Abstract
The natural killer cell receptor KIR3DS1 is associated with improved outcome in malignancies, infections, and autoimmune diseases, but data for the impact of KIR3DS1 in HSCT are inconsistent. Using genomic DNA from the National Marrow Donor Program, we performed donor KIR genotyping for 1087 patients who received an unrelated hematopoietic stem cell transplantation. A total of 33% of donors were KIR3DS1+. Compared with KIR3DS1− donors, donor KIR3DS1 was associated with lower-grade II-IV acute graft-versus-host disease (GVHD; odds ratio = 0.71; 95% confidence interval, 0.55-0.92; P = .009), but not with relapse (hazard ratio = 0.97; 95% confidence interval, 0.73-1.29; P = .82). Furthermore, grade II-IV acute GVHD, overall mortality, and transplantation-related mortality all decreased as the number of copies of donor KIR3DS1 increased (P = .007, P = .03, and P = .02, respectively), with the lowest failure rate occurring among patients homozygous for donor KIR3DS1. Selection of donors with KIR3DS1 may decrease acute GVHD without compromising relapse-free survival, separating the graft-versus-tumor effect from unwanted GVHD.
Introduction
In allogeneic hematopoietic stem cell transplantation (HSCT), natural killer (NK) cells enhance the graft-versus-tumor effect, promote bone marrow engraftment, suppress viral reactivation, and prevent graft-versus-host disease (GVHD).1 Optimizing NK-cell alloreactivity, therefore, may ultimately favor the desired graft-versus-tumor effect while minimizing the complication of GVHD. With recent advancements demonstrating a link between NK-cell function and NK-cell genetics,2,3 donor genotyping may be useful for predicting NK activity and for selecting stem cell donors for maximum NK benefit. Identification of the specific genes impacting the salutary effects may facilitate donor selection while offering insight to their biologic functions.
The killer Ig-like receptor (KIR) gene locus is a highly polymorphic family of genes encoding activating and inhibitory cell surface receptors expressed on NK cells and a subset of T cells. Previous genetic association studies have identified individual activating KIR, KIR haplotypes, and combinations of inhibitory KIR and their cognate human leukocyte antigen (HLA) class I ligands to be significantly associated with clinical outcomes after HSCT.4–8 Studies evaluating activating KIR and GVHD have been conflicting.9–13 To examine the role of activating KIR against GVHD, we evaluated a large cohort of HSCT donor-recipient pairs, hypothesizing that the presence of donor-activating KIR might lead to a lower threshold for NK activation, less GVHD, and improved survival for HSCT recipients.
Methods
The Center for International Blood and Marrow Transplant Research (CIBMTR) provided clinical data, HLA genotyping, and donor cell lines or genomic DNA for 1087 stem cell donors from 10 of 10 (62%) or 9 of 10 (30% HLA-C; 8% HLA-B) HLA allele-matched unrelated myeloablative transplantations performed from 1995 to 2002 for acute myeloid leukemia (AML; n = 306)/myelodysplastic syndrome (MDS; n = 154), chronic myelogenous leukemia (n = 390), and acute lymphoblastic leukemia (n = 237) patients with a median age of 36 years (range, 0.6-65.9 years). Donor KIR genotyping was performed using previously described methods.14 KIR3DS1+ and KIR3DL1+ genotyping defined KIR3DS1 heterozygosity; KIR3DS1+ and KIR3DL1− genotyping defined homozygosity and was confirmed by haplotype assignment. Cyclosporine A was used for GVHD prophylaxis in 70% of cases. A total of 97% of allografts were of bone marrow origin, and 19% were T-cell depleted. The median follow-up for all survivors is 7.1 years. Cox regression was used to examine the association between KIR and HSCT outcome. Two-sided P values were derived from adjusted regression models and were estimated using the Wald test. Donor KIR3DS1 was modeled as both a binary variable (positive vs negative) and a continuous linear variable to test for a KIR3DS1 dose effect via a trend test. No adjustments were made for multiple comparisons. Models were adjusted for age, disease severity, donor/patient sex, T-cell depletion, stem cell source, conditioning, and presence of HLA mismatch. All patients and donors provided informed consent in accordance with the Declaration of Helsinki, and all studies were approved through the CIBMTR institutional review boards.
