Abstract
Background:
Age is a major risk for cardiovascular diseases. Although mitochondrial reactive oxygen species (ROS) have been proposed as one of the causes of aging, their role in cardiac aging remains unclear. We have previously shown that overexpression of catalase targeted to mitochondria (mCAT) prolongs murine median lifespan by 17-21%.
Methods and Results:
We used echocardiography to study cardiac function in aging cohorts of wild type (WT) and mCAT mice. Changes found in WT mice recapitulate human aging: age-dependent increases in left ventricular mass index (LVMI) and left atrial dimension, worsening of the myocardial performance index (MPI), and a decline in diastolic function. Cardiac aging in mice is accompanied by accumulation of mitochondrial protein oxidation, increased mitochondrial DNA mutations and deletions and mitochondrial biogenesis, increased ventricular fibrosis, enlarged myocardial fiber size, decreased cardiac SERCA2 protein and activation of the calcineurin-NFAT pathway. All of these age-related changes were significantly attenuated in mCAT mice. Analysis of survival of 130 mice demonstrated that echocardiographic cardiac aging risk scores were significant predictors of mortality. The estimated attributable risk to mortality for these two parameters was 55%.
Conclusion:
This study shows that cardiac aging in the mouse closely recapitulates human aging and demonstrates the critical role of mitochondrial ROS in cardiac aging and the impact of cardiac aging on survival. These findings also support the potential application of mitochondrial antioxidants in ROS-related cardiovascular diseases.
Keywords: aging, diastole, mitochondria, oxidant stress, survival
Cardiovascular diseases are highly prevalent in the geriatric population. Age is a major risk factor for cardiovascular disease, at least in part because it prolongs exposure to hypertension, diabetes, hypercholesterolemia and other cardiovascular risks. However, intrinsic cardiac aging, the slowly progressive structural changes and functional declines with age, also makes the heart more susceptible to stress and contributes to increased cardiovascular mortality and morbidity in the elderly.
Epidemiological data from the Framingham Study and the Baltimore Longitudinal Study on Aging showed that in healthy populations there is an age-dependent increase in the prevalence of echocardiographic left ventricular hypertrophy (LVH), a decline in diastolic function, relatively preserved systolic function (ejection fraction) at rest, but a decline in exercise capacity (maximal ejection fraction) and an increased prevalence of atrial fibrillation1. LVH is well known to increase the risk of coronary heart disease, stroke and sudden death. Diastolic heart failure, heart failure with diastolic dysfunction but preserved systolic function, is a major contribution to congestive heart failure and exercise intolerance in the elderly population. Given demographic projections, heart failure is likely to become the major cause of hospital admissions and mortality in North America2.
According to the mitochondrial variant of the free radical theory of aging, reactive oxygen species (ROS) produced mainly in the mitochondria attack mitochondrial constituents, causing mitochondrial DNA (mtDNA) damage and mitochondrial dysfunction, leading to further production of ROS, increases in oxidative damage to lipids and proteins and declines of cellular and organ function that contribute to death3. We have recently shown that while both point mutations and deletions in mtDNA accumulate with aging, mtDNA deletions are likely to drive the aging process4. As a vital organ rich in mitochondria and high in oxygen utilization, the heart is especially prone to oxidative damage. Together with the fact that cardiovascular diseases are the leading cause of death in humans, we hypothesized that in the absence of other cardiovascular risks, cardiac aging would be a predictor of mortality.
In this report we have made use of a transgenic mouse model in which catalase is overexpressed and targeted to mitochondria (mCAT) in order to 1) clearly define cardiac aging phenotypes in a murine model of aging and investigate their plausible molecular mechanisms; 2) investigate the impact of reductions in mitochondrial ROS on cardiac aging; and 3) investigate the impact of cardiac aging on all-cause mortality.
Methods
See the online supplement for additional detail.
Animal Longevity Cohort and Echocardiography
C57Bl6 mice in the longevity cohort were maintained as described7. This study comprised a cross-sectional sample of 170 mice from this cohort with n=20-30 mice in each group. Mice were not subjected to any invasive experimentation other than echocardiography. Echocardiography was performed with Acuson CV-70 (Siemens) using standard imaging planes: M-mode, conventional and Tissue Doppler imaging. All protocols were approved by the University of Washington Institutional Animal Care and Use Committee.
Biochemical and Molecular Analysis
Gene expression, mitochondrial DNA copy number and mutation frequency assays were performed using quantitative PCR. Mitochondrial protein carbonyl and calcineurin activity assays were determined using commercial kits. Measurement of cardiac angiotensins was performed with a tandem quadrupole mass spectrometry coupled to a HPLC. For study of calcium-transients, cardiomyocytes were isolated by an enzymatic method, then loaded with Fluo-4 and electrically stimulated at 1Hz. Ca-transient fluorescence was collected using an IonOptix system5.
Statistics and Survival Analysis
Continuous variables with normal distribution are presented as mean ± standard error of the mean. Those significantly skewed are presented as boxplots (median, 25% and 75%) and an “x” within the box to indicate the mean. Standard t-tests, ANOVA and linear regression were applied as appropriate to detect the statistical differences between genotypes or age groups. P-values are shown uncorrected for multiple-testing. To be more conservative, a Bonferroni correction (n=2) can be applied to t-test comparisons between young wild-type (WT) vs. old WT and old WT vs. old mCAT groups, after which p<0.025 would be considered significant. To investigate the effect of cardiac aging on survival, we chose variables representing structure (LVMI) and function (MPI), a priori, to create a cardiac aging risk score. Univariate Cox regression was used to verify a strong association between these variables and mortality. We then included both age-adjusted functional decline (MPI) and structural changes (LVMI) in a multivariate analysis to generate the semi-parametric maximum likelihood estimate of the best fitting linear combination of MPI and LVMI score in predicting subsequent mortality (ß2 MPI + ß3 LVMI in model 1 Table 1). We define this linear combination to be the “cardiac aging risk score”; Kaplan Meier curves stratified on the risk score tertiles are used to illustrate the differences in risk as the score increases. Log rank test p-values serve as descriptive statistics for the strength of the differences in survival between tertiles. To approximate the attributable risk fraction of cardiac aging on mortality, we calculated the difference between the incidence of the all-cause mortality rate of mice with risk scores in the highest tertile and the incidence in those with scores in the middle and lowest tertiles6.
