] [Google Scholar]
- 4.Gastaldelli A, Ferrannini E, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA: Thiazolidinediones improve beta-cell function in type 2 diabetic patients. Am J Physiol Endocrinol Metab 2007; 292: E871– E883 [DOI] [PubMed] [Google Scholar]
- 5.Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O'Neill MC, Zinman B, Viberti G: ADOPT Study Group. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006; 355: 2427– 2443 [DOI] [PubMed] [Google Scholar]
- 6.Bajaj M, Suraamornkul S, Piper P, Hardies LJ, Glass L, Cersosimo E, Pratipanawatr T, Miyazaki Y, DeFronzo RA: Decreased plasma adiponectin concentrations are closely related to hepatic fat content and hepatic insulin resistance in pioglitazone-treated type 2 diabetic patients. J Clin Endocrinol Metab 2004; 89: 200– 206 [DOI] [PubMed] [Google Scholar]
- 7.Bajaj M, Suraamornkul S, Romanelli A, Cline GW, Mandarino LJ, Shulman GI, DeFronzo RA: Effect of a sustained reduction in plasma free fatty acid concentration on intramuscular long-chain fatty Acyl-CoAs and insulin action in type 2 diabetic patients. Diabetes 2005; 54: 3148– 3153 [DOI] [PubMed] [Google Scholar]
- 8.DeFronzo RA: Pharmacologic therapy for type 2 diabetes mellitus. Ann Intern Med 1999; 131: 281– 303 [DOI] [PubMed] [Google Scholar]
- 9.Miyazaki Y, Glass L, Triplitt C, Matsuda M, Cusi K, Mahankali A, Mahankali S, Mandarino LJ, DeFronzo RA: Effect of rosiglitazone on glucose and non-esterified fatty acid metabolism in type II diabetic patients. Diabetologia 2001; 44: 2210– 2219 [DOI] [PubMed] [Google Scholar]
- 10.Miyazaki Y, He H, Mandarino LJ, DeFronzo RA: Rosiglitazone improves downstream insulin receptor signaling in type 2 diabetic patients. Diabetes 2003; 52: 1943– 1950 [DOI] [PubMed] [Google Scholar]
- 11.DeFronzo RA, Barzilai N, Simonson DC: Mechanism of metformin action in obese and lean noninsulin-dependent diabetic subjects. J Clin Endocrinol Metab 1991; 73: 1294– 1301 [DOI] [PubMed] [Google Scholar]
- 12.Vilsbøll T: On the role of the incretin hormones GIP and GLP-1 in the pathogenesis of type 2 diabetes mellitus. Dan Med Bull 2004; 51: 364– 370 [PubMed] [Google Scholar]
- 13.Amylin Pharmaceuticals. Byetta (exenatide) injection [package insert]. San Diego, CA: 2008ref type: generic [Google Scholar]
- 14.Egan JM, Clocquet AR, Elahi D: The insulinotropic effect of acute exendin-4 administered to humans: comparison of nondiabetic state to type 2 diabetes. J Clin Endocrinol Metab 2002; 87: 1282– 1290 [DOI] [PubMed] [Google Scholar]
- 15.Vilsbøll T, Toft-Nielsen MB, Krarup T, Madsbad S, Dinesen B, Holst JJ: Evaluation of beta-cell secretory capacity using glucagon-like peptide 1. Diabetes Care 2000; 23: 807– 812 [DOI] [PubMed] [Google Scholar]
- 16.DeFronzo RA, Tobin JD, Andres R: Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979; 237: E214– E223 [DOI] [PubMed] [Google Scholar]
- 17.Matsuda M, DeFronzo RA: Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999; 22: 1462– 1470 [DOI] [PubMed] [Google Scholar]
- 18.Defronzo RA: Banting Lecture 2009: From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009; 58: 773– 795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeFronzo RA: Lilly Lecture 1987: The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes 1988; 37: 667– 687 [DOI] [PubMed] [Google Scholar]
- 20.Gastaldelli A, Ferrannini E, Miyazaki Y, Matsuda M, DeFronzo RA: San Antonio metabolism study. Beta-cell dysfunction and glucose intolerance: results from the San Antonio metabolism (SAM) study. Diabetologia 2004; 47: 31– 39 [DOI] [PubMed] [Google Scholar]
- 21.Abdul-Ghani MA, Jenkinson CP, Richardson DK, Tripathy D, DeFronzo RA: Insulin secretion and action in subjects with impaired fasting glucose and impaired glucose tolerance: results from the Veterans Administration Genetic Epidemiology Study. Diabetes 2006; 55: 1430– 1435 [DOI] [PubMed] [Google Scholar]
- 22.Weyer C, Bogardus C, Mott DM, Pratley RE: The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest 1999; 104: 787– 794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferrannini E, Gastaldelli A, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA: Beta-cell function in subjects spanning the range from normal glucose tolerance to overt diabetes: a new analysis. J Clin Endocrinol Metab 2005; 90: 493– 500 [DOI] [PubMed] [Google Scholar]
- 24.Jallut D, Golay A, Munger R, Frascarolo P, Schutz Y, Jéquier E, Felber JP: Impaired glucose tolerance and diabetes in obesity: a 6-year follow-up study of glucose metabolism. Metabolism 1990; 39: 1068– 1075 [DOI] [PubMed] [Google Scholar]
- 25.Bunck MC, Diamant M, Cornér A, Eliasson B, Malloy JL, Shaginian RM, Deng W, Kendall DM, Taskinen MR, Smith U, Yki-Järvinen H, Heine RJ: One-year treatment with exenatide improves beta-cell function, compared with insulin glargine, in metformin-treated type 2 diabetes patients: a randomized, controlled trial. Diabetes Care 2009; 32: 762– 768 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.