Results and discussion
Among the 1087 donors, 33% (n = 362) were KIR3DS1+, consistent with the gene frequency in the white population.5 There were no statistically significant differences between the KIR3DS1+/− groups with respect to disease, disease status, age, GVHD prophylaxis, degree of HLA matching, patient/donor gender or ethnicity, graft type, and cytomegalovirus serostatus (Table 1). The presence of donor KIR3DS1 was associated with a statistically significant decrease in grade II-IV acute GVHD (odds ratio [OR] = 0.71; 95% confidence interval [CI], 0.55-0.92; P = .009) and a suggestion of decreased transplantation-related mortality (TRM; hazard ratio [HR] = 0.85; 95% CI, 0.70-1.02,P = .08), leading to a trend for reduced overall mortality (OM; HR = 0.86; 95% CI, 0.73-1.01, P = .07). Because there was a suggestion that the effect of donor KIR3DS1 was different among patients with MDS (P = .20 grade II-IV acute GVHD, P = .04 TRM, P = .11 OM), we examined the associations of KIR3DS1 with outcomes within each separate disease group. Among patients with diagnoses other than MDS, the presence of donor KIR3DS1 was significantly associated with lower TRM (HR = 0.77; 95% CI, 0.63-0.95, P = .01), lower OM (HR = 0.81; 95% CI, 0.68-0.96, P = .02), and less grade II-IV acute GVHD (OR = 0.66; 95% CI, 0.50-0.88 P = .004). As summarized in supplemental Table 1 (available on the Blood website; see the Supplemental Materials link at the top of the online article), the effect was largest among patients with AML, consistent with prior studies demonstrating a disease bias in KIR-driven NK effects in HSCT.8,15
Table 1.
Patient and transplantation characteristics
| Donor KIR genotype |
P* | ||
|---|---|---|---|
| KIR3DS1 present, % | KIR3DS1 absent, % | ||
| No. of patients | 362 (33) | 725 (67) | |
| Disease | .38 | ||
| AML | 90 (25) | 216 (30) | |
| MDS | 54 (15) | 100 (14) | |
| Chronic myelogenous leukemia | 138 (38) | 252 (35) | |
| Acute lymphoblastic leukemia | 80 (22) | 157 (22) | |
| Median age, y (range) | 37.5 (0.8-60.8) | 35.3 (0.6-65.9) | .19 |
| Disease status | .38 | ||
| Early | 94 (26) | 187 (26) | |
| Intermediate | 185 (47) | 345 (45) | |
| Advanced | 83 (23) | 193 (27) | |
| HLA match | .70 | ||
| 10/10 | 219 (60) | 451 (62) | |
| 9/10; B-mismatch | 27 (7) | 59 (8) | |
| 9/10; C-mismatch | 116 (32) | 215 (30) | |
| GVHD prophylaxis | .78 | ||
| Cyclosporine A | 252 (70) | 499 (69) | |
| Non–cyclosporine A | 42 (12) | 78 (11) | |
| T-cell depletion | 68 (19) | 148 (20) | |
| Patient/donor sex | .86 | ||
| Male/male | 144 (40) | 279 (38) | |
| Male/female | 58 (16) | 127 (18) | |
| Female/male | 89 (25) | 186 (26) | |
| Female/female | 71 (20) | 133 (18) | |
| Patient/donor ethnicity | .51 | ||
| White | 332 (92) | 673 (93) | |
| Nonwhite | 30 (8) | 52 (17) | |
| Graft type | .91 | ||
| Bone marrow | 350 (97) | 700 (97) | |
| Peripheral blood | 12 (3) | 25 (3) | |
| CMV serostatus | .75 | ||
| Donor+/recipient+ | 67 (19) | 116 (16) | |
| Donor+/recipient− | 103 (28) | 212 (29) | |
| Donor−/recipient− | 130 (36) | 271 (37) | |
| Donor−/recipient+ | 59 (16) | 123 (17) | |
| Missing | 3 (1) | 3 (< 1) | |
AML indicates acute myeloid leukemia; MDS, myelodysplastic syndrome; HLA, human leukocyte antigen; GVHD, graft-versus-host disease; and CMV, cytomegalovirus.
P values by χ2 test, except for age (2-sample t test).