Table 1.
Cox regression models predicting mortality
| Predictors | Hazard Ratio |
p | 95% | Conf. Interval |
|---|---|---|---|---|
| Univariate Cox model | ||||
| Age (months) | 1.18 | <0.001 | 1.11 | 1.26 |
| Male vs. female | 1.20 | 0.46 | 0.74 | 1.94 |
| MPI* (0.1 unit) | 1.35 | <0.001 | 1.16 | 1.58 |
| LVMI† (mg/g) | 1.44 | 0.002 | 1.15 | 1.80 |
| Multivariate Cox model 1 | ||||
| Age (months) | 1.15 | <0.001 | 1.07 | 1.23 |
| MPI* (0.1 unit) | 1.26 | 0.005 | 1.07 | 1.48 |
| LVMI† (mg/g) | 1.12 | 0.41 | 0.86 | 1.45 |
| Multivariate Cox model 2 | ||||
| Age (months) | 1.16 | <0.001 | 1.09 | 1.24 |
| Cardiac aging score T2‡ | 1.80 | 0.067 | 0.96 | 3.39 |
| Cardiac aging score T3‡ | 2.88 | 0.003 | 1.43 | 5.82 |
The hazard ratio (HR) rises by a factor of 1.35 for every 0.1 unit difference in MPI
HRs associated with LVMI are calculated as for MPI.
Cardiac aging risk score = ß2 MPI + ß3 LVMI, calculated from the multivariate Cox model 1, and presented as middle (T2) or high (T3) vs. lower (T1) tertiles.
Results
MCAT attenuates the cardiac aging phenotype
There were no differences in body weight or food consumption between mCAT and WT mice7. There were no significant differences with age or genotype in blood pressure, heart rate, pulse pressure, fasting plasma glucose or total-cholesterol in the mouse longevity cohort (STab 1). This is consistent with a low incidence of conventional cardiovascular risks in these C57Bl6 mice8.
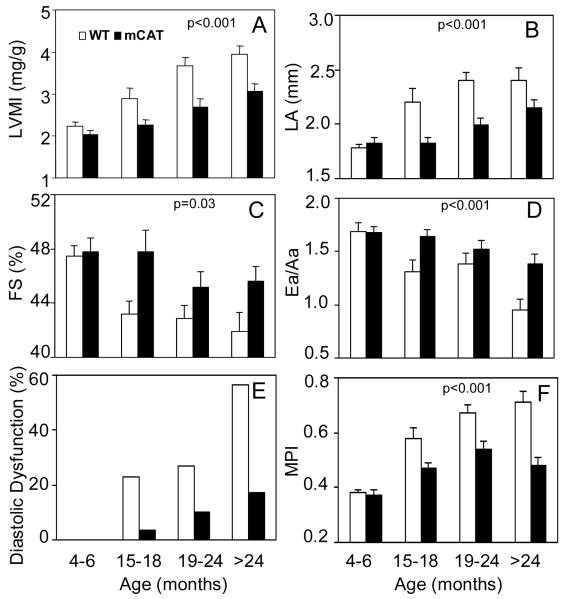
Echocardiography was performed in 90 mCAT and 80 WT littermate mice across a wide range of ages in a mouse longevity cohort. Despite the absence of cardiovascular risk factors, we found significant age-dependent linear trends for all parameters (p <0.001 for all except for FS, p=0.005). Left ventricular mass index (LVMI, Fig. 1A) was 76% higher in the oldest group compared to the young adult group. Left atrial dimension was significantly increased by 35% with age (Fig. 1B). Systolic function measured by fractional shortening showed a 12% decline from young adult to the oldest group (Fig. 1C). Tissue Doppler Imaging revealed an age-dependent decline in Ea/Aa, from 1.69 ± 0.3 in young adult to 0.95 ± 0.4 in the oldest (Fig. 1D). The prevalence of diastolic dysfunction, defined as Ea/Aa <19, was dramatically increased to 55% in the oldest age group (Fig. 1E). The myocardial performance index (MPI) was significantly increased (worsened) with age (Fig 1F), consistent with the age-related declines in systolic and diastolic function. These abnormalities mimic closely the age-related echocardiographic changes in human cardiac aging previously reported in healthy human populations1.
Figure 1. Echocardiography of mice from different age groups in the longevity cohort.
Age related changes in: A) LV mass index (mg/g body weight); B) LA dimension (mm); C) Fractional shortening (%); D) Ea/Aa measured by tissue Doppler imaging of the mitral annulus; E) Proportion of mice with diastolic dysfunction, defined as Ea/Aa < 1; F) Myocardial Performance index (MPI). There were significant linear increase across ages for all outcomes (p <0.001 for all except for FS where p=0.005, fit linearly to a continuous age function). MCAT mice had a significantly lower rate of progression with age in all of the above measurements when compared to WT (p<0.001 for all except for FS, p=0.03, for an interaction between age-slope and genotype). 90 mCAT and 80 littermate WT were examined, approximately 20 mice in each age and genotype group.
The age-related changes in all of the above echocardiographic findings were significantly delayed and attenuated in age-matched mCAT littermates. In the oldest age group, mCAT significantly reduced the age-related increase of LVMI by 39%, the percent of mice with diastolic dysfunction by 69%, the MPI by 67% and the age-related decrease in Ea/Aa by 59% (Fig. 1). The genotype difference in the linear rate of change with age (the genotype-by-slope interaction) was highly significant for all outcomes (p<0.001 for all except fractional shortening, p=0.03).