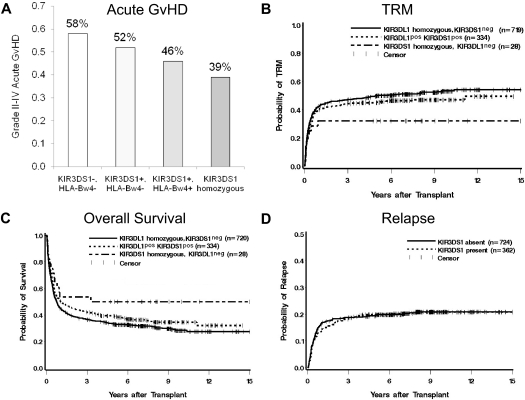
Lending support to the association with improved outcome, we found a dose effect for donor KIR3DS1: as the copy number of donor KIR3DS1 increased from 0 to 2, grade II-IV acute GVHD, OM, and TRM decreased. Specifically, the hazard of GVHD among patients with 1 donor copy (heterozygous) or 2 donor copies (homozygous) of KIR3DS1 was progressively lower than that among patients with 0 donor copies (OR = 0.75, OR = 0.46, trend test P = .007; Figure 1A). This progressive reduction was similarly seen for the hazards of TRM and OM (HR = 0.87, HR = 0.52 for TRM, P = .02, Figure 1B; HR = 0.88, HR = 0.63 for OM, P = .03, Figure 1C). The risk of relapse was similar between groups with and without donor KIR3DS1 (HR = 0.97; 95% CI, 0.73-1.29, P = .82; Figure 1D); there was no effect of KIR3DS1 on grade III-IV acute GVHD (OR = 1.01; 95% CI, 0.75-1.37, P = .95) or chronic GVHD (HR = 0.94; 95% CI, 0.76-1.15, P = .55).
Figure 1.
Influence of donor KIR3DS1 on HSCT outcomes. (A) Incidence of grade II-IV acute GVHD (aGVHD) based on donor KIR3DS1 copy number and presence/absence of recipient HLA-Bw4 KIR epitope. Grade II-IV aGVHD was lower among HLA-Bw4+ recipients with a KIR3DS1+ donor. Donor KIR3DS1 homozygosity is associated with the lowest rate of grade II-IV aGVHD (39%). (B) Probability of TRM stratified by donor KIR3DS1 copy number. TRM is similarly affected by donor KIR3DS1 copy number; the lowest TRM is associated with donor KIR3DS1 homozygosity (31%, P = .02). (C) Kaplan-Meier survival analysis demonstrating an association of overall survival with donor KIR3DS1 copy number (P = .03). (D) There is no association of donor KIR3DS1 with risk of relapse (P = .82).
Because KIR3DS1 defines a KIR B haplotype, we investigated the possibility that KIR3DS1 is not directly responsible for lower rates of acute GVHD, but rather a marker of a partial or full KIR haplotype that contains the causative immune-regulatory locus. Donor KIR B haplotypes as a group (AB, BX; n = 691) were associated with a lower rate of grade II-IV acute GVHD compared with the donor AA haplotype (OR = 0.85; 95% CI, 0.66-1.11, P = .22), but the effect was weaker than the association of KIR3DS1 alone, and not statistically significant. The frequent KIR2DS2-containing donor KIR B haplotypes and donor KIR AA haplotypes were associated with a comparable rate of acute grade II-IV GVHD (HR = 1.03; 95% CI, 0.76-1.39, P = .87). Of the other activating KIR examined, only KIR2DS1, with its strong positive linkage disequilibrium with KIR3DS1,14 was associated with decreased grade II-IV acute GVHD (OR = 0.83; 95% CI, 0.63-1.08, P = .16); but after adjusting for donor KIR3DS1, the association disappeared (OR = 1.12). The specific link between donor KIR3DS1 and GVHD suggests that individual activating KIR may play distinct biologic roles in HSCT, consistent with prior reports of associations between donor KIR2DS2 with relapse-free survival for AML12 and donor KIR2DS4/2DS2 with less cytomegalovirus reactivation.16
Although the ligand for KIR3DS1 is unknown, the high degree of structural homology in the extracellular domains of KIR3DL1 and KIR3DS1 suggests that KIR3DS1 may similarly bind HLA-Bw4 with subsequent clinical impact.17–22 We therefore tested the hypothesis that recipient HLA-Bw4 enhances the impact of donor KIR3DS1 on GVHD. Surprisingly, independent of donor KIR3DS1, patients with HLA-Bw4 had a suggestively lower rate of grade II-IV acute GVHD compared with patients lacking HLA-Bw4 (OR = 0.81; 95% CI, 0.64-1.04, P = .10), indicating a protective effect of the HLA-Bw4 epitope alone. Accordingly, GVHD protection was greater among HLA-Bw4+ recipients with KIR3DS1+ donors, compared with HLA-Bw4− recipients (OR = 0.67 and OR = 0.78, respectively). A formal test of interaction, however, failed to demonstrate a synergistic effect between KIR3DS1 and HLA-Bw4 (P = .57).
Using a large cohort of patients, we demonstrate that individual donor activating KIR, recipient HLA class I ligands, and donor KIR gene copy number all impact KIR-driven NK effects. We also show that not all KIR B haplotypes have equivalent clinical impact, and we propose that future studies consider specific B haplotype subsets or individual KIR genes in their analyses. Clinically, these data support the selection of unrelated donors with KIR3DS1 as a strategy for protection from GVHD without compromising relapse-free survival.
Acknowledgments
The authors thank Dr Mary Horowitz (CIBMTR) for assistance in study design, and Ms Clara Pinto and Ms Reenat Hassan for technical assistance.
This work was supported in part by the National Institutes of Health (UO1 AI 069197 and NIH KL2RR024996) and the Clinical and Translation Science Center at Weill Cornell Medical College.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: J.M.V. and K.C.H. conceived and designed the project, performed the data analysis and interpretation, and prepared the manuscript; T.A.G. collected the data and performed the statistical analysis; S.S. collected and assembled the data; J.P., M.M., and E.P. assisted in data collection and assembly; B.D. assisted with data analysis; and all authors approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Katharine C. Hsu, Box 336, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, New York, NY10065; e-mail: hsuk@mskcc.org.
References
- 1.Ljunggren HG, Malmberg K. Prospects for the use of NK cells in immunotherapy of human cancers. Nat Rev Immunol. 2007;7(5):329–339. doi: 10.1038/nri2073. [DOI] [PubMed] [Google Scholar]
- 2.Fernandez N, Treiner E, Vance R, Jamieson A, Lemieux S, Raulet D. A subset of natural killer cells achieves self-tolerance without expressing inhibitory receptors specific for self-MHC molecules. Blood. 2005;105(11):4416–4423. doi: 10.1182/blood-2004-08-3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu J, Heller G, Chewning J, Kim S, Yokoyama W, Hsu K. Hierarchy of the human natural killer cell response is determined by class and quantity of inhibitory receptors for self-HLA-B and HLA-C ligands. J Immunol. 2007;179(9):5977–5989. doi: 10.4049/jimmunol.179.9.5977. [DOI] [PubMed] [Google Scholar]
- 4.Leung W, Iyengar R, Turner V, et al. Determinants of antileukemia effects of allogeneic NK cells. J Immunol. 2004;172(1):644–650. doi: 10.4049/jimmunol.172.1.644. [DOI] [PubMed] [Google Scholar]
- 5.Hsu K, Keever-Taylor C, Wilton A, et al. Improved outcome in allogeneic hematopoietic stem cell transplantation in acute myelogenous leukemia (AML) predicted by donor KIR genotype and recipient HLA genotype in T-cell depleted HLA-identical sibling transplants. Blood. 2005;105(12):4878–4884. doi: 10.1182/blood-2004-12-4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsu K, Gooley T, Malkki M, et al. KIR ligands and prediction of relapse after unrelated donor hematopoietic cell transplantation for hematologic malignancy. Biol Blood Marrow Transplant. 2006;12(8):828–836. doi: 10.1016/j.bbmt.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Cook M, Briggs D, Craddock C, et al. Donor KIR genotype has a major influence on the rate of cytomegalovirus reactivation following T-cell replete stem cell transplantation. Blood. 2006;107(3):1230–1232. doi: 10.1182/blood-2005-03-1039. [DOI] [PubMed] [Google Scholar]
- 8.Miller J, Cooley S, Parham P, et al. Missing KIR-ligands is associated with less relapse and increased graft versus host disease (GVHD) following unrelated donor allogeneic HCT. Blood. 2007;109(11):5058–5061. doi: 10.1182/blood-2007-01-065383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gagne K, Brizard G, Gueglio B, et al. Relevance of KIR gene polymorphisms in bone marrow transplantation outcome. Hum Immunol. 2002;63(4):271–280. doi: 10.1016/s0198-8859(02)00373-7. [DOI] [PubMed] [Google Scholar]