To confirm the protective effects of mCAT on cardiac aging, we performed a cross sectional study of an additional 42 old WT and mCAT C57BL6 mice (ages 27-29 months), in which cardiac tissue harvest was also performed within 24 hours after echocardiography. The protective effects of mCAT on echocardiographic parameters were fully reproduced in this independent data set (STab 2).
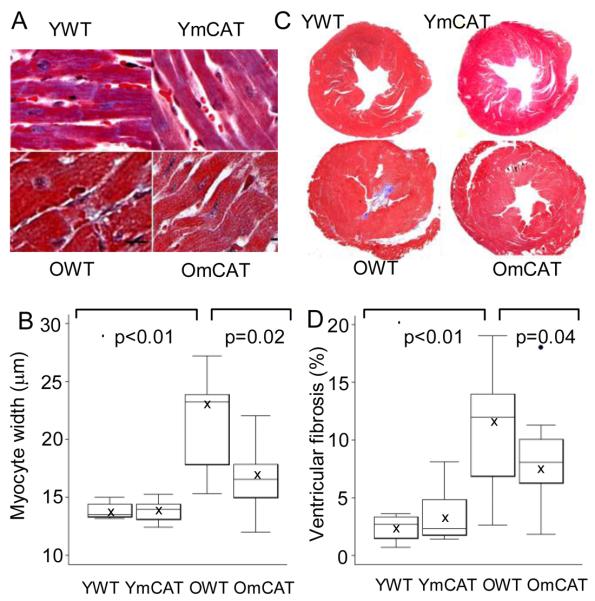
Histological analysis of tissue showed that compared with young WT (4 months old), old WT mice had significantly larger myocardial fiber width (Fig 2A, B) as well as a higher cross-sectional area with fibrosis (Fig 2C, D). Both cardiomyocyte hypertrophy and ventricular fibrosis were significantly attenuated in age-matched old mCAT mice. There was no genotype difference in young adults. Moreover, myocardial fiber width by image analysis was highly correlated with LVMI determined by echocardiography (R2=0.73, p<0.01, SFig 1).
Figure 2. Cross-sectional study of cardiac pathology.
A) Old WT (OWT) mice had larger myocardial fiber width than old mCAT (OmCAT) mice (trichrome stain, 400x, scale bar:10μm). B) Quantitative analysis showed a significant increase in myocardial fiber-width in aged heart, which was significantly attenuated in OmCAT mice. C) OWT mice had more fibrosis (blue; trichrome , 20x) than OmCAT mice, as shown by quantitative image analysis of the percent fibrotic area in aged hearts, which was significantly attenuated in OmCAT mice (D).
MCAT protects against mitochondrial oxidative damage and DNA mutations in the aged heart
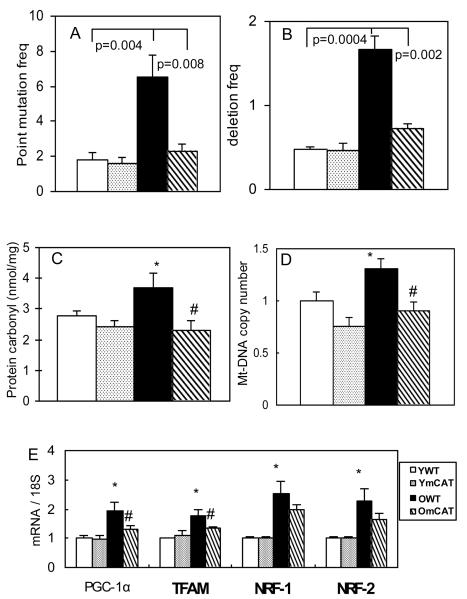
As an extension to our earlier findings that mCAT protected cardiac mitochondrial aconitase enzyme from oxidative damage7, we further found that protein carbonyls, an indicator of oxidative damage, increased significantly in cardiac mitochondria with age, and this was significantly reduced in old mCAT mice (Fig 3C). Aging is associated with increased mtDNA mutations and deletions4,10, as well as extensive morphological damage in mitochondria shown by electron micrographs (SFig 2 ). Using the newly developed “random mutation capture assay”, we found that cardiac mtDNA point mutation and deletion frequencies both significantly increased by approximately 3-fold with aging11. The mCAT genotype significantly reduced DNA point mutation and deletion frequencies to levels more comparable to those of young animals (Fig 3A,B). As oxidative stress has been shown to induce mitochondrial biogenesis12, we found that mitochondrial DNA copy number increased in aged heart (Fig 3D) and was accompanied by upregulation of transcription factors involved in mitochondrial biogenesis (Fig 3E). Most of these changes were significantly reduced in old mCAT, including an attenuated increase in mitochondrial DNA copy number, and reduced induction of level of PGC-1α and TFAM, but not NRF-1 and NRF-2 mRNA (Fig 3D,E).
Figure 3. Mitochondrial DNA mutations, oxidative damage and biogenesis in aged heart and the protective effect of mCAT.
A) Mitochondrial DNA point mutation and B) deletion frequencies (both per million) increased significantly with age and were better preserved in OmCAT mouse hearts. C) Mitochondrial protein carbonyls (nmol/mg) increased significantly in old age, while OmCAT mice were better protected from oxidative damage to mitochondrial proteins. D) Mitochondrial DNA copy number (normalized to young WT) increased significantly with age, and old mCAT mice had significantly lower mitochondrial DNA copy number. E). Genes involved in mitochondrial biogenesis, PGC-1α, TFAM, NRF-1 and NRF-2, were upregulated in aged heart, and changes in PGC-1α and TFAM were attenuated in old mCAT mice (*p<0.025 between young vs. old WT, #p<0.025 between old WT vs. mCAT; n=9-12 each group; old: 26-28 months, young:4-5 months old).
Plausible mechanisms of cardiac hypertrophy in the aged heart and the effect of mCAT
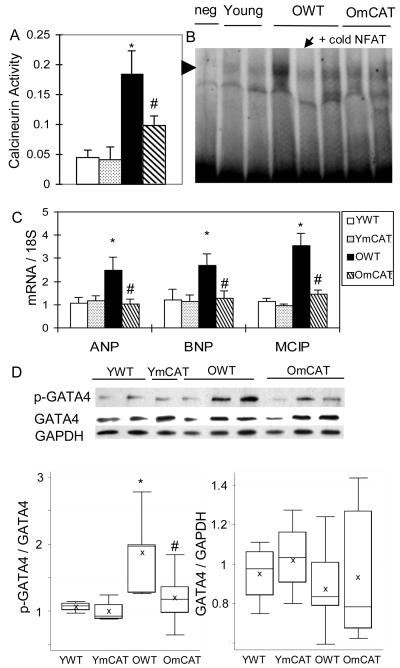
To investigate the potential mechanisms of aging-associated cardiac hypertrophy, we measured the activation of calcineurin-NFAT and phospho-ERK1/2 pathways, which are known to mediate pathological and compensated hypertrophy, respectively13. Both the phosphorylation of ERK1/2 and the total ERK1/2 protein levels did not change significantly with age (data not shown). However, we found that the calcineurin-NFAT pathway was activated in cardiac aging. Activity of calcineurin, a phosphatase that activates NFAT by dephosphorylation, was found to increase significantly with age (Fig 4A). Downstream to calcineurin, NFAT3 activity was increased in aged heart as shown by stronger DNA binding activity (Fig 4B) as well as increased transcription of NFAT target genes, including modulatory calcineurin interacting protein-1 (MCIP-1) and atrial and brain natriuretic peptides (ANP and BNP) (Fig 4C). Furthermore, we also found that GATA4, a cofactor of NFAT314, was activated in aged heart, as shown by increased phosphorylation of GATA4 at serine-105 (Fig 4D). The age-dependent activation of calcineurin-NFAT and GATA4 were significantly reduced in old mCAT hearts (Fig 4A-D).
Figure 4. Activation of calcineurin-NFAT and GATA4 in age-dependent left ventricular hypertrophy and the effect of mCAT.
A) Calcineurin phosphatase activity (nmol phosphate / 5 ug cardiac protein) increased significantly in aged heart and was partially protected in old mCAT hearts. B) Mobility shift assay showed that NFAT DNA-binding activity increased in old WT and the increase was less obvious in old mCAT hearts. Competitive DNA binding with excess unlabelled NFAT probe (arrow) using the leftmost old WT sample confirmed the specificity of the bands (arrowhead). C) The relative mRNA levels of ANP, BNP and MCIP, target genes of NFAT, increased significantly in old WT but not old mCAT hearts. D) Age-dependent increased phosphorylation of GATA-4 at Ser105 was reduced in old mCAT heart. *p<0.025 between YWT and OWT, # p<0.025 between OWT and OmCAT.
Plausible mechanisms of diastolic dysfunction in the aged heart and effect of mCAT
Several factors have been reported to cause diastolic dysfunction, including impaired myocardial relaxation due to decrease calcium reuptake and increased myocardial stiffness15. Increased fibrosis in the aged hearts and amelioration of this in mCAT mice were previously described above.
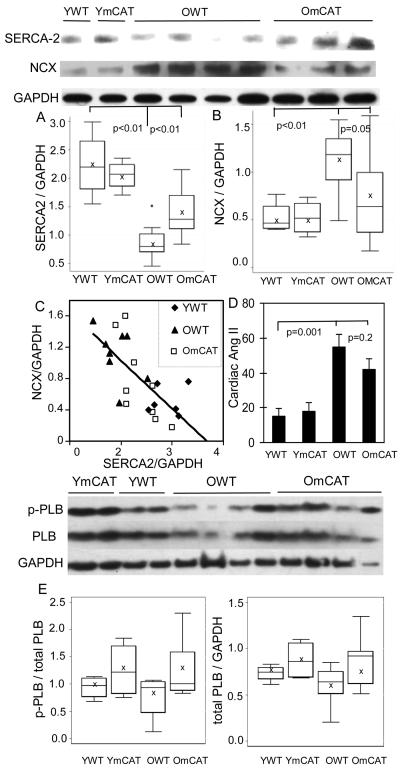
Sarco(endo)plasmic reticulum Ca ATP-ase (SERCA-2) and the sodium calcium exchanger (NCX) are key proteins in calcium reuptake to assist myocardial relaxation during the diastolic phase. These were examined by Western blots in the aged hearts. Quantitative analysis revealed that SERCA-2 protein decreased by 52% in the aged WT heart compared with young WT heart (p<0.01). SERCA2 was much better preserved in the old mCAT heart compared to old WT littermates (Fig. 5A). NCX protein increased by 103% in the aged WT heart compared with the young WT heart (Fig 5B). The increase was significantly less in old mCAT hearts compared to old WT hearts. Interestingly, we found an inverse correlation between the level of NCX and SERCA-2 proteins (Fig 5C). This is a novel observation and suggests that NCX may be up-regulated in the aged heart to compensate for the decline in SERCA-2. Levels of total and phosphorylated phospholamban, which inhibits SERCA-2 activity in its dephosphorylated form, did not change significantly with age (Fig 5E).
Figure 5. Calcium handling proteins and cardiac angiotensin-II.
Western blots of calcium handling proteins were quantified relative to GAPDH. A) SERCA2 protein was significantly reduced in aged WT heart, and better preserved in old mCAT heart; B) NCX protein was significantly increased in aged heart, and lower in old mCAT compared with old WT; C) Linear regression demonstrates an inverse correlation between SERCA2 and NCX protein levels (all ages: β= −0.61, R2=0.49, p<0.01; old mice only: β= −0.82, R2=0.52, p=0.001). D) Cardiac angiotensin II concentration (fmol/mg total protein) measured by mass spectrometry was significantly increased with age in both WT and mCAT mice. E) Phosphorylated and total protein levels of phospholamban did not change significantly with age or mCAT expression.