- 10.De Santis D, Bishara A, Witt CS, et al. Natural killer cell HLA-C epitopes and killer cell immunoglobulin-like receptors both influence outcome of mismatched unrelated donor bone marrow transplants. Tissue Antigens. 2005;65(6):519–528. doi: 10.1111/j.1399-0039.2005.00396.x. [DOI] [PubMed] [Google Scholar]
- 11.Yabe T, Matsuo K, Hirayasu K, et al. Donor killer immunoglobulin-like receptor (KIR) genotype-patient cognate KIR ligand combination and antithymocyte globulin preadministration are critical factors in outcome of HLA-C-KIR ligand-mismatched T cell-replete unrelated bone marrow transplantation. Biol Blood Marrow Transplant. 2008;14(1):75–87. doi: 10.1016/j.bbmt.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 12.Cooley S, Trachtenberg E, Bergemann T, et al. Donors with group B KIR haplotypes improve relapse-free survival after unrelated hematopoietic cell transplantation for acute myelogenous leukemia. Blood. 2008;113(3):726–732. doi: 10.1182/blood-2008-07-171926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verheyden S, Schots R, Duquet W, Demanet C. A defined donor activating natural killer cell receptor genotype protects against leukemic relapse after related HLA-identical hematopoietic stem cell transplantation. Leukemia. 2005;19(8):1446–1451. doi: 10.1038/sj.leu.2403839. [DOI] [PubMed] [Google Scholar]
- 14.Hsu KC, Liu X, Selvakumar A, Mickelson E, O'Reilly RJ, Dupont B. Killer Ig-like receptor haplotype analysis by gene content: evidence for genomic diversity with a minimum of six basic framework haplotypes, each with multiple subsets. J Immunol. 2002;169(9):5118–5129. doi: 10.4049/jimmunol.169.9.5118. [DOI] [PubMed] [Google Scholar]
- 15.Savani B, Mielke S, Adams S, et al. Rapid natural killer cell recovery determines outcome after T-cell-depleted HLA-identical stem cell transplantation in patients with myeloid leukemias but not with acute lymphoblastic leukemia. Leukemia. 2007;21(10):2145–2152. doi: 10.1038/sj.leu.2404892. [DOI] [PubMed] [Google Scholar]
- 16.Zaia JA, Sun JY, Gallez-Hawkins GM, et al. The effect of single and combined activating killer immunoglobulin-like receptor genotypes on cytomegalovirus infection and immunity after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2009;15(3):315–325. doi: 10.1016/j.bbmt.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gumperz J, Litwin V, Phillips J, Lanier L, Parham P. The Bw4 public epitope of HLA-B molecules confers reactivity with NK cell clones that express NKB1, a putative HLA receptor. J Exp Med. 1995;181(3):1133–1144. doi: 10.1084/jem.181.3.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin MP, Gao X, Lee JH, et al. Epistatic interaction between KIR3DS1 and HLA-B delays the progression to AIDS. Nat Genet. 2002;31(4):429–434. doi: 10.1038/ng934. [DOI] [PubMed] [Google Scholar]
- 19.Alter G, Rihn S, Walters K, et al. HLA class I subtype-dependent expansion of KIR3DS1+ and KIR3DL1+ NK cells during acute human immunodeficiency virus type 1 infection. J Virol. 2009;83(13):6798–6805. doi: 10.1128/JVI.00256-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stewart C, Laugier-Anfossi F, Vély F, et al. Recognition of peptide-MHC class I complexes by activating killer immunoglobulin-like receptors. Proc Natl Acad Sci U S A. 2005;102(37):13224–13229. doi: 10.1073/pnas.0503594102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khakoo S, Thio C, Martin M, et al. HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science. 2004;305(5685):872–874. doi: 10.1126/science.1097670. [DOI] [PubMed] [Google Scholar]
- 22.Alter G, Martin M, Teigen N, et al. Differential natural killer cell-mediated inhibition of HIV-1 replication based on distinct KIR/HLA subtypes. J Exp Med. 2007;204(12):3027–3036. doi: 10.1084/jem.20070695. [DOI] [PMC free article] [PubMed] [Google Scholar]