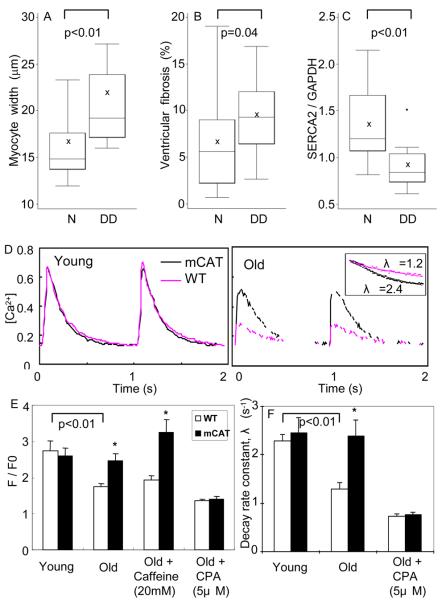
To further investigate plausible mechanisms of diastolic dysfunction in the aged heart, we analyzed the relationships of myocardial fiber width, ventricular fibrosis and SERCA-2 protein to diastolic dysfunction. Univariate analysis showed that cardiomyocyte hypertrophy and ventricular fibrosis were significantly associated with the presence of diastolic dysfunction (Fig 6A, B). These findings were consistent with the fact that both ventricular hypertrophy and fibrosis would increase myocardial stiffness. The level of SERCA-2 protein was dramatically decreased in mice with age-related diastolic dysfunction (Fig 6C). Multivariate regression modeling showed that SERCA-2 was the strongest predictor of Ea/Aa, an indicator of diastolic function (p=0.01, STab 3).
Figure 6. Plausible mechanisms of diastolic dysfunction in aged heart.
A) Myocardial fiber width was significantly greater in old mice with diastolic dysfunction (DD), compared with those with normal diastolic function (N). B) Ventricular fibrosis (%) was higher in old mice with diastolic dysfunction. C) SERCA2 protein was significantly decreased in mice with diastolic dysfunction. D) Representative calcium transients of isolated cardiomyocytes from young and old mouse hearts loaded with Fluo-4 and paced at 1 Hz. The inset illustrates OWT vs. OmCAT decay rates. E) Quantitative analysis showed significant decreases in Ca-transient amplitude (F/F0) and decay rate constant λ (s−1) in old WT, and protection in old mCAT cardiomyocytes. Sarcoplasmic reticulum Ca2+ load was also significantly higher in OmCAT cardiomyocytes, as shown by mobilization of Ca2+ by caffeine. The protective effect of mCAT was completely abolished by treating the old cardiomyocytes with cyclopyazonic acid, a SERCA-2 inhibitor.*p<0.025
To further investigate the role of SERCA2 in aging, we examined [Ca2+]i transients in ventricular myocytes isolated from mouse hearts, loaded with the Ca2+ indicator dye Fluo-4, and paced at 1Hz. Compared to young cardiomyocytes, old WT cardiomyocytes had significantly lower [Ca2+]i transients amplitude as well as slower rates of decay, while old mCAT cardiomyocytes had significantly preserved Ca-transients amplitude and decay rates (Fig 6D,E). Stimulation with caffeine, which opens ryanodine receptors and unload the SR calcium5, showed that old WT cardiomyocytes had significantly lower SR calcium loads. This is consistent with the lower [Ca2+]i transient amplitude observed in old WT cardiomyocytes. Inhibition of SERCA2 using cyclopyazonic acid (5 μM) completely abolished the beneficial effects of mCAT on calcium transient amplitude and rate of decay (Fig 6E, F), suggesting that the protective effect of mCAT in aged cardiomyocytes is mediated by better preservation of SERCA2 activity. Overall, the decline of ventricular SERCA2 thus appeared to be the most important factor associated with age-dependent cardiac diastolic dysfunction, presumably by causing impaired myocardial relaxation.
Increased cardiac angiotensin II in aging
Angiotensin II (Ang II) is known to induce cardiomyocyte hypertrophy and apoptosis, increase cardiac fibrosis and impair cardiomyocyte relaxation16, compatible with all of the above aging-related changes. We therefore measured the cardiac Ang II octapeptide in young and old LV tissue using mass spectrometry. Cardiac Ang II concentrations increased significantly with age (Fig 5D). mCAT did not appear to reduce the age-related increase in cardiac Ang II, suggesting that the mechanism of mCAT protection is downstream of Ang II.
Cardiac aging phenotype as predictors of follow-up mortality
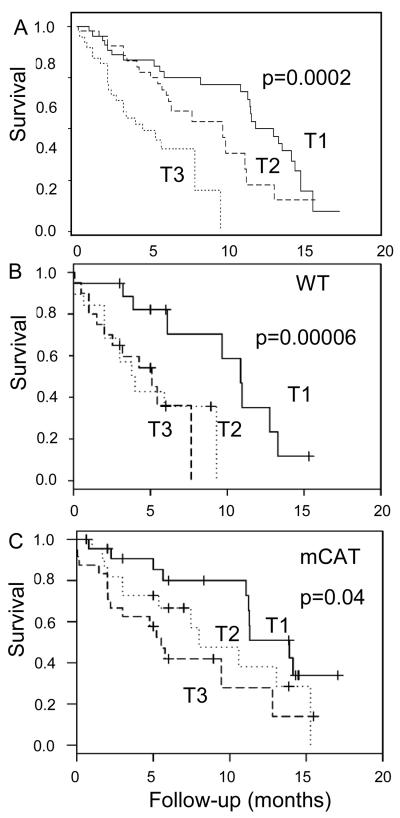
To investigate whether the cardiac aging phenotype determined by echocardiography is a predictor of follow-up mortality, we performed survival analysis of 130 old WT and mCAT mice that underwent echocardiography at mid-life (ages 16-24 mo) in the longevity cohort. Univariate Cox regression analyses verifies that age, MPI and LVMI were significant predictors of mortality (Table 1). A multivariate Cox model was used to estimate the best linear combination of these variables (ß2 MPI + ß3 LVMI) to create a cardiac aging risk score for each mouse based on these variables. Cox regression adjusted for age at the time of echocardiography (Cox model 2) verifies that this risk score provides a biologically meaningful measure of the risk of death; mice in the highest tertile (T3) had a significantly increased hazard of all-cause mortality, with a hazard ratio of 2.88 (95% CI 1.43, 5.82, p=0.003) compared with mice in the lowest tertile of cardiac aging score (T1)(Table 1 and Fig. 7A). As evidence for our hypothesis, we found a marked difference in the distribution of cardiac aging risk scores between mCAT and WT mice: at mid-life the proportions of mCAT mice in T1, T2 and T3 are 91%, 48% and 24%, vs. 9%, 52% and 76% for WT (χ2=39.4, p<10−7). Kaplan Meier analysis based on tertiles of cardiac risk scores calculated separately for WT and mCAT mice also stratified each of these genotypes into high and low mortality risk groups (Fig 7B and 7C, respectively).
Figure 7. Kaplan Meier analysis of follow-up mortality stratified by cardiac aging risk score tertiles.
Cardiac aging risk score was calculated as a linear combination of MPI and LVMI obtained from the multivariate Cox model 1 (ß2 MPI + ß3 LVMI). Mice with the higher tertile of cardiac aging score (T3) had significantly shorter survival than those with the lower tertile (T1), when scores were analyzed for WT and mCAT mice together (A) or separately for WT (B) and mCAT (C) mice.
To study the relative contribution of cardiac aging to mortality, we estimated the incidence of all-cause mortality in mice that reached their natural end-of-life. The incidence of mortality in the highest tertile of cardiac aging score T3 vs. those in the lower two tertiles of cardiac aging score were 16.2 % and 7.3%, respectively. The attributable risk fraction indicated that 55% of mortality risk was attributable to the cardiac aging risk score. This is the first such analysis of cardiac functional parameters with aging that has been reported. Thus, cardiac aging is a significant predictor of mortality in mice, despite the absence of hypertension, diabetes or hypercholesterolemia, and the cardiac aging phenotype appears to contribute to approximately half of the mortality risk in the mouse longevity cohort.
Discussion
Our results demonstrate that overexpression of catalase targeted to mitochondria (mCAT) protects mice from cardiac aging, providing direct evidence for the role of mitochondrial ROS in the aging of this vital organ. Several lines of evidence support this conclusion. In WT mice from the longevity cohort, we found age-dependent LVH and a decline in cardiac performance (especially diastolic function), concomitant with the accumulation of oxidized mitochondrial proteins, mtDNA mutations, increased ventricular fibrosis, cardiomyocyte hypertrophy, a decline of SERCA2 protein and activation of the calcineurin-NFAT pathway in the aged heart. These age-related alterations took place in the absence of significant cardiovascular risks, such as diabetes, hypertension or hypercholesterolemia, suggesting that these findings are primary changes of cardiac aging, rather than secondary to other diseases. MCAT littermates were partially protected from all of the above age-related cardiac alterations, suggesting that these aging changes are related in substantial part to chronic ROS exposure and emphasizing the role of mitochondrial ROS in cardiac aging. Interestingly, we did not observe any additional benefit in protection against cardiac aging in mice with higher versus lower mCAT cardiac expression (STab 2), which is consistent with our previous observation that lifespan is not associated with the level of mCAT expression7. We have suggested that the location of the catalase, i.e., targeting to mitochondria, is more important than the magnitude of catalase expression. Here we show that mtDNA point mutations and deletions in the heart increased significantly with age, but that the antioxidant function of mCAT protects cardiac mtDNA from these age-related mutations. These age-associated mtDNA point mutations had a spectrum similar to that observed in oxidative DNA damage4. Furthermore, as oxidative stress has been shown to induce mitochondrial biogenesis12, we also found that mt-DNA copies increased in aged heart, with upregulation of transcription factors involved in mitochondrial biogenesis. These phenomena were reduced in old mCAT mice, suggesting that mitochondrial biogenesis in the aging heart is mediated by oxidative damage to mitochondria.
To investigate the signaling in age-associated cardiac hypertrophy, we examined two major pathways of cardiac hypertrophy: MEK1/2-ERK1/2 and calcineurin pathways, which are central regulators of compensated and pathological hypertrophy, respectively13. While we did not find increased expression or phosphorylation of ERK1/2 in aging, we found that the calcineurin-NFAT pathway was significantly activated in the aged heart. Calcineurin activity was increased by approximately 4-fold in the aged heart (Fig 4A). This phosphatase activates the transcription factor NFAT. Activated NFAT translocates into nucleus where it interacts with the transcription factor (GATA4) to initiate transcription of hypertrophic fetal genes, such as ANP and BNP. EMSA showed that NFAT DNA binding activity is increased in aged heart (Fig 4B), consistent with increased mRNA levels of ANP, BNP and MCIP1 (Fig 4C). Furthermore, we found an age-dependent increase in GATA4 phosphorylation at Ser105 (Fig 4D), which has been reported to enhance its activity of DNA binding and transcription activation14. The activation of the calcineurin-NFAT pathway in age-associated cardiac hypertrophy was partially protected by mCAT, suggesting an interaction between ROS and hypertrophic signaling mediators. Though myocardial ROS has been implicated in generation of cardiac hypertrophy and dysfunction, as shown by the anti-hypertrophic effect of several antioxidants17,18, the in vivo evidence for direct links between hypertrophic mediators and ROS has been scant19. ROS might cause oxidative and nitrative modification of signaling proteins and may thereby modulate signal transduction, transcriptional and translational regulation20. However, further studies are needed to elucidate the exact mechanism of these interactions.
Upstream to the above signaling pathways, Ang II induces cardiomyocyte hypertrophy and apoptosis, increases cardiac fibrosis and impairs cardiomyocyte relaxation16, all consistent with our observed age-related changes. Inserra et al. proposed that Ang II is a crucial mediator of cardiovascular aging21. They recently showed that long-term inhibition of Ang II reduces cardiac pathology and prolongs rat survival22. Consistent with this, we found that cardiac Ang II concentrations increased significantly with age. Cardiac Ang II levels in old mCAT mice were similar to old WT mice despite mCAT protection against cardiac aging, suggesting that mCAT acts downstream of Ang II. Ang II is known to promote superoxide generation through NADPH oxidase, uncouple endothelial nitric oxide synthase and stimulate mitochondrial H2O2 production in vascular endothelial cells in vitro23. While blocking Ang II signaling provides survival benefit in patients with heart disease, the efficacy of such drugs to retard intrinsic cardiac aging remains unknown.
Diastolic dysfunction is well documented in human cardiac aging. Impaired myocardial relaxation due to functional decline in calcium handling proteins and increased myocardial stiffness related to cardiac hypertrophy and fibrosis are common causes of diastolic dysfunction. Among these, the decline in SERCA2 protein is the strongest predictor of diastolic dysfunction in aged mice (STab 3). We and others have found that old WT cardiomyocytes have a lower amplitude and prolonged decay rate of calcium transients24, and we have shown that old mCAT cardiomyocytes have calcium transient profiles more comparable to young cardiomyocytes (Fig 6E). Significantly lower SR calcium loads in old WT cardiomyocytes (shown by caffeine stimulation, Fig 6D) is best explained by chronic reduction of SERCA2 protein levels in old hearts, since dephosphorylated phospholamban did not change significantly with age (Fig 5E) and the beneficial effect of mCAT was completely abolished when SERCA2 was inhibited by cyclopyazonic acid (Fig 6E). Our data also shows that NCX is up-regulated in aged heart when SERCA2 is decreased. Such declines in SERCA2 and increases in NCX expression have been previously reported in cardiac aging in response to Ang II, as well as in heart failure25-27. Ren et al. reported that overexpression of catalase in cardiomyocytes attenuated aging-induced cardiomyocyte relaxation dysfunction by increasing NCX rather than preserving SERCA228. In contrast, mCAT preserves SERCA2 in old mice and up-regulation of NCX is less apparent.
Although mice are the best studied experimental model of mammalian aging, little evidence has directly linked the senescence of vital organs with survival. Echocardiographic data of our mouse longevity cohort suggested that both cardiac hypertrophy (increased LVMI) and functional decline (increased MPI) are significant predictors of mortality. We generated the cardiac aging risk score for each mouse in the cohort, estimated by the linear regression model of LVMI and MPI (Cox model 1, Table 1) and showed that the cardiac aging risk score is an independent predictor of mortality. Estimation by an epidemiological approach suggested that approximately half of natural death in the cohort was attributable to cardiac aging. Study of the end-of life pathology of this longevity cohort has indicated that cardiac pathology was a contributing cause of death in about half of C57BL6 mice29, consistent with the estimated attributable risk fraction for cardiac aging by echocardiography and suggesting that cardioprotection likely contributes to the lifespan extension previously observed in mCAT mice7. Such demonstration of improved organ function during aging may be even more relevant to human health than observations of extended longevity30, and this report shows that the murine model of cardiac aging is well suited for this approach. In conclusion, our data emphasizes the roles of mitochondrial ROS in cardiac aging, which is an independent predictor of mortality in mouse longevity cohort. This study also suggests potential clinical applications of mitochondrial-targeted antioxidant drugs in cardiovascular diseases, despite the disappointing results from non-targeted antioxidants.
Supplementary Material
Acknowledgments
Funding Sources
This work was supported by National Institute of Health Grants P30 AG013280 and P01 AG001751.
Footnotes
Disclosures: None
References
- 1.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease. Circulation. 2003;107:346–54. doi: 10.1161/01.cir.0000048893.62841.f7. [DOI] [PubMed] [Google Scholar]
- 2.Murphy SW. Diastolic Dysfunction. Curr Treat Options Cardiovasc Med. 2004;6:61–68. doi: 10.1007/s11936-004-0015-6. [DOI] [PubMed] [Google Scholar]
- 3.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–95. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Vermulst M, Bielas JH, Kujoth GC, Ladiges WC, Rabinovitch PS, Prolla TA, Loeb LA. Mitochondrial point mutations do not limit the natural lifespan of mice. Nat Genet. 2007;39:540–3. doi: 10.1038/ng1988. [DOI] [PubMed] [Google Scholar]
- 5.Dilly KW, Rossow CF, Votaw VS, Meabon JS, Cabarrus JL, Santana LF. Mechanisms underlying variations in excitation-contraction coupling across the mouse left ventricular free wall. J Physiol. 2006;572:227–41. doi: 10.1113/jphysiol.2005.102020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Etches V, Frank J, Di Ruggiero E, Manuel D. Measuring population health: a review of indicators. Annu Rev Public Health. 2006;27:29–55. doi: 10.1146/annurev.publhealth.27.021405.102141. [DOI] [PubMed] [Google Scholar]
- 7.Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, Wallace DC, Rabinovitch PS. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909–11. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- 8.Zheng F, Plati AR, Potier M, Schulman Y, Berho M, Banerjee A, Leclercq B, Zisman A, Striker LJ, Striker GE. Resistance to glomerulosclerosis in B6 mice disappears after menopause. Am J Pathol. 2003;162:1339–48. doi: 10.1016/S0002-9440(10)63929-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khouri SJ, Maly GT, Suh DD, Walsh TE. A practical approach to the echocardiographic evaluation of diastolic function. J Am Soc Echocardiogr. 2004;17:290–7. doi: 10.1016/j.echo.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 10.Vermulst M, Wanagat J, Kujoth GC, Bielas JH, Rabinovitch PS, Prolla TA, Loeb LA. DNA deletions and clonal mutations drive premature aging in mitochondrial mutator mice. Nat Genet. 2008;40:392–4. doi: 10.1038/ng.95. [DOI] [PubMed] [Google Scholar]
- 11.Bielas JH, Loeb LA. Quantification of random genomic mutations. Nat Methods. 2005;2:285–90. doi: 10.1038/nmeth751. [DOI] [PubMed] [Google Scholar]
- 12.St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jager S, Handschin C, Zheng K, Lin J, Yang W, Simon DK, Bachoo R, Spiegelman BM. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 13.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7:589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 14.Liang Q, Wiese RJ, Bueno OF, Dai YS, Markham BE, Molkentin JD. The transcription factor GATA4 is activated by extracellular signal-regulated kinase 1- and 2-mediated phosphorylation of serine 105 in cardiomyocytes. Mol Cell Biol. 2001;21:7460–9. doi: 10.1128/MCB.21.21.7460-7469.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure: Part II: causal mechanisms and treatment. Circulation. 2002;105:1503–8. doi: 10.1161/hc1202.105290. [DOI] [PubMed] [Google Scholar]
- 16.Rosenkranz S. TGF-beta1 and angiotensin networking in cardiac remodeling. Cardiovasc Res. 2004;63:423–32. doi: 10.1016/j.cardiores.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 17.Date MO, Morita T, Yamashita N, Nishida K, Yamaguchi O, Higuchi Y, Hirotani S, Matsumura Y, Hori M, Tada M, Otsu K. The antioxidant N-2-mercaptopropionyl glycine attenuates left ventricular hypertrophy in in vivo murine pressure-overload model. J Am Coll Cardiol. 2002;39:907–12. doi: 10.1016/s0735-1097(01)01826-5. [DOI] [PubMed] [Google Scholar]
- 18.Matsushima S, Ide T, Yamato M, Matsusaka H, Hattori F, Ikeuchi M, Kubota T, Sunagawa K, Hasegawa Y, Kurihara T, Oikawa S, Kinugawa S, Tsutsui H. Overexpression of mitochondrial peroxiredoxin-3 prevents left ventricular remodeling and failure after myocardial infarction in mice. Circulation. 2006;113:1779–86. doi: 10.1161/CIRCULATIONAHA.105.582239. [DOI] [PubMed] [Google Scholar]
- 19.McKinsey TA, Kass DA. Small-molecule therapies for cardiac hypertrophy: moving beneath the cell surface. Nat Rev Drug Discov. 2007;6:617–35. doi: 10.1038/nrd2193. [DOI] [PubMed] [Google Scholar]
- 20.Liu H, Colavitti R, Rovira II, Finkel T. Redox-dependent transcriptional regulation. Circ Res. 2005;97:967–74. doi: 10.1161/01.RES.0000188210.72062.10. [DOI] [PubMed] [Google Scholar]
- 21.Inserra F, Romano L, Ercole L, de Cavanagh EM, Ferder L. Cardiovascular changes by long-term inhibition of the renin-angiotensin system in aging. Hypertension. 1995;25:437–42. doi: 10.1161/01.hyp.25.3.437. [DOI] [PubMed] [Google Scholar]
- 22.Basso N, Cini R, Pietrelli A, Ferder L, Terragno NA, Inserra F. Protective effect of long-term angiotensin II inhibition. Am J Physiol Heart Circ Physiol. 2007;293:H1351–8. doi: 10.1152/ajpheart.00393.2007. [DOI] [PubMed] [Google Scholar]
- 23.Doughan AK, Harrison DG, Dikalov SI. Molecular Mechanisms of Angiotensin II Mediated Mitochondrial Dysfunction. Linking Mitochondrial Oxidative Damage and Vascular Endothelial Dysfunction. Circ Res. 2007 doi: 10.1161/CIRCRESAHA.107.162800. [DOI] [PubMed] [Google Scholar]
- 24.Lim CC, Apstein CS, Colucci WS, Liao R. Impaired cell shortening and relengthening with increased pacing frequency are intrinsic to the senescent mouse cardiomyocyte. J Mol Cell Cardiol. 2000;32:2075–82. doi: 10.1006/jmcc.2000.1239. [DOI] [PubMed] [Google Scholar]
- 25.Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part III: cellular and molecular clues to heart and arterial aging. Circulation. 2003;107:490–7. doi: 10.1161/01.cir.0000048894.99865.02. [DOI] [PubMed] [Google Scholar]
- 26.Houser SR, Piacentino V, 3rd, Weisser J. Abnormalities of calcium cycling in the hypertrophied and failing heart. J Mol Cell Cardiol. 2000;32:1595–607. doi: 10.1006/jmcc.2000.1206. [DOI] [PubMed] [Google Scholar]
- 27.Domenighetti AA, Wang Q, Egger M, Richards SM, Pedrazzini T, Delbridge LM. Angiotensin II-mediated phenotypic cardiomyocyte remodeling leads to age-dependent cardiac dysfunction and failure. Hypertension. 2005;46:426–32. doi: 10.1161/01.HYP.0000173069.53699.d9. [DOI] [PubMed] [Google Scholar]
- 28.Ren J, Li Q, Wu S, Li SY, Babcock SA. Cardiac overexpression of antioxidant catalase attenuates aging-induced cardiomyocyte relaxation dysfunction. Mech Ageing Dev. 2007;128:276–85. doi: 10.1016/j.mad.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Treuting PM, Linford NJ, Knoblaugh SE, Emond MJ, Morton JF, Martin GM, Rabinovitch PS, Ladiges WC. Reduction of age-associated pathology in old mice by overexpression of catalase in mitochondria. J Gerontol A Biol Sci Med Sci. 2008;63:813–22. doi: 10.1093/gerona/63.8.813. [DOI] [PubMed] [Google Scholar]
- 30.Butler RN, Miller RA, Perry D, Carnes BA, Williams TF, Cassel C, Brody J, Bernard MA, Partridge L, Kirkwood T, Martin GM, Olshansky SJ. New model of health promotion and disease prevention for the 21st century. Bmj. 2008;337:a399. doi: 10.1136/bmj.a399. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.